Abstract
Ischemia/reperfusion of mesenteric vessels is a useful model for acute vascular insufficiency and the early stages of multiorgan failure, conditions associated with high morbidity and mortality. Epidermal growth factor (EGF) is a potent mitogen that shows potential for use in intestinal injury. We therefore examined its influence on this model. Male Sprague-Dawley rats received human recombinant EGF (2 mg/kg i.p., n = 14) or saline (n = 16); 25 minutes before arterial clamping of the superior mesenteric artery (ischemic period) for 60 minutes followed by a final 60-minute reperfusion period. Additional rats were not operated on (controls, n = 7) or had sham operation (laparotomy only, n = 10). Ischemia/reperfusion caused macroscopic damage affecting 56%, 51 to 67% (median, interquartile range), of small intestinal length and intraluminal bleeding. Malondialdehyde levels (free radical marker) increased eightfold compared to nonoperated animals (2400, 2200 to 2700 μmol/mg protein versus 290, 250 to 350 μmol/mg protein, P < 0.01) and myeloperoxidase levels (marker for inflammatory infiltrate) increased 15-fold (3150, 2670 to 4180 U/g tissue versus 240, 190 to 250 U/g tissue, P < 0.01). Pretreatment with EGF reduced macroscopic injury to 11%, 0 to 15%; prevented intraluminal bleeding; and reduced malondialdehyde and myeloperoxidase levels by ∼60% and 90% (all P < 0.01 versus non-EGF-treated). Mesenteric ischemia/reperfusion also damaged the lungs and kidneys and increased serum tumor necrosis factor-α levels (circulating cytokine activity marker). EGF pretreatment also reduced these changes. These studies provide preliminary evidence that EGF is a novel therapy for the early treatment or prevention of intestinal damage and multiorgan failure resulting from mesenteric hypoperfusion.
Multiple organ failure is a severe, life-threatening condition that usually occurs as a result of major trauma, burns, or fulminant infections. Whatever the initiating event, once established, multiple organ failure has a high mortality (up to 80%). 1 The pathophysiological mechanisms underlying this condition are unclear although important contributory factors probably include hypoxia, increased intestinal permeability, bacterial translocation, endotoxemia, and uncontrolled systemic inflammatory responses. 2
Several studies suggest that the splanchnic circulation is particularly vulnerable to hypoperfusion, as occurs in low-flow states such as hemorrhagic shock and that this hypoperfusion is out of proportion to the overall reduction in cardiac output. 3 Although it is obvious that tissue ischemia initiates a series of events that can ultimately lead to cellular dysfunction and necrosis, resumption of blood flow can paradoxically create more tissue injury, possibly because of production of oxygen-derived cytotoxic products. 4 The use of ischemia/reperfusion models of injury, therefore, not only have relevance to acute vascular disruption (thrombosis, embolism), but also to the pathogenesis of development of multiorgan failure.
Epidermal growth factor (EGF) is a 53 amino acid peptide that is produced by the salivary glands and Brunners’ glands of the duodenum. It is a potent stimulant of proliferation and healing of the gastrointestinal tract, acting as a cytoprotective agent and also stabilizing cells against noxious agents such as indomethacin. 5 In addition, we have shown that EGF can prevent hepatic injury induced by carbon tetrachloride 6 and multiorgan injury induced by thioacetamide. 7 However, because the pathophysiological relevance of administration of chemical toxins is limited, we decided to examine a more clinically relevant rat model that reproduces acute ischemia/reperfusion injury.
Materials and Methods
EGF and Tumor Necrosis Factor (TNF)-α
Human recombinant (hr)-EGF expressed in Saccharomyces cerevisiae was obtained from HeberBiotc S.A. (Havana, Cuba). This product consists of a 60:40 mixture of EGF1–52 and EGF1–51 and is as biologically active as the full-length EGF1–53 form. 8 Before administration, EGF was diluted in 0.9% saline and sterilized by passage through a 0.22-μm filter. Solutions were always freshly prepared under sterile conditions. The hr-TNF-α (with specific activity of 107 U/mg), used in the TNF-α bioassay, was purchased from Boehringer Mannheim, GmbH, Mannheim, Germany.
Animal Ethics and Protocol
Ethics
Animal care and experimentation fulfilled the criteria for standards issued by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Local and national regulatory approval was obtained. Animals were acclimatized for ∼10 days before the study.
Induction of Ischemia/Reperfusion Injury
Nonfasted male Sprague-Dawley rats (age, 8 weeks; weight, 220 to 250 g) were induced with ketamine-HCl (10 mg/kg i.p.; Parke-Davis, Barcelona, Spain) and xylazine (3 mg/kg i.p.; Bayer, Malmo, Sweden). Additional doses of this combination were used for maintenance of anesthesia as required. The surgical procedures were conducted using a thermal blanket (40°C) to maintain body temperature.
Experimental Protocol
Intestinal ischemia/reperfusion injury was produced by causing complete occlusion of the anterior mesenteric artery followed by a period of reperfusion. 9 Briefly, the abdomen was shaved and opened through a midline abdominal incision. The anterior mesenteric artery was identified and clamped for 60 minutes, using surgical microvascular clamps (Moria clamps; Fine Science Tools, Foster City, CA), followed by releasing the clamps for a further 60-minute period. During ischemia/reperfusion, abdominal organs were covered with moistened gauze in warm (38°C) saline solution. At the end of the reperfusion period, the animals were killed. Before performing these procedures, the animals also received a 1-ml i.p. injection of EGF (2 mg/kg in saline, n = 14) or saline alone (n = 16), 25 minutes before arterial clamping. This dose of EGF was chosen because it is similar to values that influenced CCl4-induced liver injury 6 and a small dose-ranging pilot study, examining whether the gross macroscopic injury was influenced by EGF, had failed to demonstrate any major beneficial effect when administered at 400 μg/kg (damaged area ∼50%, which was similar to that seen in animals undergoing ischemia/reperfusion but not given EGF).
To distinguish between the effects of ischemia/reperfusion from changes because of nonspecific surgical stress, a further group of 10 rats (sham-ischemic) was subjected to abdominal incision and their organs exposed for 120 minutes but with no clamping of the mesenteric artery. In addition, to facilitate comparisons of laboratory data, samples were also taken from seven intact nonoperated rats of the same litter for use as a reference control.
Collection and Analyses of Samples
At the end of the study period, animals were killed and blood samples collected by cardiac puncture. The thoracic and abdominal organs were inspected, and the lungs, kidneys, liver, spleen, and pancreas retrieved, and representative samples fixed in formalin for subsequent histological examination. Additional samples of lungs and kidneys were rapidly frozen and stored at −70°C until subsequent biochemical analyses (see below).
Small Intestine
The total lengths of the small intestines were measured and the intestinal luminal contents then flushed with 5 ml of saline and collected for subsequent analysis of hemoglobin content. The weights of the small intestines were recorded and then split longitudinally to allow a macroscopic assessment of percentage injured area. The percentage of damage was calculated by measuring (cm) all of the regions showing gross macroscopic changes, such as petechiae, and considering the whole length of the small intestine (in cm) as 100%. Eight equispaced 2-cm segments from the length of the small bowel were then collected for histological assessment and a further three 1-cm segments collected for biochemical analyses (see below).
Microscopic Assessment
The intestinal samples for histological assessment were extended onto cardboard and fixed in 10% buffered formalin. Tissues were then embedded in paraffin, cut into 5-μm sections and stained with hematoxylin and eosin (H&E). Mucosal damage of the small intestine was quantitatively assessed according to the grading system of Chiu and colleagues. 10 This system uses a scale of 0 to 5, where 0 = normal mucosa; 1 = development of subepithelial (Gruenhagen’s) spaces; 2 = extension of the subepithelial space with moderate epithelial lifting from the lamina propria; 3 = extensive epithelial lifting with occasional denuded villi tips; 4 = denuded villi with exposed lamina propria and dilated capillaries; and 5 = disintegration of the lamina propria, hemorrhage, and ulceration. The mean score of 30 to 40 villi from each of the eight segments for each animal were pooled to provide an average score for the intestine of that animal. All assessments were performed in a blinded manner.
Biochemical Assessment
Tissue Samples
Tissue fragments were analyzed for malondialdehyde (MDA) as a marker of lipid peroxidation and myeloperoxidase (MPO) activity as a marker of neutrophilic infiltration.
Intestinal tissues were homogenized in KCl-histidine buffer (pH 7.4) and the MDA content determined using the calorimetric method of Satoh. 11 Protein levels in the supernatant were also determined using the method of Lowry and colleagues 12 to allow MDA results to be expressed as μmol/mg of protein. Supernatant samples were also analyzed for MPO activity using o-dianisidine-H2O2 as a substrate for MPO as described by Bradley and colleagues. 13 Maximal chromogen absorption is at 460 nm and enzyme activity was expressed as U/g of tissue. Because the lungs and kidneys are also targets for neutrophilic infiltration after an episode of intestinal ischemia, fragments of these organs were also processed for MPO activity.
Blood Samples
Serum TNF-α concentrations were determined based on the cytotoxicity assay of Aggarwal and colleagues. 14 Briefly, monolayer cultures of the murine fibroblast cell line L929 were maintained in RPMI medium supplemented with 10% fetal calf serum at 37°C in 5% CO2 atmosphere. Cells (105) were added to each well of a 96-well, flat-bottom plate (Costar-Corning, MA) and cultured overnight. Fifty μl of Actinomycin D (4 μg/ml) and 50 μl of varying concentrations of hr-TNF-α standard or serum sample were then added to the culture plate. Twenty-four hours later, cytotoxicity was visualized by crystal violet staining and measured in a plate reader at 570 nm. The specificity of cytotoxicity produced by TNF-α has been previously determined by neutralization assays using a rabbit polyclonal TNF-α neutralizing antibody (R&D Systems, Minneapolis, MN). Detection limit is greater than 20 pg/ml.
Statistical Analyses
Data are expressed as median, interquartile ranges. Comparisons between groups were analyzed using the Mann-Whitney U-test and P < 0.05 was taken as statistically significant.
Results
Macroscopic Assessment of Small Intestine
Sham operation did not influence intestinal weight, macroscopic injury, or luminal hemoglobin compared to reference (nonoperated) animals (Figure 1 ▶ and Table 1 ▶ ).
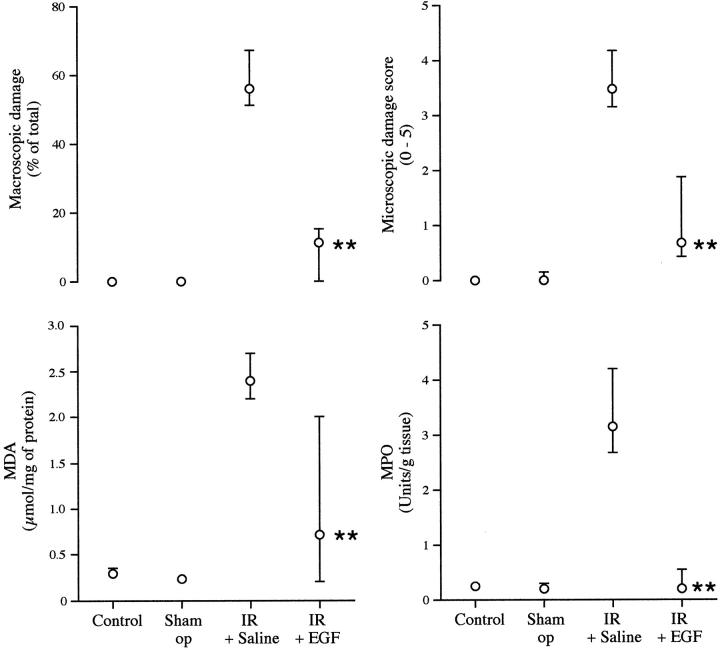
Figure 1.
Effect of EGF on the amount of small intestinal injury induced by mesenteric ischemia and reperfusion. Rats received no treatment (control, n = 7), underwent a laparotomy only (sham operation, n = 10), or laparotomy with ischemia and reperfusion (I+R). These I+R-treated animals also received either EGF (2 mg/kg i.p. in saline; n = 14) or saline alone (n = 16), 25 minutes before arterial clamping. Data are presented as median (interquartile range). Parameters shown are macroscopic damage, microscopic score, and intestinal MPO (marker for inflammatory infiltrate) and MDA levels (free radical marker). **, P < 0.01 comparing animals receiving I+R alone against animals also given EGF.
Table 1.
Effect of EGF on the Amount of Injury Induced by Mesenteric Ischemia and Reperfusion (I + R)
| Control n = 7 | Sham operation (laparotomy) n = 10 | I + R n = 16 | I + R + EGF n = 14 | |
|---|---|---|---|---|
| Small intestinal wt (g) | 11.61 (11.37–11.89) | 11.88 (11.24–12.2) | 7.65 (6.6–8.74) | 11.50† (11.15–12.15) |
| Haemoglobin levels in intestinal washout (g/dL) | 0 (0–0) | 0 (0–0) | 2.19 (2.12–3.32) | 0† (0–0) |
| MDA intestine μmol/mg protein | 0.29 (0.25–0.35) | 0.24 (0.20–0.28) | 2.40 (2.20–2.70) | 0.71† (0.21–2.00) |
| MPO intestine U/g tissue | 0.24 (0.19, 0.25) | 0.20 (0.13, 0.30) | 3.15 (2.67, 4.18) | 0.20† (0.11–0.55) |
| MPO lungs U/g tissue | 0.22 (0.11, 0.31) | 0.21 (0.16, 0.33) | 0.76 (0.64, 0.80) | 0.30† (0.16–0.35) |
| MPO kidney U/g tissue | 0.14 (0.08–0.20) | 0.12 (0.09–0.14) | 0.64 (0.47–0.77) | 0.30* (0.16–0.35) |
| Plasma TNF-α pg/mL | <20 | 57‡ (51–79) | 54 (42–67) | 22† (<20–36) |
MPO, Myeloperoxidase (marker of inflammatory infiltrate); MDA, malondialdehyde levels (free radical marker).
Data are presented as median (interquartile range).
* and † indicate P < 0.05 and < 0.01 comparing animals receiving I + R alone against animals also receiving EGF.
‡ indicates P < 0.01 of sham-operated versus control animals.
Animals who had undergone ischemia/reperfusion but did not receive EGF had a significant reduction in intestinal weight of ∼35% compared to intact or sham-operated animals (Table 1) ▶ . Ischemia/reperfusion resulted in macroscopically obvious injury affecting 56% of the intestinal length and also caused intraluminal bleeding (Figure 1 ▶ and Table 1 ▶ ).
Animals who had undergone ischemia/reperfusion and had also received EGF showed no significant reduction in intestinal weight compared to sham-operated animals (Table 1) ▶ . In these animals, macroscopic injury only affected 11% of the intestinal length, which was significantly less than that found in animals undergoing ischemia/reperfusion alone (56%, P < 0.01) (Figure 1) ▶ . Luminal hemoglobin was also below the limit of detection in these animals.
Microscopic Assessment of Small Intestine
There was severe mucosal damage, consistent with ischemic injury, ranging from loss of villi to mucosal infarction in animals that received ischemia/reperfusion alone (Figure 2A) ▶ . These changes were much less prominent in animals that had also received EGF in which the damage was usually minor, such as villus tip de-epithelialization, but with no villus destruction (Figure 2B) ▶ . Quantitative assessment showed that histological damage was reduced by ∼80% by the previous administration of EGF (Figure 1 ▶ , P < 0.01).
Figure 2.
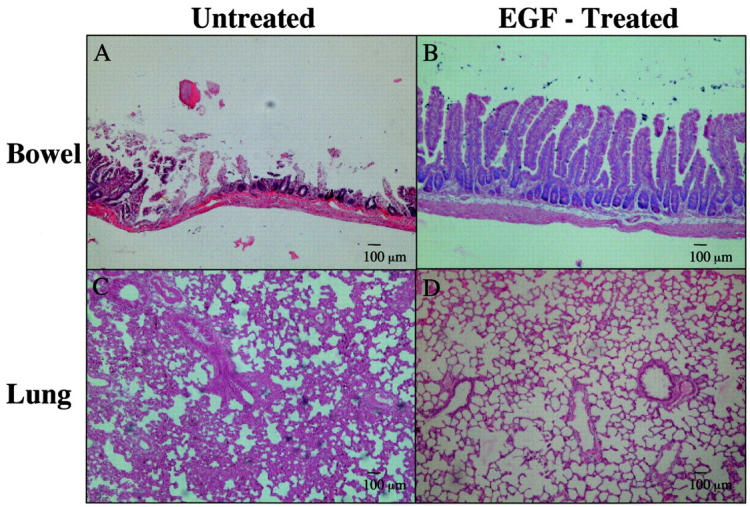
Histology of intestinal and lung tissue from animals that had received ischemia/reperfusion. Paraffin sections were stained with H&E. A: Small intestinal histology showed marked mucosal damage with extensive loss of villi and foci of almost complete mucosal necrosis. B: Intestinal injury was much less severe in animals that received EGF before the ischemia/reperfusion. C: Lung histology showed gross alveolar wall thickening, hypercellularity, and polymorph margination (individual polymorphs not seen at this magnification). D: Pretreatment with EGF markedly reduced the lung changes seen. Original magnifications, ×10; scale bars, 100 μm.
Histological Appearances of Other Organs
Animals undergoing ischemia/reperfusion showed lung changes consisting of alveolar wall thickening, hypercellularity, and neutrophilic adhesion to the endothelial surface of small pulmonary vessels (Figure 2C) ▶ . These changes appeared much less prominent in EGF-treated animals (Figure 2D) ▶ . The histology of the kidneys in all groups looked normal or showed very minor abnormalities such as occasional tubular eosinophilic deposits.
Biochemical Assessment
MDA levels were measured as a marker of lipid peroxidation. Animals that had undergone ischemia/reperfusion had an eightfold higher level of intestinal MDA compared to animals in control and sham-operated groups. Preadministration of EGF truncated this response by ∼80% (Figure 1) ▶ .
Ischemia/reperfusion caused a 15-fold increase in intestinal MPO levels (marker for neutrophilic infiltration) compared to controls. In contrast, no increase was seen in EGF-pretreated animals (Figure 1) ▶ .
MPO levels in the kidneys and lungs were increased by approximately threefold as a result of ischemia/reperfusion. As in the intestine, pretreatment with EGF markedly truncated these changes (Table 1) ▶ .
Serum Levels of TNF-α
Serum TNF-α levels were raised in animals subjected to ischemia/reperfusion but a similar rise was also seen in sham-ischemia animals (Table 1) ▶ . This showed that the rise in TNF-α was a nonspecific response to surgical stress. Pretreatment with EGF significantly reduced TNF-α levels below those seen in ischemia/reperfusion or sham-ischemia animals (Table 1) ▶ .
Discussion
We have examined the effects of pretreatment with EGF on the intestinal and extra-intestinal responses to mesenteric ischemia/reperfusion. Ischemia/reperfusion caused marked intestinal damage with ulceration, neutrophilic infiltration, and lipid peroxidation. Pretreatment with EGF markedly truncated these effects. In addition, the lung and renal injuries induced by ischemia/reperfusion were also reduced by pretreatment with EGF.
The use of mesenteric artery occlusion with reperfusion is a well-established model of intestinal injury resulting from acute vascular occlusion as occurs after embolism or thrombosis. In addition, it is used as a model for loss of the intestinal barrier function associated with hemorrhagic shock, major burns, and multiple traumas, which can result in multiorgan failure. 15 Several models have been used to mimic the early stages of multiorgan failure. Ischemia/reperfusion has the advantage of being more physiologically relevant than administration of toxic agents, such as thioacetamide, 7 because the major factors causing injury are probably internally generated proinflammatory cytokines 15,16,17 and free radical production, 4,17 rather than resulting from metabolism of an external damaging agent.
The major physiological sources of EGF production are the salivary glands, Brunner’s glands of the duodenum, and the kidney. Many studies have shown EGF to be a potent stimulant of growth for various cell types in vitro 18 and in vivo 19 and that it acts as a cytoprotective agent against gastrointestinal injury caused by a variety of noxious agents such as nonsteroidal-anti-inflammatory drug-induced gastric injury 5,20 and trinitrobenzenesulphonic acid-induced colitis. 21 In addition, we have shown recently that exogenous EGF reduced thioacetamide-induced multiorgan injury 7 in which much of the damage is thought to be mediated by free radical production. 22
The mechanisms underlying ischemia/reperfusion-induced injury and the protective effects of EGF are likely to be complex and multifactorial. During hypoxic conditions, there is an up-regulation of cell adhesion molecules, 23 facilitating recruitment of neutrophils to ischemic areas. Our studies confirmed a marked influx of inflammatory infiltrate with raised MPO levels in several organs after ischemia/reperfusion. Because EGF is known to influence cell adhesion molecule expression and function, 24,25 this may well be relevant in explaining the marked reduction of inflammatory infiltrate in the intestine of the EGF-treated animals. It is important to note, however, that the influx of inflammatory cells was not restricted to the intestine but also affected distant organs such as the lungs. This must either be because of an alteration in circulating factor(s), such as cytokines, or to the priming and activation of inflammatory cells (mainly neutrophils) at the intestinal site, that subsequently migrate to distant organs. To examine this idea further, we measured changes in circulating TNF-α because this has been reported to be one of the major cytokines raised after abdominal aortic aneurysm repair 26 and Souza and co-workers 27 have reported that TNF-α blockade (using neutralizing antibodies) is of partial benefit in reducing intestinal and lung injury caused by ischemia/reperfusion in rats. Importantly, in the current study we found that the rise in circulating TNF-α in animals undergoing laparotomy (sham-operated group) was similar to that seen in animals undergoing ischemia/reperfusion. Because lung injury and intestinal injury was only seen in the ischemia/reperfusion group, the increase in circulating TNF-α is unlikely to explain how most of the injury was caused and the reduction of plasma TNF-α concentration, seen in animals given EGF, is unlikely to be the major mechanism by which it prevented injury. Several other cytokines, such as interleukin-β, interleukin-6, and interleukin-8 are increased in such conditions 26 and further studies could potentially examine this area in more detail. Our finding of the raised TNF-α in sham-operated animals also emphasizes the importance of performing adequate controls to distinguish specific from nonspecific effects in models such as these.
Production of highly reactive oxygen species and other free radical damaging metabolites is known to occur during ischemia/reperfusion. 17,23 Uncontrolled production of such factors results in cellular damage including lipid peroxidation, as well as induction of both apoptosis and necrosis. 28,29 We found excessive free radical production in ischemia/reperfusion-treated animals, measured indirectly as markedly raised MDA levels (indicating increased lipid peroxidation). The molecular mechanisms underlying the reduction in MDA levels in EGF-treated animals may be because of several factors; It is possible that up-regulation of cellular anti-oxidant enzymes occurred in response to EGF because this has been demonstrated in rat fetal lung cells exposed to hyperoxia 30 and growth factor withdrawal from primary cultures of mouse renal tubular cells resulted in superoxide anion accumulation, resulting in cell death. 31 Further studies in this area could potentially measure changes in anti-oxidant enzyme levels in various gastrointestinal cell lines. However, it is likely that several other interrelated mechanisms are also involved: Pillai and co-workers 32 demonstrated a cytoprotective effect of heparin-binding EGF in IEC-18 cells when cultured in hypoxic conditions and considered that this was, in part, because of maintenance of cytoskeletal structure and ATP stores whereas Heck and co-workers 33 found that EGF suppressed the rise in NO and H2O2 caused by γ-interferon, lipopolysaccharide, and TNF-α and suggested that this was not caused by up-regulation of anti-oxidant enzymes but because of phosphorylation of nitric oxide synthase by EGF. A major limitation of such cell culture model systems is the absence of inflammatory cells that are likely to be of major importance in the damaging process in vivo (as demonstrated in the present study by raised MPO levels and histology). In addition, EGF also influences multiple other systems that might be important in mediating protective effects in vivo including up-regulation of prostaglandin 20 and mucus 34 production, increasing mesenteric blood flow 35,36 (which might have had relevance either before or after clamping), influencing stress-associated protein kinases 37 and affecting apoptosis, acting via the Akt survival signaling pathway. 38 Further studies could examine the changes in some of these factors in vivo although this would require a different, less damaging, protocol because of the extensive necrosis seen in the intestine of our ischemia/reperfusion-treated rats making measurement of factors in the intestinal mucosa, eg, anti-oxidative enzyme levels, of limited value.
Clinical trials of EGF are presently underway for treatment of ulcerative conditions of the bowel, such as neonatal necrotizing enterocolitis 39 and adult colitis. 40 Our studies provide preliminary evidence that EGF may also be of benefit for injury associated with mesenteric vascular hypoperfusion such as occurs during high-abdominal aortic aneurysm repair, acute vascular occlusion, and in the prevention of multiple organ failure. 15 If patients at high risk of vascular hypoperfusion can be identified at an early stage of their admission to hospital, rapid intervention with EGF may maintain organ viability. Further studies appear warranted.
Footnotes
Address reprint requests to Professor R. J. Playford, Department of Gastroenterology, Imperial College Faculty of Medicine, Hammersmith Hospital, Ducane Rd., London, UK W12 ONN. E-mail: r.playford@ic.ac.uk.
Supported in part by the Wellcome Trust Grants 047231 and 054787.
References
- 1.Zimmerman JE, Knuas WA, Sun X, Wagner DP: Severity stratification and outcome prediction for multisystem organ failure and dysfunction. World J Surg 1996, 20:401-405 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN: Current treatment of severely burned patients. Ann Surgery 1996, 1:14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeill JR, Stark RD, Greenway CV: Intestinal vasoconstriction after haemorrhage: role of vasopressin and angiotensin. Am J Physiol 1970, 219:1342-1347 [DOI] [PubMed] [Google Scholar]
- 4.Parks DA, Granger DN: Contributions of ischaemia and reperfusion to mucosal lesion formation. Am J Physiol 1986, 250:G749-G753 [DOI] [PubMed] [Google Scholar]
- 5.Playford RJ, Marchbank T, Calnan DP, Calam J, Royston P, Batten JJ, Hansen HF: Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology 1995, 108:92-101 [DOI] [PubMed] [Google Scholar]
- 6.Berlanga J, Caballero ME, Remirez D, Torres A, Valenzuela C, Lodos J, Playford RJ: Epidermal growth factor protects against carbon tetrachloride-induced hepatic injury. Clin Sci 1998, 94:219-223 [DOI] [PubMed] [Google Scholar]
- 7.Caballero ME, Berlanga J, Ramirez D, Lopez-Saura P, Gozalez R, Floyd DN, Playford RJ: Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut 2001, 48:34-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calnan DP, Fagbemi A, Berlanga-Acosta J, Marchbank T, Sizer T, Lakhoo K, Edwards AD, Playford RJ: Potency and stability of C-terminal truncated human epidermal growth factor. Gut 2000, 47:622-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baykal A, Kaynaroglu V, Demirpence E, Kilinc K, Sayek I, Sanac Y: Experimental study of the effect of adrenaline tolerance on intestinal ischaemia-reperfusion. Br J Surg 1998, 85:947-950 [DOI] [PubMed] [Google Scholar]
- 10.Chiu CJ, McArdle AH, Brown R, Scott MJ, Gurd FN: Intestinal mucosal lesion in low-flow states: a morphological, hemodynamic and metabolic reappraisal. Arch Surg 1970, 101:478-483 [DOI] [PubMed] [Google Scholar]
- 11.Satoh K: Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 1978, 90:37-43 [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with the folin phenol reagent. J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 13.Bradley PP, Priebat DA, Christensen RD, Rothstein G: Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982, 78:206-209 [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN: Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem 1985, 260:2345-2354 [PubMed] [Google Scholar]
- 15.Biffl WL, Moore EE: Splanchnic ischaemia/reperfusion and multiple organ failure. Br J Anaesth 1996, 77:59-70 [DOI] [PubMed] [Google Scholar]
- 16.Granger DN, Korthuis RJ: Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol 1995, 57:311-332 [DOI] [PubMed] [Google Scholar]
- 17.Kong SE, Blennerhassett LR, Heel KA, McCauley RD, Hall JC: Ischaemic-reperfusion injury to the intestine. Aust NZ J Surg 1998, 68:554-561 [DOI] [PubMed] [Google Scholar]
- 18.Conteas CN, Majumdar APN: The effects of gastrin, epidermal growth factor and somatostatin on DNA synthesis in a small intestinal crypt cell line (IEC-6). Proc Soc Exp Biol Med 1987, 184:307-311 [DOI] [PubMed] [Google Scholar]
- 19.Goodlad RA, Lee CY, Wright NA: Cell proliferation in the small intestine and colon of intravenously fed rats: effects of urogastrone-epidermal growth factor. Cell Prolif 1992, 25:393-404 [DOI] [PubMed] [Google Scholar]
- 20.Uribe JM, Barret KM: Nonmitogenic actions of growth factors: an integrated view of their role in intestinal physiology and pathophysiology. Gastroenterology 1997, 112:255-268 [PubMed] [Google Scholar]
- 21.Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE: Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology 1994, 107:12-17 [DOI] [PubMed] [Google Scholar]
- 22.Mesa ML, Carrizosa R, Martine Zhonduvilla C, Benito M, Fabregat I: Changes in rat liver gene expression induced by thioacetamide. Protective role of S-adenosyl-L-methionine by a gluthatione dependent mechanism. Hepatology 1996, 23:600-606 [DOI] [PubMed] [Google Scholar]
- 23.Carden DL, Granger DN: Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000, 190:255-266 [DOI] [PubMed] [Google Scholar]
- 24.Dwir O, Kansas GS, Alon R: An activated L-selectin mutant with conserved equilibrium binding properties but enhanced ligand recognition under shear flow. J Biol Chem 2000, 275:18682-18691 [DOI] [PubMed] [Google Scholar]
- 25.Solic N, Davies DE: Differential effects of EGF and amphiregulin on adhesion molecule expression and migration of colon carcinoma cells. Exp Cell Res 1997, 234:465-476 [DOI] [PubMed] [Google Scholar]
- 26.Bown MJ, Nicholson ML, Bell PR, Sayers RD: Cytokines and inflammatory pathways in the pathogenesis of multiple organ failure following abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2001, 22:485-495 [DOI] [PubMed] [Google Scholar]
- 27.Souza DG, Cassali GD, Poole S, Teixeira MM: Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br J Pharmacol 2001, 134:985-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999, 15:233-249 [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval M, Zhang XJ, Liu X, Mannick EE, Clark DA, Miller MJ: Peroxynitrite-induced apoptosis in T84 and RAW 264.7 cells: attenuation by L-ascorbic acid. Free Radic Biol Med 1997, 22:489-495 [DOI] [PubMed] [Google Scholar]
- 30.Price LT, Chen Y, Frank L: Epidermal growth factor increases antioxidant enzyme and surfactant system development during hyperoxia and protects fetal lungs in vitro from hyperoxic toxicity. Pediatric Res 1993, 34:577-585 [DOI] [PubMed] [Google Scholar]
- 31.Lieberthal W, Triaca V, Koh JS, Pagano PJ, Levine JS: Role of superoxide in apoptosis induced by growth factor withdrawal. Am J Physiol 1998, 275:F691-F702 [DOI] [PubMed] [Google Scholar]
- 32.Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE: Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res 1999, 87:225-231 [DOI] [PubMed] [Google Scholar]
- 33.Heck DE, Laskin DL, Gardner CR, Laskin JD: Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem 1992, 267:21277-21280 [PubMed] [Google Scholar]
- 34.Saroseik J, Bilski J, Murty VLN, Slomiany A, Slomiany BL: Role of epidermal growth factor in the maintenance of physico-chemical characteristics of oral and gastric mucus coat. Biochem Biophys Res Commun 1988, 152:1421-1427 [DOI] [PubMed] [Google Scholar]
- 35.Gan BS, Hollenberg MD, MacCannel KL, Lederis K, Winkler ME, Derynck R: Distinct vascular actions of epidermal growth factor-urogastrone and transforming growth factor α. J Pharmacol Exp Ther 1987, 242:331-337 [PubMed] [Google Scholar]
- 36.Tepperman BL, Soper BD: Effect of epidermal growth factor, transforming growth factor α and nerve growth factor on gastric mucosal integrity and microcirculation in the rat. Regul Pept 1994, 50:13-21 [DOI] [PubMed] [Google Scholar]
- 37.Logan SK, Falasca M, Hu P, Schlessinger J: Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-jun N-terminal kinase signalling pathway. Mol Cell Biol 1997, 17:5784-5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL: Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. Requirement for Akt activation. J Biol Chem 1999, 274:17612-17618 [DOI] [PubMed] [Google Scholar]
- 39.Sullivan PB, Lewidon PJ, Oppenheimer SJ, Cheng C, Goodlad RA, Wright NA: A pilot study of the safety and efficacy of epidermal growth factor in the treatment of necrotising enterocolitis. Gut 1997, 41(Suppl 3):A69 [Google Scholar]
- 40.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ: Epidermal growth factor enemas are effective in the treatment of left-sided ulcerative colitis. Gastroenterology 2001, 120(Suppl 1):A11. [DOI] [PubMed] [Google Scholar]