Abstract
The late stages of progression of prostate carcinoma are typically characterized by an androgen-insensitive, rapidly proliferative state. Some late-stage tumors are composed predominantly of neuroendocrine cells. Virtually no animal models of a neuroendocrine/small cell variant of prostate carcinoma are available for experimental studies. We report a human neuroendocrine/small cell prostate carcinoma xenograft that was developed from a nodal metastasis of a human prostate carcinoma and that has been propagated as serial subcutaneous implants in severe combined immunodeficient mice for >4 years. Designated LuCaP 49, all tumor passages exhibit a neuroendocrine/small cell carcinoma phenotype—insensitivity to androgen deprivation, expression of neuroendocrine proteins, lack of expression of prostate-specific antigen or androgen receptor, and an unusually rapid growth (a doubling time of 6.5 days) for prostate cancer xenografts. Genetically this tumor exhibits loss of heterozygosity for the short arm of chromosome 8 and has a complex karyotype. This xenograft should prove to be useful in the investigation of mechanisms underlying the androgen-insensitive state of progressive prostate carcinoma.
The emergence of tumor from an androgen-suppressible state to an androgen-independent state poses challenges for managing patients with prostate cancers. 1 Several different androgen-independent states have been characterized. Inactivation of androgen receptors (ARs) by mutation occurs, but rarely. 2 Furthermore, AR mutations do not necessarily lead to a loss of AR function and do not represent the most frequent mechanism whereby prostate cancer evolves to a hormone-independent state. 2 There is evidence that several different growth factors, ie, basic fibroblast growth factor, epidermal growth factor, and insulin-like growth factor (IGF), can stimulate prostate epithelial cell proliferation independent of AR activation. 3 ARs and/or the molecular targets of androgens can also be activated through a signal cascade that is independent of an intact AR and that involves MAP kinase and her2/neu signaling. 4,5 A subset of androgen-nonresponsive prostate carcinomas is composed predominantly, even exclusively, of neuroendocrine (NE) cells. These tumor cells have lost the phenotype and functional features of untreated primary prostate carcinomas. Specifically, these tumors no longer express such markers of functional differentiation as prostate-specific antigen (PSA) and AR. Furthermore, these tumors grow more rapidly than do the far more common acinar prostate adenocarcinomas. 6,7 Although NE tumor cells are known to not be under androgen regulation, the molecular mechanisms that control the growth of these cells are unknown.
The availability of model tumors propagated in animals would facilitate the investigation of prostate carcinoma and the wide range of clinical states and rates of clinical progression that is observed in humans. However, the initiation and maintenance of explanted human prostate carcinoma is problematic. Primary prostate carcinomas transplanted into immunocompromised mice or rats rarely survive serial transplantation. Consequently, there are relatively few animal models. 5,8-19 Likewise, very few prostate carcinomas have been successfully initiated and maintained as cell cultures and/or cell lines. 8,20-28 Accordingly, most in vitro studies of prostate carcinoma have been limited to a small number of cell lines—DU145, 21 PC3, 22 and LnCaP. 23 Although the majority of reported tumors were derived from metastases, and some of them are either androgen insensitive and/or do not express PSA, with only four clear-cut exceptions 18,19,29 none of them exhibit a NE phenotype. We report and characterize a human prostate carcinoma xenograft that has an exclusively NE phenotype.
Materials and Methods
History
The patient from whom this tumor was obtained was a 71-year-old male diagnosed with clinical stage B-II prostate carcinoma 4 years before obtaining the tumor. At the time of initial diagnosis the serum PSA was 50 ng/ml. He was treated with external beam radiation therapy, receiving 45 Gy to the pelvis and 68.4 Gy to the prostate. No androgen ablation therapy was given. After a serum PSA nadir of 2.9 ng/ml, the serum PSA subsequently rose to 7.5 ng/ml. In an attempt to cure the patient, a radical cystoprostatectomy with bilateral pelvic lymph node dissections was done. The specimen contained a bulky (∼50 ml) prostate carcinoma that replaced most of the prostate parenchyma, extended into the bladder neck and apex of the prostate, and had metastasized to several pelvic lymph nodes and to the omentum.
Tumor Tissue Preparation
Fresh tumor tissue was processed immediately on receipt of the cystoprostatectomy, pelvic lymph nodes, and omentum from the operating room under protocol no. 00-3449-A01 approved by the University of Washington Institutional Review Board. Portions of apparent tumor were frozen in OCT; frozen sections were made, stained, and histologically analyzed. Using a frozen section of a block of omental tissue as a template for tumor location, pieces of tumor-enriched tissue adjacent to the tissue from which the frozen sections were taken were processed under sterile conditions. These cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum.
Establishment of Xenograft in Vivo
Six-week-old athymic nude mice (Simonsen Laboratories, Gilroy, CA) and/or Fox Chase CB.17 severe combined immunodeficient (SCID) mice (Charles River Laboratories, Wilmington, MA) were implanted subcutaneously with 20 to 40 mg of tissue fragments from the omental metastasis. Growth was observed in 5 weeks. Tumor tissue from the mice was serially passaged at 9 weeks after implantation. This xenograft, which we named LuCaP 49, has been maintained by serial passage in Fox Chase CB.17 SCID mice. Tumor propagation was less efficient in nude mice. Thirty-two SCID mice were used in the present experiments.
PSA Serum Levels in Mice
Blood was drawn from the tail vein every 14 days. Serum was separated by centrifugation. Serum PSA levels were determined using the PSA IMX assay (Abbott Laboratories, Abbott Park, IL).
Histology and Immunohistochemistry
Sections of formaldehyde-fixed, paraffin-embedded xenograft tissue were stained with hematoxylin and eosin for histological characterization and for expression of antigens using an indirect avidin-biotin immunoperoxidase technique with biotinylated secondary antibodies and a diaminobenzidine (DAB) detection system, which was enhanced with nickel-chloride for some immunostains (Ni-DAB). This immunostaining procedure produces a dark blue/black reaction product. All primary antibodies were monoclonal but for anti-PSA, which was a rabbit anti-PSA polyclonal antibody (Table 1) ▶ . The negative control consisted of normal mouse serum diluted (1:1000) in phosphate-buffered saline.
Table 1.
Characteristics of Antibodies Used for Immunohistochemistry
| Antigen | Vendor | Clone or lot no. | Dilution |
|---|---|---|---|
| Adrenocorticotrophic hormone | ICN Pharm (Aurora, OH) | PAb 395 | 1:4,000 |
| Androgen receptor | Biogenex (San Ramon, CA) | F39.4.1 | 1:500 |
| Calcitonin | DAKO (Carpinteria, CA) | PAb A576 | 1:100 |
| CD44 | Novocastra (Newcastle, UK) | F10-44-2 | 1:500 |
| CD57 | Pharmingen (San Diego, CA) | Leu 7 | 1:2,000 |
| Chromogranin | Boehringer (Indianapolis, IN) | LK2H10 | 1:2,000 |
| Gastrin | DAKO | PAb A568 | 1:2,000 |
| Glucagon | INCSTAR (Stillwater, MN) | PAb 20076 | 1:2,000 |
| Insulin | DAKO | Pab A564 | 1:2,000 |
| Keratin, low MW | DAKO | 35βH11 | 1:4,000 |
| Keratin, high MW | DAKO | 34βE12 | 1:16,000 |
| MIB1-1 (Ki67) | Immunotech (Marseille, France) | KI67 | 1:50 |
| Neuron-specific enolase | DAKO | BBS/NC/VI_HI4 | 1:10 |
| Pancreatic polypeptide | Chemicon (Temecula, CA) | Pab RB939 | 1:200 |
| Prostate-specific antigen | DAKO | Cat. no. A562 | 1:32,000 |
| Prostatic acid phosphatase | DAKO | PASE/4LJ | 1:8,000 |
| Somatostatin | DAKO | Pab 566 | 1:1,000 |
| Synaptophysin | ICN Pharm. | SY38 | 1:500 |
| Vascular endothelial growth factor | R & D Systems | 26503.11 | 1:25 |
| Vimentin | DAKO | V9 | 1:4,000 |
PAb, rabbit polyclonal antibody.
Ultrastructure
Fragments of fresh xenograft tissue were minced into 1-mm fragments in an aqueous solution of 2% phosphate-buffered formaldehyde and 1% aqueous glutaraldehyde using scalpel blades. The tissue fragments were washed in distilled water and fixed in a 1% aqueous solution of osmium tetroxide for 1 hour. After dehydration in graded ethanols to propylene oxide, the tissue fragments were placed in an epoxy resin solution and allowed to polymerize. Silver ultrathin sections were cut and stained with uranyl citrate and lead acetate and examined under a transmission electron microscope.
Apoptosis and Mitotic Rate
One-μm-thick sections of tumor that were prepared for ultrastructural examination were stained with azure blue. Apoptotic bodies and metaphase mitoses were tabulated in randomly selected 6.25 × 10−2 mm2 microscopic fields (×40 objective). Successive tabulations were accumulated until the marginal change in the coefficient of variance of the running mean value of apoptotic bodies was <5%.
Growth in Intact Males, Intact Females, Castrate Males, and Castrate Females
Response to castration was measured in xenografts implanted into intact male and female mice. Tumor volume was allowed to reach 150 to 200 mm3. At this time the animals were castrated. Growth rates were determined in intact males, intact females, castrate males, and castrate females. Serum PSA levels were measured weekly using the Abbott IMX PSA Assay. Tumor size was measured biweekly using calipers.
Cytogenetics
The sample of tumor that was characterized cytogenetically came from a xenograft. The xenograft was an 8-mm diameter round piece of tissue. Incision of this mass yielded 1 ml of bloody fluid containing dispersed cells and solid tissue. Culture of this bloody fluid for 24 hours in RPMI 1640 medium supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), gentamicin (0.4 μg/ml), fungizone (0.2 μg/ml), and 20% fetal bovine serum yielded viable cells. Two hours before harvesting, the cultures were exposed to colcemid (0.2 μg/ml) followed by hypotonic treatment in 0.074 mol/L KCl for 30 minutes. The cells were fixed in three changes of a 3:1 solution of methanol and glacial acetic acid. The metaphase cells were Giemsa-trypsin-Giemsa banded. No cells grew from the solid pieces of tissue, which were placed in a separate set of culture plates.
Allelotyping
DNA was prepared from frozen xenograft tissues as described previously. 30,31 Polymerase chain reaction was used to amplify 13 sequences containing highly polymorphic microsatellite repeat markers at loci of interest for prostate cancer on chromosome 8, and five highly polymorphic microsatellite repeat markers at loci of interest for prostate cancer on chromosome 10. The markers that were used to map loss of heterozygosity on chromosome 8, with their map positions (reported as the number of bp between the locus and the chromosomal p-terminus) and cytogenetic positions were: D8S541 (17593000, 8p21.3), NEFL (25827850, 8p21), D8S540 (31728500, 8p12), D8S513 (34930000, 8p12), D8S535 (34951000, 8p12), D8S505 (35581150, 8p12), D8S87 (37930866, 8p12), D8S1121 (38485488, 8p12), D8S255 (40706050, 8p11.2), D8S531 (66200000, 8q11.2), and D8S519 (not determined, 8q11.21). The makers used to map chromosome 10 were D10S674 (19000000, 10p13), D10S211 (22479800, 10p12), D10S89 (27138000, 10p12), D10S111 (31461000, 10p12), and D10S219 (81922000, 10q22).
Primer sequences, physical map positions, and cytogenetic localizations were obtained using the Internet from public databases maintained by the National Center for Biotechnology Information for the Human Genome Sequencing Project (http://www.ncbi.nlm.nih.gov/genome/seq/). Polymerase chain reactions were performed as previously described. 30,31 Aliquots of each reaction were electrophoresed on 6% acrylamide/7 mol/L urea sequencing gels and visualized by autoradiography. Allelic loss was scored when the ratio of allelic signal intensities in tumor tissue was 50% or less of that for the same alleles in normal tissue from this patient.
cDNA Library
Total RNA was isolated from minced LuCaP 49 xenograft tissue using Trizol (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. A cDNA library (named PRCA3) was constructed using oligo-dT priming with directional cloning into the pSport vector (Life Technologies, Inc.) according to previously published methods. 32-34 The average insert size was 1.2 kb. Individual cDNA clones were randomly selected and subjected to single-pass DNA sequencing to produce a library of expressed sequence tags (ESTs) as we have previously described. 35 Clustering and database annotation of ESTs was done using Cross_Match, RepeatMasker, and Phrap. Details are reported in a companion publication. 36
Results
Establishment of Xenograft
The tissue fragments that were implanted in five Fox Chase CB.17 SCID mice have been serially passaged as subcutaneous implants for greater than 5 years. The phenotype has been maintained throughout all passages. Tumor cells that were cultured in RPMI 1640 supplemented with 10% fetal calf serum in an attempt to establish a cell line did not survive the first passage.
Histology
Primary Tumor
The major histological component of the primary tumor mass was a NE/small cell carcinoma that was composed of a uniform population of round to slightly spindle-shaped cells with scanty cytoplasm and nuclei that had a fine pattern of heterochromatin. Admixed with the major component were microscopic foci of duct/acinar prostate carcinoma, Gleason’s combined score 3 + 3 = 6 of 10. This minor component of the tumor represented <5% of the tumor mass. The tumor metastases were composed exclusively of NE/small cell carcinoma (Figure 1) ▶ .
Figure 1.
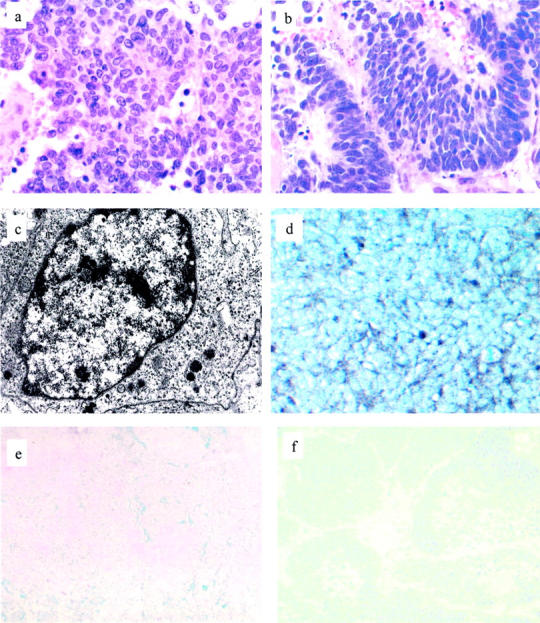
Histology, ultrastructure, and immunophenotype of tumor. a: The major component of the primary tumor consisted of sheets of histologically undifferentiated carcinoma cells with a high nuclear:cytoplasmic ratio. (H&E; original magnification, ×400). b: The xenograft tumor cells resemble the small cell carcinoma component of the primary tumor, having a high nuclear:cytoplasmic ratio and a fine pattern of heterochromatin (H&E; original magnification, ×400). c: Ultrastructurally, small clusters of membrane-bound, electron-dense granules averaging 100 μm in diameter are found in the periphery of the cytoplasm of xenograft tumor cells (EM; original magnification, ×20,000). d: Most xenograft tumor cells express synaptophysin, which is localized to the periphery of the cytoplasm (synaptophysin immunostain, Ni-DAB, nuclear green counterstain; original magnification, ×400). Neither the primary NE tumor (e) nor the xenograft (f) expresses PSA (PSA immunostain, Ni-DAB, nuclear green counterstain; original magnifications, ×200).
Xenograft
The majority of the xenograft tumor mass consisted of tumor cells; <5% of cells were mouse stromal cells. The tumor was multifocally necrotic. Cytologically, the tumor was a pure NE/small cell carcinoma that was composed of a uniform population of cells having a high nuclear: cytoplasmic ratio and a fine stippled pattern of uniformly distributed heterochromatin. This cytology is characteristic of cells that have a NE immunophenotype. These cells were cytologically identical to the pure NE/small cell carcinoma component of the primary tumor, and different from the non-NE, adenocarcinoma component of the primary tumor (Figure 1) ▶ .
Immunophenotype
The immunophenotype of the xenograft was characteristic of NE/small cell prostate carcinoma and essentially identical to that of the NE/small cell carcinoma component of the primary tumor with the exception that chromogranin immunoreactivity was only faint (Table 2) ▶ .
Table 2.
Immunophenotypes of LuCaP 49
| Antigen | Original prostate tumor | Xenograft | |
|---|---|---|---|
| Acinar component | Neuroendocrine component | ||
| Adrenocorticotrophic hormone | ND | ND | Absent |
| Androgen receptor | Uniformly+ | Absent | Absent |
| CD44 | Absent | Absent | Absent |
| CD57 | Uniformly + | Uniformly+ | Variably+ |
| Calcitonin | ND | ND | Absent |
| Chromogranin | Absent | Uniformly+ | Focally+ |
| Gastrin | ND | ND | Absent |
| Glucagon | ND | ND | Absent |
| Insulin | ND | ND | Absent |
| Keratin, low MW | Uniformly + | Uniformly+ | Uniformly+ |
| Keratin, high MW | Absent | Absent | Absent |
| MIBI/Ki67 | < 5% of cells | > 95% of cells | > 75% of cells |
| Neuron-specific enolase | Absent | Uniformly+ | Uniformly+ |
| Pancreatic polypeptide | ND | ND | Absent |
| PSA | Uniformly + | Absent | Absent |
| PSAP | Uniformly+ | Absent | Absent |
| Somatostatin | ND | ND | Absent |
| Synaptophysin | Absent | Uniformly+ | Uniformly+ |
| Vascular endothelial growth factor | ND | ND | Variably+ |
| Vimentin | Absent | Absent | Absent |
ND, not done.
Specifically, the acinar component and the NE/small cell carcinoma of the primary component of the primary tumor were PSA+/chromogranin− and PSA−/chromogranin+, respectively. In the xenograft low molecular weight keratin was localized to the cytoplasm of all tumor cells, which failed to express high molecular weight keratin. Most xenograft cells exhibited a perinuclear pattern of low molecular weight keratin expression, which is typical of the distribution of intermediate filaments in NE cells. There was no apparent expression of AR by tumor cells, with the exception of rare (<1%) cells that were lightly immunostained. CD57 was localized to the majority of tumor cells in the NE component of the primary tumor and in a significant number of cells in the xenograft. Immunostains for CD44 and PSA localized reaction product only to the interstitium and necrotic regions of the xenograft. This pattern of immunoreactivity was regarded as nonspecific. Greater than 75% of xenograft tumor cells expressed nuclear MIB1/Ki67 immunoreactivity. A majority of tumor cells expressed synaptophysin as a fine, granular reaction product that was predominantly localized in the peripheral cytoplasm of cells. Negative controls localized reaction product only to the interstitium and to necrotic regions in a nonspecific manner. Finally, the large majority of tumor cells expressed vascular endothelial growth factor in a membranous distribution (Figures 1 and 2) ▶ ▶ .
Figure 2.
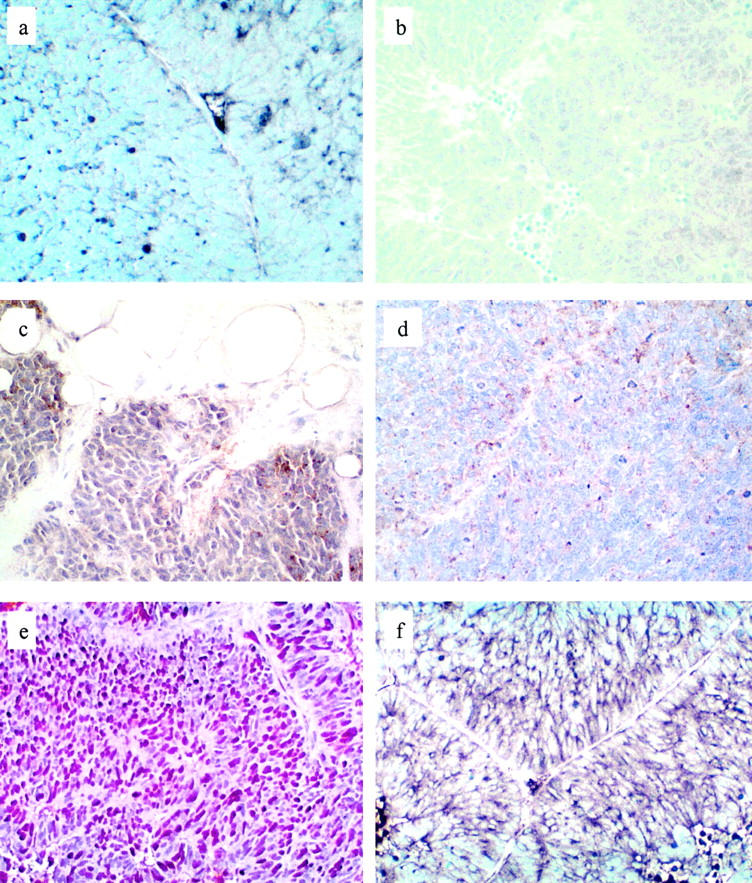
Immunophenotype of xenograft. a: Faint, focal chromogranin A immunoreactivity is seen as a granular reaction product in the cytoplasm of some tumor cells. Compare this stain with the PSA immunostain (Figure 1f) ▶ and the negative control (b), in both of which there is virtually no reaction product (chromogranin immunostain, Ni-DAB, nuclear green counterstain; original magnification, ×400). b: Negative control (normal mouse ascites immunostain, Ni-DAB, nuclear green counterstain; original magnification, ×200). CD 57 immunoreactivity in a majority of tumor cells in the primary tumor (c) and in ∼40% of tumor cells in the xenograft (d) (CD57 immunostain, Ni-DAB, hematoxylin counterstain; original magnification, ×400). The majority (>75%) of xenograft tumor cells express MIB 1 in the nuclei (e) (MIB-1 immunostains, DAB, hematoxylin counterstain; original magnification, ×200) and vascular endothelial growth factor in the cell membrane (f) (vascular endothelial growth factor immunostains, Ni-DAB, nuclear green counterstain; original magnification, ×2100).
The tumor was also analyzed for expression of specific neuropeptides—adrentocorticotrophic hormone, calcitonin, gastrin, glucagons, insulin, pancreatic polypeptide, and somatostatin. None of these were expressed immunohistochemically.
Ultrastructure
The majority of the ∼100 xenograft tumor cells that were analyzed had a fine pattern of heterochromatin and minimal cytoplasm, which contained small numbers of 50- to 150-nm diameter, dense-core, membrane-bound granules. Rare cell membrane-associated electron-dense contacts between adjacent cells were present (Figure 1) ▶ . These features are specific for a NE phenotype.
Apoptosis Rate
The number of apoptotic bodes and of metaphase mitoses per 10−2 mm2 field (or per 65 xenograft tumor cells) was 1.5 ± 0.5 (or 2.3% of tumor cells) and 2.0 ± 0.3 (or 3.1% of tumor cells), respectively.
Cytogenetics
The metaphases displayed a highly abnormal human karyotype with multiple structural and numerical aberrations. Only a small minority of chromosomes in any given metaphase was structurally normal. A number of clonal marker chromosomes were seen. However, none were observed in every cell that was analyzed. The majority of the metaphases contained a near diploid chromosome complement with a range of 32 to 49 and a mode of 38 (15 cells). Two cells with a near tetraploid chromosome complement were found.
Allelotyping
Only one allele was detectable for all polymorphic sequences mapping to the 8p chromosomal region. Two alleles were observed for the D8S531 locus, which maps to 8q11. These observations are consistent with complete loss of the short arm of chromosome 8 in LuCaP 49. In contrast, two alleles were observed for four of five markers examined mapping to chromosome 10, suggesting that two copies of chromosome 10 were retained in the xenograft tissue.
Serum PSA
No serum PSA was detected in xenograft bearing host mice of any gender or state of castration.
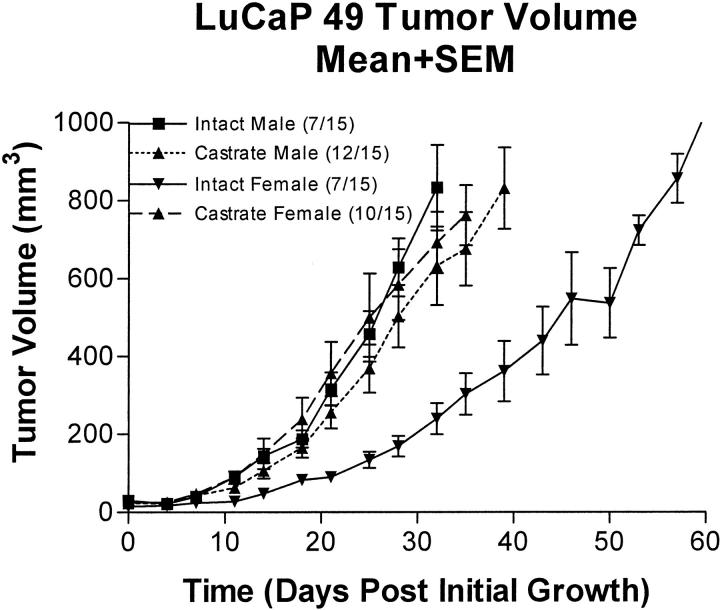
Growth in Intact Males, Intact Females, Castrate Males, and Castrate Females
Tumor doubling times are reported in Table 3 ▶ . Growth data were standardized for individuals to first measurable tumor volume so that exponential growth could be determined. Time to first measurable tumor growth varied from 21 to 49 days (median, 21 days) after implantation in individual animals. Growth rates, expressed in doubling time, were obtained by fitting the standardized tumor volume data (Figure 3) ▶ to the exponential growth equation of N = N0ek•x, where N = tumor volume for time x, and the geometric doubling time = 0.6932/k. Goodness of fit, r2, was 0.990, 0.912, 0.966, and 0.988 for intact males, castrate males, intact females, and castrate females, respectively. Growth data beginning at day 15 after tumor implantation is censored because of death of animals with the largest tumors. Using an unpaired t-test to compare xenograft growth rates with that in intact male mice, the only group of animals showing a significant difference was the noncastrate female mice group (P = 0.0203).
Table 3.
Doubling Times of LuCaP 49 in Mice with Different Hormonal States
| Intact males | Castrate males | Intact females | Castrate females | |
|---|---|---|---|---|
| Number of animals | 7 | 11 | 5 | 9 |
| Mean doubling time (days) | 7.7 | 9 | 12.3 | 10.2 |
| Standard deviation | 2.4 | 1.7 | 3.4 | 5.6 |
Median tumor volume fitted to the exponential growth equation of N = N0ek•x, where N = tumor volume for time x, and the geometric doubling time = 0.6932/k, where k = a time constant that is unique for each set of animals.
Figure 3.
Growth rate of tumor in intact and castrate male and female mice.
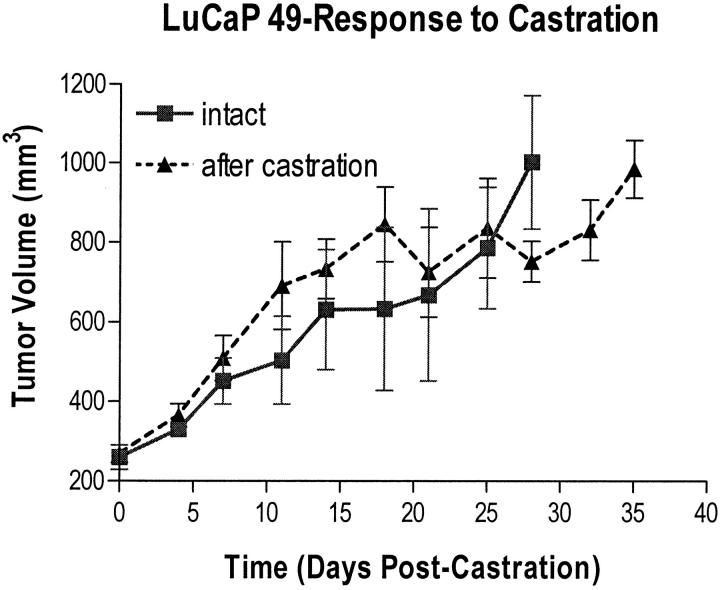
Xenograft Response to Castration in Males
Response of tumors in male mice to castration (nontreated castrates) is shown in Figure 4 ▶ . Tumor volume did not regress after castration, but continued unaffected in an androgen-independent manner until endpoint (the maximum tumor size allowed in our institutional protocol was 1000 mm3).
Figure 4.
Response of LuCaP 49 to castration of male mice. Note the drop in tumor volume between days 15 and 18 because of a disproportionate number of animals that died with large tumors. These data are censored beginning at day 15 after castration because animals with unusually large tumors died because of their tumors burden.
Xenograft Gene Expression
Analysis of the cDNA library by sequencing, assembling, and annotating randomly selected cDNA clones provided a general assessment of gene expression diversity and abundance in the LuCaP 49 xenograft. Searchers against the public DNA sequence databases identified 1423 distinct genes expressed in LuCaP 49. A comparison of LuCaP 49 ESTs with ESTs acquired from normal prostate tissue and prostate adenocarcinomas identified several genes that are regarded as being restricted in expression to NE cells. These sequences encode neuronal gamma enolase (NSE), secretogranin I (or chromogranin B), secretogranin II (chromogranin C), secretogranin III, tryptophan hydroxylase, and synaptophysin. Of interest, no EST’s of chromogranin A were found. This correlates with the virtual absence of chromogranin A immunoreactivity in the xenograft. The sequences have been entered into the Prostate Expression Database (PEDB). 33,37 Details of the cDNA library and a more extensive characterization of genes expressed in the LuCaP 49 xenograft are provided in a companion article. 36
Discussion
Prostate glands are composed predominantly of secretory and basal epithelial cells. Representing <1% of prostate epithelial cells is a third epithelial cell type—the NE cell. Characteristic of NE cells throughout the body, the prostate NE cell contains neurosecretory granules that package peptides and express the neurosecretory granule proteins chromogranin and synaptophysin. The function of the NE cells in the prostate is unknown. Although most investigators report that NE cells do not express markers of prostate secretory epithelial cell differentiation, ie, PSA, prostate-specific acid phosphatase (PSAP) or AR, 6,38,39 some investigators have observed expression of AR 6 and of PSA. 40,41 The prostate NE cells have >2-fold higher proliferative rate, as measured by MIB1 immunostaining 42 than do prostate secretory cells.
Approximately one third of prostate cancers will recur as metastatic tumor after primary therapy. Although hormonal therapy, specifically androgen blockade, initially retards the growth of most tumors, within several years the typical prostate carcinoma escapes androgen blockade and progresses. Virtually all prostate carcinomas contain some malignant cells that have a NE phenotype. 43 However, only a minority of tumors that escape androgen blockade have a predominantly NE phenotype.
The literature is conflicted regarding the clinical importance of NE differentiation in prostate cancer. Some investigators have evidence that the percentage of NE cells in a prostate carcinoma correlates directly with 1) the rate of tumor progression, 44-46 2) with adverse outcome, 43,47 and/or 3) with surrogate markers of adverse outcome such as tumor grade 48 or stage. 49 Other investigators have not found a convincing correlation between the number of NE cells and a more rapid clinical course 50 or with patient prognosis. 6,29,51,52 Finally, there is recent evidence that NE differentiation becomes more pronounced in androgen-nonresponsive tumors 53 and that NE differentiation in this situation might have prognostic significance. 54-56 The conclusions from these studies is that NE differentiation seems to be clinically important only in a subset of prostate cancers, and that in that subset a better understanding of the molecular and cell biology of NE differentiation may provide valuable insight into managing these patients.
There are several mechanisms that might explain a more aggressive course of prostate carcinomas that have a predominantly NE phenotype. Lack of AR expression could impart a hormonally insensitive state to the tumor. 38 There is evidence that some neuropeptides might have a role in tumor invasion. Vasoactive intestinal polypeptide (VIP) and a somatostatin analog (RC-160) increase the extent of invasion of basement membrane in vitro by prostate cancer cell lines. 57 A third possible mechanism is that the more rapid growth of prostate carcinomas that have a NE phenotype results from expression of anti-apoptotic factor bcl-2 by cells adjacent to foci of NE differentiation. 58 However, the mechanism(s) that lead to a more aggressive course by NE variants of prostate carcinoma in the clinical setting is unknown.
The development of animal models and successfully propagated xenografts of human NE prostate carcinomas would facilitate investigations of the molecular biology and behavior of the NE variant of prostate cancer. Although there are multiple, continually propagated xenografts of the common, ductal/acinar type of prostate carcinoma, only two well-characterized xenografts of the NE/small cell carcinoma variant of prostate carcinoma have been reported. The first, UCRU-PR-2, was obtained from a primary small cell carcinoma of the prostate. 19 This tumor had a mean doubling time of 12.5 to 14.7 days (doubling time varied with the implantation site), had a NE immunophenotype (NSE+, PSA−), and secreted peptide hormones adrenocorticotrophic hormone, β-endorphin, and somatostatin. 59 Since there have been no publications concerning UCRU-PR-2 after the late 1980’s, this tumor xenograft presumably no longer exists. A more recently reported xenograft of a NE/small cell prostate carcinoma named WISH-PC2 was established from a curettage of a prostate carcinoma. WISH-PC2 is similar to LuCaP 49 in many respects. It grows in an androgen-independent environment and the immunophenotype is similar—PSA-negative, prostate-specific membrane antigen-negative, prostatic acid phosphatase-negative, AR-negative, synaptophysin-negative, and NSE-positive. 18 A third possible NE xenograft was a recurrence in the host rodent of xenograft PAC120 that was derived from a histologically conventional human prostate adenocarcinoma. Although the putative NE recurrence was hormonally independent and described as having an NE-like histology, the cytology of the tumor cells do not have the typical fine heterochromatin pattern of NE cells; and, no assessment of NE differentiation by immunohistochemistry was undertaken. 60
Two additional, stable xenografts that have an NE component have been reported—PC-295 and PC-310. 29 These xenografts differ from LuCaP 49 in that they were not derived from a pure primary NE carcinoma and only 10% of cells had an NE phenotype. Furthermore androgen suppression resulted in an increase in the NE tumor cell fraction, which was postmitotic in that the NE cells lacked MIB-1 immunoreactivity. Because many of the functional and phenotypic features differ from LuCaP 49, these latter tumors seem to represent a phenomenon different from that we are reporting.
This report characterizes such a successfully propagated xenograft, which we have named LuCaP 49. Because the NE variant occurs in <1% of prostate carcinomas, to obtain a human tumor sample that is enriched for NE prostate carcinoma cells depends on a rare opportunity and a tumor tissue procurement program that optimizes obtaining fresh samples of tumor tissue and that is on-call full-time. We have had such a prostate cancer tissue procurement program since 1995.
The phenotype of LuCaP 49 is virtually identical to the NE/small cell component of the primary tumor from which it was derived, is similar to that of the WISH-PC2 and UCRU-PR-2 prostatic small cell carcinoma xenografts, 18,19,59 and is typical of NE prostate carcinomas. One somewhat discrepant feature is the lack of intense chromogranin immunoreactivity. That the primary tumor expressed chromogranin and that the xenograft expressed only faint, focal chromogranin immunoreactivity at 5 years after xenograft initiation is inexplicable but for the observation that this is a genetically unstable tumor, as evidenced by the cytogenetics, and, therefore, has a labile phenotype. Overall the xenograft has a NE/small cell phenotype—positive for synaptophysin, CD57, and neuron-specific enolase (NSE) and absence of immunoreactive PSA, PSAP, and/or AR. Synaptophysin is a neurosecretory/neural vesicle-associated protein that is only expressed in neural and NE cells. 61,62 Finally, there is precedent for a NE tumor not expressing chromogranin while expressing synaptophysin. 63 None of the specific neuropeptides looked for immunohistochemically in the xenograft—adrentocorticotrophic hormone, calcitonin, gastrin, glucagons, insulin, pancreatic polypeptide, and somatostatin—were found. Finally, demonstration of vascular endothelial growth factor expression provided further support that the xenograft has an aggressive, NE-like phenotype. 64
We have also characterized the molecular genotype of LuCaP 49. Classical cytogenetic analysis revealed multiple aberrations of different chromosomes. Clonal changes were seen, although note were present in all metaphases analyzed. These observations are consistent with marked chromosomal instability in this xenograft tumor.
We analyzed chromosomes 8 and part of 10 for genetic changes that are characteristic of prostate adenocarcinoma. In early stages most prostate carcinomas have genetic abnormalities in 8p 31,65 and 10p. 30,66 Many higher stage prostate carcinomas have lost genetic material in 10p and 10q. 30 The candidate tumor suppressor gene PTEN is located in 10q and is mutated in both prostate cancer cell lines and primary prostate carcinomas. 67,68 These aberrations are either mutations or loss of genetic material. Using microsatellite markers that spanned chromosome 8 we found evidence for loss of 8p, but retention of 8q and of chromosome 10. These findings suggest that there is at least one tumor suppressor gene in 8p that plays a role in NE differentiation of prostate carcinoma. We also have evidence that the molecular events of tumorigenesis of prostate NE carcinoma differ from the molecular changes that characterize progressive prostate cancer of the typical histological type in lacking mutations in 8q and chromosome 10. There are several possible interpretations of our data. Changes in 8p may be the most important for a rapidly proliferating, progressive tumor in an androgen suppression environment. An alternative explanation is that the changes in 8q and 10 may be important only in the early stage of prostate tumorigenesis with a non-NE phenotype. We cannot distinguish between these explanations.
The analysis of ESTs from the cDNA library of LuCaP 49 provide molecular data that provide further confirmation that LuCaP 49 has a NE phenotype. We observed the expression of several genes that are known to be expressed in this variant of prostate cancer, namely, keratins 8 and 18, and, in addition, neuronal gamma enolase (NSE), chromogranin B, chromogranin C, secretogranin III, and synaptophysin. The chromogranins and synaptophysin are constituents of the neurosecretory granule membrane.
Finally, functional features are similar to those of the other reported xenografts of human prostatic small cell carcinomas WISH-PC2 18 and UCRU-PR-2. 19 These tumors had doubling times of 13.5 and of 12.5 to 15.7 days, respectively. The doubling time of LuCaP 49 ranged between 5 and 11 days. The LuCaP 49 xenograft also accurately reflects the functional state of human NE prostate carcinoma in being unresponsive to androgen deprivation. In contrast, the WISH-PC2 xenograft is affected by androgen. In contrast to not being responsive to androgen, the growth of LuCaP was slower in the intact female mice than in oophorectomized female mice. This suggests that estrogen in gonadally intact female mice inhibits hormone-independent prostate cancer, as we have already observed in some of our ductal-acinar prostate carcinoma xenografts. 69
A final relevant comment concerns the recently reported tumor that was produced transgenically in the mouse under cryptidin, SV40 T-Ag induction.70 There was immunohistochemical evidence that the mouse prostate NE cells were induced to proliferate and form a hormonally, nonresponsive prostate tumor that metastasized widely. However, the degree to which the tumor faithfully manifests a NE phenotype homologous with that of human NE/small cell carcinoma is not clear because the histology and degree of expression of NE markers chromogranin and synaptophysin is not detailed.
In summary, we have initiated and maintained a xenograft that has a phenotypic profile that accurately reflects that NE phenotype of the NE/small cell carcinoma variant of human prostate carcinoma. Furthermore, we have obtained new genotypic and cytogenetic data for this variant of prostate carcinoma. This xenograft, which has been successfully maintained for more than 4 years, should be a valuable tool for modeling therapies for men with prostate carcinomas that either progress in an androgen-insensitive state and/or that have a NE/small cell phenotype.
Acknowledgments
We thank Tracie Evans, Farinaz Shokri, Annabelle Abrenica, Carol Daubenmier, and Wai Ling Mak for excellent technical support in conducting the immunostains.
Footnotes
Address reprint requests to Lawrence D. True, M.D., Department of Pathology, Box 35-6100, University of Washington Medical Center, Seattle, WA 98195. E-mail: ltrue@u.washington.edu.
Supported in part by the Richard M. Lucas Foundation, the CapCURE Foundation, the George M. O’Brien Prostate Cancer Research Center (grant PO50DK47656), the American Cancer Society (to J. A. M.), and the National Institutes of Health (grant R01CA09468 to J. A. M.).
References
- 1.Denis LJ, Griffiths K: Endocrine treatment in prostate cancer. Semin Surg Oncol 2000, 18:52-74 [DOI] [PubMed] [Google Scholar]
- 2.Wallen MJ, Linja M, Kaartinen K, Schleutker J, Visakorpi T: Androgen receptor gene mutations in hormone-refractory prostate cancer. J Pathol 1999, 189:559-563 [DOI] [PubMed] [Google Scholar]
- 3.Djakiew D: Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate 2000, 42:150-160 [DOI] [PubMed] [Google Scholar]
- 4.Craft N, Shostak Y, Carey M, Sawyers CL: A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the her2/neu tyrosine kinase. Nat Med 1999, 5:280-285 [DOI] [PubMed] [Google Scholar]
- 5.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C: From Her2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA 1999, 96:5458-5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahamsson PA: Neuroendocrine cells in tumour growth of the prostate. Endocr Related Cancer 1999, 6:503-519 [DOI] [PubMed] [Google Scholar]
- 7.Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG: Small cell carcinoma of the prostate. Part 1. A clinicopathologic study of 20 cases. Cancer 1987, 59:1803-1809 [DOI] [PubMed] [Google Scholar]
- 9.Hoehn W, Schroeder FH, Reimann JF, Joebsis AC, Hermanek P: Human prostatic adenocarcinoma: some characteristics of a serially transplantable line in nude mice (PC 82). Prostate 1980, 1:95-104 [DOI] [PubMed] [Google Scholar]
- 10.Ito YZ, Mashimo S, Nakazato Y, Takikawa H: Hormone dependency of a serially transplantable human prostatic cancer (HONDA) in nude mice. Cancer Res 1985, 45:5058-5063 [PubMed] [Google Scholar]
- 11.Graham SD, Poulton SH, Linder J, Woodard BH, Lyles KW, Paulson DF: Establishment of a long-term adenocarcinoma of the prostate cell line in the nude mouse. Prostate 1985, 7:369-376 [Google Scholar]
- 12.Hoehn W, Wagner M, Riemann JF, Hermanek P, Williams E, Walther R, Schrueffer R: Prostatic adenocarcinoma PC EW, a new human tumor line transplantable in nude mice. Prostate 1984, 5:445-452 [DOI] [PubMed] [Google Scholar]
- 13.van Steenbrugge GJ, Groen M, Bolt-de Vries J, Romijn JC, Schroeder FH: Human prostate cancer (PC-82) in nude mice: a model to study androgen regulated tumor growth. Prog Clin Biol Res 1985, 185:A23-A50 [PubMed] [Google Scholar]
- 14.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL: Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med 1997, 3:402-408 [DOI] [PubMed] [Google Scholar]
- 15.Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH: Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: luCaP 23. Clin Cancer Res 1996, 2:1039-1048 [PubMed] [Google Scholar]
- 16.Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG: CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res 1996, 56:3042-3046 [PubMed] [Google Scholar]
- 17.Van Weerden WM, de Ridder CM, Verdaasdonk CL, Romijn JC, van der Kwast TH, Schroder FH, van Steenbrugge GJ: Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol 1996, 149:1055-1062 [PMC free article] [PubMed] [Google Scholar]
- 18.Pinthus JH, Waks T, Schindler DG, Harmelin A, Said JW, Belldegrun A, Ramon J, Eshhar Z: WISH-PC2: a unique xenograft model of human prostatic small cell carcinoma. Cancer Res 2000, 60:6563-6567 [PubMed] [Google Scholar]
- 19.Van Haaften-Day C, Raghavan D, Russell P, Wills EJ, Gregory P, Tilley W, Horsfall DJ: Xenografted small cell undifferentiated cancer of prostate: possible common origin with prostatic adenocarcinoma. Prostate 1987, 11:271-279 [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Schroder FH: Human prostatic carcinoma in cell culture: preliminary report on the development and characterization of an epithelial cell line (EB 33). Urol Res 1974, 2:111-121 [DOI] [PubMed] [Google Scholar]
- 21.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF: Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 1978, 21:274-281 [DOI] [PubMed] [Google Scholar]
- 22.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW: Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979, 17:16-23 [PubMed] [Google Scholar]
- 23.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA: The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 1980, 37:115-132 [PubMed] [Google Scholar]
- 24.Claas FH, van Steenbrugge GJ: Expression of HLA-like structures on a permanent human tumor line PC-93. Tissue Antigens 1983, 21:227-232 [DOI] [PubMed] [Google Scholar]
- 25.Iizumi T, Yazaki T, Kanoh S, Kondo I, Koiso K: Establishment of a new prostatic carcinoma cell line (TSU-Pr1). J Urol 1987, 137:1304-1306 [DOI] [PubMed] [Google Scholar]
- 26.Muraki J, Addonizio JC, Choudhury MS, Fischer J, Eshghi M, Davidian MM, Shapiro LR, Wilmot PL, Nagamatsu GR, Chiao JW: Establishment of new human prostatic cancer cell line (JCA-1). Urology 1990, 36:79-84 [DOI] [PubMed] [Google Scholar]
- 27.Narayan P, Dahiya R: Establishment and characterization of a human primary prostatic adenocarcinoma cell line (ND-1). J Urol 1992, 148:1600-1604 [DOI] [PubMed] [Google Scholar]
- 28.Sramkoski RM, Pretlow TG, II, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW: A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim 1999, 35:403-409 [DOI] [PubMed] [Google Scholar]
- 29.Noordzij MA, van Weerden WM, de Ridder CM, van der Kwast TH, Schroder FH, van Steenbrugge GJ: Neuroendocrine differentiation in human prostatic tumor models. Am J Pathol 1996, 149:859-871 [PMC free article] [PubMed] [Google Scholar]
- 30.Trybus TM, Burgess AC, Wojno KJ, Glover TW, Macoska JA: Distinct areas of allelic loss on chromosomal regions 10p and 10q in human prostate cancer. Cancer Res 1996, 56:2263-2267 [PubMed] [Google Scholar]
- 31.Prasad MA, Trybus TM, Wojno KJ, Macoska JA: Homozygous and frequent deletion of proximal 8p sequences in human prostate cancers: identification of a potential tumor suppressor gene site. Genes Chromosom Cancer 1998, 23:255-262 [PubMed] [Google Scholar]
- 32.Gubler U, Hoffman BJ: A simple and very efficient method for generating cDNA libraries. Gene 1983, 25:263-269 [DOI] [PubMed] [Google Scholar]
- 33.Nelson PS, Clegg N, Eroglu B, Hawkins V, Bumgarner R, Smith T, Hood L: The prostate expression database (PEDB): status and enhancements in 2000. Nucleic Acids Res 2000, 28:212-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PS, Ng WL, Schummer M, True LD, Liu AY, Bumgarner RE, Ferguson C, Dimak A, Hood L: An expressed-sequence-tag database of the human prostate: sequence analysis of 1168 cDNA clones. Genomics 1998, 47:12-25 [DOI] [PubMed] [Google Scholar]
- 35.Clegg N, Eroglu B, Ferguson C, Arnold H, Moorman A, Nelson PS: Digital expression profiles of the prostate androgen-response program. J Steroid Chem Mol Biol 2002, 80:13-23 [DOI] [PubMed] [Google Scholar]
- 36.Clegg N, Ferguson C, True LD, Arnold H, Mooreman A, Quinn JE, Vessella RL, Nelson PS: Molecular characterization of prostatic small-cell neuroendocrine carcinoma. Prostate, in press [DOI] [PubMed]
- 37.Hawkins V, Doll D, Bumgarner R, Smith T, Abajian C, Hood L, Nelson PS: PEDB: the prostate expression database. Nucleic Acids Res 1999, 27:204-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonkhoff H, Stein U, Remberger K: Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol 1993, 423:291-294 [DOI] [PubMed] [Google Scholar]
- 39.di Sant’Agnese PA: Divergent neuroendocrine differentiation in prostatic carcinoma. Semin Diagn Pathol 2000, 17:149-161 [PubMed] [Google Scholar]
- 40.Aprikian AG, Cordon-Cardo C, Fair WR, Reuter VE: Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 1993, 71:3952-3965 [DOI] [PubMed] [Google Scholar]
- 41.Cohen RJ, Glezerson G, Haffejee Z: Prostate-specific antigen and prostate-specific acid phosphatase in neuroendocrine cells of prostate cancer. Arch Pathol Lab Med 1992, 116:65-66 [PubMed] [Google Scholar]
- 42.Helpap B, Kollermann J, Oehler U: Neuroendocrine differentiation in prostatic carcinomas: histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol Int 1999, 62:133-138 [DOI] [PubMed] [Google Scholar]
- 43.di Sant’Agnese PA: Neuroendocrine differentiation in carcinoma of the prostate: diagnostic, prognostic, and therapeutic implications. Cancer 1992, 70:254-268 [DOI] [PubMed] [Google Scholar]
- 44.Oesterling JE, Hauzeur CG, Farrow GM: Small cell anaplastic carcinoma of the prostate: a clinical, pathological and immunohistological study of 27 patients. J Urol 1992, 147:804-807 [DOI] [PubMed] [Google Scholar]
- 45.Ro JY, Tetu B, Ayala AG, Ordonez NG: Small cell carcinoma of the prostate. II Immunohistochemical and electron microscopic studies of 18 cases. Cancer 1987, 59:977-982 [DOI] [PubMed] [Google Scholar]
- 46.Cohen RJ, Glezerson G, Hafffejee Z: Neuro-endocrine cells—a new prognostic parameter in prostate cancer. Br J Urol 1991, 68:258-262 [DOI] [PubMed] [Google Scholar]
- 47.Weinstein MH, Partin AW, Veltri RW, Epstein JI: Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol 1996, 27:683-687 [DOI] [PubMed] [Google Scholar]
- 48.Bohrer MH, Schmoll J: Immunohistochemical and morphometric studies on neuroendocrine differentiation of prostate carcinomas. Verh Dtsch Ges Pathol 1993, 77:107-110 [PubMed] [Google Scholar]
- 49.Allen FJ, Van Velden DJ, Heyns CF: Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol 1995, 75:751-754 [DOI] [PubMed] [Google Scholar]
- 50.Tan MO, Karaoglan U, Celik B, Ataoglu O, Biri H, Bozkirli I: Prostate cancer and neuroendocrine differentiation. Int Urol Nephrol 1999, 31:75-82 [DOI] [PubMed] [Google Scholar]
- 51.Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, Mahlamaki E, Schraml P, Moch H, Willi N, Elkahloun AG, Pretlow TG, Gasser TC, Mihatsch MJ, Sauter G, Kallioniemi OP: Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst 1999, 91:1758-1764 [DOI] [PubMed] [Google Scholar]
- 52.Casella R, Bubendorf L, Sauter G, Moch H, Mihatsch MJ, Gasser TC: Focal neuroendocrine differentiation lacks prognostic significance in prostate core needle biopsies. J Urol 1998, 160:406-410 [PubMed] [Google Scholar]
- 53.Stratton M, Evans DJ, Lampert IA: Prostatic adenocarcinoma evolving into carcinoid: selective effect of hormonal treatment? J Clin Pathol 1986, 39:750-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krijnen JL, Bogdanowicz JF, Seldenrijk CA, Mulder PG, van der Kwast TH: The prognostic value of neuroendocrine differentiation in adenocarcinoma of the prostate in relation to progression of disease after endocrine therapy. J Urol 1997, 158:171-174 [DOI] [PubMed] [Google Scholar]
- 55.Guate JL, Escaf S, Menendez CL, del Valle M, Vega JA: Neuroendocrine cells in benign prostatic hyperplasia and prostatic carcinoma: effect of hormonal treatment. Urol Int 1997, 59:149-153 [DOI] [PubMed] [Google Scholar]
- 56.Jiborn T, Bjartell A, Abrahamsson PA: Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology 1998, 51:585-589 [DOI] [PubMed] [Google Scholar]
- 57.Hoosein NM, Logothetis CJ, Chung LW: Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analog RC-160 on the invasive capacity of human prostatic carcinoma cells. J Urol 1993, 149:1209-1213 [DOI] [PubMed] [Google Scholar]
- 58.Segal NH, Cohen RJ, Haffejee Z, Savage N: BCL-2 proto-oncogene expression in prostate cancer and its relationship to the prostatic neuroendocrine cell. Arch Pathol Lab Med 1994, 118:616-618 [PubMed] [Google Scholar]
- 59.Jelbart ME, Russell PH, Fullerton M, Russell P, Funder J, Raghavan D: Ectopic hormone production by a prostatic small cell carcinoma xenograft line. Mol Cell Endocrinol 1988, 55:167-172 [DOI] [PubMed] [Google Scholar]
- 60.de Pinieux G, Legrier ME, Poirson-Bichat F, Courty Y, Bras-Goncalves R, Dutrillaux AM, Nemati F, Oudard S, Lidereau R, Broqua P, Junien JL, Dutrillaux B, Poupon MF: Clinical and experimental progression of a new model of human prostate cancer and therapeutic approach. Am J Pathol 2001, 159:753-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiedenmann B, Franke WW: Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38, 000 characteristic of presynaptic vesicles. Cell 1985, 41:1017-1028 [DOI] [PubMed] [Google Scholar]
- 62.Wiedenmann B, Huttner WB: Synaptophysin and chromogranins/secretogranins—widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol 1989, 58:95-121 [DOI] [PubMed] [Google Scholar]
- 63.Mucci NR, Akdas G, Manely S, Rubin MA: Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum Pathol 2000, 31:406-414 [DOI] [PubMed] [Google Scholar]
- 64.Borre M, Nerstrom B, Overgaard J: Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res 2000, 6:1882-1890 [PubMed] [Google Scholar]
- 65.Bova GS, Carter BS, Bussemakers MJ, Emi M, Fujiwara Y, Kyprianou N, Jacobs SC, Robinson JC, Epstein JI, Walsh PC, Isaacs WB: Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res 1993, 53:3869-3873 [PubMed] [Google Scholar]
- 66.Ittmann M: Allelic loss on chromosome 10 in prostate adenocarcinoma. Cancer Res 1996, 56:2143-2147 [PubMed] [Google Scholar]
- 67.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R: PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275:1943-1947 [DOI] [PubMed] [Google Scholar]
- 68.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D: Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997, 57:4997-5000 [PubMed] [Google Scholar]
- 69.Corey E, Quinn JE, Emond MJ, Buhler KR, Brown LG, Vessella RL: Inhibition of androgen-independent growth of prostate cancer xenografts by 17beta-estradiol. Clin Cancer Res 2002, 8:1003-1007 [PubMed] [Google Scholar]
- 70.Garabedian EM, Humphrey PA, Gordon JI: A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci USA 1998, 95:15382-15387 [DOI] [PMC free article] [PubMed] [Google Scholar]