Abstract
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)/Apo2 ligand selectively kills neoplastic cells, including thyroid carcinoma cells (Mitsiades et al: Thyroid carcinoma cells are resistant to FAS-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res 2000, 60:4122–41299). We investigated the mechanisms regulating Apo2L/TRAIL-induced apoptosis in thyroid carcinoma cells, as well as the impact of insulin-like growth factor (IGF)-1, interferon-γ, and TNF-α. We found that the emergence of resistance to Apo2L/TRAIL, after prolonged incubation with this cytokine, was associated with increased levels of FLICE inhibitory protein (FLIP), and was overcome by cycloheximide and bisindolylmaleimide, that specifically down-regulated FLIP expression, as well as by transfection of a FLIP anti-sense oligonucleotide. IGF-1 activated Akt; up-regulated the caspase inhibitors FLIP, cIAP-2, XIAP, and survivin; and attenuated Apo2L/TRAIL-induced apoptosis. This effect was inhibited by the IGF-1 receptor neutralizing antibody aIR3, the PI-3K inhibitor wortmannin, and the heat shock protein-90 chaperone inhibitor geldanamycin. Transfection of constitutively active Akt protected from TRAIL. Conversely, interferon-γ and TNF-α had a sensitizing effect. We conclude that FLIP may negatively regulate Apo2L/TRAIL-induced apoptosis in thyroid carcinomas. Microenvironmental paracrine survival factors, such as IGF-1, up-regulate caspase inhibitors, including FLIP, and protect from Apo2L/TRAIL in a PI-3K/Akt-dependent manner. T helper-1 cytokines and compounds that selectively abrogate the IGF-1 signaling pathway may be helpful adjunct agents in Apo2L/TRAIL-based anti-cancer therapeutic regimens.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) 1 or Apo-2L 2 is a member of the TNF family that triggers apoptosis of cancer cells by interacting with two cell-surface death receptors (DR), DR4 (or TRAIL-R1), 3 and DR5 (or TRAIL-R2). 4-7 Transfection experiments have shown that both DR4 and DR5 can initiate caspase-mediated apoptosis via a stretch of 80 amino acids in the cytoplasmic domain, termed the death domain (DD). 3-5 TRAIL mRNA has been detected in a wide range of normal fetal and adult tissues, 2 suggesting the existence of a protective mechanism against Apo2L/TRAIL-mediated cytotoxicity in normal cells. Indeed, Apo2L/TRAIL induces apoptosis in a wide range of neoplastic cells, 3,8-15 but spares normal cells, 5,14 both in vitro and in vivo in studies in mice and nonhuman primates. 13,16 On the contrary, FasL, another member of the TNF family, has not demonstrated a promising anti-cancer efficacy profile, because many cancer cell lines are resistant to its apoptosis-inducing activity, as is the case for all studied thyroid carcinoma lines. 11,17 Moreover, FasL exhibits significant toxicity against normal tissues. 18 In 2000, concern was raised by a report suggesting that recombinant TRAIL killed normal human hepatocytes in vitro. 19 This finding was not reproduced in subsequent studies using clinical-grade recombinant human Apo2L and is now attributed to nonoptimized recombinant ligand preparations. 20 Therefore, the Apo2L/TRAIL pathway represents a potentially promising target for anti-cancer therapy.
Thyroid cancer is diagnosed in ∼17,000 new patients each year in the United States. Although radioactive iodine remains an efficient treatment for the subset of differentiated tumors that have retained the ability to accumulate it, a poor prognosis is still associated with less differentiated, anaplastic, and medullary carcinomas. The Apo2L/TRAIL receptors DR4 and DR5 are expressed in normal 21 and neoplastic thyrocytes. 11,15 We recently reported that Apo2L/TRAIL effectively induces apoptosis in most thyroid carcinoma cell lines, by triggering a caspase cascade originating at caspase-10. 11 In agreement with studies in other models, 3,4,22-25 we demonstrated that Apo2L/TRAIL triggers caspase-10 recruitment to the death receptor signaling complex in SW579 cells. 11 On the other hand, in contrast to its apical role in the Fas pathway, 15 caspase-8 was not recruited to this signaling complex, but was only secondarily activated in the cytoplasm and amplified the apoptotic signal. 11 Our studies suggested that recombinant Apo2L/TRAIL is a potential effective new agent against thyroid cancer.
In anticipation of the clinical use of Apo2L/TRAIL-induced apoptosis as an anti-cancer modality, we investigated the regulation of the corresponding signaling pathway in thyroid carcinomas and attempted to identify methods to overcome potential resistance. Moreover, we evaluated the effect of growth/survival factors, such as insulin-like growth factor (IGF)-1, basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF), and inflammatory cytokines, such as interferon (IFN)-γ and TNF-α, on Apo2L/TRAIL-induced apoptosis in thyroid carcinoma cells. This study identifies a role for the anti-apoptotic protein FLICE inhibitory protein (FLIP) in the regulation of Apo2L/TRAIL-induced apoptosis in thyroid carcinomas. We also provide evidence that specific survival factors can attenuate Apo2L/TRAIL-induced cell death in human neoplasias. In agreement with our studies on Fas-mediated apoptosis, IFN-γ and TNF-α also sensitized thyroid carcinoma cells to Apo2L/TRAIL. These findings could set the framework for the rational design of Apo2L/TRAIL-based anti-cancer combination therapies.
Materials and Methods
Cell Lines
The SW579 cell line, derived from a poorly differentiated human thyroid adenocarcinoma (poorly differentiated carcinoma with nuclear features of papillary carcinoma and squamous differentiation), was purchased from American Type Culture Collection (Manassas, VA) and, as we have previously shown, is very sensitive to Apo2L/TRAIL-induced apoptosis. A TRAIL-resistant line was isolated from the SW579 cells by continuous incubation with TRAIL (1000 ng/ml) for 6 months. The anaplastic thyroid carcinoma cell line FRO was a generous gift of Dr James A. Fagin (University of Cincinnati School of Medicine, Cincinnati, OH). 26 All cells were grown in Dulbecco’s modified Eagle’s medium (BioWhittaker, Walkersville, MD) with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (FCS) (Life Technologies, Inc., Gaithersburg, MD), unless stated otherwise.
Materials
Recombinant human TRAIL was obtained from Immunex Corporation (Seattle, WA), in a leucine zipper (LZ) form that promotes and stabilizes the formation of trimers, as previously reported; 13 for comparison, several experiments were repeated using the recombinant Apo2L form from Genentech Inc. (South San Francisco, CA). 20 The goat polyclonal antibodies for DR4, DR5, DcR1, and mouse monoclonal antibody for tubulin were from Santa Cruz Biotechnologies (Santa Cruz, CA); the anti-DcR2 rabbit polyclonal antibody was from Imgenex (San Diego, CA); cycloheximide, geldanamycin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Sigma Chemical Co. (St Louis, MO); IGF-1, bFGF, EGF, IFN-γ, and TNF-α were from R&D Systems (Minneapolis, MN; bisindolylmaleimide (BIM) III and wortmannin were from Calbiochem (La Jolla, CA); rabbit polyclonal antibody for caspase-10 was from Research Diagnostics Inc. (Flanders, NJ); the IGF-1 receptor neutralizing antibody aIR3 was from Oncogene Research (Cambridge, MA); and the enhanced chemiluminescence (ECL) kit, which includes the peroxidase-labeled anti-mouse and anti-rabbit secondary antibodies, was from Amersham (Arlington Heights, IL).
Flow Cytometric Analysis
Cell surface expression of TRAIL receptors was evaluated by flow cytometry as previously described. 14,23 For each cell line, 106 cells were incubated with 5.0 μg of the appropriate anti-TRAIL receptor Ab (goat polyclonal antisera for DR4, DR5, DcR1, and rabbit for DcR2) or control (goat or rabbit IgG, respectively) for 45 minutes. Cells were then washed with phosphate-buffered saline (PBS) and incubated for 45 minutes with 2.0 μg of the appropriate fluorescein isothiocyanate-conjugated secondary antibody (donkey anti-goat or goat anti-rabbit IgG, respectively) (Jackson Immunoresearch Laboratories, West Grove, PA). Cells were then washed, fixed with 1% formaldehyde-PBS, and analyzed on a EPICS-XL-MCL flow cytometer (Coulter, Hialeah, FL).
Survival and Death Assays
3-(4,5-Dimethylthiazol-2-yl)−2,5-Diphenyltetrazolium Bromide (MTT) Colorimetric Assay
Cells were plated in 24-well plates and grown to 70 to 80% confluence. Subsequently, the cells were washed in Hanks’ balanced salt solution and incubated for 18 hours with TRAIL (at the indicated concentrations), in serum-free Dulbecco’s modified Eagle’s medium at 37°C. In some experiments, cycloheximide (CHX) (10 μg/ml) or BIM III (20 μmol/L) was added to inhibit protein synthesis and protein kinase C (PKC) activity, respectively. In other experiments, the cells were pretreated with combinations of IGF-1 (100 ng/ml), bFGF (10 ng/ml), and EGF (10 ng/ml) for 6 hours, or IFN-γ (500 U/ml) and TNF-α (50 ng/ml) for 48 hours in serum-free medium. At the end of the 18-hour treatment with TRAIL, cell survival was calculated with the MTT assay as previously described. 27 Each experiment was repeated at least three times. Every experimental condition was repeated at least in sextuplicate wells for every experiment.
Western Blotting Analysis
Immunoblotting analysis was performed as previously described. 27 The proteins were visualized with the enhanced chemiluminescence technique (Amersham Pharmacia Biotech, Piscataway, NJ).
Transfection of Anti-Sense FLIP and Control Oligonucleotides
To delineate the role of FLIP as a negative regulator of Apo2L/TRAIL-induced apoptosis in thyroid carcinoma cells, we transfected the Apo2L/TRAIL-resistant line SW579-TR (that was found to express high levels of FLIP) with fully phosphorothioated single-stranded anti-sense oligonucleotide directed against the human FLIP translation initiation codon (sequence, 5′-GACTTCAGCAGACATCCTAC-3′) or control phosphorothioate oligodeoxynucleotide (sequence, 5′-TGGATCCGACATGTCAGAGA-3′), as previously described by Perlman and colleagues. 28 SW579-TR cells were plated in 24-well plates and transfected with the help of Oligofectamine (Life Technologies, Inc.). Forty-eight hours later, LZ-TRAIL (200 ng/ml) was added to appropriate wells and the cells were incubated for an additional 18 hours. Cell death was quantified by MTT as above.
Detection of Akt Activity
SW579 cells were treated with IGF-1 (100 ng/ml) for 0.5 or 1 hour, in the presence (4-hour preincubation) or absence of geldanamycin (1 μmol/L). Akt was immunoprecipitated and its enzymatic activity was assessed with the Akt Kinase Assay Kit (Cell Signaling Technology, Beverly, MA) using a GSK-3 fusion protein as substrate, according to the instructions of the manufacturer.
Transfection of Constitutively Active Akt
SW579 cells were plated on 24-well plates and 24 hours later were washed with Hanks’ balanced salt solution and transfected with a construct encoding the myristoylated, constitutively active form of Akt (Upstate Biotechnologies, Lake Placid, NY), or the empty vector, with the help of Superfect (Qiagen, Valencia, CA) according to the instructions of the manufacturer. Forty-eight hours later, the cells were treated with LZ-TRAIL (50 ng/ml). Eighteen hours later, cell survival was quantified with the MTT assay.
Caspase-8 Activity Colorimetric Assay
Caspase-8 activity was quantified with a colorimetric kit from Clontech (Palo Alto, CA), according to the instructions of the manufacturer.
Statistical Analysis
Quantitative comparisons were examined with the analysis of variance method, followed by the Duncan’s test. Statistical significance was set at 0.05.
Results
Generation and Characterization of a TRAIL-Resistant Clone of the TRAIL-Sensitive Papillary Thyroid Carcinoma Cells SW579
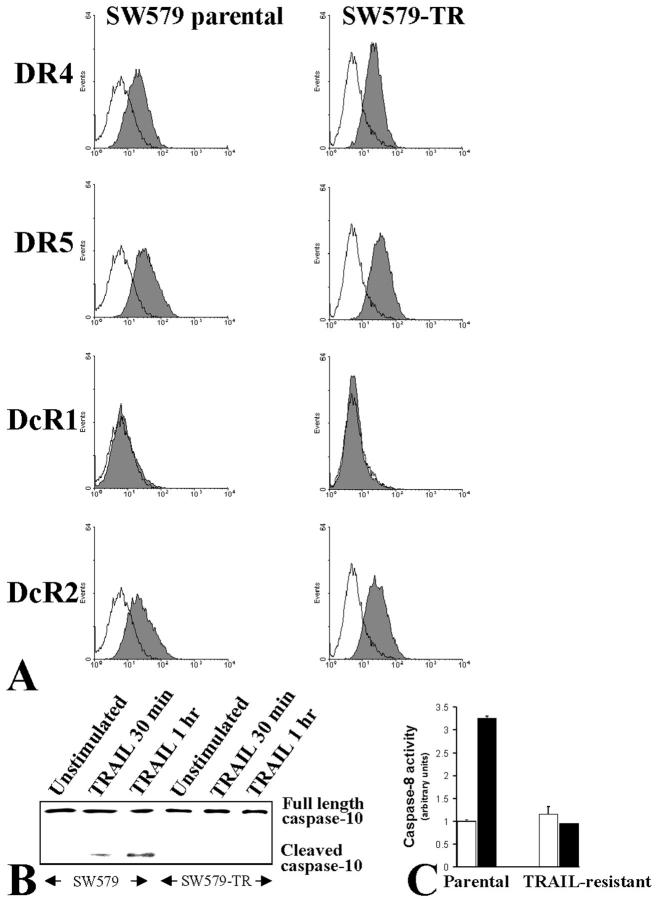
Our previous studies demonstrated that the overwhelming majority of thyroid carcinoma cell lines are sensitive to recombinant Apo2L/TRAIL. 11 However, potential emergence of resistant clones in the setting of future clinical use of Apo2L/TRAIL-induced apoptosis in cancer patients cannot be ruled out. To investigate the mechanism of emergence of resistance to Apo2L/TRAIL-induced apoptosis in thyroid carcinoma, we isolated a TRAIL-resistant clone (SW579-TR) derived from the TRAIL-sensitive SW579 cells by continuous incubation with LZ-TRAIL (1000 ng/ml) for 6 months. The resistant phenotype was stable during passage in culture without added TRAIL, as well as when cells were frozen in liquid nitrogen and thawed more than 6 months later. Flow cytometric analysis revealed that the pattern of cell surface expression of TRAIL receptors in SW579-TR cells was identical to that of the parental cells (Figure 1A) ▶ , suggesting that their resistant phenotype may be associated with intracellular regulation.
Figure 1.
A: Flow cytometric analysis of TRAIL receptors in TRAIL-sensitive SW579 and TRAIL-resistant SW579-TR cells was performed to determine the cell surface expression of TRAIL receptors DR4, DR5, DcR1, and DcR2 (shaded peaks). Control antibody staining appears as unshaded peaks. B: Immunoblotting for caspase-10 in TRAIL-sensitive parental (SW579) and TRAIL-resistant (SW579-TR) thyroid carcinoma cells. Caspase-10 was cleaved on TRAIL treatment in parental SW579 cells, but not in TRAIL-resistant SW579-TR. C: Caspase-8 activity was determined by use of the ApoAlert caspase-8 activity colorimetric assay kit in TRAIL-sensitive parental (SW579) and TRAIL-resistant (SW579-TR) thyroid carcinoma cells treated with (black bars) or without (white bars) 100 ng/ml of TRAIL for 4 hours. TRAIL induced caspase-8 activation in parental, but not in resistant cells.
To characterize the intracellular localization of the apoptotic block in SW579-TR cells, we assessed the involvement of caspases-10 and -8 in SW579 and SW579-TR cells after treatment with TRAIL. Our previous studies have demonstrated that, in agreement to other models, 3,4,22-25 Apo2L/TRAIL primarily triggers caspase-10 recruitment to the death receptor signaling complex and activation in SW579 cells, with subsequent secondary activation of caspase-8 in the cytoplasm. 11 In the present study, we found that, contrary to SW579 cells, caspases-10 and -8 are not activated in SW579-TR cells (Figure 1, B and C) ▶ . These data collectively support that the resistance of SW579-TR cells to TRAIL is because of events occurring between the cell surface receptor(s) and caspase-10. This is the level of the death receptor pathway where FLIP may exert its anti-apoptotic action.
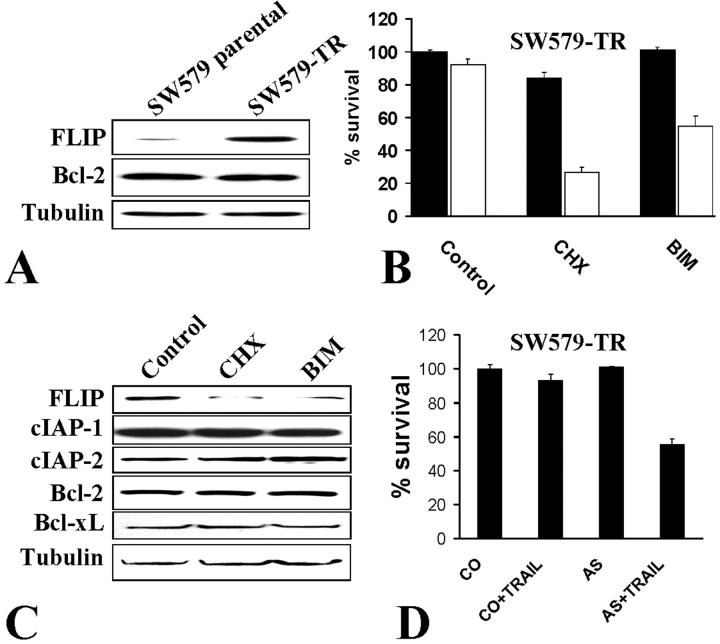
We then investigated the presence of intracellular apoptosis inhibitors in SW579 and SW579-TR cells by immunoblotting and found that SW579-TR cells express higher levels of FLIP, but not of Bcl-2 (Figure 2A) ▶ , suggesting that high levels of FLIP may contribute to the resistance of the SW579-TR cells to TRAIL.
Figure 2.
Protective role of FLIP against TRAIL-induced apoptosis in thyroid carcinoma cells. A: TRAIL-resistant SW579-TR cells express higher levels of FLIP, but not Bcl-2, than the parental, TRAIL-sensitive cells. B: Pretreatment of SW579-TR cells with cycloheximide (CHX, 10 μg/ml) or bisindolylmaleimide (BIM III, 20 μmol/L) restored sensitivity to LZ-TRAIL (300 ng/ml) (no TRAIL, black bars; TRAIL treatment, white bars). Cell survival was quantified with the MTT assay. C: CHX and BIM III specifically down-regulated FLIP protein levels in SW579-TR cells after 8 hours of treatment, but not those of cIAP-1, cIAP-2, Bcl-2, or Bcl-xL. Tubulin levels are shown for comparison. D: Indirect confirmation of the anti-apoptotic role of FLIP, SW579-TR cells were sensitized to LZ-TRAIL (300 ng/ml) by FLIP anti-sense oligonucleotides (AS), but not by control oligonucleotides (CO). Cell survival was quantified with the MTT assay.
Inhibition of Protein Synthesis and PKC Activity Sensitize SW579-TR Cells to TRAIL and Down-Regulate FLIP Expression
We and others have previously demonstrated that the protein synthesis inhibitor cycloheximide can sensitize thyroid carcinoma cells to Fas-mediated apoptosis. 11,29 We now tested the effect of cycloheximide on SW579-TR cells. We found that cycloheximide sensitized them to TRAIL-induced apoptosis (Figure 2B) ▶ . This finding excludes the possibility that the resistance of SW579-TR cells to TRAIL may be because of an inactivating mutation of cell surface TRAIL receptors, such as those found in some cases of nasopharyngeal 30 and head and neck cancers. 31 In contrast, these data indicate that in SW579-TR cells, the TRAIL apoptotic pathway is otherwise potentially functional but it is inhibited by the presence of an intracellular short-lived apoptotic inhibitor(s). FLIP could be such an intracellular inhibitor, as its expression is down-regulated in many tumor models by cycloheximide. 32-34
Previous studies have shown that the PKC inhibitor, bisindolylmaleimide (BIM) III can sensitize cells to Fas-mediated apoptosis by down-regulating FLIP expression. 32,34 As our data raised the possibility that FLIP may play an inhibitory role in Apo2L/TRAIL-induced apoptosis in SW579-TR cells, we tested the impact of BIM III in our model and found that it, too, can restore sensitivity to Apo2L/TRAIL (Figure 2B) ▶ .
Furthermore, we performed immunoblotting analysis and confirmed that, indeed, both cycloheximide and BIM III down-regulate FLIP expression levels, but not those of Bcl-2, Bcl-xL, cIAP-1, or cIAP-2 (Figure 2C) ▶ . These data suggest a pivotal role for FLIP in the regulation of Apo2L/TRAIL-induced apoptosis in thyroid carcinoma cells, in comparison to other inhibitors of apoptosis.
Down-Regulation of FLIP with Anti-Sense Oligonucleotides Overcomes TRAIL Resistance
To directly demonstrate the role of FLIP in the resistance of thyroid carcinoma cells to TRAIL, SW579-TR cells were treated with FLIP anti-sense or control oligonucleotides, as previously described by Perlman and colleagues, 28 and subsequently treated with TRAIL (200 ng/ml). We found that the presence of the FLIP anti-sense oligonucleotide restored sensitivity to TRAIL (Figure 2D) ▶ .
Sensitivity of Thyroid Carcinoma Cells to TRAIL Is Serum-Dependent
An extensive body of evidence has documented that in several tumor models the response to anti-cancer therapies may be modulated by paracrine interactions within the local tumor microenvironment or by endocrine mechanisms mediated by circulating factors. 35 We therefore investigated the effect of soluble paracrine factors on the TRAIL sensitivity of thyroid carcinoma cells, by adding serum in our experimental model. We evaluated the apoptotic activity of TRAIL against SW579 cells after 18 hours of incubation both in medium containing 10% FCS and in serum-free medium. Administration of TRAIL in serum-free conditions resulted in significantly higher cytotoxicity (LD50 = 50 ng/ml) than in the presence of serum (LD50 = 382 ng/ml) (Figure 3A) ▶ . This finding suggests that factor(s) present in serum, possibly growth/survival factor(s), can attenuate TRAIL-induced apoptosis. Furthermore, the evaluation of the cytotoxic effect of TRAIL against any given cell line should be performed within the context of the medium and serum conditions under which the experiment takes place to avoid mislabeling TRAIL-sensitive cells as resistant.
Figure 3.
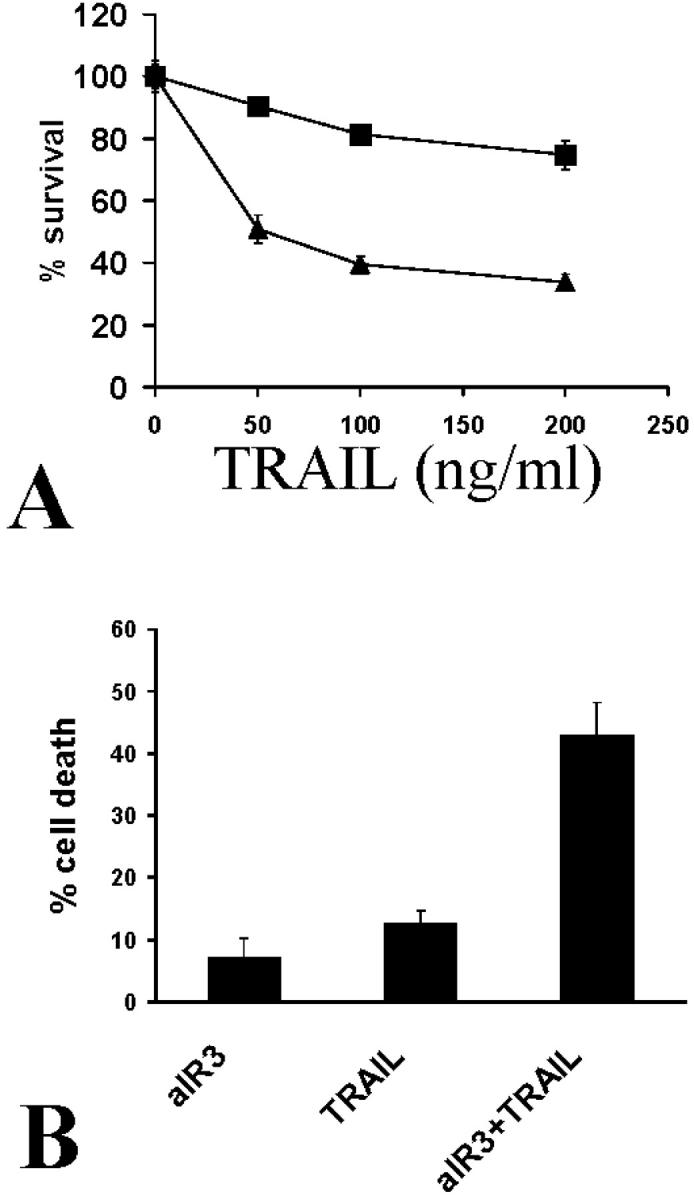
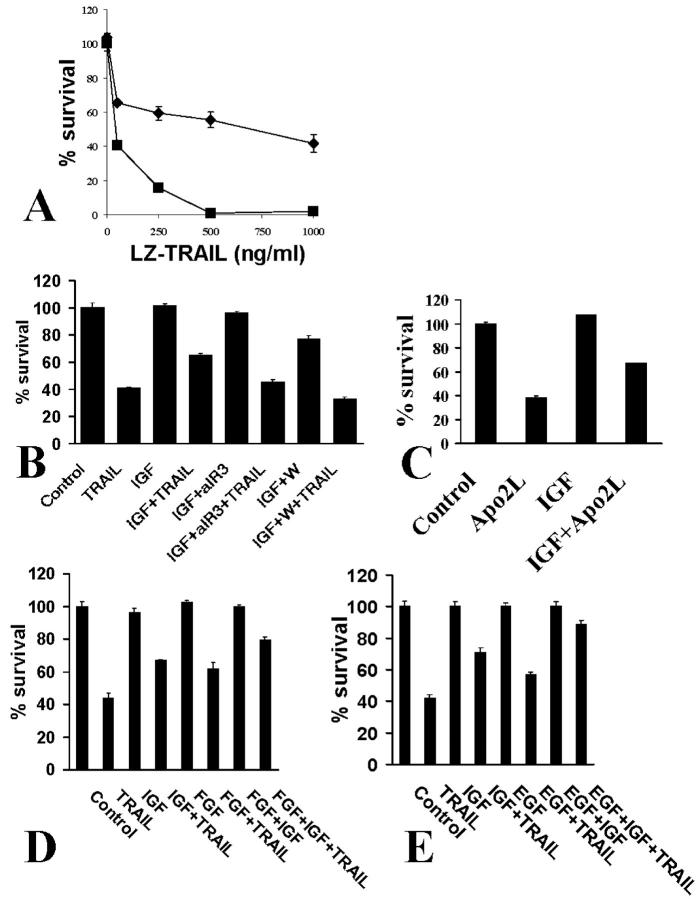
A: Treatment of SW579 cells with recombinant LZ-TRAIL (50 to 200 ng/ml) for 18 hours resulted in higher survival in the presence of 10% FCS (squares) that in serum-free medium (triangles). Cell survival was quantified with the MTT assay. B: The IGF-1 receptor-neutralizing antibody aIR3 (2 μg/ml) potentiated the apoptosis-inducing effect of LZ-TRAIL (50 ng/ml) against SW579 cells in 10% FCS (MTT assay).
IGF-1, bFGF, and EGF Protect Thyroid Carcinoma Cells from Apo2L/TRAIL-Induced Apoptosis in a PI-3K-Dependent Manner
We proceeded to further characterize the regulation of TRAIL-induced apoptosis in thyroid carcinoma cells. IGF-1 is a growth/survival factor that is produced by stromal cells in thyroid carcinomas 36 and is present in high concentrations in the serum. Thus it could locally regulate the response of neoplastic thyrocytes to TRAIL, as well as partially explain the anti-apoptotic activity that we detected in serum. The IGF-1 receptor-neutralizing antibody aIR3 restored the sensitivity of SW579 cells treated with LZ-TRAIL in the presence of 10% FCS (Figure 3B) ▶ , suggesting that IGF-1 could be one of the serum factors that confer protection from TRAIL. Indeed, pretreatment with IGF-1 had a protective effect against LZ-TRAIL-induced apoptosis in SW579 cells (Figure 4A) ▶ and in FRO cells. This effect was again specifically inhibited by the IGF-1 receptor-neutralizing antibody aIR3 (Figure 4B) ▶ , confirming that it was mediated through the IGF-1R. The protective effect of IGF-1 was also blocked by the PI-3K inhibitor wortmannin (Figure 4B) ▶ , suggesting that the signaling pathway for this effect involves PI-3K. IGF-1 had a similar protective effect against apoptosis induced by the Apo2L form of the ligand as well (Figure 4C) ▶ , which is the form that has been shown to be nontoxic for normal hepatocytes. 20
Figure 4.
Effect of survival factors on TRAIL-induced apoptosis in thyroid carcinoma cells. A: IGF-1 (100 ng/ml), a growth/survival factor present in serum and produced locally in thyroid carcinomas by stromal cells in vivo, protected SW579 cells from recombinant LZ-TRAIL (50 to 1000 ng/ml) (no IGF-1, squares; IGF-1, diamonds). B: This protective effect was inhibited by the IGF-1 receptor-neutralizing antibody aIR3 (2 μg/ml) and by the PI-3K inhibitor wortmannin (W) (5 μmol/L). Cell survival was quantified with the MTT assay. IGF-1 alone had only minimal proliferative effect within the short incubation period of the experiment (18 hours). C: IGF-1 (100 ng/ml) also lowered the proapoptotic activity of the Apo2L form of the ligand (100 ng/ml). D: bFGF (10 ng/ml) also partially attenuated LZ-TRAIL (50 ng/ml)-induced apoptosis, an effect that was potentiated by IGF-1 (100 ng/ml). Cell survival was quantified with the MTT assay. E: EGF (10 ng/ml) also partially attenuated LZ-TRAIL (50 ng/ml)-induced apoptosis, an effect that was potentiated by IGF-1 (100 ng/ml). Cell survival was quantified with the MTT assay.
Two more paracrine growth/survival factors, bFGF and EGF, demonstrated a similar protective effect on TRAIL-induced apoptosis in SW579 cells (Figure 4, D and E) ▶ and potentiated the effect of IGF-1.
Inhibition of Constitutive PI-3K Activity Sensitizes Thyroid Carcinoma Cells to TRAIL-Induced Apoptosis
Having demonstrated that the PI-3K inhibitor wortmannin attenuated the anti-apoptotic effect of IGF-1, we investigated its effect when applied alone to thyroid carcinoma cells. We found that wortmannin could sensitize both SW579 and SW579-TR cells to subtoxic doses of TRAIL (Figure 5) ▶ , suggesting that constitutive PI-3K activity is present in those cells and has an anti-apoptotic function. The fact that this sensitizing effect was present even in the TRAIL-resistant line offers hope that PI-3K-inhibiting therapies could overcome established resistance to apoptosis in a clinical setting.
Figure 5.
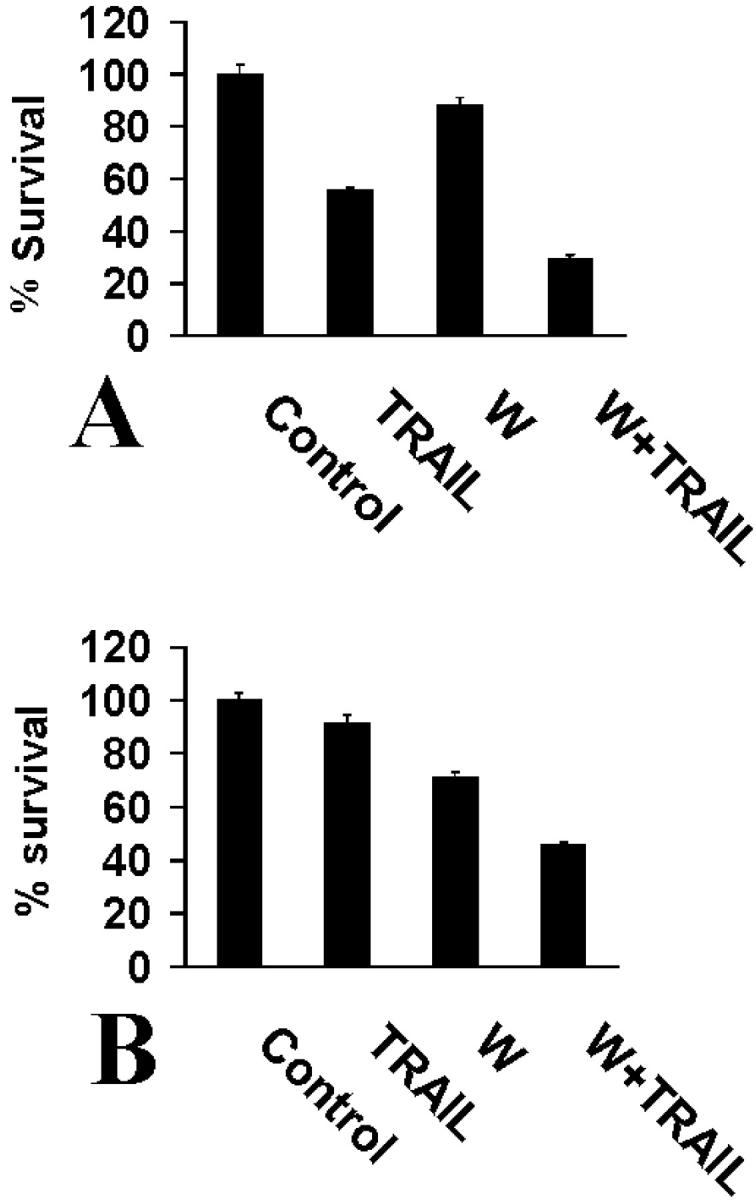
A: Wortmannin (W) (5 μmol/L) increased the sensitivity of parental SW579 cells to LZ-TRAIL (50 ng/ml), suggesting that constitutive PI-3K activity is present in thyroid carcinoma cells and has an anti-apoptotic role. B: Wortmannin (5 μmol/L) exerted a similar sensitizing effect on the TRAIL-resistant SW579-TR cells treated with a higher concentration of LZ-TRAIL (300 ng/ml). Cell survival was quantified with the MTT assay.
Characterization of the Anti-Apoptotic Effect of IGF-1 on Thyroid Carcinoma Cells: Impact on Akt Activity and Protein Levels of Apoptosis Mediators
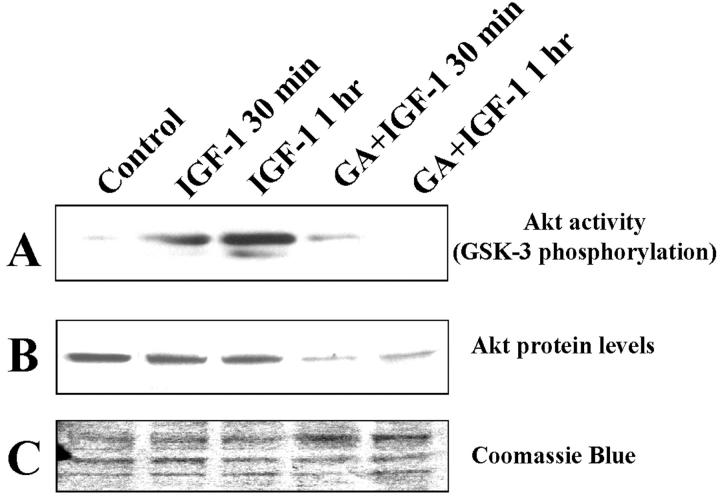
Despite a well-established anti-apoptotic role in other models 37 and its wide expression in thyroid tissue, 36 the role of IGF-1 on thyroid carcinoma apoptosis has not been studied extensively. In this study, we found that IGF-1 treatment induced the enzymatic activity of Akt in SW579 cells, as evidenced by its ability to phosphorylate a GSK-3 fusion protein (Figure 6A) ▶ . We also investigated the effect of the heat shock protein-90 chaperone inhibitor geldanamycin. 38 Previous studies have demonstrated that geldanamycin reduces the intracellular levels and activity of several kinases, including Akt. 39,40 We found that geldanamycin lowered Akt protein levels and inhibited IGF-1-induced Akt activation (Figure 6) ▶ .
Figure 6.
Akt enzymatic activity (A) and protein levels (B) in SW579 cells after treatment with IGF-1 (100 ng/ml) for 0.5 or 1 hour, in the presence or absence of 1 μmol/L of geldanamycin (GA). Cells were pretreated with geldanamycin for 4 hours. C: Equal loading of the gel in B was confirmed by Coomassie Blue staining of the membrane.
By flow cytometric analysis, we also found that IGF-1 treatment does not modify the cell surface expression of TRAIL receptors (Figure 7A) ▶ . We then investigated the effect of IGF-1 on a panel of apoptotic inhibitors in SW579 cells. We found that IGF-1 up-regulates FLIP, cIAP-2, XIAP, and survivin, but not cIAP1 or the anti-apoptotic members of the Bcl-2 family Bcl-2, Bcl-xL, A1/Bfl-1, and Mcl-1 (Figure 7B) ▶ . Nevertheless, IGF-1 down-regulated Bax protein levels, which could be an additional mechanism of protection from apoptosis.
Figure 7.

A: Flow cytometric analysis for TRAIL receptors (DR4, DR5, DcR1, and DcR2, shaded peaks) in SW579 cells treated with or without IGF-1 (100 ng/ml) for 8 hours revealed no changes. Control antibody staining appears as unshaded peaks. B: Treatment of SW579 cells with IGF-1 (100 or 300 ng/ml) for 8 hours up-regulated the apoptosis inhibitors FLIP, cIAP2, XIAP, and survivin, but not cIAP1 or the anti-apoptotic members of the Bcl-2 family Bcl-2, Bcl-xL, A1, and Mcl-1. IGF-1 also down-regulated the proapoptotic Bax.
Forced Expression of Constitutively Active Akt Attenuates TRAIL-Induced Apoptosis
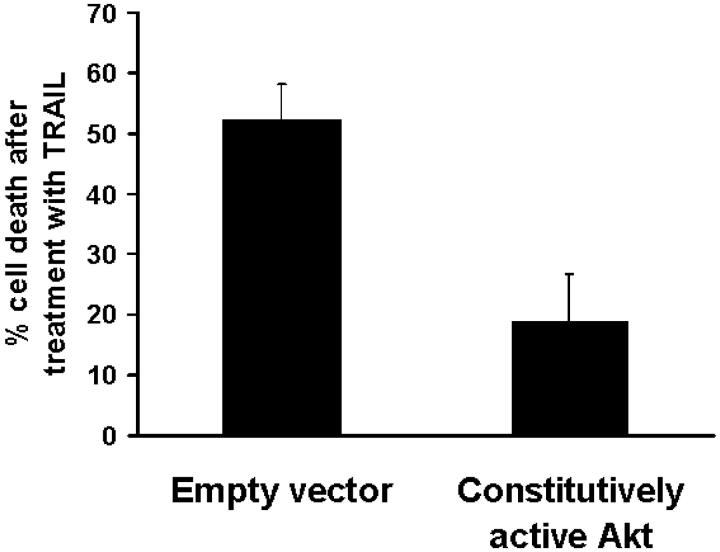
Having demonstrated that IGF-1 activates Akt and lowers sensitivity to TRAIL-induced apoptosis, we investigated the direct effect of Akt activation in our model. We found that transfection of a construct encoding constitutively active Akt in thyroid carcinoma cells protected them from TRAIL (Figure 8) ▶ , suggesting that the anti-apoptotic effect of IGF-1 in our model could be mediated (at least in part) by activation of Akt.
Figure 8.
Transfection of constitutively active Akt lowered the sensitivity of SW579 cells to LZ-TRAIL (50 ng/ml), as compared to the empty vector. Cell death was quantified with the MTT assay.
Geldanamycin Overcomes the Anti-Apoptotic Effect of IGF-1
In our effort to describe clinically achievable methods to sensitize neoplastic cells to Apo2L/TRAIL, we studied further the effects of the heat shock protein-90 chaperone inhibitor geldanamycin, which, as we already demonstrated, decreases Akt activity in IGF-1-treated cells. In agreement with that result, we found that geldanamycin overcame the protective effect of IGF-1 and increased the sensitivity of thyroid carcinoma cells to LZ-TRAIL (Figure 9A) ▶ and Apo2L (Figure 9B) ▶ . These data suggest that geldanamycin or similar agents could be helpful adjunct agents in Apo2L/TRAIL-based combination therapies.
Figure 9.
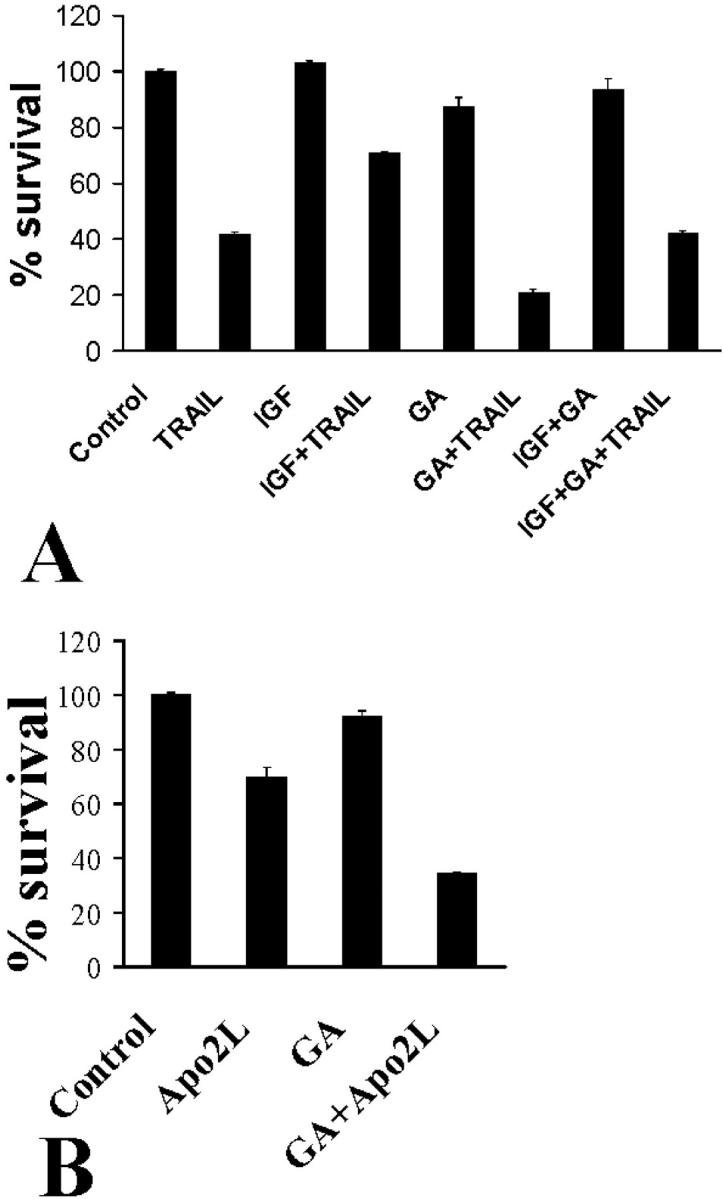
Impact of geldanamycin (GA) (1 μmol/L) on TRAIL-induced apoptosis in thyroid carcinoma cells. A: SW579 cells were pretreated with IGF-1 (100 ng/ml) for 6 hours, in the presence or absence of geldanamycin, and then incubated with LZ-TRAIL (50 ng/ml) for an additional 18 hours. Geldanamycin overcame the protective effect of IGF-1 against TRAIL-induced apoptosis. Moreover, geldanamycin alone had a sensitizing effect on TRAIL-induced apoptosis. B: Geldanamycin also sensitized SW579 cells to the Apo2L form of the ligand (50 ng/ml).
IFN-γ and TNF-α Sensitize Thyroid Carcinoma Cells to TRAIL
We have previously reported that thyroid carcinoma cells can be sensitized to Fas-mediated cell death after treatment with IFN-γ and TNF-α. 11 Although these cytokines may stimulate the apoptotic pathway at various levels, one direct effect of IFN-γ was a strong increase in Fas expression at the protein level. 11 We now investigated the effect of these cytokines on TRAIL-induced apoptosis in thyroid carcinoma. We found a strong sensitizing effect of IFN-γ, and a less pronounced effect of TNF-α, on TRAIL-induced apoptosis in SW579 (Figure 10A) ▶ and FRO cells. A similar effect was also found in SW579-TR cells (Figure 10B) ▶ , leading to the observation that despite the emergence of resistance to TRAIL, the sensitizing effect of cytokines remains intact in these cells. IFN-γ pretreatment also sensitized SW579-TR cells to TRAIL-induced cleavage of caspase-10 and caspase-8 (not shown).
Figure 10.

A: SW579 cells were sensitized by a 48-hour pretreatment with IFN-γ (500 IU/ml) and TNF-α (50 ng/ml) to an additional 18-hour incubation with a low, subtoxic concentration of LZ-TRAIL (20 ng/ml). B: TRAIL-resistant SW579-TR cells were sensitized by a 48-hour pretreatment with IFN-γ (500 IU/ml) and TNF-α (50 ng/ml) to an additional 18-hour incubation with LZ-TRAIL (300 ng/ml).
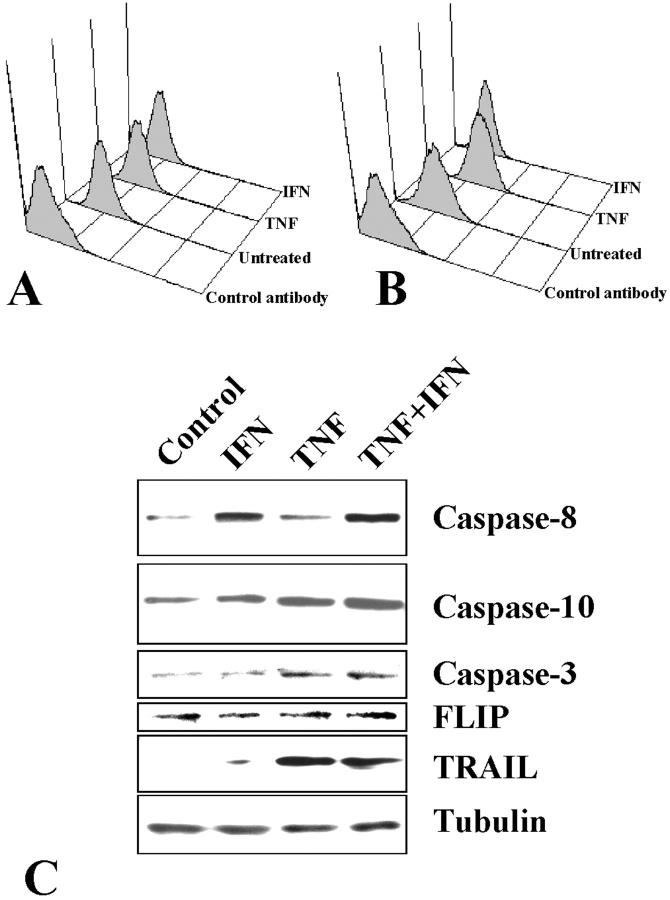
Flow cytometry analysis showed that the only change in apoptosis-inducing TRAIL receptor cell surface levels by cytokine treatment of SW579 cells that correlated with their sensitizing effect was the up-regulation of DR5 by TNF-α (Figure 11, A and B) ▶ . IFN-γ also up-regulated caspase-8, whereas TNF-α had a more pronounced effect on caspases-10 and -3, as shown by immunoblotting (Figure 11C) ▶ . Interestingly, TNF-α stimulated the protein expression of TRAIL itself (IFN-γ had only a minor effect). This finding suggests that under cytokine stimulation, thyroid carcinoma cells can produce the death ligand TRAIL.
Figure 11.
A and B: Flow cytometric analysis of TRAIL receptors DR4 (A) and DR5 (B) after a 48-hour treatment with or without IFN-γ (500 IU/ml) or TNF-α (50 ng/ml) in SW579 cells. Control antibody staining is also shown. C: Evaluation of the protein levels of caspase-8, caspase-10, caspase-3, FLIP, and TRAIL in SW579 cells after a 48-hour treatment with or without IFN-γ (500 IU/ml) or TNF-α (50 ng/ml). IFN-γ (500 IU/ml) up-regulated caspase-8 and TNF-α (50 ng/ml) and up-regulated caspases-10 and -3. Additionally, TNF-α induced the expression of TRAIL itself.
Discussion
We investigated the regulation of Apo2L/TRAIL-induced apoptosis in thyroid carcinoma cells by both intracellular and paracrine factors. We demonstrate that the emergence of resistance to TRAIL is associated with up-regulation of FLIP protein levels. More importantly, sensitivity is restored by down-regulation of FLIP, via either the protein synthesis inhibitor cycloheximide, the PKC inhibitor BIM III, or a FLIP anti-sense oligonucleotide. The growth/survival factor IGF-1 lowered the sensitivity of thyroid carcinoma cells to TRAIL in a PI-3K-dependent manner, activated Akt and increased the protein levels of several apoptosis inhibitors, including FLIP. The anti-apoptotic effect of IGF-1 was mimicked by transfection of constitutively active Akt. On the other hand, the T helper1-derived inflammatory cytokines IFN-γ and TNF-α had a sensitizing effect on TRAIL-induced apoptosis.
Although recombinant Apo2L/TRAIL is very potent against thyroid carcinoma cells in vitro, 11 we cannot rule out that future clinical use of this apoptotic pathway in cancer patients might eventually encounter emergence of refractoriness. Furthermore, the rational design of sensitization strategies could be useful from a clinical standpoint because it could conceivably lead to Apo2L/TRAIL-based combination therapies with enhanced anti-tumor activity. Therefore, we investigated the mechanisms regulating the sensitivity of thyroid carcinoma cells to Apo2L/TRAIL. We established a model of TRAIL resistance by isolating a resistant subline from a TRAIL-sensitive parental papillary carcinoma line. The TRAIL-resistant SW579-TR cells did not exhibit differences in cell surface TRAIL receptor expression in comparison to the parental cells, but had higher levels of the anti-apoptotic protein FLIP and did not exhibit activation of caspases-10 and -8 on TRAIL treatment. The protein synthesis inhibitor cycloheximide and the PKC inhibitor BIM III down-regulate FLIP protein levels 32,34 and overcome resistance to Fas-induced apoptosis in many models. 11,17,32,34 We now found that both cycloheximide and BIM III overcame the resistance of SW579-TR cells to TRAIL. Importantly, both inhibitors specifically down-regulated FLIP protein levels, and treatment with a FLIP anti-sense oligonucleotide restored sensitivity to TRAIL. These data establish FLIP as a major candidate for the role of anti-apoptotic regulator in TRAIL-induced signaling in thyroid carcinomas. Although the anti-apoptotic role of FLIP in Fas-mediated apoptosis is well-documented, 41-44 its impact on the TRAIL pathway is more controversial and may be subject to tissue-specific variations. Elevated FLIP levels correlate with Apo2L/TRAIL-resistance in some occasions, 8,44,45 but not in others. 46 Most primary papillary thyroid carcinoma cell lines are sensitive to Apo2L/TRAIL, yet all lines tested to date are resistant to FasL and this resistance can be overcome by cycloheximide. 11 This differential pattern of sensitivity could mean that two different intracellular inhibitors regulate the two pathways. Alternatively, it is possible that whereas FLIP can potentially inhibit both pathways in thyroid carcinomas, the threshold for FLIP-dependent resistance is higher for the Apo2L/TRAIL pathway than for the Fas pathway, perhaps because of differential affinity of FLIP for the adaptor molecules of the respective receptor-caspase death signaling complexes.
The local microenvironment exerts a major role on cancer cell fate via the production of growth/survival factors and cytokines. 35,47 IGF-1 is strongly expressed by stromal cells in thyroid carcinomas, 36 and is also present in the serum. IGF-1 and serum had a strong protective effect against TRAIL-induced cell death, in agreement with our previous findings in multiple myeloma cells. 48 bFGF and EGF also had anti-apoptotic effects, which were potentiated by IGF-1. These data suggest that paracrine factors produced by the local microenvironment could modulate the response of neoplastic cells to TRAIL/Apo2L. Moreover, the PI-3K inhibitor wortmannin had a sensitizing effect even when used alone, suggesting that constitutive PI-3K activity is present and has an anti-apoptotic role in thyroid carcinomas. Interestingly, this sensitizing effect was present in both TRAIL-sensitive and -resistant cells.
IGF-1 did not modulate cell surface TRAIL receptor expression, but up-regulated FLIP, cIAP-2, XIAP, and survivin. Our work thus highlights that paracrine factors, such as IGF-1, may regulate sensitivity to apoptosis by modifying caspase inhibitor expression. The increase in FLIP expression on IGF-1 treatment is consistent with its protective role on Apo2L/TRAIL-induced apoptosis that we described earlier. cIAP-2 is an inhibitor of caspase-8 activation and apoptosis induced by TNF-α 49 and its expression is under the positive regulatory control of nuclear factor-κB in several models. 49,50 Nuclear factor-κB may also up-regulate the expression of XIAP, 51 a potent inhibitor of caspases-3, -7, and -9. 52 Survivin preferentially binds to and inhibits effector caspases, such as caspase-3 and caspase-7. 53 Additionally, Bax, a proapoptotic member of the Bcl-2 family, was found in this study to be down-regulated by IGF-1-treatment, which could also potentially contribute to resistance to apoptosis.
Taken together, our data identify at least two intracellular signaling pathways as important modulators of Apo2L/TRAIL-induced apoptosis, namely the PI-3K/Akt and PKC pathways. Akt regulates proliferation and survival in normal and neoplastic thyrocytes 54-56 and has recently been reported to up-regulate FLIP 57 and protect from Apo2L/TRAIL 58 in other models. Our work, in addition to extending this concept to thyroid carcinomas, identifies specific extracellular paracrine factors, such as IGF-1, as physiological modulators of Apo2L/TRAIL-induced apoptosis via the PI-3K/Akt pathway. Moreover, transfection of constitutively active Akt attenuated TRAIL-induced apoptosis in our model. In the case of PKC, our work suggests that it confers protection from TRAIL-induced apoptosis in a FLIP-dependent manner.
Our study suggests that approaches inhibiting the activity and the intracellular signaling pathway of IGF-1 and other survival factors could be helpful adjuncts in the clinical use of TRAIL. PI-3K inhibitors, such as wortmannin, could exert such an effect. Additionally, novel agents of the ansamycin family, that inhibit the heat shock protein-90 molecular chaperone, have been reported to deplete cells of several kinases, including Akt 39,40 and are currently being evaluated clinically. 59 In our studies, geldanamycin lowered Akt protein levels and enzymatic activity after stimulation, sensitized cells to TRAIL and overcame the protective effect of IGF-1. It should be noted that similar results were obtained with both the LZ-TRAIL and the Apo2L forms of the ligand.
Another class of potentially helpful adjunct agents are the inflammatory cytokines IFN-γ and TNF-α, that, as previously reported, can sensitize both normal 60 and neoplastic thyrocytes 11 to Fas-mediated apoptosis; IFN-γ is produced by tumor-infiltrating lymphocytes, whereas TRAIL participates in cell-mediated immunity. 61-64 The sensitizing effect of cytokines may be mediated, at least in part, by the up-regulation of DR5, similar to their stimulating effect on Fas expression that we previously described, 11 and/or the increase in caspase expression, similar to a report on nonmalignant thyrocytes. 65 It should be noted that this sensitizing effect was still present in the TRAIL-resistant subline, a finding that has obvious clinical implications. Another interesting observation was that TNF-α induced the production of the death ligand TRAIL itself, in agreement with a similar report by Bretz and colleagues, 21 who also demonstrated that this effect renders the cancer cells cytotoxic against lymphocytes in a TRAIL-dependent manner. This immune evasion hypothesis resembles a counterattack mechanism that we have already described in thyroid carcinoma cells because of their constitutive expression of FasL. 17
In conclusion, we have demonstrated an inhibitory role for FLIP and the IGF-1/Akt pathway in Apo2L/TRAIL-induced apoptosis of thyroid carcinoma cells. Our studies emphasize the complex effects of circulating or paracrine factors present in the local microenvironment and set the framework for the future design of pharmacological interventions to increase the sensitivity of thyroid carcinomas to this apoptotic pathway.
Footnotes
Address reprint requests to Vassiliki Poulaki, M.D., Ph.D., Massachusetts Eye and Ear Infirmary, Harvard Medical School, 325 Cambridge St., Boston, MA 02114. E-mail: poulakiv@hotmail.com.
Supported by the Propondis Foundation.
References
- 1.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG: Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3:673-682 [DOI] [PubMed] [Google Scholar]
- 2.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A: Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996, 271:12687-12690 [DOI] [PubMed] [Google Scholar]
- 3.Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM: The receptor for the cytotoxic ligand TRAIL. Science 1997, 276:111-113 [DOI] [PubMed] [Google Scholar]
- 4.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM: An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277:815-818 [DOI] [PubMed] [Google Scholar]
- 5.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A: Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277:818-821 [DOI] [PubMed] [Google Scholar]
- 6.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT: TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 1997, 16:5386-5397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J: Characterization of two receptors for TRAIL. FEBS Lett 1997, 416:329-334 [DOI] [PubMed] [Google Scholar]
- 8.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ: Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 1998, 161:2833-2840 [PubMed] [Google Scholar]
- 9.Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S: Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res 1999, 59:734-741 [PubMed] [Google Scholar]
- 10.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M: Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res 2001, 61:2704-2712 [PubMed] [Google Scholar]
- 11.Mitsiades N, Poulaki V, Tseleni-Balafouta S, Koutras DA, Stamenkovic I: Thyroid carcinoma cells are resistant to FAS-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res 2000, 60:4122-4129 [PubMed] [Google Scholar]
- 12.Yu R, Mandlekar S, Ruben S, Ni J, Kong AN: Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in androgen-independent prostate cancer cells. Cancer Res 2000, 60:2384-2389 [PubMed] [Google Scholar]
- 13.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH: Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999, 5:157-163 [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC: TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 2001, 98:795-804 [DOI] [PubMed] [Google Scholar]
- 15.Mitsiades N, Poulaki V, Mitsiades CS, Koutras DA, Chrousos GP: Apoptosis induced by FasL and TRAIL/Apo2L in the pathogenesis of thyroid diseases. Trends Endocrinol Metab 2001, 12:384-390 [DOI] [PubMed] [Google Scholar]
- 16.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH: Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999, 104:155-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsiades N, Poulaki V, Mastorakos G, Tseleni-Balafouta ST, Kotoula V, Koutras DA, Tsokos M: Fas ligand expression in thyroid carcinomas: a potential mechanism of immune evasion. J Clin Endocrinol Metab 1999, 84:2924-2932 [DOI] [PubMed] [Google Scholar]
- 18.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S: Lethal effect of the anti-Fas antibody in mice. Nature 1993, 364:806-809 [DOI] [PubMed] [Google Scholar]
- 19.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC: Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med 2000, 6:564-567 [DOI] [PubMed] [Google Scholar]
- 20.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A: Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med 2001, 7:383-385 [DOI] [PubMed] [Google Scholar]
- 21.Bretz JD, Rymaszewski M, Arscott PL, Myc A, Ain KB, Thompson NW, Baker JR, Jr: TRAIL death pathway expression and induction in thyroid follicular cells. J Biol Chem 1999, 274:23627-23632 [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu Z: The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol 2000, 20:6638-6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M: Ewing’s sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and express DR4 and DR5 receptors. Cancer Res 2001, 61:2704-2712 [PubMed] [Google Scholar]
- 24.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, Gazdar A, Blenis J, Arnott D, Ashkenazi A: Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem 2001, 276:46639-46646 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ: Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA 2001, 98:13884-13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonsky R, Knauf JA, Elisei R, Wang JW, Su S, Fagin JA: Identification of rapid turnover transcripts overexpressed in thyroid tumors and thyroid cancer cell lines: use of a targeted differential RNA display method to select for mRNA subsets. Nucleic Acids Res 1997, 25:3823-3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsiades N, Poulaki V, Kotoula V, Leone A, Tsokos M: Fas ligand is present in tumors of the Ewing’s sarcoma family and is cleaved into a soluble form by a metalloproteinase. Am J Pathol 1998, 153:1947-1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM: FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med 1999, 190:1679-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arscott PL, Knapp J, Rymaszewski M, Bartron JL, Bretz JD, Thompson NW, Baker JR, Jr: Fas (APO-1, CD95)-mediated apoptosis in thyroid cells is regulated by a labile protein inhibitor. Endocrinology 1997, 138:5019-5027 [DOI] [PubMed] [Google Scholar]
- 30.Ozoren N, Fisher MJ, Kim K, Liu CX, Genin A, Shifman Y, Dicker DT, Spinner NB, Lisitsyn NA, El-Deiry WS: Homozygous deletion of the death receptor DR4 gene in a nasopharyngeal cancer cell line is associated with TRAIL resistance. Int J Oncol 2000, 16:917-925 [DOI] [PubMed] [Google Scholar]
- 31.Pai SI, Wu GS, Ozoren N, Wu L, Jen J, Sidransky D, El-Deiry WS: Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res 1998, 58:3513-3518 [PubMed] [Google Scholar]
- 32.Willems F, Amraoui Z, Vanderheyde N, Verhasselt V, Aksoy E, Scaffidi C, Peter ME, Krammer PH, Goldman M: Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood 2000, 95:3478-3482 [PubMed] [Google Scholar]
- 33.Kreuz S, Siegmund D, Scheurich P, Wajant H: NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol 2001, 21:3964-3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller CM, Scott DW: Distinct molecular mechanisms of Fas resistance in murine B lymphoma cells. J Immunol 2000, 165:1854-1862 [DOI] [PubMed] [Google Scholar]
- 35.Koutsilieris M, Mitsiades C, Sourla A: Insulin-like growth factor I and urokinase-type plasminogen activator bioregulation system as a survival mechanism of prostate cancer cells in osteoblastic metastases: development of anti-survival factor therapy for hormone-refractory prostate cancer. Mol Med 2000, 6:251-267 [PMC free article] [PubMed] [Google Scholar]
- 36.Vella V, Sciacca L, Pandini G, Mineo R, Squatrito S, Vigneri R, Belfiore A: The IGF system in thyroid cancer: new concepts. Mol Pathol 2001, 54:121-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butt AJ, Firth SM, Baxter RC: The IGF axis and programmed cell death. Immunol Cell Biol 1999, 77:256-262 [DOI] [PubMed] [Google Scholar]
- 38.Pratt WB: The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 1998, 217:420-434 [DOI] [PubMed] [Google Scholar]
- 39.Nimmanapalli R, O’Bryan E, Bhalla K: Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 2001, 61:1799-1804 [PubMed] [Google Scholar]
- 40.Clarke PA, Hostein I, Banerji U, Stefano FD, Maloney A, Walton M, Judson I, Workman P: Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene 2000, 19:4125-4133 [DOI] [PubMed] [Google Scholar]
- 41.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A: The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med 1999, 190:1025-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Lobito AA, Shen F, Hornung F, Winoto A, Lenardo MJ: Inhibition of Fas-mediated apoptosis by the B cell antigen receptor through c-FLIP. Eur J Immunol 2000, 30:155-163 [DOI] [PubMed] [Google Scholar]
- 43.Medema JP, de Jong J, van Hall T, Melief CJ, Offringa R: Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med 1999, 190:1033-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J: Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388:190-195 [DOI] [PubMed] [Google Scholar]
- 45.Mitsiades N, Mitsiades CS, Poulaki V, Anderson KC, Treon SP: Intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human multiple myeloma cells. Blood 2002, 99:2162-2171 [DOI] [PubMed] [Google Scholar]
- 46.Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P: Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res 1999, 59:2747-2753 [PubMed] [Google Scholar]
- 47.Mitsiades CS, Koutsileris M: Molecular biology and cellular physiology of refractoriness to androgen ablation therapy in advanced prostate cancer. Exp Opin Invest Drugs 2001, 10:1099-1115 [DOI] [PubMed] [Google Scholar]
- 48.Mitsiades CS, Mitsiades N, Poulaki V, Richardson PG, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Munshi NC, Treon SP, Anderson KC: Activation of NF-kB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene (in press) [DOI] [PubMed]
- 49.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS: NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281:1680-1683 [DOI] [PubMed] [Google Scholar]
- 50.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW: Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA 1997, 94:10057-10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J: Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med 1998, 188:211-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW: NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem 2000, 275:33777-33781 [DOI] [PubMed] [Google Scholar]
- 53.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC: IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998, 58:5315-5320 [PubMed] [Google Scholar]
- 54.Saito J, Kohn AD, Roth RA, Noguchi Y, Tatsumo I, Hirai A, Suzuki K, Kohn LD, Saji M, Ringel MD: Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid 2001, 11:339-351 [DOI] [PubMed] [Google Scholar]
- 55.De Vita G, Berlingieri MT, Visconti R, Castellone MD, Viglietto G, Baldassarre G, Zannini M, Bellacosa A, Tsichlis PN, Fusco A, Santoro M: Akt/protein kinase B promotes survival and hormone-independent proliferation of thyroid cells in the absence of dedifferentiating and transforming effects. Cancer Res 2000, 60:3916-3920 [PubMed] [Google Scholar]
- 56.Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M: Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 2001, 61:6105-6111 [PubMed] [Google Scholar]
- 57.Panka DJ, Mano T, Suhara T, Walsh K, Mier JW: Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem 2001, 276:6893-6896 [DOI] [PubMed] [Google Scholar]
- 58.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS: Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem 2001, 276:10767-10774 [DOI] [PubMed] [Google Scholar]
- 59.Munster PN, Srethapakdi M, Moasser MM, Rosen N: Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res 2001, 61:2945-2952 [PubMed] [Google Scholar]
- 60.Bretz JD, Arscott PL, Myc A, Baker JR, Jr: Inflammatory cytokine regulation of Fas-mediated apoptosis in thyroid follicular cells. J Biol Chem 1999, 274:25433-25438 [DOI] [PubMed] [Google Scholar]
- 61.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K: Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001, 7:94-100 [DOI] [PubMed] [Google Scholar]
- 62.Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL: Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol 1999, 163:5358-5366 [PubMed] [Google Scholar]
- 63.Johnsen AC, Haux J, Steinkjer B, Nonstad U, Egeberg K, Sundan A, Ashkenazi A, Espevik T: Regulation of APO-2 ligand/trail expression in NK cells-involvement in NK cell-mediated cytotoxicity. Cytokine 1999, 11:664-672 [DOI] [PubMed] [Google Scholar]
- 64.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H: Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol 1999, 163:1906-1913 [PubMed] [Google Scholar]
- 65.Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stoppacciaro A, Ruco L, Farina F, Zummo G, De Maria R: Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol 2000, 1:483-488 [DOI] [PubMed] [Google Scholar]