Abstract
Inflammatory breast carcinoma (IBC) is characterized by florid tumor emboli within lymphovascular spaces called lymphovascular invasion. These emboli have a unique microscopic appearance of compact clumps of tumor cells retracted away from the surrounding endothelial cell layer. Using a human SCID model of IBC (MARY-X), we, in previous studies, demonstrated that the tumor cell embolus (IBC spheroid) forms on the basis of an intact and overexpressed E-cadherin/α,β-catenin axis that mediates tumor cell-tumor cell adhesion. In the present study we examine the mechanism behind the apparent lack of binding of the tumor embolus to the surrounding endothelium. We find that this lack of tumor cell binding is because of markedly decreased sialyl-Lewisx/a (sLex/a) carbohydrate ligand-binding epitopes on its overexpressed MUC1 and other surface molecules that bind endothelial E-selectin. Decreased sLex/a is because of decreased α3/4-fucosyltransferase activity in MARY-X. The decreased sLex/a fail to confer electrostatic repulsions between tumor cells, which further contributes to the compactness of the MARY-X spheroid by allowing the E-cadherin homodimeric interactions to go unopposed. MARY-X spheroids were retrovirally transfected with FucT-III cDNA, significantly raising their levels of fucosyltransferase activity and surface sLex/a. In parallel experiments, enzymatic transfers with a milk α1,3-fucosyltransferase and an α2,3-sialyltransferase (ST3GalIV) were performed on the MARY-X spheroids and increased surface sLex/a. The addition of sLex/a by either manipulation caused disadherence of the MARY-X spheroids and the disruption of the E-cadherin homodimers mediating cell adhesion. Our findings support the cooperative relationship of sLex/a underexpression and E-cadherin overexpression in the genesis of the lymphovascular embolus of IBC.
Our screening methods for certain types of breast cancer such as inflammatory breast cancer (IBC) have not impacted the disease’s age-adjusted 5 year mortality because these types of breast cancer do not remain organ-confined before they are detected. 1 One telltale sign of lack of organ confinement is the presence of lymphovascular invasion, a finding that is most pronounced in IBC. 2 Lymphovascular invasion is defined by tumor emboli within lymphovascular spaces. 3 These emboli have a unique microscopic appearance of compact clumps of tumor cells retracted away from the surrounding endothelial cell layer lining the lymphovascular space. The molecular determinants of this phenotype remain undefined. Recently our laboratory established a human SCID model of IBC (MARY-X) that exhibited florid lymphovascular tumor emboli in vivo and compact spheroids in vitro. 4,5 We have characterized, in part, the molecular basis of the IBC phenotype using this model. Our initial studies indicated that MARY-X overexpressed both E-cadherin and MUC1. 4 We chose to focus on E-cadherin initially and demonstrated that both the tumor cell emboli and the in vitro spheroids formed on the basis of an intact and overexpressed E-cadherin/α,β-catenin axis that mediated tumor cell adhesion analogous to the embryonic blastocyst. 5 Disruption of the E-cadherin/α,β-catenin axis with either anti-E-cadherin antibodies or E-cadherin dominant-negative mutant approaches caused complete disadherence of the tumor emboli in vivo. Furthermore in addition to these two manipulations, trypsin proteolysis and Ca++ depletion caused complete disadherence of the spheroids in vitro. In the present study we decided to investigate the role of overexpressed MUC1 and a possible mechanism that might explain the apparent lack of binding of the tumor embolus to the surrounding endothelium.
Materials and Methods
Establishment of MARY-X
In previous studies we established MARY-X, a human xenograft, from a patient with IBC. MARY-X exhibited the phenotype of florid local lymphovascular emboli formation in nude/SCID mice. 4 Mincing fragments of MARY-X in tissue culture produced a suspension of compact multicellular spheroids. 5 The spheroids were 99% human; exhibited a very high cell density (103 cells/120-μm diameter); remained viable in suspension for 2 to 4 weeks; never formed monolayers on either plastic, extracellular matrix-coated dishes, or feeder layers of fibroblast, myoepithelial, or endothelial cells; and fully recapitulated the MARY-X phenotype of lymphovascular emboli when reinjected into nude/SCID mice. The crude minced fragments of MARY-X contained spheroids ranging in size from 10 to 600 μm with peaks of 100, 150, and 200 μm in size. Spheroids of homogeneous sizes could be obtained by filtration through various sized filters (Becton-Dickinson, Franklin Lakes, NJ). A 70-μm filter was used to exclude spheroids >70 μm; the filtrate was then subsequently filtered on a 40-μm filter where 40- to 70-μm spheroids were excluded, isolated, and resuspended. Our previous studies indicated that spheroids of all sizes formed on the basis of an overexpressed E-cadherin/α,β-catenin axis. 5
Other Cell Lines and Xenografts
Comparative cell lines and xenografts used in the present study included the Colo-205 and Colo-201 (American Type Culture Collection, Rockville, MD), lines that overexpress MUC1 rich in sialyl-Lewisx/a (sLex/a) epitopes and that bind to endothelial cell E-selectin with high affinity. All lines were grown in minimal essential medium (MEM) containing 10% fetal calf serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in an air-5% CO2 atmosphere at constant humidity. Xenografts of these lines were generated by injections of 1 × 106 cells/200 λ subcutaneously into either the ventrolateral flanks or mammary fat pads of female SCID mice and allowed to grow into 1.0-cm-diameter tumors. We also used human umbilical vein endothelial cells (HUVECs) (Clonetics, San Diego, CA) to study attachment of selected tumor cell lines.
Antibodies
The antibodies used included monoclonal antibodies to E-cadherin (clone HECD-1) at a concentration of 1 to 10 μg/ml (Zymed Laboratories, San Francisco, CA); MUC-1 (clone HMPV, mouse IgG1) (PharMingen, San Diego, CA) at a concentration of 1 to 100 μg/ml; sialyl-Lewisx (sLex) (clone CSLEX-1) (UCLA Tissue Typing Laboratory, Los Angeles, CA); sialyl-Lewisa (sLea) (clone CCOL-2) (UCLA Tissue Typing Laboratory); β-actin (a gift of Dr. Judy Berliner, UCLA); and control murine IgG1 (DAKO, Glostrup, Denmark) at a concentration of 50 μg/ml.
Western Analysis
The cell lines were harvested and immediately frozen. The xenografts were excised, frozen, and pulverized with mortar and pestle to a fine powder. Both were then extracted with buffer [1% Triton X-100, aprotinin (2 μg/ml), leupeptin (2 μg/ml), NaF (100 mmol/L), sodium orthovanadate (2 mmol/L), NaCl (150 mmol/L), sodium phosphate (10 mmol/L), ethylenediaminetetraacetic acid (10 mmol/L)] for 4 hours at 4°C with gentle agitation. The samples were then centrifuged at 13,000 × g at 4°C for 15 minutes. Protein concentrations were determined using the Bio-Rad reagent (Bio-Rad, Richmond, CA). Samples containing equal protein were boiled in a 1× Laemmli buffer under reducing conditions, run on a 7.5% sodium dodecyl sulfate-polyacrylamide gel, transferred to a nitrocellulose membrane that was incubated with the primary and secondary antibodies, and signal-detected with the ECL detection system (Amersham Life Sciences, Arlington Heights, IL) according to previous methods. 6 A monoclonal antibody to β-actin was used to normalize for protein loading.
Retroviral Transfection Studies
A plasmid, pCDM8, containing the α1,3-fucosyltransferase III (FucT-III) cDNA that had been ligated into HindIII and XbaI sites of the multiple cloning region (a gift of Dr. Minoru Fukuda, The Burnham Institute, La Jolla, CA) was subjected to digestion with the same two restriction endonucleases and the liberated cDNA was subcloned into the same two restriction sites of pGEM-9Zf(−) (Promega, Madison, WI). The latter plasmid has an expanded multiple cloning region that allowed the cDNA insert to be liberated with HindIII and SalI and then further subcloned into the same two sites of the retroviral plasmid, pLNCX2 (BD Biosciences Clontech, Palo Alto, CA). A retroviral plasmid (2.0 pmol) containing FucT-III cDNA was therefore created and used to transfect a retroviral packaging cell line, RetroPack PT67 (Clontech) according to the manufacturer’s directions. Harvesting of the viral supernatants in conditioned media (Isocoves with 10% fetal calf serum) was begun at 36 hours after transfection and collected every 4 to 6 hours up to 72 hours. Twenty-five ml of viral supernatant was filter-sterilized through a 0.45-μm pore size filter and used in subsequent experiments. This approach produced high titers (106 to 107 cfu/ml) of helper-free retrovirus containing the desired constructs. The filtered undiluted retroviral supernatants were used immediately in some experiments or aliquoted and stored at −70°C for later use. For transfection of the spheroids we used a transfection strategy based on a reversible model of spheroid formation of our MARY-X spheroids. 7,8 In Ca++-depleted media the spheroids disadhere into single cells within 30 minutes and with the readdition of Ca++ (provided it is added back within 6 hours), the cells reformed compact spheroids. The reformed spheroids first begin to reform as loose aggregates of cells by 3 hours after the readdition of Ca++, tighten by 6 hours, and become fully compact by 12 hours. This strategy allows a transfection window where retroviral transfection can be achieved while the cells are in their single cell state. With this strategy 103 to 104 intact spheroids (average size, 60-μm diameter) were plated onto 60-mm dishes and disadhered in Ca++-depleted MEM. After 30 minutes when complete disadherence was achieved, 2 ml of MEM (containing 1 mmol/L CaCl2) and various titers of viral supernatant providing 1 to 100 multiplicities of infection (MOI) in the presence of polybrene (8 μg/ml) was added. Infection was performed in a humidified incubator at 37°C in an air-5% CO2 atmosphere at constant humidity throughout a 3-hour period. After this the viral supernatant was removed and the readhering spheroids were placed in MEM with 10% fetal calf serum. After 48 hours and throughout the next 72 hours the spheroids were observed for gene expression and/or phenotypic changes. The presence of sLex/a was determined by incubating the spheroids with the monoclonals anti-sialyl-Lewisx (sLex) (clone CSLEX-1) and anti-sialyl-Lewisa (sLea) (clone CCOL-2) followed by a peroxidase-conjugated goat anti-mouse specific for murine IgM (Cappel Laboratories, Sacramento, CA) and immunoperoxidase staining. In some experiments the spheroids were permeabilized with absolute methanol for 20 minutes at −20°C before antibody incubation. The retrovirally transfected spheroids, termed FucT-III-MARY-X, were measured for fucosyltransferase activity as well as monitored for disadherence, apoptosis, attachment, and adhesion.
Enzymatic Synthesis of sLex/a on the Cell Surface
This approach established by other investigators 9 again was used with our reversible model of spheroid formation. SLex/a hexasaccharide synthesis consisting of enzymatic transfer with a milk fucosyltransferase and an α2,3-sialyltransferase (ST3GalIV) (Calbiochem, La Jolla, CA) was performed on the cell surface of the MARY-X spheroids. α1,3-fucosyltransferases were partially purified from human milk by precipitation with 65% saturated ammonium sulfate followed by CM-Sepharose C-50. The enzyme eluted in the 0.2-mol/L NaCl fraction and its activity was measured as stated subsequently. MARY-X (103) spheroids (average size, 60-μm diameter) were fully disadhered to ∼105 cells. These cells were washed in phosphate-buffered saline (PBS) and incubated with 2.4 mU of the partially purified fucosyltransferases, 50 mU of recombinant ST3GalIV, 1000 nmol/L of donors, GDP-fucose, and CMP-N-acetylneuraminic acid (CMP-NANA) (Calbiochem), in 1 ml of Ca++ containing Opti-MEM (Life Technologies, Inc., Gaithersburg, MD) with 7 mmol/L of MnCl2. Opti-MEM was titrated to pH 7.0 × 1 N HCl. Incubation of the readhering spheroids was performed for 24 hours at 37°C in a humidified atmosphere of 5% CO2. The cells were then washed in PBS and studied for sLex/a expression by both Western blot and immunocytochemistry. These spheroids, termed sLex/a-MARY-X, were monitored for disadherence, apoptosis, attachment, and adhesion.
Measurement of Fucosyltransferase Activity
Wild-type MARY-X spheroids (103) (105 to 106 cells), similar numbers of FucT-III-MARY-X, Colo-201, and Colo-205 cells were homogenized on ice in extraction buffer (2% Triton X-100, 50 mmol/L β-mercaptoethanol, 50 mmol/L Tris/HCl, pH. 7.5) and centrifuged at 4°C for 30 minutes at 2000 × g. The protein concentration of the supernatants was normalized. The α3- and α4-fucosyltransferase assays were performed with different synthetic oligosaccharide acceptors, H-type-2 and H-type-1, respectively, synthesized with a hydrophobic arm (−(CH2)8-COOCH3) (Calbiochem, San Diego, CA) that allows the glycosides to be retained on a Sep-Pak C18 cartridge (Waters, Milford, MA). 10,11 The reaction mixture was prepared on ice, contained in a volume of 60 μl: 30 μg tumoral extract, 35 mmol/L Tris/HCl, pH 7.2, 4 mmol/L ATP, 20 mmol/L MnCl2, 10 mmol/L α-L-fucose, 5 μl of a 1m/ml solution of the oligosaccharide acceptors (H-type-2: Fucα1–2Galβ1–4GlcNAc-R); H-type-1: Fucα1–2Galβ1–3GlcNAc-R) and 3.5 μmol/L GDP-[14C]-fucose (10 GBq/mmol; Amersham, Piscataway, NJ). The mixture was incubated for 16 hours at 37°C and the reaction was stopped by the addition of 3 ml of cold water and centrifuged. The supernatant was added to the Sep-Pak C18 cartridge, the unreacted GDP-[14C]-fucose and its hydrolysis products were washed out with 25 ml of H2O. Radiolabeled reaction products were eluted with methanol and counted in a liquid scintillation counter.
In Vitro Spheroid Disadherence Assays
FucT-III-MARY-X and sLex/a-MARY-X were compared to wild-type MARY-X spheroids. These different spheroids were individually placed into wells of a 96-well plate containing 50 μl of MEM with 1 mmol/L of CaCl2. The wild-type spheroids were further placed into wells containing Ca++-depleted MEM, trypsin (0.05% trypsin in MEM) (Life Technologies, Inc.) or E-cadherin antibody (50 to 100 μg/ml in MEM). The spheroids were monitored for size changes and disadherence by visualization under a phase contrast microscope at successive time points throughout 2 to 144 hours. The cell density of the spheroids was determined by counting the total cells liberated.
Apoptosis Assay
Apoptosis was measured in all of the experimental groups that included FucT-III-MARY-X, sLex/a-MARY-X, and wild-type MARY-X spheroids at various time points. Apoptosis was measured by labeling the 3′OH ends of DNA using fluorescein incorporation by terminal deoxynucleotidyl transferase. Anti-fluorescein antibodies and immunoperoxidase staining were used to demonstrate fluorescein-nucleotide incorporation with the In Situ Cell Death Detection Kit, POD (Roche Molecular Biochemicals, Mannheim, Germany), which was in essence a modified terminal dUTP nick-end labeling assay. In brief, cytopreparations of spheroids were prepared on chamber slides. After fixing with 75% ethanol, slides were rinsed with PBS and incubated in a reaction mixture containing terminal transferase and fluorescein-nucleotides at 37°C for 1 hour. The specimens were then washed followed by anti-fluorescein antibody coupled to horseradish peroxidase for 30 minutes at room temperature. After additional washings, diaminobenzidine tetrachloride was added and the cells were incubated for 10 minutes. In separate experiments, the spheroids were pelleted at 100 to 200 × g, embedded in OCT or formalin-fixed and paraffin embedded, and sectioned on a cryostat. The apoptosis assay was performed as before. The apoptotic index (AI), the percent peroxidase-positive cells, was determined by counting 200 cells in random fields.
HUVEC Attachment Assay
HUVECs were plated to form a monolayer on gelatinized 96-well culture plates at 20,000 cells/well and incubated at 37°C in 5% CO2 for 24 hours. The cells were maintained in HUVEC growth medium that consisted of medium 199 with 10% heat-inactivated fetal calf serum. Selected wells were treated with 200 U/ml of tumor necrosis factor (TNF)-α, a known inducer of endothelial cell E-selectin. 12 A sensitive enzyme-linked immunosorbent assay (clone H18/7; Becton Dickinson, San Jose, CA) was used to measure the expression of E-selectin in both the untreated and treated HUVECs. FucT-III-MARY-X, sLex/a-MARY-X, and wild-type MARY-X spheroids (intact and disadhered) and selected other cell lines (eg, Colo-201 and Colo-205) were then plated at 10,000 cells (or spheroids) per well and incubated for 30 minutes at 4°C. This 30-minute incubation occurred before any appreciable degree of apoptosis occurred in the disadhered cells. Unbound cells (or spheroids) were removed by aspiration and washing the plates with PBS containing CaCl2 and MgCl2 five times. The bound cells (or spheroids) were fixed with methanol. Attachment was quantitated by counting cells under ×200 magnification using a phase contrast microscope.
E-Selectin Adhesion Assay
To determine whether the FucT-III-MARY-X, sLex/a-MARY-X, and wild-type MARY-X spheroids (intact and disadhered) and selected other cell lines (eg, Colo-201 and Colo-205) adhere specifically to E-selectin, in vitro adhesion assays to E-selectin were performed. Ninety-six-well culture plates were coated with human recombinant E-selectin obtained from Chinese hamster ovary cells (Calbiochem, San Diego, CA). Recombinant E-selectin in PBS was added to each well at a concentration of 10 μg/ml and incubated at 4°C overnight. 13 The solution was removed and 1% bovine serum albumin in PBS was added to each well for 4 hours at 4°C to block nonspecific adhesion. The next day, cells were plated at 10,000 cells/well and incubated for 30 minutes at 4°C. Unbound cells were removed by aspiration and washing the plates with PBS containing CaCl2 and MgCl2. The bound cells were fixed with methanol. Adhesion was quantitated by counting cells under ×200 magnification using a phase contrast microscope.
Studies of Murine Tumorigenicity and Histopathology
The FucT-III-MARY-X, and wild-type MARY-X spheroids were injected into female SCID mice and their tumorigenicity and pathology observed. Experiments were performed with groups of 10 mice.
Statistical Analysis
Experiments were performed with groups of 10 mice and results analyzed with standard tests of significance, including the two-tailed Student’s t-test and a one-way analysis of variance.
Institutional Certifications
Informed patient consent and approvals from the UCLA Human Subject Protection Committee, the Chancellor’s Animal Research Committee (certification ARC 95-127-11), and the UCLA Institutional Biosafety Committee (IBC) were obtained before all studies.
Results
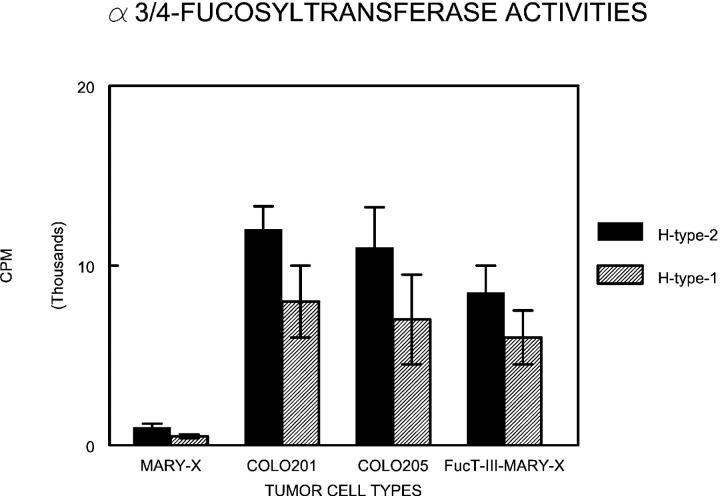
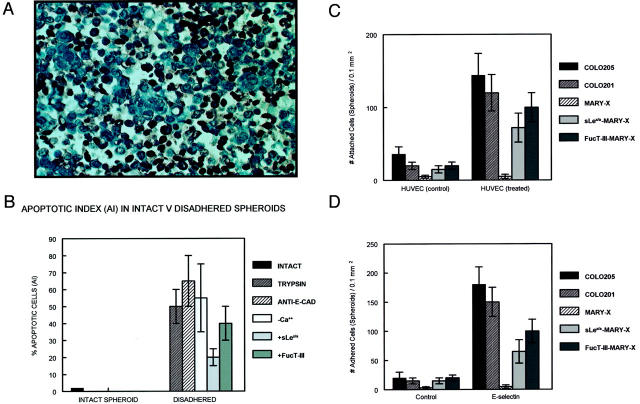
IBC manifests itself as lymphovascular emboli consisting of tight clumps of tumor cells in the central portion of vascular lumens retracted away from the endothelial layer (Figure 1A) ▶ . MARY-X also exhibited this characteristic phenotype (Figure 1B) ▶ . The lymphovascular emboli of MARY-X correspond to compact spheroids in suspension culture (Figure 2A) ▶ . Our previous studies demonstrated that these spheroids form on the basis of an intact and overexpressed E-cadherin/α,β-catenin axis. These spheroids also overexpressed MUC1 by both immunocytochemistry (Figure 2B) ▶ and Western blot (Figure 3A) ▶ . Because sLex/a epitopes on MUC1 are thought to mediate binding to endothelial adhesion molecules such as E-selectin, 13 we wondered about the sLex/a expression levels on these wild-type MARY-X spheroids. Interestingly these wild-type spheroids showed markedly decreased levels of sLex/a by both immunocytochemistry and Western blot (Figure 3B) ▶ . FucT-III retroviral transfection was successful in increasing the levels of both α3- and α4-fucosyltransferase activities that were low in the wild-type MARY-X spheroids but high in Colo-201 and Colo-205 (Figure 4) ▶ . FucT-III retroviral transfection also increased the levels of both sLex and sLea (Figure 3B) ▶ in the MARY-X spheroids, termed FucT-III-MARY-X. Enzymatic transfer of GDP-fucose with a milk fucosyltransferase and CMP-NANA with an α2,3-sialyltransferase (ST3GalIV) also increased the levels of both surface sLex and surface sLea (Figure 3B) ▶ in the MARY-X spheroids, termed sLex/a-MARY-X. The levels of sLex were greater, however, in FucT-III-MARY-X than in sLex/a-MARY-X whereas the levels of sLea were comparable in both these spheroids. The effects of both these manipulations on the MARY-X spheroids (Figure 5A) ▶ were initial enlargement of the spheroids (Figure 5B) ▶ . Spheroids increased from a mean diameter of 60 μm to a mean diameter of 80 μm. The enlargement of FucT-III-MARY-X was first observed 72 hours after transfection; the enlargement of sLex/a-MARY-X was first observed at 12 hours after the initiation of enzymatic transfer. Both groups of spheroids then underwent a progressive disadherence (Figure 5, C and D) ▶ . FucT-III-MARY-X underwent total disadherence by 120 hours whereas sLex/a-MARY-X underwent an ∼50% disadherence by 24 hours with no further disadherence noted after that point. Disadhering spheroids with either approach exhibited strong sLex (Figure 5E) ▶ and strong sLea immunoreactivity. Even fully disadhered FucT-III-MARY-X and sLex/a-MARY-X, however, maintained their expression of surface E-cadherin (Figure 5F) ▶ . These data support the concept that the negative charges on the sLex/a molecules disrupt the E-cadherin homodimers mediating cell adhesion. Disadhered cells underwent apoptosis 12 to 24 hours later (Figure 6A) ▶ . The degree of apoptosis was comparable to that observed with other MARY-X disadherence mechanisms (trypsin proteolysis, anti-E-cadherin, Ca++ depletion) noted in previous studies 8 but was greater in FucT-III-MARY-X than in sLex/a-MARY-X (Figure 6B) ▶ . Apoptosis did not begin before 6 hours. Before this time the disadhered cells were fully viable and could be evaluated for attachment and adhesion properties. The wild-type MARY-X spheroids only weakly bound to an underlying monolayer of HUVECs (Figure 6C) ▶ . This attachment was not enhanced by pretreatment of the HUVECs monolayer with 200 U/ml of TNF-α, a cytokine that increased E-selectin 20- to 50-fold (data not shown). Other cell lines examined, eg, Colo-205 and Colo-201, showed significant attachment to HUVECs, which was considerably enhanced by HUVEC’s TNF-α pretreatment (Figure 6C) ▶ . Disadhered FucT-III-MARY-X and sLex/a-MARY-X showed increased attachment to TNF-α-treated HUVECs with greater attachment exhibited by FucT-III-MARY-X (Figure 6C) ▶ . These attachment findings were mirrored by increased adhesion of disadhered FucT-III-MARY-X and sLex/a-MARY-X to plates coated with purified recombinant E-selectin with greater adhesion exhibited by FucT-III-MARY-X (Figure 6D) ▶ . FucT-III-MARY-X, when injected into mice, exhibited a marked change in tumorigenicity and the IBC phenotype (Table 1) ▶ . These collective findings support the hypothesis that the markedly decreased levels of sLex/a on the MARY-X lymphovascular embolus promote both its compact nature mediated by E-cadherin homodimers and its retraction from the vessel (Figure 7) ▶ .
Figure 1.
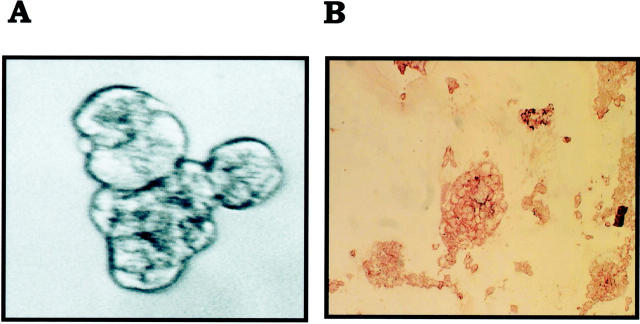
A: Lymphovascular invasion in IBC in humans is characterized by numerous tumor emboli in lymphovascular spaces. B: The presence of tumor cell-tumor cell homotypic adhesion and the lack of tumor cell-endothelial cell heterotypic adhesion is evidenced by this lymphovascular tumor embolus of MARY-X by light microscopy. H&E stain; original magnifications: ×250 (A); ×400 (B).
Figure 2.
A: Spheroids of MARY-X form tight clusters in which individual cells cannot be distinguished. B: Although this compactness is mediated by E-cadherin, these spheroids also demonstrate MUC1 immunoreactivity. A, Phase contrast (original magnification, ×200); B, anti-MUC1, immunoperoxidase.
Figure 3.
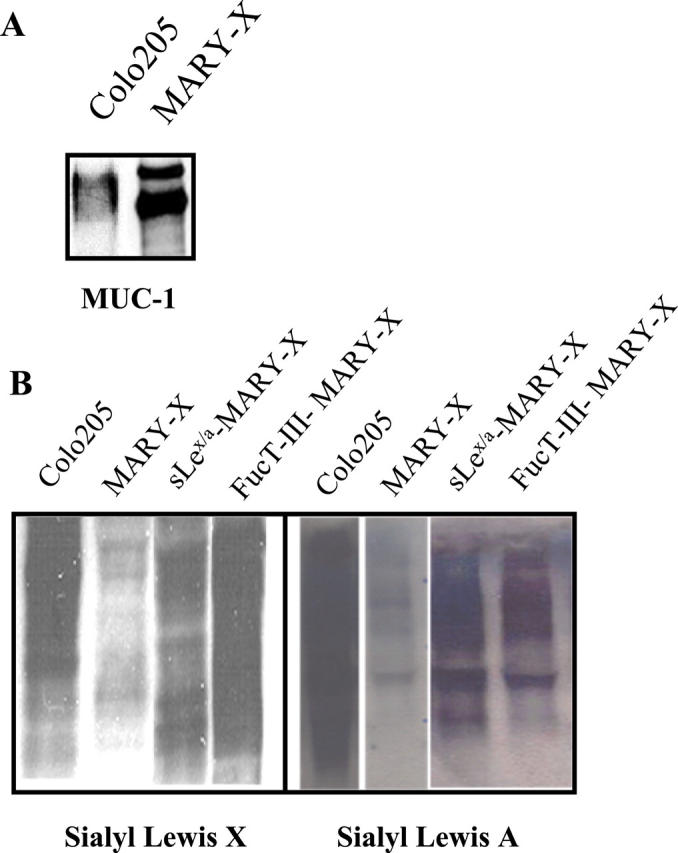
A: Like Colo-205, MARY-X spheroids overexpress MUC1 but unlike Colo-205, MARY-X exhibits markedly decreased levels of sLex and sLea by Western blot (B). Levels of sLex and sLea can be increased in MARY-X through either exogenous enzymatic fucosylation (sLex/a-MARY-X) or FucT-III transfection (FucT-III-MARY-X). A, Western blot, anti-MUC1; B, Western blot, anti-sLex and anti-sLea.
Figure 4.
Comparative assay of α3/4-fucosyltransferase activities in wild-type MARY-X, Colo-201, Colo-205, and FucT-III-MARY-X reveals low endogenous levels of both fucosyltransferases in wild-type MARY-X and increased levels after FucT-III transfection. H-type-I (Galβ1–3GlcNAc) is the oligosaccharide acceptor for α4-fucosyltransferase activity and H-type-2 (Galβ1–4GlcNAc) is the oligosaccharide acceptor for α3-fucosyltransferase activity.
Figure 5.
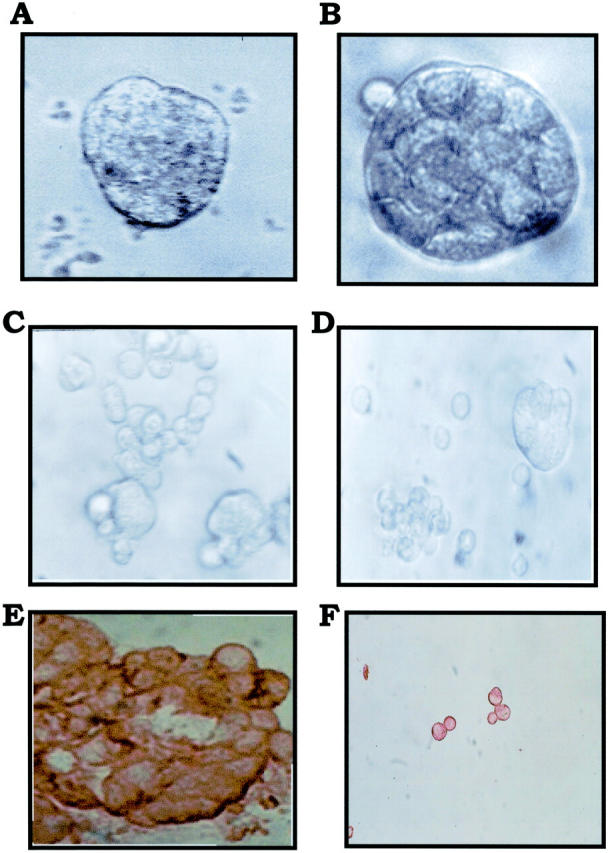
A: FucT-III-MARY-X are 60 μm in size when transfected and enlarge to 80 μm in size at 72 hours (B). SLex/a-MARY-X exhibits a similar enlargement. Subsequent FucT-III-MARY-X (C) and sLex/a-MARY-X (D) disadherence occurs with the former being complete and the latter being partial. E: Disadhering FucT-III-MARY-X exhibits strong sLex surface immunoreactivity and persistence of E-cadherin immunoreactivity even when fully disadhered (F). Original magnifications: ×200 (A–D, phase contrast); ×200 (E, anti-sLex, immunoperoxidase); ×100 (F, anti-E-cadherin, immunoperoxidase).
Figure 6.
A: Disadherence produced profound apoptosis by terminal dUTP nick-end labeling. B: Different disadherence mechanisms all increase AI, suggesting a common apoptosis mechanism. C: Attachment of Colo-205, Colo-201, and different MARY-X spheroids to stimulated and unstimulated HUVECs is depicted. D: Adhesion of these same cell types to wells coated with E-selectin is depicted. Attachment (C) and adhesion (D) assays of tumor cells and/or spheroids as detailed in Materials and Methods. Attachment and adhesion are quantitated by counting cells and/or spheroids within a 0.1-mm2 grid. Each bar represents the mean cell count ± SD of four to eight wells.
Table 1.
Differences Between Wild-Type and SLex/a-Modified Xenografts
| Wild-type MARY-X | FucT-III-MARY-X | |
|---|---|---|
| Tumorigenicity | 100% | 10% |
| LVI* | Florid | Absent |
| Metastasis | Present | Absent |
| Histopathology | Nodular | Single infiltration |
* LVI, lymphovascular invasion.
Figure 7.
Schematic depicts hypothesis of homotypic E-cadherin interactions and lack of heterotypic MUC1/sLex/a-E-selectin interactions being cooperative in nature.
Discussion
Our results offer a molecular explanation for the histological appearance of the lymphovascular emboli of IBC. More importantly our results suggest a hypothesis to explain the aggressive metastatic behavior of IBC. The compact tumor embolus is very efficient at metastatic dissemination because it is adept at implantation at its metastatic site just as the embryonal blastocyst is adept at uterine implantation. 14,15 Numerous previous studies have demonstrated that hematogenous tumor cell clumps are very efficient at metastatic dissemination and implantation. 16 Furthermore the tumor embolus is not bound to endothelial cells at its primary site so it can be dislodged fairly easily and metastasize. Our previous studies demonstrated that an intact overexpressed E-cadherin/α,β-catenin axis causes the formation of the tumor embolus and its compact nature. 5 Interestingly the embryonal blastocyst also overexpresses E-cadherin. 14,15
In the present study we demonstrate that a lack of sLex/a epitopes on adhesion molecules such as MUC1 contribute to the lack of binding of the tumor embolus to endothelium. This lack of sLex/a epitopes further supports the compactness of the tumor embolus because experimental manipulations (transfection or enzymatic transfer) that increase sLex/a epitopes create electrostatic repulsions that disrupt the E-cadherin homodimeric interactions that form the tumor embolus in the first place. The mechanism behind the decreased sLex/a epitopes on wild-type MARY-X seems to be its endogenously low level of fucosyltransferase activity that could be increased through FucT-III transfection. The accepted pathway for the biosynthesis of the sLex and sLea structures starts by sialylation of the type-2 and type-1 chain precursors, respectively, followed by fucosylation of the sialylated intermediates. 17-21 In embryogenesis, sialylation is an early phenomenon that appears at the beginning of cell differentiation and precedes fucosylation. 22 The step of fucosylation is then rate-limiting for the formation of sLex/a in many different cell types and tissues. 23 Fucosyltransferase activity is absent in early embryogenesis. 22 The analogy of the wild-type MARY-X spheroids with the embryonal blastocyst can again be made.
FucT-III transfection was more efficient at increasing surface sLex on wild-type MARY-X than enzymatic transfer with a milk fucosyltransferase and ST3GalIV. Presumably much of this surface sLex occurs on MUC1 because it is overexpressed in wild-type MARY-X but undoubtedly other sialylated surface molecules are also fucosylated by these two approaches. 24,25 FucT-III transfection was also efficient at increasing both α1,3- and α1,4-fucosyltransferase activities and at increasing surface sLea. This enzyme is nominally selective for forming α1–3 bonds but can transfer fucose to other positions. This is the mechanism that we would propose to account for the increased α1,4-fucosyltransferase activity after transfection and the increase in surface sLea. Predictably because the overall levels of sLex/a were greater in FucT-III-MARY-X than sLex/a-MARY-X, the consequences of increased sLex/a—disadherence, attachment to stimulated endothelial cells, adhesion to E-selectin 26-28 and apoptosis—were all greater in FucT-III-MARY-X than in sLex/a-MARY-X. The markedly decreased levels of sLex/a on wild-type MARY-X then promotes self-adherence rather than disadherence, aversion rather than attachment to endothelial cells, nonadhesion rather than adhesion to E-selectin, and viability rather than apoptosis. There is therefore co-operation between sLex/a underexpression and E-cadherin overexpression in the genesis of the IBC lymphovascular embolus.
It is important to realize that the spheroids of MARY-X and the lymphovascular emboli of IBC express markedly decreased levels of sLex/a and yet are quite hardy and adept at metastatic dissemination in their native wild-type state. Our findings challenge the generally accepted dogma that metastatic propensity is increased by endothelial adhesion and decreased by homotypic (E-cadherin-mediated) adhesion. When we perturbate our model by adding sLex/a either enzymatically or through gene transfer we increase sLex/a and this increase electrostatically disrupts the E-cadherin-mediated homotypic adhesions that exists among the tumor cells. It is this disruption or disadherence that induces apoptosis and not the sLex/a expression levels per se.
Our previous study concerning the overexpression rather than the loss of E-cadherin in IBC was intriguing because it viewed the E-cadherin molecule from a different perspective. Loss of epithelial differentiation in carcinomas is thought to be accompanied by higher mobility and invasiveness, which in turn is often a consequence of reduced intercellular adhesion. 29 Although this phenomenon may account for certain aspects of the invasion and metastasis process, the phenomenon has really not been observed or examined in the specific step of intravasation or the formation of tumor emboli within lymphovascular spaces. In a related recent study, 30 E-cadherin-mediated multicellular aggregates of HSC-3 (human squamous cell carcinoma) survived, proliferated, and exhibited anchorage independence and resistance to apoptosis. These multicellular aggregates required high levels of extracellular Ca++ and were inhibited with function-perturbing anti-E-cadherin antibody. In that study cadherin-mediated intercellular adhesions generated a compensatory mechanism that promoted anchorage-independent growth and suppressed apoptosis. The multicellular aggregates in that study in essence had gained a survival benefit for the tumor from the gain rather than the loss of E-cadherin function. We feel that the E-cadherin-mediated spheroid formation of MARY-X and its in vivo manifestation of lymphovascular tumor emboli are of similar survival benefit to this cancer. Tumor cell emboli are more efficient at forming metastasis than single cells; 16 tumor cell emboli are hypoxic in their centers and therefore more resistant to chemotherapy and radiation therapy than single cells; 31 tumor cell emboli probably exhibit their surface determinants in a different conformational state from those present on single cells that would escape recognition and destruction by adoptive immunotherapy strategies that are based on immunizations to single cells. So here we are dealing with one of the most aggressive of all breast cancers and yet a cancer dependent on an intact and overexpressed E-cadherin axis for metastatic efficiency.
Just as these observations on E-cadherin challenge conventional dogma concerning the general loss of this tumor suppressor gene in malignant progression, so do our present observations concerning the markedly decreased sLex/a on wild-type MARY-X. SLex/a epitopes are expressed on many cancer cells and are thought to be related to malignant progression. 32 For example, colonic carcinomas from patients at advanced stages have higher levels of sLex antigens than tissues from patients with earlier stage disease. 33 In a recent study, transfection of FucT-III into lowly metastatic murine melanoma cells, which do not normally exhibit sLex/a epitopes, increased expression of surface sLex/a and conferred increased metastatic behavior through enhanced binding to endothelial cell E-selectin and increased resistance to NK cell killing. 34 In the case of MARY-X and IBC, the markedly decreased sLex/a fosters both the E-cadherin-mediated compactness of the embolus and its lack of binding to endothelial cells. This promotes ease of metastatic dissemination. Creating sLex/a epitopes through transfection (FucT-III-MARY-X) abolishes lymphovascular invasion and metastasis. Although creating sLex/a epitopes through gene transfer increases endothelial attachment and E-selectin adhesion, this does not foster increased metastasis in MARY-X because the creation of sLex/a epitopes causes disadherence of the spheroid and apoptosis. So the absence rather than the presence of sLex/a promotes malignant progression in the setting of IBC, which again challenges conventional wisdom.
Acknowledgments
We thank Dr. Minoru Fukuda of the Burnham Institute, La Jolla, CA, for the FucT-III cDNA and for helpful discussions.
Footnotes
Address reprint requests to Sanford H. Barsky, M.D., Department of Pathology, CHS-Room 13-262, 650 Circle Dr. South, UCLA School of Medicine, Los Angeles, CA 90024. E-mail: sbarsky@ucla.edu.
Supported by the California Breast Cancer Research Program (grant 6FB-0007 to M. L. A. and grant 5JB-0104 to S. H. B.), and by the Susan G. Komen Breast Cancer Foundation (grant 99-003173 to S. H. B.).
References
- 1.Palangie T, Mosseri B, Mihura J, Campana F, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Scholl S, Asselain B, Pouillart P: Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur J Cancer 1994, 30A:921-927 [DOI] [PubMed] [Google Scholar]
- 2.Levine PH, Steinhorn SC, Ries LG, Aron JL: Inflammatory breast cancer: the experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst 1985, 74:291-297 [PubMed] [Google Scholar]
- 3.Quigley JP, Armstrong PB: Tumor cell intravasation alu-cidated: the chick embryo opens the window. Cell 1998, 94:281-284 [DOI] [PubMed] [Google Scholar]
- 4.Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH: A novel human xenograft model of inflammatory breast cancer. Cancer Res 1999, 59:5079-5084 [PubMed] [Google Scholar]
- 5.Tomlinson JS, Alpaugh ML, Barsky SH: An intact overexpressed E-cadherin/α, β-catenin axis characterizes the lymphovascular emboli of inflammatory breast cancer. Cancer Res 2001, 61:5231-5241 [PubMed] [Google Scholar]
- 6.Sternlicht MD, Kedeshian P, Shao Z-M, Safarians S, Barsky SH: The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997, 3:1949-1958 [PubMed] [Google Scholar]
- 7.Alpaugh ML, Tomlinson JS, Kasraeian S, Barsky SH: Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene 2002, 21:3631-3643 [DOI] [PubMed] [Google Scholar]
- 8.Alpaugh ML, Barsky SH: Reversible model of spheroid formation allows for high efficiency of gene delivery ex vivo and accurate gene assessment in vivo. Hum Gene Ther 2002, 13:1245-1258 [DOI] [PubMed] [Google Scholar]
- 9.Shinoda K, Morishita Y, Sasaki K, Matsuda Y, Takahashi I, Nishi T: Enzymatic characterization of human α1,3-fucosyltransferase fuc-TVII synthesized in a B cell lymphoma cell line. J Biol Chem 1997, 272:31992-31997 [DOI] [PubMed] [Google Scholar]
- 10.Mollicone R, Gibaud A, Francois A, Ratcliffe R, Oriol R: Acceptor specificity and tissue distribution of three human α-3-fucosyltransferases. Eur J Biochem 1990, 191:169-176 [DOI] [PubMed] [Google Scholar]
- 11.Palcic MM, Heerze LD, Pierce M, Hindsgaul O: The use of hydrophobic synthetic glycosides as acceptors in glycosyltransferase assays. Glycoconj J 1988, 5:49-63 [Google Scholar]
- 12.Nguyen M, Corless CL, Kraling BM, Tran C, Atha T, Bischoff J, Barsky SH: Vascular expression of E-selectin is increased in estrogen-receptor-negative breast cancer. Am J Path 1997, 150:1307-1314 [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson J, Wang JL, Barsky SH, Lee MC, Bischoff J, Nguyen M: Human colon cancer cells express multiple glycoprotein ligands for E-selectin. Int J Oncol 2000, 16:347-353 [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84:345-357 [DOI] [PubMed] [Google Scholar]
- 15.Yap AS, Brieher WM, Gumbiner BM: Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 1997, 13:119-146 [DOI] [PubMed] [Google Scholar]
- 16.Liotta LA, Kleinerman J, Saidel GM: The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 1976, 36:889-894 [PubMed] [Google Scholar]
- 17.Sears P, Wong CH: Toward automated synthesis of oligosaccharides and glycoproteins. Science 2001, 291:2344-2350 [DOI] [PubMed] [Google Scholar]
- 18.Bartek J, Muller R, Kosma P: Synthesis of a neoglycoprotein containing the Lewis X analogous trisaccharide β-d-GalpNAc-(1→4)[α-L-Fucp-(1→3)]-β-d-GlcpNAc. Carbohydr Res 1998, 308:259-273 [DOI] [PubMed] [Google Scholar]
- 19.Barstrom M, Bengtsson M, Blixt O, Norbert T: New derivatives of reducing oligosaccharides and their use in enzymatic reactions: efficient synthesis of sialyl Lewis a and sialyl dimeric Lewis x glycoconjugates. Carbohydr Res 2000, 328:525-531 [DOI] [PubMed] [Google Scholar]
- 20.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G: Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 1998, 33:151-208 [DOI] [PubMed] [Google Scholar]
- 21.Kimura H, Shinya N, Nishihara S, Kaneko M, Irimura T, Narimatsu H: Distinct substrate specificities of five human α-1,3-fucosyltransferases for in vivo synthesis of the sialyl Lewis x and Lewis x epitopes. Biochem Biophys Res Comm 1997, 237:131-137 [DOI] [PubMed] [Google Scholar]
- 22.Candelier JJ, Mollicone R, Mennesson B, Coullin P, Oriol R: Expression of fucosyltransferases in skin, conjunctiva, and cornea during human development. Histochem Cell Biol 2000, 114:113-124 [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi S, Srivastava OP, Palcic MM, Hindsgaul O, Fukuda M: Acquisition of P-selectin binding activity by en Bloc transfer of sulfo Lex trisaccharide to the cell surface: comparison to a sialyl Lex tetrasaccharide transferred on the cell surface. Arch Biochem Biophys 2000, 374:100-106 [DOI] [PubMed] [Google Scholar]
- 24.Pollevick GD, Di Noia JM, Salto ML, Lima C, Leguizamon MS, de Lederkremer RM, Frasch ACC: Trypanosoma cruzi surface mucins with exposed variant epitopes. J Biol Chem 2000, 275:27671-27680 [DOI] [PubMed] [Google Scholar]
- 25.Gallart T, Roelcke D, Blay M, Pereira A, Martinez A, Masso O, Vinas O, Cid M, Esparza J, Molina R, Barcelo J: Anti-sia-1b (anti-Gd) cold agglutinins bind the domain neuNAcα2–3gal in sialyl Lewisx, sialyl Lewisa, and related carbohydrates on nucleated cells and in soluble cancer associated mucins. Blood 1997, 90:1576-1587 [PubMed] [Google Scholar]
- 26.Ohmori K, Kanda K, Mitsuoka C, Kanamori A, Kurata-Miura K, Sasaki K, Nishi T, Tamatani T, Kannagi R: P- and E-selectins recognize sialyl 6-sulfo Lewis X, the recently identified L-selectin ligand. Biochem Biophys Res Comm 2000, 278:90-96 [DOI] [PubMed] [Google Scholar]
- 27.Galustain C, Childs RA, Yuen CT, Hasegawa A, Kiso M, Lubineau A, Shaw G, Feizi T: Valency dependent patters of binding of human L-selectin toward sialyl and sulfated oligosaccharides of Lea and Lex types: relevance to anti-adhesion therapeutics. Biochemistry 1997, 36:5260-5266 [DOI] [PubMed] [Google Scholar]
- 28.Thomas VH, Elhalabi J, Rice KG: Enzymatic synthesis of N-linked oligosaccharides terminating in multiple sialyl-Lewisx and galNAc-Lewisx determinants: clustered glycosides for studying selectin interactions. Carbohydr Res 1998, 306:387-400 [DOI] [PubMed] [Google Scholar]
- 29.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 30.Kantak SS, Kramer RH: E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem 1998, 273:16953-16961 [DOI] [PubMed] [Google Scholar]
- 31.Moore DH, Rouse MB, Massenburg GS, Zeman EM: Description of a spheroid model for the study of radiation and chemotherapy effects on hypoxic tumor cell populations. Gynecol Oncol 1992, 47:44-47 [DOI] [PubMed] [Google Scholar]
- 32.Yoon WH, Park HD, Lim K, Hwang BD: Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem Biophys Res Comm 1996, 222:694-699 [DOI] [PubMed] [Google Scholar]
- 33.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M: Carcinoma-associated expression of core 2 β-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res 1997, 57:5201-5206 [PubMed] [Google Scholar]
- 34.Ohyama C, Tsuboi S, Fukuda M: Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J 1999, 18:1516-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]