Abstract
Renin plays a central role in controlling blood pressure as it catalyzes the first step in the production of angiotensin II. The aim of this study was to isolate fragments of the human renin (hREN) promoter able to direct tissue-specific and regulated expression of a LacZ reporter gene mimicking endogenous renin. We screened several hREN promoter/LacZ constructs for transgene expression in transient embryos at E15 when renin expression begins. We found that a 12-kb hREN promoter conferred high expression in the kidney at both embryonic and adult stages and that the transgene was expressed in the same cells as endogenous renin. We explored two pathophysiological models in which renin is stimulated and showed concomitant increases in β-galactosidase and renin activities. In situ β-galactosidase staining showed renin/transgene-expressing cells are recruited in the juxtaglomerular apparatus and in the afferent arterioles as well as in larger arteries outside the kidney. Using our model, renin expression in interlobular arteries was confirmed as being striped and, for the first time, expression of renin in larger arteries outside the kidney was shown. Therefore, this strain is a suitable model to investigate renin gene pathophysiological regulations in vivo.
Renin (EC 3.4.23.15) is an aspartyl protease which catalyzes the specific cleavage of angiotensinogen to angiotensin I (AI). This is the rate-limiting step of the renin-angiotensin system (RAS) which plays a key role in the control of blood pressure and electrolyte homeostasis by controlling the level of angiotensin II. The principal site of renin synthesis is the juxtaglomerular (JG) cells of the kidney, located at the distal end of afferent arterioles of the glomerulus. 1
Surprisingly, there is limited and contradictory information concerning the cis-acting sequences in the human renin (hREN) gene and the renal trans-acting factors involved in the restricted tissue-specific expression of renin. Initial in vitro studies suggested that the 110 first nucleotides of hREN promoter were sufficient to direct specific expression in renin-producing cells such as in chorionic cells. 2-4 Additional experiments demonstrated the need for more distal specific cis-regulatory elements to obtain higher transcriptional rates. 5,6 Initial in vivo transgenic animal studies, using the entire hREN gene with a 3 kb or shorter 5′-flanking region, and 1.2 kb of the 3′-flanking region, failed to produce a renal restricted expression. 7-9 Transgenic models containing the entire hREN gene associated with more than 25 kb of 5′-flanking region and 6 kb of 3′-flanking DNA 5,10 were generated. In these transgenic lines, hREN protein production was restricted to renal cells and expression was tightly regulated by angiotensin II, sodium intake, or angiotensin converting enzyme (ACE) inhibition. The large pieces of DNA used in these studies had the advantage of insulating the transgene and allowing tissue-specific expression, but their use is limited in deciphering regulatory molecular mechanisms responsible for specific renin expression.
In the present work, we studied various lengths of hREN promoter sequences associated with a LacZ reporter gene. As renin is first expressed in the developing vessels of the kidney around E14.5 during mouse embryogenesis, embryos were screened for renal expression of the transgene at E15 to select 5′-flanking region sequence mimicking endogenous mouse renin expression. Regulated expression of the transgene was observed when 12,166 bp of the human renin promoter were linked to LacZ gene. Our results demonstrate that this transgene is expressed in a tissue-specific manner in transgenic mice, with expression of LacZ being restricted to juxtaglomerular cells in the kidney of adult transgenic mice. Furthermore, we demonstrate that LacZ expression in the kidney is regulated similarly to endogenous renin expression by salt depletion. We also show a huge increase of β-galactosidase activity in the clipped kidney in 2-kidney, 1-clip (2K1C) model.
This new model allowed us to easily describe the pattern and the kinetics of the recruitment of renin-producing cells as they concomitantly express β-galactosidase. In pathophysiological conditions where renin is needed, an increase in the number of renin-producing cells occurs by metaplastic transformation of smooth muscle cells into myoepithelioid intermediate cells. 11 Our model confirmed the renin/β-galactosidase striped expression pattern in interlobular arteries. 12 We also showed renin/β-galactosidase expression in larger arteries outside the kidney after either salt depletion or renal artery constriction. Thus, this hREN promoter/LacZ transgenic mouse strain provides an easy and suitable model for the study of hREN gene expression and regulation in an in vivo context.
Materials and Methods
Cloning and Transgene Constructs
Human renin gene 5′-flanking regions were isolated from a PWE15 human cosmid library (Stratagene) as previously described. 6 All constructs were made into the LacZ reporter gene vector pSG consisting of a p46.21D backbone 13 digested by HindIII and XmaI into which a NotI, BamHI, SpeI, PacI, SmaI, PstI, XhoI linker was introduced. A BamHI/SpeI subfragment of 5′ matrix attachment region (MAR) of lysozyme gene 14 residing in a 2955-bp fragment spanning from position −11.7 kb to −8.8 kb upstream of the lysozyme gene was inserted in the BamHI/SpeI sites of pSG creating pSG-MAR. A DNA fragment containing −5777 to +16 bp of the human renin gene promoter was isolated by HindIII digestion of pGL3–5777, 6 blunted by T4 DNA polymerase and ligated in pSG-MAR digested by XhoI and blunted by T4 DNA polymerase. Plasmid pSG-MAR-5777 was then digested by BglII to remove the −5777 to −4730 hREN promoter fragment and the −12,166 to −4730 BglII fragment was ligated creating pSG-MAR-12166. Sense and antisense constructs were screened by HindIII digestion. The nucleotide sequence of the renin promoter and promoter-LacZ junction regions of all of the constructs were sequenced to confirm no mutations had occurred during the subcloning steps.
Transgenic Mice Production
Plasmid DNA was purified by alkaline lysis followed by CsCl gradient centrifugation. The purified plasmid DNA was digested with NotI to generate a fragment containing the human renin promoter, nuclear localization sequence, LacZ reporter gene, and SV40 polyadenylation sequence. The transgene purified using Elutip-d columns (Schleicher & Schuell, Keene, NH) and the final DNA was dissolved in 10 mmol/L Tris-HCl pH 7.0, 0.1 mmol/L EDTA for microinjection into one-cell embryos derived from C57Bl/6 mice. Transgenic mice were made at the Service d’Expérimentation Animale et de Transgénèse (SEAT, Villejuif, France) of the Centre National de la Recherche Scientifique (CNRS). The day of reimplantation of microinjected eggs into C57B/16 experienced female was designated at E0. All manipulations of mice were done in accordance with policies of Ministère de l’agriculture for animal care and use.
Screening of Embryos at E15
Following sacrifice of pregnant mice by cervical dislocation, embryos were fixed in 4% paraformaldehyde, 1X PBS (w/v) for 2 hours, and rinsed with 1X PBS three times for 5 minutes. The LacZ expression was detected by incubating the embryos at 30°C overnight in PBS supplemented with 0.1% X-gal, 5 mmol/L K3Fe(CN)6, 5 mmol/L K4Fe(CN)6, 1 mmol/L MgCl2, 0.002% NP-40, 0.01% sodium deoxycholate, pH 7.0. After staining, the embryos were rinsed in PBS and postfixed at 4°C overnight in 4% paraformaldehyde in PBS. To make the embryos more visible before photography, embryos were clarified by soaking them serially in glycerol at concentrations ranging from 30 to 80%.
PCR Analysis
Transgenic mice harboring the hREN promoter-LacZ gene were identified by PCR with genomic DNA prepared from tail biopsies. Primers used to screen transgenic animals amplify a 199-bp β-galactosidase fragment. The sense primer was 5′-CGCCAGCTGGCGTAATAGCGAAGAGC-3′ and the reverse primer was 5′-GATGGGCGCATCGTAACCGTGCAT-3′.
β-Galactosidase and Renin Assays
Tissues were homogenized in appropriate volume of phosphate 0.1 mol/L buffer, pH 7. β-Galactosidase activity in the tissues was measured using β-galactosidase reporter gene assay (Roche Diagnostics).
Plasma and tissue renin concentrations were measured by enzymatic assay. 15 Briefly, renin activity was assayed by radioimmunoassay of angiotensin I produced after incubation of dilution of plasma or tissue extracts (kidney, adrenal gland, liver, brain, heart, lung and spleen) with binephrectomized rat plasma.
Renin Immunostaining
For embryos, pregnant females were sacrificed and embryos were treated as described above. No postfixation was performed before infiltration in paraffin.
For adult kidneys, mice were deeply anesthetized and the heart perfused first with 0.9% NaCl for 10 minutes then with 4% paraformaldehyde in 1X PBS for 10 minutes. Kidneys were then excised and stained with X-gal as described for the embryos. They were embedded in paraffin or incubated in 30% sucrose overnight before being frozen in isopentane cooled in dry ice.
Paraffin-embedded tissue sections (10 μm) were rehydrated and endogenous peroxidase inactivated with H2O2. They were then blocked with normal goat serum and incubated with rabbit anti-renin CAS antibody 16 (dilution 1:1000 in 1% NGS) for 90 minutes. After two washes of 5 minutes with PBS, sections were incubated for 30 minutes with biotinylated goat anti-rabbit IgG diluted 1:200. The secondary antibody was detected and amplified with ABC Elite Kit (Vector Laboratories, Burlingame, CA) using diaminobenzidine and H2O2 as a chromogen.
Colocalization of Renin and β-Galactosidase Expression
Frozen kidney sections (30 μm) were cut using a cryostat and kept at 4°C in PBS/azide 0.1%. Sections were blocked and permeabilized in PBS containing 3% normal goat serum (NGS), 1% goat anti-mouse IgG, and 0.2% Triton-X100 for 90 minutes at room temperature. The sections were then incubated overnight at room temperature with rabbit anti-renin CAS antibody (dilution 1:500) and mouse anti-β-galactosidase IgG2ak (Promega) diluted 1:1000. After five washes of 10 minutes each in PBS, the sections were then incubated for 1 hour at room temperature with FITC coupled anti-rabbit antibody (Promega) diluted 1:100, and biotinylated anti-mouse IgG2ak antibody (Promega) diluted 1:100. After five washes in PBS, sections were incubated in Texas Red-coupled streptavidin for 1 hour. Confocal microscopy was performed using a confocal scanning microscope (Leica TCS SP2). An argon laser source was used to generate excitation at 488 nm and observed emission at 520 nm for Texas Red-stained β-galactosidase. A GreNe laser source was used to generate excitation at 543 nm and observed emission at 630 nm for FITC-stained renin.
Kidney Microdissection
Microdissection of adult kidneys were performed following the protocol of Casellas et al 17 Briefly, kidneys were sliced into two parts to allow X-gal staining (as described above) and then they were treated with 5 N HCl for 1 hour at 37°C and kept overnight in water at 4°C. Tubules and vessels were then dissected.
Salt-Depletion Treatment
Wild-type (n = 3) and transgenic (n = 6) mice were treated with 14 mg/kg/day furosemide in drinking water ad libidum and a sodium-free diet (UAR-France). Animals were weighed before and after treatment. Water and food intake was measured and recorded daily (Table 1) ▶ . Blood samples were collected for plasma renin concentration. β-Galactosidase and renin content were measured in both kidneys, adrenal glands, and liver.
Table 1.
Physiological Characteristics of Control and Salt-Depleted Mice
| Mouse type | n | Body weight (g) | Water intake (ml/day) | PRA (ng Al/ml/h) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| Control WT | 3 | 24.9 ± 0.8 | 24.4 ± 0.8 | 3.8 ± 1.1 | 765 ± 110 |
| Control TG | 6 | 25.8 ± 2.1 | 26.4 ± 1.2 | 4.0 ± 0.6 | 1057 ± 221 |
| Salt-depleted WT | 3 | 26.6 ± 1.8 | 20.9 ± 1.7 | 4.9 ± 1.2 | 4978 ± 1685 |
| Salt-depleted TG | 6 | 24.0 ± 1.1 | 19.3 ± 0.8 | 6.0 ± 1.9 | 3604 ± 1655 |
Values are means ± SE. n, number of mice; WT, wild-type mice; TG, transgenic mice; PRA, plasma renin activity.
Two-Kidney, One-Clip Mice
Transgenic mice were operated under anesthesia. The left renal artery was clipped (0.12 mm opening) following the protocol of Wiesel et al 18 and the right was not disturbed. Mice were sacrificed 5 weeks after clipping. Blood samples were collected for plasma renin concentration. β-Galactosidase and renin content were measured in both kidneys.
Results
Renin Renal Expression during Ontogenesis
First we analyzed endogenous mouse renin expression by immunohistochemistry during ontogenesis in the C57Bl/6 mouse strain used to create transgenic lines. This strain carries only one renin gene, ren-1c. Renin immunohistochemistry was performed in kidneys of fetuses from E13 to E18. At E14, no renin expression was detected in the kidney (Figure 1A) ▶ . At E15, renin expression was localized in the media of interlobular arteries (Figure 1B) ▶ . From E16 to E18 (Figure 1, C–F) ▶ , widespread renin expression in interlobular arteries was observed. As seen in Figure 1F ▶ , renin is expressed along the renal interlobular arteries. At postpartum day 1 renin expression was localized to the juxtaglomerular and tubular cells, and in adult mice at 1 month (Figure 1G) ▶ , its expression was restricted to the juxtaglomerular cells.
Figure 1.
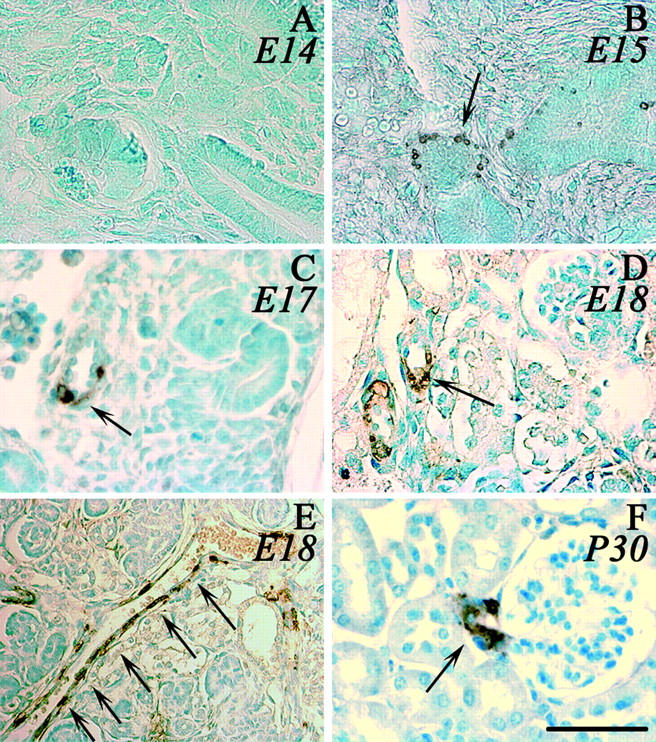
Renin expression during ontogenesis. Renin expression in the kidney of C57Bl/6 mice was detected by immunohistochemistry using the polyclonal anti-renin antibody CAS 16 detected by peroxidase staining. A–E: Photomicrographs of fetuses at different stages (A, E14; B, E15; C, E17; D, E, E18). E is photomicrograph from an interlobular artery in embryonic kidney (E18) and F from an adult kidney (P30). Arrows indicate examples of renin-expressing cells. Scale bar, 50 μm.
Screening of Transgene Renal Expression in E15 Embryos
Having shown that endogenous renin is expressed at E15, renal expression of various lengths of the human renin promoter were screened at this stage. Transgene constructs (Figure 2) ▶ containing various lengths of human renin promoter fused to a LacZ reporter gene and a matrix attachment region at the 5′ end of the transgene were injected in mouse embryos. The MAR was designed to insulate the transgene from positional effects and therefore to limit influence of insertion sites on the transgene expression. 14 The LacZ reporter gene was modified with a nuclear localization signal to target expression of β-galactosidase to the nucleus. 19 This allowed us to distinguish reporter expression from endogenous β-galactosidase expression.
Figure 2.
Schematic representation of the hREN/β-Gal transgene. Transgenes were constructed with various lengths of hREN promoter sequences (from 3 to 12 kb) associated with a LacZ reporter gene. The β-galactosidase was targeted to the nucleus with a nuclear localization sequence (NLS), and the matrix attachment region (MAR) was designed to insulate the transgene from insertion positional effects.
Expression of LacZ fused to 3 kb of the hREN promoter was tested at E15 (n = 3). No expression of the transgene or expression in inappropriate sites was found in all tested embryos (Figure 3A) ▶ . However, expression of LacZ driven by 5777 bp of the human renin promoter was restricted at E15 to the kidney (Figure 3B) ▶ and to some area of the brain (not shown) (n = 3). These findings showed that cis-regulatory elements contained in the 5777 bp were necessary to confer embryonic renal expression at E15 but were not sufficient to recapitulate a complete embryonic expression pattern.
Figure 3.
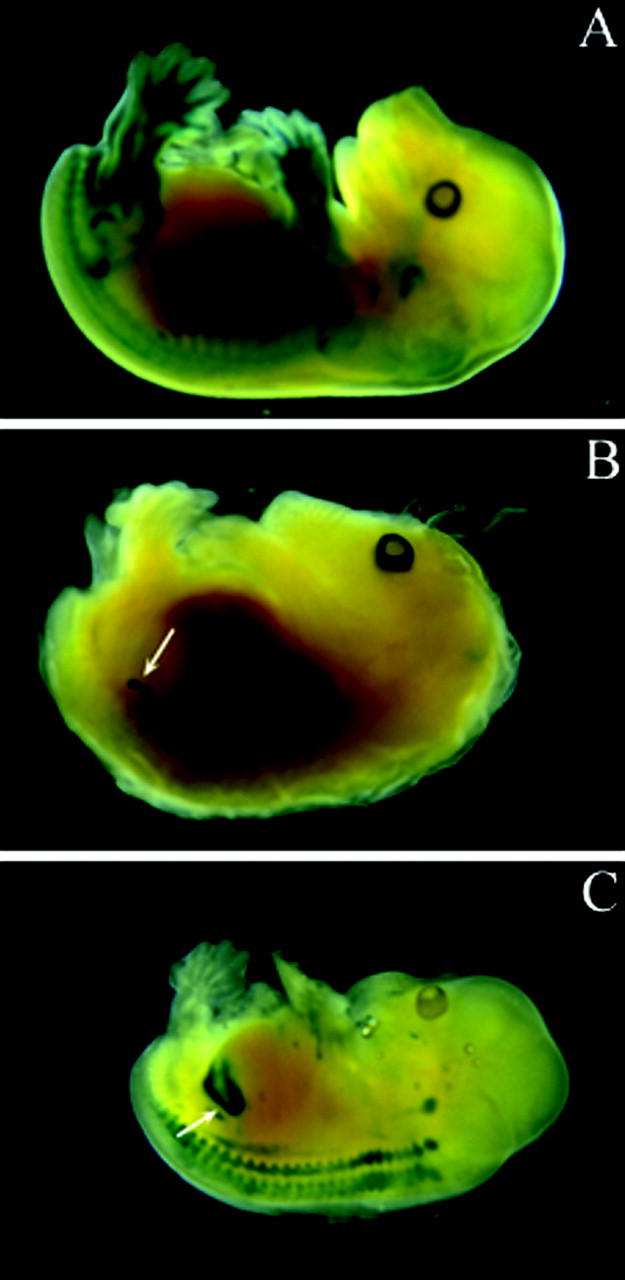
β-Galactosidase expression in E15 embryos. E15 embryos were collected from females transferred with eggs injected with pSG-MAR-2824 (A), pSG-MAR-5777 (B), or pSG-MAR-12166 transgenes (C). Embryos were stained with X-gal and screened for β-galactosidase expression sites. Renal localization was observed with pSG-MAR-5777 and pSG-MAR-12166 transgenes.
We then extended the promoter length to study whether specific renal expression was maintained and/or strengthen. We tested a fragment containing 12,166 bp of the human renin promoter fused to LacZ and studied its expression pattern at E15. As shown in Figure 3C ▶ , β-galactosidase staining was more strongly observed within the kidney (n = 4). Transgene expression along the neural axis, near the embryonic inner ear or in some area of the brain was also found in some embryos. These results showed that regulatory elements located in the promoter were sufficient and necessary to confer renal expression to a reporter gene, previously demonstrated using 4.1 kb of the 5′-flanking region of mouse renin gene. 20 Therefore, a transgenic line carrying 12,166 bp of the renin promoter linked to a LacZ reporter gene was established from one founder to study whether expression of the transgene was restricted to the kidney in adult mice.
Tissue-Specificity of Transgene Expression in Adult pSG-MAR-12166 Mice
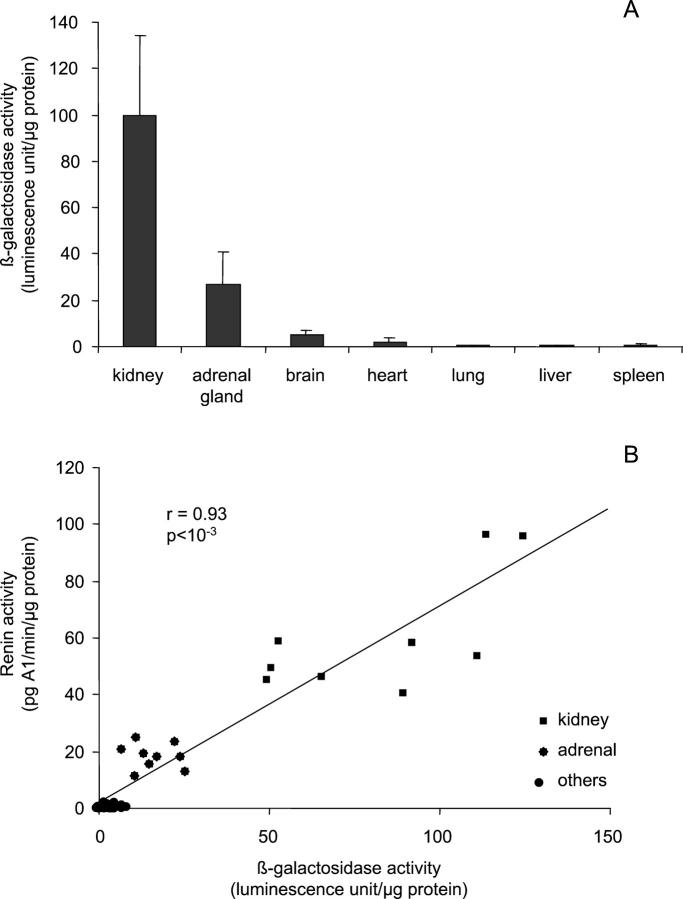
β-Galactosidase reporter activity was measured in several adult tissues (6- to 8-week-old mice): kidney, adrenal gland, brain, heart, lung, liver and spleen (Figure 4A) ▶ . The highest activity was observed in the kidney. In the adrenal gland, β-galactosidase activity was 20% of the renal activity. A weak activity (5% compared to renal expression) was observed in brain and heart and no activity was detectable in lung, liver and spleen.
Figure 4.
Tissue β-galactosidase expression in adult pSG-MAR-12166 transgenic mice. Renin and LacZ expression were measured in various tissues of transgenic mice. β-Galactosidase activity was expressed as relative β-galactosidase activity/μg protein. Renin activity was measured by RIA. A shows that β-galactosidase is mainly expressed in kidney, and to a lesser extent in adrenal gland and brain. Other tissues express very low levels or no β-galactosidase. B shows a good correlation (r = 0.93, P < 10−3) between β-galactosidase activity and mouse renin activity in these tissues.
Renin activity was measured in the same tissue extracts as β-galactosidase. As shown in Figure 4B ▶ , a strong correlation was observed between renin and β-galactosidase levels (r = 0.93). The same results were observed for male and female mice.
Cellular Specificity of Transgene Expression in Adult pSG-MAR-12166 Mice
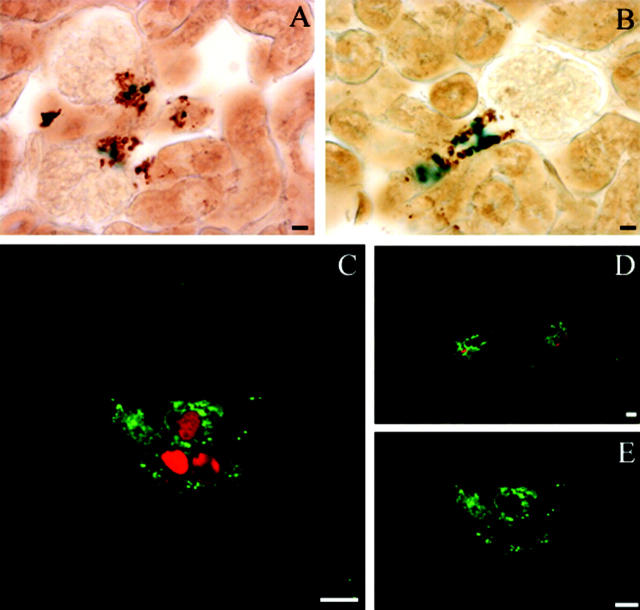
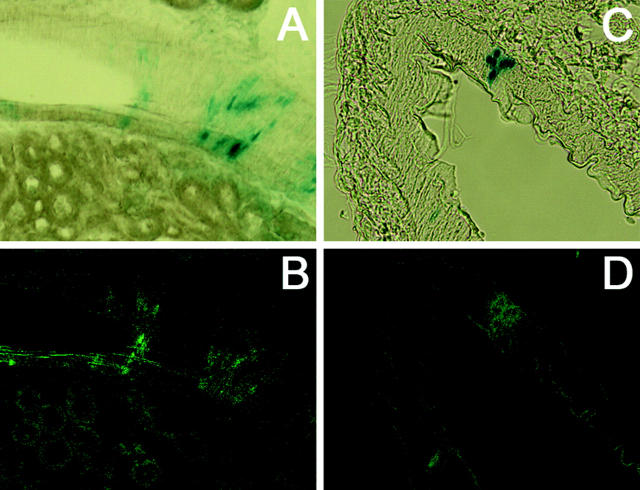
To determine in detail the precise renal expression of the transgene, double immunocytochemistry (Figure 5,A and B) ▶ and immunofluorescence (Figure 5, C–E) ▶ experiments were performed in kidney sections of adult transgenic mice to study whether the transgene was co-expressed with endogenous renin. Nuclear localization of β-galactosidase activity was observed specifically in cells immunostained for endogenous cytoplasmic renin (Figure 5, A and B) ▶ . Using confocal microscopy, coexpression was also observed (Figure 5, C and D) ▶ . Now, renin granules (Figure 5E) ▶ could clearly be observed in the cytoplasm of JG cells, suggesting that the juxtaglomerular apparatus (JGA) was not perturbed by transgene expression. These observations demonstrated co-localization of β-galactosidase driven by the hREN promoter with endogenous mouse renin in the renal JG cells of pSG-MAR-12166 mice at the adult stage.
Figure 5.
Colocalization of β-galactosidase and renin expression in juxtaglomerular cells. β-Galactosidase and renin expression were detected in kidney sections by light microscopy (A, B) or confocal microscopy (C, D, E). A and B: β-Galactosidase (blue) expression was detected by X-gal staining in the nucleus of cells expressing endogenous renin (brown) in the cytoplasm. Renin expression localization was determined by immunohistochemistry with the CAS antibody 16 detected by peroxidase staining. C and D: Projection of 8 stacked images obtained by sequential scan acquisition. A similar colocalization was observed with a nuclear localization of β-galactosidase (red) in renin-expressing cells (green). E: Typical renin granules within the JG cells are shown in a confocal section with only FITC/renin fluorescence excitation. Scale bars, 10 μm.
Salt-Depletion Treatment of Adult pSG-MAR-12166 Mice
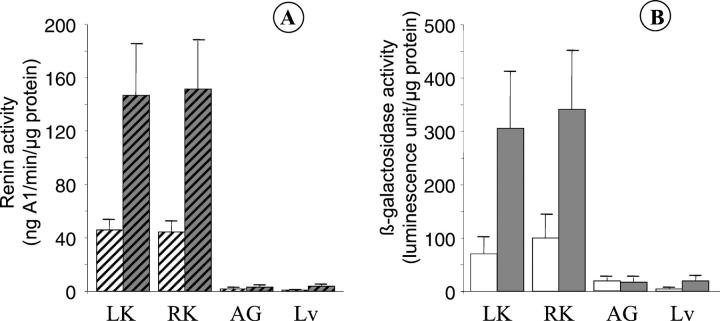
To investigate physiological regulation of the transgene, mice were salt-depleted. Wild-type and transgenic male mice were salt-deprived and treated for 1 week with furosemide (14 mg/kg/day). Treatment induced a 20% loss in body weight and a 40% increase of water intake compared to untreated animals (Table 1) ▶ . Comparable effects were observed in both wild-type and transgenic mice. These data suggest a decrease of extracellular water volume and an increase of renin activity. 21 Indeed a 3.4-fold increase of plasma renin activity was observed with a concomitant increase of renal renin activity in treated wild-type and transgenic mice (Figure 6A) ▶ . A similar 3.8-fold increase of β-galactosidase activity was observed in both kidneys (Figure 6B) ▶ . As a control, no increase of β-galactosidase activity and renin activity was observed in adrenal gland and liver.
Figure 6.
Regulation of LacZ construct in transgenic mice by salt depletion. Two groups of 6 transgenic male mice were explored. One group was used as a control (white boxes) and the other group was treated for 1 week with furosemide (14 mg/kg/d) and salt-depleted food (gray boxes). β-Galactosidase (A) and renin activity (B) were measured in kidneys (LK, left kidney; RK, right kidney), adrenal glands (AG), and liver (Lv).
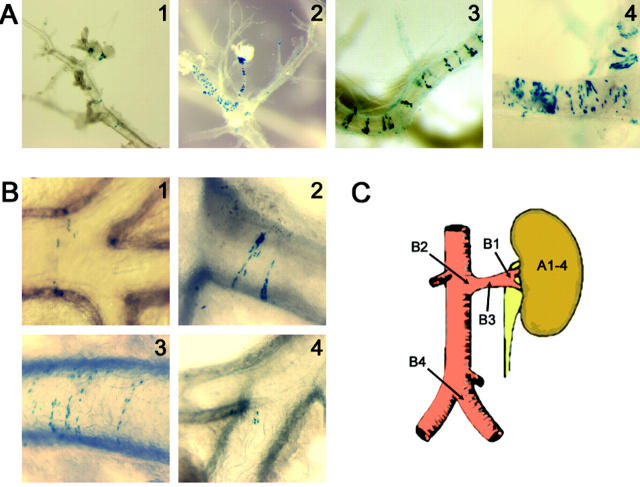
These results were visualized with dissected kidney, showing a strong β-galactosidase staining in the juxtaglomerular apparatus and along the afferent arterioles after salt depletion (Figure 7A) ▶ . Surprisingly, the staining pattern appeared as stripes in the interlobular arteries and the aorta (Figure 7) ▶ . Recruitment of β-galactosidase-expressing cells was also shown to be time dependent. After 1 week of salt depletion, staining was observed in afferent arterioles and extended along the interlobular arteries (Figure 7A) ▶ . In larger arteries (renal arteries, aorta up to iliac arteries), β-galactosidase-expressing cells were observed in the same striped pattern or as small islets after long term treatment (Figure 7B) ▶ . We observed a different pattern of expression in large arteries compared to afferent arterioles. In small afferent arterioles, β-galactosidase expression was uninterrupted starting from the glomerulus. In contrast, in larger vessels, β-galactosidase expression was repeatedly observed in discrete stripes separated by areas of non-expressing cells. To investigate expression of renin in these stripes, 30-μm-thick paraffin sections from β-galactosidase-stained kidney were immunostained with renin antibody. The same striped expression was observed for β-galactosidase (Figure 8A) ▶ and for renin (Figure 8B) ▶ . These experiments further confirm the colocalized expression of renin and β-galactosidase in the same cells, in basal condition as well as in pathophysiological conditions where the RAS is stimulated. This striped expression pattern in large vessels has not been described previously in adults. Interestingly, it resembles the pattern seen in Figure 1G ▶ for endogenous mouse renin in an artery of E18 embryonic kidney.
Figure 7.
Time course of cellular β-galactosidase expression in kidney during salt depletion. β-Galactosidase localization was observed after X-gal staining followed by microdissection. A: Identification of β-galactosidase-expressing cells in the kidneys of salt-depleted mice. 1, glomerulus from kidney of mice under normal conditions; 2, glomerulus and afferent arterioles from mice kidney after 7 days of salt depletion; 3 and 4, interlobular arteries from mice kidney depleted 7 and 9 days, respectively. A recruitment of β-galactosidase-expressing cells is seen in the juxtaglomerular apparatus, along the afferent arterioles. β-Galactosidase is also expressed in interlobular artery walls in a striped pattern. B: Expression of β-galactosidase in large extrarenal arteries during salt depletion. 1, 2 and 3, renal artery after 5, 7, or 9 days of treatment; 4, iliac arteries after 12 days of salt depletion. β-Galactosidase is expressed in large arteries in the same striped pattern. Small islets of β-galactosidase-expressing cells are seen in iliac arteries after long term treatment. β-Galactosidase staining was not seen in any parts of the aorta above the renal arteries at any stage of treatment. C represents a schematic view of abdominal aorta and a renal artery. Arrows indicate localization of renin β-galactosidase-expressing cells from panel A and B pictures.
Figure 8.
Renin and β-galactosidase expression in large intra- and extrarenal arteries. A and B show the same section of an interlobular arteries from 1-week treated mice and C and D show the same section of a iliac artery from a mice treated for 11 days. A and C show β-galactosidase expression by X-gal staining (blue stained nucleus). In B and D, renin expression is localized by immunofluorescence (green stained cells).
Two-Kidney, One-Clip Transgenic Mice
The 2K1C model was investigated in our pSG-MAR-12166 transgenic mice by clipping the left kidney artery for 5 weeks before sacrifice. Renin and β-galactosidase activity was then measured in plasma and in tissues. Despite our efforts to minimize experimental variations using stainless steel clips, a high discrepancy was observed in renin activity. Nevertheless, plasma renin concentrations were higher in clipped transgenic mice compared to unclipped transgenic mice (1880 vs. 1057 ng Al/ml/h) (Figure 9) ▶ . Both renin and β-galactosidase activities were increased (235-fold for β-galactosidase and 4.3-fold for renin; see Discussion for the apparent discrepancy) in clipped (270 pg A1/min/μg protein for renin and 306 U/μg protein for β-galactosidase) vs. unclipped kidney (62 pg A1/min/μg protein for renin and 1.3 U/μg protein for β-galactosidase). This suggests that cis-regulatory elements involved in the stimulation of renin expression in response to renal artery stenosis are present in this 12-kb fragment of the hREN promoter. As a control, we did not observe any variation of either β-galactosidase or renin activity in the adrenal gland or liver of transgenic animals (not shown).
Figure 9.
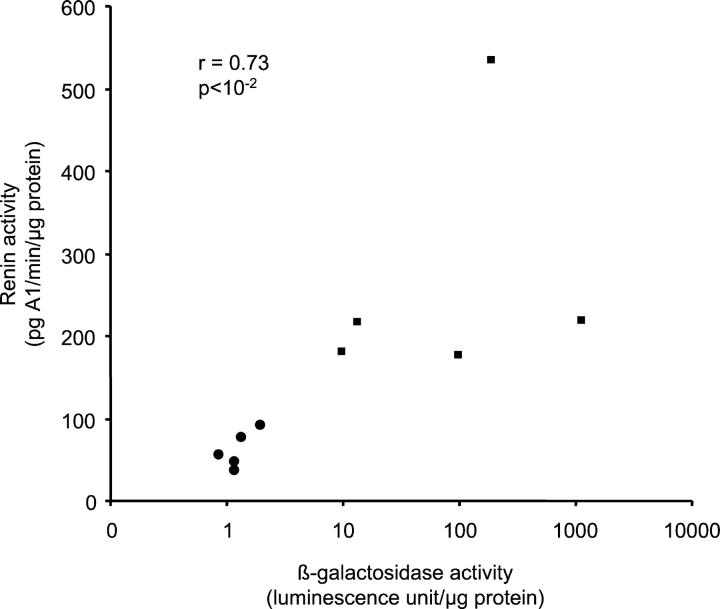
Correlation between β-galactosidase activity and renal renin activity in 2 kidney, 1-clip transgenic mice. β-Galactosidase and renin activity were measured in the clipped (▪) and contralateral kidney (•) of 5 male transgenic mice by luminescence and RIA, respectively. A strong correlation was observed (r = 0.73, P < 10−2) between β-galactosidase and renin activity in both kidneys.
At the tissue level, a punctuated β-galactosidase staining was observed in the JG apparatus of untreated mice (Figure 10A) ▶ . This staining was comparable to endogenous basal renin expression. Cells expressing β-galactosidase were recruited by the salt-depletion treatment (Figure 10B) ▶ . Even more impressive, a drastic recruitment of transgene-expressing cells was observed in 2K1C mice (Figure 10C) ▶ . Taken together, these observations suggest that β-galactosidase expression driven by the 12-kb hREN promoter reflects endogenous mouse renin regulation. The sensitivity and the facility to detect β-galactosidase allows this mouse strain to be a very powerful tool to study renin pathophysiological regulations in vivo.
Figure 10.
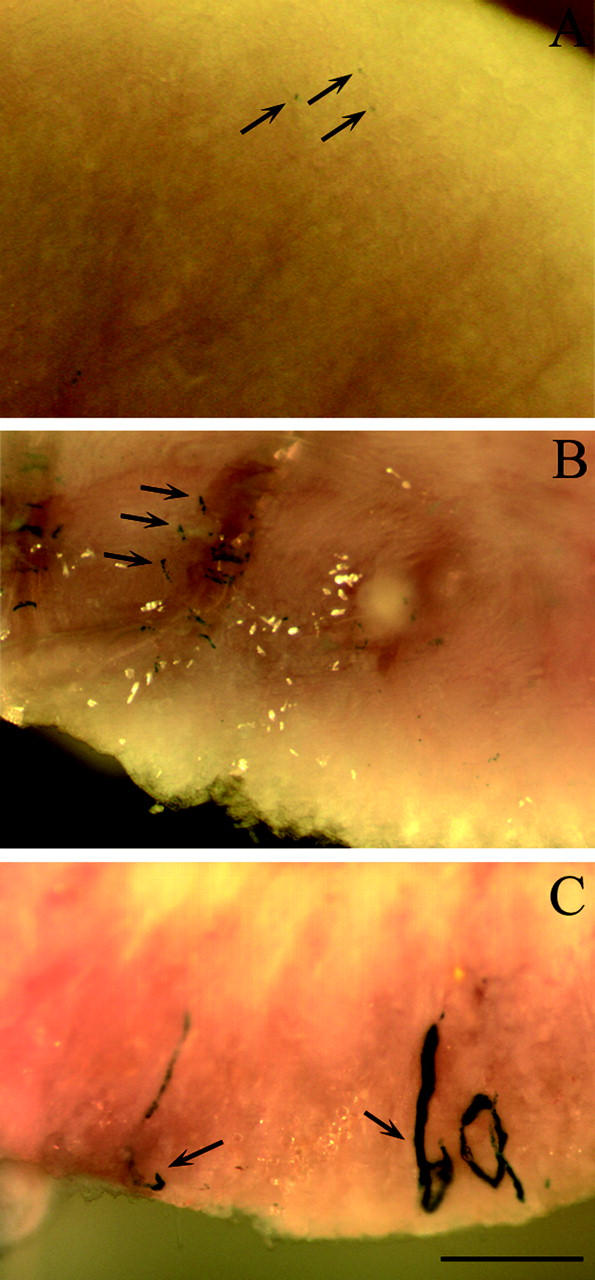
β-Galactosidase expression in kidneys or transgenic mice. Kidney from a wild-type mouse (A), from a 1-week salt-depleted mouse (B), and from a 2-kidney, 1-clip treated mouse (C) were cut into two parts and stained in toto with X-gal. β-Galactosidase activity (blue staining) is seen in the inner face of the cut kidney and is labeled by arrows. Scale bar, 1 mm.
Discussion
The elucidation of the in vivo molecular mechanisms responsible for tissue-specific regulation of renin gene expression remains largely unknown. Using a new mouse transgenic model, the aim of this work was first to determine the cis-regulatory sequences involved in renin tissue-specificity and regulated expression in vivo, and second, to obtain information concerning the recruitment of renin-expressing cells in pathophysiological conditions. For this purpose, various lengths of hREN gene promoter fragments were tested for their ability to drive expression of a LacZ reporter construct in a tissue-specific fashion. Initially, screening of the reporter protein expression driven by the hREN gene promoter was monitored at embryonic stages, eliminating the need to develop transgenic mouse strains with each promoter fragment. This strategy allowed us to screen transgene expression rapidly in several independent founders. We detected renin expression in interlobular arteries at E15 in accordance with previous report. 22 Renal β-galactosidase expression, studied at E15, was observed with at least 5777 bp of the hREN promoter which contains a 220 bp chorionic enhancer previously described. 6 Our observations and those of others 20 therefore demonstrate that the 5′ flanking region of the hREN gene can support expression of a reporter gene in the JG cells of transgenic mice. This is in contradiction to the hypothesis that the regulatory elements needed for JG cell expression are solely contained in the body of the gene. 10 Moreover, using 12,166 bp of the hREN promoter, the β-galactosidase expression in the kidney was stronger compared with the 5777 bp of the hREN promoter. Renal expression was observed in every founders we tested (at embryonic stage E15). β-Galactosidase expression in one 12,166-bp transgenic mice strain is mainly localized to the kidney but is found at a lower level in adrenal glands and brain. This localization corresponds to the renin expression pattern previously described. 23,24 At the cellular level, β-galactosidase is expressed in the nucleus of juxtaglomerular cells, whereas renin is expressed in the cytoplasm (Figure 5) ▶ .
To determine whether the 12,166-bp promoter region of the human renin promoter contains cis-regulatory elements involved in the regulation of renin expression in pathophysiological situations, two well known high renin models were studied: salt depletion and the 2-kidney, 1-clip Goldblatt model. A concomitant increase of renin and β-galactosidase activity was observed using both models. These data showed that the transgene and the endogenous renin gene responded similarly to these two different stimulatory signals suggesting that the cis-regulatory elements needed for regulated expression of the hREN gene are contained in the 12,166-bp promoter fragment. In the original experimental work of Goldblatt, 25 an increase in plasma renin concentration was found in response to renal artery stenosis. In our case, the discrepancy observed between the level of stimulation of renin versus β-galactosidase (4.3 vs. 232-fold) might be due to the long term duration of clipping. After 5 weeks, plasma renin concentration was lower than that reported at 4 weeks of clipping 18 suggesting that maximal renin stimulation was passed. Nevertheless, the stability of the β-galactosidase protein reflects accumulation over several days, in contrast to renin, which has a shorter half-life, allowing us to obtain information on renin-producing cell localization. Recruitment of renin producing cells in the afferent arteries of the glomeruli is very easy to observe by in toto tissue β-galactosidase staining. An apparently unlimited number of renin/LacZ-expressing cells can be recruited during renal artery stenosis (Figure 10) ▶ . The β-galactosidase expression observed in arteries of salt-depleted animals (Figures 7 and 8) ▶ ▶ is in agreement with a previous report 11 describing “intermediate” granulated cells within the walls of arteries and arterioles far removed from the glomerulus. A striped pattern of renin expression in interlobular renal arteries has been shown in fetuses and newborn rats. 12 Interestingly, a similar pattern of β-galactosidase expression in stripes or in rings is seen in both intrarenal arteries (Figure 7A) ▶ and in larger arteries outside the kidney (Figure 7B) ▶ during salt depletion of adult mice. Furthermore, colocalization of β-galactosidase and endogenous renin in these stripes has been confirmed (Figure 8) ▶ . These new data suggest that smooth muscle cells from vessel walls do not constitute a uniform population. Some cells express renin and some do not whereas they are all apparently exposed to the same physico-chemical and physiological environments. This is in accordance with a recent report 26 suggesting that only a subpopulation of smooth muscle cells have descended from renin precursors and are very likely the ones that undergo metaplasia to renin cells when needed. 27 Moreover, smooth muscle cells from large arteries (renal arteries, aorta, or even iliac arteries) far from the kidney were also able to express β-galactosidase in conditions of high renin synthesis (Figure 7B) ▶ . To our knowledge, this is the first time that such a phenomenon has been described, probably due to the easier localization of β-galactosidase-expressing cells compared to renin in intact kidney or vessels.
In summary, we demonstrate that β-galactosidase is expressed in a tissue- and cell-specific manner showing a comparable induction to that of the endogenous mouse renin gene in pathophysiological situations. Furthermore, observations showing that endogenous renin expression is not modified by this LacZ reporter transgene driven by the hREN promoter suggest that physiological regulation is not disturbed in this model in contrast to what was previously hypothesized using a transgene harboring the entire human renin gene. 28 This mouse strain therefore appears to be a powerful model to explore renin gene regulation and renin-expressing cells differentiation and/or recruitment in an in vivo context.
Acknowledgments
We thank Dr. Marc Lebert (CDTA, Orléans, France) and Dr. Chamsy Sarkis (CNRS UMR 9923, Paris, France) for help in transgene characterization. We also thank Dr. Pierre Meneton (INSERM U467, Paris, France) for the loan of renal clips and Dr. W. Stratling (Institut fur Kleintierforschung, Celle, Germany) for the gift of the plasmid that contains the MARs.
Footnotes
Address reprint requests to Dr Stéphane Germain, INSERM Unit 36, Collège de France, 11 place Marcelin Berthelot, 75005 Paris, France. E-mail: stephane.germain@college-de-france.fr.
Supported in part by an INSERM/Merck grant. S.F. received funds from the Ministère de la Recherche et de l’Enseignement Supérieur and from l’Académie Nationale de Médecine.
S.F. and S.G. contributed equally to this work.
References
- 1.Taugner R, Hackenthal E: The Juxtaglomerular Apparatus. 1989. Springler-Verlag, Berlin Heidelberg New York
- 2.Duncan KG, Haidar MA, Baxter JD, Reudelhuber TL: Regulation of human renin expression in chorion cell primary cultures. Proc Natl Acad Sci USA 1990, 87:7588-7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F: cis-regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res 1994, 74:764-773 [DOI] [PubMed] [Google Scholar]
- 4.Germain S, Konoshita T, Philippe J, Corvol P, Pinet F: Transcriptional induction of the human renin gene by cyclic AMP requires cyclic AMP response element-binding protein (CREB) and a factor binding a pituitary-specific trans-acting factor (Pit-1) motif. Biochem J 1996, 316:107-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Chen R, Pitarresi T, Sigmund CD, Gross KW, Sealey JE, Laragh JH, Catanzaro DF: Kidney is the only source of human plasma renin in 45-kb human renin transgenic mice. Circ Res 1998, 83:1279-1288 [DOI] [PubMed] [Google Scholar]
- 6.Germain S, Bonnet F, Philippe J, Fuchs S, Corvol P, Pinet F: A novel distal enhancer confers chorionic expression on the human renin gene. J Biol Chem 1998, 273:25292-25300 [DOI] [PubMed] [Google Scholar]
- 7.Fukamizu A, Seo MS, Hatae T, Yokoyama M, Nomura T, Katsuki M, Murakami K: Tissue-specific expression of the human renin gene in transgenic mice. Biochem Biophys Res Commun 1989, 165:826-832 [DOI] [PubMed] [Google Scholar]
- 8.Ganten D, Wagner J, Zeh K, Bader M, Michel JB, Paul M, Zimmermann F, Ruf P, Hilgenfeldt U, Ganten U, Kaling M, Bachmann S, Fukamizu A, Mullins JJ, Murakami K: Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci USA 1992, 89:7806-7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinn PL, Zhang X, Sigmund CD: JG cell expression and partial regulation of a human renin genomic transgene driven by a minimal renin promoter. Am J Physiol 1999, 277:F634-F642 [DOI] [PubMed] [Google Scholar]
- 10.Sinn PL, Davis DR, Sigmund CD: Highly regulated cell type-restricted expression of human renin in mice containing 140- or 160-kilobase pair P1 phage artificial chromosome transgenes. J Biol Chem 1999, 274:35785-35793 [DOI] [PubMed] [Google Scholar]
- 11.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y: Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney: an ultrastructural-cytochemical and autoradiographic study. Am J Pathol 1977, 87:581-602 [PMC free article] [PubMed] [Google Scholar]
- 12.Reddi V, Zaglul A, Pentz ES, Gomez RA: Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol 1998, 9:63-71 [DOI] [PubMed] [Google Scholar]
- 13.Desmarais D, Filion M, Lapointe L, Royal A: Cell-specific transcription of the peripherin gene in neuronal cell lines involves a cis-acting element surrounding the TATA box. EMBO J 1992, 11:2971-2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phi-Van L, Stratling WH: Dissection of the ability of the chicken lysozyme gene 5′ matrix attachment region to stimulate transgene expression and to dampen position effects. Biochemistry 1996, 35:10735-10742 [DOI] [PubMed] [Google Scholar]
- 15.Menard J, Catt KJ: Measurement of renin activity, concentration and substrate in rat plasma by radioimmunoassay of angiotensin I. Endocrinology 1972, 90:422-430 [DOI] [PubMed] [Google Scholar]
- 16.Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF: Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci USA 1986, 83:7552-7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casellas D, Dupont M, Kaskel FJ, Inagami T, Moore LC: Direct visualization of renin-cell distribution in preglomerular vascular trees dissected from rat kidney. Am J Physiol 1993, 265:F151-F156 [DOI] [PubMed] [Google Scholar]
- 18.Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T: Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension 1997, 29:1025-1030 [DOI] [PubMed] [Google Scholar]
- 19.Lescaudron L, Li Z, Paulin D, Fontaine-Perus J: Desmin-lacZ transgene, a marker of regenerating skeletal muscle. Neuromusc Disord 1993, 3:419-422 [DOI] [PubMed] [Google Scholar]
- 20.Jones CA, Hurley MI, Black TA, Kane CM, Pan L, Pruitt SC, Gross KW: Expression of a renin/GFP transgene in mouse embryonic, extra- embryonic, and adult tissues. Physiol Genomics 2000, 4:75-81 [DOI] [PubMed] [Google Scholar]
- 21.Abboud HE, Luke RG, Galla JH, Kotchen TA: Stimulation of renin by acute selective chloride depletion in the rat. Circ Res 1979, 44:815-821 [DOI] [PubMed] [Google Scholar]
- 22.Minuth M, Hackenthal E, Poulsen K, Rix E, Taugner R: Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol 1981, 162:173-181 [DOI] [PubMed] [Google Scholar]
- 23.Field LJ, McGowan RA, Dickinson DP, Gross KW: Tissue and gene specificity of mouse renin expression. Hypertension 1984, 6:597-603 [DOI] [PubMed] [Google Scholar]
- 24.Ekker M, Tronik D, Rougeon F: Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci USA 1989, 86:5155-5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldblatt H, Lynch J, Hanzal RF, Summerville WW: Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia J Exp Med 1934, 59:347-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA: Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 2001, 281:F345-F356 [DOI] [PubMed] [Google Scholar]
- 27.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM: Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol 1990, 259:F660-F665 [DOI] [PubMed] [Google Scholar]
- 28.Yan Y, Hu L, Chen R, Sealey JE, Laragh JH, Catanzaro DF: Appropriate regulation of human renin gene expression and secretion in 45-kb human renin transgenic mice. Hypertension 1998, 32:205-214 [DOI] [PubMed] [Google Scholar]