Abstract
Purpose
The etiology of third nerve palsy is usually diagnosed by history, motility examination, and presence of lid and pupil involvement, as well as cranial and vascular imaging. We used high-resolution magnetic resonance imaging (hrMRI) of the oculomotor nerve and affected extraocular muscles (EOMs) to investigate oculomotor palsy.
Design
Prospective, noncomparative, observational case series in an academic referral setting.
Methods
Twelve patients with non-aneurysmal oculomotor palsy of duration 0.75 to 252 months were studied. In the orbit and along the intracranial oculomotor nerve, hrMRI at 1–2mm thickness was performed. Coronal plane images of each orbit were obtained in multiple, controlled gaze positions. Structural abnormalities of the oculomotor nerve and associated changes in EOM volume and contractility were evaluated.
Results
Cases were categorized as tumor-related, congenital, diabetic, traumatic, and idiopathic according to clinical characteristics and hrMRI findings. Reduction of volume and contractility of affected EOMs were noted in six patients; however, there was no significant EOMs atrophy in two cases with diabetic oculomotor palsy, and four cases with aberrant regeneration. hrMRI demonstrated the oculomotor nerve at the midbrain and at EOMs in all cases, and in two case with previous normal neuroimaging elsewhere demonstrated contrast-enhancing tumors on the oculomotor nerve. One patient with apparently unilateral congenital inferior division oculomotor palsy had no detectable ipsilateral and a hypoplastic contralateral oculomotor nerve exiting the midbrain.
Conclusions
hrMRI provides valuable information in patients with oculomotor palsy, such as structural abnormalities of the orbit and oculomotor nerve, and atrophy and diminished contractility of innervated EOMs. This information could be helpful in diagnosis and management of oculomotor palsy.
Introduction
Palsy of the oculomotor, or third, cranial nerve is a common cause of ophthalmoplegia.1 Management of oculomotor palsy is challenging because up to six extraocular muscles (EOMs) can be partially or completely involved: the levator palpebrae superioris, superior rectus (SR), medial rectus (MR), inferior rectus (IR), inferior oblique (IO) and pupillary sphincter muscles. Etiologic diagnosis of oculomotor palsy enables prognosis and assists in management. In adults, the most common causes of oculomotor palsy are aneurysm, microvascular disease and trauma. In children, congenital factors, trauma, and inflammation are the leading causes.2
Etiology of oculomotor palsy has traditionally been ascertained by history, motility examination, presence of lid and pupil involvement, and cranial and vascular imaging. Conventionally, imaging in oculomotor palsy has mainly been for detection of intracranial aneurysm. However, it is a challenge to demonstrate the path of cranial nerves by conventional magnetic resonance imaging (MRI) because of their small size, long course, and relationship to complex bony structures. Recent progress in high-resolution MRI (hrMRI) allows us to examine nearly the entire course of cranial nerves3,4 and to study the volume, contractility, and path of the EOMs.5–8 We performed this study to explore the various etiologies of oculomotor palsy by hrMRI by examining both the third cranial nerve and the affected EOMs.
Patients and Methods
Twelve consecutive patients with oculomotor palsy were identified from an ongoing prospective hrMRI study of strabismus conducted at the Jules Stein Eye Institute, a referral facility at the University of California, Los Angeles from 1993 through 2004. No patient had intracranial aneurysm. All participants in this study had given written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Institutional Review Board.
Complete ophthalmologic evaluation was performed, including detailed history of strabismus onset, trauma and systemic illness, external and slit-lamp examinations, prism-cover testing in diagnostic gaze positions, and fundus examination. Each subject underwent multipositional hrMRI to disclose any abnormality in the path of the oculomotor nerve and determine the volume, contractility, and path of EOMs. The same physician (JLD) performed all hrMRI procedures and evaluations. Cases were categorized as tumor-related, congenital, diabetic, traumatic and idiopathic according to clinical characteristics and hrMRI findings.
Imaging was performed with a 1.5-Tesla scanner (Signa; General Electric, Milwaukee, WI). The orbits of each patient were imaged with T1-weighted MRI with an array of surface coils embedded in a transparent face mask (Medical Advances, Milwaukee, WI) to improve signal-to-noise ratio and provide fixation targets.3,9,10 The patient’s head was stabilized in the supine position by securely fastening the surface coil mask to the face with headbands and fixing the mask to the scanner gantry with foam cushions and tape. For each gaze direction, the patient fixated a small, individually illuminated, red target delivered by a fiberoptic system mounted on the face mask during the approximately 3.5-minute scan time. To demonstrate the contraction and relaxation of the EOM of interest, selected targets were used to obtain images in the EOM’s field of action and the opposite field of gaze, respectively. Contiguous 2-mm thick MRI images were obtained in axial, quasisagittal (parallel to the long axis of the orbit), and quasicoronal (perpendicular to the long axis of the orbit) planes at 2-mm thick with an 8-cm field of view and a matrix of 256 x 256 pixels, with T1 weighting and two phase encodings, providing resolution of 312 μm in scan planes. The orbital apex was demonstrated in quasicoronal planes at 2-mm thick with a 6-cm field of view, with T1 weighting and three phase encodings, providing resolution of 234 μm. Imaging of cranial nerves at the brainstem was performed in 1-mm thickness planes using the heavily T2 weighted FIESTA sequence, which provides good contrast of cranial nerves against surrounding cerebrospinal fluid.3,4 In-plane resolution was 195 μm over a 10 cm field of view (matrix 512 x 512) with 10 phase encodings.
Digital MRI images were converted to 8-bit TIFF format and were analyzed using the NIH Image Program (W. Rasband, National Institutes of Health; available by ftp from zippy.nimh.nih.gov). The oculomotor nerve was traced from midbrain to EOMs. The volume of rectus EOMs in the paretic eye was computed from six contiguous images planes extending posteriorly beginning at the globe-optic nerve junction 3, and was analyzed as the ratio to the volume of the corresponding EOM in the unaffected fellow orbit. Quasicoronal image planes are not appropriate for determination of inferior oblique muscle volume, so this EOM was not analyzed. Levator muscle volume was not determined. Contractility of EOMs was determined by increase in cross-section from relaxed to contracting gaze directions.5,9 Statistical analysis was performed using StatView 5.0 (SAS Institute, Cary, NC).
Results
Characteristics of the 12 patients with oculomotor palsy are summarized in Table 1, and include data on 7 males and 5 females with average age 37.3 ± 23.5 years (mean ± standard deviation, SD, range, 6–82). Duration of oculomotor palsy ranged from 0.75 to 252 months before enrollment in the study. These 12 patients included two cases of due to tumors, one congenital,11 two diabetic, and three traumatic cases. Four cases had no definite etiology after complete evaluation and thus were defined idiopathic. Patients 2, 3 and 12 had limitation in infraduction and adduction only, without supraduction and upper lid involvement, and hence were regarded as having inferior division palsy. The other patients had clinical signs of EOMs involvement in the territories of both superior and inferior divisions, though the severity of involvement of each EOM varied. Aberrant regeneration to the upper lid was noted in patients 6, 7, 8 and 9.
The hrMRI technique employed here readily demonstrated the path of the oculomotor nerve from midbrain to the innervated EOMs, except in the cavernous sinus. Significant abnormalities of the oculomotor nerve and atrophy of the involved EOMs were disclosed by MRI in tumor-related and congenital oculomotor palsy. Although patients 1 and 2 had previous normal neuroimaging elsewhere, hrMRI in this study demonstrated contrast-enhancing tumors on the oculomotor nerve in the subarachnoid and intraorbital regions, respectively. Neither case had signs of neurofibromatosis. The involved EOMs exhibited varying degrees of atrophy. Patient 3 had clinically unilateral congenital inferior division oculomotor palsy that proved on hrMRI to be bilateral but asymmetrical. The MRI at midbrain level showed no detectable ipsilateral oculomotor nerve and a hypoplastic contralateral oculomotor nerve. The IR and MR EOMs were smaller in the ipsilateral orbit. These cases are described in detail in the following case reports. MRI was helpful in suspected traumatic cases. Patient 6 and 8 with presumptive diagnosis of oculomotor palsy secondary to remote cranial nerve trauma were both found by hrMRI to have had direct orbital trauma resulting in occult medial wall blowout fractures. These patients had no clinical signs of restrictive motility recognized before MRI was performed. Patient 7 had traumatic oculomotor palsy, combined with superior oblique (SO) palsy not definitively diagnosed until hrMRI due to the complexity of the motility pattern.
Patients 4 and 5 had diabetic oculomotor palsy. The ipsilesional oculomotor nerve appeared normal throughout, and volumes of the affected rectus EOMs were not significantly different from those of the unaffected fellow eyes (Table 1). The mean ratios of the volumes of affected to fellow SR, IR and MR EOMs were 0.95, 1.01 and 1.00, respectively. However, contractility of affected EOMs was significantly reduced (Figure 1). Similar preservation of relative EOM volumes was observed in patients 6, 7, 8 and 9, who had acquired oculomotor palsy with clinical signs of aberrant regeneration. In these cases exhibiting aberrant regeneration with preserved EOM volume, the mean relative volumes were 1.00, 0.97 and 0.98, respectively, of the fellow EOMs (Table 1).
Table 1.
Characteristics of Subjects with Third Nerve Palsy.
| Case | Age (yrs) | Gender | Paretic Eye | Etiology | Duration (mos) | Clinical Features | Involved Division | Aberrant Regeneration | Relative Volume of More Compared to Less or Unaffected Muscle
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | IR | MR | |||||||||
| 1 | 55 | F | Right | Tumor | 48 | Markedly limited SUP, INF & moderately limited ADD, sluggish pupil, mild ptosis | Both | No | 0.702 | 0.564 | 0.608 |
| 2 | 6 | F | Right | Tumor | 12 | Markedly limited ADD & mildly limited INF, normal pupil, no ptosis | Inferior | No | 1.032 | 0.840 | 0.760 |
| 3 | 21 | F | Both | Congenital | 252 | Right eye: markedly limited INF & ADD, normal pupil, no ptosis
Left eye: slightly limited INF & ADD, normal pupil, no ptosis |
Inferior | No | 0.914 | 0.717 | 0.731 |
| 4 | 73 | M | Right | Diabetic | 0.75 | Markedly limited SUP, INF & ADD, normal pupil, mild ptosis | Both | No | 0.901 | 0.902 | 0.929 |
| 5 | 40 | M | Left | Diabetic | 2 | Moderately limited SUP, INF & ADD, normal pupil, mild ptosis | Both | No | 1.004 | 1.121 | 1.065 |
| 6 | 28 | M | Left | Traumatic | 120 | Markedly limited SUP, INF & mildly limited ADD, fixed dilated pupil, ptosis | Both | Yes | 0.982 | 1.082 | 1.035 |
| 7 | 26 | F | Right | Traumatic | 60 | Markedly limited SUP, INF & mildly limited ADD, fixed dilated pupil, ptosis | Both | Yes | 1.046 | 1.008 | 0.970 |
| 8 | 48 | M | Left | Traumatic | 6 | Markedly limited SUP, INF & ADD, fixed dilated pupil, ptosis | Both | Yes | 1.032 | 0.916 | 0.957 |
| 9 | 25 | M | Left | Idiopathic | 3 | Moderately limited SUP, INF & ADD, sluggish | Both | Yes | 0.931 | 0.905 | 0.946 |
| 10 | 82 | M | Left | Idiopathic | 7 | Markedly limited SUP, INF & ADD, sluggish pupil, mild ptosis | Both | No | 0.848 | 0.665 | 0.958 |
| 11 | 10 | M | Right | Idiopathic | 60 | Markedly limited SUP, INF &ADD, sluggish pupil, no ptosis | Both | No | 0.827 | 0.372 | 0.407 |
| 12 | 33 | F | Left | Idiopathic | 48 | Markedly limited INF & ADD, normal pupil, no ptosis | Inferior | No | 0.987 | 0.790 | 0.600 |
ADD = adduction; F = female; INF = infraduction; IR = inferior rectus muscle; M= male; MR = medial rectus muscle; SR = superior rectus muscle; SUP = supraduction.
Figure 1.
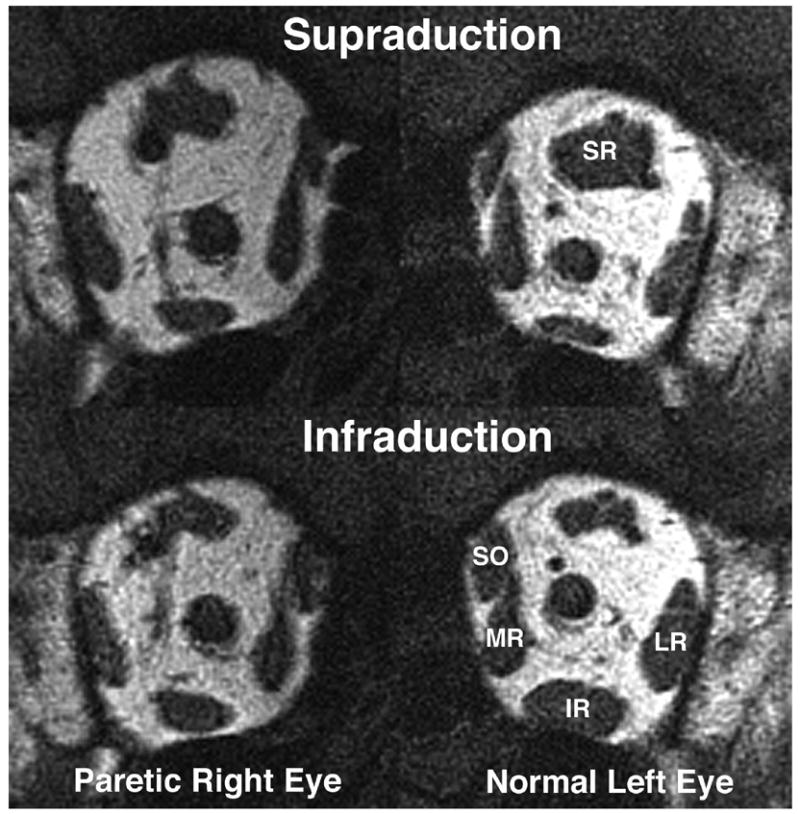
Quasicoronal T1-weighted MRI of patient 4 with right oculomotor palsy. The paretic right superior rectus muscle (SR) had less contractile thickening from infraduction to supraduction than the normal left SR. The paretic inferior rectus muscle (IR) had less contractile thickening from supraduction to infraduction than the normal left IR. LR = lateral rectus muscle; MR = medial rectus muscle; SO = superior oblique muscle.
Case Reports
Patient 1
This 55-year-old woman had a 5-year history of oblique and tilted binocular diplopia associated with right mydriasis, diminished near vision, and intermittent right blepharoptosis. Acetylcholine receptor antibody and edrophonium tests for myasthenia gravis were negative. Trials of oral prednisone and physostigmine were ineffective. Corrected visual acuity was 20/20 in each eye. In dim light, the right pupil was 5-mm in diameter and was slightly reactive to light and accommodation. The left pupil was 3.5mm and normally reactive. There were marked limitation to supraduction and infraduction with corresponding slow saccades, and moderate limitation to adduction of the right eye. There was 45Δ. exotropia (XT) in primary position that diminished in right gaze and increased in left gaze. In upward gaze, there was a left hypertropia (HT); while in downward gaze, there was a large right HT. Double Maddox rod testing demonstrated a 5 degree right incyclotorsion. The patient had elsewhere undergone two inconclusive MRI scans including the skull base in 5 mm thickness image planes, and one unremarkable magnetic resonance angiogram (MRA). High-resolution MRI of the orbits demonstrated reduction in size of branches of the right oculomotor nerve (Fig. 2 upper), as well as atrophy of all rectus EOMs innervated by these nerves (Fig. 2 lower). Ratios of EOM volume (right eye/left eye) for SR, IR and MR were 0.70, 0.56 and 0.61, respectively. At the skull base, hrMRI disclosed a 5 mm diameter, contrast-enhancing tumor of the right oculomotor nerve at the crossing point of the posterior communicating artery (Fig. 3). Concurrent MRA demonstrated that this lesion was not an aneurysm.
Figure 2.
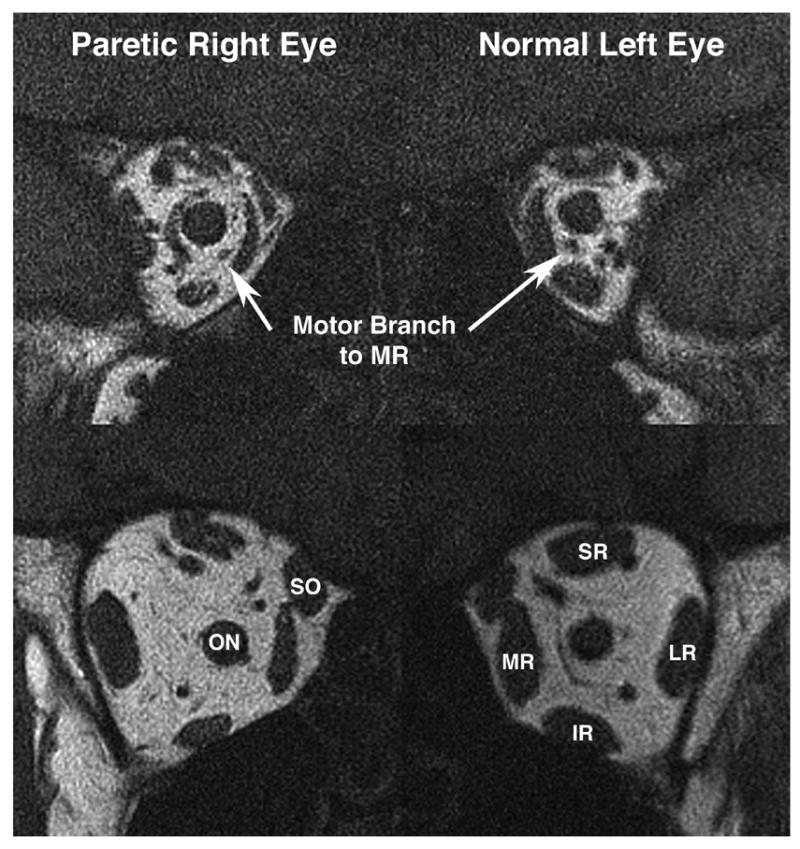
Quasicoronal T1-weighted MRI of patient 1 with right oculomotor palsy. (Upper) In deep orbit the motor nerve to the right medial rectus (MR) muscle is smaller than that to the left MR. (Lower) In mid-orbit, the right superior rectus (SR), inferior rectus (IR) and MR were atrophic, but. bulk of the superior oblique (SO) and lateral rectus (LR) were preserved. ON = optic nerve.
Figure 3.
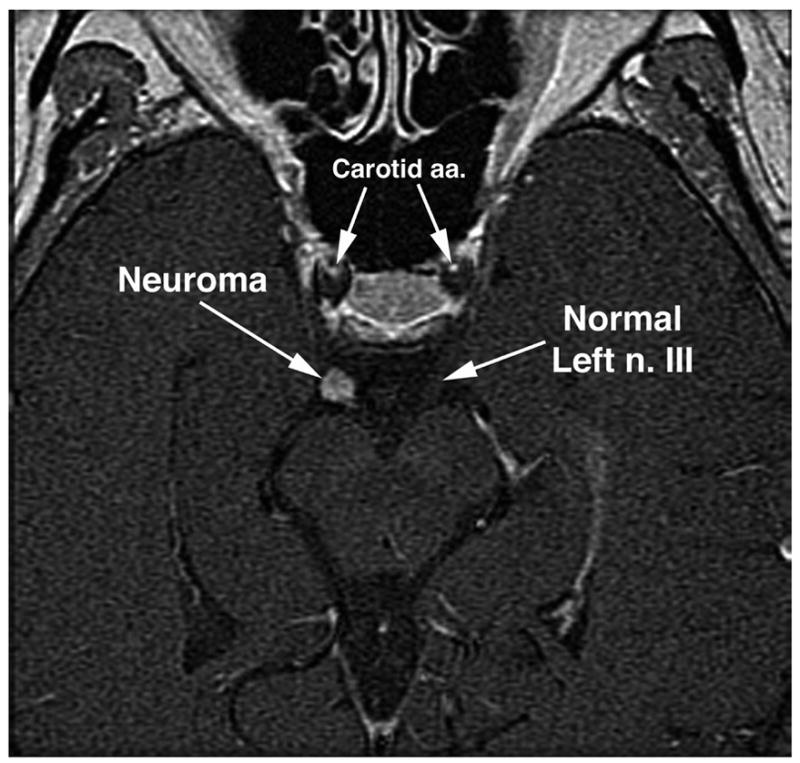
Oblique, axial T2-weighted MRI of patient 1 demonstrates 5-mm diameter contrast-enhancing tumor of the right oculomotor nerve. Carotid a.a. = carotid arteries, n. III = oculomotor nerve.
Patient 2
This 6-year-old girl had progressive adduction paresis of the right eye for 10 months. She had an extensive but inconclusive testing elsewhere, including multiple conventional MRI studies, edrophonium testing, and acetylcholine receptor antibody testing. Visual acuity was 20/25 in the right eye and 20/20 in the left eye. In primary position, she had 50Δ right XT that increased in left gaze and decreased in right gaze. The right eye demonstrated marked limitation in adduction with corresponding slow saccades, and mild limitation in infraduction. The pupils were briskly reactive to light and accommodation. There was no blepharoptosis. Under anesthesia, hrMRI demonstrated fusiform enlargement of the intra-orbital inferior division of the right oculomotor nerve that enhanced with contrast consistent with neuroma (Fig. 4). There was mild atrophy in the right IR and MR. The ratios of EOM volume (right eye/left eye) for IR and MR were 0.84 and 0.76, respectively.
Figure 4.
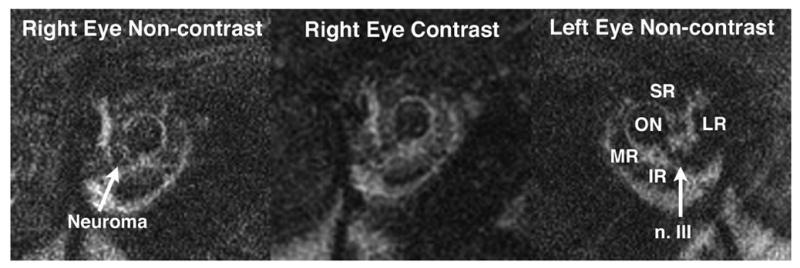
Quasicoronal MRI of the orbits of patient 2 demonstrates contrast-enhancing neuroma of the intra-orbital inferior division of the right oculomotor nerve. IR = inferior rectus muscle; LR = lateral rectus muscle; MR = medial rectus muscle; n. III = third cranial nerve; ON = optic nerve; SR = superior rectus muscle.
Patient 3
This 21-year-old woman presented with lifelong, constant, large angle XT and right HT without diplopia. There was no history of birth trauma. Corrected visual acuity was 20/80 in the right eye and 20/25 in the left eye. At distance, there was 75Δ XT and 10 Δ right HT. The XT increased to 100Δ at near. The right eye exhibited marked limitation to infraduction with normal saccade velocity, and severe limitation of adduction with saccadic slowing. In retrospect noted only after orbital hrMRI, the left eye also demonstrated minimal limitation to infraduction with normal saccade velocity, and slight limitation in adduction with a slow adducting saccade. The pupils were isocoric and normally reactive to light and accommodation without relative afferent pupillary defect. The initial clinical diagnosis was congenital right inferior division oculomotor palsy. High resolution MRI revealed absence of recognizable right oculomotor nerve at the midbrain, and hypoplasia of the left oculomotor nerve. The right IR and MR were smaller than corresponding EOMs on the left. The ratios of EOM volume (right eye/left eye) for IR and MR were 0.72 and 0.73, respectively. The right optic nerve was mildly hypoplastic. There was greater incyclotorsion of the orbital contents reflecting superior displacement of the LR pulley, nasal displacement of the SR pulley, and lateral displacement of the IR pulley. After hrMRI, the diagnosis was revised to congenital, bilateral, asymmetrical inferior division oculomotor nerve.
Patient 7
This 26-year-old woman had a 5-year history of oblique and tilted binocular diplopia since a severe head trauma resulting in coma for 6 weeks. Corrected visual acuity was 20/20 in each eye. The right palpebral fissure measured 6 mm, while the left was 10 mm. There was upper lid retraction in adduction indicating aberrant regeneration of the oculomotor nerve. The right pupil was 6.5 mm in diameter and unreactive to light and accommodation. There was marked limitation in supraduction and infraduction with corresponding slow saccades, and mild limitation of full adduction of the right eye. There was 35Δ right XT and 14Δ left HT in primary position. The XT increased in left gaze. The left HT significantly increased in right gaze and left head tilt. Double Maddox rod testing demonstrated 10 degree left excyclotorsion. Traumatic right oculomotor nerve with aberrant regeneration and possible left SO palsy was suspected. Quasicoronal hrMRI of the orbits revealed that the left SO was appreciably smaller than right SO (Fig. 5). However, volumes of the right SR, IR and MR were not significant different from those on of the left side. Ratios of EOM volume (right eye/left eye) for SR, IR and MR were 1.05, 1.01 and 0.97, respectively. Comparing the size of SR and IR between supraduction and infraduction, contractility of the SR and IR was decreased on the right side.
Figure 5.
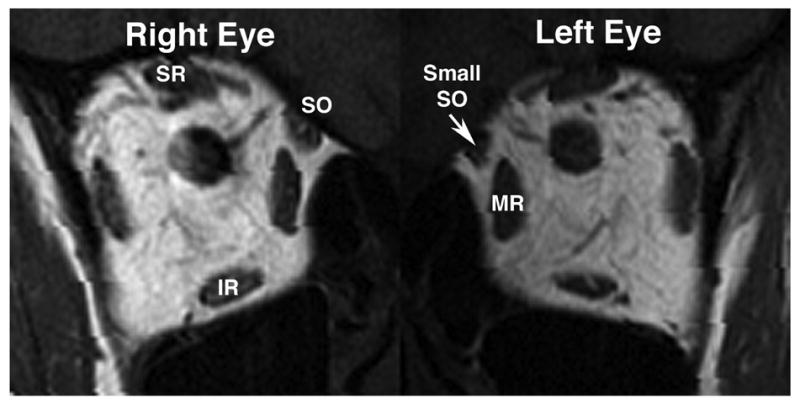
Quasicoronal MRI of the orbits of patient 7 with aberrant regeneration of right oculomotor palsy and suspected left superior oblique (SO) palsy. The left SO was smaller than right SO. In contrast, there were no significant differences in volumes of the superior rectus (SR), inferior rectus (IR) and medial rectus (MR) muscles between two orbits.
Discussion
Multiple structural causes of oculomotor palsy can be demonstrated by hrMRI. We used hrMRI to evaluate 12 patients with oculomotor palsy, often revealing unsuspected etiologies. Multipositional hrMRI of EOMs in these patients further demonstrated various degrees of EOM atrophy and decreased contractility that could be useful in surgical planning.
Two cases of presumed neuroma-related oculomotor palsy had previous neuroimaging that was reported to be normal. However, hrMRI demonstrated contrast-enhancing tumors on the oculomotor nerve in the subarachnoid and intraorbital regions, respectively, that were too small to resolve using conventional MRI technique. Although neuromas account for 8% to 10% of all intracranial tumors in the general population,12 those that involve the isolated oculomotor nerve have been considered rare. There have been only 27 well documented and histologically verified cases in the literature.13 Norman et al. reported on 5 children with isolated monocular oculomotor palsy originally believed to be idiopathic but subsequently documented to be secondary to a presumed oculomotor nerve neuroma in the subarachnoid space or cavernous sinus.14 Since neuromas usually exhibit indolent growth, these tumors tend to be small in the early disease course and easily missed in routine neuroimaging. Perhaps isolated oculomotor neuroma is not really rare, but only rarely discovered to earlier limitations on diagnostic imaging. It may be valuable to reconsider the diagnosis of chronic idiopathic oculomotor palsy, and to consider follow-up scans, especially with hrMRI.
Congenital oculomotor palsy may exist in isolation due to isolated congenital absence of the oculomotor nerve or its nucleus, or may be associated with other neurological deficits and central nervous system abnormalities. Most cases of congenital oculomotor palsy have been considered unilateral. Congenital bilateral oculomotor palsy has been rarely reported in the literature.15–17 Isolated inferior division oculomotor palsy has been considered uncommon, but is usually acquired and associated with trauma, tumor, intracranial hemorrhage, infarction, demyelinating disease, viral insult, or local orbital disease.2,18,19 Our patient 3 was a rare case of congenital isolated inferior division oculomotor palsy that was confirmed to be bilateral but asymmetrical by hrMRI. This sort of clinical presentation might be found more commonly if detailed hrMRI examination were performed.
Reduced volume of affected EOMs in oculomotor palsy was demonstrated in some current patients. Similar EOM volume reduction has been reported in other imaging studies of paralytic strabismus.3,20–22 Even though the EOMs differ from most other skeletal muscles in terms of constituent fiber types and innervation pattern, significant atrophy of some type-specific EOM fibers occurs in EOMs after denervation of the oculomotor nerve.23,24 Possible mechanisms for EOM atrophy may be due to the lack of neurotrophic influence of the motor neurons, disuse atrophy, and the passive stretch from its antagonist. In our case series, there was no significant decrease in affected EOM volume in diabetic cases and those with aberrant regeneration to the upper lid. Perhaps these pathologies do not interrupt the delivery of neurotrophic factors to EOMs innervated by the oculomotor nerve; if so, this would suggest that disuse atrophy and passive stretch are not causes of EOM atrophy generally.
Diabetic oculomotor palsy is usually self-limited with recovery within 4 months. Patient 4 had marked limitation in supraduction, infraduction and adduction; however, the duration of oculomotor palsy was only 3 weeks. Volume of EOMs might not be significantly reduced during this short period. Porter et al. demonstrated EOM fiber type-specific alterations of the MR muscle over time in oculomotor palsy.24 Although the cross-sectional area of some specific fiber types had significantly changed on the 28th day after denervation, total EOM cross-sectional area did not change significantly in this stage. Patient 5 was also diabetic and had partial recovery of affected EOM function after 2 months of onset that implied some degree of reinnervation. Reinnervation may reverse alterations in EOM fibers24 and thus preserve EOM volume. Therefore, preservation of EOM volume in diabetic oculomotor palsy might be attributed to the short time span between onset and hrMRI, and the resolving nature of the etiology.
Aberrant regeneration is frequently observed in oculomotor palsy. Random misdirection of regenerating motor axons within an injured oculomotor nerve trunk has been proposed based on histopathologic, clinical, and experimental evidence.25,26 Clinically, the upper eyelid may retract on attempted downward gaze (pseudo–von Graefe sign) or adduction (reverse Duane syndrome); the pupil, which may not react well to light, may constrict on adduction and infraduction; there may be adduction or retraction of the globe on attempted vertical gaze. Despite stereotypic patterns of movement, it is believed that the regenerating axons in the oculomotor nerve do not selectively reinnervate individual EOMs.27,28 Scherer found in regenerated oculomotor nerve that labeled axons traced from the IO to the oculomotor nerve trunk were disarrayed in the proximal portion of the oculomotor nerve so as to be distributed to SR, IR and MR.27 Therefore, a patient presenting a clinically significant aberrant regeneration to the upper lid might also have subclinical aberrant regeneration to oculorotatory EOMs. Neurotrophic factors have been shown to prevent atrophy of denervated skeletal muscle.29–31 There is no similar direct evidence for the role of neurotrophic factors in denervated EOMs, but restoration and even increase in the cross-sectional area of fibers in reinnervatated EOMs may be an indirect evidence for the neurotrophic effect of reinnervation on EOMs.24 This evidence might explain our finding of preservation of EOM volume in cases of acquired oculomotor palsy with aberrant regeneration.
Trauma could cause complex strabismus due to cranial neuropathy of multiple cranial nerves, traumatic myopathy, blow-out fracture with EOM or connective tissue entrapment, or globe displacement. In our case series, one of the three cases with traumatic oculomotor palsy had combined traumatic SO palsy, and two had occult blow-out fractures. Orbital imaging by CT or MRI may be diagnostically useful to ascertain the structural abnormalities of the orbit and remaining function of EOMs. While CT may optimally demonstrate fractures in orbital bones, the size, contractility and path of EOMs can be better imaged by hrMRI. Multipositional hrMRI is especially helpful to define the number of EOMs involved and the nature of the damage.32
Three large studies assert that 23% to 25% of cases of oculomotor palsy in adults are idiopathic.33–35 The number of idiopathic cases in pediatric patients approaches 47%.36–38 Some cases in the current series did not receive definite diagnosis of the cause of oculomotor palsy even after complete evaluation. However, hrMRI showed that one case of idiopathic oculomotor palsy with aberrant regeneration had preserved EOM volume but decreased contractility. Those idiopathic cases without aberrant regeneration had significant EOM atrophy of different degrees in severity.
In conclusion, hrMRI can provide important information in oculomotor palsy regarding anatomical abnormality, compressive lesion in the path of the oculomotor nerve, and associated cranial nerve palsy. Further, hrMRI can indicate the degree of atrophy and decreased contractility of affected EOMs. We suggest that it may be clinically valuable in selected, complex cases to employ hrMRI to visualize the path of the oculomotor nerve from brainstem to the individual EOMs.
Acknowledgments
The authors are solely responsible for the design and conduct of this study; the collection, management, analysis and interpretation of the data; and preparation, review, and approval of the manuscript.
Biographies
 Joseph L. Demer, M.D., Ph.D.
Joseph L. Demer, M.D., Ph.D.
Leonard Apt Professor of Ophthalmology, Jules Stein Eye Institute, Professor of Neurology, University of California, Los Angeles
Joseph L. Demer, M.D., Ph.D. is Leonard Apt Professor Professor and Chief of Comprehensive Ophthalmology, and Professor of Neurology, David Geffen School of Medicine at UCLA. He directs the Ocular Motility Clinical Laboratory, and the EyeSTAR Program. In 2003, Dr. Demer received the Friedenwald Award from ARVO, and a Recognition Award from the Alcon Research Institute in 2004, for his work on the extraocular muscles and orbital connective tissues. Dr. Demer chairs the ARVO Awards Committee.
 Biographic sketch
Biographic sketch
Hui-Chuan Kau, Taoyuan, Taiwan
Hui-Chuan Kau, MD, MS, is chief of Ophthalmology at Taoyuan Veterans Hospital, and Instructor in Ophthalmology at National Yang-Ming University and Taipei Veterans General Hospital in Taiwan.
Dr. Kau received medical training at National Yang-Ming University, Ophthalmology residency at Taipei Veterans General Hospital, and fellowship in Pediatric Ophthalmology at the Jules Stein Eye Institute, University of California, Los Angeles.
Dr. Kau conducts clinical research on strabismus, and laboratory research on oxidative stress in ocular diseases.
Footnotes
Supported by United States National Eye Institute EY-8313 and Research to Prevent Blindness.
Presented at: American Association for Pediatric Ophthalmology and Strabismus 31st Annual Meeting, March 9, 2005; Orlando, Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richards BW, Jones FR, Jr, Younge BR. Causes and prognosis in 4278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113:489–496. doi: 10.1016/s0002-9394(14)74718-x. [DOI] [PubMed] [Google Scholar]
- 2.Malcolm LM. Third cranial nerve palsy: diagnosis and management strategies. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia: WB Saunders; 1999. pp. 251–258. [Google Scholar]
- 3.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 4.Seitz J, Held P, Strotzer M, et al. MR imaging of cranial nerve lesions using six different high-resolution T1 and T2(*)-weighted 3D and 2D sequences. Acta Radiologica. 2002;43:349–353. doi: 10.1080/j.1600-0455.2002.430401.x. [DOI] [PubMed] [Google Scholar]
- 5.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia: WB Saunders; 1999. pp. 85–98. [Google Scholar]
- 6.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of the extra-ocular muscles a new diagnostic tool in paralytic strabismus. J Ped Opththalmol Strabismus. 1994;31:177–188. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 7.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;9:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 8.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219–1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 9.Wu TJ, Rosenbaum AL, Demer JL. Severe strabismus after scleral buckling: multiple mechanisms revealed by high-resolution magnetic resonance imaging. Ophthalmol. 2005;112:327–336. doi: 10.1016/j.ophtha.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 11.Wu TJ, Isenberg SJ, Demer JL. Magnetic resonance imaging demonstrates neuropathology in congenital inferior division oculomotor paresis. J AAPOS. doi: 10.1016/j.jaapos.2006.04.007. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller NR, editor. Walsh and Hoyt‘s Clinical Neuro-ophthalmology. Baltimore: Williams & Wilkins; 1988. [Google Scholar]
- 13.Netuka D, Benes V. Oculomotor nerve schwannoma. Br J Neurosurg. 2003;17:168–173. [PubMed] [Google Scholar]
- 14.Norman AA, Farris BK, Siatkowski RM. Neuroma as a cause of oculomotor palsy in infancy and early childhood. J AAPOS. 2001;5:9–12. doi: 10.1067/mpa.2001.112442. [DOI] [PubMed] [Google Scholar]
- 15.Good WV, Barkovich AJ, Nickel BL, et al. Bilateral congenital oculomotor nerve palsy in a child with brain anomalies. Am J Ophthalmol. 1991;111:555–558. doi: 10.1016/s0002-9394(14)73697-9. [DOI] [PubMed] [Google Scholar]
- 16.Reinecke RD. Surgical results of third cranial nerve palsies. NY State J Med. 1972;72:1255–1257. [PubMed] [Google Scholar]
- 17.Balkan R, Hoyt CS. Associated neurologic abnormalities in congenital third nerve palsies. Am J Ophthalmol. 1984;97:315–319. doi: 10.1016/0002-9394(84)90629-9. [DOI] [PubMed] [Google Scholar]
- 18.Ksiazek SM, Repka MX, Maguire A, et al. Divisional oculomotor nerve paresis caused by intrinsic brainstem disease. Ann Neurol. 1989;26:714–718. doi: 10.1002/ana.410260605. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham ET, Jr, Good WV. Inferior branch oculomotor nerve palsy. A case report J Neuroophthalmol. 1994;14:21–23. [PubMed] [Google Scholar]
- 20.Demer JL, Miller JM, Koo EY, et al. Quantitative magnetic resonance morphometry of extraocular muscles: a new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–188. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 21.Bloom JN, Cadera W, Heiberg E, et al. A magnetic resonance imaging study of horizontal rectus muscle palsies. J Pediatr Ophthalmol Strabismus. 1993;30:296–300. doi: 10.3928/0191-3913-19930901-07. [DOI] [PubMed] [Google Scholar]
- 22.Nishida Y, Hayashi O, Nishida E, et al. Volume measurement of the horizontal extraocular muscles using magnetic resonance imaging. Nippon Ganka Gakkai Zasshi. 1993;97:827–833. [PubMed] [Google Scholar]
- 23.Baker RS, Millett AJ, Young AB, et al. Effects of chronic denervation on the histology of canine extraocular muscle. Invest Ophthalmol Vis Sci. 1982;22:701–705. [PubMed] [Google Scholar]
- 24.Porter JD, Burns LA, McMahon EJ. Denervation of primate extraocular muscle: a unique pattern of structural alterations. Invest Ophthalmol Vis Sci. 1989;30:1894–1908. [PubMed] [Google Scholar]
- 25.Glaser JS, Siatkowski RM. Infranuclear disorders of eye movement. In: Glaser JS, editor. Neuro-ophthalmology. Baltimore: Williams & Wilkins; 1999. pp. 405–460. [Google Scholar]
- 26.Sibony PA, Evinger C, Lessell S. Retrograde horseradish peroxidase transport after oculomotor nerve injury. Invest Ophthalmol Vis Sci. 1986;27:975–980. [PubMed] [Google Scholar]
- 27.Scherer SS. Reinnervation of the extraocular muscles in goldfish is nonselective. J Neurosci. 1986;6:764–773. doi: 10.1523/JNEUROSCI.06-03-00764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez E, Pallini R, Gangitano C, et al. The third nerve transection and regeneration in rats with preliminary results on the sixth nerve transection and regeneration in guinea pigs. Neurol Res. 1988;10:221–224. doi: 10.1080/01616412.1988.11739845. [DOI] [PubMed] [Google Scholar]
- 29.Helgren ME, Squinto SP, Davis HL, et al. Trophic effect of ciliary neurotrophic factor on denervated skeletal muscle. Cell. 1994;76:493–504. doi: 10.1016/0092-8674(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Wang F, Hong G, et al. Protective effects of ciliary neurotrophic factor on denervated skeletal muscle. J Huazhong Univ Sci Technolog Med Sci. 2002;22:148–151. doi: 10.1007/BF02857680. [DOI] [PubMed] [Google Scholar]
- 31.Sterne GD, Coulton GR, Brown RA, et al. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J Cell Biol. 1997;139:709–715. doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortube MC, Rosenbaum AL, Goldberg RA, Demer JL. Orbital imaging demonstrates occult blow out fracture in complex strabismus. J AAPOS. 2004;8:264–273. doi: 10.1016/j.jaapos.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Miller NR. Solitary oculomotor nerve palsy in childhood. Am J Ophthalmol. 1977;83:106–110. doi: 10.1016/0002-9394(77)90197-0. [DOI] [PubMed] [Google Scholar]
- 34.Harley RD. Paralytic strabismus in children etiologic incidence and management of the third, fourth, and sixth nerve palsies. Ophthalmology. 1980;87:24–43. doi: 10.1016/s0161-6420(80)35280-9. [DOI] [PubMed] [Google Scholar]
- 35.Keith CG. Oculomotor nerve palsy in childhood. Aust N Z J Ophthalmol. 1977;15:181–184. doi: 10.1111/j.1442-9071.1987.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 36.Rucker CW. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1966;61:1293–1298. doi: 10.1016/0002-9394(66)90258-3. [DOI] [PubMed] [Google Scholar]
- 37.Green WR, Hackett ER, Schlezinger NS. Neuro-ophthalmologic evaluation of oculomotor nerve paralysis. Arch Ophthalmol. 1964;72:154–167. doi: 10.1001/archopht.1964.00970020154005. [DOI] [PubMed] [Google Scholar]
- 38.Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI. Cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981;99:76–79. doi: 10.1001/archopht.1981.03930010078006. [DOI] [PubMed] [Google Scholar]


