Abstract
Recent work has convincingly demonstrated that adult bone marrow contains cells capable of differentiating into liver epithelial cells in vivo. However, the frequency and time course with which fully functional hepatocytes emerge after bone marrow transplantation remained controversial. Here, we used the fumarylacetoacetate hydrolase knockout mouse to determine the kinetics of hepatocyte replacement after complete hematopoietic reconstitution. Single donor-derived hepatocytes were first detected 7 weeks after lethal irradiation and bone marrow transplantation. Liver disease was not required for this transdifferentiation. In the presence of selective pressure the single cells evolved into hepatocyte nodules by 11 weeks after transplantation and resulted in >30% overall liver repopulation by 22 weeks. The frequency with which hepatocytes were produced was between 10−4 and 10−6, resulting in only 50 to 500 repopulation events per liver. Hepatic engraftment was not observed without previous hematopoietic reconstitution even in the presence of liver injury. In addition, significant liver repopulation was completely dependent on hepatocyte growth selection. We conclude that hepatocyte replacement by bone marrow cells is a slow and rare event. Significant improvements in the efficiency of this process will be needed before clinical success can be expected.
Orthotopic liver transplantation is a proven therapy for many hepatic disorders, but it is associated with high morbidity, mortality, and cost. In addition, the supply of donor livers is severely limiting and many patients die while waiting for a donor organ. In some hepatic disorders, particularly inherited protein deficiencies, only the hepatocytes are involved in the pathophysiology and it is therefore not necessary to replace the entire liver. 1 These diseases are attractive targets for hepatocyte transplantation or gene transfer into hepatocytes. Hepatocyte transplantation has been performed in animal models 2-4 as well as limited human clinical trials, 5,6 but only <1% of the hepatocyte mass could be replaced. This has resulted in poor clinical efficacy of the procedure.
It has been recently shown, however, that transplanted hepatocytes can be selectively expanded in vivo in a process called therapeutic liver repopulation. 1 Hepatocyte selection can be achieved by a variety of genetic and pharmacological manipulations. 7-12 These discoveries have reinvigorated the interest in hepatocyte transplantation and raised that hope that clinically relevant degrees of hepatocyte replacement may be achievable in human patients. Even if in vivo selection of human hepatocytes could be achieved, however, the cells useful for transplantation have to be obtained from the same limited supply of organ donors used for solid organ transplantation. Thus, it would be highly desirable to have a readily available alternate source of cells.
Several recent reports have highlighted the broad developmental potential of bone marrow-derived stem cells and the term “stem cell plasticity” has been coined. Bone marrow contains hematopoietic stem cells (HSCs) 13,14 as well as mesenchymal stem cells 15,16 and multipotent adult progenitor cells. HSCs have been reported to produce not only all of the blood lineages, but also skeletal muscle, 17,18 neurons, 19,20 cardiac muscle, 21,22 pulmonary epithelium, 23 and liver epithelium. 24 The transdifferentiation of bone marrow-derived cells into hepatic cells was first described in the rat, 24 followed by reports for the mouse 25 and also the human. 26,27 Using a genetic model of hereditary tyrosinemia, a metabolic liver disease, we were able to demonstrate that bone marrow transplantation (BMT) could substitute for hepatocyte transplantation and correct the liver disease in this model. 28 We furthermore demonstrated that prospectively isolated HSCs can transdifferentiate into hepatocytes and that HSCs and liver-repopulating cells co-purified when sorting for cell surface markers. This suggested that the HSCs may indeed be plastic and be capable of producing both blood and hepatocytes.
In some reports the degree of hepatocyte replacement achieved after hematopoietic repopulation equaled or exceeded the results obtained by hepatocyte transplantation. 25 Gender-mismatched transplants and Y-chromosome in situ hybridization were the principal methods used in these experiments. In the mouse, hepatocyte replacement levels of >1% were described even in genetically normal animals without any selection other than lethal irradiation. 25 This result corresponded well to the human situation in which at least 1% donor-derived hepatocytes were routinely observed. 26,27,29 These findings suggested that routine bone marrow transplantation may achieve clinically relevant hepatocyte replacement. Some hepatic protein deficiencies, for example hemophilia A and B, would be treatable with BMT.
To determine whether similar levels of cell replacement could be detected by using a hepatocyte-specific marker that differentiates donor and host cells, we here performed detailed quantitative analysis and time course of bone marrow-transplanted fumarylacetoacetate hydrolase (FAH) knockout mice.
Materials and Methods
Mouse Strains and Animal Husbandry
As transplant recipients we used the FAHΔexon 5 strain mice previously described by this lab. 30 Transplant donors were transgenic ROSA-26 mice, a gift from P. Soriano (Fred Hutchinson Cancer Center, Seattle, WA). 31 All transplantation experiments were performed with congenic mice of the 129S4 background. All FAH mutant animals were treated with 2-(2-nitro-4-trifluoro-methylbenzyol)-1,3 cyclohexanedione (NTBC) containing drinking water at a concentration of 7.5 mg/L (a gift from S. Lindstedt, Gøtheborg, Sweden). 32,33 This provides an approximate dose of 1 mg kg−1 body weight per day. For genotyping, polymerase chain reaction was performed with a 3 primer polymerase chain reaction on 200-ng tail-cut DNA as previously described. 30 Animal care and experiments were all in accordance with the Guidelines of the Department of Animal Care at Oregon Health Sciences University.
Bone Marrow Cell Harvest and Transplantation
Donor mice were euthanized by CO2 asphyxiation, the fur saturated with 70% alcohol, and the animal moved into a sterile, laminar flow hood. Bone marrow cells were harvested from both femora by flushing the contents into a collection tube using a 27-gauge needle and RPMI supplemented with penicillin/streptomycin. Unfractionated marrow cells were counted using a Coulter Multisizer Z1 and the cell concentration was adjusted for transplantation into the retro-orbital plexus of anesthetized recipient mice.
The FAH mutant recipient mice were lethally irradiated with a total dose of 1100 cGy (experiment 1) or 1500 cGy in split doses with a 3-hour interval. One day later, cells were injected intravenously into the retro-orbital plexus of anesthetized mice using insulin syringes (Becton Dickinson, Franklin Lakes, NJ). One hundred μl of cells were injected per mouse.
Hepatocyte Selection
FAH mutant recipient mice were kept on NTBC (kindly provided by Dr. Lindstedt, Gothenburg, Sweden) for 3 to 6 weeks bone marrow transplantation. 33 To induce hepatocyte selection NTBC was then stopped until mice had dropped 30% of their initial body weight. After that NTBC was restarted until body weight recovered and then another cycle of NTBC withdrawal was initiated.
Staining of HSCs
Cells were stained as described previously. 13 For KLS (c-kit+, Lin−, Sca+) cells isolated from 129S4 ROSA26 donors, the bone marrow cells were incubated with biotinylated monoclonal antibody (mAb) specific for Sca-I (Pharmingen), then positively selected using the MACS magnetic bead system (Miltenyl Biotec, Auburn, CA). The positively selected cells were stained with phycoerythrin-conjugated lineage markers (Pharmingen), which included the following: RA3–6B2 (B220) for the B lineage marker; RM2-5 (CD2), GK1.5 (CD4), 53-7.3 (CD5), 53.6.7 (CD8), and 145-2C11 (CD3) for T cell markers; RB6-8C5 (GR-1) and M1/70 (CD11b, Mac-1) for myeloid markers; PK136 (NK1.1) for natural killer cells; and Ter119 for erythrocytes. The positively selected cells were also stained with allophycocyanin-conjugated 2B8 (c-kit, Pharmingen) and streptavidin-Cy7APC (Sav-PharRed, Pharmingen). After the final wash, cells were resuspended in a phosphate-buffered saline (PBS)/fetal calf serum buffer that contained propidium iodide (1 mg/ml) to discriminate between viable and nonviable cells.
Purification of HSCs
Isolation of HSCs was accomplished using a fluorescence-activated cell sorter manufactured by Becton Dickinson Immunocytometry Systems. Specifically, the FACSVantage SE is configured with argon, krypton, and helium-neon ion. Data parameters were collected in the list mode data file and were analyzed by the software program Flowjo (www.Treestar.com). Pure populations of sorted HSCs were resorted directly into Eppendorf tubes by an automated cell deposition unit using counter mode.
Hepatocyte Transplantation and Repopulation Time Course
Hepatocytes were isolated from congenic wild-type donors and transplanted as previously described. 9,10 FAH mutant recipient mice were either kept on NTBC until 1 day after transplant or had been off NTBC for 10 days before transplant. The livers of transplanted mice were harvested at various times after hepatocyte transplantation and evaluated by FAH immunohistochemistry as described below. At least three and as many as six animals were harvested for each time point.
Histology and Immune Histology
Liver tissues fixed in 10% phosphate-buffered formalin, pH 7.4, were dehydrated in 100% ethanol and embedded in paraffin wax at 58°C. Four-μm sections were rehydrated and stained with hematoxylin and eosin and with a polyclonal rabbit antibody to rat FAH (graciously provided by Robert Tanguay, University of Laval, Laval, Quebec) or glutamine synthetase. 34 The antibody was diluted in PBS, pH 7.4, and applied at concentrations of 1:300,000 at 37°C for 30 minutes. The glutamine synthetase antibody was used at a dilution of 1:10,000. Endogenous peroxidase activity was blocked with 3% H2O2 and methanol. Avidin and biotin pretreatment was used to prevent endogenous staining. The secondary antibody was biotinylated goat anti-rabbit IgG used at 1:250 dilution (94010, BA-1000; Vector Laboratories, Burlingame, CA). Color development was performed with the AEC detection kit from Ventana Medical Systems, Tucson, AZ (pH 8.0, 85705, catalogue no. 250-020). The staining incubation was at 37°C overnight.
β-Galactosidase Staining
The liver tissues were fixed in 2% formaldehyde and 0.2% glutaraldehyde in PBS for 30 minutes. Then the tissues were washed with PBS for two times and moved to staining medium that contained 1 mg of X-gal in buffer of 5 mmol/L K ferricyanide, 5 mmol/L K ferrocyanide, 2 mmol/L MgCl2, 0.02% Nonidet P-40, and 40 mmol/L Hepes. 35
Calculations of Sample Size and Cell Numbers
If the surface area of the sections analyzed is known, the number of total hepatocytes scored can be calculated based on assumptions about the average size of mouse hepatocytes. The images from multiple sections were analyzed by Openlab software to determine the average number of hepatocytes in tissue sections from FAH mutant mice off NTBC. Binucleated hepatocytes were counted as single cells. Nonparenchymal cells were not scored. These injured hepatocytes were larger than cells from normal tissue sections. On average 1867 ± 141 cells were present/mm2. This corresponded to an average cell diameter of ∼23.2 μm, which fits well with published estimates.
The surface area of sections was measured by scanning the glass slides along with a size standard using a Microtec 2 scanner at a resolution of 300 dpi. Adobe Photoshop 5.02 software was then used to select and count the pixels corresponding to the liver sections. The average surface area scanned was 310 ± 94 mm2 corresponding to 579,000 ± 175,000 hepatocytes. The smallest section analyzed was 98 mm2 = 182,000 cells and the largest was 480 mm2 = 896,000 hepatocytes.
When clonal hepatocyte nodules were present the number of cells scored was corrected for the increased likelihood to detect a multicell clone compared to a single cell. The correction factor is a rough estimate based on three assumptions: 1) the hepatocyte nodules are spheres; 2) all nodules in a given sample are approximately the same size; and 3) the number of cells found in the section of the largest clone in a sample represents a cross-section in the middle of that nodule. For example, a 16-cell nodule would have a sphere diameter that is 2.52 times larger than the diameter of a single cell (V = 4πr3/3). The 16-cell sphere is therefore 2.52 times more likely to be sampled in a two-dimensional tissue section than a single hepatocyte. The maximal cross-section of a 16-cell spherical nodule at the equator is 6.43 times larger than that of a single cell (A = πr2). In a two-dimensional section, a 16-cell nodule therefore would correspond to no more than ∼7 (6 to 8) visible cells. The sphere diameter and maximal cell number at the sphere equator were calculated for 2, 4, 8, 16, 32, 64, 128, and 256 cell nodules (Table 1) ▶ . The cell number in the largest clone found was used to estimate the correction factor. For example, if seven cells were found, nodules were estimated to consist of ∼16 cells, corresponding to a correction factor of 2.52 for that section.
Table 1.
Correction Factors Used for Estimation of Cell Numbers in Section
| Cells in nodule | Sphere diameter increase | Cells at equator |
|---|---|---|
| 1 | 1 | 1 |
| 2 | 1.3 | 1.6 |
| 4 | 1.6 | 2.5 |
| 8 | 2 | 4.1 |
| 16 | 2.5 | 6.4 |
| 32 | 3.2 | 10.2 |
| 64 | 4 | 16.2 |
| 128 | 5 | 25.7 |
| 256 | 6.4 | 40.8 |
| 512 | 8 | 64.8 |
| 1024 | 10.1 | 102.9 |
Results
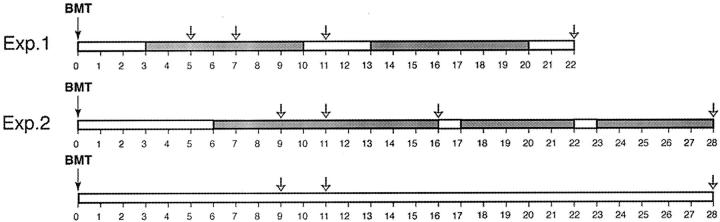
To determine the time course of liver repopulation after bone marrow transplantation we performed two independent long-term experiments. In the first experiment, FAH mutant mice received 1100 cGy total body irradiation and were given 2 × 106 bone marrow cells from congenic wild-type donors. In the second experiment, a higher dose of radiation (1500 cGy) and more cells (4 × 106) were given. This radiation dose is close to the LD50 even for bone marrow-transplanted mice and was chosen to obtain the maximum tolerated dose of total body irradiation in the context of hematopoietic rescue and to maximize the selective advantage of donor stem cells. Both groups of animals were kept on NTBC during the period of hematopoietic engraftment. In experiment 1, NTBC was continued for 3 weeks after BMT and then stopped. To maximize survival, NTBC therapy was intermittently reinstituted and withdrawn (NTBC cycling). In experiment 2 NTBC was discontinued 6 weeks after BMT in some animals and in others NTBC was continued throughout the time course. Mice were sacrificed at different times after BMT and the entire liver was harvested. The time line of the experiments are schematically shown in Figure 1 ▶ . At harvest, six separate parts (distinct lobes) were individually processed, sectioned, and stained for FAH immunoreactivity. Each section was systematically scanned at ×100 magnification for FAH-positive cells. The number and morphology of all FAH-positive hepatocytes as well as the surface area of each section were noted.
Figure 1.
Time line of the experiments. Time is indicated in weeks below the bars. Gray shading indicates time off NTBC. Harvest times are shown by the arrows. In experiment 2 (Exp. 2) some animals were kept on NTBC for the entire time, others were taken off in a cycling manner.
In experiment 1, no FAH-positive cells were found 5 weeks after BMT in 12 sections representing ∼1.2 × 106 hepatocytes (two animals, six sections each = 620 mm2 analyzed). The results are also given in Table 2 ▶ . Hematopoietic engraftment in all animals from this experiment exceeded 90% based on DNA analysis of the spleen, bone marrow, and peripheral blood (data not shown). The first donor-derived hepatocytes were detected 7 weeks after BMT (4 weeks off NTBC). Six single FAH-positive cells were found, indicating that ∼1/200,000 hepatocytes had been replaced by donor cells. Eleven weeks after BMT, the first small hepatocyte clusters consisting of 3 to 4 cells were detected. Because cell clusters are larger than single cells, the probability of finding a clonal nodule in a single histological section is greater than finding a single cell (see Materials and Methods). Based on the assumption of an average clone size of four cells and a correction factor of 2, the presence of eight small clusters indicated that one FAH+ clone emerged for every ∼290,000 hepatocytes. Because an adult mouse liver contains ∼5 × 107 hepatocytes, these numbers predict the appearance of a total of ∼170 repopulation nodules per liver. This number fits very well with the number of repopulation nodules detected by whole mount staining of liver repopulation by bone marrow from β-galactosidase-positive donors. 28 By 22 weeks after BMT repopulation nodules had become confluent and >30% of hepatocytes were FAH-positive.
Table 2.
Liver Repopulation after Standard BMT
| Mouse | Total time off NTBC | Time after BMT | FAH+clusters | Maximal cluster size | Clones/hepatocytes |
|---|---|---|---|---|---|
| 1 | Off for 2 | 5 | 0 | NA | 0 |
| 2 | Off for 2 | 5 | 0 | NA | 0 |
| 2 | Off for 4 | 7 | 3 cells | Single | 1/193,000 |
| 4 | Off for 4 | 7 | 3 cells | Single | 1/187,000 |
| 5 | Off for 7 | 11 | 2 clusters | 4 cells | 1/579,000 |
| 6 | Off for 7 | 11 | 6 clusters | 4 cells | 1/193,000 |
| 7 | Cycles, off 14 | 22 | > 30% | Confluent | ND |
| 8 | Cycles, off 14 | 22 | > 30% | Confluent | ND |
Times are given in weeks.
ND, not determined; repopulation too dense to count clones.
Higher Doses of Irradiation Do Not Enhance Hepatocyte Replacement
The results of the second experiment were very similar to the first, although a much higher dose of total body irradiation was used. Table 3 ▶ summarizes the data. Only single FAH-positive hepatocytes or small clusters were detected at the earliest time-point analyzed (9 weeks after BMT) and larger nodules were seen at 11 weeks after BMT. The estimated number of FAH+ clones corresponded well in the two experiments with ∼1/150,000 hepatocytes being donor derived at 4 months after BMT. It should be emphasized that the estimated number of FAH+ clones was similar between the two experiments and independent of the cluster size and time of harvest. It is therefore unlikely that lack of FAH immunostaining has resulted in an underestimate of the level of hepatocyte replacement. In addition, earlier work has shown that single FAH+ hepatocytes can be readily detected with our immunostaining protocol. 9 It is also important to note that the low hepatocyte replacement level cannot be explained by the use of the correction factor (Table 1) ▶ used to account for the increased likelihood of detecting nodules as compared to single cells. Even without this mathematical correction factor, replacement levels were <1/20,000.
Table 3.
Liver Repopulation after High-Dose Irradiation
| Mouse | Total time off NTBC | Time after BMT | FAH+ clusters | Maximal cluster size | Clones/hepatocytes |
|---|---|---|---|---|---|
| 9 | On | 9 | 4 | Single | 1/147,000 |
| 10 | On | 9 | 4 | Single | 1/141,000 |
| 11 | Off for 3 | 9 | 3 | Single | 1/193,000 |
| 12 | Off for 3 | 9 | 4 | 6 cells | 1/290,000 |
| 13 | On | 11 | 4 | Single | 1/145,000 |
| 14 | On | 11 | 2 | 3 cells | 1/285,000 |
| 15 | Off for 5 | 11 | 1 | Single | 1/579,000 |
| 16 | Off for 5 | 11 | 10 | 20 cells | 1/290,000 |
| 17 | Off for 10 | 16 | 15 | 50 cells | 1/304,000 |
| 18 | Off for 10 | 16 | 22 | 20 cells | 1/132,000 |
| 19 | On | 28 | 62 | 4 cells | 1/6,700 |
| 20 | On | 28 | 32 | 3 cells | 1/28,000 |
| 21 | Cycles, off for 22 | 28 | >60% | Confluent | ND |
| 22 | Cycles, off for 22 | 28 | >60% | Confluent | ND |
| KLS1 | On | 25 | 1 | Single | 1/189,000 |
| KLS2 | On | 25 | 2 | Single | 1/137,000 |
| KLS3 | On | 25 | 2 | Single | 1/169,000 |
Times are given in weeks.
KLS refers to the mice transplanted with enriched HSC. ND, not determined; repopulation too dense to count clones.
After several months of selection, the FAH-positive hepatocytes constituted a large portion of the host liver. We have previously shown that biochemical liver functions were normalized in such repopulated mice. 28 Because the number of clones determined by histological methods at early time points after BMT and β-galactosidase whole mount staining at late time points were similar, it is likely that the single FAH-positive cells found were true hepatocytes. Most of these marrow-derived hepatocytes eventually developed into large repopulation nodules of fully functional cells.
Hepatocyte Engraftment Occurs without Liver Damage
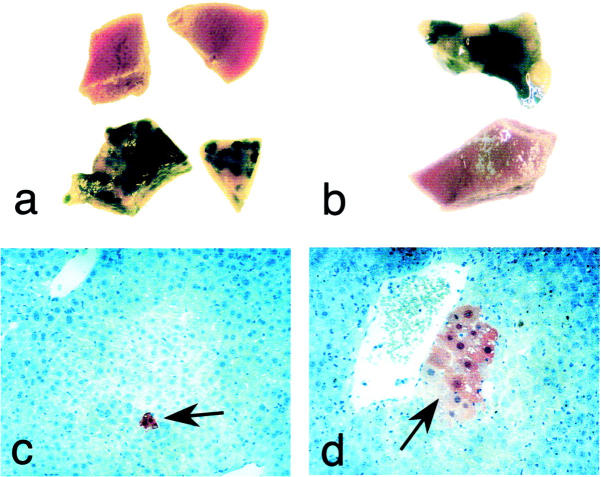
To determine whether liver injury was necessary to achieve hepatocyte replacement by bone marrow-derived cells, we kept a cohort of control animals on NTBC continuously after BMT. Interestingly, FAH-positive hepatocytes also emerged in these animals at the same time as in mice off NTBC (Table 3) ▶ . When the numbers shown in Table 3 ▶ from all animals on NTBC and those off NTBC until 16 weeks after BMT were compared, the hepatocyte replacement level was not statistically different. The average for animals on NTBC was 1/167,000 (95% confidence interval from 1/114,000 to 1/298,000) and those off NTBC was 1/243,000 (95% confidence interval 1/160,000 to 1/503,000). A two-tailed t-test showed a P value of 0.15, ie, no significant difference between the two groups. This finding suggested that no liver damage beyond total body irradiation was needed to achieve differentiation of bone marrow cells into hepatocytes. However, only single cells or very small clusters were detected in NTBC-treated animals and the overall degree of repopulation remained insignificant. Thus, the expansion of bone marrow-derived hepatocytes to functionally relevant levels was completely dependent on the powerful selective advantage of FAH+ hepatocytes (Figure 2) ▶ . The level of hepatocyte replacement in mice kept on NTBC was as high as 1/6700 cells many months after BMT (Table 3) ▶ . It is therefore possible that new bone marrow-derived hepatocytes continue to seed the liver and the replacements levels increase with time. Alternatively, the relatively high replacement level may be because of selection by the mild liver injury sometimes seen in FAH mutant mice treated with NTBC for a long term. 36
Figure 2.
Examples of liver repopulation after bone marrow transplantation. a and b: β-galactosidase whole mount staining of liver. c and d: FAH immunohistochemistry. a: Effect of NTBC withdrawal. Top: Liver samples from animals kept on NTBC for 28 weeks after BMT. Bottom: Samples from mice that were off for 22 to 28 weeks. Significant repopulation (blue clusters) was present only in animals off NTBC. b: Effects of total body irradiation. The upper liver sample was from an irradiated recipient and the lower from a control animal transplanted with the same bone marrow cells, but without total body irradiation. c: Single FAH-positive hepatocyte (arrow) found 7 weeks after BMT (original magnification, ×200). d: FAH-positive cluster 11 weeks after BMT (original magnification, ×400).
Transplantation of Enriched HSCs Does Not Enhance Hepatocyte Replacement
Earlier reports on the transdifferentiation of bone marrow cells to hepatocytes used lineage-depleted bone marrow cells. 25 Therefore, to assess whether the low engraftment efficiency observed here was because of the use of whole marrow rather than enriched stem cells, we transplanted a cohort of mice with a large number of highly enriched HSCs. The HSCs isolated from congenic Rosa26 donors were defined by the markers c-kithi, Linneg, and Sca-1+ with Linneg representing 10 different lineage markers (KLS HSCs). KLS HSCs are an enriched population of HSCs representing less than 0.1% of total adult bone marrow cells in the 129S4 mouse background. 13 Eight thousand KLS HSCs per mouse (equivalent to at least 8 × 106 unfractionated marrow cells) were transplanted into three lethally irradiated FAH mutant mice. All three mice were kept on NTBC during the whole experiment. Two months later, nucleated blood cells were tested for hematopoietic engraftment. All three animals survived the lethal irradiation and showed complete hematopoietic reconstitution. Six months after HSC transplantation and without any withdrawal of NTBC, mice were sacrificed and their liver screened for FAH-positive hepatocytes. As shown in Table 3 ▶ , the frequency of HSC-derived hepatocytes ranged from 1/137,000 to 1/189,000. These numbers closely parallel those obtained with unfractionated marrow and we therefore conclude that transplantation with highly enriched HSCs did not significantly enhance the degree of liver engraftment observed.
Hepatocyte Replacement by Bone Marrow Cells Requires Total Body Irradiation
To determine whether bone marrow cells could give rise to hepatocytes in the absence of preparative irradiation, a total of 18 mice were transplanted with 5 to 10 × 106 bone marrow cells from Rosa26 donors, but only 4 of these received preparative irradiation (Table 4) ▶ . As expected the contribution of donor cells to hematopoietic tissues was <1% in the nonirradiated animals, but >90% in the irradiated mice (data not shown). After 5 months of cycling NTBC selection, the animals were sacrificed and analyzed by whole mount β-galactosidase staining of two lobes and FAH immunostaining in the remainder of the liver. Although significant liver repopulation was detected in the irradiated animals, no FAH-positive hepatocytes were detected in the nonirradiated animals, even when serial sectioning was performed (Table 4 ▶ , Figure 2 ▶ ). Thus, the degree of hepatocyte replacement without preparative irradiation was <1/106.
Table 4.
Liver Repopulation Without Preparative Irradiation
| Mouse marked number | Cell number (×106) | Total time off NTBC | Time off NTBC before BMT | Special manipulations | Liver repopulation |
|---|---|---|---|---|---|
| 23 | 5 | 20 | No | No | |
| 24 | 5 | 20 | No | No | |
| 25 | 5 | 20 | No | No | |
| 26 | 5 | 20 | No | No | |
| 27 | 10 | 21 | 1 | Direct injection with Matrigel | No |
| 28 | 10 | 21 | 1 | Direct injection with Matrigel | No |
| 29 | 10 | 21 | 1 | Direct injection with Matrigel | No |
| 30 | 10 | 21 | 1 | No | |
| 31 | 10 | 21 | 1 | No | |
| 32 | 10 | 21 | 1 | No | |
| 33 | 10 | 21 | 1 | No | |
| 34 | 5 | 21 | No | 1.2 Gy TBI | 5–10% |
| 35 | 5 | 21 | No | 1.2 Gy TBI | ≈10% |
| 36 | 5 | 21 | No | 1.2 Gy TBI | 30–40% |
| 37 | 5 | 21 | No | 1.2 Gy TBI | 30–40% |
| 38 | 5 | 21 | No | No | |
| 39 | 5 | 21 | No | No | |
| 40 | 5 | 21 | No | No | |
| 41 | 5 | 21 | No | No |
Times are given in weeks.
TBI, total body irradiation.
We also tested some other strategies with the goal of achieving liver engraftment without previous irradiation (Table 4) ▶ . In some animals NTBC was discontinued 1 week before transplantation of bone marrow cells. This was done to determine whether liver damage at the time of transplantation could enhance direct engraftment of bone marrow hepatocyte precursors. No engraftment was observed. Furthermore, direct intrahepatic injection of bone marrow cell in a Matrigel matrix also did not result in observable repopulation.
Comparison to Hepatocyte Transplantation
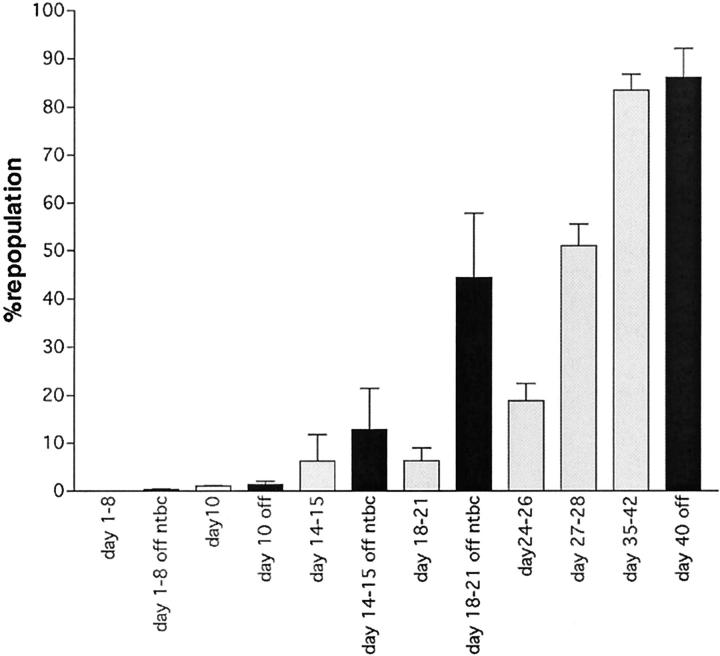
The precise kinetics of liver replacement by transplanted hepatocytes in the FAH knockout mouse were unknown and a therefore a detailed time-course experiment was performed. FAH mutant mice were transplanted with 100,000 wild-type hepatocytes by intrasplenic injection and harvested at various time points. The initial engraftment was determined by FAH immunohistochemistry on days 1 to 3 in six animals. On average 1/2485 hepatocytes were replaced by donor cells (95% confidence interval 1/1105 to 1/10,045). If one assumes that an adult mouse liver contains ∼0.5 to 1 × 108 hepatocytes, this reflects stable engraftment of ∼10,000 to 20,000 of the donor cells (10 to 20% efficiency) in keeping with previous reports. 37 The effects of liver injury at the time of transplantation was also determined. Half of the animals were on NTBC until the day after the transplantation and therefore had no hepatic injury. NTBC was removed 10 days before transplant in the other half. Figure 3 ▶ shows the kinetics of repopulation in these two groups. Although the initial engraftment was similar between the two groups, hepatocyte expansion occurred more rapidly in the group with liver injury. Significant liver repopulation of ∼50% was achieved in ∼3 weeks in mice with liver injury and in 4 weeks in those without. If kept on NTBC, liver repopulation remained at engraftment levels (data not shown).
Figure 3.
Repopulation time course using donor hepatocytes. The percentage of liver repopulation is given as a function of time. The time points of harvest after transplantation are given below each bar. The bars indicate the mean of at least three individual animals and the error bars show the 95% confidence interval. Animals were either on NTBC until the transplantation (gray bars) or had been off NTBC for 1 week at the time of transplant (black bars).
Overall, the kinetics of liver repopulation with hepatocytes differed markedly from those seen after bone marrow transplantation. Engraftment of donor cells was rapid (days) and nodule expansion could be observed as early as 10 days after transplant. Functionally significant repopulation levels were achieved in <1 month.
Discussion
The plasticity of HSCs has raised hopes that bone marrow-derived cells could be used for cell therapy in a variety of organs. In particular, the phenotypical correction of metabolic liver disease by bone marrow transplantation in a murine model suggested that BMT may be useful for the treatment of hereditary hepatic protein deficiencies. 28 Disorders amenable to this approach would include hemophilia A and B, familial hypercholesterolemia, phenylketonuria, among others. 38 Although the precise therapeutic threshold for each of these disorders is not known, it is reasonable to assume that stable replacement of ∼1% of hepatocytes may be beneficial in factor VIII and IX deficiency 39 and that substitution of ∼10% of cells would ameliorate many hyperlipidemias and disorders of amino acid metabolism. The recently published literature on the differentiation of bone marrow-derived cells into hepatocytes differs greatly in terms of the quantitative estimates of hepatocyte replacement after bone marrow transplantation. Although some reports observed replacement of only endothelial cells and not hepatocytes by donor cells in the rat 40 others have consistently reported that ∼1 to 2% of hepatocytes or more were substituted by donor-derived cells in both rodents and humans. 25-27 Furthermore, in the mouse this degree of cell replacement was achieved without any selective advantage of donor hepatocytes other than the conditioning regimen used for BMT. 25 This is remarkable when one considers that transplantation of hepatocytes themselves results in tissue replacement levels of <1%. 37 The original report on bone marrow-derived liver epithelial cells reported the appearance of hepatocytes at ∼1/1000 only after an oval cell induction regimen that causes liver injury. 24 The reported 1% cell replacement approaches the therapeutic threshold for many disorders and only 3 to 4 rounds of cell division would be needed to reach clinically significant levels for most liver disorders.
Unfortunately, the level of hepatocyte replacement observed in the work described here was quite low, on the order of only 1/150,000 that is much below the therapeutic threshold for any known hepatic disorder. In addition, the emergence of bone marrow-derived hepatocytes was rather slow, with the first cells appearing 2 months after BMT. Significant liver repopulation could be observed only when strong selective pressure was applied. In this setting, the 300 or so bone marrow-derived hepatocytes per liver were able to expand and eventually replace >30% of the hepatic parenchyma requiring at least 18 rounds of cell doubling. These results suggest that the efficiency of liver repopulation by transplanted bone marrow cells is limited at the moment. Significant enhancement of the procedure by improved engraftment and/or selection of donor cells will be required before clinical use could be considered.
We were unable to detect hepatic engraftment of bone marrow-derived cells in the absence of preparative whole body irradiation. This was true even when the liver was already injured at the time of cell transplantation or if the bone marrow cells were injected directly into hepatic parenchyma. Furthermore, hepatocytes did emerge in lethally irradiated recipients, even if they were kept on NTBC after the BMT. Thus liver damage was neither required for transdifferentiation into hepatocytes nor did it enhance engraftment significantly. Together with the slow kinetics of repopulation the requirement for irradiation suggests that hematopoietic engraftment may be needed before hepatocytic differentiation can occur. The phenotypic transition into hepatocytes may be a stochastic, low-frequency phenomenon rather than a physiological response to tissue injury.
Our results agree with some published reports, but are inconsistent with others. Similar to us, Gao and colleagues 40 also could detect only negligible hepatocyte replacement in bone marrow-transplanted rats despite complete hematopoietic reconstitution. Only endothelial cell replacement was observed. In contrast, several other studies have reported significant hepatocyte replacement within 2 months of simple bone marrow transplants. 25-27 The reasons for the major discrepancies between our findings here and other reports are unclear. Several possibilities will be discussed in the following. First, different techniques for the detection of donor-derived hepatocytes were used. Although gender-mismatched transplants and Y-chromosome in situ hybridization were used by others, we used a cell-type-specific transgene difference between donor and host cells. The FAH enzyme is expressed in 100% of wild-type hepatocytes from all three zones, but not in hematopoietic cells including Kupffer cells, bile duct epithelium, endothelial cells, or stellate cells. 9,41 In contrast, any donor-derived cell, including hematopoietic cells and endothelial cells will generate a positive Y-chromosome signal. Hence, the identification of hepatocytes by this technique relies on co-labeling with other markers specific to this cell type. It is possible that the co-labeling technique overestimates the incidence of fully differentiated hepatocytes, especially because confocal microscopy was not routinely used to verify co-localization of Y-chromosome and hepatocyte markers. 42 It is also possible that the donor-derived cells expressed only some hepatocyte markers and were not fully differentiated. Second, selection of donor bone marrow-derived hepatocytes could account for the high apparent replacement level. The required expansion from 1/100,000 cells to 1/100 would require 10 rounds of cell doubling and result in obvious, large cell clusters. However, no clustering of bone marrow-derived hepatocytes was described in mice with reportedly 1 to 2% repopulation. 25 It is therefore unlikely that the quantitative differences to our study can be explained by hepatocyte selection. In contrast, hepatocyte clustering was described in some of the examples of repopulated human livers. 26,27,29 In those cases, selective growth of donor cells may partially explain the high degree of cell replacement. Selective pressure may have been generated by the use of chemotherapy drugs for preconditioning or the diseases that affected the patients (from example hepatitis C). Third, it is conceivable that the discrepancies are because of the specific strains of mice or the species used. In their work Theise and colleagues 25 used B6D2F1 mice whereas the animals used here were of the 129S4 strain. The dose of radiation used as well as the degree of hematopoietic engraftment observed was very similar between their work and our current report. Therefore, the behavior of bone marrow-derived hepatocyte progenitors in different mouse strains would have to be very different to explain the vastly different degrees of repopulation observed.
The experiments described here do not elucidate details of the mechanism by which bone marrow-derived hepatocytes are derived. It remains unclear whether the same stem cell gives rise to both hepatocytes and hematopoietic lineages (stem cell plasticity) or whether different cells give rise to these lineages. Recently, it has been suggested that apparent stem cell plasticity could also be explained by fusion between donor and host cells. 43,44 Here, we did not determine whether bone marrow-derived hepatocytes arose via a fusion between FAH mutant host hepatocytes and wild-type hematopoietic cells. It is noteworthy, however, that the kinetics of the replacement process (rare events only after previous hematopoietic reconstitution) are not inconsistent with the hypothesis of in vivo fusion and nuclear reprogramming of the donor hematopoietic cell.
It is worthwhile to directly compare the liver replacement kinetics after BMT to those observed when wild-type hepatocytes were used as the donor cells for liver repopulation. As shown here, the engraftment was much more rapid (days instead of weeks) when hepatocytes were used and significant tissue replacement could be achieved in <1 month. In addition, preparative irradiation was not needed. Thus, hepatocytes are currently considerably more efficient than bone marrow-derived cells for hepatic repopulation.
Because of the quantitatively very different results obtained after simple BMT by different investigators, future studies in additional models will be required to determine the efficiency of liver repopulation by bone marrow cells in a variety of settings. Our results reported here clearly indicate that this process is not automatically efficient even when complete hematopoietic reconstitution has been achieved. Therefore, detailed knowledge regarding its properties will be needed before human trials or even large-animal studies can be contemplated. It should also be said, however, that bone marrow-derived hepatocytes can indeed be used for therapy of severe liver disease if selective pressure favors the donor cells. With the development of safe and efficient methods for the expansion of donor hepatocytes in the host, cell therapy with bone marrow-derived progenitors could become an important modality in the management of liver disease.
Acknowledgments
We thank Angela Major and Billie Smith for their support with histological analysis.
Footnotes
Address reprint requests to Markus Grompe, Department of Molecular and Medical Genetics, L 103, Oregon Health Sciences University, 3181 SW Sam Jackson Pk. Rd., Portland, OR 97201. E-mail: grompem@ohsu.edu.
Supported by the National Institutes of Health (grant RO1-DK48252 to M. G.) and the Juvenile Diabetes Foundation (to X. W.).
References
- 1.Grompe M, Laconi E, Shafritz DA: Principles of therapeutic liver repopulation. Semin Liver Dis 1999, 19:7-14 [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Yerneni PR, Vemuru RP, Lee CD, Yellin EL, Bhargava KK: Studies on the safety of intrasplenic hepatocyte transplantation: relevance to ex vivo gene therapy and liver repopulation in acute hepatic failure. Hum Gene Ther 1993, 4:249-257 [DOI] [PubMed] [Google Scholar]
- 3.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S: Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology 1996, 111:1092-1102 [DOI] [PubMed] [Google Scholar]
- 4.Rozga J, Holzman M, Moscioni AD, Fujioka H, Morsiani E, Demetriou AA: Repeated intraportal hepatocyte transplantation in analbuminemic rats. Cell Transplant 1995, 4:237-243 [DOI] [PubMed] [Google Scholar]
- 5.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC: Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998, 338:1422-1426 [DOI] [PubMed] [Google Scholar]
- 6.Strom SC, Chowdhury JR, Fox IJ: Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 1999, 19:39-48 [DOI] [PubMed] [Google Scholar]
- 7.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA: Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol 1998, 153:319-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL: Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263:1149-1152 [DOI] [PubMed] [Google Scholar]
- 9.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 10.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M: Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 11.Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A, Soubrane O, Gilgenkrantz H, Kahn A: Selective repopulation of normal mouse liver by Fas/CD95-resistant hepatocytes. Nat Med 1998, 4:1185-1188 [DOI] [PubMed] [Google Scholar]
- 12.De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP: Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology 2000, 119:1720-1730 [DOI] [PubMed] [Google Scholar]
- 13.Spangrude GJ, Heimfeld S, Weissman IL: Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241:58-62 [DOI] [PubMed] [Google Scholar]
- 14.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B: Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA 1992, 89:2804-2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ: Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Science 1997, 276:71-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prockop DJ: Marrow stromal cells as stem cells for nonhematopoietic tissues. Matrix Biol 1998, 16:519-5289569121 [Google Scholar]
- 17.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F: Muscle regeneration by bone marrow-derived myogenic progenitors. Hum Immunol 1998, 59:137-148 [DOI] [PubMed] [Google Scholar]
- 18.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC: Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 401:390-394 [DOI] [PubMed] [Google Scholar]
- 19.Brazelton TR, Rossi FM, Keshet GI, Blau HM: From marrow to brain: expression of neuronal phenotypes in adult mice. Science 2000, 290:1775-1779 [DOI] [PubMed] [Google Scholar]
- 20.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR: Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290:1959-1962 [DOI] [PubMed] [Google Scholar]
- 21.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P: Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 2001, 98:10344-10349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P: Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410:701-705 [DOI] [PubMed] [Google Scholar]
- 23.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ: Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105:369-377 [DOI] [PubMed] [Google Scholar]
- 24.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP: Bone marrow as a potential source of hepatic oval cells. Science 1999, 284:1168-1170 [DOI] [PubMed] [Google Scholar]
- 25.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS: Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000, 31:235-240 [DOI] [PubMed] [Google Scholar]
- 26.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA: Hepatocytes from non-hepatic adult stem cells. Nature 2001, 414:10-11 [DOI] [PubMed] [Google Scholar]
- 27.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause D: Liver from bone marrow in humans. Hepatology 2000, 32:11-16 [DOI] [PubMed] [Google Scholar]
- 28.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M: Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000, 6:1229-1234 [DOI] [PubMed] [Google Scholar]
- 29.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z: Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 2002, 346:738-746 [DOI] [PubMed] [Google Scholar]
- 30.Grompe M, Al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P: Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 1993, 7:2298-2307 [DOI] [PubMed] [Google Scholar]
- 31.Friedrich G, Soriano P: Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 1991, 5:1513-1523 [DOI] [PubMed] [Google Scholar]
- 32.Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B: Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 1992, 340:813-817 [DOI] [PubMed] [Google Scholar]
- 33.Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M: Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet 1995, 10:453-460 [DOI] [PubMed] [Google Scholar]
- 34.Smith DDJ, Campbell JW: Distribution of glutamine synthetase and carbamoyl-phosphate synthetase I in vertebrate liver. Proc Natl Acad Sci USA 1988, 85:160-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacGregor GR, Mogg AE, Burke JF, Caskey CT: Histochemical staining of clonal mammalian cell lines expressing E. coli β-galactosidase indicate heterogeneous expression of the bacterial gene. Somat Cell Mol Genet 1987, 13:253-265 [DOI] [PubMed] [Google Scholar]
- 36.Al-Dhalimy M, Overturf K, Finegold M, Grompe M: Long-term therapy with NTBC and tyrosine-restricted diet in a murine model of hereditary tyrosinemia type I. Mol Genet Metab 2002, 75:38-45 [DOI] [PubMed] [Google Scholar]
- 37.Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, Ledley FD, Chowdhury JR, Woo SL: Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci USA 1991, 88:1217-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwich AL: Inherited hepatic enzyme defects as candidates for liver-directed gene therapy. Curr Top Microbiol Immunol 1991, 168:185-200 [DOI] [PubMed] [Google Scholar]
- 39.Kay MA, High K: Gene therapy for the hemophilias. Proc Natl Acad Sci USA 1999, 96:9973-9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Z, McAlister VC, Williams GM: Repopulation of liver endothelium by bone-marrow-derived cells. Lancet 2001, 357:932-933 [DOI] [PubMed] [Google Scholar]
- 41.Ruppert S, Kelsey G, Schedl A, Schmid E, Thies E, Schütz G: Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev 1992, 6:1430-1443 [DOI] [PubMed] [Google Scholar]
- 42.Kornack DR, Rakic P: Cell proliferation without neurogenesis in adult primate neocortex. Science 2001, 294:2127-2130 [DOI] [PubMed] [Google Scholar]
- 43.Ying QL, Nichols J, Evans EP, Smith AG: Changing potency by spontaneous fusion. Nature 2002, 416:545-548 [DOI] [PubMed] [Google Scholar]
- 44.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW: Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002, 416:542-545 [DOI] [PubMed] [Google Scholar]