Abstract
Epidermolysis bullosa acquisita is an autoimmune subepidermal blistering disease associated with autoantibodies to type VII collagen, the major constituent of anchoring fibrils. Previous attempts to demonstrate the blister-inducing potential of autoantibodies to this protein have failed. To address this question, we used an in vitro model involving cryosections of human skin incubated with patients’ autoantibodies and leukocytes from healthy donors. We show that sera from 14 of 16 epidermolysis bullosa acquisita patients, in contrast to sera from healthy controls, induced dermal-epidermal separation in the cryosections. Recruitment and activation of neutrophils at the dermal-epidermal junction was necessary for split induction, whereas mononuclear cells were not required. Importantly, patients’ autoantibodies affinity-purified against a recombinant form of the noncollagenous 1 domain of type VII collagen retained their blister-inducing capacity in a dose-dependent manner, whereas patients’ IgG that was depleted of reactivity to type VII collagen lost this ability. Monoclonal antibody LH7.2 to the noncollagenous 1 domain of type VII collagen also induced subepidermal splits in the cryosections; F(ab′)2 fragments of autoantibodies to type VII collagen were not pathogenic. We demonstrate the capacity of autoantibodies to type VII collagen to trigger an Fcγ-dependent inflammation leading to split formation in cryosections of human skin.
Epidermolysis bullosa acquisita (EBA) is a chronic subepidermal blistering disease of skin and mucous membranes. It is characterized by the deposition of IgG and/or C3 at the dermal-epidermal junction (DEJ) of patients’ skin. 1 By indirect immunofluorescence (IF) microscopy on 1 mol/L NaCl-split skin, circulating autoantibodies label the dermal side. 2 Ultrastructurally, IgG deposits localize to both the lamina densa and sublamina densa of the DEJ. 3-5 Serum autoantibodies react with a 290-kd protein by immunoblotting of dermal extracts and immunoprecipitation of cellular extracts from keratinocytes and fibroblasts. 4,6 This protein is also referred to as type VII collagen, the major component of anchoring fibrils. Type VII collagen is composed of three identical α-chains, each consisting of a central collagenous triple-helical portion of 145-kd, flanked by 145-kd (NC1) and 34-kd (NC2) noncollagenous domains at the amino- and carboxy-terminus, respectively. 7 Two molecules form anti-parallel tail-to-tail dimers stabilized by disulfide bonding through a carboxy-terminal overlap between NC2 domains. 8 Epitopes recognized by the majority of EBA sera were mapped to the NC1 domain of type VII collagen. 9-12
In other autoimmune blistering diseases, including pemphigus and anti-epiligrin/laminin 5 pemphigoid, the blister-inducing capacity of patients’ autoantibodies has been demonstrated by passive transfer into neonatal BALB/c and severe combined immunodeficient (SCID) mice, respectively. 13,14 In addition, autoantibodies to β4 integrin from patients with ocular cicatricial pemphigoid were shown to induce subepidermal blisters in an in vitro conjunctival organ culture model. 15 Sera from bullous pemphigoid patients induce dermal-epidermal separation in cryosections of human skin when co-incubated with complement and leukocytes from healthy donors. 16 Although antibodies from patients with bullous pemphigoid do not cross-react with murine skin and do not induce blisters by passive transfer into neonatal mice, IgG from rabbits, immunized with recombinant murine BP180, led to a blistering disease in the mice that mimicked bullous pemphigoid. 17 However, the blister-inducing capacity of autoantibodies to type VII collagen has not yet been unequivocally demonstrated. Previous attempts to induce EBA by passive transfer of patients’ autoantibodies into neonatal BALB/c mice 18,19 or human skin grafted onto SCID mice were not successful. 20
Previously, sera from some EBA patients were shown to induce leukocyte recruitment to the DEJ using a leukocyte attachment assay. 21 Modifying this assay, we incubated cryosections of human skin with IgG preparations from EBA patients and subsequently with leukocytes from healthy donors. We demonstrate the capacity of autoantibodies from EBA sera, affinity-purified against recombinant type VII collagen, to trigger an immune complex-mediated inflammation leading to blister formation in the cryosections. This effect was shown to be mediated by autoantibodies to the NC1 domain of type VII collagen and to involve a Fcγ-dependent recruitment of leukocytes and their activation at the DEJ.
Materials and Methods
Antibodies
Serum samples were obtained from 16 patients with EBA before the initiation of treatment. Nine of the 16 patients presented with the noninflammatory type of the disease and 5 with inflammatory EBA, and in 2 patients the clinical data available did not allow a precise classification. Two patients (EBA13 and EBA14) suffered from the childhood variant of the disease (5 and 6 years, respectively), the remainder of the patients were adults (mean age, 58 years). All EBA patients were characterized by 1) blisters on the skin; 2) linear deposits of IgG at the DEJ by direct IF microscopy; 3) circulating IgG autoantibodies binding to the dermal side of 1 mol/L of NaCl-split human skin by indirect IF microscopy (titers ranging from 40 to 1280); and 4) immunoblot reactivity with type VII collagen extracted from dermis. By complement-fixation test on neonatal foreskin, 22 12 EBA sera fixed complement to the DEJ, whereas 4 sera did not. Serum samples from patients with bullous pemphigoid (n = 20), pemphigus vulgaris (n = 6), and from healthy donors (n = 20) were used as controls. Murine monoclonal antibodies (mAbs) to type IV collagen (clone CIV 22, IgG1) and to type VII collagen (clone LH7.2, IgG1) were purchased from Sigma (St. Louis, MO).
Construction of cDNA for the Type VII Collagen NC1 Domain
Amplification and cloning procedures were performed as described previously. 23 Briefly, primers for polymerase chain reactions were synthesized by Perkin-Elmer/Applied Biosystems (Foster City, CA). DNA sequence data of type VII collagen were retrieved from EMBL DNA data bank (Cambridge, UK) using the most recent accession number NM_000094. 7 The full-length 3787-bp NC1-cDNA (nucleotides 114 to 3878) was generated by applying nested polymerase chain reaction on a cDNA pool generated by reverse transcription of total RNA from human keratinocytes. Restriction sites for SalI and HindIII were introduced during the nested polymerase chain reactions using the primer pair p323 gatcaagcttatgacgctgcggcttctggtggccgcgctctg and p326 gatcgtcgacctggccctttggacaatacactgggcagggc. The NC1-cDNA was cloned into linearized pBBH2c (Invitrogen, Carlsbad, CA) resulting in the recombinant transfer vector pBBHNC1-FL. The presence of correct inserts was verified by DNA sequence analysis following the dideoxy method using a DyeDideoxy Terminator kit (Perkin Elmer) and a 377 DNA sequence analyzer (Perkin Elmer).
Heterologous Expression of Type VII Collagen NC1 in Sf21 Insect Cells
Spodoptera frugiperda 21 (Sf21) insect cells (Invitrogen) grown in Grace’s insect medium (Invitrogen) were co-transfected with the recombinant vector pBBHNC1-FL and linearized baculovirus AcMNPV DNA (Bac-N-Blue, Invitrogen) using the liposome technique resulting in recombinant baculovirus BV-NC1. For control experiments, insect cells were co-transfected with linear baculovirus DNA and transfer vector pBBH2c (lacking NC1-cDNA) resulting in recombinant baculovirus BV-0. Recombinant baculovirus clones were identified and purified by plaque assay. Recombinant NC1 and a vector-specific peptide of 52 amino acids including only the hexahistidine tag and a sequence encoded by the multicloning site, but no NC1 sequence, were expressed by infecting the cells with recombinant baculovirus BV-NC1 or BV-0 at a multiplicity of infection of 5.0 and 4.0, respectively. Two days after infection, the recombinant hexahistidine-tagged proteins were purified from Sf21 insect cells by immobilized metal chelate affinity chromatography using Ni-NTA agarose (Qiagen, Valencia, CA). For analysis of expression and purification of the recombinant protein monoclonal anti-RGSHHH-antibody (Qiagen) was used. 23 Protein concentrations were measured by the Bradford assay (Bio-Rad, Hercules, CA).
Immunoblot Analysis
Extracts of human dermis were prepared as described. 24 Recombinant NC1 or dermal extracts were fractionated by 8% and 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, respectively, transferred to nitrocellulose, and analyzed by immunoblotting. 25
Affinity-Purification of Total IgG and IgG Specific for Type VII Collagen
IgG from serum of patients and controls was isolated using Protein G Sepharose Fast Flow affinity column chromatography (Pharmacia AB, Uppsala, Sweden). 26 Antibodies from sera of EBA patients were affinity-purified using an AminoLink Plus immobilization kit following the manufacturer’s instructions (Pierce, Rockford, IL). Recombinant protein encoded by BV-0 (lacking NC1) and recombinant NC1 protein (also including the vector-specific peptide) were covalently coupled to 4% beaded agarose at pH 10 and incubated with patient’s serum for 1 hour. Antibodies were eluted with 0.1 mol/L of glycine buffer (pH 2.5), neutralized with Tris-HCl, and concentrated under extensive washing with phosphate-buffered saline (PBS) (pH 7.2) using Ultrafree 15 filters (Millipore, Bradford, MA). Flow-through fractions were subjected to protein G column chromatography and concentrations of IgG in flow-through and eluted fractions were determined by absorbance at 280 nm. Reactivity of both fractions was analyzed by indirect IF microscopy on salt-split skin and by immunoblotting with cell-derived type VII collagen or recombinant NC1.
Preparation of IgG F(ab′)2 Fragments
Immunoaffinity-purified antibodies specific to type VII collagen were subjected to pepsin digestion. 27 Completeness of fragmentation and reactivity of the F(ab′)2 preparations were tested by indirect IF microscopy on 1 mol/L salt split-skin using fluorescein isothiocyanate-labeled secondary antibodies specific to Fab (Sigma) and Fc (Serotec Ltd., Oxford, UK) portions of IgG, respectively.
Peripheral Blood Leukocytes and Complement
Peripheral blood leukocytes from healthy volunteers were isolated by a sedimentation gradient medium containing sodium diatrizoate and dextran 500 (Nycomed, Oslo, Norway). Cells were harvested, washed twice in RPMI 1640 (Life Technologies, Karlsruhe, Germany), and resuspended in the same medium. The cell suspension was kept on ice and cell viability was tested using trypan blue; preparations with a viability greater than 95% were used. To determine the activation status of leukocytes, the cells were resuspended in medium containing 0.05% nitro blue tetrazolium (Roth, Karlsruhe, Germany), before incubation with the cryosections. Subsequently, unfixed sections were examined by light microscopy for the presence of dark-blue formazan precipitates. Serum samples from healthy volunteers served as a source of human complement and were diluted twofold with RPMI. 28
Treatment of Cryostat Sections
Neonatal human foreskin, obtained from routine circumcision, was washed in cold PBS, cut in pieces of 5 × 15 mm, embedded in optimum cutting temperature compound (Sakura Finetek Europe B.V., Zoeterwonde, The Netherlands), and stored at −80°C. Four cryosections of 6 μm were placed in the center of a Superfrost Plus microscope slide (Menzel-Gläser, Braunschweig, Germany). Sera from EBA patients and controls were heat-inactivated for 30 minutes at 56°C and diluted twofold in PBS. Before the treatment of skin sections, protein G affinity-purified IgG from EBA sera was diluted in PBS to an indirect IF titer of 80 and the mAb LH7.2 was used at an indirect IF titer of 1000. When EBA sera were purified against recombinant type VII collagen NC1, flow-through and eluted fractions were adjusted to an IgG concentration of 0.5 mg/ml. Skin sections were washed with PBS for 5 minutes to remove embedding medium before incubation with 50 μl of diluted sera or antibody preparations (90 minutes at room temperature). After washing the sections with PBS twice, chambers were prepared as described 16 and the leukocyte suspension, mixed 1:1 with either diluted fresh or heated serum or medium alone, was injected into the chambers. Incubation occurred in a humidified air incubator containing 5% CO2 for 3 hours at 37°C. Subsequently, chambers were disassembled, sections were washed in PBS for 10 minutes, air-dried for 10 minutes, fixed in formalin, and stained with hematoxylin and eosin.
Results
Preparation of the Recombinant NC1 Domain of Type VII Collagen
The recombinant NC1 domain was expressed in insect cells infected with baculovirus and purified by immobilized metal chelate affinity chromatography using Ni-NTA agarose. Sera from 16 EBA patients and mAb LH7.2 reacted with the 145-kd recombinant NC1 domain by immunoblotting, in contrast to sera from patients with bullous pemphigoid (n = 20), pemphigus vulgaris (n = 6), and from healthy controls (n = 20).
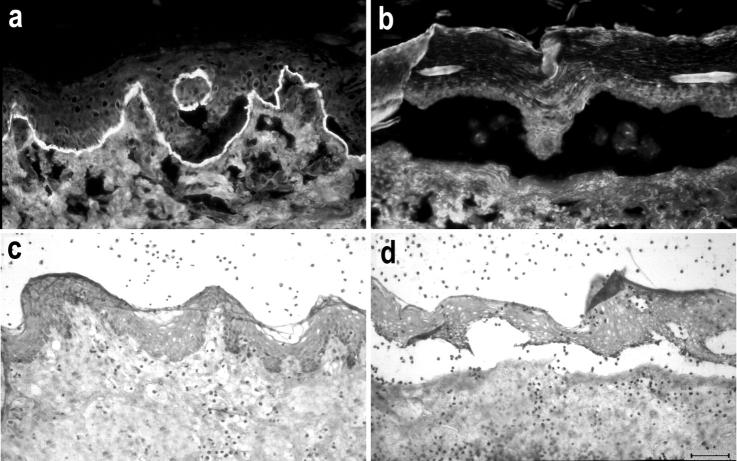
Sera from EBA Patients Recruit Neutrophils to the DEJ and Induce Subepidermal Splits in Cryosections of Human Skin
Cryosections of normal human skin were incubated with serum samples from EBA patients (n = 16) and, subsequently, with leukocytes from healthy donors. IgG from EBA sera, but not from healthy controls, bound to the DEJ as revealed by indirect IF microscopy (Figure 1a) ▶ . Addition of leukocytes resulted in the recruitment of leukocytes to the DEJ in cryosections that had been incubated with EBA sera, but not in those incubated with sera from controls (Figure 1b) ▶ . We usually observed more background attachment of leukocytes when the sections were incubated with EBA sera compared with sections that were treated with control sera. To investigate the time-dependency of neutrophil attachment and blister formation, we incubated the cryosections that had been treated with sera from patients EBA8 and EBA11 with leukocytes for 30, 60, 90, 120, and 180 minutes, respectively. Leukocyte attachment to the DEJ was strongest after an incubation time of 60 minutes (covering 65% of the length of the DEJ) and declined subsequently (30%, 14%, and almost no binding at 90, 120, and 180 minutes of incubation, respectively). Dermal-epidermal separation was first seen after an incubation time of 60 minutes (on 10% of the length of the DEJ); with longer incubation times, the extent of dermal-epidermal separation increased and reached its maximum after 120 minutes (dermal-epidermal separation on 60% of the length of the DEJ) (Figure 1e) ▶ . No dermal-epidermal separation was seen in cryosections treated with control sera (Figure 1f) ▶ . The extent of dermal-epidermal separation was dependent on the number of leukocytes. At a 2-hour incubation, splits were seen when we used 107 cells/ml. The separation became larger with the use of greater numbers of neutrophils. With numbers exceeding 3 × 107/ml, the extent of splits did not further increase, but both epidermis and dermis were unspecifically and increasingly damaged. When we used leukocyte numbers below 107/ml or when we omitted leukocytes, this abolished blister induction completely. Under the optimized assay conditions (incubation with 3 × 107 leukocytes for 120 minutes), 14 of 16 EBA sera induced dermal-epidermal separation, whereas serum from EBA13 and EBA14 did not. End-point titers of immunoblot reactivity with recombinant NC1 of those EBA sera that induced a dermal-epidermal separation ranged from 500 to 5000. End-point titers of immunoblot reactivity of sera EBA13 and EBA14, that were not able to induce separation, were 1000 and 2000, respectively. To address the question if the addition of complement augments the extent of dermal-epidermal separation in this model, we incubated cryosections that were previously treated with EBA sera with 1) leukocytes and fresh serum, 2) leukocytes and complement-inactivated (heated) serum, or 3) with leukocytes alone. Interestingly, the addition of complement did not increase the extent of dermal-epidermal separation in the cryosections compared with the extent observed when complement was heat-inactivated or omitted completely. Because complement did not play a role in this model, further experiments did not include fresh serum as a source of complement.
Figure 1.
Serum from an EBA patient recruits neutrophils to the DEJ and induces subepidermal splits. a: Cryosections of normal human skin, incubated with EBA serum, show IgG deposits at the DEJ by indirect IF microscopy. b: Subsequent addition of leukocytes leads to neutrophil recruitment at the DEJ. Activation of neutrophils, as revealed by reduction of nitro blue tetrazolium to formazan (dark precipitates), is induced by EBA serum (c), but not by normal human serum (d). e: With longer incubation times, subepidermal splits develop in the cryosections that were treated with EBA serum. f: In contrast, serum from a healthy donor does not induce neutrophil recruitment or dermal-epidermal separation. Scale bars, 40 μm.
Leukocytes at the DEJ Are Activated
To determine whether leukocytes that attach to the DEJ become activated, we incubated cryosections that had been treated with patients’ sera (EBA3, EBA10, and EBA16) with leukocytes in the presence of nitro blue tetrazolium. After a 30-minute incubation, dark-blue precipitates of reduced nitro blue tetrazolium (formazan) were found at both DEJ and stratum corneum (Figure 1c) ▶ . In sections treated with normal human serum, no recruitment of cells to the DEJ was observed and blue precipitates were restricted to the stratum corneum (Figure 1d) ▶ .
Granulocytes, but Not Mononuclear Cells, Are Required for Split Formation Induced by Autoantibodies from EBA Patients in Cryosections of Human Skin
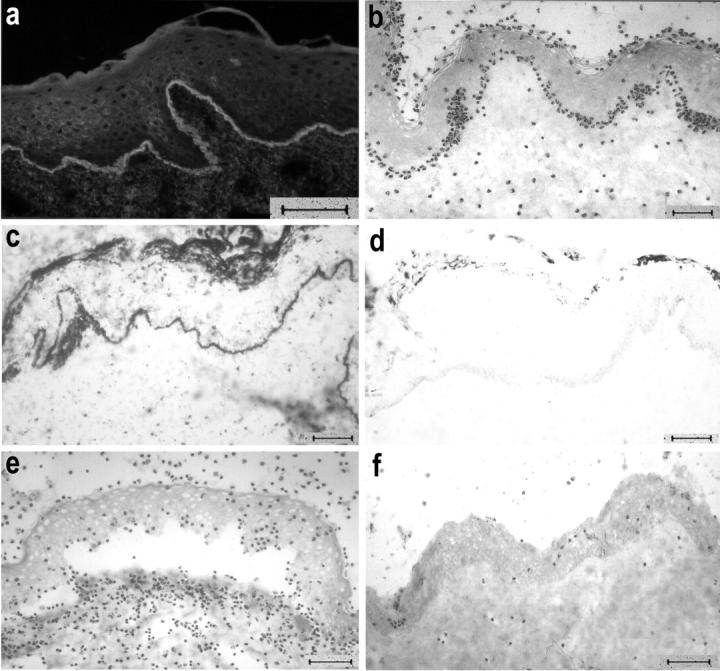
To dissect the role of different leukocyte subpopulations in the split-formation induced by EBA autoantibodies, granulocytes and peripheral blood mononuclear cells from healthy donors were separated by gradient centrifugation. The preparations were used at a density of 3 × 107 cells/ml. When incubated with the cryosections that had been treated with serum from EBA3, EBA10, and EBA16, only granulocytes (Figure 2a) ▶ , but not mononuclear cells (Figure 2b) ▶ , were recruited to the DEJ and induced subepidermal splits.
Figure 2.
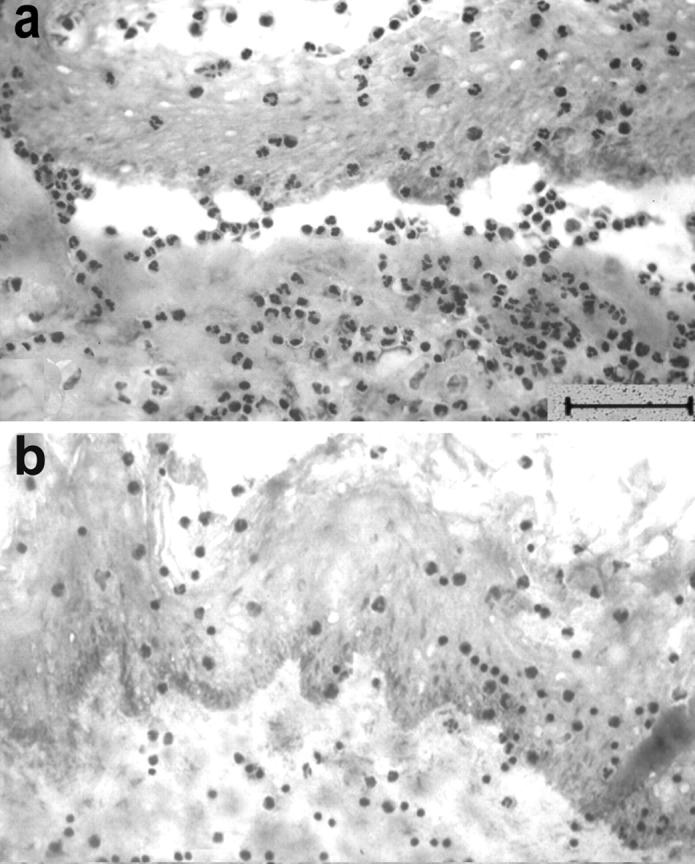
Granulocytes, but not mononuclear cells, are required for split induction by autoantibodies from EBA patients in cryosections of human skin. a: Granulocytes, which were isolated from the blood of healthy donors, mediate subepidermal cleavage in cryosections treated with an EBA serum. b: In contrast, mononuclear cells are not recruited to the DEJ and do not cause subepidermal cleavage.
IgG Purified from Serum of EBA Patients Retains Its Blister-Inducing Ability
To confirm the results obtained with patients’ sera, we purified the IgG fractions of five EBA sera by protein G column chromatography (Table 1) ▶ . IgG preparations from patients and controls were used at the same IgG concentration. When incubated with the cryosections, IgG from EBA patients, in contrast to IgG from controls, induced subepidermal splits.
Table 1.
Characterization of Affinity-Purified IgG Preparations*
| Patient | IgG preparation | Concentration† (mg/ml) | IIF titer‡ | Type VII collagen§ | rNC1¶ |
|---|---|---|---|---|---|
| EBA4 | Total IgG | 22.5 | 160 | + | + |
| EBA5 | Total IgG | 17.5 | 80 | + | + |
| EBA7 | Total IgG | 25 | 320 | + | + |
| EBA8 | Total IgG | 62.5 | 640 | + | + |
| EBA11 | Total IgG | 21.3 | 160 | + | + |
*Total IgG fractions were isolated from human sera using protein G affinity column chromatography.
†Concentrations of affinity-purified antibodies were determined using absorbance at 280 nm.
‡Antibody titer by indirect IF microscopy on 1 mol/L NaCl-split human skin.
§Immunoblot reactivity with full-length type VII collagen extracted from dermis.
¶Immunoblot reactivity with the recombinant NC1 domain of type VII collagen.
Antibodies Specific to the NC1 Domain of Type VII Collagen Induce Subepidermal Cleavage in Cryosections of Human Skin
To characterize the specificity of pathogenic autoantibodies from EBA sera, we purified antibodies from five EBA patients against a recombinant form of type VII collagen. Total IgG from the flow-through fractions was further purified by protein G column chromatography. The reactivity and specificity of antibodies eluted from NC1 coupled to agarose and of IgG purified from the flow-through fractions are summarized in Table 2 ▶ and representative patterns are shown in Figure 3 ▶ . Importantly, when incubated with the cryosections, eluted autoantibodies specific to type VII collagen induced subepidermal splits (Figure 3Ca) ▶ , whereas IgG depleted of reactivity to the NC1 domain of type VII collagen lost its blister-inducing ability (Figure 3Cb) ▶ . To exclude the possibility that baculovirus- or insect cell-specific proteins other than recombinant NC1 unspecifically preadsorb the blister-inducing capacity of EBA sera, we purified three sera (EBA7, EBA8, and EBA11) against the recombinant vector-specific peptide, encoded by BV-0, not containing any NC1 sequence. Eluted fractions of this purification were unreactive by indirect IF microscopy on salt-split skin and by immunoblotting with recombinant NC1 (Figure 4, Aa and B ▶ , lane 3), whereas IgG from the flow-through (preadsorbed serum) stained the dermal side of salt-split skin by indirect IF microscopy (Table 2 ▶ , Figure 4Ab ▶ ) and reacted with recombinant NC1 by immunoblotting (Figure 4B ▶ , lane 2). Both eluted and flow-through fractions were then incubated with the cryosections followed by incubation with leukocytes. Eluted fractions did not cause subepidermal splits, in contrast to flow-though fractions that were still pathogenic (Figure 4C) ▶ . These results demonstrate that preadsorption of type VII collagen-specific antibodies was only achieved with recombinant NC1, but not with other proteins produced by baculovirus-infected insect cells.
Table 2.
Characterization of Immunoaffinity-Purified Immunoglobulin Preparations
| Patient IgG preparation | Concentration‡ (mg/ml) | IIF titer§ | Type VII collagen¶ | rNC1∥ | |
|---|---|---|---|---|---|
| Purification against recombinant NC1 encoded by BV-NC1* | |||||
| EBA4 | |||||
| Eluted fraction | 2.7 | 320 | + | 2000** | |
| Flow-through fraction | 21 | − | − | − | |
| EBA5 | |||||
| Eluted fraction | 0.68 | 160 | + | 2000** | |
| Flow-through fraction | 3.72 | − | − | − | |
| EBA6 | |||||
| Eluted fraction | 2.56 | 160 | + | 2000** | |
| Flow-through fraction | 2.2 | − | − | − | |
| EBA7 | |||||
| Eluted fraction | 2.81 | 160 | + | 1000** | |
| Flow-through fraction | 2.11 | − | − | − | |
| EBA8 | |||||
| Eluted fraction | 0.68 | 320 | + | 5000** | |
| Flow-through fraction | 39 | − | − | − | |
| Purification against the vector-specific peptide encoded by BV-0† | |||||
| EBA7 | |||||
| Eluted fraction | 0.01 | − | N.D. | − | |
| Flow-through fraction | 10 | 320 | N.D. | + | |
| EBA8 | |||||
| Eluted fraction | 0 | − | N.D. | − | |
| Flow-through fraction | 12 | 160 | N.D. | + | |
| EBA11 | |||||
| Eluted fraction | 0.009 | − | N.D. | − | |
| Flow-through fraction | 8 | 160 | N.D. | + | |
*Antibodies to type VII collagen from patients’ sera were purified against recombinant NC1 covalently coupled to an agarose matrix.
†Patients’ sera were purified against Ni-NTA-purified lysate of Sf21 insect cells infected with BV-0.
‡Concentrations of affinity-purified antibodies were determined using absorbance at 280 nm.
§Antibody titer by indirect IF microscopy on 1 mol/L NaCl-split human skin.
¶Immunoblot reactivity with full-length type VII collagen extracted from dermis.
∥Immunoblot reactivity with the recombinant NC1 domain of type VII collagen.
**End-point titers of immunoblot reactivity with recombinant NC1.
Figure 3.
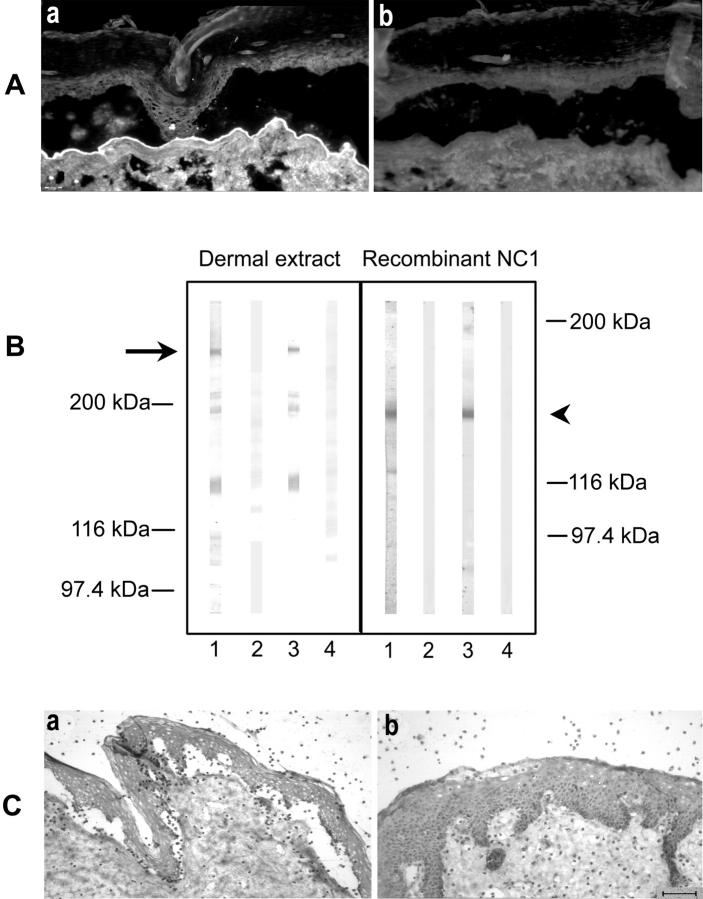
Immunoadsorption of EBA serum against the recombinant NC1 domain of type VII collagen abolishes split induction. Autoantibodies to type VII collagen from EBA serum were purified using a recombinant form of the NC1 domain covalently coupled to an agarose matrix. A: When analyzed by indirect IF microscopy, IgG eluted from recombinant NC1 retains reactivity with the dermal side of NaCl-split human skin (a), in contrast to IgG from the flow-through fraction that completely lost IF reactivity (b). B: By immunoblotting, IgG autoantibodies eluted from the column (lane 3) recognized, like a serum sample from the same patient (lane 1), both full-length type VII collagen extracted from dermis (arrow) and a recombinant form of its NC1 domain (arrowhead). In contrast, the flow-through fraction (lane 2) or normal serum (lane 4) showed no reactivity. C: When incubated with the cryosections, the patient’s autoantibodies specific to type VII collagen NC-1 induce dermal-epidermal separation (a), whereas IgG depleted of reactivity to the NC1 domain (flow-through fraction) does not (b). Scale bar, 40 μm.
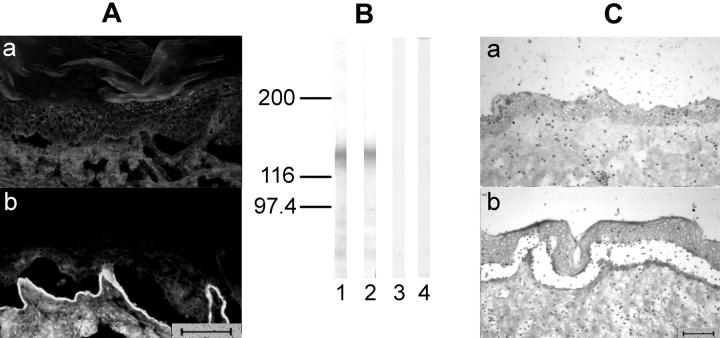
Figure 4.
Preadsorption of EBA serum against proteins from insect cells infected with recombinant BV-0, lacking NC1-cDNA, does not abolish its blister-inducing capacity. EBA serum was purified against proteins from insect cells infected with recombinant BV-0 coupled to an agarose matrix. A: By indirect IF microscopy, IgG eluted from the column that completely lost indirect IF reactivity (a), in contrast IgG from the flow-through fraction stained the dermal side of salt-split skin (b). B: By immunoblotting, like the original serum (lane 1), the flow-through fraction (lane 2), reacted with recombinant NC1, whereas the eluted fraction (lane 3), like normal human serum (lane 4), was unreactive. C: When incubated with cryosections, in contrast to the eluted fraction (a), the flow-through fraction retained its ability to induce blisters (b). Scale bars, 40 μm.
Autoantibodies to Type VII Collagen Induce a Dose-Dependent Dermal-Epidermal Separation in the Cryosections
To study the dependency of the extent of split formation on autoantibody reactivity to type VII collagen, we incubated the cryosections with different dilutions of both EBA sera and immunoaffinity-purified antibody preparations followed by incubation with leukocytes. As shown in Table 3 ▶ , EBA sera and NC1-specific antibody preparations with higher indirect IF titers induced more extensive DEJ separation compared with preparations of lesser reactivity.
Table 3.
Serum Samples and NC1-Specific Autoantibodies from EBA Patients Induce a Dose-Dependent Dermal-Epidermal Separation in Cryosections of Human Skin
| EBA sera | NC1-specific antibody preparations* | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Dilution | IIF titer† | DES (%)‡ | Patient | Dilution | IIF titer† | DES (%)‡ |
| EBA8 | 1:2 | 80 | 70 | EBA8 | 1:4 | 80 | 65 |
| 1:4 | 40 | 60 | 1:16 | 20 | 40 | ||
| 1:16 | 10 | 40 | 1:64 | 5 | 5 | ||
| 1:160 | 1 | 10 | 1:320 | 1 | 0 | ||
| EBA11 | 1:4 | 80 | 60 | EBA11 | 1:2 | 80 | 75 |
| 1:8 | 40 | 55 | 1:8 | 20 | 50 | ||
| 1:32 | 10 | 30 | 1:32 | 5 | 30 | ||
| 1:320 | 1 | 5 | 1:160 | 1 | 10 | ||
*Antibodies specific to NC1 were purified from the serum of two EBA patients by immunoaffinity column chromatography and incubated with cryosections of human skin followed by a 2-hour incubation with human leukocytes.
†Titer of antibodies by indirect IF microscopy on 1 mol/L NaCl-split human skin.
‡Length of DEJ with separation in relation to total length (percent).
A Monoclonal Antibody to the NC1 Domain of Type VII Collagen Induces Subepidermal Splits in the Cryosections
mAb LH7.2, directed to an epitope on the central portion of the NC1 domain of type VII collagen induced dermal-epidermal separation of the cryosections (Figure 5a) ▶ . In contrast, a mAb to type IV collagen that belonged to the same isotype and was used at the same IgG concentration (0.1 mg/ml) was not pathogenic (Figure 5b) ▶ . The extent of split formation induced by mAb LH7.2 was less pronounced when compared with patients’ autoantibodies to type VII collagen and split-induction required a longer incubation time with leukocytes (3 hours).
Figure 5.
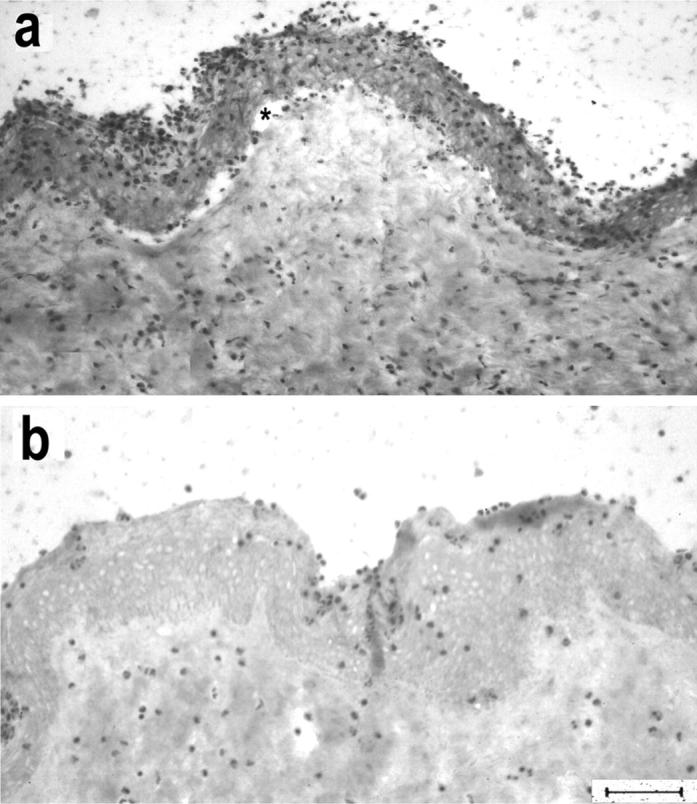
A mAb to the NC1 domain of type VII collagen induces subepidermal splits in cryosections of human skin. Incubation of cryosections with mAb LH7.2, directed to the NC1 domain, and subsequently with leukocytes leads to dermal-epidermal separation (a), in contrast to a mAb to type IV collagen that was used at the same IgG concentration (b). Asterisk indicates the cleavage. Scale bar, 40 μm.
F(ab′)2 Fragments of Blister-Inducing Autoantibodies Lose Their Pathogenicity
To address the question if the Fc portion of autoantibodies to type VII collagen is of importance for blister induction in our in vitro model, we prepared F(ab′)2 fragments of antibodies from EBA serum that had been eluted from recombinant NC1 coupled to agarose. F(ab′)2 fragments, lacking the Fc portion of the antibody, labeled the dermal side of NaCl-split skin by indirect IF microscopy (Figure 6, a and b) ▶ . Interestingly, F(ab′)2 preparations, although used at the same indirect IF titer (80) as unfragmented IgG, failed to recruit neutrophils to the DEJ and to induce subepidermal cleavage when incubated with the cryosections (Figure 6, c and d) ▶ .
Figure 6.
Dermal-epidermal separation is dependent on the Fc portion of autoantibodies to type VII collagen. Patient’s autoantibodies, which had been purified against recombinant NC1 of type VII collagen, were subsequently subjected to pepsin digestion. Resulting F(ab′)2 fragments labeled the dermal side of 1 mol/L of NaCl-split skin by indirect IF microscopy using an anti-Fab fluorescein isothiocyanate-conjugated secondary antibody (a), whereas no staining was observed using a Fc-specific conjugate (b). F(ab′)2 fragments failed to induce blisters (c), in contrast to unfragmented autoantibodies to type VII collagen (d). Scale bar, 40 μm.
Discussion
In this study, we show the capacity of autoantibodies to type VII collagen from EBA patients and of a monoclonal antibody to this protein to trigger an Fcγ-dependent inflammation leading to blister formation in cryosections of human skin. Previous attempts to induce EBA by passive transfer of patients’ autoantibodies into neonatal mice 18,19 or human skin grafted onto SCID mice had failed. 20
In a first set of experiments, we show that serum from EBA patients, in contrast to serum from healthy donors, induces recruitment and activation of neutrophils at the DEJ of cryosections and, subsequently, dermal-epidermal separation. Complement was shown not to be required for split induction or to augment the extent of dermal-epidermal separation. However, in this model, in contrast to the in vivo situation, leukocytes do not have to migrate along a chemotactic gradient from blood vessels to the DEJ. Instead, by incubating the cryosections with leukocytes, the cells are placed in close contact with the DEJ. Therefore, the role of complement in blister formation of EBA cannot be addressed using this model. Of the 16 EBA sera we tested, 2 did not induce a split. Interestingly, these sera came from two children and showed high reactivity by both indirect IF microscopy and immunoblotting of recombinant NC1. One may speculate that in these two patients, another pathogenic mechanism accounts for blister formation, which, in contrast to the majority of EBA sera, cannot be reproduced in our in vitro model. In subsequent experiments, our findings with crude EBA serum were confirmed with the use of IgG purified from patients’ sera. In contrast to IgG from healthy controls, patients’ IgG caused dermal-epidermal separation. The development of subepidermal cleavage in cryosections that strongly adhere to the glass slide might be because of a natural tension within the DEJ. Indeed, bundles of elastic microfibrils of the lamina fibroreticularis anchor the basal lamina to dermal elastic fibers. 29 Cleavage of components of the anchoring complexes by leukocyte-derived proteases may release this tension resulting in a recoil of elastic fibers and retraction of the dermis from the epidermis.
Clinically, EBA may present as an inflammatory or noninflammatory disease. Sufficient clinical data to classify our patients into these two groups was available for 14 of 16 cases included in this study. Nine patients suffered from the noninflammatory and the remaining five patients from the inflammatory type of the disease. Serum from all nine noninflammatory patients and from three patients with inflammatory disease caused dermal-epidermal separation in the cryosections. The two clinical phenotypes have in common that the patients develop subepidermal blisters. This common finding can be reproduced in our model. However, serum from patients with both variants required the presence of leukocytes to induce dermal-epidermal separation in this assay. Therefore, clinical differences in the patients are likely due to other factors that cannot be analyzed using this model. In addition, further studies, based on a larger number of patients, will have to show if serum from noninflammatory patients really have a greater split-inducing potential compared to serum from patients with inflammatory EBA.
The NC1 domain has been characterized as the immunodominant region of type VII collagen that is targeted by the majority of EBA sera. 9-12 To characterize the specificity of split-inducing autoantibodies in these sera, we produced a recombinant form of the NC1 domain expressed in baculovirus-infected insect cells. Patients’ autoantibodies were purified against recombinant NC1 that was covalently coupled to an agarose matrix. Importantly, antibodies eluted from the NC1 column retained their blister-inducing capacity, whereas IgG that was depleted of reactivity to NC1 lost its ability to recruit neutrophils and to induce blisters. In addition, we show a dependency of the extent of split formation on the titer of NC1-specific autoantibodies. The pathogenic relevance of the NC1 region was further supported by the finding that mAb LH7.2, directed to an epitope on the central portion of the NC1 domain of type VII collagen, in contrast to a mAb to type IV collagen, also induced subepidermal splits of the cryosections. The extent of splits caused by mAb LH7.2 was less pronounced than splits in sections incubated with patients’ sera. This may be because of the fact that, in contrast to patients’ autoantibodies, LH7.2 is directed to a single epitope on type VII collagen. Recently, it was reported that the blister-inducing capacity of mAbs to the ectodomain of type XVII collagen/BP180, a major target of autoantibodies in bullous pemphigoid patients, was enhanced when two mAbs were concurrently injected into human skin grafted onto athymic mice compared with the single use of these mAbs. 30 These and our own results suggest that the extent of split formation may be increased when antibodies target different epitopes on the same autoantigen.
In a further set of experiments, we studied the importance of leukocytes for dermal-epidermal separation in this model. With shorter incubation times (30 to 90 minutes), recruitment of leukocytes to the DEJ predominated over the induction of a cleavage, whereas the extent of dermal-epidermal separation gradually increased with longer incubation and reached its maximum after an incubation of 2 hours. Leukocytes at the DEJ were activated, as demonstrated by their ability to generate a respiratory burst. In cryosections that were incubated with EBA sera, we usually saw more leukocytes scattered over the section when compared to sections treated with control sera. This finding may be because of the fact that neutrophils binding to IgG complexed at the DEJ, in contrast to neutrophils incubated with sections treated with IgG from healthy controls, may release proinflammatory mediators (eg, interleukin-8) that may increase an IgG-independent adhesion of neutrophils to extracellular matrix proteins, for example mediated by integrins. 31-33 By two independent lines of evidence we demonstrate that dermal-epidermal separation of the cryosections, induced by autoantibodies to type VII collagen, is dependent on the presence of leukocytes: 1) omitting leukocytes abolished the induction of subepidermal splits; 2) in contrast to undigested antibodies, their F(ab′)2 fragments, that lacked the effector (Fc) portion, lost the blister-inducing ability when incubated with the cryosections. In contrast to granulocytes, preparations of mononuclear cells were not able to mediate subepidermal cleavage.
In summary, this study demonstrates that antibodies to the NC1 domain of type VII collagen mediate an Fcγ-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Future studies will be aimed at characterizing the factors that determine the blister-inducing potential of autoantibodies to type VII collagen, including the immunoglobulin subclass(es) of pathogenically relevant antibodies and their interaction with Fcγ receptors expressed on leukocytes. In addition, this experimental model may eventually contribute to the development of novel therapeutic strategies for this disease.
Acknowledgments
We thank Drs. Kim B. Yancey (Milwaukee, WI) and Leena Bruckner-Tuderman (Münster, Germany) for providing us with patients’ sera, Dr. Enno Schmidt (Würzburg, Germany) for helpful discussions, and Andrea Knopp (Würzburg, Germany) for assistance with the cryosection experiments.
Footnotes
Address reprint requests to Detlef Zillikens, M.D., Department of Dermatology, University of Würzburg, Josef-Schneider-Str. 2, 97080 Würzburg, Germany. E-mail: zillikens-d.derma@mail.uni-wuerzburg.de.
This work was supported by grants Zi 439/6-1 (to D. Z.) and GK 520/1 (to C. S.) from the Deutsche Forschungsgemeinschaft.
C. S. and A. K. both contributed equally to this article.
References
- 1.Woodley DT, Gammon WR, Briggaman RA: Epidermolysis bullosa acquisita 1999. Freedberg IM Eisen AZ Wolff K Austen KF Goldsmith LA Katz SI Fitzpatrick TB eds. Fitzpatrick’s Dermatology in General Medicine, ed 4 1999:pp 702-709 McGraw-Hill, New York
- 2.Gammon WR, Briggaman RA, Inman AOD, Queen LL, Wheeler CE: Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol 1984, 82:139-144 [DOI] [PubMed] [Google Scholar]
- 3.Yaoita H, Briggaman RA, Lawley TJ, Provost TT, Katz SI: Epidermolysis bullosa acquisita: ultrastructural and immunological studies. J Invest Dermatol 1981, 76:288-292 [DOI] [PubMed] [Google Scholar]
- 4.Woodley DT, Briggaman RA, O’Keefe EJ, Inman AO, Queen LL, Gammon WR: Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med 1984, 310:1007-1013 [DOI] [PubMed] [Google Scholar]
- 5.Shimizu H, Ishiko A, Masunaga T, Kurihara Y, Sato M, Bruckner-Tuderman L, Nishikawa T: Most anchoring fibrils in human skin originate and terminate in the lamina densa. Lab Invest 1997, 76:753-763 [PubMed] [Google Scholar]
- 6.Stanley JR, Rubinstein N, Klaus-Kovtun V: Epidermolysis bullosa acquisita antigen is synthesized by both human keratinocytes and human dermal fibroblasts. J Invest Dermatol 1985, 85:542-545 [DOI] [PubMed] [Google Scholar]
- 7.Parente MG, Chung LC, Ryynanen J, Woodley DT, Wynn KC, Bauer EA, Mattei MG, Chu ML, Uitto J: Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proc Natl Acad Sci USA 1991, 88:6931-6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruckner-Tuderman L, Nilssen O, Zimmermann DR, Dours-Zimmermann MT, Kalinke DU, Gedde-Dahl T, Jr, Winberg J-O: Immunohistochemical and mutation analyses demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol 1995, 131:551-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapiere JC, Woodley DT, Parente MG, Iwasaki T, Wynn KC, Christiano AM, Uitto J: Epitope mapping of type VII collagen. Identification of discrete peptide sequences recognized by sera from patients with acquired epidermolysis bullosa. J Clin Invest 1993, 92:1831-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gammon WR, Murrell DF, Jenison MW, Padilla KM, Prisayanh PS, Jones DA, Briggaman RA, Hunt SWD: Autoantibodies to type VII collagen recognize epitopes in a fibronectin-like region of the noncollagenous (NC1) domain. J Invest Dermatol 1993, 100:618-622 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Furukawa F, Imamura S: Epitope mapping for epidermolysis bullosa acquisita autoantibody by molecularly cloned cDNA for type VII collagen. J Invest Dermatol 1994, 102:706-709 [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Keene DR, Costa AM, Tahk SH, Woodley DT: The carboxy-terminus of type VII collagen mediates antiparallel dimer formation and constitutes a new antigenic epitope for epidermolysis bullosa acquisita autoantibodies. J Biol Chem 2001, 276:21649-21655 [DOI] [PubMed] [Google Scholar]
- 13.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA: Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med 1982, 306:1189-1196 [DOI] [PubMed] [Google Scholar]
- 14.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB: Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest 1996, 98:1509-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan RY, Bhol K, Tesavibul N, Letko E, Simmons RK, Foster CS, Ahmed AR: The role of antibody to human beta4 integrin in conjunctival basement membrane separation: possible in vitro model for ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci 1999, 40:2283-2290 [PubMed] [Google Scholar]
- 16.Gammon WR, Merritt CC, Lewis DM, Sams WM, Carlo JR, Wheeler CE: An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol 1982, 78:285-290 [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ: A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest 1993, 92:2480-2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigemoto T, Nashiro K, Tsuchida T, Seki Y, Tamaki K: Administration of IgG fraction of epidermolysis bullosa acquisita (EBA) serum into mice. J Dermatol 1988, 15:123-127 [DOI] [PubMed] [Google Scholar]
- 19.Borradori L, Caldwell JB, Briggaman RA, Burr CE, Gammon WR, James WD, Yancey KB: Passive transfer of autoantibodies from a patient with mutilating epidermolysis bullosa acquisita induces specific alterations in the skin of neonatal mice. Arch Dermatol 1995, 131:590-595 [PubMed] [Google Scholar]
- 20.Chen DM, Welsh EA, Woodley DT, Wynn KC, Briggaman RA, Kim YH: Passive transfer of epidermolysis bullosa acquisita antibody into xenotransfused SCID mice with human grafts. J Invest Dermatol 1992, 98:589A [Google Scholar]
- 21.Gammon WR, Inman AOD, Wheeler CE, Jr: Differences in complement-dependent chemotactic activity generated by bullous pemphigoid and epidermolysis bullosa acquisita immune complexes: demonstration by leukocytic attachment and organ culture methods. J Invest Dermatol 1984, 83:57-61 [DOI] [PubMed] [Google Scholar]
- 22.Katz SI, Hertz KC, Yaoita H: Herpes gestationis. Immunopathology and characterization of the HG factor. J Clin Invest 1976, 57:1434-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kromminga A, Scheckenbach C, Georgi M, Hagel C, Arndt R, Christophers E, Brocker EB, Zillikens D: Patients with bullous pemphigoid and linear IgA disease show a dual IgA and IgG autoimmune response to BP180. J Autoimmun 2000, 15:293-300 [DOI] [PubMed] [Google Scholar]
- 24.Zillikens D, Kawahara Y, Ishiko A, Shimizu H, Mayer J, Rank CV, Liu Z, Giudice GJ, Tran HH, Marinkovich MP, Brocker EB, Hashimoto T: A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol 1996, 106:1333-1338 [DOI] [PubMed] [Google Scholar]
- 25.Zillikens D, Herzele K, Georgi M, Schmidt E, Chimanovitch I, Schumann H, Mascaro JM, Jr, Diaz LA, Bruckner-Tuderman L, Brocker EB, Giudice GJ: Autoantibodies in a subgroup of patients with linear IgA disease react with the NC16A domain of BP1801. J Invest Dermatol 1999, 113:947-953 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt E, Reimer S, Kruse N, Jainta S, Brocker EB, Marinkovich MP, Giudice GJ, Zillikens D: Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol 2000, 115:842-848 [DOI] [PubMed] [Google Scholar]
- 27.Parham P: On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol 1983, 131:2895-2902 [PubMed] [Google Scholar]
- 28.Gammon WR, Lewis DM, Carlo JR, Sams WM, Wheeler CE: Pemphigoid antibody mediated attachment of peripheral blood leukocytes at the dermal-epidermal junction of human skin. J Invest Dermatol 1980, 75:334-339 [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T: Anchoring of basal lamina to elastic fibers by elastic fibrils. J Invest Dermatol 1977, 68:389-390 [DOI] [PubMed] [Google Scholar]
- 30.Egan CA, Taylor TB, Foutz MW, Florell SR, Meyer LJ, Petersen MJ, Zone JJ: Development of an animal model of bullous pemphigoid characterized by basement membrane zone separation and an eosinophilic inflammatory infiltrate. J Invest Dermatol 1999, 112:531A [Google Scholar]
- 31.Cassatella MA: The production of cytokines by polymorphonuclear neutrophils. Immunol Today 1995, 16:21-26 [DOI] [PubMed] [Google Scholar]
- 32.Loike JD, Cao L, Budhu S, Marcantonio EE, el Khoury J, Hoffman S, Yednock TA, Silverstein SC: Differential regulation of beta1 integrins by chemoattractants regulates neutrophil migration through fibrin. J Cell Biol 1999, 144:1047-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loike JD, el Khoury J, Cao J, Richards CP, Rascoff H, Mandeville JT, Maxfield FR, Silverstein SC: Fibrin regulates neutrophil migration in response to interleukin 8, leukotriene B4, tumor necrosis factor, and formyl-methionyl-leucyl-phenylalanine. J Exp Med 1995, 181:1763-1772 [DOI] [PMC free article] [PubMed] [Google Scholar]