Abstract
Platelet-derived growth factor (PDGF), a potent chemotactic and proliferation factor for mesenchymal-derived cells, has been demonstrated to play critical roles in kidney development. Two receptors for PDGF, PDGFR-α and PDGFR-β, have been identified and we previously analyzed the effects of blockade of PDGFR-α signal in neonatal mice. In the current study, we examined the role of PDGFR-β in glomerular development by blocking PDGFR-β signal in neonatal mice by administration of antagonistic anti-PDGFR-β monoclonal antibody. Unlike the mice injected with anti-PDGFR-α antibody, the mice injected daily with anti-PDGFR-β antibody could be kept alive at least for 2 weeks after birth but showed severe disruption of the glomerular structure, whereas no apparent deformation was observed in the collecting ducts. In the disrupted glomeruli, the number of the mesangial cells was reduced markedly. Electron microscopic analysis and immunohistochemical studies with terminal deoxynucleotidyl transferase nick-end labeling staining revealed that the capillary endothelial cells of the glomeruli in the outer cortex region underwent apoptosis. However, the glomeruli located near the medulla were less affected. Because PDGFR-β is not expressed in the endothelial cells, the effects of the blockade of PDGFR-β might have caused glomerular endothelial cell apoptosis by inducing the loss of mesangial cells and/or pericytes.
Platelet-derived growth factor (PDGF) exists as disulfide-linked dimers of four homologous polypeptide chains, PDGF-A, PDGF-B, 1 and the recently identified PDGF-C 2 and PDGF-D. 3,4 The polyfunctional effect of PDGFs on various cells suggest their roles in multiple processes such as cell proliferation, survival, and chemotaxis, as well as wound healing, 5 gastrulation, 6 glial cell development, 7 angiogenesis, 8 and atherosclerosis. 9
Each of these PDGF chains has a different receptor affinity. Although the PDGF receptor (PDGFR)-α binds and is activated by PDGF-A, -B, or -C, the PDGFR-β is activated exclusively by PDGF-BB and PDGF-DD. It is therefore conceivable that the two PDGFRs transmit functionally specific signals, and the capability of these receptors to activate PDGF-induced responses might not be identical. For example, PDGFR-β can promote chemotaxis in certain cell types such as vascular smooth muscle cells, whereas the PDGFR-α cannot. 10,11 Interestingly, exposure of these cells to PDGF-BB significantly activates their migration, whereas PDGF-AA shows inhibitory effects on the cell migration. Similarly, although both PDGFRs can trigger mitogenesis, only the PDGFR-β is efficient at driving cellular transformation, and inhibition of PDGFR-α signaling enhances this event. 12-14 Thus, in some cell types, the PDGFR-β is able to mediate PDGF-induced reactions, whereas the PDGFR-α cannot.
Vasculogenesis, development of the vascular system during the body formation, is composed of complex process, in which coordinated migration and assembly of vascular endothelial cells and smooth muscle cells play pivotal roles. Because PDGF is believed to be one of the most potent proliferative and chemotactic factors for vascular smooth muscle cells, it would be conceivable that the PDGF pathway could be involved in vascular morphogenesis. To test this hypothesis, the developmental process of the glomerulus or corpuscle of the kidney offers an ideal experimental system, as developmental assembly of endothelial, mesangial, and epithelial cells into glomerular vascular capillaries requires a coordinated and temporally and spatially defined series of steps in an anatomically ordered sequence. The vascular network or tuft is surrounded by a layer of visceral epithelial cells and the parietal layer of the Bowman capsule. During embryogenesis, a cleft-like structure, in which glomerular vasculature develops, is formed at the pole of the S-shaped body directly opposite the pole destined to join the ureteric duct. As compared with that of tubular structures, however, the molecular basis of glomerular development has, thus far, received less attention. 15
PDGF-B and PDGFR-β mutant mice had virtually identical phenotype during embryonic stage, 16,17 both showing markedly abnormal glomerular development with an absence of mesangial cells and glomerular capillary tufts. Although the absence of mesangial cells was correlated with endothelial hyperplasia and an increase in capillary diameter, 18 the glomerular components except for mesangial cells and endothelial cells were almost unchanged, and basement membrane and podocytes appeared within normal limits. Although these observations suggested that the PDGFR-β signal is critical in the embryonic stage, the search for the molecular mechanism behind the role of PDGFR-β has been elusive because of embryonic lethality. One way to get around the limitation would be a tissue-specific gene disruption. An example of this is the bacteriophage site-directed recombination system (Cre-lox). Although the Cre-lox system has been used in vivo, the major problem is the lack of truly tissue-specific promoters and the long time it takes to create the mice. Another method to avoid the problem is to use antagonistic reagents or antibodies. This strategy depends on specific reagent or antibody, for example specific neutralized monoclonal antibody.
Accordingly, we introduced a new strategy by administration of antagonistic rat monoclonal anti-murine PDGFR antibodies into neonatal mice to dissect the signal transduction pathways from the two PDGFRs, which could be responsible for such distinct cellular actions. For this purpose, we have created two antagonistic rat monoclonal anti-murine PDGFR antibodies, APA5 and APB5, with high specificity for PDGFR-α and PDGFR-β, respectively.
We have already reported that the blockade of the PDGFR-α pathway by administration of APA5 in newborn mice interfered with the formation of the hair canal, dermal mesenchyme, and hair follicles. 19 In the present study, we administered APB5, an antagonistic anti-murine PDGFR-β monoclonal antibody, to neonatal or adult mice to analyze the function of the PDGFR-β. The neonatal mice injected with APB5 were kept alive at least 2 weeks from birth. We found that in those mice the blockade of PDGFR-β signals caused deformation of the glomerular capillary and apoptosis of the glomerular endothelial cells. In contrast, little change was observed in adult mice that had already completed formation of the glomerular vascular system. These results suggest that the PDGFR-β pathway could play an essential role in vasculogenesis as far as renal vascular development is in progress, but would have less significance after the assembly of the vascular component is completed.
Materials and Methods
Administration of Rat Monoclonal Antibody to Mice
Pregnant ICR female mice were purchased from Japan SLC Inc. (Shizuoka, Japan). The mice were kept in a temperature-controlled facility on a 14-hour light/10-hour dark cycle with free access to food and water.
The preparation of APB5, a rat monoclonal anti-murine PDGFR-β antibody (IgG2a), and its antagonistic effects on the PDGFR-β signal transduction pathway in vivo and in vitro were described previously. 9 An isotype-matched irrelevant rat IgG was used for the control study. These antibodies were purified by 50% ammonium sulfate precipitation.
The bearing day of the newborn mice were defined as 0 day post partum (dpp) and the mice were injected intraperitoneally everyday with 100 μg of either APB5 or control IgG until 13 dpp. The dose usage of the antibodies was based on the animal weight and deduced from our previous study with adult mice. 9 The mice were killed at 3, 9, and 13 dpp (Figure 1) ▶ . For adult mice study, the ICR female mice at 8 weeks of age were injected intraperitoneally every other day with 1 mg of each antibody for 3 weeks as reported previously. 9
Figure 1.
The experimental protocol was designed to study whether APB5 affects the vascular system in the kidney glomeruli. The ICR pregnant mice were purchased. The bearing day of the newborn mice was defined as 0 dpp and athe mice were injected intraperitoneally with 100 μg of APB5 or control irrelevant rat IgG daily. Then 1, 3, 9, and 13 dpp mice were killed, and the kidneys were removed.
Histochemistry
Briefly, each mouse was killed and the kidneys were removed. The kidneys were fixed with ethyl Carnoy’s solution and embedded in paraffin. Sections (3-μm thick) were stained with periodic acid-methenamine-silver (PAM).
For hematoxylin and eosin (H&E) staining, the kidneys of neonatal mice were fixed in 4% paraformaldehyde before paraffin embedding. Then the tissues were routinely slide-mounted. The slides were stained with Meyer’s H&E (Wako Pure Chemical Industries, Osaka, Japan).
Terminal Deoxynucleotidyl Transferase Nick-End Labeling (TUNEL) Assay
The apoptotic cells were detected with TUNEL assay using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostic, Mannheim, Germany), according to the specifications and instructions recommended by the manufacturer. To quantify apoptosis, the sections from APB5-treated mice (n = 5) or control mice (n = 5) were applied to TUNEL staining followed by counterstaining with Meyer’s hematoxylin solution. The cells that existed within 50 μm from the surface of kidney cortex from each mouse were examined. For each mouse, five microscopic fields at a magnification of ×200 were randomly selected and the proportion of the TUNEL-positive cells to the total cells was calculated and subjected to statistical analysis. The apoptotic rate was presented as the number of apoptotic cells per 1000 nuclei. Furthermore we calculated the ratio of the number of glomeruli containing apoptotic cells to the total number of glomeruli in the cortex.
Immunohistochemistry
The tissue preparation was conducted as described previously. 9 Briefly, the kidney was removed, snap-frozen in O.C.T. compound (Sakura Finetek, Tokyo, Japan), and sectioned at 6 μm. This section was subjected to staining with a mouse monoclonal antibody, 3B4, against vimentin labeled with a horseradish peroxidase/enhanced polymer one-step staining (EPOS) system (DAKO A/S, Glostrup, Denmark). Immunoreactivity was detected using diaminobenzidine. Sections were counterstained with Meyer’s hematoxylin solution.
To detect apoptotic cells and endothelial cells, sections were subjected to double staining with TUNEL and anti-Flk1 antibody, respectively. After labeling first with TUNEL as described above, the sections were blocked by the avidin/biotin-blocking kit (Vector Laboratories, Inc., Burlingame, CA). After further blocking with phosphate-buffered saline containing with 0.1% bovine serum albumin and 10% serum, the sections were stained with biotinylated anti-murine Flk1 antibody. 20 Flk1-positive signals were amplified by the Vectastain Elite ABC kit (Vector Laboratories, Inc.) and reacted with Texas-red avidin (ICN Biomedicals Inc., Aurora, OH). Specimens were observed with a Zeiss microscope (Jena, Germany) equipped with proper filters.
Electron Microscopy
ICR mouse kidney tissue samples were cut into 1-mm cubes and fixed for 4 hours at 4°C in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer. They were postfixed in 1% buffered osmium tetroxide, dehydrated through graded ethanols, and embedded in epoxy resin. Thin sections (80 nm) were cut with a diamond knife, collected on 300-mesh copper or nickel grids and double-stained with uranyl acetate and lead citrate before examination using an electron microscope (H-700; Hitachi, Tokyo, Japan).
Statistical Analysis
Apoptotic rate data were expressed as means ± SD and were analyzed by Student’s t-test using Statview software (Abacus Concepts Inc., Berkeley, CA).
Results
Blockade of PDGFR-β Pathway Disrupted Glomerular Development in Neonatal Mice
We first examined whether the blockade of the signal transduction pathway through the PDGFR-β could affect glomerular capillary formation in vivo. According to the experimental protocol (Figure 1) ▶ , 100 μg of APB5 or irrelevant IgG was administered intraperitoneally to the neonatal mice daily. The mice were killed at 3, 9, or 13 dpp and kidneys were subjected to histochemical analysis. When glomeruli from the mice killed at 1 dpp were studied by PAM staining, no difference was observed between the mice injected with APB5 (Figure 2B) ▶ and with irrelevant IgG (Figure 2A) ▶ . In contrast, at 3 dpp, the glomerular cell population decreased in the mice that had been injected with APB5 (Figure 2, D and F) ▶ . The glomeruli with deformed capillaries in the representative section are indicated by the arrow in Figure 2D ▶ . These phenotypes were similar to the findings reported previously on the homozygous PDGFR-β-deficient mouse embryo. 16 When APB5-treated mice were killed at 9 dpp, the capillary tufts were almost lost in the glomeruli located in the outer cortex (Figure 2, H and J) ▶ . In contrast, injection with control IgG caused none of such changes in the mice killed either at 3 dpp or at 9 dpp (Figure 2; C, E, G, and I) ▶ .
Figure 2.
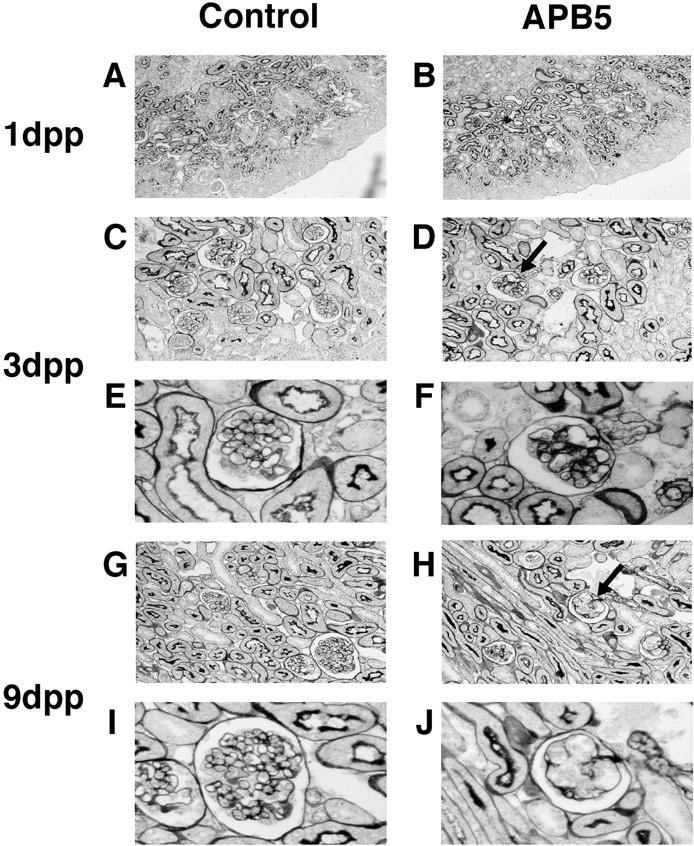
Photomicrographs of the kidney from neonatal mice. The kidneys from the mice injected with rat monoclonal antibodies were stained with PAM at 1 dpp (A and B), 3 dpp (C–F), and 9 dpp (G–J) according to Figure 1 ▶ . The kidneys of the mice administered with control irrelevant rat IgG and APB5 are shown in A, C, E, G, I, and in B, D, F, H, and J, respectively. A and B: No obvious changes were detected between control and APB5-treated mice at 1 dpp. D and F: The capillary tufts began to rupture in the kidney of the mouse administered with APB5 (indicated by the arrow in D and high magnification in F). C and E: No apparent deformation was observed in the kidney of the mouse treated with control IgG. H and J: At 9 dpp, the rupture of the glomerular capillary was more marked in the mouse administered with APB5 than at 3 dpp and glomerular cells decreased (deformed glomeruli are indicated by the arrow in H). G and I: The mouse injected with control IgG showed no apparent glomerular deformation. Original magnifications: ×100 (A and B); ×200 (C, D, E, and F), ×1000 (E, F, I, and J).
To further study the effect of APB5 administration on glomerular morphogenesis in the mice killed at 9 dpp, we conducted histochemical analysis with anti-vimentin antibody. The glomeruli with deformed capillaries in the representative section are indicated in Figure 3, A and B ▶ . Interestingly, the glomeruli found in the region near the medulla were less affected (Figure 3, A and C) ▶ . We also estimated the proportion of the glomeruli with deformed capillary tufts. The proportions of the glomeruli affected by APB5 administration were 27.7% (91 of 328 glomeruli examined in total) and 70.0% (505 of 726 glomeruli examined) at 3 dpp and 9 dpp, respectively. Administration of APB5 did not cause a significant change either in the size of the kidney or in the body weight throughout the experimental period (data not shown).
Figure 3.
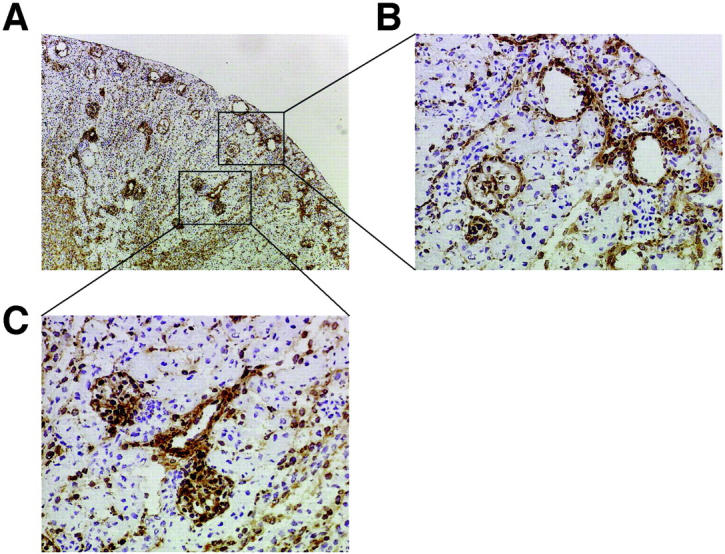
Photomicrographs of the kidney immunostained with anti-vimentin antibody. The kidney was obtained from 9 dpp mice injected with 100 μg per day of APB5. A: The fresh-frozen section was immunostained with anti-vimentin antibody. The outer cortex area and the region near the medulla are indicated by the rectangles. B: The glomeruli in the outer cortex. Notice the rupture of glomerular capillaries. C: The glomeruli in the region near the medulla that were less affected by APB5 injection. Original magnifications: ×100: (A); ×400 (B and C).
PAM Staining of Adult Mice Kidney
We further studied whether APB5 could cause deformation of adult mouse glomeruli. For this purpose, 1 mg of APB5 or control irrelevant rat IgG was administered intraperitoneally to each mouse on alternate days from 8 to 11 weeks of age. At the end of the experiment, the animals were killed and the freshly removed kidneys were fixed and stained with PAM. As shown in Figure 4 ▶ , the glomeruli of the APB5-treated adult mice (Figure 4, B and D) ▶ showed no apparent deformation as compared with those of control mice (Figure 4, A and C) ▶ .
Figure 4.
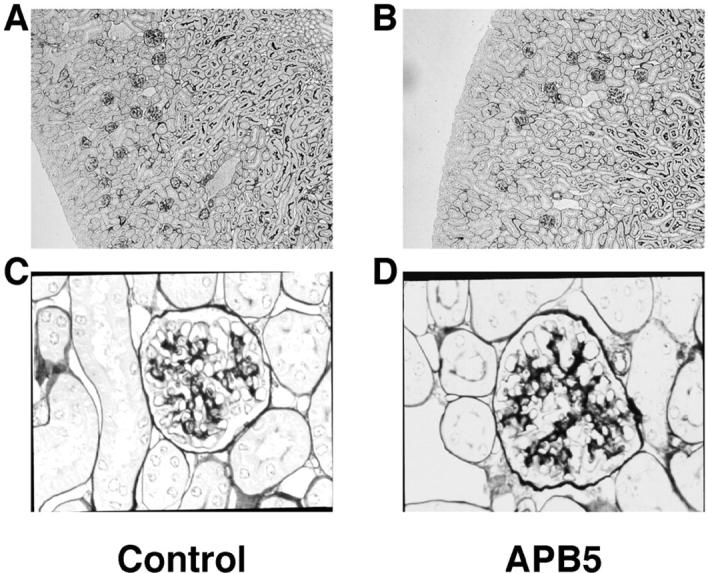
Histochemical analysis of the kidneys from the adult mice injected with APB5 for 3 weeks. The 8-week-old mice were injected with 1 mg of irrelevant rat IgG (n = 3) or APB5 (n = 3) intraperitoneally for 3 weeks on alternate days. At 11 weeks of age, the mice were killed and the kidney specimens were fixed and stained with PAM. Representative sections of the kidneys from the mice injected with irrelevant rat IgG (A and C) or APB5 (B and D) are presented. Original magnifications: ×100 (A and B); ×400 (C and D).
Induction of Apoptosis in the Glomerular Endothelial Cells by PDGFR-β Blockade
To clarify which cell types were involved in APB5-induced glomerular deformation, we studied glomeruli under electron microscopy. At 3 dpp, as far as we could examine, virtually no mesangial cells could be found in the glomeruli in the mice injected with APB5 and the apoptotic bodies were detected in the glomerular endothelial cells (Figure 5) ▶ . In striking contrast, no apoptotic body was observed in the podocytes (Figure 5, A and B) ▶ . At 9 dpp, although blood cells were still observed in the lumen, glomerular capillary tufts were almost completely disrupted (Figure 5D) ▶ . No obvious changes were detected in the kidney from the mice injected with control IgG either at 3 dpp or at 9 dpp (data not shown).
Figure 5.
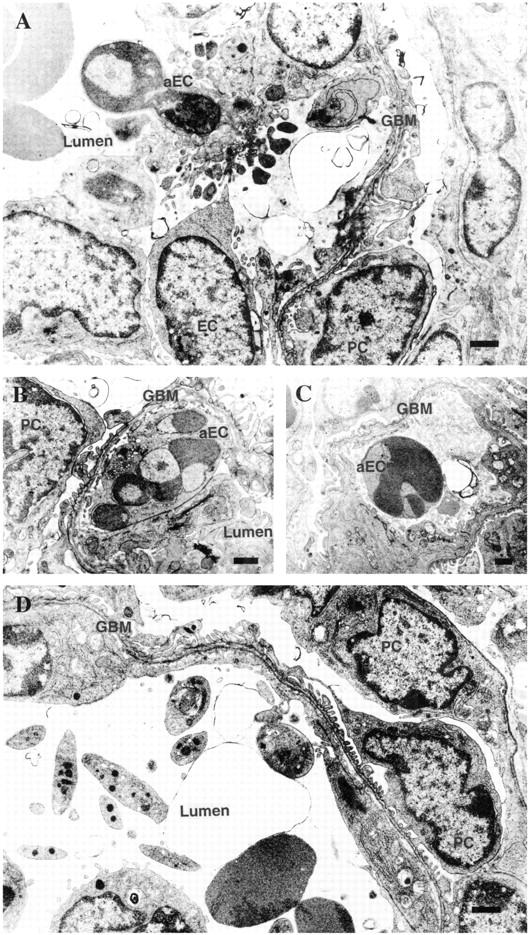
Electron microphotographs of kidney glomeruli from the mice injected with APB5 for 3 days (A–C) or 9 days (D). A: An apoptotic glomerular endothelial cell with many buddings and apoptotic bodies. B: An apoptotic glomerular endothelial cell containing fragmented nuclei with peripherally condensed chromatin. C: An apoptotic cell seen in the glomerular capillary lumen. D: At 9 dpp, glomerular capillary tufts were almost destroyed and blood cells (red blood cells, leukocytes, and platelets) were observed inside, presumably reflecting glomerular endothelial cell loss via apoptosis at younger ages. aEC, apoptotic endothelial cell; EC, glomerular endothelial cell; GBM, glomerular basement membrane; PC, podocyte. Scale bars, 1 μm.
To detect the cells undergoing apoptosis, we also conducted an in situ TUNEL assay. In accordance with the results above, the APB5-treated mice killed at 3 dpp presented a large number of TUNEL signals (Figure 6B) ▶ . In contrast, the control IgG-treated mouse kidney showed only a few TUNEL signals (Figure 6A) ▶ . To examine whether glomerular endothelial cells were responsible for the apoptotic change, we further applied the sequential sections of the TUNEL-labeled slides to H&E staining (Figure 6, C and D) ▶ . Approximately 95% of the signals were detected in the glomeruli in the outer cortex region, whereas very few signals were found in the glomeruli near the medulla. As shown in Figure 6, D and E ▶ , a sequential slide of Figure 6B ▶ , most of the cells corresponding to the TUNEL-positive signals were located in the glomeruli in the outer cortex. To quantify the apoptotic rate, we counted the apoptotic cells and whole nuclei in the outer cortex. In the mice injected with APB5 (n = 5), 18.57 ± 1.427 apoptotic cells were observed per 1000 nuclei. In striking contrast, in the mice injected with irrelevant rat IgG (n = 5), only 4.018 ± 1.510 apoptotic cells were detected per 1000 nuclei (P < 0.0001) (Figure 6F) ▶ . We further estimated the ratio between the number of glomeruli containing apoptotic cells and the total number of glomeruli in the kidney cortex. As shown in Figure 6G ▶ , the ratio in the mice injected with APB5 (n = 5), 30.44 ± 3.988%, was obviously higher than in the control mice (n = 5), 14.63 ± 2.299% (P < 0.0001). To further confirm that glomerular endothelial cells were undergoing apoptosis, we performed double staining with anti-Flk1 antibody and TUNEL (Figure 6, H to K) ▶ . The Flk1-positive endothelial cells were completely overlapped with TUNEL-positive cells as shown in the merged image (Figure 6, J and K) ▶ . In Figure 6K ▶ , at higher magnification, two glomeruli were observed and TUNEL-positive glomerular endothelial cells were labeled yellow. It was concluded that blockade of the PDGFR-β pathway in neonatal mice induced apoptosis in glomerular endothelial cells.
Figure 6.
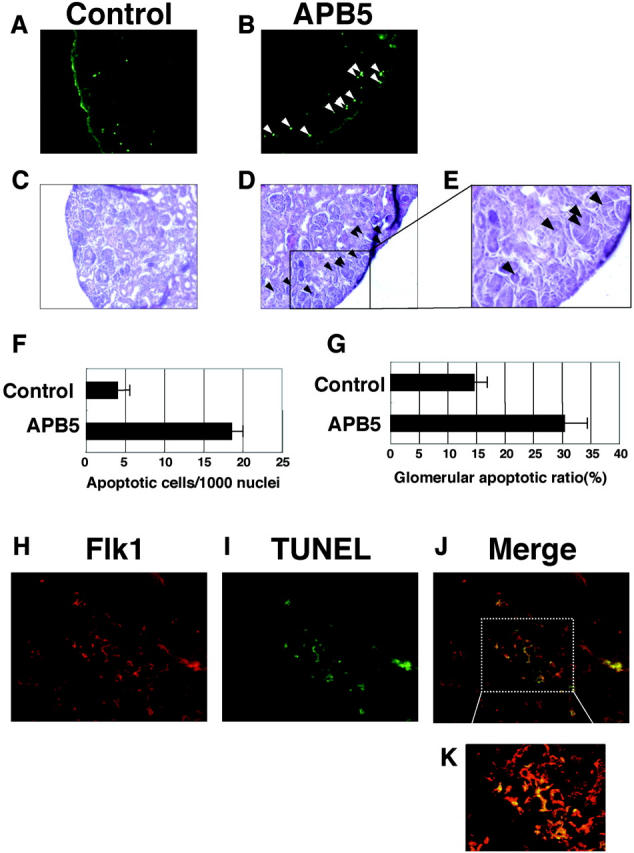
Micrographs of glomeruli stained with TUNEL and anti-Flk1 antibody. The neonatal mice were injected everyday with irrelevant IgG (A and C) or APB5 (B and D) and killed at 3 dpp. A and B: The frozen sections of the kidneys were subjected to TUNEL analysis as described in Material and Methods. The mouse kidney injected with APB5 presented a larger number of green signals (B) than the control mouse kidney (A). More than 95% of TUNEL-positive signals were exclusively detected in the glomeruli localized in the outer cortex. TUNEL signals are depicted by the white arrowheads. The sequential sections (C and D) of the TUNEL-stained slides (A and B) from control and APB5-treated mice were applied to H&E staining, respectively. In D, the TUNEL-positive cells in B are indicated by the black arrowheads. E: Magnified image of the rectangle area in D. F: Quantitative analysis of the effect of APB5 on apoptosis induction in the outer cortex (<50 μm from the surface). The number of apoptotic cells per 1000 nuclei is presented. In the mice treated with APB5 (n = 5), the proportion of apoptotic cells is increased significantly as compared to the control animal (n = 5) (P < 0.0001). Data are mean ± SD G: The ratio of the glomeruli containing TUNEL-positive cells to the total glomeruli in the cortex could be observed in the mice treated with APB5 (n = 5) or with control IgG (n = 5) (P < 0.0001). H–K: Double-histochemical preparation (Flk1 and TUNEL) of the glomeruli from the mice injected with APB5. H: The glomerular endothelial cells were stained with anti-Flk1 antibody (red; Texas red). I: Apoptotic cells were stained with TUNEL (green; fluorescein). J: Merged-image of H and I. K: High magnification of the rectangle area in J. Original magnifications: ×200 (A–D); ×400 (E, H–J); ×600 (K).
Discussion
In the current study, we examined the molecular mechanisms for glomerular development by blocking the PDGFR-β signal in the neonatal mouse by administration of an antagonistic monoclonal antibody. The neonatal mice injected daily with anti-PDGFR-β antibody could be kept alive for at least for 2 weeks with apparently unaffected collecting ducts, but showed severe deformation of the glomerular structure. The number of the mesangial cells was reduced markedly and the glomerular capillary formation was disrupted. Electron microscopic analysis and the TUNEL-Flk1 double staining revealed that the endothelial cells of the glomerular capillary underwent apoptosis.
We first asked whether APB5, an antagonistic rat anti-murine PDGFR-β antibody, could block the signal transduction pathway through PDGFR-β in vivo and disrupt glomerular vasculogenesis in the neonatal mice. When the mice that had been injected with APB5 were killed at 3 dpp, the glomerular capillaries were distended with a decrease in cell density (Figure 2, D and F) ▶ . In the mice injected with APB5 and killed at 9 dpp, the capillary tufts were almost lost in the glomeruli in the region of outer cortex, whereas those located in the region near the medulla were less affected (Figure 3) ▶ . We also tested whether APB5 could also affect the glomerular structure in the adult mice. For this purpose, APB5 or control IgG was administered from 8 to 11 weeks in ICR mice. In contrast to the neonatal mice, the adult mice injected with APB5 showed no apparent glomerular deformation (Figure 4) ▶ . In many mammalian species, including human, rat, and mouse, nephrogenesis is completed during the perinatal period. Thus, mice are born with the nephrons in different developmental stages and nephrogenesis still continues until several days after birth. 15 Our observations would propose the proof that the nephrons near the medulla are considered to be more matured than those located in the outer cortex. The glomeruli found near the medulla were associated with the cell components assembled almost completely and therefore might have been less affected by the antibody. In contrast, those in the outer cortex would be at an immature stage, in which cell assembly might be still in progress. This could have made the glomeruli in the outer cortex more prone to the blockade of the PDGF signal pathway. These results indicate that the signals via the PDGFR-β is critically involved in the glomerular vasculogenesis presumably mediating the migration and proliferation of the mesangial cells, and in the neonates, the PDGFR-β pathway plays a more significant role in vasculogenesis of the glomeruli located in the outer cortex than of those near the medulla where glomerular tuft formation might have been already completed.
Furthermore, to study which cells could be affected by APB5, we studied the kidney from the mice injected with APB5 until 3 dpp under electron microscopy. In the glomeruli associated with deformed tufts, the mesangial cells were hardly detected, and apoptotic bodies were observed in the glomerular endothelial cells (Figure 5) ▶ . We also conducted in situ TUNEL analysis, which showed that a larger number of TUNEL signals were detected in the APB5-treated mouse kidney than in the control IgG-treated mouse kidney (Figure 6; A to D) ▶ . In the APB5-treated mice, ∼95% of the signals were detected in the glomeruli in the outer cortex region, whereas very few signals were found in the glomeruli near the medulla (Figure 6; B, D, and E) ▶ . To estimate the apoptotic rate, we counted the apoptotic cells and whole nuclei in the outer cortex. The rate in the APB5-treated mice was significantly higher than in the control rat IgG-treated mice (P < 0.0001) (Figure 6F) ▶ . We also calculated the ratio between the number of glomeruli containing apoptotic cells and the total number of glomeruli in the kidney cortex. As shown in Figure 6G ▶ , the ratio in the mice injected with APB5 was significantly higher than in the control mice (P < 0.0001). To further confirm that glomerular endothelial cells were responsible for apoptotic change in the mouse kidney, we performed double staining with anti-Flk1 antibody and TUNEL (Figure 6; H to K) ▶ . The Flk1-positive glomerular endothelial cells were completely overlapped with TUNEL-positive cells as shown in the merged image (Figure 6, J and K) ▶ . In Figure 6K ▶ , two glomeruli were observed and TUNEL-positive glomerular endothelial cells were labeled yellow. Taken together, it was concluded that blockade of the PDGFR-β pathway in neonatal mice induced apoptosis in glomerular endothelial cells.
It has been reported previously that PDGF-B is produced by endothelial cells and that PDGFR-β is present on pericytes/vascular smooth muscle cells. 21-24 However, as determined by immunoblot analysis, PDGFR-β was not expressed in murine glomerular endothelial cells (Xu et al, unpublished observation). It is therefore unlikely that APB5 exerted direct action on the endothelial cells. Taken together, our current data would indicate that: blockade of the PDGFR-β pathway during the stage of glomerular neovascularization results in a decrease in the mesangial cell density because of inhibition of mesangial cell migration, which then induces apoptosis in the glomerular endothelial cells thereby leading to glomerular deformation; and because the presence or association of mesangial cells were required for the viability of the glomerular endothelial cells, there would be some signals directed from mesangial cells toward other cells including glomerular endothelial cells.
In summary, this study demonstrated that blockade of PDGFR-β signals induces glomerular endothelial cell apoptosis in the newborn mice. Hellstrom and colleagues 24 have reported that PDGFR-β-deficient mice showed no apoptotic signals in the glomerular endothelial cells. To interpret the discordance, it could be conceivable that the mesenchymal cells including smooth muscle progenitor cells and immature pericytes of the PDGFR-β-deficient mice did not differentiate enough to form integrated vascular structure at the delivery. On the other hand, in the neonatal mice in our study, these cells might have differentiated to a certain extent and played a role in glomerular capillary formation in the outer cortex. The functional blockade of PDGFR-β pathway could prohibit the proliferation and migration of those cells abruptly and result in the endothelial cell apoptosis. Another possible explanation would be that some anti-apoptotic factors were prevented from endothelial apoptosis in the PDGFR-β-deficient mice. It has been reported that vascular endothelial growth factor expression is increased in PDGFR-β-deficient mice. 18 It is of importance to study and identify these factors or signals. Nehls and colleagues 25,26 and Hellstrom and colleagues 18 have reported that lack of pericytes leads to endothelial cell hyperplasia by morphological studies on the brain of the mouse embryo. It has been proposed that pericytes control vessel sprouting and branching. Our electron microscopic and TUNEL analysis showed glomerular endothelial cells had apoptotic status. It is conceivable that the mesangial cells, which migrate to the central region of the vascular tuft of the glomerulus, might play a significant role in maintenance of the glomerular endothelial cell viability either by supporting the structure of the tuft secreting extra cellular matrices or by sending some humoral factors to the endothelial cells that are essential for endothelial cell stability. It is to be further investigated the molecular mechanism for the regulatory role of mesangial cells on function and structure of the glomerulus.
Acknowledgments
We thank Drs. Noriaki Kume, Hisanori Horiuchi, Hidenori Arai, Makoto Tanaka, and Motoko Yanagita for valuable discussion; and Hideo Uchiyama and Akiko Kato for their excellent technical assistance.
Footnotes
Address reprint requests to Masayuki Yokode, M.D., Department of Geriatric Medicine, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan. E-mail: yokode@kuhp.kyoto-u.ac.jp.
Supported by the Ministry of Education, Science, Sports, and Culture of Japan (research grants 04263104, 054040439, 0557052, 04304051, 08407026, and 9578); the International Scientific Research Program (grants 05044163, 07044254, and 09044293) from the Japanese Ministry of Education, Science, Sports, and Culture; a research grant for health sciences from the Japanese Ministry of Health and Welfare (grants 5A-2 and A8-1 for cardiovascular diseases); Grants-in-Aid for Scientific Research on Priority Areas (grants 09281103 and 09281104); Grants-in-Aid for Creative Basic Research (grant 09 NP 0601); the HMG-CoA Reductase Research Fund; the Japanese Foundation of Metabolism and Diseases; Takeda Medical Research Foundation; and the Research Fellowships of Japan Society for the Promotion of Science for Young Scientist and Research for the Future Program.
References
- 1.Heldin CH, Ostman A, Ronnstrand L: Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta 1998, 1378:F79-F113 [DOI] [PubMed] [Google Scholar]
- 2.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U: PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2000, 2:302-309 [DOI] [PubMed] [Google Scholar]
- 3.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS: PDGF-D, a new protease-activated growth factor. Nat Cell Biol 2001, 3:517-521 [DOI] [PubMed] [Google Scholar]
- 4.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U: PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 2001, 3:512-516 [DOI] [PubMed] [Google Scholar]
- 5.Davidson JM, Aquino AM, Woodward SC, Wilfinger WW: Sustained microgravity reduces intrinsic wound healing and growth factor responses in the rat. EMBO J 1999, 13:325-329 [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran RK, Wikramanayake AH, Uzman JA, Govindarajan V, Tomlinson CR: Disruption of gastrulation and oral-aboral ectoderm differentiation in the Lytechinus pictus embryo by a dominant/negative PDGF receptor. Development 1997, 124:2355-2364 [DOI] [PubMed] [Google Scholar]
- 7.Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD: Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development 1999, 126:457-467 [DOI] [PubMed] [Google Scholar]
- 8.Risau W, Drexler H, Mironov V, Smits A, Siegbahn A, Funa K, Heldin CH: Platelet-derived growth factor is angiogenic in vivo. Growth Factors 1992, 7:261-266 [DOI] [PubMed] [Google Scholar]
- 9.Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T: Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation 2001, 103:2955-2960 [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Pierce JH, Fleming TP, Greenberger JS, LaRochelle WJ, Ruggiero M, Aaronson SA: Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci USA 1989, 86:8314-8318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama N, Morisaki N, Saito Y, Yoshida S: Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem 1992, 267:22806-22812 [PubMed] [Google Scholar]
- 12.Heidaran MA, Beeler JF, Yu JC, Ishibashi T, LaRochelle WJ, Pierce JH, Aaronson SA: Differences in substrate specificities of alpha and beta platelet-derived growth factor (PDGF) receptors. Correlation with their ability to mediate PDGF transforming functions. J Biol Chem 1993, 268:9287-9295 [PubMed] [Google Scholar]
- 13.Yu JC, Gutkind JS, Mahadevan D, Li W, Meyers KA, Pierce JH, Heidaran MA: Biological function of PDGF-induced PI-3 kinase activity: its role in alpha PDGF receptor-mediated mitogenic signaling. J Cell Biol 1994, 127:479-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Deuel TF, Kim HR: Platelet-derived growth factor (PDGF) receptor-alpha activates c-Jun NH2-terminal kinase-1 and antagonizes PDGF receptor-beta-induced phenotypic transformation. J Biol Chem 2000, 275:19076-19082 [DOI] [PubMed] [Google Scholar]
- 15.Tisher CC, Madsen KM: Anatomy of the kidney. Brenner BM eds. Brenner and Rector’s The Kidney, ed 6 2000:pp 3-67 W. B. Saunders Company, Philadelphia
- 16.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 1994, 8:1888-1896 [DOI] [PubMed] [Google Scholar]
- 17.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994, 8:1875-1887 [DOI] [PubMed] [Google Scholar]
- 18.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C: Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001, 153:543-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takakura N, Yoshida H, Kunisada T, Nishikawa S, Nishikawa SI: Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol 1996, 107:770-777 [DOI] [PubMed] [Google Scholar]
- 20.Kataoka H, Takakura N, Nishikawa S, Tsuchida K, Kodama H, Kunisada T, Risau W, Kita T, Nishikawa SI: Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ 1997, 39:729-740 [DOI] [PubMed] [Google Scholar]
- 21.Holmgren L, Glaser A, Pfeifer-Ohlsson S, Ohlsson R: Angiogenesis during human extraembryonic development involves the spatiotemporal control of PDGF ligand and receptor gene expression. Development 1991, 113:749-754 [DOI] [PubMed] [Google Scholar]
- 22.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-R beta signaling controls mesangial cell development in kidney glomeruli. Development 1998, 125:3313-3322 [DOI] [PubMed] [Google Scholar]
- 23.Betsholtz C, Karlsson L, Lindahl P: Developmental roles of platelet-derived growth factors. BioEssays 2001, 23:494-507 [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom M, Kaln M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 25.Nehls V, Denzer K, Drenckhahn D: Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res 1992, 270:469-474 [DOI] [PubMed] [Google Scholar]
- 26.Nehls V, Schuchardt E, Drenckhahn D: The effect of fibroblasts, vascular smooth muscle cells, and pericytes on sprout formation of endothelial cells in a fibrin gel angiogenesis system. Microvasc Res 1994, 48:349-363 [DOI] [PubMed] [Google Scholar]