Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR)-mediated secretion of an electrolyte-rich fluid is a major but incompletely understood function of the salivary glands. We provide molecular evidence that guanylin, a bioactive intestinal peptide involved in the CFTR-regulated secretion of electrolyte/water in the gut epithelium, is highly expressed in the human parotid and submandibular glands and in respective clinically most relevant tumors. Moreover, in the same organs we identified expression of the major components of the guanylin signaling pathway, ie, guanylin-receptor guanylate cyclase-C, cGKII, and CFTR, as well as of the epithelial Cl−/HCO3− anion exchanger type 2 (AE2). At the cellular level, guanylin is localized to epithelial cells of the ductal system that, based on its presence in the saliva, is obviously released into the salivary gland ducts. The guanylin-receptor guanylate cyclase-C, cGKII, CFTR, and AE2 are all confined exclusively to the apical membrane of the same duct cells. These findings implicate guanylin as intrinsic regulator of electrolyte secretion in the salivary glands. We assume that duct epithelial cells synthesize and release guanylin into the saliva to regulate electrolyte secretion in the ductal system by an intraductal luminocrine signaling pathway. Moreover, the high expression of guanylin in pleomorphic adenoma and Warthin tumors (cystadenolymphoma), the most common neoplasms of salivary glands, predicts guanylin as a significant marker in tumor pathology.
The ductal system in the salivary glands is the site of modification and secretion of an electrolyte-rich fluid, the mechanism of secretion, however, is poorly understood. 1 Because the electrolyte secretion in these glands is impaired in cystic fibrosis (CF), 2 it became evident that the cystic fibrosis transmembrane conductance regulator (CFTR) protein as the CF gene product is immediately involved in the secretory function. 2,3 In various epithelial cells, CFTR acts as a Cl− channel 2,3 and serves as regulator of various transporters including Na+-HCO3− co-transporter and Cl−/HCO3− exchanger. 4-6 Intracellularly, CFTR is regulated by the cAMP/cAMP-dependent protein kinase pathway. 3 However, the role of cGMP or the identity of peptides using this second messenger for salivary gland secretory function remained enigmatic. 1 In this respect, recent studies in the intestine demonstrated that the newly discovered peptides guanylin and uroguanylin 7,8 regulate via cGMP epithelial electrolyte/water transport. 8,9 Both peptides are structurally related to heat-stable enterotoxins (STa) that cause secretory diarrhea when secreted into the intestine by pathogenic strains of Escherichia coli. 7,8 Guanylate cyclase-C (GC-C), originally identified as the STa-receptor, 10 represents the genuine receptor for the endogenous ligand guanylin 11 that elicits an increase in intracellular cGMP on stimulation. 7,9
Since the discovery that the guanylin or STa effect on Cl− conductance or on epithelial HCO3− secretion disappears in the small intestine of transgenic CF-mice 9,12 it became evident that CFTR is decisively involved in the guanylin-mediated Cl− and HCO3− secretion. In addition to the well-established cAMP/cAMP-dependent protein kinase pathway in the regulation of CFTR, 3 the recently detected cGMP-dependent protein kinase II (cGKII) 13 also phosphorylates and thus activates the CFTR Cl− channel 13,14 in terms of epithelial net chloride and water secretion. The physiological impact of cGKII has been reinforced by studies in cGKII-deficient mice demonstrating lack of guanylin and STa effect on Cl− conductance in the intestinal epithelium; 14 however, in the intestine cGKII- or CFTR-independent signaling pathways may also exist for the guanylin-mediated regulation of anion secretion. 15,16 Moreover, it is well documented that CFTR serves as regulator for a Cl−/HCO3− exchanger 5 to promote luminal HCO3−-secretion as demonstrated in the submandibular gland or in the pancreas, 5,6 although the molecular identity of the anion exchanger as yet remained undefined.
In view of the fact that CFTR is highly expressed in salivary glands 17 and that, as demonstrated in the intestinal epithelium 9,12 and pancreas, 18 the peptide guanylin regulates CFTR function, we comparatively analyzed in the present study the human parotid and submandibular glands for expression and cell-specific localization of guanylin, guanylin receptor GC-C, cGKII, and CFTR. The investigations particularly included the anion exchanger isoform AE2 as the major Cl−/HCO3− exchanger candidate of epithelial HCO3− secretion. 19,20 Localization studies at the cellular level were particularly aimed to identify the cellular origin of guanylin and its target cells and membranes to analyze the intercellular signaling route of guanylin in situ. Expression of guanylin and its localization in distinct cells of pleomorphic adenoma and Warthin tumors of the parotid gland may indicate the potential role of guanylin in the pathology of salivary gland neoplasms.
Materials and Methods
Tissues and Tissue Preparation
Tissues of salivary glands (parotid gland, n = 12; submandibular gland, n = 4) used in this study were obtained after operation in patients suffering from pleomorphic adenoma or Warthin tumor (cystadenolymphoma) of the salivary glands. The operations were performed at the Department of Otorhinolaryngology, Philipps University (Marburg, Germany). Neoplastic tissues were resected from the center of the respective tumors to exclude contamination with normal tissue. Normal tissues were taken outside the tumors and were both macroscopically and histologically normal. After resection, the tissues were fixed in different fixatives for immunohistochemistry or immediately frozen in liquid nitrogen for Western blot and reverse transcriptase-polymerase chain reaction (RT-PCR) analyses. Gastrointestinal tissues from guinea pig (n = 3) and rat (n = 3) served as reference organs that were treated similarly.
RT-PCR Analyses
Table 1 ▶ show the primers that were constructed and used based on the GenBank cDNA sequences (see respective database accession numbers).
Table 1.
Primers Constructed and Used Based on GenBank Sequences
| Primer sets | Corresponding positions in the cDNA of the respective proteins | |
|---|---|---|
| Guanylin | 5′-AGT GGG CAC AAG GAG TAT GG | 334–353 |
| (no. M97496) | 5′-TGG CAG TTC TGA CTC ACC TG | 457–476 |
| GC-C | 5′-TGA AAT CTG TGC CTA CGC TG | 9–28 |
| (no. M73489) | 5′-TAG GTT GAG CTG CTG GGA GT | 120–139 |
| cGKII | 5′-CTG GGA ACC TCA CCA CTG AT | 67–86 |
| (no. X94612) | 5′-GGA AAA TTC AGG GGG TTT GT | 424–443 |
| CFTR | 5′-CGA CAG GGT GAA GCT CTT TC | 4464–4483 |
| (no. M28668) | 5′-TCT GGC TTG CAA AAC ACA AG | 4824–4843 |
| AE2 | 5′-CAC GCT CAT CAT CTC CAA GA | 3121–3140 |
| (no. X62137) | 5′-ATG CAG CCG CTC ATA GAA CT | 3477–3496 |
The primers displayed no homology to any previous reported sequences. Using the RNeasy kit (Qiagen, Hilden, Germany), total RNA was isolated from tissues and reverse-transcribed into cDNA with oligo(dT) primer (Promega, Madison, WI). PCR was performed in a thermocycler (MWG-Biotech, Ebersberg, Germany) using 50 ng of template cDNA. The PCR products were subsequently separated in a 1.8% agarose gel. The product length was identified by staining with ethidium bromide and the expected sizes of ∼131 bp for guanylin, ∼143 bp for GC-C, ∼377 bp for cGKII, ∼380 bp for CFTR, and ∼376 bp for AE2 were obtained. Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was used as control. The amplification of genomic DNA was excluded by appropriate controls.
Peptide Synthesis, Immunization Procedure, and Antisera
The following peptides were synthesized as detailed 21 referring to the published human proguanylin sequence: 22 proguanylin-(25-37), proguanylin-(34-46), proguanylin-(101-115). Rabbits (New Zealand White, five for each antigen) and four chickens (for proguanylin-(34-46) were immunized subcutaneously with the respective peptides. 21,23 The titer of the generated antibodies were checked by enzyme-linked immunosorbent assay. Out of the antibodies raised, the antibodies K39 [directed against proguanylin-(25-37)], K42 [directed against proguanylin-(34-46)], K605, and K606 [each directed against proguanylin-(101-115)] and the chicken antibody H9 [directed against proguanylin-(34-46)] exhibited the highest titers. Except for H9, these antibodies have been used and characterized in detail. 18,21,23,24
From the human GC-C sequence 25 GC-C-(31-45) was synthesized in a linear form and GC-C-(1009-1023) was synthesized as an octameric multiple antigenic peptide on a SMPS 350 automated multiple peptide synthesizer (Zinsser, Frankfurt, Germany) using the standard Fmoc protocol. 21 The purity and sequence of the synthesized peptides were checked by reverse-phase high performance liquid chromatography (C18; Vydac, Hesparia, CA), capillary zone electrophoresis (Biofocus 3000, Bio-Rad), mass spectrometry (Sciesx API III, Perkin-Elmer), automated Edman degradation (model 473A protein sequencer, Applied Biosystems), and amino acid analysis (Aminoquant 1090L, Hewlett-Packard). GC-C-(31-45) (0.5 mg per rabbit) was conjugated to keyhole limpet hemocyanin (Sigma) with carbodiimide as coupling reagent. GC-C-(1009-1023) (0.5 mg of multiple antigenic peptide per rabbit) was dissolved in saline (1 mg/ml). Rabbits (New Zealand White, five for each antigen) were immunized subcutaneously with the respective peptides. The antibodies K735 recognizing GC-C-(31-45) and K741 recognizing GC-C-(1009-1023) were generated according to the protocol published. 21,23
From the human cGMP-dependent protein kinase II (cGKII), 26 from the CFTR protein, 27 and from the anion exchanger AE2 sequence 28 the peptides cGKII-(146-158), cGKII-(734-749), CFTR-(122-136), CFTR-(843-857), and AE2-(1214-1236) were synthesized (Eurogentec, Belgium) using a standard Fmoc protocol on a Rainin Symphony multiple peptide synthesizer. These peptides were coupled to keyhole limpet hemocyanin using m-maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce). For each peptide conjugate two SPF-rabbits (Charles River-Iffa Credo) were immunized (Eurogentec) intradermally with 0.2 mg of peptide conjugate per animal emulsified in Freund’s adjuvant 1:1 (v/v). Booster injections were given every 2 weeks and bleeding was 10 days after each booster injection. The antisera with the highest titers thus generated were EG(1)-PKIIA directed against cGKII-(146-158), EG(2)-PKIIB directed against cGKII-(734-749), EG(1)-CFTR122 directed against CFTR-(122-136), EG(1)-CFTR843 directed against CFTR-(843-857), and EG(1)-AE2 and EG(2)-AE2 both directed against AE2-(1214-1236). The antibody BS-CAII against carbonic anhydrase II (CAII) was purchased from The Binding Site (Birmingham, England).
Extraction of Guanylin from Salivary Glands
Frozen tissues were powdered and boiled in 1 mol/L of acetic acid for 10 minutes. The homogenates were centrifuged at 20,000 × g for 20 minutes at 4°C and the supernatants were filtered through a 0.45-μm (pore size) filter. To concentrate the protein content, total tissue extracts were applied to an octadecasilyl (C18) Sep-Pak cartridge (Waters, MA). The column was washed with 0.01 mol/L of HCl and material was eluted with 30% (v/v) 2-propanol/30% (v/v) methanol/0.01 mol/L HCl. 21,23 The eluted protein fractions were lyophilized and stored at −80°C until use.
Detection of Guanylin in Saliva
Saliva samples were obtained from 10 healthy persons by collecting in sterile tubes. The saliva production was not stimulated. The unpooled samples were centrifuged at 1000 × g for 10 minutes at 4°C, the supernatants were filtered (0.45 μm) and extracted in octadecasilyl (C18) Sep-Pak cartridges as described above.
For detection of immunoreactive guanylin in saliva, extracts of 10-ml samples were separated electrophoretically on 16.5% tricine-sodium dodecyl sulfate-polyacrylamide gels and immunoblotted with guanylin antibodies. The concentration of guanylin in saliva was measured with a guanylin-specific monoclonal antibody 29 by enzyme-linked immunosorbent assay (ELISA) according to the established protocol. 29
Protein Preparation from Tissues and Western Blot Procedure
Proteins from salivary glands were extracted using a Tris-HCl buffer containing 100 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.4, 10% glycerol, 1% Triton X-100, 2 μg/ml leupeptin, 2 μg/ml pepstatin, 1 mmol/L phenylmethyl sulfonyl fluoride for GC-C and cGKII, and 10 mmol/L KCl, 1.5 mmol/L MgCl2, 10 mmol/L Tris-HCl, pH 7.4, 1 mmol/L phenylmethyl sulfonyl fluoride, and 2 μg/ml each of leupeptin and pepstatin for CFTR and AE2. For Western blot analysis, 25 μg of total protein (measured by the Lowry method) from salivary glands was separated on 8 to 12% sodium dodecyl sulfate-polyacrylamide gels dependent on the molecular mass of the various membrane proteins. To detect guanylin, a 16.5% tricine-sodium dodecyl sulfate-polyacrylamide gel was used according to the protocol published. 18 Separated proteins were transferred onto hydrophobic polyvinylidene fluoride-based membranes and incubated with the various antisera at dilutions also used for immunohistochemistry (see below). The respective immunoreactive proteins were visualized after incubation with alkaline phosphatase-conjugated goat anti-rabbit or anti-chicken IgG (each diluted 1:30,000; Sigma) using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as chromogens (Sigma). The immunoreaction in Western blot was specifically blocked after preincubation of the antibodies with the corresponding peptide immunogens. Any crossreactions with the second goat anti-rabbit or anti-chicken antibodies were excluded by appropriate controls. 21,23
Immunohistochemistry
Tissues were fixed overnight in 4% aqueous formalin or in Bouin’s fixative and embedded in paraffin. The sections (4 μm) were immunostained by the avidin-biotin-peroxidase complex technique as detailed: 30,31 sections were incubated with the respective antisera [K39, K42, K605, K606, diluted 1:1000 to 1:3000; H9, 1:7000; K735 and K741, 1:500; EG(1)-PKIIA and EG(2)-PKIIB, EG(1)-AE2 and EG(2)-AE2, EG(1)-CFTR122 and EG(1)-CFTR843, each diluted 1:2000; BS-CAII, 1:1000] overnight at 4°C, followed by incubation with biotinylated anti-rabbit IgG (Jackson ImmunoResearch) or anti-chicken IgY (IgG) (Dianova, Hamburg, Germany) for 30 minutes diluted 1:200. The sections were then incubated for 30 minutes with a preformed complex of biotin-peroxidase/streptavidin (Jackson ImmunoResearch), diluted in phosphate-buffered saline (PBS) (final concentrations: biotin-peroxidase, 0.7 μg/ml; streptavidin, 5 μg/ml). The antigen-antibody-binding sites were visualized by incubation of the sections in 0.7 mmol/L diaminobenzidine hydrochloride/0.002% H2O2 in 0.05 mol/L Tris-HCl (pH 7.6). PBS was used as diluent for the antisera and as rinsing solution.
Specificity Controls
Method-dependent nonspecificities were excluded by running controls as described. 30,31 Specificities of the antibodies were tested by preadsorption of all antisera with homologous and heterologous antigenic peptides (6.25 to 100 μg/ml of the antiserum). 30,31 Preadsorption of the antisera with homologous antigens at concentrations as low as 6.25 μg/ml completely blocked immunostaining in the salivary glands, whereas preadsorption of the antisera with heterologous antigens at concentrations up to 100 μg/ml had no effect on immunostaining.
Results and Discussion
Expression of Guanylin and Its Affiliated Signaling and Effector Proteins in the Human Parotid and Submandibular Glands
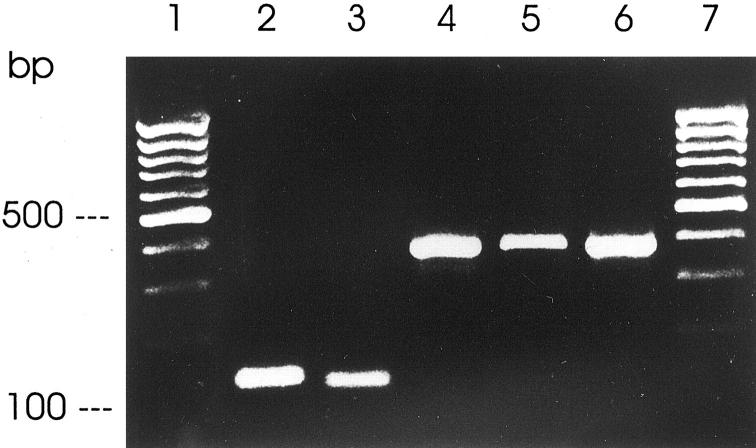
RT-PCR analyses showed that guanylin is highly expressed in the human salivary glands (Figure 1) ▶ . Although recent studies failed to detect expression of the guanylin receptor GC-C in the opossum submandibular gland, 32 the present RT-PCR analyses clearly revealed that not only the guanylin receptor GC-C but also cGKII and CFTR as key proteins of the guanylin-signaling pathway 11-13 are expressed in the human parotid and submandibular glands (Figure 1) ▶ . Likewise, expression of AE2 was also found in both exocrine glands (Figure 1) ▶ .
Figure 1.
RT-PCR analyses of gene expression of guanylin (lane 2), GC-C (lane 3), cGKII (lane 4), CFTR (lane 5), and AE2 (lane 6) in the human submandibular gland with amplification products of correct molecular sizes. One hundred-bp DNA ladder is indicated (lanes 1 and 7).
To verify the presence of the translated proteins in the human parotid and submandibular glands, we raised a bulk of region-specific antisera against guanylin and molecular domain-specific antisera against the various affiliated signaling cascade and effector proteins and used them in Western blotting analyses and immunohistochemistry.
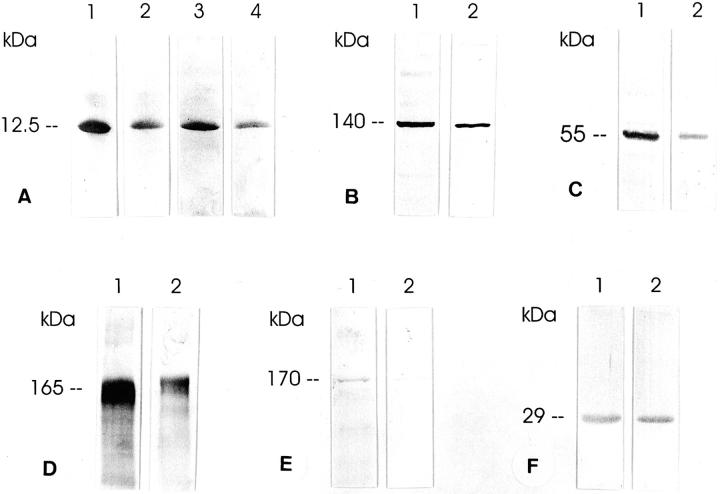
In Western blots, all guanylin antisera recognizing different epitopes of proguanylin concurrently identified the immunoreactive peptide of ∼12.5-kd molecular mass (Figure 2) ▶ that corresponds to that deduced from the respective cDNA sequence. 22
Figure 2.
Western blot analyses of guanylin (A), GC-C (B), cGKII (C), CFTR (D), AE2 (E), and CAII (F) in extracts of the human salivary glands. A: Immunoreactive guanylin of ∼12.5-kd molecular mass was identified by the region-specific antibodies K605 (lanes 1 and 2) and K42 (lanes 3 and 4) in the human parotid (lanes 1 and 3) and submandibular (lanes 2 and 4) glands. B–F: GC-C (antibody K735), cGKII [antibody EG(1)-PKIIA], CFTR [antibody EG(1)-CFTR843], AE2 [antibody EG(1)-AE2], and CAII (antibody BS-CAII) were detected in extracts of the parotid (lanes 1) and submandibular (lanes 2) glands with molecular masses as indicated.
Moreover, the antisera K735 and K741, directed against the extracellular and the intracellular domain of GC-C, respectively, identified the immunoreactive protein at ∼140 kd (Figure 2) ▶ , although the same antisera detected GC-C in the range of ∼130 kd in intestinal extracts (data not shown). This is quite conceivable because both immunoreactive protein bands of respective molecular masses have already been attributed to GC-C. 33,34 These different molecular sizes of GC-C in the intestine versus salivary glands may be because of tissue-specific heterogeneities of GC-C that already have been documented in the digestive tract. 34
The expression of cGKII has been reported mainly in the intestine, where it was identified as an 86-kd protein. 13 The region-specific cGKII antisera coincidentally recognized cGKII in the range of ∼55 kd (Figure 2) ▶ in the human parotid and submandibular glands, although the same antisera detected cGKII as a 86-kd protein in the intestine. 18 Of note, in the rat parotid and submandibular glands the same antisera identified the immunoreactive cGKII protein at 86 kd and additionally at 55 kd (data not shown). In view of the fact that cGKII has a distinct propensity for proteolytic decomposition, 35 the 55-kd cGKII immunoreactive protein may reflect an enzymatic fragment, most likely generated during preparation for Western blotting. Remarkably, in cGKII-transfected cells cGKII has been detected at 50 to 60 kd and 86 kd 35 indicating that the 55-kd immunoreactive protein identified in human salivary gland extracts may certainly represent a physiological molecular variant of cGKII.
Because cGKII serves as a cGMP-dependent alternative regulator of CFTR in the intestine, 13 it is of particular significance that this protein is expressed in the salivary glands where CFTR is also highly expressed. 17 The antisera EG(1)-CFTR122 and EG(1)-CFTR843 specifically identified CFTR as a strongly immunoreactive protein in the expected 3 range of 160 to 170 kd (Figure 2) ▶ . Moreover, two different antisera identified AE2 at ∼170 kd and, in addition, distinctly at ∼140 kd (Figure 2) ▶ . Indeed, AE2 of respective molecular masses has been reported to occur as glycosylated and unglycosylated forms. 19,20 Western blot with the CAII antibody BS-CAII revealed an immunoreactive protein of ∼29 kd (Figure 2) ▶ that is exactly in line with the molecular mass of CAII as shown previously. 36
Cell-Specific Localization of Guanylin in the Human Salivary Glands
The guanylin antisera K39, K42, K605, K606, and H9 directed against different epitopes coincidentally localized guanylin to epithelial cells of the ductal system of the parotid gland as well as of the submandibular gland (Figure 3) ▶ . The acinar cells and the myoepithelial cells completely lacked any guanylin immunoreactivity. Within the ductal system, the guanylin immunoreactive cells were located in the intercalated, intralobular (striated), and interlobular (excretory) ducts. However, the various segments of the ductal network were heterogeneous with respect to their guanylin immunoreactivity; in the interlobular ducts only the secretory columnar cells exhibited immunoreactivity for guanylin, the basally situated reserve cells in the interlobular ducts 37 were unreactive for guanylin. Although the intercalated ducts showed a faint immunoreactivity for guanylin, the striated ducts displayed the strongest guanylin immunoreactivity (Figure 3) ▶ which is why this ductal segment was considered as the main source of guanylin in the salivary glands.
Figure 3.
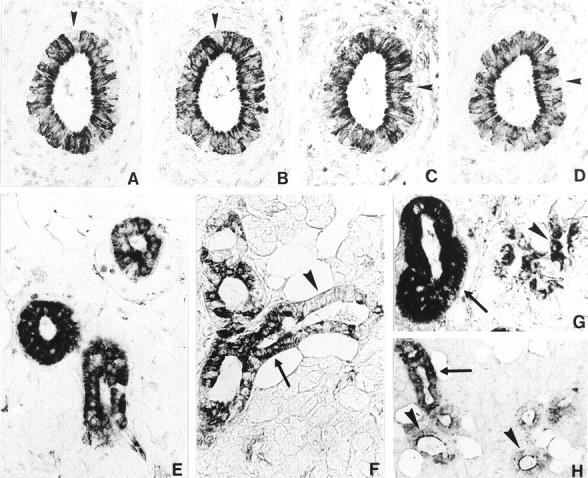
Cellular localization of guanylin in the human parotid gland. A–D: Four serial paraffin sections of the human parotid gland immunostained for guanylin by the region-specific antibodies K42 (A), K605 (B), K39 (C), and K606 (D) using the avidin-biotin-peroxidase complex technique. All antibodies coincidentally localize guanylin to the same duct cells. Note that some basally situated cells (A–D, arrowheads) in the interlobular ducts are completely unreactive for guanylin. E and F: Guanylin immunoreactivities of varying densities are demonstrated in ducts of the same lobule (E) immunostained by the antibody H9. Even in the same ducts (F) immunostained by the antibody K605, intercellular differences of guanylin immunoreactivities exist showing strongly (arrow) and faintly immunoreactive or unreactive (arrowhead) epithelial cells. G: Guanylin immunoreactivity is not only present in the striated ducts (arrow), but also in the intercalated ducts (arrowhead). H: In some ducts guanylin immunoreactivity is distributed within the cytoplasm of the epithelial cells (arrow), but in others this immunoreactivity is strongly concentrated toward the apical pole of the respective cells (arrowheads). Original magnifications: ×360 (A–G); ×180 (H).
Remarkably, all guanylin antisera produced a granular immunoreactivity pattern assuming localization of the peptide in small secretory vesicles of the respective cells that have been already identified in these cells by electron microscopy. 38 Notably, distinct intercellular differences exist between the epithelial cells even of the same duct with respect to the density of guanylin immunoreactivity that may reflect intercellular differences in expression or secretion of guanylin. Moreover, in some ducts immunoreactivity for guanylin was localized in the whole cytoplasm of the epithelial cells whereas in other ducts strong guanylin immunoreactivity was concentrated at the apical pole of the secretory cells (Figure 3) ▶ . This peculiar distribution pattern of guanylin at the cellular level may assume a luminally directed release of guanylin.
Detection of Guanylin in the Saliva
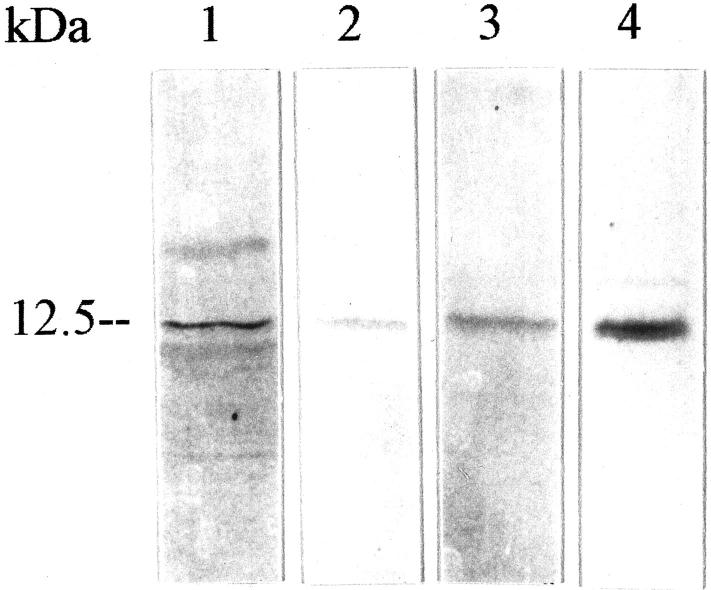
The potential release of guanylin into the saliva was substantiated by Western blotting studies. The region-specific guanylin antisera coincidentally identified a strongly labeled band of correct molecular mass 18,22 that co-migrated exactly with the immunoreactive guanylin as in salivary gland tissue extracts (Figure 4) ▶ . To measure the range of guanylin concentration in human saliva, a sensitive ELISA with a detection sensitivity of 5 pmol/ml was developed. ELISA analyses with a monoclonal guanylin antibody, which was already successfully used in ELISA experiments, 29 revealed a high concentration of guanylin in the range from 9.8 to 25.3 nmol per ml saliva (n = 10) (mean ± SE; 15.08 ± 5.65 nmol/ml). This concentration is considerably higher than the reported guanylin concentration in the circulation. 24 Based on these findings we conclude that salivary duct cells release the bioactive peptide guanylin into the saliva. Such a luminal secretion route is quite conceivable because the guanylin-receptor GC-C is immediately localized to the apical membrane of neighbored duct epithelial cells (see below).
Figure 4.
Western blot analyses of guanylin in human parotid gland (lanes 1 and 4) and saliva (lanes 2 and 3) with the antibodies K42 (lanes 1 and 2) and K 605 (lanes 3 and 4). Note the co-migration of the respective guanylin immunoreactivities.
Cell- and Membrane-Specific Localization of the Guanylin Receptor GC-C, cGKII, CFTR Cl− Channel, and AE2
The immunolabeling characteristics of all antisera against GC-C, cGKII, CFTR, and AE2 were verified in gastrointestinal specimens used as reference organs. Except for AE2, they all localized the respective proteins in the brush border of enterocytes (data not shown), exactly where they have been reported to occur. 13,17,34 With regard to AE2, the region-specific AE2 antisera identified the respective protein at the basolateral membrane of gastric parietal cells; this particular localization of AE2 is completely in accordance with previous findings. 19,20
The human parotid and submandibular glands were investigated immunohistochemically for the cellular localization of GC-C, cGKII, CFTR, and AE2 with particular focus on their organization at distinct functional membrane domains of the respective cells. In both organs, the molecular domain-specific GC-C antisera K735 and K741 coincidentally localized the guanylin-receptor to the epithelial cells of striated and interlobular ducts where GC-C was specifically confined to the apical cell membrane (Figure 5) ▶ . In no case was GC-C immunoreactivity found at the basolateral membrane domain. This site of location of GC-C unequivocally pleads for a luminal activation of this receptor by guanylin in the ductal system.
Figure 5.
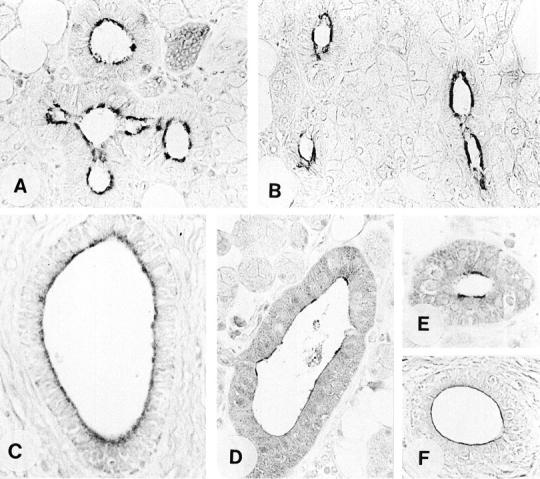
Immunohistochemical localization of GC-C (A, B), cGKII (C), CFTR (D, E), and AE2 (F) in the human parotid gland by the avidin-biotin-peroxidase complex technique. Note that immunoreactivity for all components is exclusively confined to the apical membrane of the duct cells [A, antibody K735; B, K741; C, EG(1)-PKIIB; D, EG(1)-CFTR122; E, EG(1)-CFTR843; F, EG(1)-AE2]. Original magnifications, ×360.
Coincident with the cellular distribution of GC-C, region-specific antisera localized CFTR at the apical membrane domain of the striated and interlobular duct cells (Figure 5) ▶ . This finding fully corresponds to previous data showing that CFTR is confined to the apical pole of striated duct epithelial cells of rat submandibular gland. 39
Based on the data in the intestine, cGKII is co-expressed with CFTR to activate the CFTR Cl− channel function. 13 In accordance with this finding, the present immunohistochemical studies revealed that cGKII is co-localized with GC-C and CFTR to the striated and interlobular duct epithelial cells of the human salivary glands (Figure 5) ▶ . Notably, the immunoreactivity for cGKII was concentrated to the apical membrane of the respective cells where GC-C and CFTR were also confined. This type of spatial organization at the cellular level may be of functional significance as previous studies could show that targeting of cGKII to the apical membrane and its close proximity to CFTR is required for phosphorylation and activation of CFTR Cl− channel function. 40
Corresponding to the localization of GC-C, cGKII, and CFTR, AE2 was detected in the striated and interlobular segments of the ductal network where it was also confined to the apical membrane of the respective duct cells (Figure 5) ▶ despite the fact that the same antibodies identify the protein at the basolateral membrane of gastric parietal cells. 18-20 Previous studies in the human salivary glands yielded contradictory data by localization of AE2 to apical or basolateral membranes of duct cells or to basolateral membrane of acinar cells. 41,42 In contrast to these findings, in the present study no immunoreactivity for AE2 was found at the basolateral membrane of the duct cells and in no case was immunoreactivity found in the acinar cells. Both antisera against AE2 exhibited a clear immunostaining restricted to the apical membrane of striated and interlobular duct cells. This specific localization of AE2 is in accordance with the functionally characterized apical Cl−/HCO3− exchanger in ducts of the salivary glands. 1,42,43 In this respect, the human parotid gland produces a final secretion that becomes HCO3−-rich and Cl−-poor as secretion rate is increased 1 which argues additionally for a luminal Cl−/HCO3− exchanger. Based on our findings, AE2 is at least one anion exchanger isoform candidate involved in Cl−/HCO3− exchange in the salivary glands. The co-localization of AE2 and CFTR in the apical membrane of duct cells is completely in line with the fact that CFTR ultimately regulates luminal Cl−/HCO3− exchanger in the submandibular gland, 6 provided that CFTR is correctly folded and positioned in the plasma membrane. 5,6 Intracellularly, the transportable bicarbonate ions are generated from hydration of CO2 catalyzed by carbonic anhydrase. 1 In line with previous findings, 44 we detected CAII in the same ductal segments of the salivary glands (data not shown) where AE2 has also been localized suggesting a functional coupling of both proteins as verified for AE1 and CAII. 45
Expression of Guanylin in Pleomorphic Adenoma and in Warthin Tumors
The salivary glands are afflicted by a greater variety of neoplasms than any other organ system in the body. 46 Approximately 80% of all salivary gland tumors occur in the parotid gland, where pleomorphic adenoma and Warthin tumors are the most common salivary neoplasms. 37 Although the pleomorphic adenoma originates from epithelial cells, 47 the cellular origin of the Warthin tumor is uncertain, 37 although current theory suggests that the tumor arises from heterotopic salivary tissue trapped within a lymph node during embryogenesis. 46
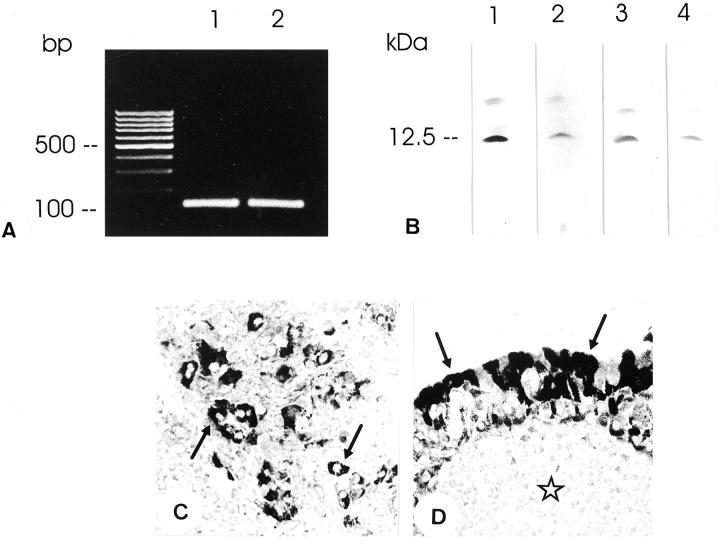
RT-PCR analyses showed that guanylin is expressed not only in normal salivary gland tissues, but also in pleomorphic adenoma (n = 3) and in Warthin tumors (n = 3) of the parotid gland (Figure 6) ▶ . The existence of guanylin in these tumors at the translational level was verified by Western blotting analyses. In extracts of tumors, guanylin antisera identified the immunoreactive peptide of ∼12.5-kd molecular mass that co-migrated with the immunoreactive band observed in normal tissues (Figure 6) ▶ . Using immunohistochemistry, all region-specific guanylin antisera displayed cytoplasmic immunoreactivity of guanylin exclusively in neoplastic epithelial cells in pleomorphic adenomas (n = 7) and in Warthin tumors (n = 3) (Figure 6) ▶ . The fact that guanylin was found in duct cells of the salivary glands and in epithelial cells of gland tumors supports the view that this part of the gland is the origin of a neoplastic proliferation. 37 With regard to the uncertain cellular origin, the presence of guanylin in Warthin tumors may indicate that this tumor type originates from the duct cells. Previous studies have assumed a role of guanylins in tumor tissues. Although the localization of guanylin is reduced 48 or absent 49 in intestinal tumors in mice and humans, high expression of guanylin is seen in the present study in the salivary gland tumors. These differences may be because of tumor-specific expression and function of guanylins. Although our data suggest similar electrolyte/water secretion regulatory effects of guanylin in the salivary glands as in the intestine or pancreas, the nonsecretory anti-tumor actions of guanylin 50,51 may be nevertheless different in intestinal versus salivary gland tumors.
Figure 6.
A: RT-PCR analysis of gene expression of guanylin in the normal human parotid gland (lane 1) and in pleomorphic adenoma (lane 2) of the same patient with amplification products of correct molecular size. One hundred-bp DNA ladder is indicated (lane L). B: Western blot analysis of guanylin (lanes 1 and 2, antibody K605; lanes 3 and 4, antibody K42) in normal parotid gland (lanes 1 and 3) and in pleomorphic adenoma (lanes 2 and 4) of the same patient. Note the predominant guanylin immunoreactivity at ∼12.5 kd in both tissue extracts. C: Immunohistochemistry in pleomorphic adenoma localizes strong guanylin immunoreactivity (antibody K605) in tumor epithelial cells (arrows). D: In Warthin tumors guanylin immunoreactivity is present in the outer layer of oncocytes (arrows) (antibody K605). No immunoreactivity is detected in the lymphoid stroma (asterisk). Original magnifications, ×360 (C, D).
Conclusions
In the salivary glands, the regulation of composition and secretion of the saliva is incompletely understood although the role of various neural and humoral stimulants using cAMP or Ca2+ has been documented in this instance. 1 However, the function of cGMP in the salivary gland secretory activity and particularly regulatory peptides using this second messenger remained as yet undefined. 1 Based on the present findings it is evident that not only guanylin but also its receptor GC-C, cGKII, CFTR, and AE2 are expressed in the salivary glands assuming functional coupling of these proteins in terms of guanylin-regulated electrolyte secretion. Thus, in line with its functional role in the intestine and pancreas 9,12,14,18 we expect that guanylin regulates CFTR Cl− channel function via GC-C, cGMP, and cGKII also in salivary glands. The integrity and specificity of the guanylin-signaling cascade have been clearly documented by studies showing that the guanylin effect on Cl− conductance primarily disappears in transgenic CF-mice and in cGKII-deficient mice. 12,14-16 Therefore, CFTR as a key protein in electrolyte secretion 2,3 was considered as a major effector of the guanylin signaling pathway. 12 In addition, the regulatory effect of CFTR on bicarbonate secretion in the submandibular glands by activation of the luminal Cl−/HCO3− exchange is functionally well characterized 6 although the molecular identity of the anion exchanger isoform involved as yet remains unclear. In this respect, the expression of AE2 and particularly its co-localization with CFTR to the apical membrane of salivary gland duct cells may indicate that AE2 is the CFTR-dependent Cl−/HCO3− exchanger isoform significantly promoting bicarbonate secretion in the salivary glands. Based on the presence of guanylin in secretory duct cells and in the saliva and the localization of the guanylin receptor GC-C, cGKII, CFTR, and AE2 restricted to the apical membrane of the duct cells, we assume that guanylin as an intrinsic regulatory peptide exerts its function by a luminocrine route of signaling. Hence, the duct epithelial cells may predictably release guanylin intraductally (luminally) into the saliva to activate its receptor that is immediately located on the apical membrane of the same or neighbored duct epithelial cells. Thus, in addition to the ion-transporting characteristics, one function of human salivary gland duct cells is apparently synthesis and secretion of the regulatory peptide guanylin that by its high expression in salivary gland tumors may also be of diagnostic value as marker in tumor pathology. 47 Because the conventional histology-based diagnoses of epithelial and myoepithelial tumors of salivary glands mostly bear difficulties, we assume that guanylin may be useful in differentiation and identification of the cellular origin of the tumors.
Acknowledgments
We thank Mrs. A. Fiedler, Ch. Merte-Grebe, and T. Seitz for their expert technical assistance.
Footnotes
Address reprint requests to Prof. Dr. Y. Cetin, Abt. Molekulare Zellbiologie, Institut für Anatomie und Zellbiologie, Philipps-Universität Marburg, Robert-Koch-Str. 6, D-35033 Marburg, Germany. E-mail: cetiny@mailer.uni-marburg.de.
Supported by grants from the Deutsche Forschungsgemeinschaft, Stiftung P. E. Kempkes Marburg, and Research Pool of the Philipps University Marburg.
References
- 1.Cook DI, van Lennep EW, Roberts ML, Young JA: Secretion by the major salivary glands. Johnson LR eds. Physiology of the Gastrointestinal Tract. 1994:pp 1061-1117 Raven Press, New York
- 2.Quinton PM: Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 1999, 79:3-22 [DOI] [PubMed] [Google Scholar]
- 3.Fuller CM, Benos DJ: CFTR! Am J Physiol 1992, 263:C267-C286 [DOI] [PubMed] [Google Scholar]
- 4.Shumaker H, Amlal H, Frizzell R, Ulrich CD, Soleimani M: Pancreatic bicarbonate secretion: role of CFTR and the sodium-bicarbonate cotransporter. Am J Physiol 1999, 276:C16-C25 [DOI] [PubMed] [Google Scholar]
- 5.Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, Muallem S: Regulation of Cl−/HCO3− exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 3T3 and HEK 293 cells. J Biol Chem 1999, 274:3414-3421 [DOI] [PubMed] [Google Scholar]
- 6.Lee MG, Choi LY, Luo X, Strickland E, Thomas PJ, Muallem S: Cystic fibrosis transmembrane conductance regulator regulates luminal Cl−/HCO3− exchange in mouse submandibular and pancreatic ducts. J Biol Chem 1999, 274:14670-14677 [DOI] [PubMed] [Google Scholar]
- 7.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE: Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA 1992, 89:947-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, Duffin KL, Siegel NR, Currie MG: Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci USA 1993, 90:10464-10468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M: A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 1997, 505:411-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz S, Green CK, Yuen PST, Garbers DL: Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 1990, 63:941-948 [DOI] [PubMed] [Google Scholar]
- 11.Garbers DL: Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell 1992, 71:1-4 [DOI] [PubMed] [Google Scholar]
- 12.Cuthbert AW, Hickman ME, MacVinish LJ, Evans MJ, Colledge WH, Ratcliff R, Seale PW, Humphrey PPA: Chloride secretion in response to guanylin in colonic epithelia from normal and transgenic cystic fibrosis mice. Br J Pharmacol 1994, 112:31-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, de Jonge HR: Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci 1997, 22:307-312 [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fässler R: Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 1996, 274:2082-2086 [DOI] [PubMed] [Google Scholar]
- 15.Joo NS, London RM, Kim HD, Forte LR, Clarke LL: Regulation of intestinal Cl- and HCO3- secretion by uroguanylin. Am J Physiol 1998, 274:G633-G644 [DOI] [PubMed] [Google Scholar]
- 16.Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, de Jonge HR: Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology 2000, 118:108-114 [DOI] [PubMed] [Google Scholar]
- 17.Trezise AEO, Buchwald M: In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 1991, 353:434-437 [DOI] [PubMed] [Google Scholar]
- 18.Kulaksiz H, Schmid A, Hönscheid M, Eissele R, Klempnauer J, Cetin Y: Guanylin in the human pancreas: a novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system. Histochem Cell Biol 2001, 115:131-145 [DOI] [PubMed] [Google Scholar]
- 19.Kopito RR: Molecular biology of the anion exchanger gene family. Int Rev Cytol 1990, 123:177-199 [DOI] [PubMed] [Google Scholar]
- 20.Alper SL: The band 3-related AE anion exchanger gene family. Ann Rev Physiol 1991, 53:549-564 [DOI] [PubMed] [Google Scholar]
- 21.Cetin Y, Kuhn M, Kulaksiz H, Adermann K, Bargsten G, Grube D, Forssmann WG: Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase-activating peptide. Proc Natl Acad Sci USA 1994, 91:2935-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Sauvage FJ, Keshav S, Kuang WJ, Gillett N, Henzel W, Goeddel DV: Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci USA 1992, 89:9089-9093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cetin Y, Kulaksiz H, Redecker P, Bargsten G, Adermann K, Grube D, Forssmann WG: Bronchiolar nonciliated secretory (Clara) cells: source of guanylin in the mammalian lung. Proc Natl Acad Sci USA 1995, 92:5925-5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn M, Kulaksiz H, Adermann K, Rechkemmer G, Forssmann WG: Radioimmunoassay for circulating human guanylin. FEBS Lett 1994, 318:205-209 [DOI] [PubMed] [Google Scholar]
- 25.de Sauvage FJ, Camerato TR, Goeddel DV: Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem 1991, 266:17912-17918 [PubMed] [Google Scholar]
- 26.Fujii M, Ogata T, Takahashi E, Yamada K, Nakabayashi K, Oishi M, Ayusawa D: Expression of the human cGMP-dependent protein kinase II gene is lost upon introduction of SV 40 T antigen or immortalization in human cells. FEBS Lett 1995, 375:263-267 [DOI] [PubMed] [Google Scholar]
- 27.Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC: Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989, 245:1066-1073 [DOI] [PubMed] [Google Scholar]
- 28.Gehrig H, Müller W, Appelhans H: Complete nucleotide sequence of band 3 related anion transport protein AE2 from human kidney. Biochem Biophys Acta 1992, 1130:326-328 [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Adermann K, Forssmann WG, Kuhn M: Regulated, side-directed secretion of proguanylin from isolated rat colonic mucosa. Endocrinology 1999, 140:5022-5029 [DOI] [PubMed] [Google Scholar]
- 30.Cetin Y, Bargsten G, Grube D: Mutual relationships between chromogranins A and B and gastrin in individual gastrin cells. Proc Natl Acad Sci USA 1992, 89:2912-2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cetin Y, Aunis D, Bader MF, Galindo E, Jörns A, Bargsten G, Grube D: Chromostatin, a chromogranin A-derived bioactive peptide, is present in human pancreatic insulin (β) cells. Proc Natl Acad Sci USA 1993, 90:2360-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause WJ, London RM, Freeman RH, Forte LR: The guanylin and uroguanylin peptide hormones and their receptors. Acta Anat 1997, 160:213-231 [DOI] [PubMed] [Google Scholar]
- 33.Vaandrager AB, Schulz S, de Jonge HR, Garbers DL: Heat stable enterotoxin activation of immunopurified guanylyl cyclase C. Modulation by adenine nucleotides. J Biol Chem 1993, 268:2174-2179 [PubMed] [Google Scholar]
- 34.Scheving LA, Russell WE, Chong KM: Structure, glycosylation, and localization of rat intestinal guanylyl cyclase C: modulation by fasting. Am J Physiol 1996, 271:G959-G968 [DOI] [PubMed] [Google Scholar]
- 35.Vaandrager AB, Edixhoven M, Bot AGM, Kroos MA, Jarchau T, Lohmann S, Genieser HG, de Jonge HR: Endogenous type II cGMP-dependent protein kinase exists as a dimer in membranes and can be functionally distinguished from the type I isoforms. J Biol Chem 1997, 272:11816-11823 [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm B, Keppler C, Hoffbauer G, Lottspeich F, Linder D, Meinhardt A, Aumüller G, Seitz J: Cytoplasmic carbonic anhydrase II of rat coagulating gland is secreted via the apocrine export mode. Histochem Cytochem 1998, 46:505-511 [DOI] [PubMed] [Google Scholar]
- 37.Speight PM, Tinkler S: McGee JOD Isaacson PG Wright NA eds. Textbook of Pathology. 1992:pp 1067-1081 Oxford University Press, New York
- 38.Leeson CR: Structure of salivary glands. Code CF eds. Handbook of Physiology, Section 6: Alimentary Canal. 1967:pp 463-495 American Physiological Society, Washington DC
- 39.Webster P, Vanacore L, Nairn AC, Marino CR: Subcellular localization of CFTR to endosomes in a ductal epithelium. Am J Physiol 1994, 267:C340-C348 [DOI] [PubMed] [Google Scholar]
- 40.Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EME, Bot AGM, Edixhoven M, Boomaars WEM, Lohmann SM, de Jonge HR: Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proc Natl Acad Sci USA 1998, 95:1466-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez JJ, Vazquez M, Idoate MA, Montuenga L, Martinez-Anso E, Castillo JE, Garcia N, Medina JF, Prieto J: Anion exchanger immunoreactivity in human salivary glands in health and Sjögren’s syndrome. Am J Pathol 1995, 146:1422-1432 [PMC free article] [PubMed] [Google Scholar]
- 42.Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F: Immunolocalization of anion exchanger AE2 and Na+-HCO3− cotransporter in rat parotid and submandibular glands. Am J Physiol 1999, 277:G1288-G1296 [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Xu X, Diaz J, Muallem S: Na+, K+, and H+/HCO3− transport in submandibular salivary ducts. Membrane localization of transporters. J Biol Chem 1995, 270:19599-19605 [PubMed] [Google Scholar]
- 44.Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H: Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 1994, 35:646-650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vince JW, Reithmeier RA: Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl−/HCO3− exchanger. J Biol Chem 1998, 273:28430-28437 [DOI] [PubMed] [Google Scholar]
- 46.Rice DH: Salivary gland disorders. Neoplastic and nonneoplastic. Med Clin North Am 1999, 83:197-218 [DOI] [PubMed] [Google Scholar]
- 47.Seifert G: Diagnose und Prognose der Speicheldrüsentumoren. Mund Kiefer GesichtsChir 1997, 1:252-267 [DOI] [PubMed] [Google Scholar]
- 48.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB: Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun 2000, 273:225-230 [DOI] [PubMed] [Google Scholar]
- 49.Cohen MB, Hawkins JA, Witte DP: Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab Invest 1998, 78:101-108 [PubMed] [Google Scholar]
- 50.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, Boddupalli SS, Currie MG, Forte LR: Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res 2000, 60:5151-5157 [PubMed] [Google Scholar]
- 51.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA: Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci USA 2001, 98:7846-7851 [DOI] [PMC free article] [PubMed] [Google Scholar]