Abstract
RB1-inducible coiled-coil 1 (RB1CC1) is a nuclear DNA-binding protein that can induce RB1 (retinoblastoma 1) expression. RB1CC1 is abundantly expressed in human musculoskeletal and cultured osteosarcoma cells. The present study analyzed the expression of RB1CC1 and RB1 in osteosarcoma cells and in musculoskeletal cells of human embryos to evaluate the contribution of both genes to the maturational process of musculoskeletal cells. The amount of RB1CC1 message was closely related to RB1 expression in various osteosarcoma cell lines. RB1CC1 expression was difficult to detect in immature proliferating chondroblasts or myogenic cells in human embryos, but became obvious and prominent concomitantly with the maturation of osteocytes, chondrocytes, and skeletal muscle cells. RB1CC1 expression in these musculoskeletal cells increased with RB1 expression, which is linked to the terminal differentiation of many tissues and cells. In addition, the introduction of wild-type RB1CC1 decreased the formation of macroscopic colonies in the cell growth assay. Accordingly, both RB1CC1 and RB1 genes preferentially co-expressed and contributed to the maturation of human embryonic musculoskeletal cells, and may regulate the proliferative activity and maturation of tumor cells derived from these tissues.
We identified RB1CC1 (RB1-inducible coiled-coil 1) as a novel nuclear DNA-binding protein that can induce RB1 (retinoblastoma 1) expression. 1 Our previous study 1 also demonstrated abundant RB1CC1 expression in skeletal muscle tissues and in one human osteosarcoma cell line, U-2 OS. Thus, the RB1CC1 gene product is expected to play significant roles on human musculoskeletal cells; however, the expressional status of RB1CC1 has not been precisely evaluated in human musculoskeletal tissue yet.
We examined two osteosarcoma cell lines in a preliminary study. U-2 OS with intact RB1 protein expressed high levels of RB1CC1, whereas Saos-2 without intact RB1 did not. RB1 is best known for its function in the control of cell cycle progression. 2,3 Mutations of this gene alter biological behaviors and are related to a poor prognosis in patients with musculoskeletal sarcomas. 4-6 Products of the RB1 gene family are linked to the terminal differentiation of many tissues. 7 Therefore, the status of RB1CC1 and RB1 expression in human musculoskeletal cells should be examined from the viewpoint of a correlation between differentiation and proliferation.
To evaluate the correlation between RB1CC1 and RB1 during the maturation of human musculoskeletal cells, we compared the expression of these two genes in human embryonic tissues and various human osteosarcoma cell lines.
Materials and Methods
Cell Culture
Ten human osteosarcoma cell lines listed below were maintained at 37°C under a humidified 5% CO2 atmosphere in Iscove’s modified Dulbecco’s medium supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml; Gibco BRL, Paisley, Scotland), and 10% heat-inactivated fetal calf serum (Biological Industries, Kibbutz Beth Hemak, Israel). The medium was exchanged every 3 to 4 days. Saos-2, U-2 OS, and HOS cell lines were purchased from the American Type Culture Collection, Rockville, MD. SARG and IOR/OS9 cell lines have been characterized. 8,9 IOR/OS10, 14, 15, 18, and MOS cell lines were obtained from surgical specimens of high-grade human osteosarcomas at the Istituto Ortopedici Rizzoli, Bologna, Italy. 10
Isolation of cDNA Fragments
Total RNA was extracted from osteosarcoma cell lines using Trizol reagent (Gibco). Complementary DNA was synthesized from U-2 OS total RNA using SuperScript II reverse transcriptase and Oligo d(T)12–18 primer (Gibco). Human RB1CC1 and RB1 primers were designed based on the cDNA sequences (RB1CC1, GenBank accession no. AB059622; RB1, GenBank accession no. NM_000321). The primer sequences for RB1CC1 were CC1-S, 5′-CTA GAA CAA CTT GAA GAG CA-3′ and CC1-A, 5′-CGA GCT CGA TCT TCA GAA AG-3′ (224-bp amplified region). Those of RB1 were RB1-S, 5′-ACC AGA TCA TGT CAG AGA GA-3′ and RB1-AS, 5′-ACT GCT GGG TTG TGT CAA AT-3′ (340-bp amplified region). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer pairs were obtained from Clontech, Palo Alto, CA (983-bp product). The reverse-transcribed products were amplified by polymerase chain reaction (PCR) using the appropriate set of primers. The PCR cycling conditions were 95°C for 20 seconds, 55°C for 20 seconds, and 72°C for 30 seconds using GeneAmp PCR System 9600 (Perkin Elmer, Norwalk, CT). Amplified products were identified by 2% agarose gel electrophoresis after staining with ethidium bromide. The reverse transcriptase-PCR fragments were cloned using the Original TOPO-TA Cloning Kit (Invitrogen, Groningen, The Netherlands), and sequenced using BigDye Terminator Cycle Sequencing FS Ready Reaction Kits and an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, CA), according to the manufacturers’ protocols. To confirm the specificity of isolated cDNA fragments, their nucleotide sequences were searched in public databases using the BLAST program.
Northern Blots
We assessed RB1CC1 and RB1 expression in various human osteosarcoma cell lines by Northern blotting. Equal amounts of total RNAs (20 μg) were resolved by electrophoresis on 1% agarose gels containing 20 mmol/L 3-morpholino propanesulfonic acid (MOPS) buffer, 5 mmol/L sodium acetate, 1 mmol/L ethylenediaminetetraacetic acid, and 6% formaldehyde then transferred to nylon membranes (Roche, Mannheim, Indianapolis, IN) in 10× standard saline citrate. Blots were cross-linked with ultraviolet light. To analyze RNAs, cloned fragments corresponding to both genes were digested with the appropriate restriction enzymes, excised from the gels, labeled with [α-32P] dCTP (3000 Ci/mmol; Amersham, Piscataway, NJ) by random priming, then hybridized with the membranes at 42°C overnight in 50% formamide containing 1% sodium dodecyl sulfate, 1 mol/L NaCl, 200 mg/ml sonicated herring sperm DNA, and 10% dextran sulfate. After hybridization, the blots were washed twice at 42°C in 2× standard saline citrate containing 1% sodium dodecyl sulfate, and once with 0.2× standard saline citrate containing 1% sodium dodecyl sulfate for 5 minutes at 65°C. Autoradiography was finally performed using a Kodak BioMax and intensifying screens (Kodak, New Haven, CT). GAPDH hybridization was used as a reference to confirm the amounts of mRNA in each lane.
Human Embryonic Materials
Five normal embryos were originally obtained from women who had spontaneously aborted at an estimated 4 to 8 weeks after ovulation according to Carnegie’s criteria. 11 These embryos were suitable to evaluate the development of human musculoskeletal cells but not for hemopoietic islets in the bone marrow because it is not significant until the fourth to the fifth month. 11-13 The embryos were selected from the surgical pathology file at our university hospital and all of them were fixed in 10% buffered formalin and embedded in paraffin. Serially sliced 4-μm sections were deparaffinized before use. All experiments using these embryos proceeded with respect to human rights under the regulations of Japanese Legal Controls and the Ethics Committee of our institution.
Detection of RB1CC1 mRNA
RB1CC1 message was detected by in situ hybridization using an RB1CC1-specific cRNA probe. RB1CC1 cDNA fragment cloned into pCR II-TOPO vector (Invitrogen) was linearized, then run-off incorporation of digoxigenin-labeled UTP was performed using a T7/Sp6 RNA labeling kit (Roche). Embryonic tissue sections were digested with pepsin (2.5 mg/ml in 0.1 N HCl) at 37°C for 30 minutes, and dehydrated in absolute ethanol. The sections were hybridized overnight with 1 μg/ml of digoxigenin-labeled RB1CC1-specific cRNA probe in hybridization solution at 37°C, then washed three times in 1× Tris-buffered saline containing 0.05% Tween 20. All reagents used until this step were supplied by Kreatech (Amsterdam, Netherlands) and the protocol was followed according to the supplier’s recommendations. Subsequently, the sections were incubated with 0.2 μg/ml of anti-digoxigenin mouse IgG1 (Roche) for 60 minutes, followed by biotin-labeled secondary antibody. Finally, sections were exposed to peroxidase conjugated with streptavidin using DAKO Universal Quick Stain kit (DAKO Japan, Kyoto, Japan). Peroxidase activity was detected using the chromogen diaminobenzidine and the sections were counterstained with methyl green. The positive control was an RNA-positive control poly-A oligo-probe (Kreatech), and negative controls used an RB1CC1-specific sense RNA probe.
Immunohistochemical Detection of RB1CC1 and RB1
RB1CC1 and RB1 expression in the musculoskeletal tissues of human embryos was detected using a three-step streptavidin-biotin immunoperoxidase stain and anti-human RB1CC1 rabbit polyclonal antibody (anti-RBICC-642 1 ) or anti-RB1 mouse monoclonal antibody (G3-245; PharMingen, San Diego, CA), respectively, as the primary antibodies. Before staining, deparaffinized embryonic tissue sections were exposed to 0.3% H2O2, autoclaved at 120°C for 1 minute to unmask antigen, and rinsed with 1× Tris-buffered saline. The sections were then incubated with the primary antibody at 4°C overnight, and stained using streptavidin-biotin-peroxidase complex. Primary antibody was replaced with normal rabbit or mouse antisera for negative controls.
Cell Growth Assay
The colony formation assay using the EBC-1 cell line, which expresses low levels of endogenous RB1CC1 and RB1, was performed. The cells were grown to ∼70% confluence in 60-mm dishes, and transfected with pCR-RBICC (2 μg), RB1CC1 expression vector; 1 or pCR3.1 control vector (2 μg, Invitrogen) by Lipofectamine Plus reagent (Gibco). G418 (800 μg/ml) was added to the culture medium 48 hours after transfection. The cells were incubated for 14 days, fixed with 10% acetate/10% methanol for 15 minutes, and stained with 0.4% crystal violet in 20% ethanol for 15 minutes to visualize the colonies.
Results
Synchronized Expression of Both RB1CC1 and RB1 in Various Human Osteosarcoma Cell Lines
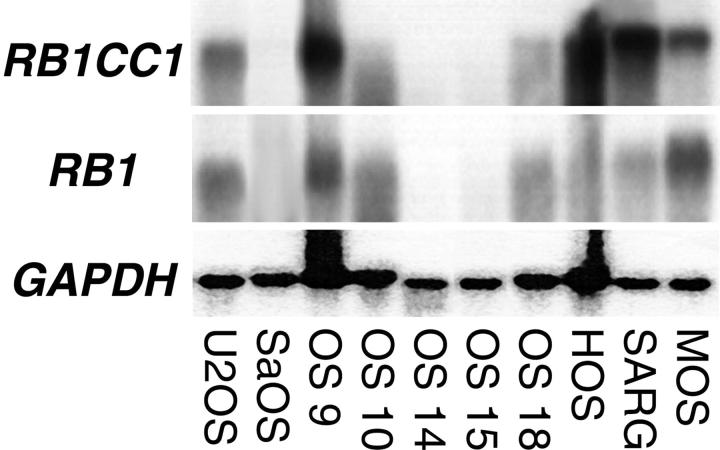
Northern blotting revealed that RB1CC1 and RB1 were co-expressed in 7 of 10 human osteosarcoma cell lines. The expression levels of these two genes also correlated in these seven lines. Neither RB1CC1 nor RB1 was significantly expressed in the remaining three cell lines, IOR/OS14, 15, and Saos-2 (Figure 1) ▶ , in which the RB1 gene was abnormal.
Figure 1.
Northern blots of human RB1CC1, RB1, and GAPDH. The 6.6-kb and 4.7-kb signals, respectively, corresponding to the human RB1CC1 and RB1, were noted with comparatively similar intensities in 7 of 10 human osteosarcoma cell lines.
Synchronized Expression of Both RB1CC1 and RB1 in the Developmental Musculoskeletal Cells of Human Embryos
Various developmental stages of musculoskeletal cells were evident in the embryonic tissues estimated as 4 to 8 weeks old after ovulation. These included proliferating chondrocytes, hyperplastic chondrocytes, osteoblasts, and immature and mature myogenic cells. RB1CC1 expression was histologically detected in cells of the developing long bones and in the surrounding connective tissues such as skeletal muscles in all five human embryos (Figures 2 and 3) ▶ ▶ . However, the degree of RB1CC1 expression varied considerably in cell nuclei of these tissue elements. In the cartilaginous tissue, expression was prominent in hypertrophic chondrocytes at sites of enchondral ossification (Figure 3A2) ▶ , but poor in small, immature chondrocytes near the joint surface and in actively proliferating chondrocytes (Figure 3A1) ▶ . Osteoblasts in and around the bone matrix expressed RB1CC1 prominently regardless of their location (Figure 3, A3 and A4) ▶ , but poorly in immature cells without active matrix formation such as preosteoblasts in the periosteum (Figure 3A4) ▶ . In 4- to 8-week-old postovulatory embryos, hemopoietic centers were located in the liver and spleen, and hemopoietic progenitor cells were barely detected in their developing bone marrow spaces. 11-13 Accordingly, there was no practical problem in the distinction between osteoblasts and other progenitor cells in the bone marrow of such embryos. Within the skeletal muscle lineage, RB1CC1 expression was quite weak in single nucleated myogenic precursor cells, but significantly increased with formation of the myotubes and mature skeletal muscle fibers. Myoblasts fusing to the myotube also emitted intense signals (Figure 3A5) ▶ . In other mesenchymal components, RB1CC1 expression was not significant. In situ hybridization detected abundant RB1CC1 messages in osteoblasts forming bone matrix, hypertrophic chondrocytes, and in mature skeletal muscle cells (Figure 2; C to E) ▶ , but very low levels in proliferating chondrocytes (Figure 2B) ▶ . The amount and localization of the RB1CC1 message varied in a similar manner to those of RB1CC1 protein in the musculoskeletal tissues of human embryos. RB1 protein was detected in the nuclei of all types of musculoskeletal cells that possessed abundant RB1CC1 expression, and the intensity of RB1 staining was relevant to the amount of RB1CC1 expression (Figure 3; B1 to B5) ▶ .
Figure 2.
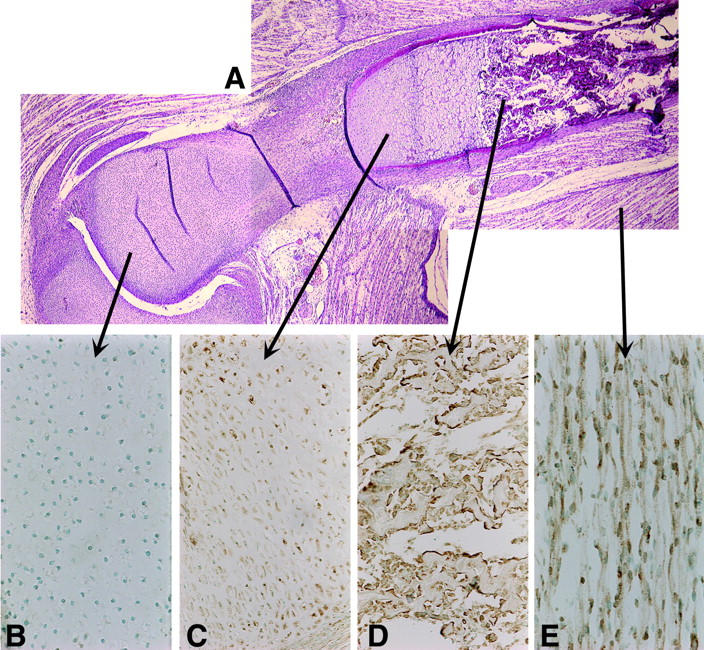
RB1CC1 expression in musculoskeletal tissues. H&E staining (A) and in situ hybridization of RB1CC1 (B–E). RB1CC1 mRNAs (brown) are present in various musculoskeletal cells in human embryos. They are scarcely expressed in proliferating chondrocytes (B), but are abundant in hypertrophic chondrocytes (C), osteoblasts (D), and muscles (E). Original magnifications: ×200 (B, D, E); ×100 (C).
Figure 3.
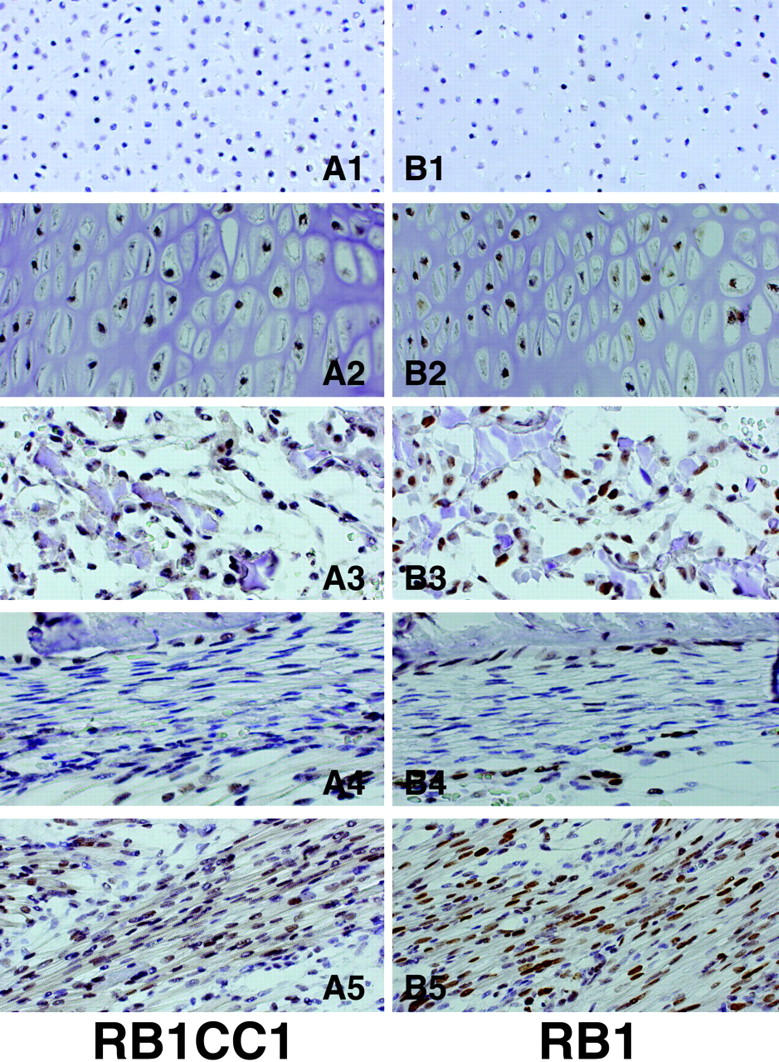
Expression of RB1CC1 and RB1 in musculoskeletal tissues. Immunohistochemistry of RB1CC1 (A), and of RB1 (B) in long bones and the surrounding skeletal muscles of human embryos. The intensity of RB1CC1 and RB1 expression increased concomitantly with maturation of musculoskeletal tissue elements. Proliferating chondrocytes (A1, B1); hypertrophic chondrocytes (A2, B2); osteoblasts in bone trabeculae (A3, B3); periosteal osteoblasts attaching to bone surface (A4, B4); multinucleated skeletal muscles (A5, B5) are shown. Gene expression is shown in brown. Original magnifications, ×200 (A1–5, B1–5).
Cell Growth Suppression by RB1CC1 Introduction
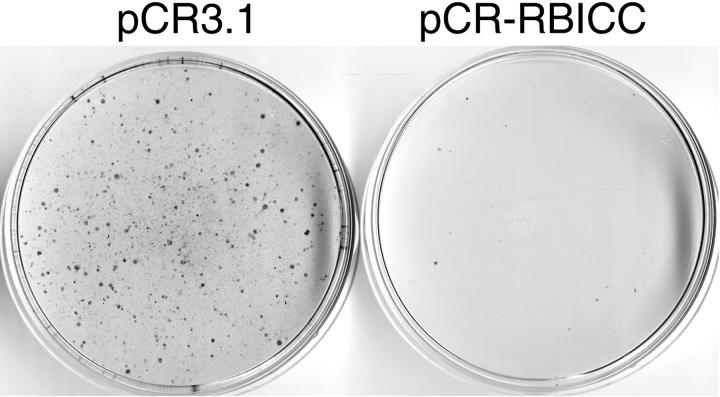
The introduction of wild-type RB1CC1 suppressed the formation of macroscopic colonies of EBC-1 cells. On the other hand, the control vector without RB1CC1 exerted no growth suppression of EBC-1 cells. There was an 86% decrease in colony number on RB1CC1 transgene delivery (Figure 4) ▶ .
Figure 4.
Cell growth suppression by RB1CC1 introduction. The introduction of wild-type RB1CC1 suppressed the formation of macroscopic colonies of EBC-1 cells, which expresses low levels of endogenous RB1CC1 and RB1. The cells were transduced with the vector carrying the neomycin resistance gene alone (pCR3.1), or in combination with RB1CC1 gene (pCR-RBICC), and then selected in the presence of G418 for 14 days.
Discussion
Human RB1CC1 is a novel nuclear DNA-binding protein that is a key regulator of RB1 gene expression: RB1CC1 expression correlates closely with that of RB1 in various cancer cell lines and normal human tissues, and introduction of wild-type RB1CC1 induced significant RB1 expression in human leukemic cells. 1 Our previous study 1 also demonstrated abundant RB1CC1 expression in normal skeletal muscles and in U-2 OS human osteosarcoma cells. In addition, RB1CC1 expression was quite low in the osteosarcoma cell line, Saos-2, in which the RB1 gene is abnormal. Therefore, we proposed that both RB1CC1 and RB1 are correlatively expressed, interact each other, and play any biologically important roles in human musculoskeletal cells as well as in osteogenic neoplastic cells. We then analyzed the manner of RB1CC1 and RB1 gene expressions in 10 osteosarcoma cell lines, and found RB1CC1 expression in 7 of them and little in the remainder. Expressional levels of RB1CC1 and RB1 were related in all 10 human osteosarcoma cell lines. These findings were similar to those of other cancer cells and normal human tissues in the previous study. 1
In situ hybridization and immunohistochemical studies of nonneoplastic musculoskeletal systems revealed abundant RB1CC1 expression in hypertrophic chondrocytes, osteoblasts, deep-seated periosteal osteoblasts, and muscles, but less RB1CC1 was expressed in proliferating chondrocytes and other immature mesenchymal cells. The intensity of RB1 immunoreactivity in cell nuclei correlated with that of RB1CC1, and the expression of both concomitantly increased with maturation of the musculoskeletal mesenchymal cells. In consequence, like the findings from various human osteosarcoma cell lines, the degrees of expression of both RB1CC1 and RB1 were related in various musculoskeletal cells, and an increase of both was preferentially associated with the maturation of cellular components of the musculoskeletal tissues in human embryos.
The RB1 family is best known for its function in the control of cell cycle progression, and ∼80% of human cancers are associated with dysregulation of the RB1 pathway. 3,4 In musculoskeletal neoplasms such as osteosarcoma, chondrosarcoma, leiomyosarcoma, and rhabdomyosarcoma, RB1 mutations alter the biological behavior of cell proliferation and lead to a poor prognosis. 4-6 However, whether or not RB1 plays crucial roles on the maturation of musculoskeletal cells has not yet been clarified. Novel roles for the RB1 family of proteins have emerged, because it has been linked with regulation of the terminal differentiation of many tissues and cell types. 7 A more precise definition of RB1 function in the proliferation and differentiation of musculoskeletal cell lineages is required. To the best of our knowledge, this study is the first to provide evidence of RB1 expression in the developing musculoskeletal systems of human embryos. Our histological studies suggested that RB1 is involved with the terminal differentiation of human embryonal musculoskeletal cells, after stabilizing the cell cycle processes.
RB1CC1, a putative transcription factor, has been implicated in the regulation of RB1. 1 However, information about RB1CC1 is scarce, and whether RB1CC1 controls RB1 directly as a transcription factor, or indirectly through some other molecules, remains to be determined. Evidence shows that RB1CC1 is intimately related with RB1 status in cancer cell lines as well as in various normal human tissues, and therefore, it was considered to be a key regulator of RB1 gene expression. 1 As shown in the present study, the introduction of wild-type RB1CC1 to EBC-1 cells, which have a low level of RB1CC1 expression, decreased the formation of macroscopic colonies, indicating that RB1CC1 can suppress proliferation of some types of cells. Together, the findings of the present study suggest that the two nuclear proteins, RB1CC1 and RB1, were preferentially co-expressed in terminally differentiated mesenchymal tissues, and may play some fundamental biological roles such as suppressing the cell proliferation in the development of musculoskeletal cell lineages of human embryos.
In conclusion, this study showed that both RB1CC1 and RB1 are expressed in a similar manner in musculoskeletal cells, and that RB1CC1 is also important in the musculoskeletal development of human embryos, along with RB1. RB1CC1 may play some crucial roles on the expression and function of RB1, and stabilize the cell cycle processes, in musculoskeletal cells. Thus, RB1CC1 and RB1 preferentially co-expressed and contribute to the development of musculoskeletal cell lineages.
Footnotes
Address reprint requests to Tokuhiro Chano, M.D., Ph.D., Department of Clinical Laboratory Medicine, Shiga University of Medical Science, Tsukinowa-cho, Otsu, Shiga 520-2192, Japan. E-mail: chano@belle.shiga-med.ac.jp.
Supported by grant-in-aids for Japan Society for the Promotion of Science Fellows (no. 4808; to T. C.) and Scientific Research (B) (no. 13470520), The Ministry of Education, Science, Sports and Culture of Japan, and grant from the Japan Orthopaedics and Traumatology Foundation, Inc (no. 0110).
References
- 1.Chano T, Ikegawa S, Kontani K, Okabe H, Baldini N, Saeki Y: Identification of RB1CC1, a novel human gene that can induce RB1 in various human cells. Oncogene 2002, 21:1295-1298 [DOI] [PubMed] [Google Scholar]
- 2.Kaelin WG, Jr: Function of the retinoblastoma protein. BioEssays 1999, 21:950-958 [DOI] [PubMed] [Google Scholar]
- 3.Taya Y: RB kinases and RB-binding proteins: new points of view. Trends Biochem Sci 1997, 21:14-17 [DOI] [PubMed] [Google Scholar]
- 4.Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, Yamamuro T: Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 1994, 54:3042-3048 [PubMed] [Google Scholar]
- 5.Wunder JS, Czitrom AA, Kandel R, Andrulis IL: Analysis of alterations in the retinoblastoma gene and tumor grade in bone and soft-tissue sarcomas. J Natl Cancer Inst 1991, 83:194-200 [DOI] [PubMed] [Google Scholar]
- 6.Stratton MR, Williams S, Fisher C, Ball A, Westbury G, Gusterson BA, Fletcher CD, Knight JC, Fung YK, Reeves BR, Cooper CS: Structural alterations of the RB1 gene in human soft tissue tumours. Br J Cancer 1989, 60:202-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipinski MM, Jacks T: The retinoblastoma gene family in differentiation and development. Oncogene 1999, 18:7873-7882 [DOI] [PubMed] [Google Scholar]
- 8.Scotlandi K, Serra M, Landuzzi L, Baldini N: SARG: a new human osteosarcoma cell line. Expression of bone markers and of major histocompatibility antigens. Ann Oncol 1992, 3(Suppl 2):S29-S31 [DOI] [PubMed] [Google Scholar]
- 9.Scotlandi K, Serra M, Manara MC, Nanni P, Nicoletti P, Landuzzi L, Maurici D, Baldini N: Human osteosarcoma cells, tumorigenetic in nude mice, express b1 integrins and low level of alkaline phosphatase activity. Int J Oncol 1993, 3:963-969 [DOI] [PubMed] [Google Scholar]
- 10.Ferracini R, Di Renzo MF, Scotlandi K, Baldini N, Olivero M, Lollini PL, Cremona O, Campanacci M, Comoglio PM: The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995, 10:739-749 [PubMed] [Google Scholar]
- 11.O’Rahilly R, Muller F: The Embryonic Human Brain. 1994. Wiley-Liss, New York
- 12.Leslie BA: Developmental Anatomy: A Textbook and Laboratory Manual of Embryology, 7th ed. 1965. WB-Saunders, Philadelphia
- 13.Kurt JE: Human Developmental Anatomy. 1988. Wiley-Liss, New York