Abstract
Knowledge of the role of cell-cycle regulators in the pathogenesis of acquired immune deficiency syndrome-related non-Hodgkin’s lymphomas (AIDS-NHLs) is scarce. Here we analyzed 86 systemic AIDS-NHLs and 20 AIDS-primary central nervous system lymphomas for expression of p27Kip1, a negative regulator of cell-cycle progression belonging to the Kip family of cyclin-dependent kinase inhibitors. In parallel, we investigated the relationship between p27Kip1, the lymphoma proliferation index, Epstein-Barr virus status, expression of cellular cyclin D3 and cyclin D1, and B-cell differentiation stage. We report that AIDS-immunoblastic lymphomas (AIDS-IBLs), either systemic or primarily localized to the central nervous system, consistently express p27Kip1 protein (19 of 24 and 10 of 14, respectively) despite the high proliferative rate of the lymphoma clone, suggesting a failure of p27Kip1 to inhibit the cell cycle in AIDS-IBL. Conversely, the remaining systemic AIDS-NHLs and AIDS-primary central nervous system lymphomas preferentially fail to express p27Kip1. Expression of p27Kip1 in Epstein-Barr virus-positive AIDS-NHLs generally associates with latent membrane protein 1 (LMP1) expression and is related to a late stage of B-cell differentiation, characterized by the BCL-6−/MUM1+/syn-1± phenotypic profile, whereas it seems to be unrelated to the expression of cellular cyclins. In B cells in vitro, induction of LMP-1 expression under the control of inducible promoters up-regulates expression of p27Kip1, thus providing a putative mechanistic explanation for the association between LMP1 and p27Kip1 observed in vivo. Overall, these data show that AIDS-IBL pathogenesis is characterized by loss of the inverse relationship between p27Kip1 positivity and tumor growth fraction that is otherwise generally observed in normal lymphoid tissues and in most other types of NHLs.
Acquired immune deficiency syndrome-related non-Hodgkin’s lymphomas (AIDS-NHLs) represent a significant source of morbidity and mortality among human immunodeficiency virus (HIV)-infected individuals. All AIDS-NHLs are characterized by extreme clinical aggressiveness, display a predilection for extranodal sites, and derive from B cells. Pathologically, however, AIDS-NHLs are markedly heterogeneous. 1,2 The pathological spectrum of AIDS-NHLs includes systemic AIDS-NHLs, primary central nervous system lymphomas (AIDS-PCNSLs), primary effusion lymphoma (AIDS-PEL), and plasmablastic lymphoma (AIDS-PBL) of the oral cavity. 1-7 Systemic AIDS-NHLs are histologically classified into AIDS-related Burkitt’s lymphoma (AIDS-BL) and AIDS-related diffuse large cell lymphomas that include AIDS-related large noncleaved cell lymphoma (AIDS-LNCCL) and AIDS-related immunoblastic lymphoma (AIDS-IBL). 4,5,8 AIDS-PCNSLs are classified into AIDS-LNCCL and AIDS-IBL. 2,4,5
The pathological heterogeneity of AIDS-NHL is consistent with the existence of multiple molecular pathways selectively associated with a given type of AIDS-NHL and implicated in the pathogenesis of these disorders. Despite their pathological heterogeneity, 9 most cases of AIDS-NHL are histogenetically related to germinal center (GC) or post-GC B cells, as indicated by the expression pattern of several B-cell phenotypic markers corresponding to different physiological stages of mature B-cell development. 1,10
The biological research performed on AIDS-NHLs during the last decade has led to significant discoveries that have clarified several topics regarding the pathogenesis of these lymphomas. 2 However, knowledge of the role of cell-cycle regulators in AIDS-lymphoma development and growth is more limited. 11 The cellular cyclins contribute to cell-cycle control by forming complexes with catalytic subunits termed cyclin-dependent kinases (CDKs). 12,13 The activation of the CDK/cyclin complex is controlled by CDK inhibitors that regulate cell cycle. 12 A major inhibitor of the CDK/cyclin complex is represented by p27Kip1, a nuclear phosphoprotein belonging to the Kip family of CDK inhibitors. 14-18 In physiological conditions, expression of p27Kip1 is highest in quiescent cells and declines as cells re-enter the cell cycle. In lymphoid tissues, p27Kip1 is expressed in nonproliferating lymphocytes, whereas activated lymphocytes, eg, GC cells, score consistently negative for p27Kip1 expression. 19 The inverse relationship between p27Kip1 expression and cell proliferation that is physiologically observed in normal lymphoid tissues is also encountered in most subtypes of NHL of immunocompetent hosts. 19-21
The aims of the present study were to establish the expression pattern of p27Kip1 in systemic AIDS-NHL and AIDS-PCNSL and to address the relationship between p27Kip1 expression, proliferation index, B-cell differentiation stage, viral status for Epstein-Barr virus (EBV), and expression of cellular cyclin D3 and cyclin D1 in these lymphomas.
Materials and Methods
Neoplastic Samples
This study was based on 106 AIDS-NHLs. All cases were of B-cell origin and included 86 systemic AIDS-NHLs and 20 AIDS-PCNSLs. Systemic AIDS-NHLs were classified according to the revised European-American classification of lymphoid neoplasms (REAL). 22 AIDS-BLs (from 29 patients) include cases displaying the histological features of classical endemic BLs and the so-called Burkitt-like lymphomas. 22 AIDS-LNCCLs (from 32 patients) usually contain large noncleaved cells that are intermediate in size between those of Burkitt’s and IBL. They have scant to moderately abundant cytoplasm and round nuclei containing one or more small distinct nucleoli adjacent to the nuclear membrane. Occasionally LNCCLs contain variable proportions or are composed entirely of neoplastic cells containing cleaved or multilobated nuclei. 9 AIDS-IBLs (from 24 patients) contain large neoplastic cells that usually have abundant acidophilic cytoplasm with evidence of plasmacytoid differentiation. All AIDS-PCNSLs were classified as LNCCL or IBL (a total of 16 patients). 9 AIDS-PCNSL cases containing a mixture of large noncleaved cells and large cells, immunoblastic plasmacytoid were classified separately as LNCCL/IBL (four patients). 23 Thus, the subject of the analysis is mainly a series of diffuse large-cell lymphoma patients. Tissues were fixed in Bouin solution or neutral-buffered formalin. In most cases, a portion of unfixed tissue was snap-frozen in liquid nitrogen and stored at −80°C. Detailed characterization of part of these cases has been reported previously. 1,10
Sixteen samples of IBL (similar in morphology and immunophenotype to the AIDS-IBL) from nonimmunosuppressed (HIV-seronegative) patients were also included in the study for comparative purposes.
Nonneoplastic Samples
Nonneoplastic lymph node (n = 93), nasopharynx (n = 30), and tonsil (n = 4) samples from a total of 100 HIV-infected patients with persistent generalized lymphadenopathy were also included in the study. The histopathological pattern of lymph nodes and tonsils was predominantly represented by hyperplastic changes of the lymphoid follicles. The nasopharynx exhibited the nasopharyngeal lymphoid tissue hypertrophy pattern related to HIV infection. 24
Immunohistochemical Studies
Immunohistochemistry was performed by the avidin-biotin-peroxidase complex (ABC-px) or alkaline phosphatase anti-alkaline phosphatase methods. 25,26
The expression of p27Kip1 was investigated with the monoclonal antibody (mAb) Kip-1 (Transduction Laboratories, Lexington, KY) or the mAb 1B4 (Novocastra Laboratories Ltd., Newcastle on Tyne, UK). The reactivity pattern of both antibodies recognizing p27Kip1 was tested in selected cases and was superimposable.
The proliferation index was assessed using the MIB-1 mAb (Immunotech, Marseille, France) directed against the Ki-67 nuclear proliferation antigen.
In selected cases (ie, p27Kip1-positive cases and a fraction of p27Kip1-negative cases), the expression of cyclin D1 and D3 was assayed. Cellular cyclin D1 was detected with the mAbs AM29 (Zymed Laboratories, San Francisco, CA); cyclin D3 was detected with the mAb DCS-22 (NeoMarkers, Inc., Fremont, CA).
All these antigens were tested on paraffin-embedded sections from cell blocks with a previous step of antigen retrieval. For p27Kip1 assessment, sections were treated twice in a microwave oven for 5 minutes in citrate buffer (pH 6); for Ki-67, sections were first treated with trypsin (Sigma Chemical Co., St. Louis, MO) (0.33 mg/ml) for 1 minute and then twice for 5 minutes in citrate buffer (pH 6) in a microwave oven at 650 W; for cyclin D1, Bouin-fixed sections were treated with trypsin (Sigma Chemical Co.) (0.2 mg/ml) for 5 minutes whereas formalin-fixed sections were treated for 30 minutes in citrate buffer (pH 7) in microwave oven at 250 W; for cyclin D3, sections were treated for 30 minutes in TEC (Tris-ethylenediaminetetraacetic acid-citrate) solution (pH 7.8) in a microwave oven at 250 W.
Immunocytochemical staining for p27Kip1, Ki-67, and cyclin D3 was performed using the ABC method 25 (ABC-Elite kit; Vector, Burlingame, California), whereas immunocytochemical staining for cellular cyclin D1 was performed on an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to the company’s protocols.
The expression of BCL-6, MUM1/IRF4, and CD138/syndecan-1 (syn-1), which are well-defined phenotypic markers of B-cell lymphoma histogenesis, 1,10 was tested on paraffin-embedded tissue sections as previously described. 10
In all AIDS-NHLs tested, the percentage of neoplastic cells showing positive staining for the different antigens (nuclear staining for p27Kip1, Ki-67, cyclins D1 and D3, BCL-6,s and MUM1; cytoplasmic staining for syn-1) was assessed in each case. The percentage of antigen-positive neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10 to 24%, 25 to 49%, 50 to 74%, and ≥75%. Only definite and unambiguous staining on unequivocal malignant cells was accepted as positive.
All neoplastic samples included in this study were subjected to determination of tumor infection by EBV as previously described. 27,28 The expression of EBV-encoded latent membrane protein 1 (LMP1) was tested on paraffin-embedded tissue sections of EBER-positive samples as previously described. 1,10 Immunostaining for LMP1 was performed with a LMP1-specific antibody (Dakopatts A/S, Glostrup, Denmark). 1,10 The percentage of LMP1-positive neoplastic cells was assigned to one of the following categories: 0, less than 10%, 10 to 24%, 25 to 49%, 50 to 74%, and ≥75%.
Expression of p27Kip1 in Nontransformed Lymphoid Tissues and Neoplastic Samples
The expression of p27Kip1 in reactive B cells within lymphoid tissues and in AIDS-NHLs was referred to the stages of physiological B-cell differentiation as defined by the combined expression of BCL-6, MUM1, and syn-1. 10 The expression pattern of p27Kip1 was also compared with that of LMP1 in AIDS-NHL. Serial sections were used to compare the immunoreactivity of these antibodies.
Cell Lines and Cell Transfection
Ramos cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal bovine serum. To generate cells expressing LMP1, 20 μg of plasmid DNA (pMEP4 or pMEP4-LMP1; EBV-based episomallly replicating vector) were first mixed with 1.0 × 107 cells in 0.4 ml of IMDM medium with 10% fetal bovine serum and then transfected into Ramos cells by electroporation using the Bio-Rad Gene Pulser apparatus (960 μF, 200 V) (Bio-Rad Laboratories, Hercules, CA). After transfection, cells were transferred to T25 flasks, incubated at 37°C for 48 hours, and then selected for transfected cells in IMDM with 10% fetal bovine serum containing 450 μg/ml of hygromycin and 0.5 mmol/L of ethylenediaminetetraacetic acid. Approximately 2 weeks later, the hygromycin-resistant populations were collected for analysis. For LMP1 induction, hygromycin and ethylenediaminetetraacetic acid were withdrawn from cell culture by washing with phosphate-buffered saline (PBS) for three times, then 1 μmol/L of cadmium chloride (CdCl2) was added to the cell culture, and the cells were harvested at various time points as indicated.
Western Blot Analysis
Proteins were extracted from cells by lysing with RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, 1.0% Nonidet P-40, 0.5% deoxycholat (DOC), 0.1% sodium dodecyl sulfate), separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose filters, and immunoassayed with anti-LMP1 (1:250, DAKO monoclonal anti-EBV, LMP), anti-p27Kip1 (1:1000, Transduction Laboratories), or anti-β-actin (1:1000, Sigma) antibodies. Secondary antibodies (anti-mouse Ig, horseradish peroxidase-linked whole antibody; Amersham Pharmacia Biotech, Piscataway, NJ) were used at 1: 3000 dilution and the results were developed by enhanced chemiluminescence (Amersham).
Molecular Studies
Genomic DNA was isolated by cell lysis followed by digestion with proteinase K and purified by salting-out extraction and precipitation by ethanol. 29 Mutations of the p53 gene (exons 5 through 9) were investigated by a combination of polymerase chain reaction-single chain conformation polymorphism and DNA direct sequencing, as previously reported. 30 DNA hypermethylation in the CpG islands of the p16INK4a and p73 genes was determined by methylation-specific polymerase chain reaction using previously described primers and strategies. 31,32
Statistical Methods
Fisher exact test was used to compare differences in discrete data, whereas correlations were computed by means of Spearman (r) rank-order coefficients. 33
Results
Relationship between p27Kip1 Expression and B-Cell Differentiation Stage as Defined by MUM1, BCL-6, and syn-1 in Nontransformed Lymphoid Tissues
Expression of p27Kip1 was referred to the stages of mature B-cell differentiation identified by the coordinated expression of BCL-6, MUM1, and syn-1 in reactive B cells within lymphoid tissues from HIV-infected individuals with persistent generalized lymphadenopathy (Figure 1, A to D) ▶ . According to this model, expression of p27Kip1 occurs early before B-cell entry in the GC, because it is found in resting B cells of the mantle zone but not in centroblasts and centrocytes of the GC (Figure 1A) ▶ . On B-cell activation and cell-cycle entry, topographically corresponding to the phases of B-cell transit through the GC, B cells undergo a decrease in p27Kip1 expression, switch-off BCL-6 and start to express MUM1 (Figure 1, B and C) ▶ . On GC exit, immunoblasts are still p27Kip1-negative (Figure 1A) ▶ , MUM1-positive, but lack plasma cell morphology. Finally, differentiation to plasma cells associates with expression of p27Kip1 and syn-1 (Figure 1D) ▶ though retaining expression of MUM1 (Figure 1, A to D) ▶ .
Figure 1.
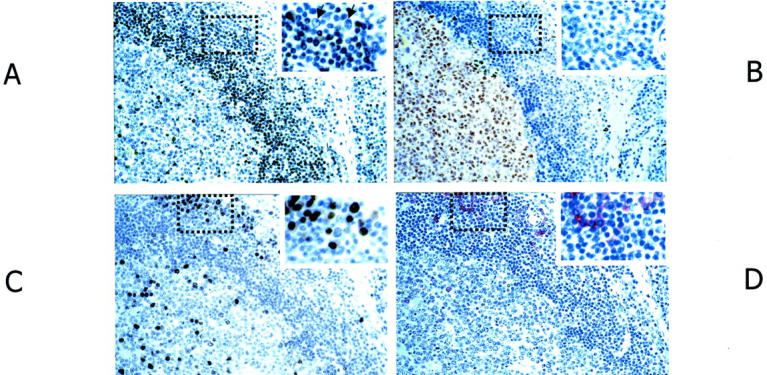
Relationship between p27Kip1 expression and B-cell differentiation stage as defined by BCL-6, MUM1, and syn-1 in nontransformed lymphoid tissues. Serial sections from hyperplastic lymph node from an HIV-infected individual with persistent generalized lymphadenopathy. A: A follicle with a large GC (left) and perifollicular/interfollicular areas (right) is shown. Numerous small lymphocytes in the follicular mantle zone exhibit nuclear staining (brown) for p27Kip1. Several plasma cells in the perifollicular areas (dotted area and inset) also score positive. p27Kip1 is absent in large blast cells in the perifollicular area (inset, arrows). B: Within the same follicle, numerous GC cells (centroblasts and centrocytes) exhibit nuclear staining (brown) for BCL-6, whereas B cells in the mantle zone and perifollicular/interfollicular areas score negative (see also dotted area and inset). C: Expression of MUM1 is restricted to a small subset of cells located in the GC and to a group of perifollicular plasma cells (dotted area and inset); the staining is nuclear (brown). D: In the same field, cells with a plasma cell morphology show a strong cytoplasmic and membrane staining (red) with the anti-syn-1 mAb. They are present within the perifollicular areas (dotted area and inset). Conversely, intrafollicular or extrafollicular cell populations other than plasma cells are devoid of syn-1 staining. Thus, the coordinated expression of BCL-6, MUM1, and syn-1 in reactive B cells shows that the expression of p27Kip1 occurs early before B-cell entry in the GC, because it is found in mantle zone B cells but not in GC B cells. During the phases of B-cell transit through the GC, B cells undergo a decrease in p27Kip1 expression, switch off BCL-6, and start to express MUM1. Finally, on GC exit and differentiation to plasma cells, B cells express p27Kip1 and syn-1. Paraffin-embedded tissue sections, immunoperoxidase method (A–C), alkaline phosphatase anti-alkaline phosphatase method (D), hematoxylin counterstain. Original magnifications, ×250.
p27Kip1 and Ki-67 Expression in AIDS-NHL
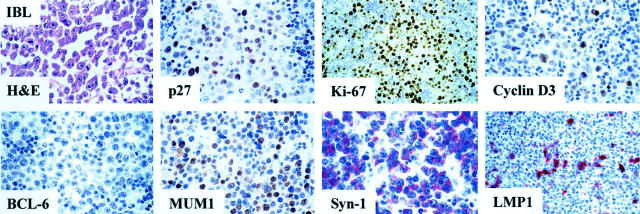
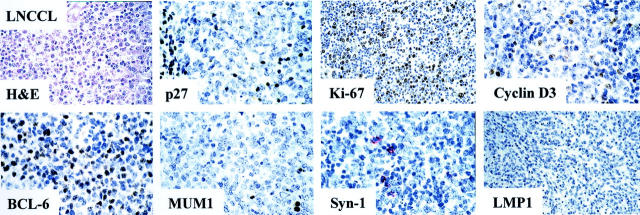
Results of the expression pattern of p27Kip1 in systemic AIDS-NHL are detailed in Table 1 ▶ . Representative examples are shown in Figures 2 and 3 ▶ ▶ . Overall, expression of p27Kip1 was detected in 24 of 86 (28%) cases of systemic AIDS-NHL and clustered with AIDS-IBL (19 of 24, 79%) (Figure 2) ▶ , whereas it was restricted to 4 of 29 (14%) cases of AIDS-BL (not shown) and to 1 of 33 (3%) cases of AIDS-LNCCL (Table 1) ▶ . Difference between AIDS-IBL and all other systemic AIDS-NHLs was statistically significant (P < 0.01). In the majority of positive cases, nuclear p27Kip1 staining consistently was of moderate to strong intensity.
Table 1.
p27Kip1 Protein Expression in Acquired Immune Deficiency Syndrome-Related Non-Hodgkin’s Lymphomas (AIDS-NHL)
| Percentage of neoplastic cells p27Kip1-positive | ||||||
|---|---|---|---|---|---|---|
| 0% | <10% | 10–24% | 25–49% | 50–74% | >75% | |
| Systemic AIDS-NHL* | ||||||
| BL (n = 29) | 25 | 0 | 0 | 0 | 2 | 2 |
| LNCCL (n = 33) | 32 | 1 | 0 | 0 | 0 | 0 |
| IBL (n = 24) | 5 | 2 | 1 | 5 | 5 | 6 |
| AIDS-PCNSL† | ||||||
| LNCCL (n = 2) | 1 | 0 | 0 | 1 | 0 | 0 |
| LNCCL/IBL (n = 4) | 2 | 1 | 0 | 1 | 0 | 0 |
| IBL (n = 14) | 4 | 0 | 2 | 3 | 5 | 0 |
Abbreviations: BL, Burkitt’s lymphoma; LNCCL, large noncleaved cell lymphoma; IBL, immunoblastic lymphoma; PCNSL, primary central nervous system lymphoma.
*P value <0.01 comparing p27Kip1 expression (negative versus positive) for AIDS-IBL versus all other systemic AIDS-NHL.
†P value = 0.61 comparing p27Kip1 expression (negative versus positive) for AIDS-IBL versus all other AIDS-PCNSL.
Figure 2.
Relationship between p27Kip1 expression and B-cell differentiation stage as defined by MUM1, BCL-6, and syn-1 in AIDS-related IBL. The figure shows a p27Kip1-positive IBL case that is associated with the BCL-6−/MUM1+/syn-1+ phenotypic pattern reflecting post-GC B cells. LMP1 is expressed by a fraction of large tumor cells. The staining is cytoplasmic and intense. In the figure numerous immunoblastic-plasmacytoid tumor cells show nuclear immunoreactivity with the anti-Ki-67 (MIB1) mAb. Furthermore, neoplastic cells display strong nuclear immunoreactivity with the anti-cyclin D3 mAb. Paraffin-embedded tissue sections, immunoperoxidase method (p27Kip1, Ki-67, cyclin D3, BCL-6, MUM1), alkaline phosphatase anti-alkaline phosphatase method (syn-1, LMP1), hematoxylin counterstain. Original magnifications, ×250.
Figure 3.
Relationship between p27Kip1 expression and B-cell differentiation stage as defined by MUM1, BCL-6, and syn-1 in AIDS-related large noncleaved cell lymphoma (LNCCL). The figure shows a p27Kip1-negative LNCCL case that is associated with the BCL-6+/MUM1−/syn-1− phenotypic pattern reflecting GC B cells. LMP1 is not expressed by tumor cells. In the figure several large tumor cells show nuclear immunoreactivity with the anti-Ki-67 (MIB1) mAb. Only few neoplastic cells display weak nuclear immunoreactivity with the anti-cyclin D3 mAb. Paraffin-embedded tissue sections, immunoperoxidase method (p27Kip1, Ki-67, cyclin D3, BCL-6, MUM1), alkaline phosphatase anti-alkaline phosphatase method (syn-1, LMP1), hematoxylin counterstain. Original magnifications, ×250.
Expression of the proliferation marker Ki-67 among cases of systemic AIDS-NHL occurred both in the presence and in the absence of p27Kip1 expression (Tables 1 and 2) ▶ ▶ . In most AIDS-IBL (17 of 24, 71%), the proliferative index of the neoplastic population was high (Ki-67 > 50%) (Table 2 ▶ , Figure 2 ▶ ) regardless of the high p27Kip1 expression. In the remaining systemic AIDS-IBL cases (7 of 24, 29%), the proliferative index of the neoplastic population was lower (25 to 49%, six cases and 10 to 24%, one case) (Table 2) ▶ and the expression of p27Kip1 was extremely variable ranging from negative (0%, two cases) to positive (25 to 49%, one case; 50 to 74%, one case; ≥75%, three cases). In contrast to AIDS-IBL, the histological types AIDS-BL and AIDS-LNCCL generally failed to express p27Kip1 (Figure 3) ▶ and displayed an inverse relationship between the proliferative index and p27Kip1 positivity (Tables 1 and 2) ▶ ▶ .
Table 2.
Ki-67 Expression in Acquired Immune Deficiency Syndrome-Related Non-Hodgkin’s Lymphomas (AIDS-NHL)
| Percentage of neoplastic cells Ki-67-positive | ||||||
|---|---|---|---|---|---|---|
| 0% | <10% | 10–24% | 25–49% | 50–74% | >75% | |
| Systemic AIDS-NHL | ||||||
| BL (n = 29) | 0 | 0 | 0 | 0 | 6 | 23 |
| LNCCL (n = 33) | 0 | 1 | 1 | 7 | 13 | 11 |
| IBL (n = 24) | 0 | 0 | 1 | 6 | 5 | 12 |
| AIDS-PCNSL | ||||||
| LNCCL (n = 2) | 0 | 1 | 0 | 0 | 0 | 1 |
| LNCCL/IBL (n = 4) | 0 | 3 | 0 | 0 | 1 | 0 |
| IBL (n = 14) | 0 | 4 | 0 | 1 | 9 | 0 |
Abbreviations: BL, Burkitt’s lymphoma; LNCCL, large noncleaved cell lymphoma; IBL, immunoblastic lymphoma; PCNSL, primary central nervous system lymphoma.
The results observed in systemic AIDS-NHL were also confirmed in AIDS-PCNSL (Table 1) ▶ . In particular, expression of p27Kip1 was detected in 13 of 20 (65%) cases of AIDS-PCNSL, including 10 of 14 AIDS-IBL (71%), 1 of 2 AIDS-LNCCL, and 2 of 4 AIDS-LNCCL/IBL. In the majority of positive cases, nuclear p27Kip1 staining was of moderate to strong intensity. Analogous to systemic AIDS-IBL, the proliferation index of the majority of AIDS-PCNSL with IBL morphology (10 of 14, 71%) was high (Ki-67 > 50%) regardless of p27Kip1 expression (Tables 1 and 2) ▶ ▶ .
For comparative purposes, 16 IBL cases from nonimmunosuppressed patients were included in the study. Expression of p27Kip1 was detected in 2 of 16 (12.5%) cases, whereas expression of the proliferation marker Ki-67 occurred in all cases. The proliferation index was high (Ki-67 > 50%) regardless of p27Kip1 expression.
Relationship between p27Kip1 Expression, LMP1, and Phenotypic Profile in AIDS-NHL
Among systemic AIDS-NHLs carrying EBV infection (38 of 73 cases, 52%), expression of the EBV-encoded LMP1 antigen was detected in 10 of 38 cases (26%) (Table 3) ▶ . Most cases of LMP1-positive systemic AIDS-NHL were morphologically classified as AIDS-IBL (9 of 10, 90%) (Table 3 ▶ and Figure 2 ▶ ), scored positive for p27Kip1 (6 of 10, 60%) (Table 3) ▶ , expressed both MUM1 and syn-1 (8 of 10, 80%), and stained negative for BCL-6 (10 of 10, 100%) (Table 4 ▶ and Figure 2 ▶ ). Conversely, expression of LMP1 was absent in 28 EBV-positive systemic AIDS-NHLs, which usually expressed BCL-6 (21 of 28, 75%) and failed to express p27Kip1(20 of 28, 71%) (Figure 3) ▶ . Overall, among systemic AIDS-NHL carrying EBV infection, p27Kip1 positivity correlates with expression of LMP1 (r = 0.24, P = 0.02) and preferentially associates with the BCL-6−/MUM1+/syn-1+ profile (Table 4) ▶ .
Table 3.
Relationship between p27Kip1 Expression and Latent Membrane Protein 1 (LMP1) in Acquired Immune Deficiency Syndrome-Related Non-Hodgkin’s Lymphomas (AIDS-NHL) Carrying EBV Infection
| LMP1+ | LMP1− | |||
|---|---|---|---|---|
| p27Kip1 + | p27Kip1 − | p27Kip1 + | p27Kip1 − | |
| Systemic AIDS-NHL | ||||
| BL (n = 12) | 0 | 0 | 1 | 11 |
| LNCCL (n = 10) | 0 | 1 | 1 | 8 |
| IBL (n = 16) | 6 | 3 | 6 | 1 |
| AIDS-PCNSL | ||||
| LNCCL (n = 2) | 0 | 0 | 1 | 1 |
| LNCCL/IBL (n = 4) | 2 | 2 | 0 | 0 |
| IBL (n = 14) | 10 | 4 | 0 | 0 |
Abbreviations: BL, Burkitt’s lymphoma; LNCCL, large noncleaved cell lymphoma; IBL, immunoblastic lymphoma; PCNSL, primary central nervous system lymphoma.
Table 4.
Relationship between p27Kip1 Expression and B-Cell Differentiation Stage as Defined by MUM1, BCL-6, and syn-1 in Acquired Immune Deficiency Syndrome-Related Non-Hodgkin’s Lymphomas (AIDS-NHL)
| Major phenotypic patterns | ||||||||
|---|---|---|---|---|---|---|---|---|
| BCL6+/MUM1−/syn-1− | BCL6+/MUM1+/syn-1− | BCL6−/MUM1+/syn-1− | BCL6−/MUM1+/syn-1+ | |||||
| p27+ | p27− | p27+ | p27− | p27+ | p27− | p27+ | p27− | |
| Systemic AIDS-NHL | ||||||||
| BL (n = 29) | 4 | 21 | 0 | 4 | 0 | 0 | 0 | 0 |
| LNCCL (n = 33) | 0 | 26 | 1 | 4 | 0 | 1 | 0 | 0 |
| IBL (n = 24) | 0 | 0 | 0 | 0 | 3 | 3 | 16 | 2 |
| AIDS-PCNSL | ||||||||
| LNCCL (n = 2) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| LNCCL/IBL (n = 4) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| IBL (n = 14) | 0 | 0 | 0 | 0 | 2 | 1 | 8 | 2 |
| EBV-positive (n = 58) | 1 | 17 | 3 | 3 | 4 | 4 | 18 | 4 |
| LMP1-positive (n = 28) | 0 | 0 | 1 | 0 | 2 | 3 | 14 | 4 |
Abbreviations: BL, Burkitt’s lymphoma; LNCCL, large noncleaved cell lymphoma; IBL, immunoblastic lymphoma; PCNSL, primary central nervous system lymphoma.
Minor phenotypic patterns included: BCL6−/MUM1−/syn1− in one systemic NHL (with LNCCL morphology) that scored negative for p27Kip1; BCL6−/MUM1−/syn1+ in one PCNSL (with IBL morphology) that scored negative for p27Kip1; BCL6+/MUM1+/syn1+ in two PCNSL (with LNCCL/IBL morphology) one of which scored positive for p27Kip1; and BCL6+/MUM1−/syn1+ in one PCNSL (with LNCCL/IBL morphology) that scored negative for p27Kip1.
With respect to AIDS-PCNSL, expression of LMP1 was detected in 18 of 20 cases. All LMP1-positive AIDS-PCNSLs were morphologically classified as AIDS-IBL or LNCCL/IBL. Most of these cases expressed p27Kip1 (12 of 18, 67%), MUM1 (14 of 18, 78%) and/or syn-1 (13 of 18, 72%), and stained negative for BCL-6 (14 of 18, 78%). Expression of LMP1 was negative in the AIDS-PCNSL with AIDS-LNCCL morphology.
All IBL from nonimmunosuppressed patients were EBV-negative and, in most cases, displayed the BCL-6−/MUM1+/syn-1− phenotype.
LMP1 Induces the Expression of the CDK Inhibitor p27Kip1
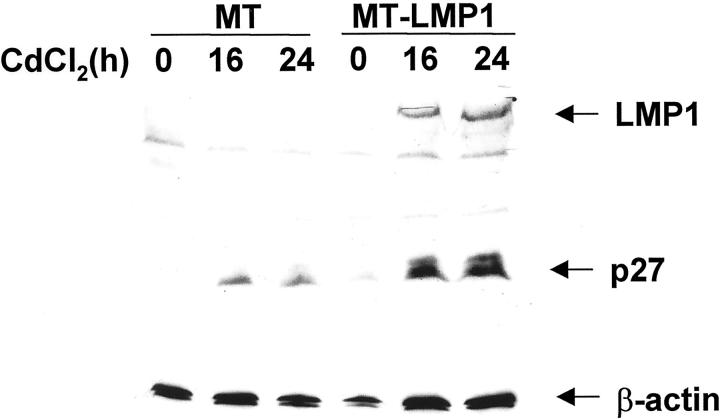
To determine whether LMP1 expression affects the expression of p27Kip1 in B cells, we established a Ramos cell line (MT-LMP1) stably transfected with a vector expressing LMP1 under the control of the CdCl2-inducible metallothionein promoter (MT). Figure 4 ▶ shows that, on treatment with CdCl2, LMP1 expression was readily detected in cells transfected with MT-LMP1, but not in control cells transfected with the same vector lacking LMP1. As shown in Figure 4 ▶ , induction of LMP1 expression in B cells is associated with induction of p27Kip1 expression. Overall, these results show that LMP1 is capable of inducing p27Kip1 expression in B cells.
Figure 4.
Induction of LMP1 expression activates p27Kip1 expression. Ramos cells were stably transfected with an expression vector (MT-LMP1) in which LMP1 expression was dependent on the metallothionein promoter, or with empty control vector (MT). The stably transfected cells were treated with CdCl2 for 16 hours or 24 hours as indicated, and then LMP1 expression (top), p27Kip1 expression (middle), and β-actin expression (bottom, as a loading control) were analyzed by Western immunoblotting using specific antibodies. The results show that p27Kip1 is induced on LMP1 induction. A modest p27Kip1 induction is observed also in control cells because of CdCl2 treatment.
Cyclin D1 and Cyclin D3 Expression in Systemic AIDS-NHL
Cyclin D1 was tested in 71 systemic AIDS-NHLs (26 AIDS-BLs, 24 AIDS-LNCCLs, 21 AIDS-IBLs). All cases were considered negative for cyclin D1 expression. Sixty-three cases did not show any staining. Conversely, eight cases (1 of 26 AIDS-BLs, 3 of 24 AIDS-LNCCLs, and 4 of 21 AIDS-IBLs) displayed cyclin D1 positivity in scattered and occasional cells.
Cyclin D3 was tested in 55 systemic AIDS-NHLs (16 AIDS-BLs, 16 AIDS-LNCCLs, 23 AIDS-IBLs). Cyclin D3 scored positive in a large fraction of cases (47 of 55, 85%), including 14 of 16 (87.5%) AIDS-BLs, 15 of 16 (94%) AIDS-LNCCLs, and 18 of 23 (78%) AIDS-IBLs. Cyclin D3 positivity occurred both in the presence (4 AIDS-BLs, 15 AIDS-IBLs) and in the absence of p27Kip1 expression (10 AIDS-BLs, 15 AIDS-LNCCLs, and 3 AIDS-IBLs) (Figures 2 and 3) ▶ ▶ . Cyclin D3 was also tested in 10 AIDS-PCNSLs (2 AIDS-LNCCLs, 6 AIDS-IBLs, 2 AIDS-LNCCL/IBLs). Cyclin D3 could be observed only in two AIDS-IBLs that scored positive also for p27Kip1 expression (not shown).
Molecular Alterations of the p53, p16INK4a, and p73 Genes
Mutations of the p53 tumor suppressor gene were detected in 4 of 22 tested cases of AIDS-NHL. CpG island hypermethylation of the p16INK4a and p73 genes were observed in 7 of 21 and 2 of 17 AIDS-NHLs, respectively. The occurrence of molecular lesions of p53, p16INK4a, and p73 genes in AIDS-NHL did not show a preferential association with a given expression pattern of p27Kip1 expression.
Discussion
In this study, we analyzed a large panel of systemic AIDS-NHLs and AIDS-PCNSLs for the expression of p27Kip1 in relation to the lymphoma proliferation index, B-cell differentiation stage, EBV status, and expression of cellular cyclins D3 and D1. We report that AIDS-IBLs, either systemic or primarily localized to the central nervous system, consistently express p27Kip1 despite the high proliferative rate of the lymphoma clone, suggesting a failure of p27Kip1 to inhibit the cell cycle in AIDS-IBL cells. The co-existence of p27Kip1 expression and high proliferative index is a selective feature of AIDS-IBLs among systemic AIDS-NHLs and AIDS-PCNSLs and may be because of concomitant expression of the EBV-encoded LMP1 and/or to peculiarities in the differentiation stage of the lymphoma clone.
The role of EBV in determining the co-existence of p27Kip1 and high proliferative index in AIDS-IBLs is supported by the observation that LMP1 is generally expressed by p27Kip1-positive AIDS-IBLs in vivo and is able to induce p27Kip1 expression in B cells in vitro. Conceivably, up-regulation of p27Kip1 may be mediated by regulation of BCL-6 expression. In fact, LMP1 is able to down-regulate expression of BCL-6, 1,34 that, in turn, physiologically exerts a negative effect on p27Kip1 expression. 35 Thus, by removing a negative regulator of p27Kip1, namely BCL-6, LMP1 allows for up-regulation of p27Kip1 expression. According to this model, it may be argued that LMP1 is able to stimulate the lymphoma growth and cell-cycle progression despite the high expression of the cell-cycle inhibitor p27Kip1. In particular, it may be envisaged that expression of LMP1 by AIDS-IBLs induces and simultaneously overcomes the p27Kip1-mediated inhibition of cell growth. The precise molecular mechanism allowing LMP1 to overcome p27Kip1-mediated cell-cycle inhibition in lymphoma cells is not known and may be related to currently unexplored molecular derangements proper of the tumor clone. However, because some p27Kip1-positive AIDS-IBL cases are LMP1-negative, it is conceivable that EBV infection may determine the co-existence of p27Kip1 and high proliferative index in AIDS-IBL also by mechanisms that are independent of LMP1 expression.
The co-existence of p27Kip1 expression and high proliferative index in AIDS-IBL may also be related to the differentiation stage of the lymphoma clone, that is generally characterized by the BCL-6−/MUM1+/syn-1+ phenotype of preterminally and terminally differentiated B cells. Indeed, in normal physiology, expression of p27Kip1 associates with B cells that have exited the GC and differentiate to plasma cells, as indicated by acquisition of the BCL-6−/MUM1+/syn-1+ phenotype. Conversely, GC centroblasts and centrocytes, characterized by the BCL-6+/MUM1−/+/syn-1− phenotype, fail to express p27Kip1. The hypothesis that p27Kip1 expression in AIDS-IBL may be related to the tumor differentiation stage is reinforced by the notion that, among AIDS large-cell lymphomas, co-expression of p27Kip1 and Ki-67 is virtually restricted to AIDS-IBL (this study) and to primary effusion lymphoma, 10,11 a rare lymphoma type associated with HHV-8 infection and constituted of preterminal B cells displaying the BCL-6−/MUM1+/syn-1+ phenotype typical also of AIDS-IBL.
Co-expression of p27Kip1 and Ki-67 is a preferential feature of AIDS-IBL also considering tissue-based large B-cell lymphomas of the immunocompetent host. In fact, with the exception of few B-diffuse large-cell lymphomas, 36 the blastic variant of mantle cell lymphoma 21 and a fraction of Burkitt’s lymphoma, 36 NHL of the immunocompetent host generally display an inverse relation between p27Kip1 and growth fraction. 19-21 In particular, our data indicate that regulation of p27Kip1 differs markedly in IBL of immunocompetent hosts compared to AIDS-IBL, because IBL of immunocompetent hosts display the inverse relation between p27Kip1 expression and growth fraction and, accordingly, score negative for p27Kip1 in virtually all cases. Differences in p27Kip1 expression in AIDS-IBL versus IBL of immunocompetent hosts may reflect differences in the virological features and/or the differentiation stage of the lymphoma. In fact, expression of the EBV-encoded LMP-1, which is capable of up-regulating p27Kip1, is restricted to AIDS-IBL, whereas it is consistently negative in IBL of immunocompetent hosts. Also, AIDS-IBLs generally display the BCL-6−/MUM1+/syn-1+ phenotype, that associates with p27Kip1 expression in B-cell physiology, whereas IBL of immunocompetent hosts show a lesser degree of differentiation, identified by the BCL-6−/MUM1+/syn-1− phenotype that normally fails to express p27Kip1.
Footnotes
Address reprint requests to Prof. Antonino Carbone, Division of Pathology, Centro di Riferimento Oncologico, Istituto di Ricovero e Cura a Carattere Scientifico, Istituto Nazionale Tumori, Via Pedemontana Occidentale, Aviano I-33081, Italy. E-mail: acarbone@cro.it.
Supported in part by the Istituto Superiore di Sanità, III Programma Nazionale di Ricerca Sull’AIDS-Progetto Patologia Clinica e Terapia dell’AIDS; the Ministero della Sanita’ (RF 1999); the Associazione Italiana per la Ricerca sul Cancro; and the National Institutes of Health (grant CA-37295).
References
- 1.Carbone A, Gaidano G, Gloghini A, Larocca LM, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R: Differential expression of BCL-6, CD138/syndecan-1 and EBV-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood 1998, 91:747-755 [PubMed] [Google Scholar]
- 2.Gaidano G, Carbone A, Dalla-Favera R: Pathogenesis of AIDS-related lymphomas. Molecular and histogenetic heterogeneity. Am J Pathol 1998, 152:623-630 [PMC free article] [PubMed] [Google Scholar]
- 3.Levine AM: AIDS-related malignancies: the emerging epidemic. J Natl Cancer Inst 1993, 85:1382-1397 [DOI] [PubMed] [Google Scholar]
- 4.Gaidano G, Carbone A: AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol 1995, 90:235-243 [DOI] [PubMed] [Google Scholar]
- 5.Knowles DM: Molecular pathology of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma. Semin Diagn Pathol 1997, 14:67-82 [PubMed] [Google Scholar]
- 6.Dal Maso L, Rezza G, Zambon P, Tagliabue G, Crocetti E, Vercelli M, Zanetti R, Falcini F, Tonini G, Mangone L, De Lisi V, Ferretti S, Tumino R, Stanta G, Vitarelli S, Serraino D, Franceschi S: Non-Hodgkin lymphoma among young adults with and without AIDS in Italy. Int J Cancer 2001, 19:430-435 [DOI] [PubMed] [Google Scholar]
- 7.Goedert JJ, Coté TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, Jaffe ES, Biggar RJ: Spectrum of AIDS-associated malignant disorders. Lancet 1998, 351:1833-1839 [DOI] [PubMed] [Google Scholar]
- 8.: Non-Hodgkin’s lymphoma pathologic classification project: National Cancer Institute sponsored study of classification of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. Cancer 1982, 49:2112-2135 [DOI] [PubMed] [Google Scholar]
- 9.Knowles DM, Pirog C: Pathology of AIDS-related lymphomas and other AIDS-defining neoplasms. Eur J Cancer 2001, 37:1236-1250 [DOI] [PubMed] [Google Scholar]
- 10.Carbone A, Gloghini A, Larocca LM, Capello D, Pierconti F, Canzonieri V, Tirelli U, Dalla-Favera R, Gaidano G: Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood 2001, 97:744-751 [DOI] [PubMed] [Google Scholar]
- 11.Carbone A, Gloghini A, Bontempo D, Monini P, Tirelli U, Volpe R, Browning PJ, Gaidano G: Proliferation in HHV-8 positive primary effusion lymphoma is associated with expression of HHV-8 cyclin but independent of p27Kip1. Am J Pathol 2000, 156:1209-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grana X, Reddy EP: Cell cycle control in mammalian cells: role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11:211-219 [PubMed] [Google Scholar]
- 13.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 14.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 15.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 16.Reynisdóttir I, Polyak K, Iavarone A, Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 1995, 9:1831-1845 [DOI] [PubMed] [Google Scholar]
- 17.Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A: Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ 1996, 7:135-146 [PubMed] [Google Scholar]
- 18.Cheng M, Sexl V, Sherr CJ, Roussel MF: Assembly of cyclin D-dependent kinase and titration of p27/KIP1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc Natl Acad Sci USA 1998, 95:1091-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Beato M, Sáez AI, Martínez-Montero JC, Mateo MS, Sánchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin dependent kinase inhibitor p27KIP1 in lymphoid tissue. p27KIP1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 20.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G: Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas. Prognostic implications. Blood 1998, 92:770-777 [PubMed] [Google Scholar]
- 21.Quintanilla-Martinez L, Thieblemont C, Fend F, Kumar S, Pinyol M, Campo E, Jaffe ES, Raffeld M: Mantle cell lymphomas lack expression of p27 Kip1 a cyclin-dependent kinase inhibitor. Am J Pathol 1998, 153:175-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri S, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 23.Larocca LM, Capello D, Rinelli A, Nori S, Antinori A, Gloghini A, Cingolani A, Migliazza A, Saglio G, Camilleri-Broet S, Raphael M, Carbone A, Gaidano G: The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B-cells. Blood 1998, 92:1011-1019 [PubMed] [Google Scholar]
- 24.Carbone A, Gloghini A, Vaccher E, Barzan L, Tirelli U: Nasopharyngeal lymphoid tissue masses in patients with human immunodeficiency virus-1 (letter). Cancer 1995, 76:527-528 [DOI] [PubMed] [Google Scholar]
- 25.Hsu S-M, Raine L, Fanger H: A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol 1981, 75:734-738 [DOI] [PubMed] [Google Scholar]
- 26.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, Macdonald S, Pulford KAF, Stein H, Mason DY: Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal antialkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 27.Carbone A, Gaidano G, Gloghini A, Pastore C, Saglio G, Tirelli U, Dalla-Favera R, Falini B: BCL-6 protein expression in AIDS-related non-Hodgkin’s lymphomas: inverse relationship with Epstein-Barr virus-encoded latent membrane protein-1 expression. Am J Pathol 1997, 150:155-165 [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone A, Gloghini A, Gaidano G, Cilia AM, Bassi P, Polito P, Vaccher E, Saglio G, Tirelli U: AIDS-related Burkitt’s lymphoma. Morphologic and immunophenotypic study of biopsy specimens. Am J Clin Pathol 1995, 103:561-567 [DOI] [PubMed] [Google Scholar]
- 29.Miller SA, Dykes DD, Polesky HF: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988, 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, Magrath IT, Knowles DM, Dalla-Favera R: p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA 1991, 88:5413-5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corn PG, Kuerbitz SJ, van Noesel MM, Esteller M, Compitello N, Baylin SB, Herman JG: Transcriptional silencing of the p73 gene in acute lymphoblastic leukemia and Burkitt’s lymphoma is associated with 5′ CpG island methylation. Cancer Res 1999, 59:3352-3356 [PubMed] [Google Scholar]
- 32.Esteller M, Tortola S, Toyota M, Capella G, Peinado MA, Baylin SB, Herman JG: Hypermethylation-associated inactivation of p14ARF is independent of p16INK4a methylation and p53 mutational status. Cancer Res 2000, 60:129-133 [PubMed] [Google Scholar]
- 33.Armitage P, Berry G: Statistical Methods in Medical Research 1987. Blackwell Scientific Publications, Oxford
- 34.Cattoretti G, Zhang J, Cleary AM, Lederman S, Gaidano G, Carbone A, Chaganti RSK, Dalla-Favera R: Downregulation of BCL-6 gene expression by CD40 and EBV latent membrane protein-1 (LMP-1) and its block in lymphoma carrying BCL-6 rearrangements. Blood 1997, 90:175A [Google Scholar]
- 35.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM: B-cell represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000, 13:199-212 [DOI] [PubMed] [Google Scholar]
- 36.Sánchez-Beato M, Camacho FI, Martínez-Montero JC, Sáez AI, Villuendas R, Sánchez-Verde L, García JF, Piris MA: Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpressison. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 1999, 94:765-772 [PubMed] [Google Scholar]