Abstract
Interleukin-8 (IL-8) has recently been shown to contribute to human melanoma progression by functioning as a mitogenic and angiogenic factor. In the present study, we investigated whether targeting IL-8 by a fully human anti-IL-8 antibody (ABX-IL8) could be a potential therapeutic strategy to control angiogenesis, growth, and metastasis of melanoma. The human melanoma cells A375SM (high IL-8 producer) and TXM-13 (intermediate IL-8 producer) were injected subcutaneously into nude mice, which were then treated with ABX-IL8 (1 mg/3 times weekly, i.p., for 3 weeks). Tumor growth of both melanomas in ABX-IL8-treated mice was significantly inhibited when compared with control IgG-treated animals. ABX-IL8 treatment also suppressed experimental metastasis when the melanoma cells were injected intravenously. IL-8 blockade by ABX-IL8 significantly inhibited the promoter activity and the collagenase activity of matrix metalloproteinase-2 in human melanoma cells, resulting in decreased invasion through reconstituted basement membrane in vitro. In vivo, ABX-IL8 treatment resulted in decreased expression of matrix metalloproteinase-2, and decreased vascularization (angiogenesis) of tumors concomitant with increased apoptosis of tumor cells. Moreover, in an in vitro vessel formation assay, ABX-IL8 directly interfered with the tubule formation by human umbilical vein endothelial cells. Taken together, these results point to the potential utility of ABX-IL8 as a modality to treat melanoma and other solid tumors either alone or in combination with conventional chemotherapy or other anti-tumor agents.
The incidence of malignant melanoma has increased in recent years, more than that of any other cancer in the United States. 1 Patients with advanced malignant melanoma have a poor prognosis, many dying from distant metastasis. 2,3 Considering that human malignant melanoma is highly resistant to conventional treatment, new treatment strategies are essential if the metastatic potential of this disease is to be blocked.
Recent studies have shown that the aggressive nature of human melanomas is related to several abnormalities in growth factors, cytokines, and their receptors. For example, human melanoma cells constitutively secrete the cytokine interleukin-8 (IL-8). 4 In fact IL-8, originally discovered as a chemotactic factor for leukocytes, may play an important role in the progression of human melanomas. 5-13 Several reports have demonstrated that the expression levels of IL-8 correlate with disease progression in human melanomas in vivo. 6-13 Serum levels of IL-8 are also elevated in patients with malignant melanoma. 10,11 Moreover, we have previously shown that transfection of nonmetastatic and IL-8-negative melanoma cells with the IL-8 gene rendered them highly tumorigenic and increased their metastatic potential in nude mice. 13
The role of IL-8 in melanoma progression has previously been primarily attributed to its ability to act as an autocrine growth factor for melanoma cells 4 and to induce haptotatic migration. 14 More recently, it has been shown that IL-8 also exhibits potent angiogenic activities both in vitro and in vivo. 15-18 The angiogenic activity of IL-8 produced by monocytes and macrophages was first demonstrated by Koch and colleagues. 15 They found that human recombinant IL-8 was potently angiogenic when implanted in a rat cornea and induced proliferation and chemotaxis of human umbilical vein endothelial cells (HUVECs). The involvement of IL-8 in tumor angiogenesis was first demonstrated in human bronchogenic carcinoma. 18 Tumor cell-derived IL-8 induces endothelial cell chemotaxis in vitro and corneal neovascularization in vivo. These observations have been confirmed in many other types of human tumors including melanomas. 19 Now IL-8 is considered to be one of the most potent angiogenic factors secreted by melanoma cells. The question of how IL-8 exerts its angiogenic activity, however, remains unknown. We have recently demonstrated that metastatic melanoma cells producing IL-8 or primary cutaneous melanoma (IL-8-negative) transfected with the IL-8 gene displayed up-regulation of matrix metalloproteinase (MMP)-2 expression and activity and increased invasiveness through Matrigel-coated filters. 13 Activation of MMP-2 by IL-8 can enhance the invasion of host stroma by tumor cells and increase angiogenesis and, hence, metastasis. In addition, IL-8 has been shown recently to act directly on vascular endothelial cells and to serve as a survival factor. 20 Thus, multiple mechanisms seem to be involved in IL-8 action, including direct effects on tumor and vascular endothelial cell proliferation, angiogenesis, and migration. These observations suggest that IL-8 could be a mediator of angiogenesis, tumor growth, and metastasis in melanoma and offered a potential target for immunotherapies against human melanomas.
In the present study, we used a fully human anti-IL-8 antibody (ABX-IL8, obtained from Abgenix, Inc., Fremont, CA) to neutralize the IL-8 secreted by melanoma cells and examine its effect on tumor growth. ABX-IL8 did not inhibit the proliferation of melanoma cells in vitro. However, ABX-IL8 suppresses the tumorigenicity and metastatic potential of metastatic human melanoma A375SM and TXM-13 cells in vivo. ABX-IL8 displayed potent inhibition of MMP-2 activity in melanoma cells, and inhibited the invasion of tumor cells through basement membrane in vitro. Inhibition of tumor growth and metastasis by ABX-IL8 in vivo correlated with decreased vascularization of melanomas in nude mice that was at least partially because of decreased MMP-2 expression. These results suggest that blocking of IL-8 by ABX-IL8 suppresses angiogenesis and metastasis of human melanoma. Thus, the human IL-8 neutralizing antibody ABX-IL8 may be beneficial for melanoma therapy either alone or in combination with other chemotherapeutic or anti-angiogenic agents.
Materials and Methods
Cell Lines and Culture Conditions
The human melanoma cell lines were originally isolated from different human patients. The A375 cell line was established from a lymph node metastasis. The highly metastatic A375SM line was established from a pool of lung metastases produced by A375 cells grown subcutaneously in nude mice. TXM-13 cells were isolated from the brain metastasis of a human patient.
All cell lines were maintained in cell culture as monolayers in Eagle’s minimal essential medium (MEM) supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, Hepes buffer, and penicillin-streptomycin, and incubated at 37°C with 5% CO2. All cultures were free of mycoplasma and pathogenic murine viruses.
ABX-IL8
ABX-IL8 is a human IgG2 monoclonal antibody directed against human IL-8 that was generated using Abgenix’s proprietary XenoMouse mice. The XenoMouse technology is one in which the murine heavy and light chain loci have been inactivated and subsequently replaced with a majority of human heavy- and kappa light-chain immunoglobulin loci. When immunized, these mice produce fully human antibodies. The mice used for this immunization contained only the human IgG2 heavy chain sequences and human kappa light chain. ABX-IL8 binds to human IL-8 with high affinity (kd = 2 × 1010 mol/L) and fails to cross-react with a panel of closely related chemokines. ABX-IL8 blocks the binding of IL-8 to IL-8 receptors and inhibits IL-8-dependent neutrophil activation, migration, and degranulation. 21 Chemopure human IgG control antibody was purchased from Jackson ImmunoResearch (West Grove, PA) and was used at the same concentration of ABX-IL8 in all experiments.
Effect of ABX-IL8 on Proliferation
Ninety-six-well plates containing 2000 cells/well from three melanoma cell lines treated with 100 μg/ml of ABX-IL8, IgG control antibody, or CMEM were cultured for 5 days and analyzed by MTT assay, which determines relative cell numbers based on the conversion of MTT to formazan in viable cells. MTT (40 μg/ml) was added to each well and incubated for 2 hours. The medium was removed and 100 μl of dimethyl sulfoxide was added to lyse cells and solubilize formazan. Absorbance was determined on a microplate reader.
Animals
Male athymic BALB/c nude mice were purchased from the Animal Production Area of the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). The mice were housed in laminar flow cabinets under specific pathogen-free conditions and used at 8 weeks of age. Animals were maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health.
In Vivo Tumor Growth and Metastasis
To prepare tumor cells for inoculation, cells in exponential growth phase were harvested by brief exposure to a 0.25% trypsin/0.02% ethylenediaminetetraacetic acid solution (w/v). The flask was sharply tapped to dislodge the cells and supplemented medium was added. The cell suspension was pipetted to produce a single-cell suspension. The cells were washed and resuspended in Ca2+/Mg2+-free Hanks’ balanced salt solution (HBSS) to the desired cell concentration. Cell viability was determined by trypan blue exclusion, and only single-cell suspensions of >90% viability were used. Subcutaneous tumors were produced by injecting 5 × 105 tumor cells/0.2 ml HBSS over the right scapular region of the mice. Growth of subcutaneous tumors was monitored by weekly examination of the mice and measurement of tumors with calipers. The mice were killed 2 months after injection, and tumors were processed for hematoxylin and eosin (H&E) staining.
For experimental lung metastasis, 0.5 to 1 × 106 tumor cells in 0.2 ml of HBSS were injected into the lateral tail vein of nude mice. The mice were killed after 60 days, and the lungs were removed, washed in water, and fixed with Bouin’s solution for 24 hours to facilitate counting of tumor nodules as described previously. 22 The number of surface tumor nodules was counted under a dissecting microscope. Sections of the lungs were stained with H&E to confirm that the nodules were melanoma and to monitor the presence of micrometases.
Both the subcutaneous and intravenous groups were treated every other day with either l mg or 100 μg of ABX-IL8 as indicated or with control IgG antibody by intraperitoneal injection.
Zymography
MMP-2 activity was determined on substrate-impregnated gels as described before 13 with minor modifications. Metastatic A375SM and TXM-13 cells (5 × 103) were plated in six-well plates and allowed to attach for 24 hours. Cells were treated with 100 μg/ml of ABX-IL8, 100 μg/ml of IgG, or CMEM for 5 days. Treatment for 5 days was found to be optimal for the antibody to affect MMP-2 activity. On day 5, CMEM was removed and replaced with serum-free medium overnight. The supernatant was collected, the volume adjusted for cell number, loaded, and separated on gelatin-impregnated (1 mg/ml; Difco, Detroit, MI) sodium dodecyl sulfate/8% polyacrylamide gels under nonreducing conditions, followed by 30 minutes of shaking in 2.5% Triton X-100 (BDH, Poole, UK). The gels were then incubated for 16 hours at 37°C in 50 mmol/L Tris, 0.2 mol/L NaCl, 5 mmol/L CaCl2, and 0.02% Brij 35 (w/v) at pH 7.6. At the end of the incubation, the gels were stained with 0.5% Coomassie G 250 (Bio-Rad, Richmond, CA) in methanol/acetic acid/H2O (30:10:60). The intensity of the various bands was determined on a computerized densitometer (type 300A; Molecular Dynamics, Sunnyvale, CA).
Invasion Assay through Matrigel
Invasion of highly metastatic A375SM and TXM-13 cells was measured by plating 5 × 103 cells on six-well plates and allowed to attach for 24 hours. After 5 days of treatment with 100 μg/ml of ABX-IL8, 100 μg/ml of IgG, or CMEM, cells were released from the plates by a brief exposure to trypsin-ethylenediaminetetraacetic acid (Life Technologies, Inc., Rockville, MD), counted, and centrifuged. Biocoat Matrigel invasion chambers (Becton-Dickinson, Mountain View, CA) were primed according to the manufacturer’s directions. A solution of 10% fetal bovine serum-CMEM was placed in the lower well to act as a chemoattractant, and 2.5 × 103 cells in 500 μl of serum-free medium with appropriate antibody were placed in the upper chamber of the Matrigel plate and incubated at 37°C for 22 hours. Cells on the lower surface of the filter were stained with Diff-Quick (American Scientific Products, McGraw Park, IL) and quantified with an image analyzer (Optomax V) attached to an Olympus CK2 microscope. The data were expressed as the average number of cells from 10 fields migrated to the lower surface of the filter in each of three experiments performed ± SD.
Transient Transfection and Luciferase Assays: Effect of ABX-IL8 on MMP-2 Promoter
The MMP-2 promoter construct was generated by cutting the MMP-2 promoter region, −390 to + 290, 23 out of p682 basic (CAT-driven MMP-2 promoter) 13 at the HindII/XbaI sites and ligating the region into pGEM-9Zf(−) vector (Promega, Madison, WI) using the same sites. The MMP-2 promoter region was then removed via the SpeI/SalI sites and ligated into the pGL3-Enhancer (Promega). 24 Melanoma cells were treated with 100 μg/ml of ABX-IL8, 100 μg of IgG, or CMEM for 4 days and then transfected with 10 ng of pB-actin-RL 22 and 2 μg of plasmid DNA consisting of either luciferase basic vector, SV40-positive control, or MMP-2 promoter vector, using 10 μl of Lipofectin Reagent (Life Technologies, Inc.). The medium was changed and treatments added after 12 hours. Cells were lysed and analyzed using Dual Luciferase Assay (Promega) and Ascent Lumiskan plate reader and software. 24
Effect of ABX-IL8 on Vessel-Like Tube Formation by HUVECs
The basement membrane-like substrate (Matrigel) induces HUVECs to rapidly form vessel-like tubes in vitro. 25 Because HUVECs express the receptors for IL-8 and IL-8 was shown to act as a survival factor for human endothelial cells, we analyzed the effect of ABX-IL8 on HUVEC tube formation. To that end, 24-well plates were coated with reconstituted Matrigel (Becton-Dickinson) following the manufacturer’s directions. HUVECs were pretreated with medium containing 100 μg/ml of ABX-IL8, 100 μg/ml of IgG, or MCBD (FIND) medium alone for 4 days. Treatment for 4 days was found to be optimal for the antibody to affect tube formation. Cells were briefly trypsinized, and 2 × 104 cells were added to each well and incubated at 37°C, 5% CO2, for 18 hours. Pictures were captured with bright-field microscopy using a Sony digital camera equipped with an Optimas 6.2 program.
In Situ Terminal dUTP Nick-End Labeling (TUNEL) Assay
Tissues were fixed in 10% buffered formalin solution and then embedded in paraffin. Thin sections (4 μm) were prepared and the TUNEL assay was performed using a commercial kit according to the manufacturer’s protocol (Promega). Briefly, tissue sections were deparaffinized and fixed at room temperature for 5 minutes in 4% paraformaldehyde. Cells were stripped of proteins by incubation for 10 minutes with 20 μg/ml of proteinase. The tissue sections were then permeabilized by incubating with 0.5% Triton X-100 in phosphate-buffered saline (PBS) for 5 minutes at room temperature. After being rinsed twice with PBS for 5 minutes, the slides were incubated with terminal deoxynucleotidyl transferase buffer for 10 minutes. Terminal deoxynucleotidyl transferase and buffer were then added to the tissue sections and incubated in a humid atmosphere at 37°C for 1 hour. The slides were washed for 5 minutes three times with PBS. Prolong solution (Molecular Probes, Eugene, OR) was used to mount the coverslips. Immunofluorescence microscopy was performed using a ×40 objective (Zeiss Plan-Neofluar) on an epifluorescence microscope equipped with narrow bandpass excitation filters mounted on a filter wheel (Lud1 Electronic Products, Hawthorne, NY) to select for green fluorescence. Images were captured using a cooled charge-coupled device camera (Photometrics, Tucson, AZ) and SmartCapture software (Digital Scientific, Cambridge, UK) on a Macintosh computer. Images were further processed using Adobe PhotoShop software (Adobe Systems, Mountain View, CA). Quantitation of TUNEL was determined by conventional diaminobenzidine staining. Results are presented as mean percentage ± SD of apoptotic cells from the total number of cells counted in eight fields per slide.
Immunohistochemistry
For CD31 and MMP-2 staining, sections of frozen tissues were prepared from tumor xenografts. The slides were then rinsed twice with PBS, and endogenous peroxidase was blocked by the use of 3% hydrogen peroxide in PBS for 12 minutes. The samples were then washed three times with PBS and incubated for 10 minutes at room temperature with a protein-blocking solution consisting of PBS (pH 7.5) containing 5% normal horse serum and 1% normal goat serum. Excess blocking solution was drained and the samples were incubated for 18 hours at 4°C with a 1:100 dilution of monoclonal rat anti-CD31 (1:100) antibody or anti-MMP-2 (1:100) (PharMingen, San Diego, CA). The samples were then rinsed four times with PBS and incubated for 60 minutes at room temperature with the appropriate dilution of peroxidase-conjugated anti-mouse IgG1, anti-rabbit IgG, or anti-rat IgG. The slides were rinsed with PBS and incubated for 5 minutes with diaminobenzidine (Research Genetics, Huntsville, AL). The sections were then washed three times with distilled water and counterstained with Gill’s hematoxylin. Sections (4 μm thick) of formalin-fixed, paraffin-embedded tumors were also stained with H&E for routine histological examination.
Statistical Analysis
The in vitro data were analyzed for significance by the Student’s t-test (two-tailed) and the in vivo data were analyzed by the Mann-Whitney test.
Results
Suppression of Tumorigenicity and Metastasis in Human Melanoma Cells by ABX-IL8
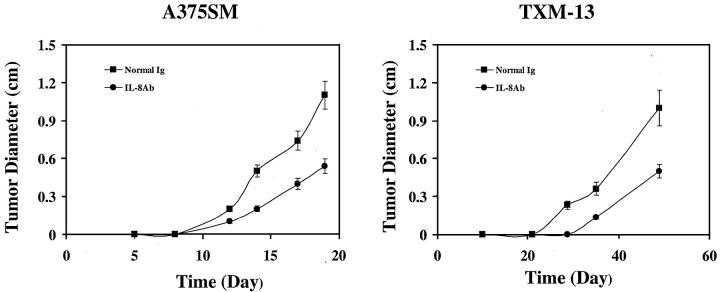
ABX-IL8 acts as a neutralizing antibody to IL-8. It binds to human IL-8 with high affinity and blocks the binding of IL-8 to its receptors. 21 In addition, ABX-IL8 inhibits IL-8-dependent neutrophil activation, migration, and degranulation. 21 In the first set of experiments, we determined the effect of ABX-IL8 on the tumor growth of human melanoma cells in nude mice. A375SM (high IL-8 producer) and TXM-13 (intermediate IL-8 producer) melanoma cells (5 × 105) were injected subcutaneously into nude mice (n = 5). Three days later, animals injected with tumor cells were subsequently injected with 1 mg of ABX-IL8 Ab or control IgG intraperitoneally every other day for 30 days. Tumor cells in the animals treated with control IgG grew progressively and produced large tumors reaching the size of 1.1 to 1.2 cm in mean diameter after 20 and 50 days, for A375SM and TXM-13, respectively (Figure 1) ▶ . In contrast, treatment with ABX-IL8 reduced tumor growth to ∼0.5 cm in mean diameter during the same periods of time for both A375SM and TXM-13 melanoma cells (Figure 1) ▶ .
Figure 1.
Effect of ABX-IL8 on tumor growth of human melanoma cells in nude mice. A375SM and TXM-13 cells (5 × 105) were injected subcutaneously into nude mice (n = 5). Three days later the mice were treated with ABX-IL8 (1 mg/3 times weekly, i.p.) or control IgG. The diameter of the subcutaneous tumors was determined every 3 to 5 days. Note that ABX-IL8 caused a significant reduction in A375SM and TXM-13 tumor growth. Data from one of two representative experiments.
To determine the effect of ABX-IL8 on metastasis of human melanoma cells, the A375SM (5 × 105) and TXM-13 (1 × 106) cells were injected intravenously into nude mice to produce experimental lung metastasis. Three days later, animals injected with tumor cells were also injected with ABX-IL8 or control IgG intraperitoneally every other day for 60 days. Two different doses of ABX-IL8 (100 μg or 1 mg) were used to treat animals injected with TXM-13 cells. As shown in Table 1 ▶ , both the incidence and number of lung metastasis of TXM-13 cells were reduced in ABX-IL8-treated mice, when compared with the control IgG-treated group. The higher dose of ABX-IL8 (1 mg) was more effective at inhibiting tumor metastasis. Therefore, we treated mice harboring the more aggressive A375SM cells with a 1 mg/dose of ABX-IL8 as described above. In IgG-treated mice, A375SM cells produced numerous lung metastases (median, 55; range, 30 to 65), whereas treatment with ABX-IL8 significantly inhibited the ability of A375SM cells to form metastasis in nude mice (median, 21; range, 0 to 48; P < 0.01). Collectively, these data demonstrate that treatment of mice with ABX-IL8 leads to suppression of tumor growth and metastasis.
Table 1.
Experimental Lung Metastasis of A375SM and TXM-13 Cells in Nude Mice after Treatment with ABX-IL8
| Cell line | Treatment | Metastasis | ||
|---|---|---|---|---|
| Median | Range | Incidence | ||
| TXM-13 | IgG | 6 | 1–19 | 5/5 |
| ABX-IL8 (1 mg) | 0* | 0–1 | 2/5 | |
| ABX-IL8 (100 μg) | 1* | 1–2 | 4/5 | |
| A375SM | IgG | 55 | 30–65 | 10/10 |
| ABX-IL8 (1 mg) | 21* | 0–48 | 8/10 | |
A375SM and TXM-13 cells were injected intravenously into groups of nude mice. Mice were treated with ABX-IL8 or control IgG. Experimental lung metastasis was determined 60 days after tumor inoculation.
*, P < 0.01 as determined by Mann-Whitney U test.
In Vitro Growth of Melanoma Cells Treated with ABX-IL8 Antibody
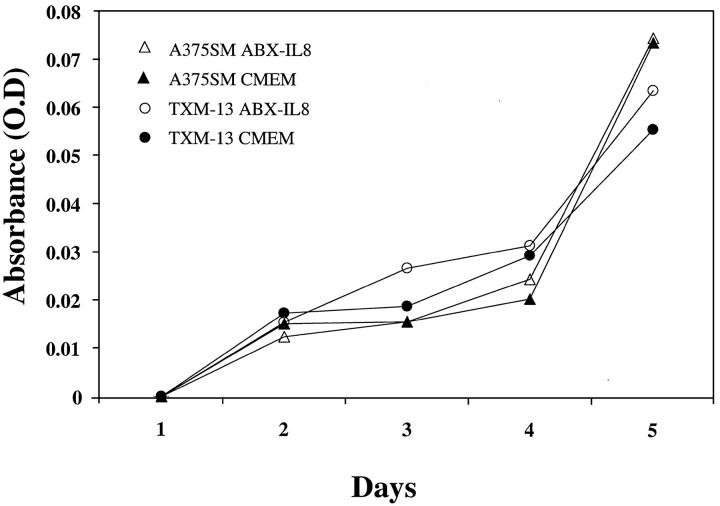
IL-8 has previously been shown to be an autocrine growth factor in various tumor cells. 4,26-29 Therefore, we tested whether ABX-IL8 had a direct effect on cell growth and proliferation of both melanoma cell lines in vitro. A375SM and TXM-13 melanoma cells were seeded into 96-well plates (5 × 103 cells/well) for 24 to 96 hours in the presence of 100 μg/ml of ABX-IL8 or control IgG. Cell proliferation was determined by MTT assay. The addition of IL-8-neutralizing antibody ABX-IL8 did not alter the cell growth in vitro (Figure 2) ▶ . These data suggest that ABX-IL8 did not have a direct effect on cell proliferation in vitro and that the inhibition in tumor growth and metastases formation observed in vivo may not be because of differences in cell division time.
Figure 2.
Effect of ABX-IL8 on proliferation of melanoma cells in vitro. A375SM and TXM-13 melanoma cells were treated with 100 μg/ml of ABX-IL8 or control IgG for 1 to 4 days. Cell proliferation was determined by the MTT assay. This is one of two representative experiments.
Down-Regulation of MMP-2 Activity in Human Melanoma Cells by ABX-IL8 In Vitro
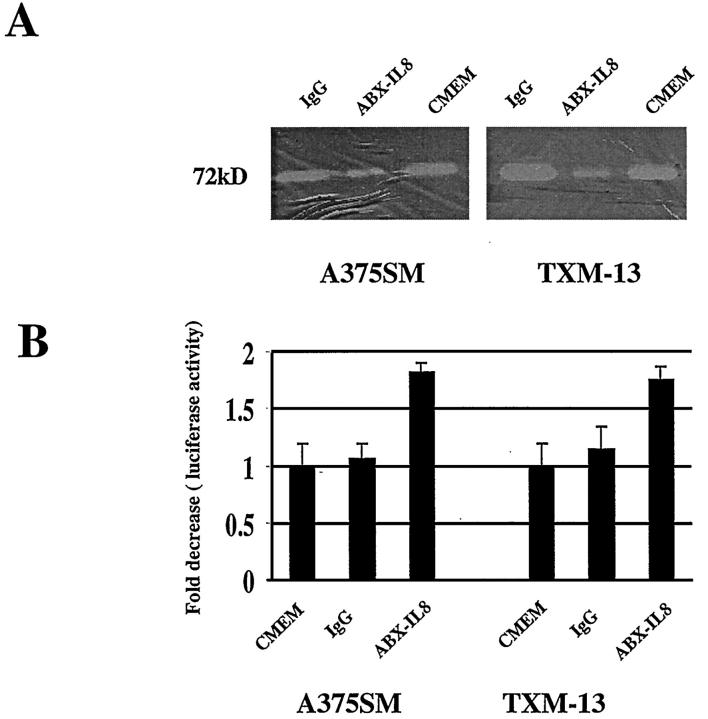
The metastatic potential of tumor cells depends on proper vascularization of the tumor and their ability to degrade type IV collagen. We recently demonstrated that IL-8 exerts its angiogenic activity through the induction of the metalloproteinase MMP-2 in melanoma cells. Activation of MMP-2 by melanoma cells may provide a mechanism for the increase in their metastatic potential. We therefore tested whether neutralizing IL-8 by ABX-IL8 had any effect on MMP-2 activity by melanoma cells. A375SM and TXM-13 cells were treated with ABX-IL8 (100 μg/ml) or control IgG for 5 days, and MMP-2 activity was analyzed by zymography after the volume of supernatant was normalized for cell number. As shown in Figure 3A ▶ , the 72-kd MMP-2 collagenase activity of ABX-IL-8-treated A375SM and TXM-13 cells was significantly decreased, compared with either IgG-treated or CMEM-treated control cells. As MMP-2 is the major metalloproteinase expressed and secreted by melanoma cells, we observed a faint band of MMP-9 activity in all melanoma cells tested regardless of treatment with ABX-IL8 (data not shown).
Figure 3.
A: Down-regulation of MMP-2 activity in melanoma cells by ABX-IL8 treatment in vitro. A375SM and TXM-13 melanoma cells were treated with ABX-IL8 (100 μg/ml) for 5 days and analyzed for MMP-2 activity by zymography. Note that ABX-IL8 suppressed MMP-2 activity in melanoma cells. B: Effect of ABX-IL8 on MMP-2 promoter activity. Melanoma cells were treated with 100 μg/ml of ABX-IL8, 100 μg/ml of control IgG, or CMEM for 4 days, and then the PGL3-MMP-2 reporter plasmid was co-transfected with the pB-Actin-RL reporter gene into the melanoma cells. Fold decrease in luciferase activity was calculated relative to the luciferase activity in untreated A375SM or TXM-13 cells, which were assigned the value of 1.
To examine the effect of ABX-IL8 on MMP-2 transcription, we next analyzed the promoter activity of MMP-2 in ABX-IL8-treated and control melanoma cells. A375SM and TXM-13 cells were transfected with MMP-2 promoter-driven luciferase reporter gene and treated with ABX-IL8 (100 μg/ml) or control IgG for 48 hours. Consistent with a decreased MMP-2 collagenase activity, MMP-2 promoter activity in ABX-IL8-treated A375SM and TXM-13 cells was decreased by 2.0 fold when compared to IgG-treated and untreated cells (Figure 3B) ▶ , respectively. These results suggest that IL-8 may directly regulate MMP-2 expression at the transcriptional level and that blocking IL-8 by ABX-IL8 suppressed MMP-2 expression in melanoma cells.
Suppression of Human Melanoma Cell Invasion by ABX-IL8
We next analyzed whether the decreased expression of MMP-2 in ABX-IL8-treated cells correlated with their ability to invade through the basement membrane, an important component in the process of tumor invasion and metastasis. To that end, 2.5 × 103 A375SM and TXM-13 melanoma cells that had been treated with 100 μg/ml of ABX-IL8 or control IgG for 5 days, were subsequently placed in the upper compartment of an invasion chamber in the presence of 100 μg/ml of ABX-IL8 or control IgG. After 22 hours of incubation, the cells on the lower surface of the filter were counted. As shown in Table 2 ▶ , A375SM and TXM-13 cells treated with ABX-IL8 exhibited a significant decrease in invasion through Matrigel-coated filter, when compared with IgG-treated or untreated cells (184 ± 3 versus 45 ± 10 for TXM-13, P < 0.05; and 125 ± 43 versus 12 ± 1, P < 0.01 for A375SM). These results indicate that blockade of IL-8 in melanoma cells by ABX-IL8 inhibited their ability to penetrate through the basement membrane. Collectively, our data indicate that inactivation of MMP-2 by ABX-IL8 in melanoma cells, may account for the decrease in metastatic potential.
Table 2.
Invasion of ABX-IL8-Treated Melanoma Cells through Matrigel-Coated Filters
| Cell lines | Treatment | Invasion cells (number/field ± SD) |
|---|---|---|
| TXM-13 | IgG | 184 ± 3 |
| TXM-13 | ABX-IL8 | 45 ± 10 (P < 0.05) |
| A375SM | IgG | 125 ± 43 |
| A375SM | ABX-IL8 | 12 ± 1 (P < 0.01) |
ABX-IL8-treated and untreated melanoma cells were assayed for their potential to penetrate through Matrigel-coated filters. Melanoma cells (2 × 105) were placed in the upper chamber. After 22 hours of incubation, the cells on the lower surface of the filter were stained and counted.
Decreased Expression of MMP-2 in Tumors Treated with ABX-IL8
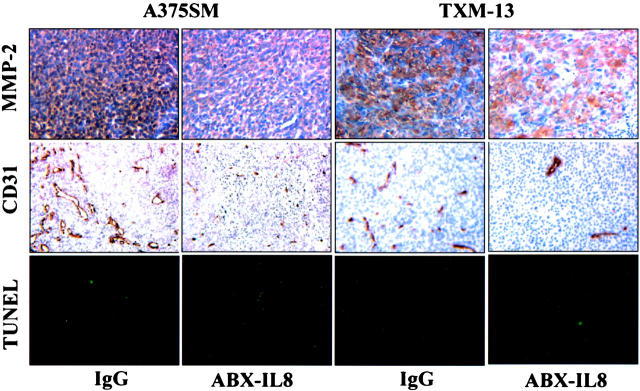
To determine whether ABX-IL8 suppressed the expression of MMP-2 in vivo, we performed immunohistochemical analysis on the melanoma derived from A375SM and TXM-13. The protein expression of MMP-2 in vivo was evaluated by immunohistochemistry using anti-MMP-2 antibodies. As shown in Figure 4 ▶ , MMP-2 staining was observed in IgG-treated A375SM and TXM-13 tumors, but was significantly decreased in ABX-IL8-treated tumors. Thus, blocking of IL-8 by ABX-IL8 antibody in melanoma cells inhibited the expression of MMP-2 gene in vivo as well as in vitro.
Figure 4.
MMP-2 expression, tumor MVD, and apoptosis (TUNEL) in subcutaneous melanoma xenografts. A375SM and TXM-13 cells were injected subcutaneously into groups of nude mice (n = 5). Mice were treated once a week with l mg of ABX-IL8 or control IgG antibody by intraperitoneal injection. Thirty to 60 days later, the resulting subcutaneous tumors with similar size were resected and processed for immunohistochemical analysis. Blood vessels were counted using a light microscope after immunostaining of sections with anti-CD31 antibodies. TUNEL assay and MMP-2 staining were performed as described in Materials and Methods.
Inhibition of Tumor Angiogenesis in ABX-IL8-Treated Mice
Because both IL-8 and MMP-2 are important angiogenic factors, we determined whether blockade of IL-8 by ABX-IL8 could result in suppression of tumor angiogenesis. Tumor-associated neovascularization as indicated by microvessel density (MVD) was determined by immunohistochemistry using anti-CD31 antibodies. As shown in Figure 4 ▶ , we found a significant reduction in tumor MVD per field after treatment with ABX-IL8 as compared with control IgG-treated tumors. The mean number of MVD was 16 ± 3 in ABX-IL8-treated A375SM tumors; and 14 ± 5 in ABX-IL8-treated TXM-13 tumors. In contrast, the mean number of MVD was 34 ± 4 and 41 ± 9 for control IgG-treated A375SM and TXM-13 tumors, respectively. Moreover, the number of TUNEL-positive tumor cells was inversely correlated with MVD in the studied tumors. The number of tumor cells undergoing apoptosis was higher in the ABX-IL8-treated animals than in tumors in IgG control mice (Figure 4) ▶ . The percentage of apoptotic cells was 21.9 ± 6.7% in ABX-IL8-treated A375SM; and 20.9 ± 6.1% in ABX-IL8-treated TXM-13 tumors. In contrast, the percentage of apoptotic cells was 1 ± 0.1% and 2.6 ± 1.8% for control IgG-treated A375SM and TXM-13 tumors, respectively. The above data indicate that ABX-IL8 treatment significantly decreased tumor-associated neovascularization and subsequently increased apoptosis of tumor cells.
Interference of Vessel-Like Tube Formation by HUVECs
Because HUVECs express the receptors for IL-8, and because IL-8 can act in an autocrine manner to affect their proliferation, we next analyzed the ability of ABX-IL8 to disrupt the Matrigel-induced formation of vessel-like tubes by HUVECs. To that end, HUVECs were pretreated with 100 μg/ml of ABX-IL8, 100 μg/ml of control IgG, or CMEM alone for 4 days and then plated on Matrigel to induce vessel-like tube formation. As shown in Figure 5 ▶ , CMEM-pretreated HUVECs formed lumen-like structures and anastomosing tubes with multicentric junctions. Similar endothelial cell structural morphogenesis occurred in IgG-pretreated HUVECs. In contrast, HUVECs pretreated with ABX-IL8 did not form typical vessel-like tube on Matrigel (Figure 5) ▶ . Furthermore, when ABX-IL8 (100 μg/ml) was added to the CMEM after tube formation by HUVECs had occurred, the morphogenesis of pre-existing vessel-like tubes was not altered. Our results thus demonstrate that ABX-IL8 directly inhibits de novo formation of capillary-like networks, but not pre-existing tubule networks.
Figure 5.
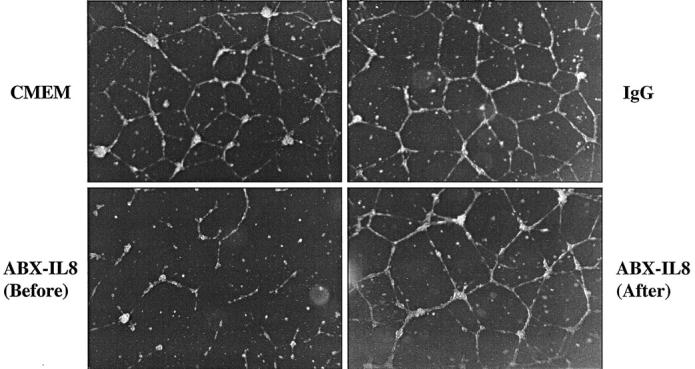
Effect of ABX-IL8 on vessel-like formation by HUVECs. HUVECs were pretreated with 100 μg/ml of ABX-IL8 [ABX-IL8 (before)], 100 μg/ml of IgG, or CMEM alone for 4 days, and then plated on Matrigel. In the panel labeled ABX-IL8 (after), 100 μg/ml of ABX-IL8 was added to the pre-existing tubes. This is a representative experiment of three performed.
Discussion
IL-8 has been shown to promote the growth, invasiveness, motility, angiogenesis, and metastatic potency of melanoma cells. 4-13,19 In this study, we demonstrate that blockade of IL-8 by neutralizing anti-IL-8 antibodies significantly inhibited the growth and metastasis of human melanoma cells in nude mice by suppressing angiogenesis and invasion.
Since the first report showing that IL-8 acts as a growth factor, 4 many recent studies have demonstrated that a variety of human tumor cells produce IL-8 and express the receptors for IL-8, thereby forming an autocrine growth stimulatory loop. However, the IL-8 dependence of cell growth in vitro varied widely among different tumor types. Some tumor cell lines were apparently dependent on IL-8 for proliferation, whereas others were not. 4,29 In our present study, we found that growth of A375SM and TXM-13 melanoma cells in vitro was not affected by the production of IL-8. This is based on our observation showing that ABX-IL8, an IL-8 neutralizing antibody, did not block the growth of these cells in vitro. However, in vivo, treatment with ABX-IL8 increased the number of apoptotic tumor cells (TUNEL-positive), and resulted in significant suppression of tumorigenicity and metastasis. These data indicate that the role of IL-8 in the progression of human melanoma is more complex than simple autocrine growth stimulation.
Indeed, we show that IL-8 has a significant effect on angiogenesis. Growth and metastasis of human melanoma cells depend on their ability to develop an adequate vasculature. In fact, the progression of neoplasms from the benign to malignant state is often associated with a switch to an angiogenic phenotype, representing an increase in proangiogenic molecules produced by the tumor cells and organ-specific environments. 30,31 Melanoma cells secrete a variety of proangiogenic molecules, including basic fibroblast growth factor, 32 vascular endothelial growth factor, 33 and IL-8. 4-10 We found that blockade of IL-8 by ABX-IL8 suppressed angiogenesis of human melanoma cells grown in nude mice. All of the control tumors were highly vascularized and produced large tumors, whereas the ABX-IL8-treated mice produced smaller tumors. The retarded tumor growth directly correlated with decreased blood vessel formation. One mechanism for the inhibition of angiogenesis could be attributable to down-regulation of MMP-2. MMP-2, the 72-kd type IV collagenase, is a member of a family of matrix metalloproteinases. The proteolytic effect of MMPs facilitates the migration of endothelial cells through the altered extracellular matrix toward the source of the angiogenic stimulus; in this manner, MMP-2 is an integral component of the angiogenesis pathway. 34 Moreover, in an in vitro endothelial cell morphogenesis assay (vessel-like tube formation assay), we found that ABX-IL8 directly inhibited the formation of capillary-like network by HUVECs. IL-8 receptor expression on endothelial cells has been demonstrated previously. 35 For example, human microvascular endothelial cells express the IL-8 receptors, which allows direct interaction of IL-8 with endothelial cells. 20 IL-8 may promote angiogenesis by supporting the survival of endothelial cells or inducing endothelial cell migration. 36 Thus, the concomitant decrease in expression of IL-8 and MMP-2, which serve as survival factors or migration factors for endothelial cells, could have contributed to the decrease in vessel density in ABX-IL8-treated tumors. It should be noted that treatment with ABX-IL8 did not affect the existing vessel-like tube formation in vitro and disrupted only the formation of the newly formed blood vessels in vivo. Although the effect of ABX-IL8 on melanoma cells is very well documented in our studies, its effect on angiogenesis needs to be further established. Nevertheless, ABX-IL8 may act on both tumor and vascular endothelial cells to affect tumor growth and metastasis of melanoma.
The potential of antibody therapy represented by ABX-IL8 fits recent discoveries. Antibody immunotherapy provides a novel approach for the treatment of a broad spectrum of diseases including cancer. 37-41 Cetuximab (IMC-C225), a mouse-human chimeric anti-EGFR monoclonal antibody, and ABX-EGF have been shown to inhibit the proliferation of a variety of cultured human tumor cell lines that overexpress EGFR and to inhibit tumor growth in several xenograft models. 40,41
Currently, both of these antibodies are being evaluated in clinical trials. The therapeutic modalities to control tumor growth and metastasis of human melanoma are very limited. 2 The idea of using fully humanized antibodies to neutralize IL-8 is especially appealing because multiple dose regimens of the antibody could be administrated to the patients with little risk of mounting an immune reaction. A phase II single-dose clinical trial and phase I/II multiple-dose clinical trial with ABX-IL8 have been conducted in patients with moderate to severe plaque psoriasis. Both trials were designed as dose-escalating trials to examine the safety of administering a range of dose levels of ABX-IL8 by intravenous infusion. ABX-IL8 was shown to be safe and well tolerated in both the single-dose and the multiple dose trials. No serious or unexpected adverse events have been reported, no immunogenicity has been detected, and there has been no evidence of cytokine release syndrome in either of the trials. Our studies should promote a serious consideration for initiating a phase I/II clinical trial with ABX-IL8 in patients with stage III melanoma. Because ABX-IL8 did not completely inhibit tumor growth and metastasis of melanoma, it should most likely be used in combination with chemotherapy or other anti-cancer agents to increase its efficacy. In addition, ABX-IL8 should be considered as a treatment modality for other solid tumors in which IL-8 plays an angiogenic role, including bladder, 42 prostate, 43,44 ovarian, 45 and lung 46 cancers.
Acknowledgments
We thank Dr. Corazon Bucana for her help in immunohistochemistry and TUNEL analyses, Patherine Greenwood for excellent preparation of this manuscript, and Mr. Walter Pagel for scientific editing.
Footnotes
Address reprint requests to Menashe Bar-Eli, Ph.D., Department of Cancer Biology, Box 173, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: mbareli@mail.mdanderson.org.
Supported in part by the National Institutes of Health (grant CA 76098) and the Eli Milbauer Memorial Research Fund.
This work is dedicated to Eli Milbauer who died from malignant melanoma.
References
- 1.: American Cancer Society: Cancer Facts and Figures, 2000. American Cancer Society,
- 2.Dooley T: Recent advances in cutaneous melanoma oncogenesis research. Oncol Res 1994, 6:1-9 [PubMed] [Google Scholar]
- 3.Fidler IJ: The biology of melanoma metastasis. Balch CM Houghton AN Milton GW Sober AJ Soong SJ eds. Cutaneous Melanoma, 1992, vol 2.:pp 112-129 JB Lippincott, Philadelphia [Google Scholar]
- 4.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM: IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol 1993, 15:2667-2675 [PubMed] [Google Scholar]
- 5.Bar-Eli M: Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999, 67:12-18 [DOI] [PubMed] [Google Scholar]
- 6.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ: Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994, 54:3242-3247 [PubMed] [Google Scholar]
- 7.Singh RK, Gutman M, Reich R, Bar-Eli M: Utraviolet-B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin-8. Cancer Res 1995, 55:3669-3674 [PubMed] [Google Scholar]
- 8.Singh RK, Varney ML, Bucana CD, Johansson SL: Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res 1999, 9:383-387 [DOI] [PubMed] [Google Scholar]
- 9.Nurnberg W, Tobias D, Otto F, Henz BM, Schadendorf D: Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol 1999, 189:546-551 [DOI] [PubMed] [Google Scholar]
- 10.Kunz M, Goebeler M, Brocker EB, Gillitzer R: IL-8 mRNA expression in primary malignant melanoma mRNA in situ hybridization: sensitivity, specificity, and evaluation of data. J Pathol 2000, 192:413-415 [DOI] [PubMed] [Google Scholar]
- 11.Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U: Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumor load. Melanoma Res 1995, 5:179-181 [DOI] [PubMed] [Google Scholar]
- 12.Ugurel S, Rappl G, Tilgen W, Reinhold U: Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 2001, 19:577-583 [DOI] [PubMed] [Google Scholar]
- 13.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M: Expression of interleukin-8 by human melanoma cells upregulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997, 151:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JM, Taraboletti G, Matsushima K, Van Damme J, Mantovani A: Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun 1990, 169:165-170 [DOI] [PubMed] [Google Scholar]
- 15.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, Dipietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 16.Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE: Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology 1994, 62:134-139 [DOI] [PubMed] [Google Scholar]
- 17.Hu DE, Hori Y, Fan TP: Interleukin-8 stimulates angiogenesis in rats. Inflammation 1993, 17:135-143 [DOI] [PubMed] [Google Scholar]
- 18.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM: Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994, 179:1409-1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphal JR, Van’t Hullenaar R, Peek R, Willems RW, Crickard K, Crickard U, Askaa J, Clemmensen I, Ruiter DJ, De Waal RM: Angiogenic balance in human melanoma: expression of VEGF, bFGF, IL-8, PDGF and angiostatin in relation to vascular density of xenografts in vivo Int J Cancer 2000, 86:768-776 [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M: Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol 1997, 17:4015-4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang XD, Corvalan JR, Wang P, Roy CM, Davis CG: Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J Leukoc Biol 1999, 66:401-410 [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M: Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. EMBO J 1998, 17:4358-4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huhtala P, Tuuttila A, Chow LT, Lohi J, Keski-Oja J, Tryggvason K: Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem 1991, 266:16485-16490 [PubMed] [Google Scholar]
- 24.Gershenwald JE, Sumner W, Calderone T, Wang Z, Huang S, Bar-Eli M: Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene 2001, 20:3363-3375 [DOI] [PubMed] [Google Scholar]
- 25.Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, Zain M, Kleinman HK: Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol 1992, 153:614-625 [DOI] [PubMed] [Google Scholar]
- 26.Galffy G, Mohammed KA, Dowling PA, Nasreen N, Ward MJ, Antony VB: Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res 1999, 59:367-371 [PubMed] [Google Scholar]
- 27.Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A: Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother 1998, 47:47-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brew R, Erikson JS, West DC, Flanagan BF, Christmas SE: Interleukin-8 as a growth factor for human colorectal carcinoma cells in vitro. Biochem Soc Trans 1997, 25:S264-S268 [DOI] [PubMed] [Google Scholar]
- 29.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K: Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res 1999, 5:3711-3721 [PubMed] [Google Scholar]
- 30.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis (review). Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 31.Fidler IJ, Ellis LM: The implications of angiogenesis for the biology and therapy of cancer metastasis (minireview). Cell 1994, 79:185-188 [DOI] [PubMed] [Google Scholar]
- 32.Becker D, Meier CB, Herlyn M: Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J 1989, 8:3685-3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salven P, Heikkila P, Joensuu H: Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer 1997, 76:930-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 25:327-336 [DOI] [PubMed] [Google Scholar]
- 35.Schonbeck U, Brandt E, Petersen F, Flad HD, Loppnow H: IL-8 specifically binds to endothelial but not to smooth muscle cells. J Immunol 1995, 154:2375-2383 [PubMed] [Google Scholar]
- 36.Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE: Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology 1994, 62:134-139 [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Smolen J: Monoclonal antibody therapy. Adv Protein Chem 2001, 56:369-421 [DOI] [PubMed] [Google Scholar]
- 38.Illidge TM, Bayne MC: Antibody therapy of lymphoma. Expert Opin Pharmacother 2001, 2:953-961 [DOI] [PubMed] [Google Scholar]
- 39.Mendelsohn J, Baselga J: The EGF receptor family as targets for cancer therapy. Oncogene 2000, 27:6550-6565 [DOI] [PubMed] [Google Scholar]
- 40.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A: Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999, 15:1236-1243 [PubMed] [Google Scholar]
- 41.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG: Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol 2001, 38:17-23 [DOI] [PubMed] [Google Scholar]
- 42.Inoue K, Slaton JW, Kim SJ, Perrotte P, Eve BY, Bar-Eli M, Radinsky R, Dinney CP: Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res 2000, 15:2290-2299 [PubMed] [Google Scholar]
- 43.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL: Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 1998, 51:161-167 [DOI] [PubMed] [Google Scholar]
- 44.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP: Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res 2000, 6:2104-2119 [PubMed] [Google Scholar]
- 45.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ: Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst 1998, 18:447-454 [DOI] [PubMed] [Google Scholar]
- 46.Yatsunami J, Tsuruta N, Ogata K, Wakamatsu K, Takayama K, Kawasaki M, Nakanishi Y, Hara N, Hayashi S: Interleukin-8 participates in angiogenesis in non-small cell, but not small cell carcinoma of the lung. Cancer Lett 1997, 120:101-108 [DOI] [PubMed] [Google Scholar]