Abstract
Inflammatory diseases are characterized by the leukocyte infiltration into tissues. L-selectin on lymphocytes and its endothelial glycosylated ligands are instrumental in the initiation of lymphocyte extravasation. Immunohistochemical stainings with monoclonal antibodies against functionally active glycan-decorated L-selectin ligands, ie, sialyl-Lewis x (sLex, 2F3, and HECA-452) or sulfated extended core 1 lactosamine (MECA-79), were performed on more than 400 specimen representatives for thyroiditis, myocarditis, psoriasis, vasculitis, ulcerative colitis, and their corresponding noninflamed tissues. The endothelial expression of sLex or sulfo sLex glycans in postcapillary venules was either absent or low in control tissues. The de novo induction of endothelial expression of sLex or sulfo sLex glycans was detected in all inflamed tissues. Furthermore, each organ carried its own modification of sLex or sulfo sLex glycans, ie, zip code. Our results suggest that these zip code glycans may provide means for organ selective leukocyte traffic that could be used in selective leukocyte traffic inhibition.
Inflammatory diseases, such as acute kidney and heart allograft rejections or bronchial asthma, are characterized by a heavy infiltration of lymphocytes and monocytes, but not of granulocytes, leading finally to necrotic changes at the inflammatory site. 1-4 The recruitment of lymphocytes across the microvascular postcapillary endothelium 5-7 is a complex cascade, taking its origin from the initial tethering and rolling of leukocytes that are mediated by the members of the selectin family and their glycosylated ligands. These interactions are followed by a chemokine-mediated activation of integrins, leading to a firm adhesion of rolling leukocytes and, subsequently, to transendothelial migration into inflammatory sites. 8,9
The tethering and rolling phases of lymphocyte extravasation have been proposed to be initiated by L-selectin. This molecule recognizes endothelial ligands, such as CD34, podocalyxin, MAdCAM-1, and sgp200, provided that these ligands are decorated with α2,3-sialylated, α1,3-fucosylated, and sulfated lactosamines. 1,5-7,10-12 These sulfated sialyl Lewis x (sLex) glycans are necessary for the L-selectin-mediated binding of lymphocytes to endothelium and are constantly expressed at least on the CD34 glycans of lymph node high endothelium. 13 Under normal conditions, the flat endothelium in parenchymal organs does not express properly glycosylated modifications of L-selectin ligands 14 and no extravasation occurs. However, the induction of sLex- or sulfo sLex-decorated L-selectin ligands onto the postcapillary microvascular endothelium occurs both in rodents and humans undergoing heart and kidney allograft rejection and also in the early specimens taken from bronchial asthma pa-tients. 2-4,14 Moreover, the enzymatically synthesized multivalent sLex glycans can prevent the selectin-dependent lymphocyte adhesion to properly glycosylated endothelium ex vivo, pointing out a putative mean to inhibit the inflammatory reaction. 14,15
Here we expand these previous observations and analyze the endothelial sLex or sulfo sLex expression patterns in various inflammatory diseases. For this purpose, a large series of histological biopsies (>400) from patients with ulcerative colitis, psoriasis, thyroiditis, myocarditis, and vasculitis and their corresponding noninflamed tissues were collected. The endothelium of control noninflamed tissues did not express essentially any sLex or sulfo sLex epitopes whereas on the endothelium of inflamed organs they were prominently expressed. Most importantly, the up-regulated sLex or sulfo sLex expression patterns of inflamed tissues were clearly different between every organ analyzed, and when combined with our previous data, it could be postulated that every inflamed organ expressed its own specific endothelial sLex or sulfo sLex zip code regulating lymphocyte traffic to a given organ. This thorough analysis of the endothelial sLex and sulfo sLex expression patterns in inflammatory diseases should warrant the more detailed analysis of the authentic glycosylations on the relevant endothelial glycoproteins acting as L-selectin ligands. 13
Materials and Methods
Histological Specimens
Formalin-fixed, paraffin-embedded archived blocks of normal and diseased human tissues and corresponding slides were obtained from the Department of Pathology, Helsinki University Central Hospital. The selection of human samples from archives was based on the primary pathological reports; all specimens were also re-evaluated independently to be representative for a given type of inflammation or serve as relevant controls of normal tissues by three observers (JR, OT, and TP). The ethical committee of the Helsinki University Central Hospital had approved the study plan.
Altogether, nearly 1000 histological specimens were identified from the records of the Department of Pathology and screened through for further study. One hundred twenty-two histological specimens with lymphocytic thyroiditis, 130 with ulcerative colitis, 126 with psoriasis, 108 skin biopsies with vasculitis, and 57 with myocarditis were screened, and 50 cases from each disease were chosen for the study. As control specimens, 131 samples with goiter, 150 samples with noninflammatory colon, 98 samples of normal skin, and 20 samples with myocardial hypertrophia or myofibrosis were screened, and 30 cases from each organ were selected.
Immunohistochemistry
The glycans on selectin ligands were identified by immunohistochemistry with three different monoclonal antibodies (mAbs) each recognizing specific glycosylation modifications (Table 1) ▶ . mAb HECA-452 16 (rat IgM) and mAb MECA-79 17 (rat IgM) were kindly provided by Dr. S. Jalkanen (University of Turku, Turku, Finland). mAb 2F3 18 (IgM) was from Pharmingen (San Diego, CA). As a positive control for the detection of endothelial cells, anti-human CD34, class II (mIgG1) (1:25; DAKO, Glostrup, Denmark) and as negative controls mAbs 7C7 (mouse IgM) and TIB-146 (rat IgM), both kindly provided by Dr. S. Jalkanen, were used. For immunohistochemical analysis, previously described protocols was used. 3,4 In short, tissue specimens originally embedded in paraffin were cut into 5-μm sections, deparaffinized, and rehydrated, followed by a microwave pretreatment and a serum blocking. Vector Vecstain IgG mouse and rat kits were used for immunoperoxidase stainings according to the manufacturer’s instructions (Vector Laboratories, Inc., Burlingame, CA).
Table 1.
Monoclonal Antibodies Used in this Study
| mAb | Concentration | Epitope recognized |
|---|---|---|
| 7C7 | 1.2 μg/ml | Control for murine IgM antibodies |
| TIB-146 | 10 μg/ml | Control for rat IgM antibodies |
| 2F3 | 0.6 μg/ml | Sialyl Lewis x, 6–, 6′–, 6, 6′–sulfo sialyl Lewis x |
| HECA-452 | 2 μg/ml | Sialyl Lewis x |
| MECA-79 | 1 μg/ml | Extended core 1 6–sulfated lactosamine |
| CD34 | 2 μg/ml | CD34 glycoprotein |
Every CD34+ vessel from a field of view using ×400 magnification was calculated and because the size of this field was known the number of vessels was converted to vessels/mm2. Ten to 20 high-power fields were analyzed and the mean ± SEM was calculated. The intensity of the staining was scored either positive or negative for each vessel. After determining the number of mAb 2F3-, HECA-452-, or MECA-79-reactive vessels this was divided by the number of CD34+ vessels from the same specimens yielding the percentage of sLex- or sulfo sLex-reactive vessels. Only the small capillaries/venules were included because the arteries or major vessels did not react with the antibodies used.
Statistical Analysis
Fifty cases from inflamed specimens and 30 cases from specimens with normal histology entered the statistical analysis. The results were first analyzed by the Kruskal-Wallis one-way analysis of variance by ranks. This test is basically a comparison of medians of several unpaired groups and the null hypothesis is that the medians are equal; P values <0.05 were considered as significant. The Kruskal-Wallis test indicated that we could use the nonparametric Mann-Whitney U-test for the multiple comparisons of medians in control and inflammation groups. The null hypothesis is that the medians are equal. In the Mann-Whitney U-test, differences were considered to be significant at P < 0.05.
Results
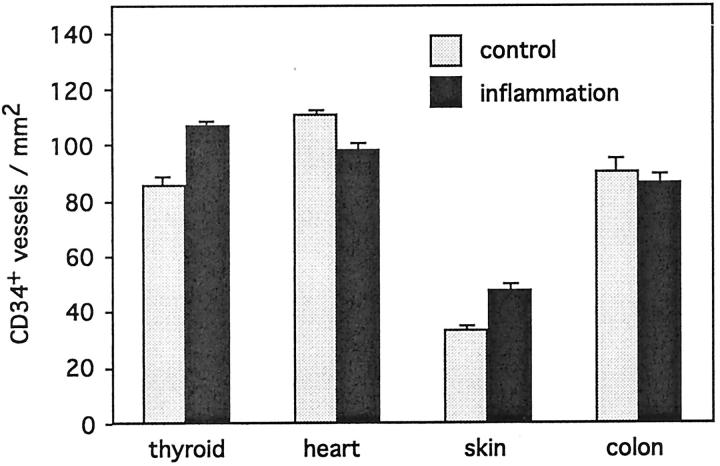
The expression of an endothelial CD34 was analyzed in all specimens for two reasons. First, mAb CD34 was used as a marker to determine the number of vessels/mm2, because this molecule has been shown to be expressed on all vascular endothelia, and second, because the CD34 glycoprotein is one of the prime protein ligands for L-selectin carrying sulfo sLex glycans. In control specimens representing noninflamed thyroid, heart, and colon, the number of CD34+ vessels was 86 ± 3.0/mm2, 111 ± 1.6/mm2, and 91 ± 4.2/mm2, respectively (mean ± SEM) (Figure 1) ▶ , whereas the vascularity in noninflamed skin biopsies was only 34 ± 1.8/mm2. The numbers of anti-CD34+ vessels were modified only marginally in the inflammatory tissues when compared to their control noninflamed tissue samples (107 ± 2.0/mm2 in thyroiditis, 99 ± 1.8/mm2 in myocarditis, 87 ± 2.9/mm2 in colitis, 47 ± 1.9/mm2 in psoriasis, 49 ± 2.6/mm2 in vasculitis).
Figure 1.
The number of postcapillary vessels/mm2 in control and inflammatory tissue specimens. The expression of endothelial CD34 glycoprotein in thyroid gland, heart, skin, and colon specimens with noninflammatory histology (gray bars) and with the signs of inflammation (black bars).
High endothelial venules (HEVs) in lymph nodes express specific sulfo sLex-decorated versions of L-selectin ligands, such as CD34 glycoprotein, fulfilling the most if not all of the requirements for L-selectin recognition. However, the same endothelial protein backbone is not properly glycosylated in any other organ under normal conditions. Because there is not a single antibody available to recognize all known glycodecorations of L-selectin ligands, we needed to probe the differently decorated L-selectin ligands with a panel of mAbs. The reacting epitopes of antibodies used in this study are shown in Table 1 ▶ . The original antigens in the immunization yielding mAbs 2F3 and HECA-452 sLex decorated glycolipids and stromal components of human lymph nodes, respectively. 16,18 mAbs 2F3 and HECA-452 detect α2,3-sialylation and α1,3-fucosylation of lactosamine, but they do not distinguish between sulfated and nonsulfated epitopes. MECA-79 was originally defined to recognize an antigen-entitled peripheral lymph node adressin (PNAd). 17,19 This is actually a family of glycoproteins, most of which are able to bind L-selectin. In humans only CD34 and podocalyxin have been identified at the molecular level among the glycoprotein species reacting with MECA-79. 20 Later the recognizing epitope of MECA-79 was shown to recognize an extended sulfated core 1 lactosamine structure. This antibody does not recognize α2,3-sialylation or α1,3-fucosylation, but is however able to block in vitro and in vivo lymphocyte homing to murine lymph nodes. 17,19
The sLex moiety and its sulfated forms are of importance in the extravasation process leading to leukocyte invasion into different human tissues. To assess whether distinct inflammatory diseases differ from each other in the relation to their endothelial glycosylation, the profiles of 50 specimens representing inflammation and 30 control specimens from five different noninflammatory conditions were studied by immunohistochemistry using two different sLex mAbs and one sulfation-dependent mAb with relevant controls.
Thyroid Gland
In the thyroid gland (Figure 2 ▶ ; A to F), the anti-sLex antibody mAb 2F3 did not react with the endothelium in control goiter specimens with noninflammatory histology. As noninflamed controls, both the diffuse and multinodular types of thyroid hyperplasias but not adenomas were included. In thyroiditis specimens, the percentage of mAb 2F3-reactive vessels was significantly increased to 26.3 ± 2.4% (mean ± SEM) (Figure 3A) ▶ when compared to control specimens (P < 0.001, Mann-Whitney U-test). It should be noted that only capillaries and venules expressed sulfo sLex-decorated L-selectin ligands whereas arteries or arterioles did not.
Figure 2.
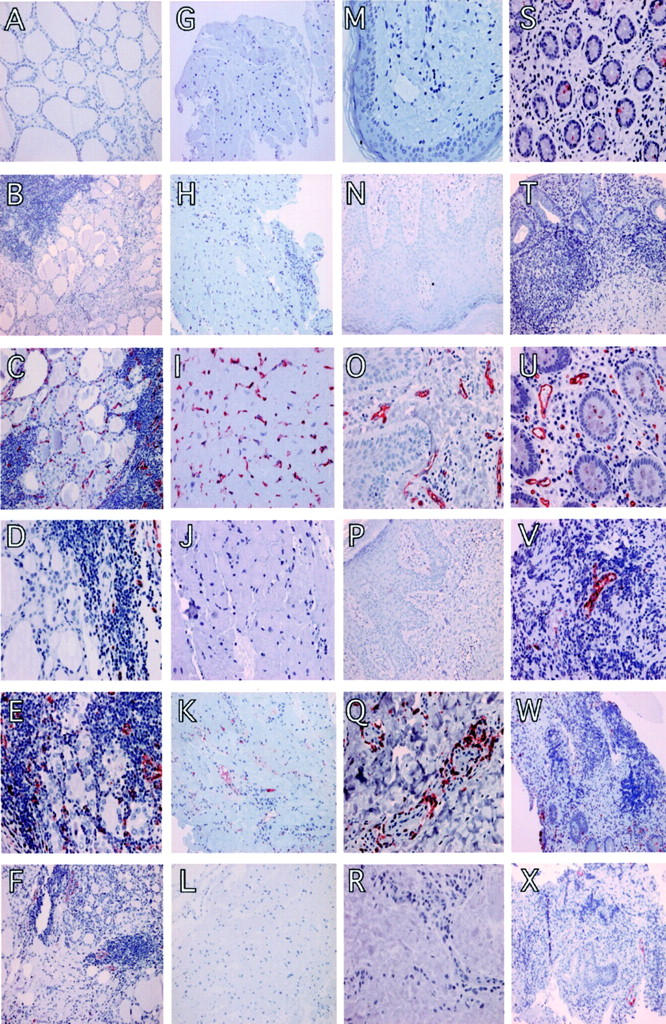
The histological distribution of sulfo sLex-decorated L-selectin ligands in both noninflamed and inflamed tissues of various organs. Thyroid gland: A, mAb 7C7, a mouse IgM control mAb, showed no endothelial reactivity in noninflamed thyroid gland; B, mAb TIB-146, a rat IgM control mAb, showed no endothelial reactivity in thyroiditis; C, anti-CD34 antibody showing a strong positive reaction with the endothelium of all capillaries and venules within a specimen with histological signs of thyroiditis; D, anti-sLex mAb, mAb 2F3, showing endothelial reactivity in thyroiditis; E, mAb HECA-452 stained strongly capillaries in thyroiditis; F, a vascular endothelial reactivity with mAb MECA-79 in several vessels in the inflammatory focus of thyroiditis. Heart: G, mAb 7C7, a mouse IgM control mAb, showed no endothelial reactivity in a heart specimen with noninflamed histology; H, mAb TIB-146, a rat IgM control mAb, showed no endothelial reactivity in myocarditis; I, anti-CD34 antibody showing a strong positive reaction with the endothelium of all capillaries and venules within a specimen with noninflamed histology; J, anti-sLex mAb, mAb2F3, showing no endothelial reactivity in myocarditis; K, mAb HECA-452 stained strongly a small proportion of capillaries during myocarditis; L, mAb MECA-79 showed no endothelial reactivity in the mild myocarditis. Skin: M, mAb TIB-146, a rat IgM control mAb, showed no endothelial reactivity in a skin specimen with noninflamed histology; N, mAb 7C7, a mouse IgM control mAb, showed no endothelial reactivity in psoriasis; O, anti-CD34 antibody showing a strong positive reaction with the endothelium of capillaries and venules within psoriasis; P, anti-sLex mAb, mAb 2F3, showing no endothelial reactivity in a psoriatic lesion with local; Q, mAb HECA-452 stained strongly capillaries within a skin specimen with histological signs of psoriasis; R, no endothelial reactivity with mAb MECA-79 shown in the inflammatory focus of vasculitis. Colon: S, mAb TIB-146, a rat IgM control mAb, showed no endothelial reactivity in a colon specimen with noninflamed histology; T, mAb 7C7, a mouse IgM control mAb, showed no endothelial reactivity in a ulcerative colitis specimen; U, anti-CD34 antibody showing a strong positive reactivity with the endothelium of capillaries and venules within a colon specimen with noninflamed histology; V, anti-sLex mAb, mAb 2F3, showing a strong endothelial reactivity in a colitis specimen; W, mAb HECA-452 stained strongly a proportion of capillaries during colitis; X, a vascular endothelial reactivity with mAb MECA-79 shown in several vessels in the inflammatory focus of colitis. Original magnifications: ×200 (A, C, F, G, H, K, L, N, P, T, W, X); ×100 (B); ×400 (D, E, I, J, M, O, Q, R, S, U, V).
Figure 3.
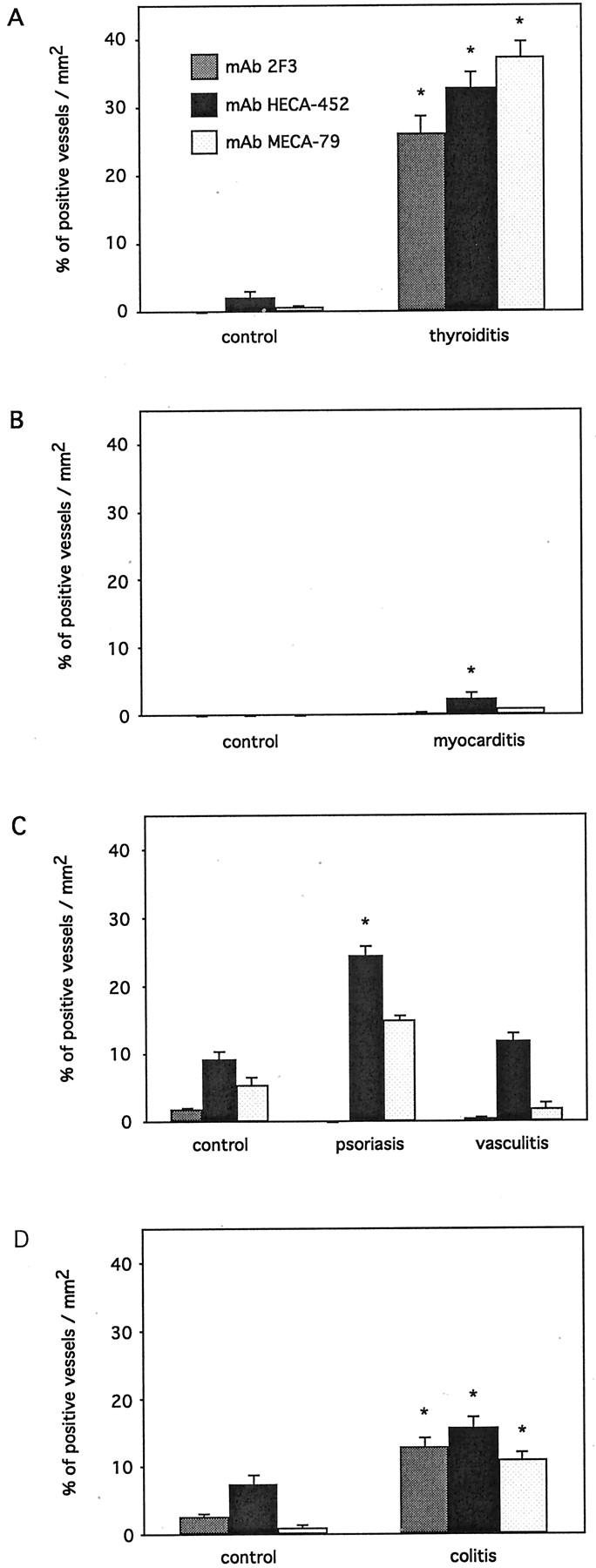
The percentages of vessels expressing sulfo sLex epitopes analyzed with two anti-sLex antibodies, mAbs 2F3 and HECA-452, and an anti-sulfated extended core 1 lactosamine antibody, mAb MECA-79 in various organs. In the Mann-Whitney U-test, differences between the expression of sulfo sLex decorations in control and inflamed specimens were considered significant at P < 0.05 and marked with an asterisk. A: Thyroiditis. B: Myocarditis. C: Psoriasis and vasculitis. D: Colitis.
The second anti-sLex antibody used, mAb HECA-452, reacted positively only in 2.1 ± 0.9% of vessels in goiter specimens with noninflammatory histology. Again, a strong induction in the endothelial mAb HECA-452 reactivity was found in thyroiditis specimens (32.8 ± 2.4%) and this increase in the expression level of mAb HECA-452 positively reactive endothelia was significant when compared to controls (P < 0.001, Mann-Whitney U-test) (Figure 2, A to F ▶ , and Figure 3A ▶ ).
To analyze the sulfated variants of functionally active L-selectin ligands on the endothelium, the antibody recognizing the extended core 1 polylactosamine structure, mAb MECA-79, was used. Although only 0.6 ± 0.3% of the vessels reacted positively with mAb MECA-79 in specimens with noninflamed histology, a strong endothelial reactivity was observed in thyroiditis specimens (37.5 ± 2.3%) (P < 0.001, Mann-Whitney U-test).
Heart
In myocarditis, a similar analysis of the endothelial expression of various glycosylation variants was performed (Figure 2 ▶ ; G to L). Although specimens with noninflamed myocardium remained essentially negative with all of the three antibodies used (Figure 3B) ▶ , myocarditis lesions showed some slight, but yet significant endothelial up-regulated expressions of sLex epitopes, detected with mAb HECA-452, rising from 0.0 ± 0.0% to 2.5 ± 0.9% (mean ± SEM, P = 0.017).
Skin
The control noninflamed skin specimens expressed a small proportion of positively stained sulfo sLex vessels (Figure 2 ▶ , mol/L-R), especially when detected with mAb HECA-452 (9.2 ± 1.3%, mean ± SEM) (Figure 3C) ▶ . In the psoriasis specimens with active skin lesions, the endothelial reactivity with mAb HECA-452 (24.6 ± 1.3%) was significantly elevated (P < 0.001, Mann-Whitney U-test). Concomitantly, the endothelial reactivity with mAb 2F3 remained totally negative.
In the skin biopsy specimens with vasculitis, the mAb 2F3 and MECA-79 reactivities remained essentially at a background level, and the staining with mAb HECA-452 was practically not altered either (9.2 ± 1.3% to 11.8 ± 1.2%).
Colon
In the colon, the endothelial sulfo sLex expression was already present in control specimens with noninflamed histology (Figure 2, S–X ▶ ). However, the endothelial expression levels analyzed with all three mAbs were even more enhanced in colitis specimens. The percentage of mAb 2F3-expressing vessels increased from 2.6 ± 0.5% to 12.8 ± 1.4%, the mAb HECA-452-reactive vessels from 7.5 ± 1.4% to 15.8 ± 1.7%, and mAb MECA-79 from 1.0 ± 0.4% to 11.0 ± 1.1% (mean ± SEM) (Figure 3D) ▶ . All these elevations in the expression of endothelial sulfo sLex variants were statistically significant between the noninflamed controls and the inflammatory specimens (P < 0.001 for each pair, Mann-Whitney U-test).
Putative Endothelial Zip Codes
In addition to the de novo-induced or -enhanced endothelial sLex or sulfo sLex expression shown in this study, we propose that each organ also possessed its own sLex- or sulfo sLex-specific zip code. A zip code could be determined by combining the percentages of reactive vessels for each of the three mAbs used, as shown in Table 2 ▶ . Thus the glycosylation zip code for endothelium in thyroid gland would be 00-02-00 during noninflamed conditions and 26-33-38 during thyroiditis. Similarly the sulfo sLex-specific zip code for heart endothelium is normally 00-00-00, 00-003-00 during mild myocarditis and 38-25-45 during severe allograft rejections (Table 2) ▶ .
Table 2.
The Organ-Specific Endothelial Zip Codes
| 2F3-HECA-MECA | Origin of biopsies | ||
|---|---|---|---|
| Normal | Inflamed | ||
| Skin | 02-09-06 | 00-25-15 | Psoriasis |
| 01-12-02 | Vasculitis | ||
| Thyroid gland | 00-02-00 | 26-33-38 | Thyroiditis |
| Heart | 00-00-00 | 00-03-00 | Myocarditis |
| 38-25-45 | Fulminant heart allograft rejection | ||
| Colon | 03-08-01 | 13-16-11 | Ulcerative colitis |
| Lungs | 00-00-00 | 05-05-06 | Bronchial asthma |
First two digits represent the percentages of mAb 2F3-reactive vessels, second two digits for mAb HECA-452-reactive vessels, and third two digits for mAb MECA-79-reactive vessels. Data pooled from this study as well as from our earlier works. 3,4,19
Discussion
The present study with more than 400 histological specimens from inflammatory diseases and their relevant noninflamed control tissues showed that control specimens did not essentially express sulfoglycans when detected by a panel of mAbs 2F3, HECA-452, and MECA-79. In contrast, the relevant tissue specimens obtained from the inflammatory sites from patients with thyroiditis, myocarditis, psoriasis, vasculitis, or ulcerative colitis showed significant increases of the endothelial expressions of the glycan decorations being detectable with one, two, or all three mAbs 2F3, HECA-452, and MECA-79. Because this triplet is a representative of functionally active L-selectin ligands, we suggest that the de novo-induced glycans expressed on endothelial glycoproteins could be of importance and involved both in the lymphocyte traffic and in the generation of lymphocytic infiltration, the two common hallmarks for all of these inflammatory conditions.
These observations are well in line with previously published data and extend further the earlier results with both rodent and human inflammatory lesions, such as allograft rejection. 2,4,14,19,21 In rat kidney and heart allograft studies, the venous endothelium expressed de novo sLex glycans at the onset of rejection. This phenomenon did presumably promote the lymphocyte extravasation into tissue parenchyma as shown with anti-sLex antibodies, the L-selectin-IgG fusion protein, as well as with the ex vivo Stamper-Woodruff lymphocyte-binding assay. 14,15,22 In addition, after synthesizing multivalent sLex polylactosamine glycans with different organ specificities, we were able to show that these extended sialyl diLex glycans were able to inhibit lymphocyte binding to heart endothelium with 10-fold lower IC50 values when compared to the lymphocyte binding to lymph node endothelium in the same animals. 23 Thus, the manipulation of the regulation of sLex ligand expression could provide means to a site-specific immunosuppression, by inhibiting lymphocyte traffic only to a given organ, but not interfering the common normal host immunity in lymph nodes.
For this study, we selected five different inflammatory diseases and compared the endothelial expression of sLex- and sulfo sLex-decorated L-selectin ligands in inflamed tissues to their noninflamed counterparts. In lymphocytic thyroiditis, there is a marked and intense infiltrate of lymphocytes and plasma cells in thyroid gland that finally and virtually ends up in replacing the actual thyroid parenchyma and, thus, the histology of a late-stage disease may resemble the histology of lymphatic tissue. Myocarditis is defined as an inflammatory involvement of the heart muscle characterized by a leukocyte infiltrate and the necrosis or degeneration of myocytes. In psoriasis, the most typical lesions are well-demarcated pink plaques, which have a characteristic marked epidermal thickening and a superficial lymphocytic infiltrate, but the most diagnostic sign is the great number of neutrophils forming small aggregates. The term vasculitis is applied to any blood vessel inflammation. The classic histological feature is an aggregation of neutrophils within and about venules, which can in severe cases lead to a lymphocyte infiltration of venule walls. Ulcerative colitis is a common recurrent disease expressing both active inflammatory ulceration areas and healing areas, both showing a microscopic evidence of inflammation. Histologically, the active phase is characterized by crypt abscesses and ulcerations extending down to the muscular layer and surrounded by a prominent mucosal infiltrate of inflammatory cells. 24
Recently, a novel sulfotransferase (HEC-GlcNAc6ST or LSST) responsible for synthesizing the 6 sulfation of GlcNAc has been cloned. 25,26 The gene deletion of this high endothelial venule-specific enzyme results in a significantly hampered lymphocyte traffic to lymph nodes in vivo. 27 Because such sulfations could be of a crucial importance for the recruitment of lymphocytes into the site of inflammation, the presence and the role of such enzymes warrant study in future. The endothelial expressions of MECA-79 epitopes, sulfated extended core 1 lactosamine structures, 28 have been shown earlier to be induced during inflammation at the sites of acute transplant rejection, bronchial asthma, as well as inflamed muscle or skin lesions. 3,29,30 Here we expand these observations and show that endothelial mAb MECA-79 reactivity was present in many sites of inflammations, such as thyroiditis, psoriasis, ulcerative colitis, asthma, and organ transplant rejections.
In addition to the de novo-induced or -enhanced endothelial sLex or sulfo sLex expression shown in this study, we propose that each organ could also possess its own sLex- or sulfo sLex-specific zip code. When expressed as a percentage of reactive vessels for each of the three mAbs used, we could designate these zip codes as shown in Table 2 ▶ . These induced expression levels of glycans that fulfill the criteria of L-selectin ligands suggest that these glycans could be crucial in the initiation of leukocyte extravasation into sites of inflammations. To test this hypothesis either an indirect Stamper-Woodruff type of ex vivo lymphocyte-binding assay could be performed in each organ, but this would require the use of fresh and not formalin-fixed tissues. Another more direct approach could be to isolate the endothelial glycoproteins, such as CD34, known to act as L-selectin ligands and directly analyze with mass spectrometry the O-glycans on both normal and inflamed tissues and testing their ability to support L-selectin-dependent leukocyte rolling. We have already began the latter approach and characterized the glycans on CD34 of tonsillar high endothelial venules. 13
Furthermore, we were able to observe differences even between the inflammations of the same organ, such as between psoriasis and vasculitis in the skin. Nonetheless, whether this observation was caused by the severity of the inflammatory incidence or by the difference in the putative pathogenesis of lymphocytic infiltration remains to be defined. However, our observations about inducible organ- and inflammation-specific zip codes could be understood as an extension to the previously reported organ-specific peptides and their ligands. 31-34 Both the glycan and peptide specificities of various organs could serve as the means of targeted interventions for re-circulating cells, whether or not these cells are leukocytes or malignant tumor cells during hematogenous metastasis.
The down-regulation of the endothelial expression of sulfo sLex-bearing glycoforms is taking place concomitantly with the inflammation resolving. 2 As previously shown, of six or more human α1,3-fucosyltransferases, FucTVII is the one capable of synthesizing sLex glycans at the level of endothelial expression and, thus, linked to the induction of heart allograft rejections. 23,35,36 The studies with L-selectin-deficient mice as well as with FucTVII-null or FucTIV and FucTVII double-null animals have demonstrated and supported the importance of L-selectin in the extravasation of lymphocytes into lymph nodes and inflamed tissues. 37-39
Taken together, we show here that the de novo induction of endothelial sLex or sulfo sLex glycans is a common event in all organs analyzed here and, thus, suggests that these glycans play a crucial role in the early-state induction of tissue inflammation. In addition, because each organ was shown to carry its own zip code, ie, a specific modification of sLex or sulfo sLex glycans, it suggests that this phenomenon could putatively provide a mean for the organ-selective inhibition of leukocyte trafficking.
Acknowledgments
We thank Eriika Wasenius for excellent technical assistance and Maaret Helintö for expertise in statistics.
Footnotes
Address reprint requests to Risto Renkonen, Biomedicum and Haartman Institute, P.O. Box 63 (Haartmaninkatu 8), FIN-00014 University of Helsinki, Helsinki, Finland. E-mail: risto.renkonen@helsinki.fi.
Supported in part by research grants from the Academy of Finland and the Technology Development Center, Helsinki; the Sigrid Juselius Foundation; grants from the Helsinki University Central Hospital Fund; and a grant from the Heart Association, Helsinki, Finland.
References
- 1.Rosen SD: Endothelial ligands for L-selectin. From lymphocyte recirculation to allograft rejection. Am J Pathol 1999, 155:1013-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toppila S, Paavonen P, Nieminen M, Hayry P, Renkonen R: Endothelial L-selectin ligands are likely to recruit lymphocytes into rejecting human heart transplants. Am J Pathol 1999, 155:1303-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toppila S, Paavonen T, Laitinen A, Laitinen L, Renkonen R: Endothelial L-selectin ligands in bronchial asthma but not in other chronic inflammatory lung diseases. Am J Respir Cell Mol Biol 2000, 23:492-498 [DOI] [PubMed] [Google Scholar]
- 4.Kirveskari J, Paavonen T, Hayry P, Renkonen R: De novo induction of endothelial L-selectin ligands during kidney allograft rejection. J Am Soc Nephrol 2000, 11:2358-2365 [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Williams M, Youngman K, Rott L, Briskin M: Lymphocyte trafficking and regional immunity. Adv Immunol 1999, 72:209-253 [DOI] [PubMed] [Google Scholar]
- 6.Lowe JB: Glycosylation, immunity, and autoimmunity. Cell 2001, 104:809-812 [DOI] [PubMed] [Google Scholar]
- 7.Vestweber D, Blanks JE: Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 1999, 79:181-213 [DOI] [PubMed] [Google Scholar]
- 8.Palframan RT, Jung S, Cheng CY, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH: Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med 2001, 194:1361-1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC: CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med 2001, 194:1541-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paavonen T, Renkonen R: Selective expression of sialyl-Lewisx and sialyl Lewisa, putative ligands for L-selectin, on peripheral lymph node high endothelial venules. Am J Pathol 1992, 141:1259-1264 [PMC free article] [PubMed] [Google Scholar]
- 11.Varki A: Selectin ligands: will the real ones please stand up? J Clin Invest 1997, 99:158-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmerich S, Rosen SD: Carbohydrate sulfotransferases in lymphocyte homing. Glycobiology 2000, 10:849-856 [DOI] [PubMed] [Google Scholar]
- 13.Satomaa T, Renkonen O, Helin J, Kirveskari J, Mäkitie A, Renkonen R: O-glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood 2002, 99:2603-2611 [DOI] [PubMed] [Google Scholar]
- 14.Turunen J, Majuri M, Seppo A, Tiisala S, Paavonen T, Miyasaka M, Lemström K, Penttilä L, Renkonen O, Renkonen R: De novo expression of endothelial sialyl Lewis a and sialyl Lewis x during cardiac transplant rejection. Superior capacity of a tetravalent sLex-oligosaccharide in inhibiting L-selectin-dependent lymphocyte adhesion. J Exp Med 1995, 182:1133-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renkonen O, Toppila S, Penttilä L, Salminen H, Helin J, Maaheimo H, Costello C, Turunen J, Renkonen R: Enzyme-assisted synthesis of a tetravalent sialyl Lewis x glycan, derived from a linear polylactosamine. Glycobiology 1997, 7:453-461 [DOI] [PubMed] [Google Scholar]
- 16.Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJLM, Butcher EC: High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol 1988, 130:147-155 [PMC free article] [PubMed] [Google Scholar]
- 17.Streeter PR, Rouse BTR, Butcher EC: Immunohistological and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol 1988, 107:1853-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmori K, Takada A, Ohwaki I, Takahashi N, Furukawa Y, Maeda M, Kiso M, Hasegawa A, Kannagi M, Kannagi R: A distinct type of sialyl Lewis X antigen defined by a novel monoclonal antibody is selectively expressed on helper memory T cells. Blood 1993, 82:2797-2805 [PubMed] [Google Scholar]
- 19.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ: The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol 1993, 143:1688-1698 [PMC free article] [PubMed] [Google Scholar]
- 20.Sassetti C, Tangemann K, Singer M, Kershaw D, Rosen S: Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med 1998, 187:1965-1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalkanen S, Saari S, Kalimo H, Lammintausta K, Vainio E, Leino R, Duijvestijn A, Kalimo K: Lymphocyte migration into the skin: the role of lymphocyte homing receptor (CD44) and endothelial cell antigen (HECA-452). J Invest Dermatol 1990, 94:786-792 [DOI] [PubMed] [Google Scholar]
- 22.Toppila S, Lauronen J, Mattila P, Turunen J, Penttilä L, Paavonen T, Renkonen O, Renkonen R: L-selectin ligands in rat high endothelium. Multivalent sialyl Lewis x glycans are high affinity inhibitors of lymphocyte adhesion. Eur J Immunol 1997, 27:1360-1365 [DOI] [PubMed] [Google Scholar]
- 23.Toppila S, Renkonen R, Penttilä L, Natunen J, Salminen H, Helin J, Maaheimo H, Renkonen O: Enzymatic synthesis of α3′sialylated and multiply α3fucosylated biantennary polylactosamines. A bivalent sialyl diLex-saccharide inhibited lymphocyte endothelial adhesion organ selectively. Eur J Biochem 1999, 261:208-215 [DOI] [PubMed] [Google Scholar]
- 24.Cotran R, Kumar V, Collins T: Pathologic Basis of Disease, ed 6 1999. WB Saunders Co., Philadelphia
- 25.Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo FR, Huang CC, Kannagi R, Rosen SD, Hemmerich S: Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J Cell Biol 1999, 145:899-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh JC, Izawa D, Tanaka T, Miyasaka M, Lowe JB, Fukuda M: A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis(x), an L-selectin ligand displayed by CD34. Immunity 1999, 11:79-89 [DOI] [PubMed] [Google Scholar]
- 27.Hemmerich S, Bistrup A, Singer M, van Zante A, Lee J, Tsay D, Peters M, Carminati J, Brennan T, Carver-Moore K, Leviten M, Fuentes M, Ruddle N, Rosen S: Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 2001, 15:237-247 [DOI] [PubMed] [Google Scholar]
- 28.Yeh J-C, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindgaul O, Marth JD, Lowe JB, Fukuda M: Novel sulfated lymphocyte homing receptors and their control by a core 1 extension β1,3-N-acetylglucosaminyltransferase. Cell 2001, 105:957-969 [DOI] [PubMed] [Google Scholar]
- 29.Jimi T, Wakayama Y, Murahashi M, Inoue M, Shibuya S, Yamashita S, Misugi N, Kobayashi T: Expression of selectin families and their ligand sialyl Lewis X in the muscles of inflammatory myopathies: an immunohistochemical study. Intern Med 1999, 38:632-635 [DOI] [PubMed] [Google Scholar]
- 30.Lechleitner S, Kunstfeld R, Messeritsch-Fanta C, Wolff K, Petzelbauer P: Peripheral lymph node addressins are expressed on skin endothelial cells. J Invest Dermatol 1999, 113:410-414 [DOI] [PubMed] [Google Scholar]
- 31.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R: Tumor targeting with a selective gelatinase inhibitor. Nature Biotechnol 1999, 17:768-774 [DOI] [PubMed] [Google Scholar]
- 32.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E: Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 2000, 60:722-727 [PMC free article] [PubMed] [Google Scholar]
- 33.Ruoslahti E: Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol 2000, 10:435-442 [DOI] [PubMed] [Google Scholar]
- 34.Ruoslahti E, Rajotte D: An address system in the vasculature of normal tissues and tumors. Annu Rev Immunol 2000, 18:813-827 [DOI] [PubMed] [Google Scholar]
- 35.Niemela R, Natunen J, Majuri ML, Maaheimo H, Helin J, Lowe JB, Renkonen O, Renkonen R: Complementary acceptor and site specificities of Fuc-TIV and Fuc-TVII allow effective biosynthesis of sialyl-TriLex and related polylactosamines present on glycoprotein counterreceptors of selectins. J Biol Chem 1998, 273:4021-4026 [DOI] [PubMed] [Google Scholar]
- 36.Roos C, Kolmer M, Mattila P, Renkonen R: Composition of Drosophila melanogaster proteome involved in fucosylated glycan metabolism. J Biol Chem 2002, 277:3168-3175 [DOI] [PubMed] [Google Scholar]
- 37.Tedder TF, Steeber DA, Pizcueta P: L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med 1995, 181:2259-2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maly P, Thall AD, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB: The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 1996, 86:643-653 [DOI] [PubMed] [Google Scholar]
- 39.Homeister JW, Thall A, Petryniak B, Maly P, Rogers CE, Smith PL, Kelle RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB: The α(1,3)fucosyltransferase FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 2001, 15:115-126 [DOI] [PubMed] [Google Scholar]