Abstract
Recent reports have indicated that enzymes such as cathepsins D and B are translocated from lysosomal compartments to the cytosol early during apoptosis. We have previously noted that a translocation of cathepsins D and B occur before cytochrome c release and caspase activation in cardiomyocytes and human fibroblasts during oxidative stress-induced apoptosis. In the present report, we use a microinjection technique to investigate if cytosolic location of the cathepsins D and B are important for induction of apoptosis. We found that microinjection of cathepsin D into the cytosol of human fibroblasts caused apoptosis, which was detected as changes in distribution of cytochrome c, cell shrinkage, activation of caspases, chromatin condensation, and formation of pycnotic nuclei. No apoptosis was, however, induced by microinjection of cathepsin B. Moreover, apoptosis was prevented in fibroblasts pretreated with a caspase-3-like inhibitor, and also when microinjected with cathepsin D mixed with the cathepsin D inhibitor, pepstatin A. These results show that cytosolic cathepsin D can act as a proapoptotic mediator upstream of cytochrome c release and caspase activation in human fibroblasts.
Apoptosis, or programmed cell death, occurs through activation of a cell suicide process that is regulated by many different intracellular and extracellular events. During apoptosis, the cell is degraded through activation of proteases and endonucleases. The class of proteases known as caspases plays an essential role in the induction and execution of apoptosis, and, when activated, they acquire the ability to cleave key intracellular substrates that results in the biochemical and morphological changes associated with apoptosis. 1 Besides the caspases, lysosomal proteases such as cathepsins (cats) D, B, and L have been shown to act as mediators of apoptosis in a number of cell systems. 2-5
During apoptosis, various proteins that are normally sequestered in the mitochondria are released to the cytosol, including cytochrome c (cyt c), apoptosis-inducing factor, and procaspases 2 and 9. In the cytosol, cyt c can undergo complexation with cytosolic apoptosis protein-activating factor 1 (Apaf-1), and, in the presence of dATP or ATP, this leads to activation of procaspase-9 and the caspase cascade. 6 Microinjection of cyt c into the cytosol, without the presence of any other apoptosis-inducing stimuli, has been found to cause apoptosis in several different types of cells. 7-10 Moreover, the proapoptotic effect of microinjected cyt c was prevented by caspase inhibitors and by overexpression of Bcl-2 and Bcl-XL. 7,9
Increased expression or activity of cat D has been observed in apoptotic cells after activation of Fas/APO-1 2 and after exposure to oxidative stress 11,12 or Adriamycin. 13 We have noted previously, that during oxidative stress-induced apoptosis, cat D was translocated from lysosomal structures to the cytosol, and that the release of cat D preceded the release of cyt c and the loss of mitochondrial membrane potential. If we pretreated the cultures with the cat D inhibitor pepstatin A before oxidative stress exposure, no cyt c release or caspase-3 activation could be detected, and apoptosis was inhibited. 11,12,14,15
Because several investigations imply lysosomal proteases as mediators of apoptosis and few deal with their intracellular localization, we used a microinjection technique to determine whether cytosolic location of cat D and cat B is important for induction of apoptosis.
Materials and Methods
Cells and Culture Conditions
Human foreskin fibroblasts (AG-1518, passages 14 to 20; Coriell Institute, Camden, NJ) were cultured in Eagle’s minimal essential medium supplemented with 2 mmol/L glutamine, 50 IU/ml penicillin-G, 50 μg/ml streptomycin, and 10% fetal bovine serum (Gibco, Paisley, UK). Twenty-four hours before the experiments, the cells were trypsinized and seeded into 35-mm Petri dishes (Costar, Cambridge, MA) at a density of 10,000 cells/cm2. The caspase-3-like protease inhibitor Ac-DEVD-CHO (25 μmol/L; Calbiochem, San Diego, CA) was added to cultures 1 hour before microinjection of cat D.
Microinjection
Microinjection was performed on the stage of a Zeiss Axiovert (Zeiss, Gena, Germany) inverted microscope, using a pressure injector from Eppendorf (model 5246; Eppendorf, Hamburg, Germany) and an Injectman micromanipulator (Eppendorf). Eppendorf microloaders were used to fill the microinjection needles (Femtotips II, Eppendorf), that had an inner diameter of less than 0.5 μm. All injectates contained 1 mg/ml dextran-conjugated Alexa Fluor 488 or 0.25 mg/ml Alexa Fluor 546 (molecular weight, 10,000; Molecular Probes, Eugene, OR) in Dulbecco’s phosphate-buffered saline (PBS) (pH 5.5 or 7.0). Freshly prepared Alexa Fluor containing 0.25 mg/ml of cat D (C 8696, diluted in PBS, pH 5.5 or 7.0; Sigma, Stockholm, Sweden), 3 mg/ml of cyt c (C 7752, diluted in PBS, pH 7.3; Sigma), 0.25 mg/ml of cat B (C 8571, diluted in PBS, pH 5.5; Sigma) or 3.1 mg/ml of active caspase-3 (diluted in PBS, pH 7.3; Becton Dickinson, Mountain View, CA) was injected into the cytoplasm of cells (pressure 100 hPa, 1.5 seconds). We also used inactivated cat D, which was incubated overnight at 37°C before injection, or inhibited cat D that was mixed with 5 μmol/L of pepstatin A before injection. In each experiment, 100 to 300 cells in each dish were injected, and the results are presented as average values for at least four dishes.
The volume injected was estimated by injecting 33P as orthophosphate 16 (Amersham Pharmacia Biotech, Buckinghamshire, UK) diluted 1:50 in dextran-conjugated Alexa Fluor (1 mg/ml). Thereafter, the cells that had received a microinjection were counted, and the activity of 33P was determined using vials containing 10 ml of Ready Safe (Beckman, Fullerton, CA) and a liquid scintillation counter (1217 Rackbeta; Wallac, Turku, Finland). The 33P activity was compared with a standard curve obtained the same day. The injection volume was calculated to 4.9 ± 0.3 × 10−12 1 (n = 3), and the activity of the injected cat D (see below) was subsequently calculated to 0.7 × 10−6 au/h·cell and for cat B to 6.8 × 10−4 au/h·cell.
Cat D and B Activity Measurement
Cat D activity was determined as described by Barett 17 using hemoglobin as a substrate. To determine such activity in cell cultures, the fibroblasts were pelleted and washed in PBS and then exposed to three cycles of freezing and thawing to rupture the plasma membrane. In the injection solution the activity of cat D was determined after dilution to 1:50 in PBS. The sample was mixed with 2 mmol/L of sodium phosphate buffer (pH 6.5), 250 μl of 4% (w/v) hemoglobin, and 250 μl of 1 mol/L sodium formate buffer (pH 3.5), and then incubated at 45°C for 60 minutes. The reaction was terminated by adding 5 ml of 3% trichloric acid, and the precipitate was filtered. The amount of peptides released was analyzed using the method described by Lowry and colleagues 18 with some modifications. 17 We calculated the cat D activity of a single cell in a Petri dish to be 1.3 × 10−6 au/h·cell.
Cat B activity was determined as described by Barrett. 17 In short, culture medium was withdrawn, and the cells were washed in PBS and lysed in 340 mmol/L of sodium acetate buffer (pH 5.0) containing 0.1% Triton X-100, 60 mmol/L acetic acid, 4 mmol/L ethylenediaminetetraacetic acid, and 8 mmol/L dithiothreitol. The cell lysate was incubated for 15 minutes at 30°C with the cat B-specific substrate z-Arg-Arg-AMC (20 μmol/L, Sigma). The reaction was stopped by adding a buffer consisting of 100 mmol/L of sodium monochloric acetate, 30 mmol/L of sodium acetate, and 70 mmol/L of acetic acid. The fluorescence of liberated AMC was analyzed at λex 380 nm and λem 435 nm in a spectrofluorometer. We calculated the cat B activity of a single cell in a Petri dish to be 13.2 × 10−4 au/h·cell.
Detection of Apoptotic Morphology
After microinjection, all fluorescent cells were counted, and the percentage of apoptotic cells was calculated based on the number of rounded/shrunken or detached fluorescent cells detected. Nuclear size was analyzed in cells mounted in Vectashield medium supplemented with 4,6-diamidino-2-phenylindole (1.5 μg/ml; Vector Laboratories, Burlingame, CA). Cells in Petri dishes were examined in a Nikon photomicroscope (Nikon, Tokyo, Japan), and the cells were photographed using a digital video camera (Hamamatsu, Tokyo, Japan) mounted on the microscope. For ultrastructural analysis, ∼90% of the cells in a marked circle (diameter, ∼5 mm) in the center of a dish were injected with dextran-conjugated Alexa Fluor alone or together with cat D or cyt c. The cultures were then prepared as previously described. 19 Briefly, fibroblasts were fixed in situ in the plastic culture dishes by adding 2% glutaraldehyde (vacuum distilled; Agar Scientific, Essex, UK) in 0.1 mol/L of sucrose-sodium cacodylate-HCl buffer (pH 7.2) and postfixed in osmium tetroxide (Johnson Matthey Chemicals, Roystone, UK). Dehydration, en bloc staining with uranyl acetate, repeated dehydration, and embedding in Epon-812 (Fluka AG, Buchs, Switzerland) were also done in the culture dishes. Thin sections of the cured blocks were cut with a diamond knife, stained with lead citrate, and then examined and photographed in a JEOL 1200-EX electron microscope (Tokyo, Japan) at 100 kV.
Assessment of DNA Fragmentation
Apoptotic cells were also identified by the terminal dUTP nick-end labeling technique using the ApopTag in situ apoptosis detection kit according to the instructions of the manufacturer (Intergen, Purchase, NY). The cells were fixed for 15 hours in 1% paraformaldehyde at 4°C and then permeabilized for 5 minutes in ethanol and acetic acid (2:1) at −20°C. Thereafter, the cultures were incubated with terminal deoxynucleotidyl transferase enzyme for 1 hour at 37°C and then with anti-digoxigenin-rhodamine/fluorescein for 30 minutes, and were subsequently mounted in Vectashield medium. The cells were examined in a Nikon photomicroscope, using green exciting light and a 590-nm barrier filter or blue exciting light and a 520-nm barrier filter, and photographed with a digital camera.
Immunofluorescence Detection of Cat D and Cyt c
Microinjected fibroblasts were fixed in 4% formaldehyde in PBS for 20 minutes at 4°C and then processed for immunocytochemistry as described earlier. 19 The cells were incubated with a polyclonal rabbit anti-human cat D antibody (dilution 1:100; DAKO, Glostrup, Denmark), followed by a goat anti-rabbit IgG Texas Red conjugate (1:200; Vector Laboratories) or a monoclonal mouse-anti-human cyt c (1:50; Pharmingen, San Diego, CA), and subsequently a mouse anti-IgG fluorescein isothiocyanate (FITC) conjugate (1:50, Calbiochem). Thereafter, the cells were rinsed in PBS and distilled water and mounted in Vectashield medium. The cells were examined in a Nikon photomicroscope, using green exciting light and a 590-nm barrier filter or blue exciting light and a 520-nm barrier filter, and photographed with a digital camera. Controls incubated without anti-cat D antibodies or anti-cyt c antibodies did not stain.
Detection of Activated Caspases
A FITC conjugate of the cell-permeable pan caspase inhibitor VAD-FMK (CaspACETM* FITC-VAD-FMK In Situ Marker; Promega, Madison, WI) was used to detect caspase activity in microinjected fibroblasts. The structure of this FITC conjugate allows delivery of the inhibitor into the cell, where it binds to activated caspases. Briefly, 4, 8, or 24 hours after microinjection, the cells were incubated with the conjugate (10 μmol/L) in culture medium for 30 minutes at 37°C, rinsed in PBS five times, examined in a Zeiss Axiovert inverted fluorescence microscope, and photographed with a digital camera. All microinjected Alexa Fluor red fluorescent cells were counted and the percentage of caspases activated, green fluorescent cells, were calculated.
Statistical Analysis
We repeated all experiments at least four times. The results were analyzed statistically using the Mann-Whitney U-test. P values ≤0.05 were considered significant.
Results
Microinjection of Cat D Induces Apoptotic Morphology
To mimic the physiological conditions that prevail during the release of cat D, we microinjected this protein at pH 5.5, and we included dextran-conjugated Alexa Fluor (red fluorescence) to identify the injected cells. The activity of the cat D introduced into each cell was estimated to be approximately half of the activity detected in one normal fibroblast (ie, 0.7 × 10−6 au/h·cell was injected). Under these conditions, cell shrinkage was observed 2 hours after the microinjection. The number of apoptotic cells increased with time, and 8 hours after the microinjection 28% of the cells exhibited shrinkage and pycnotic nuclei (Figure 1) ▶ and DNA fragmentation (detected using the terminal dUTP nick-end labeling technique; data not shown). Because it has been reported that cat B is also released to the cytosol during apoptosis, the effect of microinjected cat B was investigated. 5,12 As for microinjected cat D, the activity of the cat B introduced into each cell was estimated to be approximately half of the activity detected in one normal fibroblast (6.8 au/h·cell was injected). However, 6 hours and 24 hours after microinjection, no apoptotic changes could be detected.
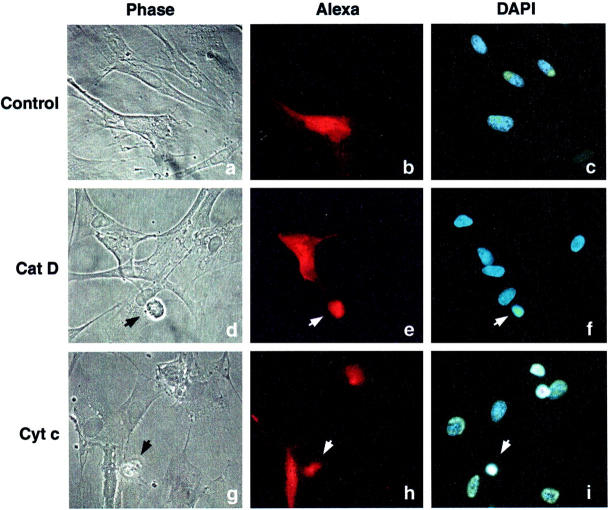
Figure 1.
Light and fluorescence microscopy of fibroblasts 8 hours after microinjection of cat D or cyt c. Apoptotic fibroblasts were detected as shrunken cells with pycnotic nuclei by light and fluorescence microscopy. a–c: Cells microinjected with Alexa Fluor alone. No apoptosis was detected. d–f: Cells microinjected with Alexa Fluor together with 0.25 mg/ml of cat D, or g–i, together with 3 mg/ml of cyt c. Microinjected cells were visualized with Alexa Fluor (b, e, and h; red fluorescence) and nuclear morphology by 4,6-diamidino-2-phenylindole staining (c, f, and i). Arrows show microinjected apoptotic cells.
We also microinjected cyt c into fibroblasts at the concentration (3 mg/ml) used by Li and collaborators 7 to induce apoptosis in human 293 kidney cells and HeLa cells. Using the same pressure and time as for microinjection of cat D, we found that the onset of apoptosis was more rapid after injection of cyt c (Figure 1) ▶ . More precisely, after 6 hours, 42% of the fibroblasts injected with cyt c were apoptotic as compared to 25% of the cells with cat D. Almost no apoptotic morphology (2%) could be detected after microinjection with the vehicle PBS and Alexa Fluor.
To confirm the morphological characteristics of apoptosis discerned by light microscopy, we analyzed injected cells by transmission electron microscopy. Compared to control cells (Figure 2A) ▶ , 6 hours after microinjection with cyt c (Figure 2B) ▶ or cat D (Figure 2, C and D) ▶ the fibroblasts showed condensed chromatin, pycnotic nuclei, and convoluted nuclear membranes, as well as aggregation of condensed mitochondria around the nucleus. Moreover, all injected cells contained an increased number of autophagic vacuoles. This may be because of cellular damage induced by the microinjection procedure and/or the Alexa dye, however no shrinkage or nuclear changes were seen in microinjected control cells (Figure 2A) ▶ .
Figure 2.
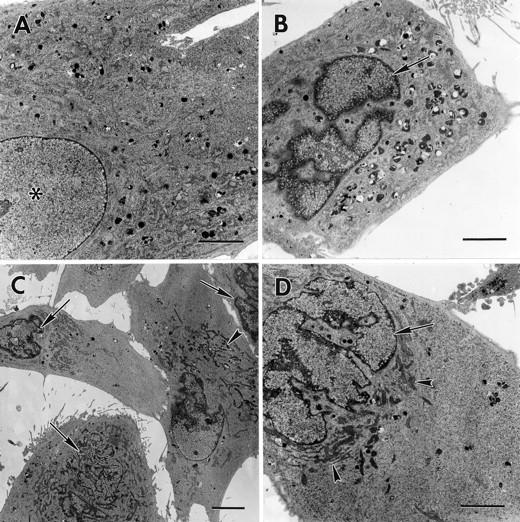
Transmission electron microscopy of microinjected fibroblasts. The micrographs show fibroblasts 6 hours after microinjection with Alexa Fluor alone (A) or with Alexa Fluor and 3 mg/ml of cyt c (B), or with Alexa Fluor and 0.25 mg/ml of cat D (C and D). Note in A a normal nucleus (asterisk) and in B–D apoptotic morphology-like partially fragmented nuclei, nuclei with condensed chromatin (arrows), and condensed mitochondria around the nucleus (arrowheads). Original magnifications: ×2000 (A, B, and D); ×1000 (C). Scale bars: 4 μm (A, B, and D); 10 μm (C).
Distribution of Cyt c and Cat D in Microinjected Fibroblasts
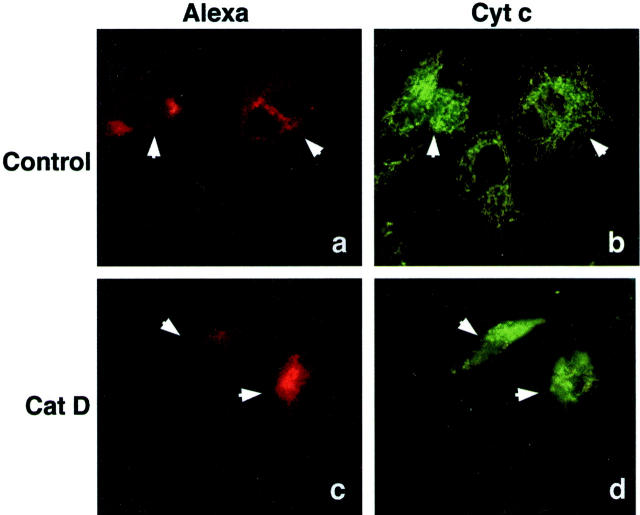
Release of cyt c from mitochondria is a well-known event that triggers activation of caspases during apoptosis. Therefore, we subjected cat D-microinjected fibroblasts to immunofluorescence staining to examine the intracellular localization of cyt c. Staining of control cells for cyt c revealed the presence of the protein in vermiform mitochondria. However, 2 hours after microinjection of cat D, the staining pattern had become more diffuse, indicating that cyt c had been translocated from the mitochondria to the cytosol. The mitochondria that still contained cyt c were seen as punctiform structures aggregated around the nucleus (Figure 3) ▶ . Similar results have been shown in HeLa cells prone to apoptosis because of overexpression of Bax, 20 or to ultraviolet exposure. 21
Figure 3.
Cyt c location by immunofluorescence staining of fibroblasts. Micrographs of fibroblasts 2 hours after microinjection with Alexa Fluor alone (a and b) or with Alexa Fluor together with 0.25 mg/ml of cat D (c and d). Microinjected cells detected by the red fluorescence of Alexa Fluor (a and c), and cyt c by immunofluorescence staining with a FITC-conjugated secondary antibody (b and d). Arrows indicate microinjected cells.
Immunocytochemistry of cat D in control fibroblasts showed a granular staining pattern, indicating the presence of this protein in lysosomal compartments, and the same pattern was seen 2 and 4 hours after microinjection of cat D (data not shown). These results demonstrate that microinjection of cat D did not cause lysosomal damage or translocation of endogenous cat D. Microinjection of cyt c or active caspase-3 also had no effect on the granular staining of cat D for up to 2 hours. However, 4 hours after cyt c or active caspase-3 injection, a diffuse cat D staining was revealed, indicating translocation of cat D from the lysosomal compartment to the cytosol. In addition, 6 hours after microinjection of cat D, we could detect lysosomal leakage of cat D to the cytosol. This may be a secondary effect because of mitochondrial malfunction and increased oxidative stress in the cells.
Cat D-Induced Apoptosis Involves Caspase Activation
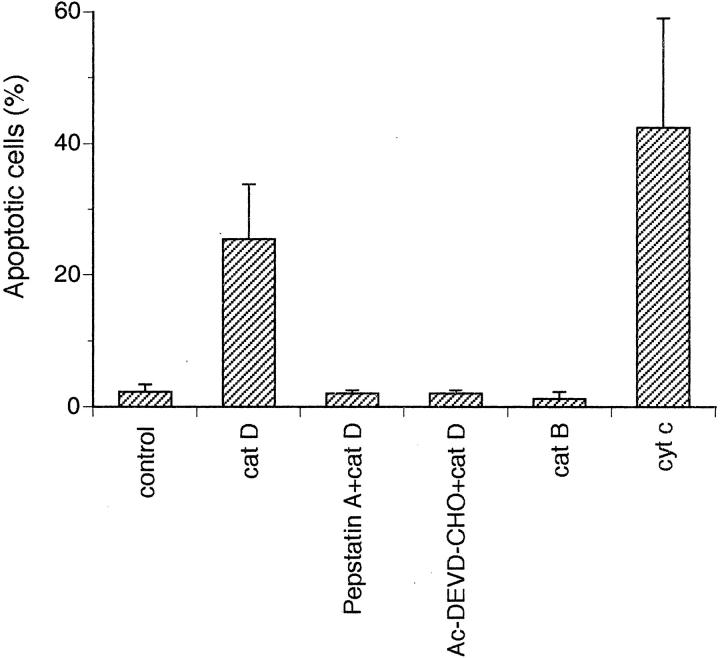
Other investigators have reported that apoptosis induced by microinjection of cyt c involves caspase activation. 7,9 We used a FITC conjugate of the pan caspase inhibitor VAD-FMK to study activation of caspases. This methodology revealed caspase activation 4 hours after microinjection of cat D or cyt c in most of the cells showing apoptotic morphology (Figure 4) ▶ . Eight hours after microinjection of cat D, 21.6 ± 5.2% of the cells showed caspase activity (controls, 2.3 ± 0.8%), and 24 hours after injection 37.45 ± 7.5% of the cells contained active caspases (controls, 2.9 ± 1.4%). To further explore the role of caspases in cat D-induced apoptosis, we pretreated cells for 1 hour with Ac-DEVD-CHO, a caspase-3-like protease inhibitor, before injecting cat D. Analysis of cell morphology, 6 hours after injection of cat D, showed that such treatment provided complete protection against apoptosis (Figure 5) ▶ . Microinjection of cat D increased the number of apoptotic cells 11-fold and microinjection of cyt c 18-fold compared to cells microinjected with cat D inhibited by pepstatin A, cat B, or only PBS that did not induce apoptosis (Figure 5) ▶ . Injection of inactivated cat D also failed to induce apoptosis (data not shown).
Figure 4.
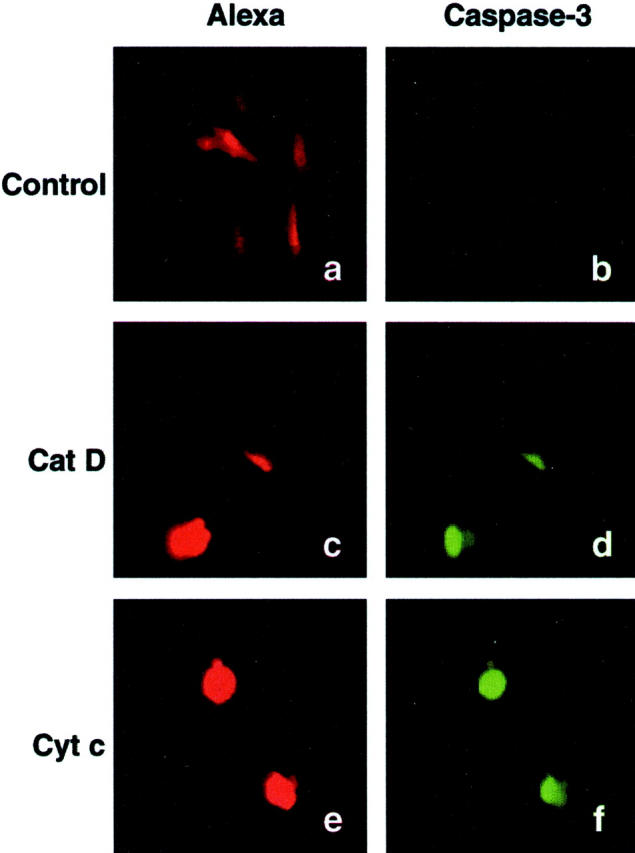
Caspase-3-like protease activity was detected 4 hours after microinjection, using a FITC conjugate of the caspase inhibitor VAD-FMK. Fibroblasts were microinjected with Alexa Fluor alone (a and b) or with Alexa Fluor together with 0.25 mg/ml of cat D (c and d) or with Alexa Fluor together with 3 mg/ml cyt c (e and f). The red Alexa Fluor fluorescence shows microinjected cells (a, c, and e), and FITC fluorescence indicates caspase activity (b, d, and f).
Figure 5.
The histogram shows the percentage of apoptosis detected as shrunken or detached fibroblasts by fluorescence microscopy performed 6 hours after microinjection. The fibroblasts were microinjected with Alexa Fluor alone or together with 0.25 mg/ml of cat D (active or inhibited by 5 μmol/L of pepstatin A) or pretreated with Ac-DEVD-CHO and then injected with cat D. Fibroblasts were also injected with Alexa Fluor together with 0.25 mg/ml of active cat B or 3 mg/ml of cyt c. Values represent mean percentages ± SD of apoptotic cells from at least four separate experiments.
Cat D-Mediated Apoptosis Is Not Dependent on pH
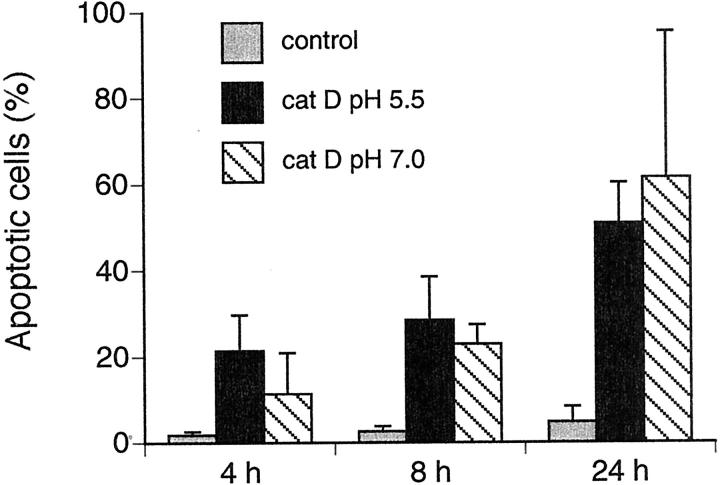
Within a cell, cat D is normally localized to lysosomes and endosomes, where the pH ranges from 4 to 6. To determine whether the effect of microinjected cat D is pH-dependent, we compared the frequency of apoptosis in cells injected with cat D at pH 5.5 and 7.0. As shown in Figure 6 ▶ , no significant difference in the rate of apoptosis was detected 4, 8, and 24 hours after injection of cat D at pH 5.5 compared to at pH 7.0. Control cells injected at pH 5.5 and 7.0 showed almost no apoptotic morphology.
Figure 6.
The histogram shows the percentage of apoptotic fibroblasts after microinjection with cat D, detected as shrunken or detached cells by fluorescence microscopy. Fibroblasts were injected with Alexa Fluor and 0.25 mg/ml of cat D at pH 5.5 or 7.0, and controls were microinjected with Alexa Fluor alone at pH 5.5. Values represent mean percentages ± SD of apoptotic cells from at least four separate experiments. The number of apoptotic cells is not significantly different between the groups microinjected with cat D at pH 5.5 or at pH 7.0.
Discussion
The cat D gene is transcriptionally activated by p53 13 and the participation of cat D in apoptosis has been demonstrated in several different systems. For example, cells pretreated with pepstatin A, cat D −/− fibroblasts, and anti-sense cat D RNA-treated cells have lower rates of apoptosis after exposure to different apoptotic triggers as compared to controls. 2,13,15 Cat D is normally found in lysosomes, but we have observed that it is translocated to the cytosol at the onset of apoptosis induced by oxidative stress. 22 In the present study, we used a microinjection technique as a means of mimicking cat D release, to ascertain whether cat D found in the cytosol plays a role in the induction of apoptosis.
Our results show that microinjection of cat D in fibroblasts induces cyt c release, caspase activation, nuclear changes, and apoptotic morphology. Therefore, we suggest that translocation of cat D to the cytosol plays an important role in induction of apoptosis. The mechanisms by which cat D causes these effects are not known, but there are several possibilities. For example, cat D could directly affect mitochondria, facilitating the release of cyt c. In most apoptosis model systems, release of cyt c from mitochondria is necessary for the activation of downstream caspases. Our previous research has shown that inhibition of cat D during exposure to oxidative stress prevents the release of cyt c, decrease in mitochondrial membrane potential, and activation of caspase-3 in rat cardiac myocytes. 11,15 In the present experiments, cat D-injected fibroblasts exhibited a more diffuse immunostaining of cyt c than control cells did, which indicates translocation of cyt c from the mitochondria to the cytosol. This suggests cat D to be a mediator of cyt c release. However, Stoka and colleagues 23 reported that lysosomal extracts alone have no effects on cyt c release from isolated mitochondria. Moreover, they found that the proapoptotic protein Bid, was cleaved to tBid in the presence of lysosomal extracts, and that cyt c was released from mitochondria incubated with tBid. Bid is a proapoptotic member of the Bcl-2 family and tBid like the proapoptotic member Bax, can trigger the release of cyt c from mitochondria. It is possible that cat D might be acting on a substrate such as Bid upstream of mitochondrial events and caspase activation.
Another possibility is that cathepsins directly activate caspases. Ishisaka and colleagues 3,4 illustrated the participation of cat L in a direct activation of caspase-3. On the other hand, Stoka and co-workers 23 recently showed that none of six different cathepsins (B, H, K, L, S, and X) were able to directly activate procaspases. Analysis of the temporal relationship of our results show that microinjection of cat D first causes cyt c release and then caspase activation, and that the ensuing apoptosis is dependent on caspases.
Eight hours after microinjection of cat D, 28% of the cells showed typical apoptotic morphology. However, under the same experimental conditions, injection of inactivated cat D or injection of cat D inhibited by pepstatin A did not induce apoptosis. In addition, apoptosis was detected in fibroblasts microinjected with cyt c, which agrees with results published by Li and colleagues. 7 Compared to cells microinjected with cat D, those microinjected with cyt c showed a higher rate of apoptosis after both 6 and 24 hours. According to Li and colleagues. 7 the amount of cyt c microinjected into the cytosol in their system is larger than the total amount normally present in one cell of either the MCF7F or the human kidney 293 cell line. In our experimental system, we estimated the injected cat D activity to be approximately half of that found in a single fibroblast; in other words, the amount of cat D we introduced was probably comparable to the physiological level of cat D in the cytosol during apoptosis. These findings, together with the results indicating that the release of cat D precedes the release of cyt c in the chain of events comprising apoptosis, may explain why injection of cat D gave rise to comparatively less apoptosis than injection of cyt c did. Gucciardi and colleagues 5 have recently shown that translocated cat B contributes to tumor necrosis factor-α-mediated apoptosis in hepatocytes. They also show that cat B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis. 24 Foghsgaard and colleagues 25 show similar results in tumor necrosis factor-α-induced apoptosis in fibrosarcoma cells, where cat B has the role of the dominant execution protease. The same study shows that primary murine hepatocytes and fibroblasts are minimally dependent on cat B during tumor necrosis factor-α-induced apoptosis. Previously, we have also discovered that oxidative stress-induced apoptosis is not dependent on cat B in fibroblasts. Pretreatment of fibroblasts with the cat B inhibitor CA 074-Me did not inhibit oxidative stress-induced apoptosis, and we have also noticed a rapid decline in cat B activity. 12 Accordingly, microinjection of cat B does not induce apoptosis which agrees with our earlier findings.
Lysosomes are acidic organelles, and human cat D is optimally active against most substrates between pH 3 to 4. 17 However, other in vitro studies indicated that cat D displays significant activity at a pH greater than 6.5. 26,27 In light of those findings, and because cat D released from lysosomes is exposed to a higher pH in the cytosol, we felt that it was important to determine whether cat D could induce apoptosis at pH values higher than 5.5. Our results indicate that cat D also induces apoptosis at pH 7.0, and therefore it might be able to proteolytically cleave substrates outside the lysosomal compartment.
We have earlier found that cats B and L are translocated from lysosomes to the cytosol simultaneously with cat D during oxidative stress. 12 It was therefore of interest to study lysosomal stability during microinjection to make sure that no lysosomal leakage occurred that could affect apoptosis induction. We observed granular lysosomal-like immunofluorescence staining of cat D in fibroblasts injected with cat D, cyt c, or caspase-3, as well as in control cells. This indicates that cytosolic cat D does not seem to affect the lysosomal membranes from the outside, nor does it cause translocation of endogenous cat D from lysosomes to the cytosol. However, after apoptosis induction, ie, 4 hours after injection of cyt c and caspase-3 or 6 hours after injection of cat D, a diffuse immunostaining of cat D was observed suggesting lysosomal damage at a late time point. We interpret this lysosomal destabilization as a secondary event in the apoptosis process.
In conclusion, the present study demonstrates that cytosolic location of cat D is important for the initiation of apoptosis. Furthermore, the cat D target is probably cytosolic, and acts upstream of cyt c translocation and caspase-3 activation.
Acknowledgments
We thank Dr. Uno Johansson for technical assistance and Dr. Brett Garner for expert linguistic help.
Footnotes
Address reprint requests to Karin Roberg, Division of Pathology II, Faculty of Health Sciences, Linköping University, S-58185 Linköping, Sweden. E-mail: karin.roberg@pat.liu.se.
Supported by grants from the Swedish Cancer Foundation (no. 2703), the Swedish Society for Medical Research, and Östergötlands läns landsting.
References
- 1.Cohen GM: Caspases: the executioners of apoptosis. Biochem J 1997, 326:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deiss LP, Galinka H, Barissi H, Cohen O, Kimichi A: Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-α. EMBO J 1996, 15:3861-3870 [PMC free article] [PubMed] [Google Scholar]
- 3.Ishisaka R, Utsumi T, Yabuki M, Kanno T, Furuno T, Inoue M, Utsumi K: Activation of caspase-3-like protease by digitonin-treated lysosomes. FEBS Lett 1998, 435:233-236 [DOI] [PubMed] [Google Scholar]
- 4.Ishisaka R, Utsumi T, Kanno T, Arita K, Katunuma N, Akiyama J, Utsumi K: Participation of a cathepsin-L-type protease in the activation of caspase-3. Cell Struct Funct 1999, 24:465-470 [DOI] [PubMed] [Google Scholar]
- 5.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ: Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000, 106:1127-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarlett JL, Murphy MP: Release of apoptogenic proteins from the mitochondrial intermembrane space during the mitochondrial permeability transition. FEBS Lett 1997, 418:282-286 [DOI] [PubMed] [Google Scholar]
- 7.Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC: Cell-specific induction of apoptosis by microinjection of cytochrome c. J Biol Chem 1997, 272:30299-30305 [DOI] [PubMed] [Google Scholar]
- 8.Zhivotovsky B, Orrenius S, Brustugun OT, Doskeland SO: Injected cytochrome c induces apoptosis. Nature 1998, 391:449-450 [DOI] [PubMed] [Google Scholar]
- 9.Brustugun OT, Fladmark KE, Doskeland SO, Orrenius S, Zhivotovsky B: Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ 1998, 5:660-668 [DOI] [PubMed] [Google Scholar]
- 10.Chang SH, Phelps PC, Berezesky IK, Ebersberger ML, Trump BF: Studies on the mechanism and kinetics of apoptosis induced by microinjection of cytochrome c in rat kidney tubule epithelial cells (NRK-52E). Am J Pathol 2000, 156:637-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Öllinger K: Inhibition of cathepsin D prevents free radical induced apoptosis in rat cardiomyocytes. Arch Biochem Biophys 2000, 73:346-351 [DOI] [PubMed] [Google Scholar]
- 12.Kågedal K, Johansson U, Öllinger K: The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. EMBO J 2001, 15:1592-1594 [DOI] [PubMed] [Google Scholar]
- 13.Wu GS, Saftig P, Peters C, El-Deiry WS: Potential role for cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene 1998, 162:2177-2183 [DOI] [PubMed] [Google Scholar]
- 14.Roberg K, Johansson U, Öllinger K: Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic Biol Med 1999, 27:1228-1237 [DOI] [PubMed] [Google Scholar]
- 15.Roberg K: Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab Invest 2001, 81:149-158 [DOI] [PubMed] [Google Scholar]
- 16.Mellgren G, Vintermyr OK, Bøe R, Døskeland SO: Hepatocyte DNA replication is abolished by inhibitors selecting protein phosphatase 2A rather than phosphatase 1. Exp Cell Res 1993, 205:293-301 [DOI] [PubMed] [Google Scholar]
- 17.Barrett AJ: Cathepsin D and other carboxyl proteases. Barrett AJ eds. Proteases in Mammalian Cells and Tissue. 1977:pp 209-248 Elsevier/North-Holland Biochemical Press, Amsterdam
- 18.Lowry OH, Langemann H, Krenger W, Kempf A: Protein measurement with the Folin phenol reagent. J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 19.Brunk UT, Dalen H, Roberg K, Hellqvist HB: Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med 1997, 23:616-626 [DOI] [PubMed] [Google Scholar]
- 20.Desagher S, Martinou J-C: Mitochondria as the central control point of apoptosis. Trends Cell Biol 2000, 10:369-377 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JC, Waterhouse NJ, Jusin P, Evans GI, Green DR: The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2000, 2:156-162 [DOI] [PubMed] [Google Scholar]
- 22.Roberg K, Öllinger K: Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am J Pathol 1998, 152:1151-1156 [PMC free article] [PubMed] [Google Scholar]
- 23.Stoka V, Turk B, Schendel SL, Kim TH, Cirma T, Snipas SJ, Ellerby LM, Bredersen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS: Lysosomal protease pathway to apoptosis. Cleavage of Bid, not-caspases, is the most likely route. J Biol Chem 2001, 276:3149-3157 [DOI] [PubMed] [Google Scholar]
- 24.Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ: Cathepsin B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis and liver injury. Am J Pathol 2001, 159:2045-2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jäättelä M: Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol 2001, 153:999-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenessey A, Nacharaju P, Ko LW, Yen SH: Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem 1997, 69:2026-2038 [DOI] [PubMed] [Google Scholar]
- 27.Nagy Z, Esiri MM, Smith AD: The cell division cycle and the pathophysiology of Alzheimer’s disease. Neuroscience 1998, 87:731-739 [DOI] [PubMed] [Google Scholar]