Abstract
The development of bleomycin-induced lung injury, a model of pulmonary fibrosis, results from inflammatory cell infiltration, a process highly regulated by the expression of multiple adhesion molecules. At present, the identity and role of the adhesion molecules involved in the fibrotic process are unknown. Therefore, bleomycin-induced pulmonary fibrosis was examined in mice lacking L-selectin (L-selectin−/−) expression, intercellular adhesion molecule-1 (ICAM-1) expression, or both. After 16 days of intratracheal bleomycin challenge, collagen deposition was inhibited in both L-selectin−/− and ICAM-1−/− mice when compared with wild-type littermates. Interestingly, collagen deposition was virtually eliminated in L-selectin/ICAM-1−/− mice relative to either the L-selectin−/− or ICAM-1−/− mice. Decreased pulmonary fibrosis was associated with reduced accumulation of leukocytes, including neutrophils and lymphocytes. Decreased mRNA expression of proinflammatory cytokines and transforming growth factor (TGF)-β1 paralleled the inhibition of collagen deposition. The present study indicates that L-selectin and ICAM-1 play a critical role in pulmonary fibrosis by mediating the accumulation of leukocytes, which regulate the production of proinflammatory cytokines and TGF-β1. This suggests that these adhesion molecules are potential therapeutic targets for inhibiting human pulmonary fibrosis.
Pulmonary fibrosis comprises a diverse group of diseases characterized by inflammatory infiltrates, disruption of alveolar structure, and excessive synthesis and deposition of connective tissue. 1 Idiopathic pulmonary fibrosis is a chronic and often fatal disorder with a 5-year survival rate of ∼50%. In addition, pulmonary fibrosis is frequently associated with certain connective tissue diseases, including systemic sclerosis and dermatomyositis/polymyositis. The pathogenesis of pulmonary fibrosis remains unknown, and conventional treatment with immunosuppressive therapy has been disappointing. To examine the underlying pathophysiology and to test new therapeutic approaches, bleomycin-induced lung injury is widely used as an established animal model of pulmonary fibrosis. 1,2
Intratracheal administration of bleomycin induces acute alveolitis and interstitial inflammation, characterized by the sequential recruitment of leukocytes in the first week. 3,4 Subsequent to these inflammatory responses, fibrotic responses characterized by fibroblast proliferation and extracellular matrix synthesis occur in the second week. 3 It is generally assumed that leukocytes infiltrating the lung are involved in the evolution of pulmonary fibrosis by secreting reactive oxygen species, fibrogenic cytokines, and growth factors. 1 In general, leukocyte recruitment into inflammatory sites is achieved using constitutive or inducible expression of multiple adhesion molecules. 5,6 L-selectin (CD62L) which primarily mediates leukocyte capture and rolling on the endothelium is constitutively expressed by most leukocytes. 7 In vitro, L-selectin binds to several glycosylated mucin-like proteins expressed by high endothelial venules. 7 Cytokine-inducible ligands for L-selectin have also been described for peripheral endothelial cells, but their identity is unknown. 8,9 L-selectin-deficient (L-selectin−/−) mice have reduced trauma- and tumor necrosis factor-α (TNF-α)-induced leukocyte rolling, decreased leukocyte recruitment into an inflamed peritoneum, decreased delayed-type hypersensitivity responses, delayed rejection of allogeneic skin transplants, and resistance to lipopolysaccharide (LPS)-induced septic shock. 10-13 Intercellular adhesion molecule-1 (ICAM-1, CD54) is constitutively expressed at low levels by endothelial cells and is rapidly up-regulated during inflammation, resulting in increased leukocyte-endothelial cell adhesion. 14 Leukocytes express β2 integrins, including lymphocyte function-associated antigen-1 (LFA-1, CD11a/CD18), which interact with ICAM-1. ICAM-1/β2 integrin interactions promote leukocyte rolling, but also mediate firm adhesion and the transmigration of leukocytes at sites of inflammation. 6,15 ICAM-1−/− mice have significantly reduced numbers of infiltrating neutrophils during peritonitis, reduced susceptibility to LPS-induced septic shock, delayed skin wound repair, and impaired delayed-type hypersensitivity reactions, although allogeneic skin graft rejection is normal. 12,16-18 Furthermore, L-selectin and ICAM-1 function synergistically to mediate optimal leukocyte rolling and entry into inflammatory sites, which is essential for the generation of inflammatory responses in vivo. 10,15,18
Despite a critical role of inflammatory cell infiltration in the bleomycin-induced pulmonary fibrosis, studies investigating the contribution of adhesion molecules to this model are limited. Bleomycin-induced pulmonary fibrosis is inhibited in transgenic mice overexpressing soluble E-selectin that inhibits the binding of leukocytes to E-selectin on the endothelium. 19 Administration of monoclonal antibody (mAb) against α4 integrins reduces pulmonary fibrosis and decreases the number of inflammatory cells in the bronchoalveolar lavage (BAL). 20 In addition, in vivo treatment with anti-CD11a or CD11b mAb significantly diminishes pulmonary fibrosis: this effect is accompanied by decreased lymphoid infiltration. 21 By contrast, another study has suggested that the antagonism of ICAM-1 and LFA-1 by mAbs does not attenuate bleomycin-induced pulmonary fibrosis, although the same treatment decreases neutrophil infiltration in the BAL. 22 Thus, the in vivo contribution of ICAM-1 and L-selectin to bleomycin-induced pulmonary fibrosis remains unclear.
The role of L-selectin and ICAM-1 in lung inflammation is complex and dependent on the models of inflammation induced in the lung. L-selectin is not required for neutrophil emigration into the alveolar space and subsequent edema formation in bacterial pneumonia. 23 By contrast, L-selectin is required for the development of airway hyperresponsiveness and lymphocyte migration into the inflamed lung during an allergic inflammatory response. 24,25 Radiation-induced, endotoxin-induced, or antigen-dependent allergic pulmonary inflammation is attenuated in ICAM-1−/− mice. 25-28 By contrast, ICAM-1 loss increases mortality in Escherichia coli or Klebsiella pneumonia. 29,30 Therefore, the contribution of L-selectin or ICAM-1 to lung inflammation varies according to the stimuli used for initiating inflammation. To directly assess roles of ICAM-1 and L-selectin in lung fibrosis, bleomycin-induced pulmonary fibrosis was investigated using mice lacking either L-selectin, ICAM-1, or both adhesion molecules. The results of this study suggest that ICAM-1 and L-selectin significantly contribute to bleomycin-induced pulmonary fibrosis by regulating the influx of leukocytes and their subsequent production of proinflammatory cytokines and growth factors.
Materials and Methods
Animals
L-selectin−/− mice were produced as described. 11 A lack of L-selectin expression in L-selectin−/− mice was confirmed by flow cytometric analysis of blood leukocytes stained with phycoerythrin-conjugated anti-L-selectin mAb (MEL14; Beckman Coulter, Inc., Miami, FL). L-selectin was expressed on the majority of blood leukocytes from wild-type littermates, but not from L-selectin−/− mice (Figure 1) ▶ . Similar results were obtained using leukocytes from the spleen and peripheral lymph nodes (data not shown). ICAM-1−/− mice 16 expressing residual amounts of ICAM-1 splice variants in the thymus and spleen but not in other organs including lung 31 were from the Jackson Laboratory (Bar Harbor, ME). Mice lacking both L-selectin and ICAM-1 were generated as described. 15 All mice were backcrossed between 5 and 10 generations onto the C57BL/6 genetic background. Mice used for experiments were 12 to 16 weeks old. Age-matched wild-type littermates and C57BL/6 mice (Jackson Laboratory) were used as controls with equivalent results so all control results were pooled. To verify that the size of body or lungs was similar for mutant and wild-type littermates, the weight of body or lungs was measured (n = 10 for each genotype). The body weight was similar for L-selectin−/− (29.2 ± 3.3 g), ICAM-1−/− (28.4 ± 2.9 g), L-selectin/ICAM-1−/− (29.3 ± 2.7 g), and wild-type (28.9 ± 3.4 g) littermates. The weight of both lungs was also similar for L-selectin−/− (140.7 ± 26.4 mg), ICAM-1−/− (138.2 ± 22.6 mg), L-selectin/ICAM-1−/− (138.0 ± 27.9 mg), and wild-type (140.7 ± 31.5 mg) littermates. All mice were healthy, fertile, and did not display evidence of infection or disease. All mice were housed in a specific pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science.
Figure 1.
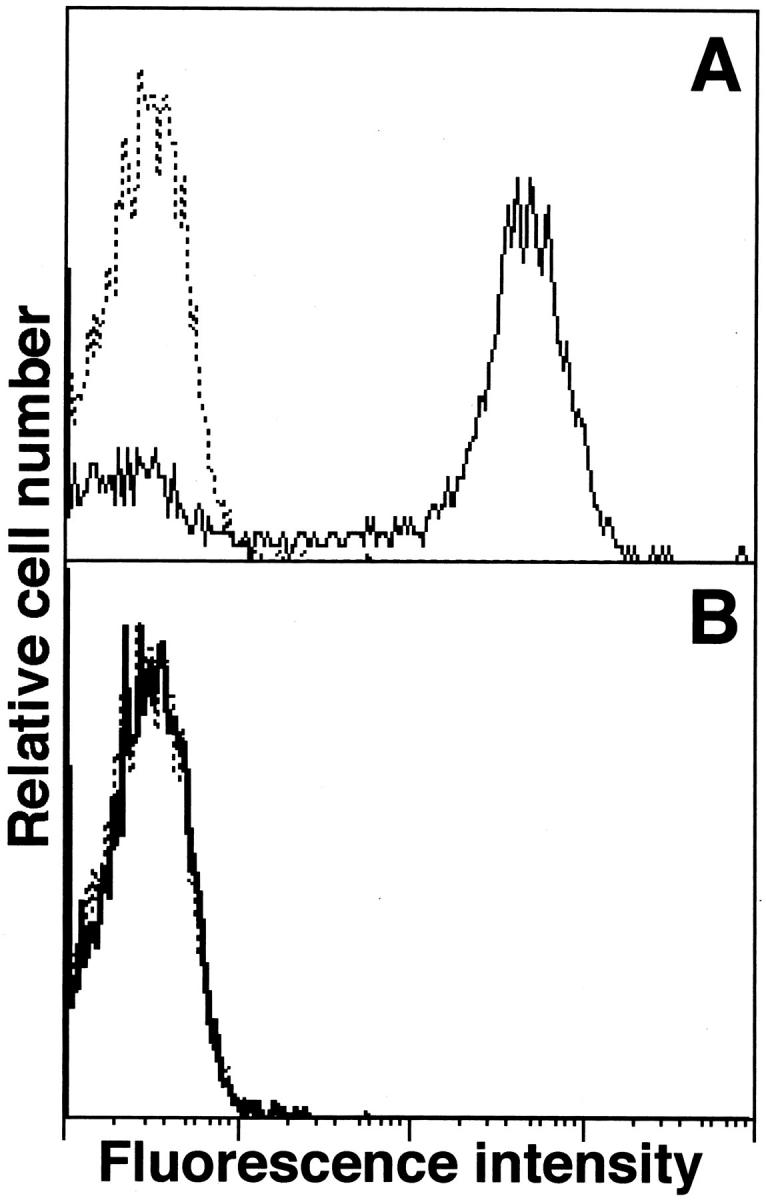
Immunofluorescence analysis of L-selectin expression by blood leukocytes from wild-type (A, thin line) and L-selectin−/− (B, solid line) littermates with flow cytometric analysis. Dotted histograms represent immunofluorescent staining with unreactive, isotype-matched control Abs. These results represent those obtained with 5 mice of each genotype.
Bleomycin Administration
Bleomycin sulfate (Nippon Kayaku, Tokyo, Japan) was administered to mice anesthetized by inhalation of diethyl ether. Using aseptic techniques, a single incision was made at the neck and the muscle covering the trachea was snipped to expose the tracheal rings. A single intratracheal instillation of bleomycin sulfate (8 mg/kg) in 250 μl of sterile saline was performed using a 27-gauge needle.
Preparation of BAL
BAL cells were prepared as described elsewhere. 32 Briefly, at 2, 4, 8, 12, and 16 days postinstillation, the mice were sacrificed and the lungs lavaged with saline before fixation. BAL fluid was collected as follows: 1 ml of saline was instilled three times and withdrawn from the lungs via an intratracheal cannula. In each mouse examined, approximately 2.5 ml of BAL fluid was retrieved. A 500-μl aliquot of the recovered BAL fluid was analyzed for total and differential leukocyte counts after lysis of erythrocytes. A total leukocyte count was performed using a hemocytometer in the presence of trypan blue. Cell differential counts were determined after cytospin centrifuge with May-Giemsa staining. A total of 200 cells were counted from randomly chosen high power microscopic fields for each sample and 5 to 10 mice of each genotype were examined. Neutrophils were identified morphologically in the BAL cells, as described previously. 33
Hydroxyproline Assay
Hydroxyproline is a modified amino acid uniquely found at a high percentage in collagen. Therefore, the tissue hydroxyproline content of lungs was assessed as a quantitative measure of collagen deposition as previously described. 34 Briefly, lungs were harvested 16 days after bleomycin administration. The lung vasculature was perfused free of blood by slowly injecting 3 ml of phosphate-buffered saline (PBS) into the right ventricle. The right lung was then excised and homogenized in 2 ml of PBS, pH 7.4, with a Tissue Tearor (Iuchi, Osaka, Japan). Each sample (0.5 ml) was desiccated overnight at 110°C, and then digested in 1 ml of 6 N HCl for 8 hours at 120°C. Samples were again desiccated for 6 hours at 120°C. Fifty μl of citrate/acetate buffer (5% citric acid, 7.24% sodium acetate, 3.4% NaOH, 1.2% glacial acetic acid, pH 6.0) and 1 ml of chloramine T solution (1.13 g of chloramine T, 8 ml of 1-propanol, 8 ml of H2O, 64 ml of citrate/acetate buffer) were added to each sample and the samples were left at room temperature for 20 minutes. Then, 1 ml of Ehrlich’s solution (10.13 g of p-(dimethylamino)benzaldehyde, 41.85 ml of 1-propanol, 17.55 ml of 70% perchloric acid) was added and incubated for 15 minutes at 65°C. Samples were cooled for 10 minutes, spun at 3100 × g for 5 minutes, and read at 550 nm on a spectrophotometer. A hydroxyproline standard solution of 0 to 4 mg/ml was used to generate a standard curve. Eight to 10 mice of each genotype were examined. All reagents were purchased from Wako Co., Osaka, Japan.
Histological Examination and Immunohistochemistry
The same mice were used for histological evaluation of fibrosis and measurement of hydroxyproline content, whereas separate mice were used for analysis of BAL components. After the right lung of each mouse was removed for hydroxyproline assay, the left lung was inflated and fixed with 4% paraformaldehyde. The lung was ligated at the bronchi, excised, and further fixed by immersion in 4% paraformaldehyde for 24 hours, at which time they were changed to 70% alcohol before paraffin embedding. Six-μm sections were stained with hematoxylin and eosin (H&E) to evaluate alveolitis and with Azan-Mallory stain to identify collagen deposition in the lung. In the lung sections stained with H&E, leukocyte infiltration was also assessed. Five sections of the entire lung stained with H&E were chosen randomly from each mouse. Leukocyte infiltration was evaluated by averaging the numbers of leukocytes present in more than 30 successive microscopic fields (0.07 mm2) from each mouse. Each section was examined independently by three investigators in a blinded fashion, and the mean was used for analysis. Eight to 10 mice of each genotype were examined.
For immunohistochemistry, frozen sections of lung were acetone-fixed and then incubated with 10% normal rabbit serum in PBS (10 minutes, 37°C) to block nonspecific staining. Sections were then incubated with rat mAb specific for mouse ICAM-1 (Beckman Coulter, Inc.). Rat IgG (Southern Biotechnology Associates Inc., Birmingham, AL) was used as a control for nonspecific staining. Sections were incubated sequentially (20 minutes, 37°C) with a biotinylated rabbit anti-rat IgG secondary Ab (Vectastain ABC method, Vector Laboratories, Burlingame, CA), then horseradish peroxidase-conjugated avidin-biotin complexes (Vectastain ABC method, Vector Laboratories). Sections were washed three times with PBS between incubations. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, and then counterstained with methyl green. Eight mice of each genotype were examined.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) in Bleomycin-Challenged Lung
Lungs were harvested 8 days after bleomycin administration, and total RNA was isolated from frozen lung specimens using a RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). RNA yield and purity were determined by spectrophotometry. RNA was then reverse transcribed into cDNA and amplified using the Reverse Transcription System (Promega, Madison, WI). Amplification was performed in a PCR thermal cycler MP (Takara, Kusatsu, Japan) for the appropriate number of cycles with denaturation at 94°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 60 seconds. The final extension was performed for 10 minutes, and then for 5 minutes at 5°C. The optimal number of PCR cycles for each primer set were as follows: 30 cycles for TNF-α, interleukin (IL)-1β, transforming growth factor (TGF)-β1, and β-actin; 40 cycles for IL-6 and interferon (IFN)-γ. The PCR products were electrophoresed on 2% agarose gels and stained with ethidium bromide. The density of the product was calculated using NIH Image 1.62 software and compared with that of β-actin to quantitate the PCR products. Five mice of each genotype were examined. The primers for TNF-α, IL-1β, IL-6, IFN-γ, TGF-β1, and β-actin were designed according to previous studies and were synthesized by Bex Co. (Tokyo, Japan). The sense and antisense primers used were as follows: TNF-α primer, 5′-AGC CCA CGT AGC AAA CCA CCA A-3′ and 5′-ACA CCC ATT CCC TTC ACA GAG CAA T-3′; IL-1β primer, 5′-TCA TGG GAT GAT GAT GAT AAC CTG CT-3′ and 5′-CCC ATA CTT TAG GAA GAC ACG GAT T-3′; IL-6 primer, 5′-CTG GTG ACA ACC ACG GCC TTC CCT A-3′ and 5′-ATG CTT AGG CAT AAC GCA CTA GGT T-3′; IFN-γ primer, 5′-GAA AGC CTA GAA AGT CTG AAT AAC T-3′ and 5′-ATC AGC AGC GAC TCC TTT TCC GCT T-3′; TGF-β1 primer, 5′-GAA GCC ATC CGT GGC CAG AT-3′ and 5′-GAC GTC AAA AGA CAG CCA CT-3′; and β-actin primer, 5′-GTG GGG CGC CCC AGG CAC CA-3′ and 5′-GCT CGG CCG TGG TGG TGA AGC-3′.
Statistical Analysis
The Mann-Whitney U-test was used for determining the level of significance of differences in sample means and Bonferroni’s test was used for multiple comparisons.
Results
Pulmonary Fibrosis Is Inhibited in the Absence of L-Selectin and/or ICAM-1
In bleomycin-induced pulmonary fibrosis, leukocytes accumulate mainly during the first week with subsequent fibrotic responses occurring in the second week. 3,4 To estimate the fibrotic changes in the lung, H&E and Azan-Mallory staining was performed 16 days after bleomycin injection. Bleomycin-treated wild-type littermates exhibited consolidation which consisted of subpleural foci of collapsed alveolar walls with dense inflammatory infiltration, and inflammatory response of cells surrounded the elevated collagen deposition that was revealed by Azan-Mallory staining of collagen (Figure 2, A and B) ▶ . These pathological changes induced by bleomycin were reduced in each adhesion molecule-deficient mouse compared with wild-type littermates. Low level collagen deposition and inflammatory cell infiltration into the lung were similar in L-selectin−/− and ICAM-1−/− mice. By contrast, fibrotic changes were essentially absent in L-selectin/ICAM-1−/− mice, although small inflammatory lesions were still observed. Lung sections from saline-treated mutant and wild-type littermates showed no significant pulmonary consolidation, fibrosis, and inflammatory cell infiltration.
Figure 2.
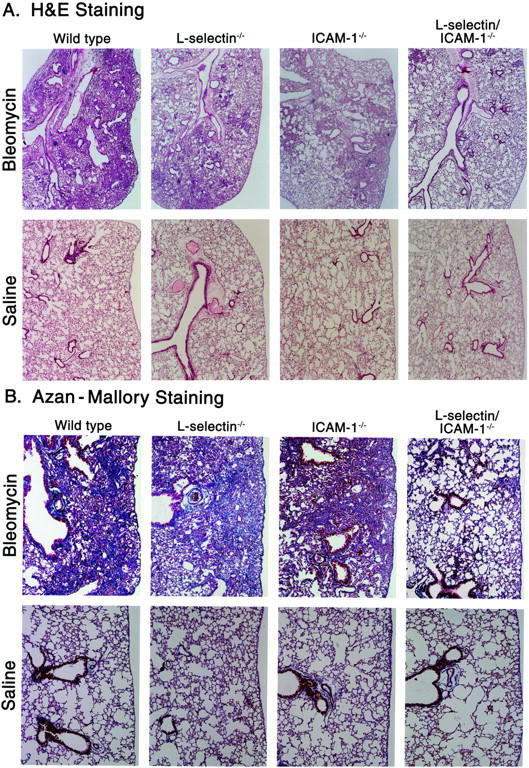
Representative histological sections of lungs from mutant and wild-type littermates at 16 days following intratracheal bleomycin administration. Mutant and wild-type littermates that received intratracheal saline injection served as controls. The lung sections were stained with hematoxylin and eosin (H&E) to evaluate alveolitis (A) and with Azan-Mallory to identify collagen deposition (B). In this preparation, collagen stains blue. These results represent those obtained with 8–10 mice of each genotype. Magnifications: A, ×40; B, ×100.
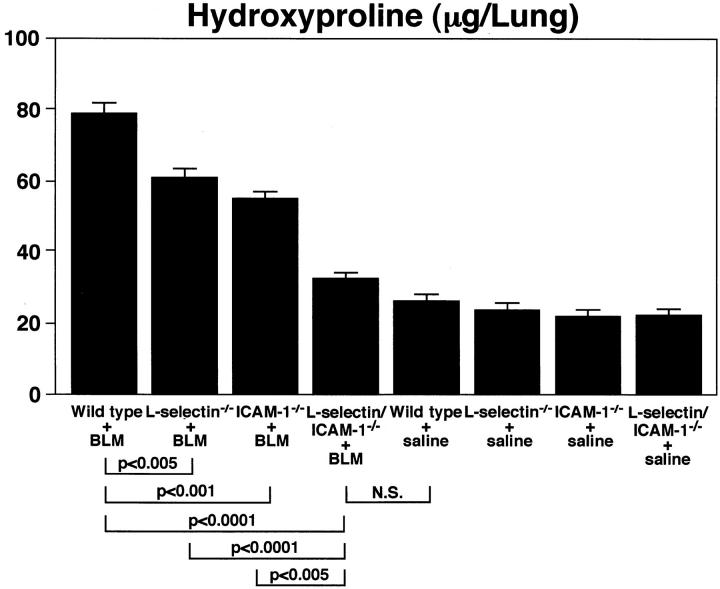
Pulmonary fibrosis was further assessed by quantitatively measuring the hydroxyproline content in the lungs. Sixteen days following bleomycin administration, the hydroxyproline content of lungs from wild-type littermates was increased almost 2.5-fold (P < 0.0001) compared with that of saline-treated wild-type control mice (Figure 3) ▶ . The hydroxyproline content was significantly reduced in bleomycin-treated L-selectin−/− (20% decrease, P < 0.005), ICAM-1−/− (28%, P < 0.001), and L-selectin/ICAM-1−/− (50%, P < 0.0001) mice compared with treated wild-type littermates. The loss of both ICAM-1 and L-selectin resulted in a significant reduction in hydroxyproline content relative to the loss of either L-selectin (P < 0.0001) or ICAM-1 (P < 0.005) alone. Remarkably, the hydroxyproline content in lungs from treated L-selectin/ICAM-1−/− mice was not significantly different from that of saline-treated control mice. The hydroxyproline content of lungs from saline-treated mice was similar for L-selectin−/−, ICAM-1−/−, L-selectin/ICAM-1−/−, and wild-type littermates. Thus, both L-selectin and ICAM-1 play important roles in the development of pulmonary fibrosis. Furthermore, the concurrent loss of L-selectin and ICAM-1 function resulted in almost complete elimination of bleomycin-induced collagen deposition.
Figure 3.
Bleomycin-induced pulmonary fibrosis in mutant and wild-type littermates. Pulmonary fibrosis was assessed by quantitatively measuring the hydroxyproline content of lungs 16 days after intratracheal bleomycin (BLM) administration. Mutant and wild-type littermates that received intratracheal saline injection served as controls. Although not indicated, hydroxyproline content in bleomycin-treated lungs from single-deficient mice and wild-type littermates was significantly increased compared to that of control lungs (P < 0.0001). All values represent the mean ± SEM of results obtained from 8 to 10 mice in each group.
Effect of L-Selectin and/or ICAM-1 Loss on Leukocyte Infiltration
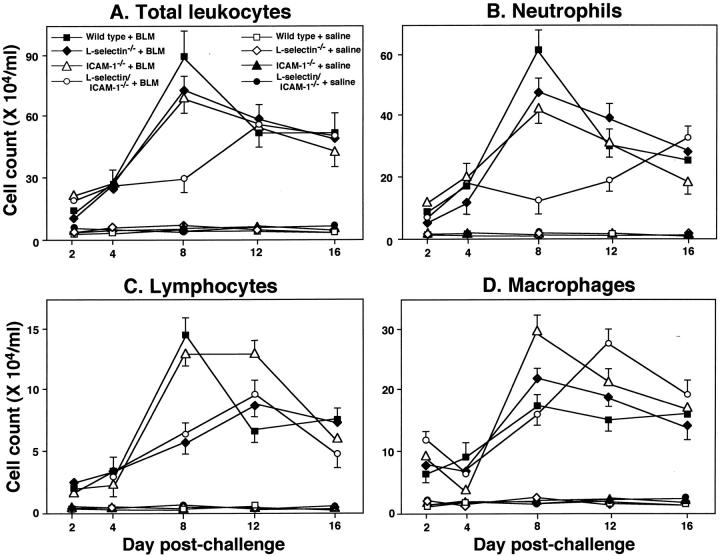
The effects of cell adhesion molecule loss on leukocyte infiltration into the lung were evaluated in the bleomycin-induced inflammatory process. For this purpose, the inflammatory cell populations in the BAL were assessed at 2, 4, 8, 12, and 16 days after bleomycin challenge (Figure 4) ▶ . The total number of leukocytes in the BAL reached a maximum 8 days after bleomycin challenge in wild-type littermates (Figure 4A) ▶ . The leukocyte influx at day 8 following challenge was significantly reduced in L-selectin−/− (20% decrease, P < 0.05), ICAM-1−/− (22%, P < 0.05), and L-selectin/ICAM-1−/− (66%, P < 0.0001) mice compared with wild-type littermates. Although leukocyte influx was inhibited similarly in ICAM-1−/− and L-selectin−/− mice, L-selectin/ICAM-1−/− mice exhibited a significant reduction in leukocyte numbers relative to either of the single deficient mice (P < 0.0001). The total number of leukocytes in L-selectin/ICAM-1−/− mice did not significantly increase after 8 days of challenge, but thereafter gradually increased, which was in contrast of the kinetics of the response found in other mutant mice and wild-type littermates. No significant increase in leukocyte numbers in BAL fluid following saline treatment was detected in mutant and wild-type littermates; there was no significant difference between mutant and wild-type littermates. Thus, while either L-selectin or ICAM-1 deficiency alone modestly reduced the peak increase in leukocyte accumulation, the double deficiency significantly inhibited leukocyte infiltration at this time point.
Figure 4.
Time course of total leukocytes (A), neutrophils (B), lymphocytes (C), and macrophages (D) in the BAL from mutant mice and wild-type littermates. BAL was collected at 2, 4, 8, 12, and 16 days after intratracheal bleomycin (BLM) administration. Mutant and wild-type littermates that received intratracheal saline injection served as controls. All values represent the means ± SEM of results obtained using 5–10 mice in each group.
To assess leukocyte accumulation in the interstitial compartment, we evaluated leukocyte numbers in the H&E-stained lung sections from mutant and wild-type littermates after 8 days of bleomycin challenge. The leukocyte number after 8 days of bleomycin challenge was significantly reduced in L-selectin−/− (20% decrease, P < 0.0001), ICAM-1−/− (28%, P < 0.0001), and L-selectin/ICAM-1−/− (90%, P < 0.0001) mice compared with wild-type littermates (Figure 5) ▶ . Furthermore, the leukocyte number in L-selectin/ICAM-1−/− mice was significantly decreased relative to either L-selectin−/− (P < 0.0001) or ICAM-1−/− (P < 0.0001) mice. However, the leukocyte accumulation in lungs from treated L-selectin/ICAM-1−/− mice was significantly increased compared with saline-treated control mice (P < 0.05). Lung sections from saline-treated mutant and wild-type littermates showed no significant leukocyte infiltration (Figure 5B) ▶ . Thus, like the alveolar compartment, bleomycin treatment in wild-type littermates induced significant leukocyte accumulation in the interstitial compartment that was inhibited by deficiency of cell adhesion molecules.
Figure 5.
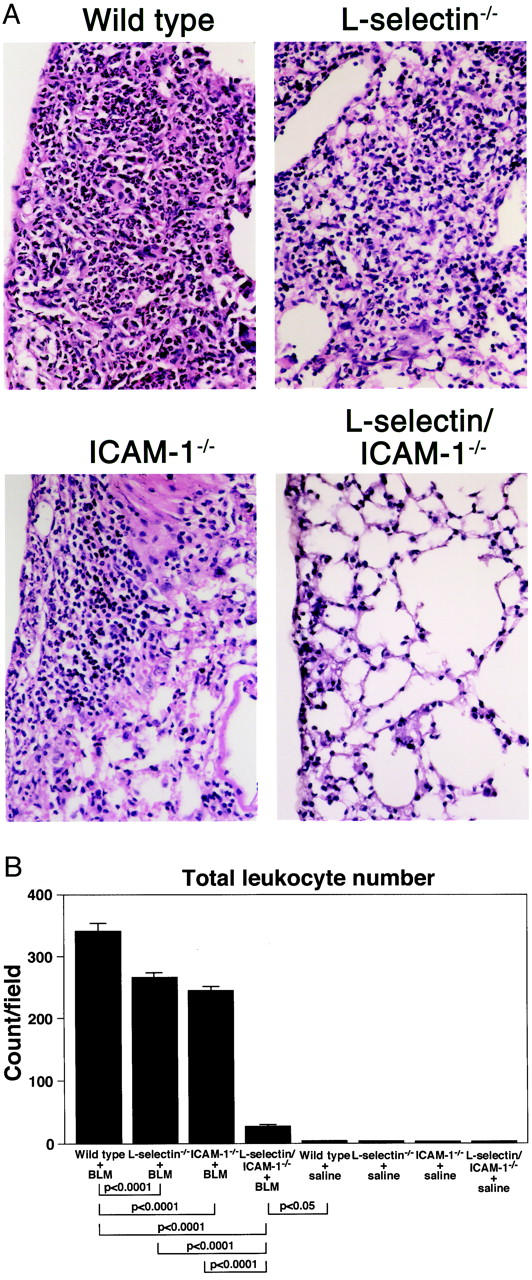
Leukocyte accumulation in the interstitial compartment of lung from mutant and wild-type littermates at 8 days following intratracheal bleomycin (BLM) administration. A: Representative histological lung sections stained with H&E. These results represent those obtained with 8–10 mice of each genotype. Magnification, ×400. B: Leukocyte numbers in the lung sections stained with H&E. Five sections of the entire lung stained with H&E were chosen randomly from each mouse. Leukocyte infiltration was evaluated by averaging the numbers of leukocytes present in more than 30 successive microscopic fields (0.07 mm2). Mutant and wild-type littermates that received intratracheal saline injection served as controls. All values represent the means ± SEM of results obtained using 8–10 mice in each group.
Effect of L-Selectin and/or ICAM-1 Loss on Infiltration of Leukocyte Subsets in the BAL
Results comparable with the total leukocyte numbers were obtained for neutrophil numbers of BAL cells, except that the neutrophil number at day 16 was significantly increased compared with wild-type littermates (P < 0.05; Figure 4B ▶ ). Similar to what was observed for total leukocyte BAL infiltration, lymphocyte numbers peaked in the BAL of wild-type littermates 8 days following bleomycin treatment (Figure 4C) ▶ . At this time, lymphocyte entry into the alveolar space was reduced by ∼60% in L-selectin−/− and L-selectin/ICAM-1−/− mice (P < 0.005). In addition, the peak lymphocyte influx in these adhesion molecule-deficient mice was shifted from 8 days to 12 days following treatment. Interestingly, normal numbers of lymphocytes entered the alveolar space in ICAM-1−/− mice 8 days following treatment and then remained at peak levels until 12 days postchallenge. ICAM-1−/− mice showed significantly elevated number of macrophages compared with wild-type littermates 8 days postchallenge (P < 0.05) while L-selectin/ICAM-1−/− mice exhibited significantly increased number after 12 days (P < 0.05; Figure 4D ▶ ). Macrophage numbers were similar for L-selectin−/− and wild-type littermates at all time points. No significant increase in numbers of neutrophils, lymphocytes, and macrophages in BAL fluid following saline treatment was detected in mutant and wild-type littermates; there was no significant difference between mutant and wild-type littermates. Thus, the importance of L-selectin or ICAM-1 function in leukocyte infiltration into the bleomycin-inflamed lung was dependent on both leukocyte class and the period of time following challenge.
ICAM-1 Expression in the Lung
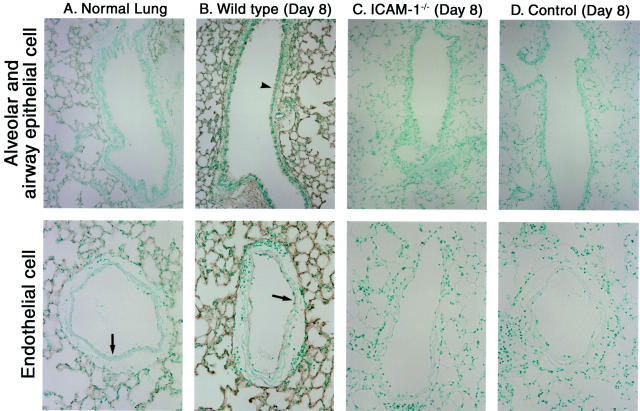
ICAM-1 expression on various types of cells, including epithelial cells, is induced by stimulation with proinflammatory cytokines in vitro. 14 Alveolar epithelial cells and lung vascular endothelial cells express ICAM-1 that is up-regulated after inflammation, including intranasal TNF-α instillation, bacterial pneumonia, and Goodpasture’s syndrome. 35-37 These results suggest that the loss of ICAM-1 expression on lung epithelial cells may contribute to the inhibited inflammation and fibrosis observed in the bleomycin-challenged ICAM-1−/− mice. Therefore, ICAM-1 expression in bleomycin-treated lung was assessed immunohistochemically. In normal lungs, ICAM-1 was expressed on alveolar epithelial cells and weakly on vascular endothelial cells, but was not detected on airway epithelial cells (Figure 6A) ▶ . After 8 days of bleomycin treatment, both alveolar epithelial cells and vascular endothelial cells up-regulated ICAM-1 expression, and airway epithelial cells expressed detectable ICAM-1 (Figure 6B) ▶ . However, the staining intensity of ICAM-1 on alveolar epithelial cells remained greater than that on vascular endothelial or airway epithelial cells. Similar results were obtained 16 days after bleomycin administration (data not shown). By contrast, ICAM-1 expression was not detected in either intact or inflamed lungs from ICAM-1−/− mice (Figure 6C ▶ and data not shown). Furthermore, staining with control polyclonal rat IgG revealed the absence of nonspecific staining in either intact or inflamed lungs from wild-type littermates (Figure 6D ▶ and data not shown). The loss of L-selectin expression did not noticeably affect ICAM-1 expression in either the intact or inflamed lungs (data not shown). Expression of other adhesion molecules on endothelium, including E-selectin and vascular cell adhesion molecule-1 (VCAM-1), was similar for L-selectin/ICAM-1−/− and wild-type littermates at any time points (data not shown). Thus, ICAM-1 was expressed on alveolar and airway epithelial cells as well as endothelial cells during bleomycin-induced inflammation.
Figure 6.
ICAM-1 expression in lung during bleomycin-induced pulmonary fibrosis. ICAM-1 expression in the normal lung (A) and in the inflamed lung of wild-type mice 8 days after intratracheal bleomycin administration (B) was assessed by immunohistochemistry using anti-ICAM-1 mAb. Arrows and arrowheads represent ICAM-1 expression on endothelial cells and airway epithelial cells, respectively. Inflamed lungs from ICAM-1−/− mice 8 days after intratracheal bleomycin administration served as negative controls (C). In addition, staining with control polyclonal rat IgG in lung sections of inflamed lungs from wild-type littermates 8 days after bleomycin treatment was shown as negative controls (D, Control). These results represent those obtained with 8 mice of each genotype. Magnifications: upper panels, ×150; lower panels, ×300.
Effect of L-Selectin and/or ICAM-1 Loss on Cytokine and Growth Factor Production
A variety of cytokines and growth factors are implicated in the development of bleomycin-induced pulmonary fibrosis, including TNF-α, IL-1β, IL-6, IFN-γ, and TGF-β1. 38-43 These cytokines are generated and released, in part, by inflammatory cells infiltrating the lungs. To assess the effects of L-selectin and/or ICAM-1 deficiency on production of these effector cytokines, their production in the lung was examined 8 days after bleomycin challenge by RT-PCR. In mutant mice and wild-type littermates treated with saline, mRNA expression of TNF-α, IL-1β, IL-6, IFN-γ, and TGF-β1 was not detected (Figure 7 ▶ and data not shown). By contrast, at 8 days after bleomycin treatment, mRNA levels of all cytokines examined were increased in the lungs from each adhesion molecule-deficient mouse and their wild-type littermates. However, L-selectin/ICAM-1−/− mice showed a significant decrease in mRNA levels for TNF-α (P < 0.01), IL-1β (P < 0.01), IL-6 (P < 0.05), and TGF-β1 (P < 0.01) relative to wild-type littermates. In contrast, IFN-γ production was similar for L-selectin/ICAM-1−/− mice and wild-type littermates. L-selectin−/− mice showed significantly decreased production of TNF-α (P < 0.05) and TGF-β1 (P < 0.05), but normal production of IL-1β, IL-6, and IFN-γ relative to wild-type littermates. On the other hand, ICAM-1−/− mice exhibited a significant reduction in production of IL-6 (P < 0.05) and TGF-β1 (P < 0.05), but normal production of TNF-α, IL-1β, and IFN-γ compared with wild-type littermates. TGF-β1 mRNA levels in L-selectin/ICAM-1−/− mice were significantly reduced relative to L-selectin−/− or ICAM-1−/− mice (P < 0.05). Thus, the combined loss of L-selectin and ICAM-1 resulted in decreased production of all cytokines examined except for IFN-γ.
Figure 7.
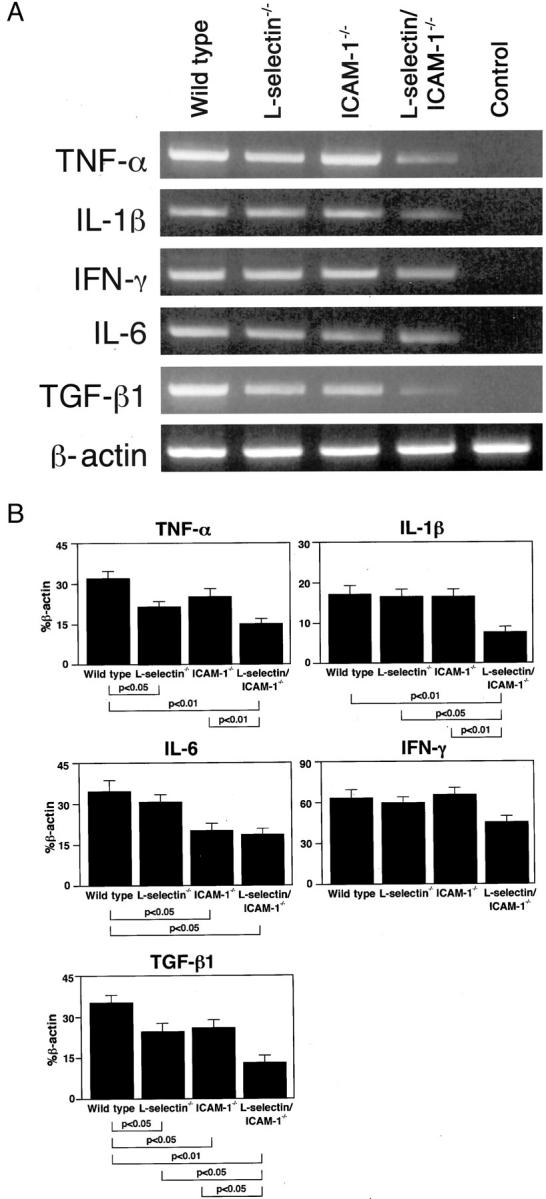
mRNA expression of TNF-α, IL-1β, IL-6, IFN-γ, and TGF-β1 in the lungs of mutant mice and their wild-type littermates 8 days after intratracheal bleomycin administration. Representative mRNA expression as assessed by RT-PCR amplification of cytokine and β-actin transcripts is shown (A). mRNA expression in wild-type littermates that received intratracheal saline injection served as controls. These results represent those obtained with 5 mice of each genotype. The amount of each cytokine PCR product was quantified and normalized to the level of β-actin (B). All values represent the means ± SEM of results obtained from 5 mice in each group.
Discussion
This is the first report showing a critical in vivo role for L-selectin or ICAM-1 in bleomycin-induced lung fibrosis using gene-targeted adhesion molecule-deficient mice. In the present study, the deficiency of either L-selectin or ICAM-1 significantly inhibited bleomycin-induced pulmonary fibrosis (Figures 2 and 3) ▶ ▶ . Furthermore, the loss of both L-selectin and ICAM-1 inhibited pulmonary fibrosis beyond that produced by loss of ICAM-1 or L-selectin alone. This is consistent with the finding that L-selectin and ICAM-1 function cooperatively to mediate optimal leukocyte rolling as well as to recruit leukocytes into inflammatory sites. 10,15,18 More importantly, bleomycin-induced pulmonary fibrosis was nearly eliminated in the absence of both ICAM-1 and L-selectin expression (Figures 2 and 3) ▶ ▶ . Therefore, the present study reveals a critical and cooperative role of L-selectin and ICAM-1 in the development of bleomycin-induced pulmonary fibrosis.
It is assumed that leukocytes and cytokines produced by leukocytes participate in the pathogenesis of pulmonary fibrosis. Consistent with this, all of the mutant mice exhibited significantly decreased numbers of total leukocytes in the BAL fluid (Figure 4A) ▶ and the interstitial compartment (Figure 5) ▶ at day 8 following bleomycin treatment. Similar results were obtained for neutrophil accumulation in the BAL since the predominant cell population was neutrophils (Figure 4B) ▶ . This suggests that neutrophil infiltration may play a role in the development of fibrosis. Consistently, the development of pulmonary fibrosis is related to the persistence of tissue neutrophil activation as assessed by positron emission tomography. 44 By contrast, depletion of polymorphonuclear leukocytes (PMN) by anti-PMN serum in hamster results in greater lung collagen synthesis induced by bleomycin treatment. 45 In this study, neutrophil number was increased in the BAL fluid from L-selectin/ICAM-1−/− mice relative to wild-type littermates at day 16 when lung fibrosis in L-selectin/ICAM-1−/− mice was significantly inhibited (Figures 2, 3, and 4B) ▶ ▶ ▶ . Therefore, the role of neutrophils in the development of lung fibrosis remained unknown in this study. Furthermore, although deficiency of cell adhesion molecule resulted in normal or augmented numbers of macrophages in the BAL (Figure 4D) ▶ , L-selectin−/− and L-selectin/ICAM-1−/− mice showed reduced numbers of lymphocytes (Figure 4C) ▶ . Collectively, our results suggest that infiltration of leukocytes, including neutrophils and lymphocytes, contributes to the bleomycin-induced pulmonary fibrosis.
Neutrophil recruitment to the lung has been shown to be either ICAM-1-dependent or independent. 1 In response to endotoxin or Pseudomonas aeruginosa, incomplete inhibition (∼60%) of neutrophil emigration by anti-ICAM-1 mAb or antisense oligonucleotides has been observed. 46,47 This observation suggests that adhesion pathways, in addition to ICAM-1, function during neutrophil emigration in the lung. The additional loss of L-selectin in ICAM-1−/− mice resulted in the almost complete elimination of neutrophil entry into the alveolar space 8 days after bleomycin treatment (Figure 4B) ▶ . Therefore, ICAM-1-independent pathways may be mediated by L-selectin after 8 days in this model. However, at later time points, neutrophil accumulation was augmented in L-selectin/ICAM-1−/− mice compared with wild-type littermates (Figure 4B) ▶ . L-selectin/ICAM-1−/− mice exhibited normal expression of E-selectin and VCAM-1 on endothelial cells during bleomycin-induced inflammation (data not shown). This suggests that other adhesion pathways or mechanical forces, which do not require expression of L-selectin and ICAM-1, are operable during the late stages of bleomycin-induced lung inflammation.
ICAM-1 is expressed on many types of cells and its expression is up-regulated by several proinflammatory cytokines. 14 Therefore, ICAM-1 expression on cells other than endothelial cells might be involved in the migration of leukocytes into the alveolar space, or in the retention of leukocytes within the alveolar space. In skin wounds, ICAM-1 is expressed strongly on endothelial cells and only weakly on epidermal cells. 18 By contrast, in normal, uninflamed lungs, ICAM-1 was expressed strongly on alveolar epithelial cells and weakly on endothelial cells, and not at all on airway epithelial cells (Figure 6A) ▶ . Bleomycin treatment up-regulated ICAM-1 expression on alveolar epithelial cells and endothelial cells, and induced detectable ICAM-1 expression on airway epithelial cells (Figure 6B) ▶ . Previous detailed studies demonstrate that type I alveolar epithelial cells that cover the vast majority of the alveolar basement membrane express abundant ICAM-1 in normal lungs whereas type II alveolar epithelial cells lack ICAM-1 expression. 35-37 However, type II cells markedly up-regulate ICAM-1 expression after induction of inflammation. 35,37 Type I cells also augment ICAM-1 expression after TNF-α treatment or in experimental Goodpasture’s syndrome, but not in bacterial pneumonia. 35-37 In addition, in vitro studies have shown that ICAM-1 expressed on lung epithelial cells is able to support neutrophil adhesion. 48 Taken together, the loss of ICAM-1 expression on alveolar type I and II epithelial cells and airway epithelial cells, as well as endothelial cells, could all contribute to the decreased level of inflammation and fibrosis observed in ICAM-1−/− mice. In fact, alveolar epithelial ICAM-1 may bind and retain leukocytes within the alveolar spaces, resulting in direct epithelial injury and the sustained release of cytokines.
Various cytokines and growth factors have been investigated in terms of their contribution to the pathogenesis of bleomycin-induced pulmonary fibrosis. Among them, TNF-α plays a key role in this model. 40,41 In addition, other proinflammatory cytokines, including IL-1β and IL-6, are involved in pulmonary fibrosis. 38,39 The loss of both ICAM-1 and L-selectin expression resulted in significantly decreased production of TNF-α, IL-1β, and IL-6 compared with wild-type littermates (Figure 7) ▶ . The ICAM-1 deficiency reduced the production of IL-6 but not TNF-α and IL-1β, while the L-selectin deficiency diminished the production of TNF-α but not IL-6 and IL-1β. Thus, L-selectin and ICAM-1 differentially and cooperatively regulate the production of proinflammatory cytokines probably by controlling the population and kinetics of inflammatory cells infiltrating into the alveolar space (Figure 4) ▶ . Several studies have shown that IFN-γ production is increased in bleomycin-induced pulmonary fibrosis, suggesting a pathogenetic role for IFN-γ. 38,42 By contrast, another report demonstrated anti-fibrotic effects of IFN-γ on bleomycin-induced pulmonary fibrosis. 49 Interestingly, lack of L-selectin and/or ICAM-1 expression did not affect IFN-γ production (Figure 7) ▶ . TGF-β, especially TGF-β1, mainly participates in fibrotic responses subsequent to inflammation since Ab-mediated neutralization of TGF-β reduces bleomycin-induced pulmonary fibrosis. 43,50 Consistent with this finding, our results showed that each adhesion molecule-deficient mouse exhibited reduced TGF-β1 production that was parallel with the degree of pulmonary fibrosis inhibition (Figures 2, 3, and 7) ▶ ▶ ▶ . Therefore, it appears that one of the major mechanisms by which loss of L-selectin and ICAM-1 expression may inhibit the fibrotic process is by reducing TGF-β1 production. Deficiency of adhesion molecules influences not only leukocyte trafficking, but also survival and the state of activation by their role in outside-in signaling. 51 Although macrophage numbers in BAL fluid from adhesion molecule-deficient mice were similar to or greater than those found in wild-type littermates (Figure 4D) ▶ , it cannot be ruled out that the decreased levels of TNF-α and TGF-β1 mRNA in L-selectin/ICAM-1−/− mice (Figure 7) ▶ may be due to a decrease in the functional state of macrophages.
To date, there have been few reports directly addressing the in vivo role of adhesion molecules in bleomycin-induced pulmonary fibrosis. The present study indicates that expression of L-selectin and ICAM-1 both contribute to pulmonary fibrosis by mediating the accumulation of leukocytes that may initiate lung inflammation by generating and releasing proinflammatory cytokines. Furthermore, loss of these molecules resulted in decreased production of TGF-β1 and the inhibition of lung fibrosis. The finding that pulmonary fibrosis was almost completely inhibited by the loss of both L-selectin and ICAM-1 expression suggests that these adhesion molecules are potential therapeutic targets for human pulmonary fibrosis.
Acknowledgments
We thank M. Matsubara and Y. Yamada for technical assistance.
Footnotes
Address reprint requests to Shinichi Sato, M.D., Ph.D., Department of Dermatology, Kanazawa University Graduate School of Medical Science, 13–1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan. E-mail: s-sato@med.kanazawa-u.ac.jp.
Supported by Kanae Foundation for Life and Socio-Medical Science (to S. S.) and National Institutes of Health grants CA54464 and CA81776 (to T. F. T.).
References
- 1.Paine R, 3rd, Ward PA: Cell adhesion molecules and pulmonary fibrosis. Am J Med 1999, 107:268-279 [DOI] [PubMed] [Google Scholar]
- 2.Bowden DH: Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest 1984, 50:487-488 [PubMed] [Google Scholar]
- 3.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL: A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol 1995, 57:782-787 [DOI] [PubMed] [Google Scholar]
- 4.Tokuda A, Itakura M, Onai N, Kimura H, Kuriyama T, Matsushima K: Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol 2000, 164:2745-2751 [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Tedder TF: Leukocyte interactions with vascular endothelium: new insights into selectin-mediated attachment and rolling. J Immunol 1995, 155:525-528 [PubMed] [Google Scholar]
- 6.Springer TA: Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 1995, 57:827-872 [DOI] [PubMed] [Google Scholar]
- 7.Tedder TF, Li X, Steeber DA: The selectins and their ligands: adhesion molecules of the vasculature. Adv Mol Cell Biol 1999, 28:65-111 [Google Scholar]
- 8.Spertini O, Luscinskas FW, Gimbrone MA, Jr, Tedder TF: Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-selectin) under non-static conditions. J Exp Med 1992, 175:1789-1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertini O, Luscinskas FW, Kansas GS, Munro JM, Griffin JD, Gimbrone MA, Jr, Tedder TF: Leukocyte adhesion molecule-1 (LAM-1, L-selectin) interacts with an inducible endothelial cell ligand to support leukocyte adhesion J Immunol 1991, 147:2565-2573 [PubMed] [Google Scholar]
- 10.Steeber DA, Tang MLK, Green NE, Zhang X-Q, Sloane JE, Tedder TF: Leukocyte entry into sites of inflammation requires overlapping interaction between the L-selectin and ICAM-1 pathways. J Immunol 1999, 163:2176-2186 [PubMed] [Google Scholar]
- 11.Arbones ML, Ord DC, Ley K, Radich H, Maynard-Curry C, Capon DJ, Tedder TF: Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin (CD62L) deficient mice. Immunity 1994, 1:247-260 [DOI] [PubMed] [Google Scholar]
- 12.Tang MLK, Hale LP, Steeber DA, Tedder TF: L-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in L-selectin-deficient mice. J Immunol 1997, 158:5191-5199 [PubMed] [Google Scholar]
- 13.Tedder TF, Steeber DA, Pizcueta P: L-selectin deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med 1995, 181:2259-2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA: Induction by IL-1 and interferon-τ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol 1986, 137:245-253 [PubMed] [Google Scholar]
- 15.Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF: Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci USA 1998, 95:7562-7567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL: Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA 1993, 90:8529-8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Gonzalo JA, St. Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Guiterrez-Ramos J-C: Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med 1994, 180:95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, Tedder TF, Sato S: Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol 2000, 157:237-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azuma A, Takahashi S, Nose M, Araki K, Araki M, Takahashi T, Hirose M, Kawashima H, Miyasaka M, Kudoh S: Role of E-selectin in bleomycin induced lung fibrosis in mice. Thorax 2000, 55:147-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Wang Y, Hyde DM, Gotwals PJ, Lobb RR, Ryan ST, Giri SN: Effect of antibody against integrin α4 on bleomycin-induced pulmonary fibrosis in mice. Biochem Pharmacol 2000, 60:1949-1958 [DOI] [PubMed] [Google Scholar]
- 21.Piguet PF, Rosen H, Vesin C, Grau GE: Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis 1993, 147:435-441 [DOI] [PubMed] [Google Scholar]
- 22.Matsuse T, Teramoto S, Katayama H, Sudo E, Ekimoto H, Mitsuhashi H, Uejima Y, Fukuchi Y, Ouchi Y: ICAM-1 mediates lung leukocyte recruitment but not pulmonary fibrosis in a murine model of bleomycin-induced lung injury. Eur Respir J 1999, 13:71-77 [DOI] [PubMed] [Google Scholar]
- 23.Doyle NA, Bhagwan SD, Meek BB, Kutkoski GJ, Steeber DA, Tedder TF, Doerschuk CM: Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest 1997, 99:526-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiscus LC, Van Herpen J, Steeber DA, Tedder TF, Tang ML: L-Selectin is required for the development of airway hyperresponsiveness but not airway inflammation in a murine model of asthma. J Allergy Clin Immunol 2001, 107:1019-1024 [DOI] [PubMed] [Google Scholar]
- 25.Keramidaris E, Merson TD, Steeber DA, Tedder TF, Tang ML: L-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol 2001, 107:734-738 [DOI] [PubMed] [Google Scholar]
- 26.Kamochi M, Kamochi F, Kim YB, Sawh S, Sanders JM, Sarembock I, Green S, Young JS, Ley K, Fu SM, Rose CE, Jr: P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am J Physiol 1999, 277:L310-L319 [DOI] [PubMed] [Google Scholar]
- 27.Hallahan DE, Virudachalam S: Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci USA 1997, 94:6432-6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatfield CA, Brashler JR, Winterrowd GE, Bell FP, Griffin RL, Fidler SF, Kolbasa KP, Mobley JL, Shull KL, Richards IM, Chin JE: Intercellular adhesion molecule-1-deficient mice have antibody responses but impaired leukocyte recruitment. Am J Physiol 1997, 273:L513-L523 [DOI] [PubMed] [Google Scholar]
- 29.O’Brien AD, Standiford TJ, Bucknell KA, Wilcoxen SE, Paine R, 3rd: Role of alveolar epithelial cell intercellular adhesion molecule-1 in host defense against Klebsiella pneumoniae. Am J Physiol 1999, 276:L961-L970 [DOI] [PubMed] [Google Scholar]
- 30.Zeni F, Parent C, Correa R, Natanson C, Freeman B, Fontana J, Quezado M, Danner RL, Fitz Y, Richmond S, Gerstenberger E, Banks SM, Eichacker PQ: ICAM-1 and CD11b inhibition worsen outcome in rats with E. coli pneumonia. J Appl Physiol 1999, 87:299-307 [DOI] [PubMed] [Google Scholar]
- 31.King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B: Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol 1996, 154:6080-6093 [PubMed] [Google Scholar]
- 32.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, 3rd, Toews GB: GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol 2000, 165:4032-4039 [DOI] [PubMed] [Google Scholar]
- 33.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR: Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol 1991, 147:22-28 [PubMed] [Google Scholar]
- 34.Schrier DJ, Phan SH, McGarry BM: The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis: a biochemical evaluation. Am Rev Respir Dis 1983, 127:614-617 [DOI] [PubMed] [Google Scholar]
- 35.Burns AR, Takei F, Doerschuk CM: Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol 1994, 153:3189-3198 [PubMed] [Google Scholar]
- 36.Hill PA, Lan HY, Nikolic-Paterson DJ, Atkins RC: Pulmonary expression of ICAM-1 and LFA-1 in experimental Goodpasture’s syndrome. Am J Pathol 1994, 145:220-227 [PMC free article] [PubMed] [Google Scholar]
- 37.Kang BH, Manderschied BD, Huang YC, Crapo JD, Chang LY: Contrasting response of lung parenchymal cells to instilled TNFα and IFNγ: the inducibility of specific cell ICAM-1 in vivo. Am J Respir Cell Mol Biol 1996, 15:540-550 [DOI] [PubMed] [Google Scholar]
- 38.Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM: T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol 1999, 65:187-195 [DOI] [PubMed] [Google Scholar]
- 39.Piguet PF, Vesin C, Grau GE, Thompson RC: Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine 1993, 5:57-61 [DOI] [PubMed] [Google Scholar]
- 40.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P: Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 1989, 170:655-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH: TNF-α-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol 1997, 158:954-959 [PubMed] [Google Scholar]
- 42.Gur I, Or R, Segel MJ, Shriki M, Izbicki G, Breuer R: Lymphokines in bleomycin-induced lung injury in bleomycin-sensitive C57BL/6 and -resistant BALB/c mice. Exp Lung Res 2000, 26:521-534 [DOI] [PubMed] [Google Scholar]
- 43.Giri SN, Hyde DM, Hollinger MA: Effect of antibody to transforming growth factor β on bleomycin induced accumulation of lung collagen in mice. Thorax 1993, 48:959-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones HA, Schofield JB, Krausz T, Boobis AR, Haslett C: Pulmonary fibrosis correlates with duration of tissue neutrophil activation. Am J Respir Crit Care Med 1998, 158:620-628 [DOI] [PubMed] [Google Scholar]
- 45.Clark JG, Kuhn C, 3rd: Bleomycin-induced pulmonary fibrosis in hamsters: effect of neutrophil depletion on lung collagen synthesis. Am Rev Respir Dis 1982, 126:737-739 [DOI] [PubMed] [Google Scholar]
- 46.Qin L, Quinlan WM, Doyle NA, Graham L, Sligh JE, Takei F, Beaudet AL, Doerschuk CM: The roles of CD11/CD18 and ICAM-1 in acute Pseudomonas aeruginosa-induced pneumonia in mice. J Immunol 1996, 157:5016-5021 [PubMed] [Google Scholar]
- 47.Kumasaka T, Quinlan WM, Doyle NA, Condon TP, Sligh J, Takei F, Beaudet A, Bennett CF, Doerschuk CM: Role of the intercellular adhesion molecule-1 (ICAM-1) in endotoxin-induced pneumonia evaluated using ICAM-1 antisense oligonucleotides, anti-ICAM-1 monoclonal antibodies, and ICAM-1 mutant mice. J Clin Invest 1996, 97:2362-2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tosi MF, Stark JM, Smith CW, Hamedani A, Gruenert DC, Infeld MD: Induction of ICAM-1 expression on human airway epithelial cells by inflammatory cytokines: effects on neutrophil-epithelial cell adhesion. Am J Respir Cell Mol Biol 1992, 7:214-221 [DOI] [PubMed] [Google Scholar]
- 49.Gurujeyalakshmi G, Giri SN: Molecular mechanisms of antifibrotic effect of interferon γ in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp Lung Res 1995, 21:791-808 [DOI] [PubMed] [Google Scholar]
- 50.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ: Transforming growth factors-β1, -β2, and -β3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997, 150:981-991 [PMC free article] [PubMed] [Google Scholar]
- 51.Longhurst CM, Jennings LK: Integrin-mediated signal transduction. Cell Mol Life Sci 1998, 54:514-526 [DOI] [PMC free article] [PubMed] [Google Scholar]