Abstract
Epithelial-mesenchymal transition (EMT) involving down-regulation of E-cadherin is thought to play a fundamental role during early steps of invasion and metastasis of carcinoma cells. The aim of our study was to elucidate the role of EMT regulators Snail, SIP1 (both are direct repressors of E-cadherin), and Twist (an activator of N-cadherin during Drosophila embryogenesis), in primary human gastric cancers. Expression of Snail, SIP1, and Twist was analyzed in 48 gastric carcinomas by real-time quantitative RT-PCR in paraffin-embedded and formalin-fixed tissues. The changes of expression levels of these genes in malignant tissues compared to matched non-tumorous tissues were correlated with the expression of E- and N-cadherin. From 28 diffuse-type gastric carcinomas analyzed reduced E-cadherin expression was detected in 11 (39%) cases compared to non-tumorous tissues. Up-regulated Snail could be found in 6 cases with reduced or negative E-cadherin expression. However, there was no correlation to increased SIP1 expression. Interestingly, we could detect abnormal expression of N-cadherin mRNA in 6 cases, which was correlated with Twist overexpression in 4 cases. From 20 intestinal-type gastric cancer samples reduced E-cadherin expression was found in 12 (60%) cases, which was correlated to up-regulation of SIP1, since 10 of these 12 cases showed elevated mRNA levels, whereas Snail, Twist, and N-cadherin were not up-regulated. We present the first study investigating the role of EMT regulators in human gastric cancer and provide evidence that an increase in Snail mRNA expression is associated with down-regulation of E-cadherin in diffuse-type gastric cancer. We detected abnormally positive or increased N-cadherin mRNA levels in the same tumors, probably due to overexpression of Twist. SIP1 overexpression could not be linked to down-regulated E-cadherin in diffuse-type tumors, but was found to be involved in the pathogenesis of intestinal-type gastric carcinoma. We conclude that EMT regulators play different roles in gastric carcinogenesis depending on the histological subtype.
E-cadherin, a homophilic Ca2+-dependent cell adhesion molecule located in adherens junctions of epithelia, plays a critical role in the suppression of tumor invasion; its loss of function coincides with increased tumor malignancy. This is supported by the findings that most epithelial cancers display down-regulated or inactivated E-cadherin and several research groups have shown that regain of functional E-cadherin suppresses invasion in many tumor cell types. 1,2 Cavallaro recently suggested that loss of E-cadherin may even actively participate in tumor progression. 3
Cadherin mediated cell adhesion also plays a critical role in early embryonic development, where numerous phenotypic changes occur through a mechanism called epithelial-mesenchymal transition (EMT). The acquisition of a fibroblastic phenotype is accompanied by the loss of E-cadherin and allows cells to dissociate from epithelial tissue to migrate freely. This is an essential event during gastrulation movements and neural crest formation, but has also been suggested to play a fundamental role during early steps of invasion and metastasis of carcinoma cells 4 and proposes the same molecules triggering EMT to be involved in tumor progression, invasion, and metastasis.
There are multiple mechanisms inactivating the E-cadherin mediated cell adhesion system in cancer, such as gene mutations, promoter hypermethylations, chromatin rearrangements, post-translational truncation 5 or modification, and the recently highlighted transcriptional repressors. 6-9
Several studies of the human E-cadherin promoter, 10 mainly carried out by Cano et al 7,11,12 revealed regulatory elements located in the 5′ proximal sequence of the promoter. Among them the E-pal element (containing two E-boxes) which acts as a repressor and can even overcome the effects of positive factors acting on the proximal promoter. 11 One of the zinc finger proteins targeting these E-boxes is the transcription factor Snail which was shown to be a strong repressor of transcription of the E-cadherin gene. 7,13 Snail was found to evoke tumorigenic and invasive properties in epithelial cells on overexpression. 7 Another zinc finger protein postulated as invasion promoter, as it can repress E-cadherin transcription via promoter binding, is SIP1 (Smad interacting protein 1). 8 Snail and SIP1 bind to partly overlapping promoter sequences and show similar silencing effects.
A further molecule known to trigger EMT mechanisms is Twist, a transcription factor containing a helix-loop-helix DNA binding domain essential for the initiation of N-cadherin expression in Drosophila. 14 Twist is possibly involved in the E- to N-cadherin switch during EMT. However, at present it is unclear whether E-cadherin transcription can be repressed directly by Twist. Forced N-cadherin expression was found to exert a dominant effect over E-cadherin function in breast cancer cells 15 and expression of N-cadherin in normal epithelial cells resulted in down-regulation of E-cadherin expression. 16 Recent studies have shown that N-cadherin enhances tumor cell motility and migration. 16-18
It has been observed that Snail expression is inversely correlated with E-cadherin mRNA levels in several epithelial tumor cell lines, eg, MDA-MB-435 breast cancer cells express high levels of Snail and E-cadherin mRNA cannot be detected in these cells, whereas MCF-7, a less transformed breast cancer cell line, still expresses E-cadherin but lacks Snail mRNA. 6 Reverse correlation of Snail and E-cadherin was also detected in hepatocellular carcinoma-derived cell lines, 19 in oral squamous cell carcinoma clones, 20 and in human primary melanocytes. 21 Very recently, Snail expression was found to be involved in progression of breast ductal tumors. 22 We chose to analyze the role of Snail and additional EMT regulators in down-regulation of E-cadherin in gastric carcinomas, where two morphologically and genetically different variants, diffuse-type and intestinal-type gastric cancer, are known, which may also show differences in the expression of the EMT regulators. The mechanism for loss of functional E-cadherin in gastric cancer includes mutations of the gene itself (50% of diffuse-type gastric carcinoma, absent in intestinal-type gastric cancer), 23-25 and promoter hypermethylation. 10 However, inactivation of E-cadherin due to mutation, hypermethylation, or loss of heterozygosity (LOH) is seen in only a fraction of cases. The underlying mechanisms of E-cadherin loss in the remaining cases is not clear. Possibly, E-cadherin mRNA expression is down-regulated by EMT regulators. In this study focusing on the distinct carcinogenetic pathways for intestinal and diffuse type gastric carcinoma, we propose a model of E-cadherin-dependent carcinogenesis that integrates EMT regulators, E-cadherin mutations, and E-cadherin expression.
Materials and Methods
Tissue Samples
A series of 48 gastric carcinomas from the Klinikum Rechts der Isar TU Munich were analyzed. The material was composed of 28 diffuse-type and 20 intestinal-type tumors and histologically verified by two experienced pathologists (I. Becker and H. Höfler) using an hematoxylin and eosin stained reference section. Only clear diffuse-type or intestinal type gastric cancers were included. Occasionally, intestinal metaplasia was seen but the sections used for the analysis did not show intestinal metaplasia. The diffuse-type cases have partly been used for other studies and the E-cadherin mutation status of the collective was determined by mutation specific monoclonal antibodies to include deletion mutants of exon 9 and 8. 26,27 Thus the collective is not randomized.
From all patients archival material from the archive of the Institute of Pathology was used for the analysis. In addition, from patients with diffuse-type tumors, fresh-frozen material was used for E-cadherin mutation analysis. Immunohistochemical analysis and quantitative real-time TaqMan PCR were performed using archival material. From all patients tumorous (containing at least 80% tumor cells) and non-tumorous tissues were analyzed after macrodissection. E-cadherin mutation analysis was done after RNA extraction from fresh-frozen material.
E-Cadherin Mutation Analysis
Total RNA from tissues was isolated using a RNA isolation kit (RNeasy, Qiagen, Hilden, Germany) according to manufacturer’s instructions. Sequences spanning the entire E-cadherin coding region were amplified in five overlapping fragments (A to E) using reverse transcription-polymerase chain reaction (RT-PCR) as described previously. 28 The amplification products were purified by agarose gel electrophoresis and directly sequenced using internal primers. The primer pairs used to amplify the complete coding region were as follows (nucleotide (nt) positions refer to an E-cadherin sequence deposited in the EMBL/GenBank Data base Libraries, accession number Z13009; the A of the ATG of the initiator Met codon is denoted nt + 1): fragment A (nt −2 to 832), primer A1: 5′-CCATGGGCCCTTGGAGCCGC, A2: 5′-CTG GAA GAG CAC CTT CCA TGA C; fragment B (nt 649-1378), primer B1: 5′-ACA GAG CCT CTG GAT AGA GAA CGC, B2: 5′-CCACATTCGTCACTGCTACG; fragment C (nt1220–1686), primer C1: 5′-CAG CGT GGG AGG CTG TAT ACA C, C2: 5′-TGT GTA CGT GCT GTT CTT CAC; fragment D (nt 1483–2170), primer D1: 5′-GTG TCC GAG GAC TTT GGC GTG, D2: 5′-TCA GAA TTA GCA AAG CAA GAA TTC C; fragment E (nt 2077–2687), primer E1: 5′-GGC GTC TGT AGG AAG GCA CAG, E2: 5′-CCA GCA CAT GGG TCT GGG.
Specificity of Antibodies: Western Blot Analysis
Extracts from E-cadherin transfected MDA-MB-435S cells were prepared and used for Western blot analysis according to a method previously published. 29 MDA-MB-435S cells are known to express N-cadherin both at the mRNA and protein level. 15 To detect E-cadherin and N-cadherin protein, monoclonal antibodies (1:2500) from Transduction Laboratories (Lexington, KY) were used (E-cadherin: clone 36, Catalog. No. C20820; N-cadherin: clone 32, Catalog No. C70320). Detection was performed using a horseradish peroxidase-coupled secondary sheep monoclonal anti-mouse antibody (1:2000) (ECL-Western, Amersham).
Immunohistochemical Analysis
2-μm sections of routine formalin-fixed and paraffin-embedded material were analyzed. After microwave-based antigen retrieval with citric acid pretreatment, sections were incubated in 1% hydrogen peroxide for 15 minutes to block endogenous peroxidase. For detection of specific immunoreactivity, the specimens were incubated with monoclonal antibodies against E-cadherin and N-cadherin (both from Transduction Laboratories) at room temperature for 1 hour. Bound antibodies were detected using the avidin-biotin-complex (ABC) peroxidase method (ABC Elite Kit, Vector, Burlingame, CA). Final staining was developed with the Sigma FAST DAB peroxidase substrate kit (Sigma, Deisenhofen, Germany). Hematoxylin was used for counterstaining. Non-tumorous epithelial cells served as positive control for E-cadherin staining. For N-cadherin, ganglion cells of Meissner’s plexus or Auerbach’s plexus served as positive control. Omitting the first antibody was used as negative control. Furthermore, lymphocytes present in the stained sections were used as negative controls.
RNA Extraction from Archival Tissues
Total RNA from gastric cancer specimens was extracted from formalin-fixed, paraffin-embedded tissue using 8-μm sections as previously described. 30 Paraffin was removed by extracting two times with xylene for 10 minutes followed by rehydration through subsequent washes with 100, 90, and 70% ethanol diluted in RNase-free water. After the final 70% ethanol wash and short hemalaun staining a minimum of 2000 cells of a defined area (tumorous or non-tumorous tissue verified by a pathologist) was scraped off using a sterile blade and transferred into a sterile 1.5-ml tube containing 200 μl of RNA lysis buffer (10 mmol/L Tris/HCl pH 8.0, 0.1 mmol/L ethylenediamine tetraacetic acid pH 8.0, 2% sodium dodecyl sulfate pH 7.3, and 500 μg/ml proteinase K). Lysis was carried out at 60°C for 14 hours or until the tissue was completely solubilized.
RNA was purified by phenol and chloroform extractions (one volume of phenol pH 4.5, chloroform, isoamylalcohol in a proportion of 25:24:1), followed by precipitation with an equal volume of isopropanol in the presence of 0.1 volume of 3 mol/L sodium acetate (pH 4.0), and 1 μl of 10 mg/ml of carrier glycogen at −20°C. The RNA pellet was washed once in 70% ethanol, air-dried, and resuspended in 10 μl of RNase-free water.
Reverse Transcription
RNA extracted from frozen and formalin-fixed, paraffin-embedded tissue sections was reverse-transcribed in a final volume of 20 μl using Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) according to manufacturer’s instructions with the following conditions: 1 mmol/L dNTPs, 40 U of RNase inhibitor (Amersham Pharmacia Biotech, Freiburg, Germany), 300 ng of random hexamers (Amersham Pharmacia Biotech), and 10 μl of RNA solution. The reactions were performed at 42°C for 45 minutes, followed by inactivation of the enzyme at 70°C for 15 minutes. The cDNA was stored at −20°C.
Real-Time Quantitative RT-PCR
Real-time quantitative RT-PCR analyses for E-cadherin, Snail, SIP1, Twist, N-cadherin and GAPDH mRNAs were performed using the ABI PRISM 7700 Sequence Detection System instrument and software (PE Applied Biosystems, Inc., Foster City, CA). Intron-spanning primers and probes for the TaqMan system were designed using Primer Express software (Perkin Elmer, Foster City, CA). The sequences of the PCR primer pairs and fluorogenic probes used for each gene are shown in Table 1 ▶ . The oligonucleotides are designated by the nucleotide position relative to GAPDH GenBank accession no. BC026907, E-cadherin GenBank accession no. Z13009, SIP1 GenBank accession no. AB056507, Snail GenBank accession no. AF155233, Twist GenBank accession no. NM 000474, and N-cadherin GenBank accession no. X57548. Probes were purchased from Perkin-Elmer. The principle of real-time RT-PCR has been described in detail elsewhere. 31,32
Table 1.
Sequence of TaqMan Primers and Probes
| Oligo | Location relative to GenBank accession no. | Sequence 5′–3′ | Size of PCR product | Temperature (°C) |
|---|---|---|---|---|
| FP GAPDH | 548F | CGTGGAAGGACTCATGACCA | 87 bp | 58 |
| RP GAPDH | 635R | GCCATCACGCCACAGTTTC | 59 | |
| P GAPDH | 591 | CAGAAGACTGTGGATGGCCCCTCC | 68 | |
| FP E-cadherin | 1765F | GAACAGCACGTACACAGCCCT | 76 bp | 59 |
| RP E-cadherin | 1841R | GCAGAAGTGTCCCTGTTCCAG | 58 | |
| P E-cadherin | 1787 | ATCATAGCTACAGACAATGGTTCTCCAGTTGCT | 67 | |
| FP SIP1 | 1250F | GCGGCATATGGTGACACACAA | 80 bp | 59 |
| RP SIP1 | 13030R | CATTTGAACTTGCGATTACCTGC | 58 | |
| P SIP1 | 1279 | CAGATCAGCACCAAATGCTAACCCAAGG | 69 | |
| FP Snail | 131F | TGCAGGACTCTAATCCAAGTTTACC | 71 bp | 59 |
| RP Snail | 202R | GTGGGATGGCTGCCAGC | 60 | |
| P Snail | 158 | TCCAGCAGCCCTACACCAGGCC | 68 | |
| FP Twist | 674F | TGTCCGCGTCCCACTAGC | 92 bp | 59 |
| RP Twist | 766R | TGTCCATTTTCTCCTTCTCTGGA | 59 | |
| P Twist | 712 | TCAGCAGGGCCGGAGACCTAGATGT | 69 | |
| FP N-cadherin | 809F | GACGGTTCGCCATCCAGAC | 66 bp | 60 |
| RP N-cadherin | 875R | TCGATTGGTTTGACCACGG | 59 | |
| P N-cadherin | 830 | ACCCAAACAGCAACGACGGGTTAGTC | 68 |
FP, forward primer; RP, reverse primer; P, probe.
Real-time RT-PCR was performed with the TaqMan Universal PCR Master Mix (PE, Applied Biosystems) using 5 μl of diluted cDNA, 300 nmol/L of the probe, and 300 nmol/L primers in a 30-μl final reaction mixture. After a 2-minute incubation at 50°C to allow for uracil N-glycosylase (UNG) cleavage, AmpliTaq Gold was activated by an incubation for 10 minutes at 95°C. Each of the 40 PCR cycles consisted of 15 seconds of denaturation at 95°C and hybridization of probe and primers for 1 minute at 60°C.
Quantitation of Expression
Relative expression levels of target sequences were determined by the standard curve method using a standard cDNA solution from MDA-MB 435S carcinoma cell line (stably transfected with E-cadherin) which was serially fivefold diluted (100 ng to 0.16 ng) and analyzed in triplicates for the genes of interest and GAPDH as housekeeping gene. The resultant data were used to generate standard curves and line equations for the genes of interest and GAPDH plotting CT values versus the relative mass of nucleic acid analyzed. Relative gene expression is calculated by first solving for the nucleic acid mass represented in the GAPDH expression using the standard curve. Next, the CT value for the gene of interest is used to calculate the relative gene expression. Finally, the gene expression value for the gene of interest is normalized to the nucleic acid mass value determined from the house keeping gene GAPDH to adjust variances in the quality of RNA and the amount of input cDNA.
Statistical Analysis
A bivariate Spearman test was chosen to assess the correlation between relative expression levels using SPSS 11.0 for Windows.
Results
Quantitative Gene Expression Analysis in Matched Formalin-Fixed, Paraffin-Embedded Tissue by Real-Time (TaqMan) PCR
To evaluate the correlation between SIP1, Snail, Twist, E- and N-cadherin expression and to elucidate their role in gastric cancer, 28 diffuse-type and 20 intestinal-type tumors were examined. To screen the mRNA expression of the genes of interest in macrodissected tumorous and non-tumorous tissue we used quantitative real-time TaqMan PCR (QRT-PCR). Since the material from paraffin-embedded sections is limited and the RNA is degraded into short pieces by the formalin fixation procedure, this highly sensitive and fast technique for detecting relative mRNA transcription levels seemed suitable considering the large number of samples. There are no antibodies available for immunohistochemical analysis of Snail, Twist, and SIP1.
All primers and hybridization probes were designed to span an intron to exclude annealing to genomic DNA and amplicon sizes were kept small (66 to 92 bp; see Table 1 ▶ ). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was included as housekeeping gene control to correct for equal amounts of matched samples. We then calculated relative amounts of mRNAs by the standard curve method in relation to GAPDH levels in tumorous tissue and compared the values with those from the corresponding non-tumorous tissue. Changes in expression levels of >2 were termed as up- or down-regulation, respectively. TaqMan runs were done twice in duplicates using cDNA from independent reverse transcription reactions.
Diffuse Type Tumors
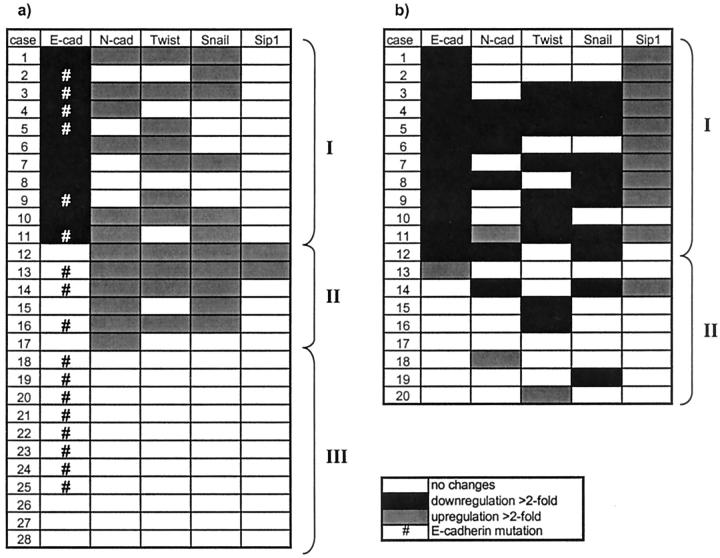
From 28 diffuse-type gastric carcinomas analyzed reduced E-cadherin expression was detected in 11 cases (39%) compared to non-tumorous tissues (Figure 1) ▶ . In 6 of 11 cases (55%), down-regulation of E-cadherin coincided with up-regulated Snail. Statistical analysis revealed a significant correlation (P = 0.049, correlation according to Spearman) between increases in Snail mRNA and E-cadherin down-regulation, although additional 5 cases (where differential expression of E-cadherin was not detected) showed up-regulated Snail mRNA in tumorous tissue.
Figure 1.
Matrix of EMT regulator expression analyzed with QRT-PCR in malignant tissues compared to non-tumorous tissue in diffuse type gastric cancer (a) and in intestinal type gastric cancer (b). Cases were arranged from top to bottom, beginning with the highest down-regulation of E-cadherin mRNA, to show the relationship to N-cadherin and EMT regulator expression.
With regard to the 11 cases showing reduced E-cadherin mRNA levels, there was no relation to up-regulated SIP1 but increased N-cadherin expression could be found in 6 cases (55%) and increased Twist expression was seen in 4 of these 6 cases (66%). Furthermore there were additional 6 cases overexpressing N-cadherin in tumorous tissue without any changes in E-cadherin expression. Statistical analysis according to Spearman could not verify a mathematical correlation (P = 0.864) between increases in N-cadherin mRNA and E-cadherin down-regulation. Interestingly, increased expression of N-cadherin is associated with up-regulated Twist mRNA levels, confirmed by statistical analysis (correlation according to Spearman) which is highly significant on the 1% level (P = 0.007).
The change of E-cadherin expression levels between tumorous tissues and non-tumorous tissues underlies a broad range (−76 to −2) as shown in Table 2 ▶ , where the cases were arranged according to the x-fold change of expression in tumors. Diffuse type case no. 1 has lost E-cadherin expression in the tumor cells analyzed.
Table 2.
Summary of Alterations in Analyzed Genes
| Case | x-Fold change of expression in TU (QRT-PCR results) | Immunohistochemistry | Sequencing E-cad mutations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E-cad | N-cad | Twist | Snail | Sip1 | N-cad TU | N-cad NT | E-cad TU* | ||
| Diffuse | |||||||||
| 1 | neg | 3 | 13 | 4 | – | n.d. | n.d. | (1) M | No mRNA |
| 2 | −76 | – | – | 3 | – | (0) | pac | (3) M | Yes |
| 3 | −29 | 5 | 3 | 3 | – | (0) | pac gan | (3) M | Yes |
| 4 | −8 | 2 | – | – | – | (0) | (3) M | Yes | |
| 5 | −4 | – | 3 | – | – | (0) | pac | (3) M/C | Yes |
| 6 | −4 | 7 | 2 | – | – | n.d. | n.d. | (3) M/C | No |
| 7 | −3 | – | 2 | 2 | – | (0) | pac | n.d. | No |
| 8 | −3 | – | – | – | – | n.d. | n.d. | n.d. | No |
| 9 | −3 | – | 4 | – | – | (0) | (3) M/C | Yes | |
| 10 | −3 | 4 | 2 | 2 | – | (0) gan | pac, gan | (3) M | No |
| 11 | −2 | 9 | – | 4 | – | (2) | pac | (3) M/C | Yes |
| 12 | – | 15 | 5 | 3 | 3 | gan | pac | (3) gran/C | No |
| 13 | – | 5 | 3 | 3 | 5 | (0) | gan | n.d. | Yes |
| 14 | – | 5 | 8 | 2 | – | (0) | (3) M | Yes | |
| 15 | – | 4 | – | 2 | – | (0) gan | pac | (3) M/C | No |
| 16 | – | 2 | 7 | 2 | – | (0) | pac, gan | (3) M/C | Yes |
| 17 | – | 2 | – | – | – | (0) | gan | (3) M/C | No |
| 18 | – | – | – | – | – | (0) gan | gan | (3) M | Yes |
| 19 | – | – | – | – | – | n.d. | n.d. | (3) M | Yes |
| 20 | – | – | – | – | – | (0) | (3) M | Yes | |
| 21 | – | – | – | – | – | (0) | (1) M | Yes | |
| 22 | – | – | – | – | – | n.d. | n.d. | n.d. | Yes |
| 23 | – | – | – | – | – | (0), gan | (3) M | Yes | |
| 24 | – | – | – | – | – | (0) pac | pac | n.d. | Yes |
| 25 | – | – | – | – | – | n.d. | n.d. | n.d. | Yes |
| 26 | – | – | – | – | – | n.d. | n.d. | n.d. | No |
| 27 | – | – | – | – | – | (0) gan | gan | (2) M | No |
| 28 | – | – | – | – | – | (0) | gan | (3) M/C | No |
| Intestinal | |||||||||
| 1 | −17 | – | – | – | 6 | (0) | (3) M | ||
| 2 | −14 | – | – | – | 3 | (0) gan | gan, pac | (3) M | |
| 3 | −12 | – | −7 | −3 | 5 | (0) gan | gan, pac | (3) M | |
| 4 | −8 | −4 | −6 | −4 | 2 | (0) | (3) M | ||
| 5 | −6 | −3 | −47 | −9 | 2 | (0) gan | gan, pac | (3) M | |
| 6 | −6 | −11 | – | – | 4 | (0) | (3) M | ||
| 7 | −5 | – | −2 | −7 | 3 | (0) | (3) M | ||
| 8 | −4 | −5 | – | −10 | 3 | (0) | gan, pac | (3) M | |
| 9 | −4 | – | −7 | −13 | 4 | (0) | (3) M | ||
| 10 | −4 | – | −7 | – | – | (0) | (3) M | ||
| 11 | −3 | 2 | −3 | −4 | 2 | (0) | (3) M | ||
| 12 | −2 | −35 | – | −10 | – | (0) | gan, pac | (3) M | |
| 13 | 3 | – | – | – | – | (0) | (3) M | ||
| 14 | – | −2 | – | −4 | 3 | (0) | (3) M | ||
| 15 | – | – | −5 | – | – | (0) | (3) M | ||
| 16 | – | – | −6 | – | – | (0) | pac | (3) M | |
| 17 | – | – | – | – | – | (0) gan | gan, pac | (3) M | |
| 18 | – | 2 | – | – | – | (0) | (3) M | ||
| 19 | – | – | – | −4 | – | (0) gan | gan, pac | (3) M | |
| 20 | – | – | 2 | – | – | (0) | gan, pac | (3) M | |
The cases were arranged according to the x-fold change of E-cadherin mRNA expression in tumors compared to nontumorous tissue, beginning with the case of highest down-regulation for diffuse-type tumors and then for intestinal type tumors. –, no changes in gene expression when comparing tumorous tissue to normal tissue. E-cad, E-cadherin; N-cad, N-cadherin; TU, tumorous tissue; NT, non-tumorous tissue; n.d., not determined, due to limited material. Immunohistochemistry scoring: 3, more than 50% tumor cells positive; 2, more than 10% tumor cells positive; 1, less than 10% positive; 0, no immunoreactivity of tumor cells detected. pac, chief and parietal cells; gan, ganglion cells, refer to the cell type of specific immunoreactivity. The cellular localization of E-cadherin can be membranous (m), cytoplasmatic (c), and/or granular (gran).
*Fricke E, Keller G, Becker I, Rosivatz E, Schott C, Höfler H, Becker KF, Luber B: Relationship between E-cadherin gene mutation, p53 accumulation, Bcl-2 expression, and Ki-67 staining in diffuse-type gastric carcinoma. Int J Cancer (submitted).
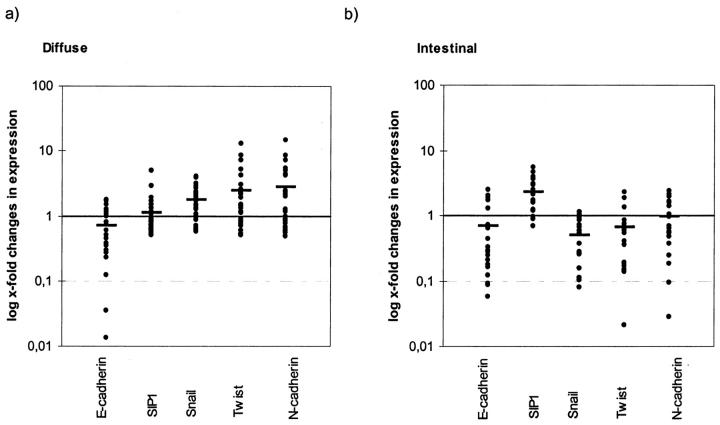
The mean of changes in expression of all 28 cases taken together should reflect the trend of transcriptional regulation occurring in diffuse type gastric cancer compared to matched non-tumorous tissue. Figure 2 ▶ shows down-regulation of E-cadherin in tumorous tissue which is accompanied by up-regulation of Snail, Twist, and N-cadherin but SIP1 is nearly equally expressed. Statistical analysis is shown in Table 3 ▶ .
Figure 2.
Changes in expression levels comparing tumor to non-tumorous samples logarithmically scaled. Horizontal bars (mean of changes in expression) represent a typical expression pattern of EMT regulators in relation to E-cadherin down-regulation. a: Diffuse type tumors show down-regulation of E-cadherin which is accompanied by up-regulation of Snail, Twist, and N-cadherin, but SIP1 is nearly equally expressed. b: The molecular profile of intestinal type gastric cancer shows that down-regulation of E-cadherin is accompanied by increases of SIP1, whereas Snail, Twist, and N-cadherin are even down-regulated, which is nearly the reverse expression pattern seen in diffuse type tumors (except for E-cadherin).
Table 3.
Correlation According to Spearman: Diffuse Type Tumors
| SpearmanRho | E-cadherin | Snail |
|---|---|---|
| E-cadherin correlation | 1.000 | 0.375* |
| Significance (pairwise) | 0.049 | |
| N | 28 | 28 |
| Snail correlation | 0.375* | 1.000 |
| Significance (pairwise) | 0.049 | |
| N | 28 | 28 |
| SpearmanRho | N-cadherin | Twist |
| N-cadherin correlation | 1.000 | 0.500** |
| Significance (pairwise) | 0.007 | |
| N | 28 | 28 |
| Twist correlation | 0.500** | 1.000 |
| Significance (pairwise) | 0.007 | |
| N | 28 | 28 |
| SpearmanRho | E-cadherin | N-cadherin |
| E-cadherin correlation | 1.000 | 0.034 |
| Significance (pairwise) | 0.864 | |
| N | 28 | 28 |
| N-cadherin correlation | 0.034 | 1.000 |
| Significance (pairwise) | 0.864 | |
| N | 28 | 28 |
*Correlation significant on the 0.05 level (pairwise).
**Correlation significant on the 0.01 level (pairwise).
In addition, we identified novel, potentially inactivating, E-cadherin mutations (Table 4) ▶ . E-cadherin gene mutations are characteristic for 50% of diffuse-type gastric cancers but absent from intestinal-type tumors. We aimed to relate expression of the EMT regulators to the integrity of the E-cadherin gene. To analyze E-cadherin gene alterations, we performed two strategies. First, we screened diffuse-type gastric cancer samples for in-frame deletions of exon 8 or 9, since these mutations can easily be detected in archival material using mutation-specific monoclonal antibodies. 26,27 Second, we sequenced the entire E-cadherin coding region after reverse transcription and PCR amplification of fresh-frozen material to identify additional mutations or to exclude sequence changes. From the 28 cases of this study, 17 harbored an E-cadherin gene mutation and 10 cases expressed the normal E-cadherin molecule (Table 4) ▶ . Case no. 1 could not be analyzed for mutations because there was no mRNA for amplification. This case was E-cadherin negative. By mutation-specific immunohistochemical analysis we found 7 cases harboring an exon 9 deletion (1138del183) and 3 cases with an exon 8 deletion (1009del129, Table 4 ▶ ). In addition, we identified 5 cases with other E-cadherin gene mutations, 4 of which are novel mutations that have not been reported before. A 24-bp in-frame deletion (1012del24) was detected in case no. 3 removing codons 338–345 from exon 8. A point mutation (1004G>A) resulting in an amino acid exchange (R335Q) was seen in patient no. 5. A 83-bp deletion removing nucleotides 1054–1137 from exon 8 was seen in patient no. 13. In patient no. 16 we found a point mutation (59G>A), resulting in a W20X premature stop codon. An identical mutation has previously been detected in a lobular breast cancer patient. 24 An in-frame deletion was detected in case no. 23 (1532del33). The only mutation in the intracellular domain was identified in patient no. 25: a duplication of exon 14 and 15 (2440ins275), resulting in a frame shift. One case with a point mutation and a 69 bp deletion from exon 10, no. 21, has been reported previously. 23
Table 4.
E-Cadherin Mutation Analysis
| Case | Exon | Nucleotide change | Predicted protein change |
|---|---|---|---|
| 1 | — | — | — |
| 2 | 9 | 1138del183 | In-frame deletion |
| 3 | 8 | 1012del24 | In-frame deletion |
| 4 | 8 | 1009del129 | In-frame deletion |
| 5 | 7 | 1004G>A | R335Q |
| 6 | No mutation | ||
| 7 | No mutation | ||
| 8 | No mutation | ||
| 9 | 8 | 1138del183 | In-frame deletion |
| 10 | No mutation | ||
| 11 | 9 | 1009del129 | In-frame deletion |
| 12 | No mutation | ||
| 13 | 8 | 1054del83 | Frame shift |
| 14 | 8 | 1009del129 | In-frame deletion |
| 15 | No mutation | ||
| 16 | 2 | 59G>A | W20X |
| 17 | No mutation | ||
| 18 | 9 | 1138del183 | In-frame deletion |
| 19 | 9 | 1138del183 | In-frame deletion |
| 20 | 9 | 1138del183 | In-frame deletion |
| 21 | 10 | 1387G>C and 1320del69 | E463Q and In-frame deletion |
| 22 | 9 | 1138del183 | In-frame deletion |
| 23 | 10 | 1532del33 | In-frame deletion |
| 24 | 9 | 1138del183 | In-frame deletion |
| 25 | 15 | 2440ins275 | Frame shift |
| 26 | No mutation | ||
| 27 | No mutation | ||
| 28 | No mutation |
Mutations have been named according to Antonarakis SE. 54 Nucleotide positions refer to an E-cadherin sequence deposited in the EMBL/GenBank Data Libraries, accession no. Z13009; the A of the ATG of the initiator Met codon is denoted nucleotide +1. Mutation analysis of case no. 1 could not be performed because of lack of E-cadherin mRNA expression.
With regard to EMT regulator expression, E-cadherin gene mutations were present in cases with down-regulated E-cadherin mRNA and/or up-regulated EMT regulators. However, most E-cadherin gene mutations are detected in cases with unchanged E-cadherin or EMT regulator mRNA levels (Figure 1a) ▶ .
Intestinal Type Tumors
As shown in Figure 1 ▶ , from 20 intestinal-type gastric cancer samples reduced E-cadherin expression was found in 12 cases (60%) compared to the matched non-tumorous samples. In contrast to diffuse type cancer samples, we found a high correlation with SIP1, since 10 of these 12 cases (83%) showed elevated SIP1 mRNA levels which is highly significant on the 1% level (correlation according to Spearman, P = 0.0003). However, neither elevated Snail nor increased N-cadherin and Twist levels were associated with down-regulated E-cadherin in the intestinal type as we observed in diffuse type cases. We could even detect down-regulation of Snail in 10 of 20 cases (50%), 9 cases (45%) were found to express lower levels of Twist. Remarkably, 6 cases (30%) were found to express lower levels of N-cadherin in tumorous tissue than in non-tumorous tissue.
The ratios of relative expression levels between tumorous and non-tumorous tissues show a broad range; however, the values for E-cadherin down-regulation (−17 to −2) are not as extreme as found in diffuse type samples (Table 2) ▶ and there is no intestinal tumor sample negative for E-cadherin.
The mean of changes in expression of all 20 cases taken together should reflect the trend of transcriptional regulation occurring in intestinal type gastric cancer compared to matched non-tumorous tissue. Figure 2 ▶ shows down-regulation of E-cadherin in tumorous tissue accompanied by increases of SIP1, whereas Snail, Twist, and N-cadherin are even down-regulated which is nearly the reverse expression pattern determined for diffuse-type tumors (except for E-cadherin). Statistical analysis is shown in Table 5 ▶ .
Table 5.
Correlation According to Spearman: Intestinal Type Tumors
| SpearmanRho | E-cadherin | SIP1 |
|---|---|---|
| E-cadherin correlation | 1.000 | 0.747** |
| Significance (pairwise) | 0.000 | |
| N | 20 | 20 |
| SIP1 correlation | 0.747** | 1.000 |
| Significance (pairwise) | 0.000 | |
| N | 20 | 20 |
| SpearmanRho | SIP1 | Snail |
| SIP correlation | 1.000 | 0.385 |
| Significance (pairwise) | 0.094 | |
| N | 20 | 20 |
| Snail correlation | 0.385 | 1.000 |
| Significance (pairwise) | 0.094 | |
| N | 20 | 20 |
| SpearmanRho | SIP1 | Snail |
| SIP correlation | 1.000 | 0.385* |
| Significance (pairwise) | 0.047 | |
| N | 20 | 20 |
| Snail correlation | 0.385* | 1.000 |
| Significance (pairwise) | 0.047 | |
| N | 20 | 20 |
*Correlation significant on the 0.01 level (pairwise).
**Correlation significant on the 0.05 level.
Immunoreactivity of E-Cadherin and N-Cadherin
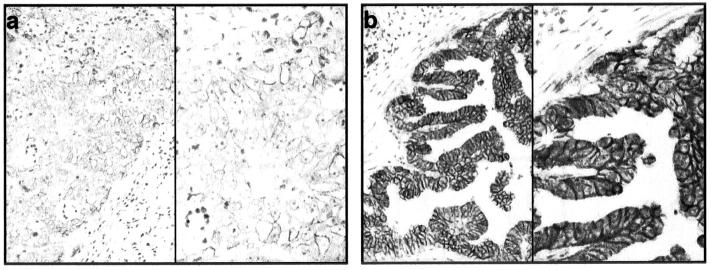
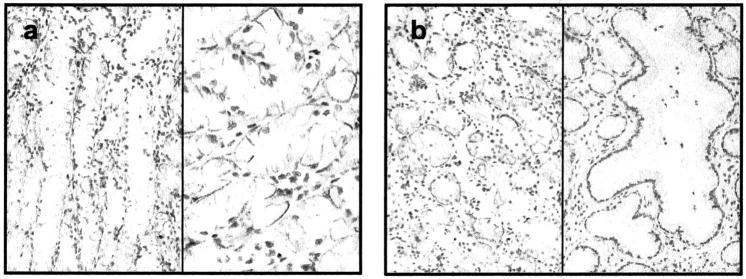
We performed immunohistochemical staining of E-cadherin and N-cadherin to analyze gene expression and to compare TaqMan results on the protein level (Table 2) ▶ and to confirm tumor cell type specific expression. The scoring is: 3, more than 50% tumor cells positive; 2, more than 10% tumor cells positive; 1, less than 10% positive; 0, no detectable immunoreactivity. The cellular localization of E-cadherin can be membranous (m), cytoplasmic (c), and/or granular (gran). The specificities of the N-cadherin and E-cadherin antibodies were confirmed by immunoblotting (Figure 3) ▶ . For immunohistochemistry of N-cadherin, ganglion cells were used as positive control (Figure 4a) ▶ for E-cadherin, non-tumorous epithelial cells served as positive control.
Figure 3.
Immunoblot analysis of N-cadherin (a) and E-cadherin expression detected by ECL (b). 15 μg of total protein from MDA MB-435s cell lysates transfected with normal E-cadherin cDNA 29 were used for the analysis. Molecular weights of the protein are indicated.
Figure 4.

Immunohistochemistry of diffuse-type gastric carcinoma with a specific monoclonal N-cadherin antibody and counterstained with hematoxylin. a: Ganglion cells were used as positive control (magnifications: left, ×100; right, ×200). b: 20% tumor cells show membranous or weak cytoplasmic staining (case no. 11; magnifications: ×100 and ×200). c: No immunodetectable protein expression though abundant N-cadherin mRNA (case no. 4; magnifications: left, ×100; right, ×200).
N-cadherin mRNA expression, in contrast to E-cadherin mRNA expression, did not always result in immunodetectable protein expression and appearance in tumor cells was found in only one single case. As shown in Figure 4b ▶ , diffuse-type case no. 11 (Table 2) ▶ which was clearly N-cadherin positive in TaqMan analysis showed appearance at the intercellular adhesion sites or dot-like cytoplasmic appearance of N-cadherin in more than 20% of tumor cells. In contrast in case no. 4, expression of N-cadherin could not be detected on the protein level by immunohistochemical staining with the antibody used although mRNA expression was seen by TaqMan analysis (Figure 4c) ▶ .
Staining of N-cadherin was detected in chief and parietal cells of non-tumorous epithelia (pac in Table 2 ▶ ) used for TaqMan analysis as “non tumorous”; furthermore, in some cases non-tumorous tissue was streaked with ganglion cells (gan in Table 2 ▶ ) expressing high levels of N-cadherin, but the tumorous counterparts analyzed for mRNA expression lacked such cell type.
The expression of E-cadherin was observed between tumor-cell boundaries in all cases studied except in diffuse-type case no. 12, where just granular and cytoplasmic appearance of E-cadherin was seen. One single case (diffuse-type no. 1), which lacked E-cadherin mRNA, showed very weak membranous staining. In all remaining cases analyzed immunohistochemical detection of E-cadherin corresponded with TaqMan results. As shown in Figure 6a ▶ intestinal type case no. 1 tumor cells expressed low levels of E-cadherin consistent with TaqMan results; strong expression of E-cadherin in tumor cells consistent with TaqMan results was seen in intestinal-type tumor cells of case no. 19 (Figure 6b) ▶ .
Figure 6.
Immunohistochemistry of intestinal-type gastric carcinomas with the specific monoclonal E-cadherin antibody and counterstained with hematoxylin. a: Tumor cells show low protein expression consistent with TaqMan results (magnifications: left, ×100; right, ×200). b: High expression of E-cadherin in tumor cells consistent with TaqMan results (magnifications: left, ×100; right, ×200).
Discussion
Recent reports have highlighted the role of epithelial mesenchymal transition (EMT) regulators Snail and SIP1 as strong repressors of E-cadherin gene expression in tumor cell lines, thus inducing tumor malignancy. 6,11,21 Additionally, Twist 14 was proposed to be involved in this process, as it is known as an activator of N-cadherin in Drosophila. 14 N-cadherin enhances cell motility of various tumor cells 16-18,33 and may even overcome strong E-cadherin-dependent cell-cell contacts. 15 E-cadherin alterations have been investigated extensively in many tumors, 1 but to our knowledge this is the first study examining expression patterns of EMT regulators in case-matched primary human gastric cancer specimens in the context of E-cadherin repression at the transcriptional level.
Comparing SIP1, Snail, Twist, E- and N-cadherin expression in diffuse type tumors and intestinal type tumors versus matched non-tumorous tissue, we found different expression patterns according to Laurén’s classification. This result proposes the existence of distinct carcinogenetic pathways for the intestinal and diffuse type carcinoma as was suggested by Tahara 34 and Correa 35 for other genes before.
For diffuse-type carcinomas we confirm down-regulation of E-cadherin in 39% of cases (Figure 1) ▶ , but we did not observe the potential SIP1 repressor function, which was proposed by Comijn et al. 8 The strict inverse correlation of Snail expression with E-cadherin expression in various well-established tumor cell lines and as it has recently been reported for hepatocellular carcinoma cell lines 19 could not be confirmed. However, our findings suggest that Snail may play a role in diffuse-type carcinoma as a repressor of E-cadherin transcription. The fact, that SIP1 is not involved in diffuse-type carcinogenesis appears to be reasonable, since these two repressor factors are known to bind partly overlapping regions in the E-cadherin promoter. 36 Although we have no evidence for direct repression of E-cadherin by Snail in a biologically functional relationship, we propose a role for this EMT regulator in diffuse type gastric carcinoma based on our data and apply to the increasing number of publications 37,38 focusing on transcriptional repression of E-cadherin via Snail in human tumors. Moreover, independent of E-cadherin, Snail has additional targets, such as desmoplakin 7 and cytokeratin-18, 37 emphasizing its role in tumor progression. An interesting aspect is the observation that Snail can interact with MeCP2, which represses transcription at methylated promoters. 39,40 Possibly, Snail supports E-cadherin inactivation through promoter hypermethylation in gastric cancer. 41 Further studies are needed to address this question.
Unexpectedly, we found up-regulated expression of N-cadherin in diffuse type gastric cancer samples, which suggests a new role for this mesenchymal, pro-migratory cadherin in gastric cancer progression, as recently reported by Yanagimoto et al 42 Similar findings were published for melanoma, breast, and prostate cancer cells before. 15,17,18,33 This abnormal expression in tumors has never been linked to Twist. Our observation that N-cadherin up-regulation is highly correlated to Twist up-regulation (Figure 1) ▶ proposes a similar function for Twist in human cancers during EMT. Whether Twist may act as indirect repressor of E-cadherin through transcriptional activation of N-cadherin or directly via binding to the E-cadherin promoter and exerting repressor function is not clear at the moment.
In intestinal type gastric cancer samples, we found SIP1 clearly associated with reduced E-cadherin transcript levels, whereas Snail1, conversely to diffuse type gastric carcinoma, seems not to be involved (Figure 1) ▶ . The highly significant correlation underlines the hypothesized repressor function of SIP1 on E-cadherin expression in human tumors, though based on our findings of higher changes in expression of E-cadherin in diffuse type tumor samples, we propose Snail is a more potent repressor of E-cadherin transcription. The observation of frequently up-regulated SIP1 in intestinal type gastric cancer and simultaneously reduced Snail mRNA levels is consistent with the findings made by Comijn et al in MDCK cell lines, where a weak down-regulation of Snail was seen on SIP1 induction. 8
Slight down-regulation of N-cadherin and Twist (Figure 1) ▶ found in intestinal type tumor samples compared to non-tumorous tissues was unexpected, as normal epithelial cells are not known to express N-cadherin. These findings can be explained by the fact that in these cases the analyzed non-tumorous tissue is streaked with ganglion cells (Table 2 ▶ , gan) known to express N-cadherin. Interestingly, we found chief and parietal cells (Figure 5 ▶ ; Table 2 ▶ , pac) of non-tumorous epithelia expressing high levels of N-cadherin, whereas the tumorous counterparts analyzed with QRT-PCR lacked the latter cell type. It’s likely that the same pattern goes for Twist, as its expression may be essential for N-cadherin transcription initiation. 14 The expression of N-cadherin in epithelial cells of the adult stomach has never been reported before, and the molecule’s function therein is unclear. However, its expression can be found in neuroendocrine complexes of fetal stomach, small intestine, and pancreas during human embryonic development. 43
Figure 5.
Immunohistochemistry of non-tumorous gastric glands counterstained with hematoxylin. a: Unexpected positive N-cadherin immunoreaction in chief and parietal cells (magnifications: left, ×100; right, ×200). b: Comparison of deep gastric glands with foveolar glands, where N-cadherin immunoreactivity is seen in chief and parietal cells but not in foveolar epithelium (magnification, ×100).
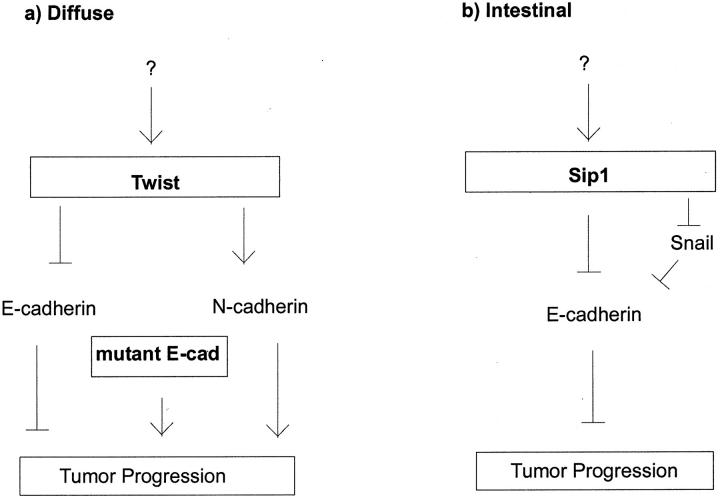
In the context of EMT regulators with respect to E-cadherin expression we propose two different pathways occurring during carcinogenesis in the diffuse or intestinal type, respectively (Figure 7, a and b) ▶ . Based on our results, we suggest the E- to N-cadherin switch, in addition to E-cadherin gene mutation (see below), may play a key role in diffuse type carcinogenesis, whereas in the intestinal type SIP1 is the main repressor of E-cadherin function which finally leads to enhanced migratory properties of tumor cells. It is presently not clear how Twist, Snail, or SIP1 are regulated themselves. It has been reported that the cytokine transforming growth factor TGF-β, which is often up-regulated in human tumors, enhances SIP1 expression 8,44 as well as Snail expression, 37,45 and Boyer et al suggested the growth factors FGF-1, EGF and TGF-α, which increase the motility of some cell lines, to activate Snail via Ras and the PI3-kinase pathway. 4 Additionally, Tan reported enhanced Snail promoter activity on ILK (integrin-linked kinase) overexpression in human colon carcinoma cells. 46 In Drosophila Twist transcription is induced by DI, a transcription factor that is homologous to the NF-κB family of proteins. 47 In addition, Twist may be regulated through subcellular localization, as it is common for bHLH molecules. 48 The upstream regulators of Snail, SIP1 and Twist in gastric cancer remain elusive.
Figure 7.
Proposed model of E-cadherin dependent carcinogenesis pathways of diffuse type (a) and intestinal type gastric cancer (b). Based on our findings, we propose a key role for the E- to N-cadherin switch, beside loss of E-cadherin function through gene mutations, in diffuse type gastric carcinoma. In intestinal type gastric carcinoma, enhanced SIP1 expression may be involved in tumor progression due to E-cadherin repression.
The expression matrix in Figure 1 ▶ shows three different subgroups (I-III) of underlying mechanisms of E-cadherin loss and thus cancer development or progression in the cases analyzed: I, the cases with down-regulated E-cadherin, partly linked to differentially expressed Snail, Twist, and N-cadherin; II, cases with normal E-cadherin expression, but up-regulated N-cadherin, which may overcome E-cadherin function; and III, the group of cases without any detectable changes in expression levels of the genes examined. In the latter group posttranslational modifications 49-51 or mutations in the E-cadherin gene may lead to its loss of function and force tumor progression.
In our study we included 17 patients with E-cadherin gene mutations. In addition to 10 patients harboring either an exon 8 or exon 9 deletion, we identified other mutations as well. With regard to E-cadherin and EMT regulator expression it seems that the mutations uncouple regulatory mechanisms: in many cases harboring such mutations E-cadherin, N-cadherin, Twist, Snail, and SIP1 mRNA expression are all unchanged. We cannot rule out, however, that hypermethylation of the E-cadherin promoter may be of importance for some of those cases harboring E-cadherin gene mutations as was suggested by Machado et al. 52
The expression matrix in Figure 1 ▶ of intestinal type tumor cases shows two subgroups: cases which have down-regulated E-cadherin due to increased SIP1 expression levels (I) and cases where the underlying mechanism of loss of E-cadherin function is not yet clear (II) and may occur through other mechanisms, as mentioned above.
To clarify the unexpected down-regulation of N-cadherin in some cases, we examined the E- and N-cadherin expression on the protein level by immunohistochemistry as well in addition to QRT-PCR analysis. Immunohistochemical analysis of the same sections with Twist, Snail, and SIP1 could not be performed, as there exist no satisfying commercially available antibodies, so far.
Immunohistochemical staining of E-cadherin was mirrored by mRNA expression (Figure 6) ▶ . The only case in which we failed to detect E-cadherin mRNA had less than 10% stained tumor cells. So this divergence may be due to the sensitivity of the method.
N-cadherin mRNA expression, in contrast to E-cadherin mRNA expression, did not result in immunodetectable protein expression (Figure 4c) ▶ , except in one case (Figure 4b ▶ , diffuse-type case no. 11). Apart from the fact of very low levels of N-cadherin in tumor cells and therefore reaching the sensitivity limits of the antibody, there are some more reasons which could explain the discrepancy. Presumably, N-cadherin is posttranslationally modified and thus makes it impossible for the antibody to bind the protein. 53 A mechanism truncating proteolytically the β-catenin binding domain is known for E-cadherin 5 and may also be the case for N-cadherin, as they share highly homologous cytoplasmic domains, where the immunogen for N-cadherin antibody originated from. However, such a mechanism would be tumor cell specific as detection of N-cadherin in ganglion cells serving as positive control always worked well (Figure 4a) ▶ . Furthermore, we have to consider, but seems rather unlikely, that the detected N-cadherin mRNA is not translated into protein. We can exclude an unspecific amplification, as the PCR product from the primers used (Table 1) ▶ was sequenced and clearly identified as N-cadherin.
We conclude that EMT regulators play critical roles in human tumorigenesis and in the down-regulation of the invasion suppressor E-cadherin. The mechanism of repression, however, remains to be elucidated.
Acknowledgments
We thank Christina Schott for excellent technical assistance and Gisela Keller for helpful discussions.
Footnotes
Address reprint requests to Dr. Karl-Friedrich Becker, Klinikum rechts der Isar der Technischen Universitaet, Institut fuer Pathologie, Trogerstrasse 18/III.Stock/R3.16, D-81675, Muenchen, Germany. E-mail: kf.becker@lrz.tum.de.
Supported in part by grant 10–1675-Be1 from the Deutsche Krebshilfe (to H. H. and K.-F. B.).
References
- 1.Hirohashi S: Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 1998, 153:333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M: E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol 2000, 156:1515-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallaro U, Christofori G: Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta 2001, 1552:39-45 [DOI] [PubMed] [Google Scholar]
- 4.Boyer B, Valles AM, Edme N: Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol 2000, 60:1091-1099 [DOI] [PubMed] [Google Scholar]
- 5.Rashid MG, Sanda MG, Vallorosi CJ, Rios-Doria J, Rubin MA, Day ML: Post-translational truncation and inactivation of human E-cadherin distinguishes prostate cancer from matched normal prostate. Cancer Res 2001, 61:489-492 [PubMed] [Google Scholar]
- 6.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia DH: The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat Cell Biol 2000, 2:84-89 [DOI] [PubMed] [Google Scholar]
- 7.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA: The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000, 2:76-83 [DOI] [PubMed] [Google Scholar]
- 8.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F: The two-handed e box binding zinc finger protein sip1 down-regulates e-cadherin and induces invasion. Mol Cell 2001, 7:1267-1278 [DOI] [PubMed] [Google Scholar]
- 9.Hemavathy K, Ashraf SI, Ip YT: Snail/Slug family of repressors: slowly going into the fast lane of development and cancer. Gene 2000, 257:1-12 [DOI] [PubMed] [Google Scholar]
- 10.Behrens J, Lowrick O, Klein-Hitpass L, Birchmeier W: The E-cadherin promoter: functional analysis of a G.C-rich region and an epithelial cell-specific palindromic regulatory element. Proc Natl Acad Sci USA 1991, 88:11495-11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigo I, Cato AC, Cano A: Regulation of E-cadherin gene expression during tumor progression: the role of a new Ets-binding site and the E-pal element. Exp Cell Res 1999, 248:358-371 [DOI] [PubMed] [Google Scholar]
- 12.Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A: A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem 2001, 276:27424-27431 [DOI] [PubMed] [Google Scholar]
- 13.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T: The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition Mol Cell Biol 2001, 21:8184-8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda H, Tsukita S, Takeichi M: Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol 1998, 203:435-450 [DOI] [PubMed] [Google Scholar]
- 15.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ: N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 1999, 147:631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR: Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 1996, 135:1643-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA: Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000, 148:779-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Satyamoorthy K, Herlyn M: N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 2001, 61:3819-3825 [PubMed] [Google Scholar]
- 19.Jiao W, Miyazaki K, Kitajima Y: Inverse correlation between E-cadherin and Snail expression in hepatocellular carcinoma cell lines in vitro and in vivo. Br J Cancer 2002, 86:98-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, Nagayama M: Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol 2001, 37:65-71 [DOI] [PubMed] [Google Scholar]
- 21.Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK: Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem 2001, 276:24661-24666 [DOI] [PubMed] [Google Scholar]
- 22.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA: Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 2002, 21:3241-3246 [DOI] [PubMed] [Google Scholar]
- 23.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H: E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994, 54:3845-3852 [PubMed] [Google Scholar]
- 24.Berx G, Becker KF, Hofler H, van Roy F: Mutations of the human E-cadherin (CDH1) gene. Hum Mutat 1998, 12:226-237 [DOI] [PubMed] [Google Scholar]
- 25.Keller G, Vogelsang H, Becker I, Hutter J, Ott K, Candidus S, Grundei T, Becker KF, Mueller J, Siewert JR, Hofler H: Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol 1999, 155:337-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker KF, Kremmer E, Eulitz M, Becker I, Handschuh G, Schuhmacher C, Muller W, Gabbert HE, Ochiai A, Hirohashi S, Hlubek F, Hofler H: Analysis of E-cadherin in diffuse-type gastric cancer using a mutation-specific monoclonal antibody. Am J Pathol 1999, 155:1803-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker KF, Kremmer K, Eulitz M, Schulz S, Mages J, Handschuh G, Wheelock MJ, Cleton-Jansen AM, Hofler H, Becker I: Functional allelic loss detected at the protein level in archival human tumors using allele-specific E-cadherin monoclonal antibodies. J Pathol 2002, 197:567-574 [DOI] [PubMed] [Google Scholar]
- 28.Becker KF, Reich U, Schott C, Becker I, Berx G, van Roy F, Hofler H: Identification of eleven novel tumor-associated E-cadherin mutations. Mutations in brief no. 215. Online. Hum Mutat 1999, 13:171. [DOI] [PubMed] [Google Scholar]
- 29.Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Hofler H, Becker KF: Tumor-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 1999, 18:4301-4312 [DOI] [PubMed] [Google Scholar]
- 30.Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H: Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol 2001, 158:419-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson UE, Heid CA, Williams PM: A novel method for real time quantitative RT-PCR. Genome Res 1996, 6:995-1001 [DOI] [PubMed] [Google Scholar]
- 32.Heid CA, Stevens J, Livak KJ, Williams PM: Real time quantitative PCR. Genome Res 1996, 6:986-994 [DOI] [PubMed] [Google Scholar]
- 33.Tran NL, Nagle RB, Cress AE, Heimark RL: N-cadherin expression in human prostate carcinoma cell lines: an epithelial-mesenchymal transformation mediating adhesion with stromal cells. Am J Pathol 1999, 155:787-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tahara E: Genetic alterations in human gastrointestinal cancers: the application to molecular diagnosis. Cancer 1995, 75:1410-1417 [DOI] [PubMed] [Google Scholar]
- 35.Correa P, Shiao YH: Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res 1994, 54:1941s-1943s [PubMed] [Google Scholar]
- 36.Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D: SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem 1999, 274:20489-20498 [DOI] [PubMed] [Google Scholar]
- 37.Nieto MA: The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002, 3:155-166 [DOI] [PubMed] [Google Scholar]
- 38.Thiery JP: Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer 2002, 2:442-454 [DOI] [PubMed] [Google Scholar]
- 39.Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT, Wu CW, Shen CY: Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 2001, 20:3814-3823 [DOI] [PubMed] [Google Scholar]
- 40.Nan X, Tate P, Li E, Bird A: DNA methylation specifies chromosomal localization of MeCP2. Mol Cell Biol 1996, 16:414-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura G, Sato K, Akiyama S, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Nishizuka S, Motoyama T: Molecular characterization of undifferentiated-type gastric carcinoma. Lab Invest 2001, 81:593-598 [DOI] [PubMed] [Google Scholar]
- 42.Yanagimoto K, Sato Y, Shimoyama Y, Tsuchiya B, Kuwao S, Kameya T: Co-expression of N-cadherin and α-fetoprotein in stomach cancer. Pathol Int 2001, 51:612-618 [DOI] [PubMed] [Google Scholar]
- 43.Gaidar YA, Lepekhin EA, Sheichetova GA, Witt M: Distribution of N-cadherin and NCAM in neurons and endocrine cells of the human embryonic and fetal gastroenteropancreatic system. Acta Histochem 1998, 100:83-97 [DOI] [PubMed] [Google Scholar]
- 44.Zwijsen A, van Grunsven LA, Bosman EA, Collart C, Nelles L, Umans L, Van de PT, Wuytens G, Huylebroeck D, Verschueren K: Transforming growth factor β signaling in vitro and in vivo: activin ligand-receptor interaction, Smad5 in vasculogenesis, and repression of target genes by the deltaEF1/ZEB-related SIP1 in the vertebrate embryo. Mol Cell Endocrinol 2001, 180:13-24 [DOI] [PubMed] [Google Scholar]
- 45.Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W: Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-β1 and Ha-Ras: steps toward invasiveness. J Cell Sci 2002, 115:1189-1202 [DOI] [PubMed] [Google Scholar]
- 46.Tan C: Inhibition of integrin linked kinase (ILK) suppresses α-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC[minus]/[minus] human colon carcinoma cells. Oncogene 2001, 20:133-140 [DOI] [PubMed] [Google Scholar]
- 47.Akimaru H, Hou DX, Ishii S: Drosophila CBP is required for dorsal-dependent twist gene expression. Nat Genet 1997, 17:211-214 [DOI] [PubMed] [Google Scholar]
- 48.Lee MS, Lowe G, Flanagan S, Kuchler K, Glackin CA: Human Dermo-1 has attributes similar to twist in early bone development. Bone 2000, 27:591-602 [DOI] [PubMed] [Google Scholar]
- 49.Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S: Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA 2002, 99:365-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pece S, Gutkind JS: E-cadherin and Hakai: signaling, remodeling, or destruction? Nat Cell Biol 2002, 4:E72-E74 [DOI] [PubMed] [Google Scholar]
- 51.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W: Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002, 4:222-231 [DOI] [PubMed] [Google Scholar]
- 52.Machado JC, Oliveira C, Carvalho R, Soares P, Berx G, Caldas C, Seruca R, Carneiro F, Sobrinho-Simoes M: E-cadherin gene (CDH1) promoter methylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene 2001, 20:1525-1528 [DOI] [PubMed] [Google Scholar]
- 53.Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM, Kosma VM: Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas: comparison of immunohistochemistry and in situ hybridization. J Clin Pathol 2001, 54:533-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonarakis SE: Recommendations for a nomenclature system for human gene mutations. Hum Mutat 1998, 11:1-3 [DOI] [PubMed] [Google Scholar]