Abstract
Proteinase-activated receptor (PAR)-2, a G-protein-coupled receptor for trypsin and mast cell tryptase, is highly expressed in the intestine. Luminal trypsin and tryptase are elevated in the colon of inflammatory bowel disease patients. We hypothesized that luminal proteinases activate PAR-2 and induce colonic inflammation. Mice received intracolonically PAR-2 agonists (trypsin, tryptase, and a selective PAR-2-activating peptide) or control drugs (boiled enzymes, inactive peptide) and inflammatory parameters were followed at various times after this treatment. Colonic administration of PAR-2 agonists up-regulated PAR-2 expression and induced an inflammatory reaction characterized by granulocyte infiltration, increased wall thickness, tissue damage, and elevated T-helper cell type 1 cytokine. The inflammation was maximal between 4 and 6 hours and was resolved 48 hours after the intracolonic administration. PAR-2 activation also increased paracellular permeability of the colon and induced bacterial trans-location into peritoneal organs. These proinflammatory and pathophysiological changes observed in wild-type mice were not detected in PAR-2-deficient mice. Luminal proteinases activate PAR-2 in the mouse colon to induce inflammation and disrupt the integrity of the intestinal barrier. Because trypsin and tryptase are found at high levels in the colon lumen of patients with Crohn’s disease or ulcerative colitis, our data may bear directly on the pathophysiology of human inflammatory bowel diseases.
Proteinase-activated receptors (PARs) are G-protein-coupled receptors that are activated by the proteolytic cleavage of their N-terminal domain. 1-3 The new N-terminal sequence that is exposed by proteolysis acts as a tethered ligand that binds to and activates the receptor. 4 Serine-proteinases such as thrombin, trypsin, tryptase, and cathepsin G are potential endogenous agonists for PARs. However, synthetic peptides corresponding to the tethered ligand sequences [PAR-activating peptides (PAR-APs)] can activate PARs selectively and are important pharmacological tools for studying PAR functions. 3 Four members of the PAR family have been cloned: PAR-1, PAR-3, and PAR-4 can be activated by thrombin, whereas PAR-2 is activated by trypsin and mast cell tryptase, but not by thrombin. 3 Activation of PARs by proteinases is entirely dependent on their proteolytic properties. PAR-3 appears to be a co-factor for the activation of PAR-4 by thrombin. 5
The discovery of this novel receptor family has highlighted a new role for proteinases, not only as degradative enzymes, but also as signaling molecules that can affect tissue functions via the PARs. This new role for proteinases and their receptors has been recently investigated in the area of inflammation. 3 When injected in vivo into the rat paw, PAR-2 agonists produce all of the classical hallmarks of inflammation: pain, swelling, heat, and redness. 6 Other studies have shown additional proinflammatory effects for PAR-2 activation, 3,7 including vasodilatation; 8 hypotension; 8,9 edema; 6,7 leukocyte rolling, adherence and extravasation; 10 and cytokine production. 11 Conversely, several studies also suggest a protective role for PAR-2 in the airways, 12 the gastric or colonic mucosa, 13 or after myocardial ischemia-reperfusion injury. 14
PAR-2 is highly expressed in the gastrointestinal tract, where it is found in endothelial cells, colonic myocytes, enterocytes (both on basolateral and apical membranes), enteric neurons, terminals of mesenteric afferent nerves, and immune cells. 15,16 However, it is unclear whether PAR-2 serves a proinflammatory or anti-inflammatory role in the gastrointestinal tract. Because proteinases able to activate PAR-2 are normally present in the intestinal lumen, and because their levels are elevated in inflammatory bowel diseases, we evaluated whether luminal proteinases can induce inflammation. We also evaluated the effects of luminal PAR-2 agonists on epithelial permeability and bacterial translocation. Because PAR-2 antagonists are not yet available, a key feature of our study was in the use of PAR-2-deficient mice (PAR-2−/−), in which the actions of PAR-2 agonists could be compared with their effects in wild-type (PAR-2+/+) animals. Here we describe, for the first time, a proinflammatory role for acute PAR-2 activation in the colon of mice, which is accompanied by increased epithelial permeability and resulting bacterial translocation. Moreover, we provide evidence that trypsin and tryptase in the lumen of mouse colon are able to induce inflammation.
Materials and Methods
Animals
Male Swiss 3T3 and C57BL6 mice were obtained respectively from Harlan (Grannat, France) and Charles River Laboratories (Quebec, Canada). PAR-2−/− mice were provided by the Johnson & Johnson Pharmaceutical Research Institute (Spring House, PA). All of the animals were housed in a temperature-controlled room; food and water were provided ad libitum. The local Animal Care and Ethic Committees approved all experimental protocols.
Chemicals
Peptides (the selective PAR-2-AP SLIGRL-NH2 and the control peptide LRGILS-NH2 inactive on PAR-2), prepared by solid-phase synthesis, were obtained from the peptide synthesis facility of the Faculty of Medicine, University of Calgary. The composition and the purity of peptides were confirmed by high-pressure liquid chromatography analysis; mass spectrometry and amino acid analysis were used to verify peptide concentration. The PAR-2-AP SLIGRL-NH2 has been shown to be a selective PAR-2 agonist. 17 Peptides were dissolved in 10% ethanol, 10% Tween 80, and 80% saline (0.9% NaCl). Trypsin from porcine pancreas (type IX, 16,700 U/mg of protein) was from Sigma Chemical Co. (St. Louis, MO) and St. Quentin (Fallavier, France). Tryptase (2.5 mU/μg protein) was purified from human lung. 18 Trypsin and tryptase were diluted in saline.
Intracolonic Injections
Mice were fasted for 12 hours. Under light halothane anesthesia, a polyethylene catheter was inserted intrarectally to 3 to 4 cm from the anus. All compounds were administrated into the distal colon through the catheter at a maximum volume of 100 μl.
Assessment of Inflammation
At different times after the intracolonic administrations, mice were killed and distal colonic tissues were excised to assess macroscopic damage using the criteria listed in Table 1 ▶ (adapted from previously described scoring systems). 19 The bowel wall thickness was measured with a caliper at 1-cm from the anus. Myeloperoxidase (MPO) activity, an index of tissue granulocyte infiltration, was assayed in tissues as described. 20,21 Other tissues from adjacent sites were fixed in neutral buffered formalin and processed by routine techniques for histological evaluation of microscopic signs of inflammation. Samples of colonic tissues were used for RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.
Table 1.
Observed Parameter for Evaluation of Macroscopic Damage Score
| Macroscopic damage score |
|---|
| 1. Erythema (0, 1 on less than 1-cm, 2 on more than 1-cm) |
| 2. Hemorrhage |
| 3. Edema |
| 4. Stricture formation |
| 5. Ulceration |
| 6. Fecal blood |
| 7. Presence of mucus |
| 8. Diarrhea |
| 9. Adhesions (0, 1 mild, 2 severe) |
NOTE: Each parameter was awarded 1 point if observed after tissue examination, with the exception of the erythema and adhesion, which were awarded a maximum of 2 according to the extension of erythema or the severity of the adhesion.
RT-PCR
For PAR-2 RT-PCR, total RNA from mouse colonic tissues was isolated using the Trizol method (Gibco Canada). RNA (2 μg) was reverse-transcribed and DNA was amplified according to the following cycle conditions: dissociation of nucleic strands at 94°C for 1 minute, annealing at 55°C for 30 seconds, and extension for 1 minute at 72°C. The primer sequences for PAR-2 were: 5′-CAA GGT GCT CAT TGG CTT TT, 3′-CAG AGG GCG ACA AGG TAG AG, and for GADPH: 5′-CGG AGT CAA CGG ATT TGG TCG TAT, 3′-AGC CTT CTC CAT GGT GGT GAA GAC. Twenty-seven cycles were performed for PAR-2 and 23 cycles for GADPH. PCR products were then separated on a 1% agarose gel with ethidium bromide, the gel was scanned under UV light and bands quantified using a gel-doc system. For RT-PCR detection of cytokine mRNA, total mRNA (5 to 10 μg) was reverse-transcripted into complementary DNA (cDNA) using Superscript II RNase H− RT (Gibco-BRL, Cergy Pontoise, France). The primers used were β-actin: 5′-GGG TCA GAA GGA TTC CTA TG-3′ and 5′-GGT CTC AAA CAT GAT CTG GG-3′; interleukin (IL)-10: 5′-ATG CAG GAC TTT AAG GGT TACT TG-3′ and 5′-AGA CAG CTT GGT CTT GGA GCT TA-3′; IL-4: 5′-TCG ACA TTT TGA ACG AGG TC-3′ and 5′-GAA AAG CCC GAA AGA GTC TC-3′; interferon-γ 5′-GCT CTG AGA CAA TGA ACG CT-3′ and 5′-AAA GAG ATA ATC TGG CTC TGC-3′; tumor necrosis factor-α 5′-TCT CAT CAG TTC TAT GGC CC-3′ and 5′-GGG AGT AGA CAA GGT ACA AC-3′; IL-1-β 5′-AGA AGG TGC TCA TGT CCT CAT-3′ and 5′-TTG ACG GAC CCC AAA AGA TG-3′. Competitive PCR analysis was performed using linearized plasmids: pQB3 for the competition with β-actin, and pMus3 for the competition with interferon-γ, IL-2, IL-4, IL-5, IL-10, and tumor necrosis factor-α. 22,23 Amplification was performed for 40 cycles consisting of denaturation for 1 minute at 94°C, primer annealing for 1 minute at 52 to 55°C, and primer extension for 1.5 minutes at 72°C. DNA products were then separated on a 3% agarose gel with ethidium bromide and the ratio between amplified molecules for the target cDNA and the competitor, ie, log (DNA competitor/target cDNA), was calculated for each graded concentration of the competitor, using an image analyzer (QuantityOne software; Biorad, Amersham Pharmacia, Orsay, France). A curve of the ratios was established according to the competitor concentrations. This allowed the calculation of the equivalence point, at which the amount of amplified target mRNA and DNA competitor are equal [log (DNA competitor/target cDNA) = 0]. This value corresponds to the concentration of the target cDNA present in the initial sample. 22,23 To quantify more accurately cytokines in samples, the number of cytokine molecules was expressed as compared with the number of cDNA molecules of the internal control β-actin in the same sample.
Assessment of in Vivo Colonic Paracellular Intestinal Permeability
Anesthetized mice were treated intracolonically with SLIGRL-NH2 or LRGILS-NH2 (100 μg/mouse) and were then perfused intracolonically with 51 Cr-ethylenediaminetetraacetic acid (EDTA) at 2.10 6 cpm/hour for 3 hours starting either immediately after PAR-2-AP (0 to 3 hours) or 3 hours after PAR-2-AP (3 to 6 hours) (in a total volume of 75 μl). After the 3-hour intracolonic perfusion period, blood was collected by cardiac puncture, and then measured for counts using a gamma counter.
Bacterial Translocation
Twenty-four hours after the intracolonic administrations, mice were killed by cervical dislocation and their organs were tested for translocated bacteria as previously described. 24 Briefly, using sterile techniques, blood was obtained by cardiac puncture, and mesenteric lymph nodes, spleen, and liver were removed and weighed. The organs were homogenized and serial dilutions of aliquots (0.1 ml) were plated onto blood agar to enumerate total aerobic and facultative bacteria and plated onto MacConkey’s agar to enumerate aerobic and facultative gram-negative enteric bacilli. The plates were incubated for 24 and 48 hours at 37°C in aerobic conditions and the number of bacterial colonies was recorded.
Immunohistochemistry
Mice were infused intracolonically with PAR-2-AP, the control peptide (100 μg/mouse each), or their vehicle, and 4, 6, and 10 hours later, tissues were harvested and processed for immunohistochemistry. 25 Frozen sections were washed with phosphate-buffered saline (PBS) containing 1% normal goat serum and 0.3% Triton X-100 for 10 minutes, and were preblocked with PBS containing 5% normal goat serum and 0.3% Triton X-100 for 30 minutes. Sections were incubated with primary antibody in PBS with 5% normal goat serum and 0.3% Triton X-100 for 24 hours at 4°C. Primary antibody B5 was raised to rat PAR-2 (30GPNSKGR↓SLIGRLDT46PYGGC, ↓ = cleavage site) conjugated to keyhole limpet hemocyanin, and was used at a 1:250 to 1:500 dilution. In control experiments, the B5 antibody was preincubated for 24 to 48 hours at 4°C with 1 or 10 μmol/L of the peptide used for immunization before staining, or was omitted. Slides were washed and incubated with secondary antibody conjugated to fluorescein isothiocyanate (1:200 dilution; Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. Tissue sections were examined using an MRC 1000 laser-scanning confocal microscope (Bio-Rad, Hercules, CA) equipped with a krypton/argon laser and attached to a Zeiss Axiovert microscope. Images were processed using Adobe Photoshop.
Bacterial Translocation
Twenty-four hours after the intracolonic administration of the PAR-2-AP or the control peptide (100 μg/mouse each), mice were killed by cervical dislocation and their organs were tested for translocated bacteria as previously described. 24 Briefly, using sterile techniques, blood was obtained by cardiac puncture, and mesenteric lymph nodes, spleen, and liver were removed and weighed. The organs were homogenized, and serial dilutions of aliquots (0.1 ml) were plated onto blood agar to enumerate total aerobic and facultative bacteria and plated onto MacConkey’s agar to enumerate aerobic and facultative gram-negative enteric bacilli. The plates were incubated for 24 and 48 hours at 37°C in aerobic conditions and the number of bacterial colonies was recorded.
Data Analysis
For all groups, significance was estimated using the appropriate version of Student’s t-test. Group data are expressed as mean ± SE, and a P value <0.05 was required to reject the null hypothesis.
Results
PAR-2 Expression in Mouse Colonic Tissues
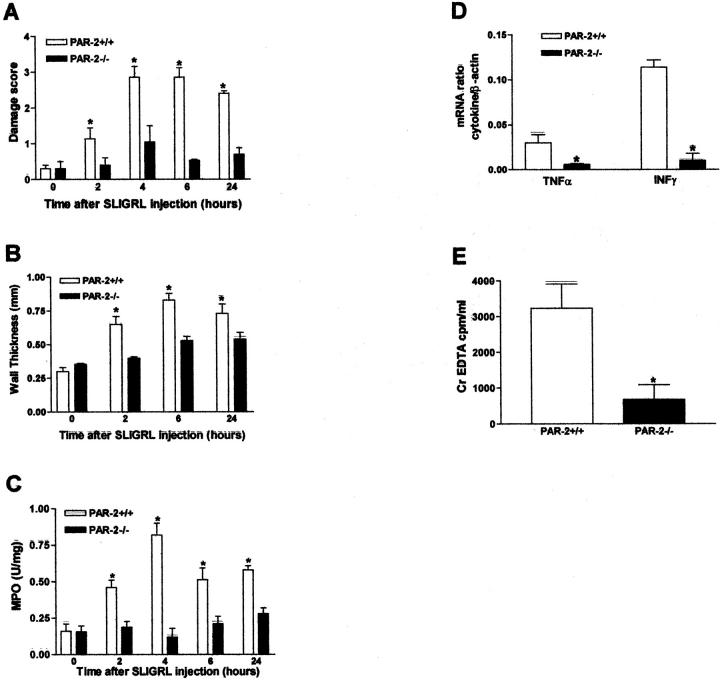
A PCR product of a predicted size of 549 bp was amplified from RNA prepared from the colons of mice, showing PAR-2 presence in those tissues. Compared to the level of expression of PAR-2 after intracolonic injection of the control peptide LRGILS-NH2 (100 μg/mouse), administration of the selective PAR-2 agonist SLIGRL-NH2 (100 μg/mouse) caused a significant increase in PAR-2 mRNA expression relative to the GAPDH RT-PCR signal (size, 306 bp) 10 hours after the peptide injection (Figure 1, A and B) ▶ . PAR-2 immunoreactivity was prominently localized to colonocytes where it was detected at the membrane and in intracellular compartments, as previously described. 25,26 Four hours after SLIGRL-NH2 injection, PAR-2 immunoreactivity markedly diminished in both villi and crypts, while 6 and 10 hours later, PAR-2 was up-regulated and was found present prominently in intracellular compartments in crypts. Staining was abolished by preabsorption of antibody with the receptor fragments or omission of primary antibody (Figure 1C) ▶ . These results show that PAR-2 activation results in down-regulation of PAR-2, possibly because of endocytosis and degradation, followed by up-regulation of mRNA and protein reflecting increase of exocytosis.
Figure 1.
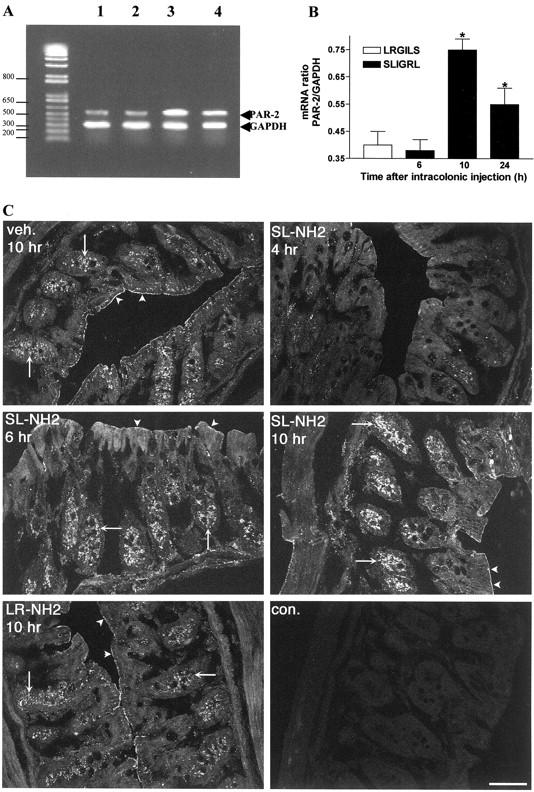
Kinetic detection of PAR-2 in mouse distal colon by RT-PCR (A, B) or immunofluorescence and confocal microscopy (C), after intracolonic administration of the PAR-2-AP SLIGRL-NH2 (100 μg/mouse), the control peptide LRGILS-NH2 (100 μg/mouse), or vehicle. PAR-2 was assessed by the amplification of a specific 549-bp PCR fragment; GAPDH was a 306-bp fragment, in colonic tissues from mice 10 hours after the intracolonic injection of LRGILS-NH2 (lane 1), or 6 hours (lane 2), 10 hours (lane 3), and 24 hours (lane 4) after the intracolonic injection of SLIGRL-NH2. In B, values are mean ± SEM, n = 8 per group; *, significantly different from LRGILS-NH2-treated group, P < 0.05. In C, PAR-2 was localized using antiserum B5 (1:250, 24 hours, 4°C; arrowheads, plasma membrane; arrow, intracellular compartments). Images are composites of 9 to 12 optical sections of 0.5 to 0.6 μm. Tissues were collected at various times after intracolonic administration of vehicle (veh), 100 μg of SLIGRL-NH2 (SL-NH2), or inactive LRGILS-NH2 (LR-NH2). Note the down-regulation of PAR-2 immunoreactivity 4 hours after SL-NH2 followed by up-regulation in crypts at 6 to 10 hours. The control shows omission of primary antibody. Scale bar, 10 μm (C).
Effects of Local Administration of PAR-2 Agonists on Colonic Inflammation Parameter
PAR-2-AP
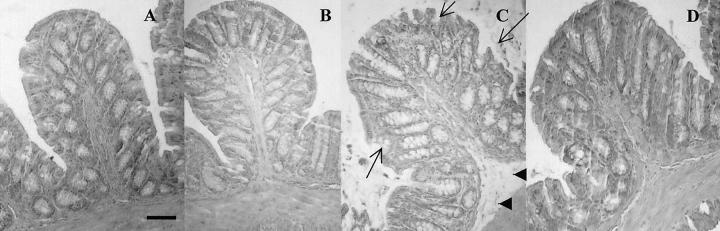
The intracolonic administration of SLIGRL-NH2 (100 μg/mouse) caused a significant increase in macroscopic damage score, wall thickness, and MPO activity in colonic tissues from C57BL6 mice (Figure 2; A, B, and C) ▶ . A maximal effect was observed from 4 to 6 hours after SLIGRL-NH2 dosing (Figure 2; A, B, and C) ▶ . At all of the observed time points, the same dose of the control peptide LRGILS-NH2 had no effect on the observed inflammatory parameters. Histological examination revealed edema in the submucosa (Figure 3C ▶ , arrowheads) and erosion of the epithelium (Figure 3C ▶ , arrows) after SLIGRL-NH2 in wild-type mice but not in PAR-2−/− mice (Figure 3D) ▶ , whereas LRGILS-NH2 did not cause significant damage compared to saline (Figure 3, B and A) ▶ . In another mouse strain (Swiss 3T3 mice), SLIGRL-NH2 also caused a significant increase in MPO activity and microscopic damage score, with a maximum effect 4 hours after peptide intracolonic administration (Figure 2, D and E) ▶ . As per the C57BL strain, the effect of SLIGRL-NH2 on MPO activity and microscopic damage score in Swiss 3T3 mice was dose-dependent from 10 μg to 100 μg/mouse, and the control peptide had no effect (Figure 2, F and G) ▶ .
Figure 2.
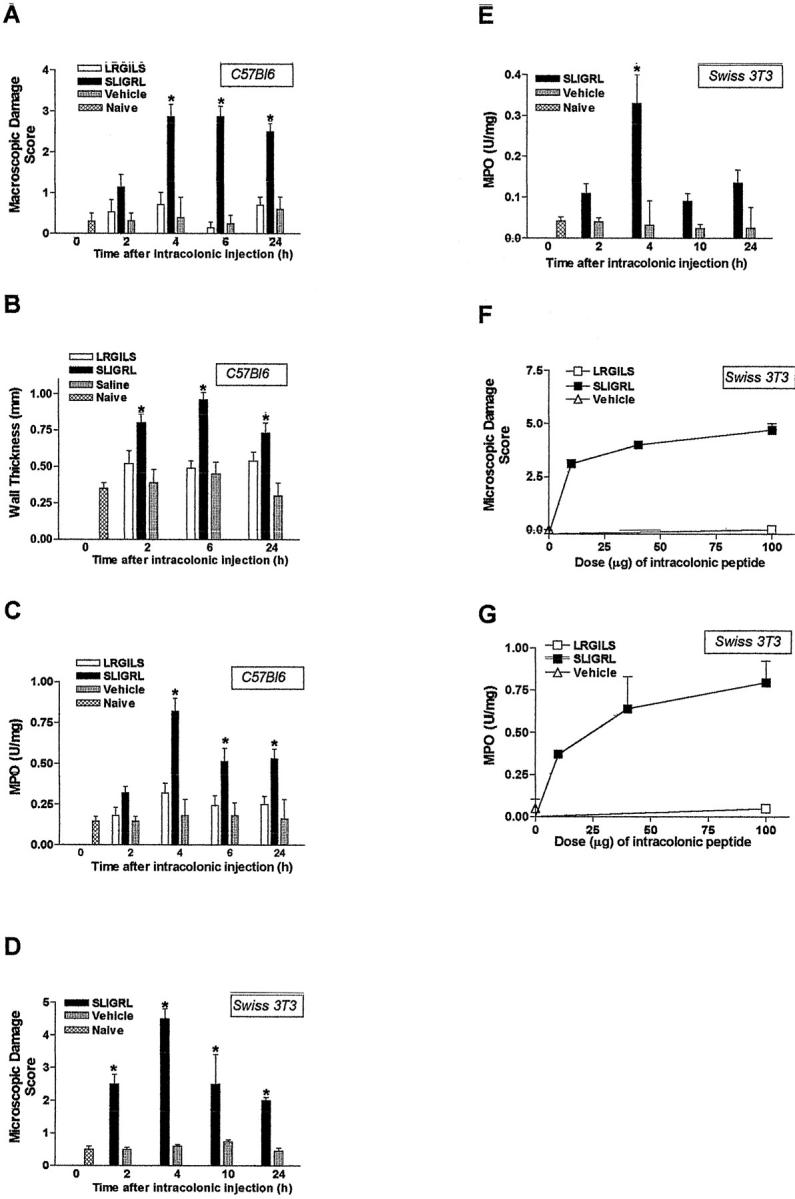
Kinetic (A–E) and dose-response curves (F and G) of inflammation induced by the intracolonic administration of the PAR-2-AP SLIGRL-NH2 (100 μg/mouse for A–E) or the control peptide LRGILS-NH2 (100 μg/mouse for A–E) in two different strains of mice: C57BL6 and Swiss 3T3. Different inflammatory parameters were followed: macroscopic (A) and microscopic (D and F) damage scores, wall thickness (B) and MPO activity (C, E, and G). Values are mean ± SEM, n = 8 per group, *, Significantly different from LRGILS-NH2-treated group, P < 0.05.
Figure 3.
Representative H&E-stained histological sections of colon from wild-type mice that have received an intracolonic injection of saline (A), LRGILS-NH2 (100 μg/mouse, 6-hour time point) (B), SLIGRL-NH2 (100 μg/mouse, 6-hour time point) (C), or PAR-2−/− mouse that have received an intracolonic injection of SLIGRL-NH2 (100 μg/mouse, 6-hour time point). Scale bar, 15 μm (applies to A–D).
Proteinases: Trypsin and Tryptase
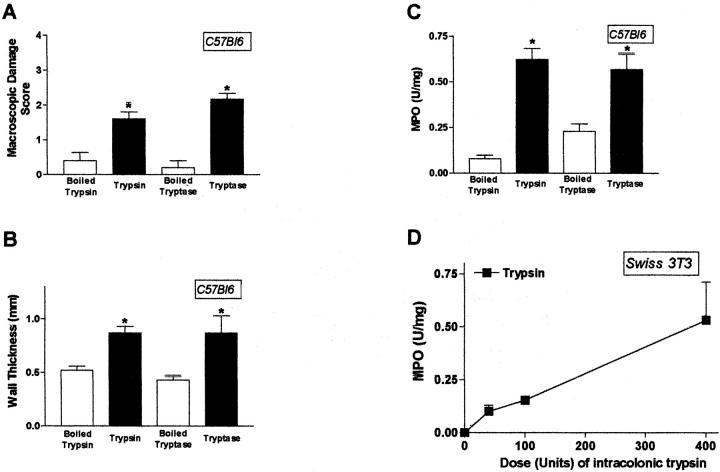
Trypsin (400 U/mouse) or human mast cell tryptase (1 μg/mouse) caused increased macroscopic damage score (70 to 90%), wall thickness (32 to 50%), and MPO activity (65 to 84%) in colonic tissues from C57BL6 mice 6 hours after their intracolonic administration (Figure 4; A, B, and C) ▶ . The maximum effect of trypsin and tryptase was observed between 4 and 6 hours (time course not shown), whereas the inactive enzymes (boiled for 10 minutes) had no effect (Figure 4; A, B, and C) ▶ at all time points. In Swiss 3T3 mice, trypsin also increased MPO activity and microscopic damage score (not shown), in a dose-dependent manner, from 10 to 400 U/mouse, 4 hours after its colonic administration (Figure 4D) ▶ . These results show that luminal administration of PAR-2 agonists (peptides and proteinases) induces inflammation in the colon of mice.
Figure 4.
Inflammation induced by the intracolonic administration of trypsin (50, 100, and 400 U/mouse) in C57BL6 and Swiss 3T3 mice and tryptase (1 μg/mouse) in C57BL6 mice. Macroscopic damage score (A), wall thickness (B), and MPO activity (C and D) were evaluated as inflammatory parameters 6 hours after the intracolonic injection. Values are mean ± SEM, n = 8 per group. *, Significantly different from boiled enzyme-treated group, P < 0.05.
Effects of Local Administration of PAR-2 Agonists on Cytokine Expression in the Colon
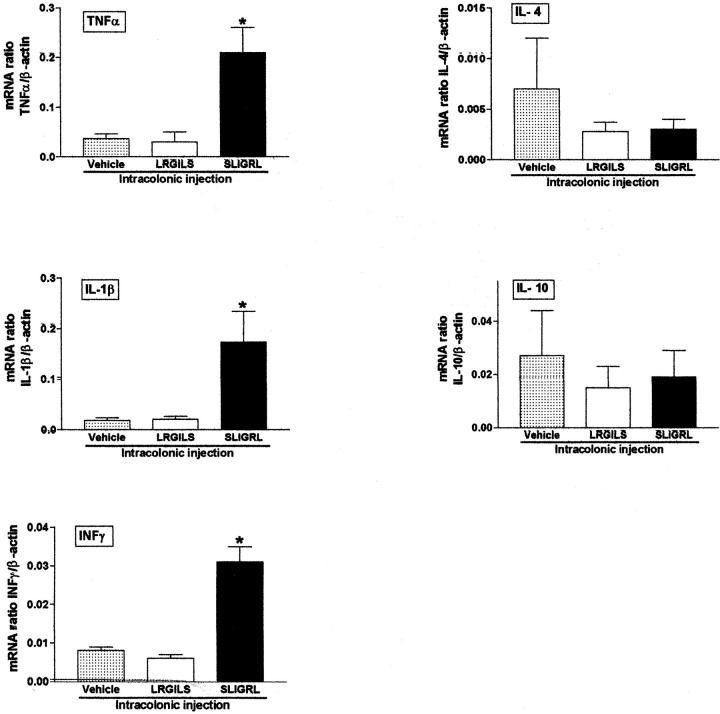
Four hours after its intracolonic administration, SLIGRL-NH2 (100 μg/mouse), but not the control peptide LRGILS-NH2, caused a significant increase in tumor necrosis factor-α, IL-1β, and interferon-γ mRNA expression in Swiss 3T3 mouse colonic tissues, whereas the expression of IL-4 and IL-10 remained unchanged (Figure 5) ▶ . Thus PAR-2 agonists induce elevation of T-helper cell type 1 (Th1) cytokines in the mouse colon.
Figure 5.
Cytokine mRNA expression in mouse colonic tissues 4 hours after the intracolonic administration of the PAR-2-AP SLIGRL-NH2 (100 μg/mouse), the control peptide LRGILS-NH2 (100 μg/mouse), or their vehicle. Values are mean ± SEM, n = 8 per group. *, Significantly different from LRGILS-NH2-treated group, P < 0.05.
Effects of Local Administration of PAR-2 Agonists on Colonic Permeability and Bacterial Translocation
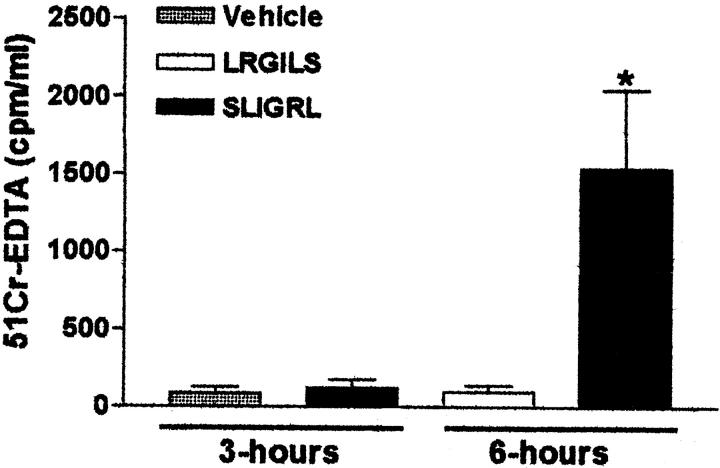
Intracolonic administration of SLIGRL-NH2, but not LRGILS-NH2 (100 μg/mouse each), significantly increased the passage of the permeability marker 51Cr-EDTA from the gut lumen to the vasculature. Permeability was unchanged between 0 and 3 hours after the peptide intracolonic administration, but was substantially increased between 3 and 6 hours after SLIGRL-NH2 dosing (Figure 6) ▶ . In contrast to LRGILS-NH2-injected mice, whose organs were sterile 24 hours after peptide treatment, bacteria translocated from the gut to the mesenteric lymph nodes, but also to the liver, spleen, and blood, after the intracolonic administration of SLIGRL-NH2 (100 μg/mouse) (Table 2) ▶ . Thus, PAR-2 agonists induce increased colonic paracellular permeability and bacterial translocation.
Figure 6.
Changes in intestinal permeability in vivo 3 and 6 hours after the intracolonic administration of the PAR-2-AP SLIGRL-NH2 (100 μg/mouse), the control peptide LRGILS-NH2 (100 μg/mouse), or their vehicle. The passage of the macromolecule 51Cr-EDTA from the gut lumen to the blood was used as an index of intestinal permeability. Values are mean ± SEM, n = 8 per group. *, Significantly different from LRGILS-NH2-treated group, P < 0.05.
Table 2.
Effect of Intracolonic Injection of PAR-2 Agonists on Bacterial Translocation
| Mice | Intracolonic injection | Colony number/g of tissue or per 100 μl of blood in | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Spleen | MLN | Blood | |||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |||
| PAR-2+/+ (n = 7) | LRGILS | Mean | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 μg | SEM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PAR-2+/+ (n = 5) | SLIGRL | Mean | 423.4 | 646.7 | 1268.0 | 1408.0 | 4740 | 4941 | 29.8 | 15.4 |
| 100 μg | SEM | 276.5 | 459.6 | 887.8 | 659.5 | 3418 | 3583 | 29.8 | 15.2 | |
| PAR-2−/− (n = 8) | SLIGRL | Mean | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 μg | SEM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
MLN, mesenteric lymph nodes.
PAR-2 Activation Is Responsible for PAR-2 Agonist-Induced Colonic Inflammation
To investigate whether the PAR-2 agonist-induced colonic inflammation was because of PAR-2 activation, we used mice deficient for the PAR-2 gene, compared to the C57BL wild-type mice. When injected into the colon of PAR-2−/− mice, SLIGRL-NH2 did not cause increased macroscopic damage score, wall thickness, or MPO activity, as observed in C57BL wild-type mice (Figure 7; A, B, and C) ▶ . Further, no elevated mRNA levels of Th1 cytokine interferon-γ or inflammatory cytokine tumor necrosis factor-α were observed in PAR-2-deficient mice compared with C57BL wild-type mice after the intracolonic administration of SLIGRL-NH2 (Figure 7D) ▶ . Finally, SLIGRL-NH2 did not cause bacterial translocation (Table 2) ▶ or increased permeability to 51Cr-EDTA in PAR-2-deficient mice, compared to wild-type mice (Figure 7E) ▶ . The intracolonic administration of trypsin or tryptase in PAR-2-deficient mice did not cause changes in macroscopic damage score, wall thickness, or MPO activity (Figure 8; A, B, and C) ▶ , confirming that the trypsin and tryptase-induced inflammatory parameters were because of PAR-2 activation. Thus, the PAR-2 agonists SLIGRL-NH2, trypsin and tryptase, induce intestinal inflammation and cause increased intestinal permeability by activation of PAR-2.
Figure 7.
Inflammation (A–C), as observed by the macroscopic damage score (A), wall thickness (B), MPO activity (C), cytokine mRNA expression (D), and intestinal permeability as observed by the passage of the macromolecule 51Cr-EDTA from the gut lumen to the blood (E) after the intracolonic administration of the PAR-2-AP SLIGRL-NH2 (100 μg/mouse), in wild-type or PAR-2-deficient (PAR-2−/−) mice. Values are mean ± SEM, n = 8 per group except for E where n = 6 per group. *, Significantly different from wild type, P < 0.05.
Figure 8.
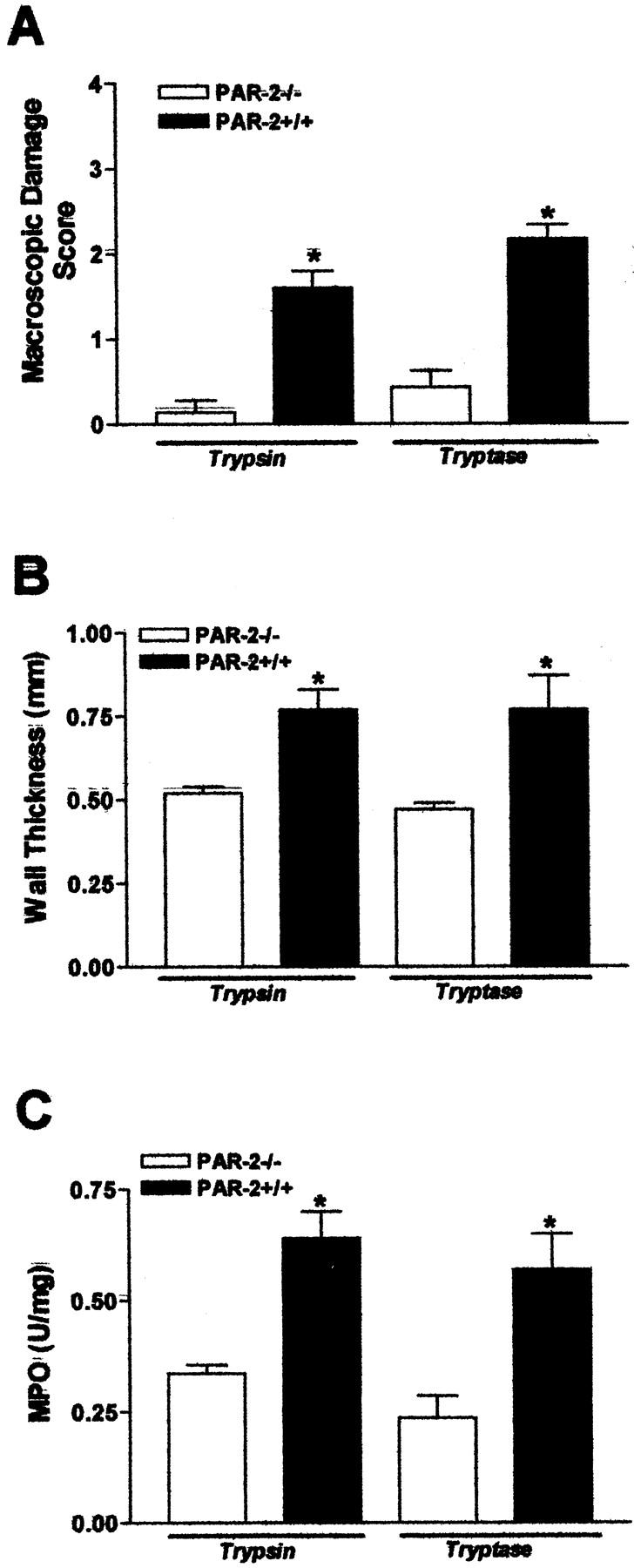
Inflammation as observed by the macroscopic damage score (A), wall thickness (B), and MPO activity (C), induced after the intracolonic administration of trypsin (400 U/mouse) or tryptase (1 μg/mouse), in wild-type or PAR-2-deficient (PAR-2−/−) mice. Values are mean ± SEM, n = 8 per group. *, Significantly different from wild type, P < 0.05.
Effects of PAR-2 Agonist Systemic Administration on Colonic Inflammation Parameters
SLIGRL-NH2 (100 μg/mouse), when injected intraperitoneally, did not induce change in both microscopic damage scores and MPO activities at 2, 4, 6, 8, or 10 hours after the peptide injection (Table 3A) ▶ . Higher intraperitoneal doses of SLIGRL-NH2 (up to 400 μg/mice) did not cause significant changes in MPO activity and microscopic damage 4 hours after its injection (time point corresponding to the maximum effect of SLIGRL-NH2 intracolonic administration) (Table 3B) ▶ . This result shows that systemic administration of PAR-2-AP did not induce colon inflammation.
Table 3.
Effect of Intraperitoneal Injection of PAR-2 Agonists on MPO Activity and Microscopic Damage Score (Mean ± SEM; n = 8)
| MPO (U/mg of protein) | Microscopic damage score | |
|---|---|---|
| A | ||
| SLIGRL-NH2 (μg/mice) | ||
| 40 | 201.3 ± 23.1 | 0.5 ± 0.12 |
| 100 | 159.2 ± 12.7 | 0.8 ± 0.10 |
| 400 | 181.2 ± 21.3 | 0.4 ± 0.17 |
| LRGILS-NH2 400 μg | 148.2 ± 30.7 | 0.5 ± 0.23 |
| B | ||
| Time after SLIGRL-NH2 (100 μg/mice) administration | ||
| 4 (h) | 132.1 ± 11.2 | 0.9 ± 0.21 |
| 6 (h) | 155.5 ± 62.74 | 0.5 ± 0.09 |
| 8 (h) | 192.4 ± 36.61 | 0.3 ± 0.06 |
| 10 (h) | 140.9 ± 75.66 | 0.7 ± 0.11 |
Discussion
Probably more than any other organ system, the gastrointestinal tract is exposed to high levels of proteinases both physiologically and during diseases. Proteinases present in the intestine derive from digestive glands (eg, trypsin), inflammatory cells (eg, tryptase), and proteinases from bacterial and viral pathogens. 27-30 Some of these proteinases, such as trypsin or mast cell tryptase, have been shown to activate PAR-2, 15,31 a receptor highly expressed throughout the gastrointestinal tract. 2 Thus, it is likely that PAR-2, via activation by intestinal proteinases, may play a prominent role in the pathogenesis of intestinal diseases. As a first step to identify this potential role, we have studied the effects of intracolonic administration of different PAR-2 agonists (PAR-2-AP, trypsin, and tryptase).
Luminal PAR-2 Agonists Induce Colonic Inflammation and Loss of Barrier Function
Our results showed, in two different strains of mice, that PAR-2 agonists present in the colonic lumen can provoke an inflammatory reaction characterized by granulocyte infiltration (elevated levels of MPO activity), tissue damage, elevated cytokine expression, and also caused changes in intestinal permeability and subsequent bacterial translocation to peritoneal organs. We confirmed that the proinflammatory effects of PAR-2 agonists were mediated by PAR-2 and not by a related receptor, observing the lack of effects of PAR-2-AP in mice lacking the gene that encodes PAR-2 (Figure 7 ▶ and Table 2 ▶ ). The elevated expression of PAR-2 mRNA and the internalization of the receptor in colonic tissues 10 hours after exposure to SLIGRL-NH2 support further the hypothesis that PAR-2 is a key factor in the pathophysiology of the changes observed (Figure 1) ▶ . The deleterious effects we observed for PAR-2 activation in the mouse colon constitute a first body of evidence to suggest an active role for PAR-2 in the pathophysiological changes observed in intestinal inflammatory diseases.
Cells Involved in PAR-2 Agonist-Induced Inflammation and Permeability Breakage
Considering the increased intestinal permeability after PAR-2-AP intracolonic administration, it is possible that the peptide reaches the circulation and could potentially influence other organ systems. However, the lack of proinflammatory effects in the mouse colon after SLIGRL-NH2 intraperitoneal injection (Table 3) ▶ supports a direct involvement of cells located on the colonic wall to induce intestinal inflammation. It has been shown that PAR-2 is expressed at the apical and basolateral membrane of rat and human enterocytes and our results confirmed the same presence of PAR-2 on mouse enterocytes (Figure 1B) ▶ . The internalization of the receptor in enterocytes after exposure to SLIGRL-NH2 (Figure 1B) ▶ , suggested that these cells are responsible, at least in part, for the PAR-2 agonist-induced intestinal changes. Moreover, it has been shown that PAR-2 agonists can signal to enterocytes, provoking the release of prostaglandin E2, arachidonic acid, and inositol 1,4,5-trisphosphate. 26 It can be hypothesized that PAR-2-mediated intestinal barrier breakdown constitutes an initial event, which then allows pathogen penetration to cause further inflammation. However, the fact that inflammation preceded paracellular permeability in our study does not support this hypothesis and might actually favor the hypothesis that inflammation might be responsible for increased intestinal permeability as previously described. 32 We cannot rule out a possible concomitant direct activation of PAR-2 on other cells present in the colon. PAR-2 expression has been described in cells implicated in inflammation such as neutrophils, eosinophils, endothelial cells, neurons, and fibroblasts, where its activation leads to proinflammatory signals (eg, cytokine expression, leukocyte rolling and adhesion, increased vascular permeability). 3 Thus, it is also possible that SLIGRL-NH2-induced colonic inflammation might be mediated by a direct activation of PAR-2 on inflammation-related cells and the subsequent release of inflammatory mediators. These inflammatory mediators (ie, cytokines) might then modify paracellular permeability and junctional protein expression as it was observed in enterocytes exposed to interferon-γ. 33 Clinical studies of inflammatory bowel disease patients suggest that the down-regulation of junctional molecules observed in ulcerative colitis 34 or Crohn’s disease patients 35 does not likely represent a primary phenomenon. 36 These studies support the view that alterations in tight junction proteins and subsequent changes in paracellular permeability may be the consequence rather than the triggering factor of colonic mucosa inflammation.
Trypsin and Tryptase Are Responsible for PAR-2 Colonic Activation
One of the major obstacles in defining a physiological role for PAR-2 resides in the question of the endogenous proteinase responsible for its activation. Under physiological conditions, trypsin is present in the intestinal lumen after feeding, but mainly in the upper gastrointestinal tract. In the colon, trypsin does not exert any digestive functions and its activity is constantly counterbalanced by the presence of trypsin inhibitors. Although trypsin is not released in the intestine on inflammation, studies have shown that the abundance of trypsin inhibitors is substantially reduced in the tissues of patients with ulcerative colitis or Crohn’s disease 37 and that trypsin proteolytic activity is increased in the intestinal lumen of these patients 38 or in animal models of colitis. 39 Tryptase, another proteinase that can activate PAR-2, constitutes the major protein released upon mucosal mast cell degranulation in humans, and is released in the setting of inflammation and allergic response into the intestinal lumen and vasculature. 40 Thus, trypsin and tryptase constitute good candidates to activate PAR-2 in the intestinal lumen in pathophysiological conditions. Our study showed that trypsin and tryptase reproduced the proinflammatory effects of the selective PAR-2-AP when injected directly into the colon. Experiments using PAR-2-deficient mice have further implicated trypsin and tryptase as possible endogenous proteinases responsible for PAR-2 activation showing that the proinflammatory effects of trypsin and tryptase were entirely mediated by PAR-2 (Figure 8) ▶ . Although our results showed that human tryptase induced colonic inflammation in mice through the activation of PAR-2, it is important to note that mucosal mast cells that are normally present in the mouse intestine lack tryptase. 41 However, it has been shown in mice that jejunal mast cells can alter reversibly the expression of chymases or tryptases according to the inflammatory state after Trichinella spiralis infection. 42 In physiological conditions, a constant balance between proteinases and their inhibitors is observed, both in the vasculature and the gut lumen, and this is particularly true for trypsin, which is primarily present in the small intestine. In pathophysiological conditions such as inflammatory bowel disease, allergy, or parasite infection, the physiological balance of proteinase activity in the gut lumen is broken. From our results, it seems that luminal PAR-2 activation rendered possible by an increased tryptic activity in the gut lumen could generate an inflammatory reaction. A very recent study has shown that treatment of ulcerative colitis patients with a tryptase inhibitor significantly improved (49% of the patients) or even caused remission (9% of the patients) of the disease. 43 This clearly suggests that tryptase is involved in the generation of inflammatory symptoms associated with ulcerative colitis and from our results, we can think that tryptase involvement in the generation of inflammation might be mediated by the activation of PAR-2.
PAR-2 Pro- or Anti-Inflammatory Agent?
Although all of the classical hallmarks of inflammation, ie, swelling (increased wall thickness), redness (erythema), impaired function, and even pain, 44 have been observed after the acute intracolonic administration of a selective PAR-2 agonist, it is also necessary to consider a possible anti-inflammatory role for PAR-2. Several studies have suggested that PAR-2 activation might promote resolution of inflammation. 3 In airway epithelia, both PAR-1-AP and PAR-2-AP elicit relaxation through the release of prostaglandin E2, thus causing a powerful bronchodilatation. 12 A recent study has shown that systemic treatment with PAR-2-AP inhibits the development of TNBS-induced colitis leading to an increased survival rate, improved macroscopic and histological damage score, and a decrease in the mucosal content of Th1 helper cell type 1 cytokines. 13 In that study, the authors also showed that in vivo treatment with a PAR-2-AP directly inhibited trinitrobenzensulfonic acid-induced interferon-γ secretion and CD44 expression on lamina propria T lymphocytes. 13 The apparent discrepancy between our results documenting a proinflammatory role for PAR-2-AP and anti-inflammatory effects of PAR-2-AP observed by Fiorucci and colleagues 13 in the TNBS-induced colitis model, may be explained by the nature of inflammation. The TNBS-induced colitis model is a model of chronic intestinal inflammation and the authors have looked at the damage 7 days after the induction of colitis, whereas in our study, an acute exposure to PAR-2-AP provoked an intestinal inflammation that was maximal between 4 and 6 hours after its induction. It is well known that some inflammatory mediators provoke an inflammatory reaction when administered acutely, but can be anti-inflammatory in the setting of chronic inflammation. 45-47 Another explanation for proinflammatory versus anti-inflammatory effects of PAR-2 activation is that inflammatory response may result from local activation of PAR-2, whereas systemic activation of PAR-2 may lead to an anti-inflammatory effect. In fact, local activation of PAR-2 (intracolonic administration), but not systemic injection (intraperitoneal) was responsible for the proinflammatory effects in the colon, whereas only a systemic injection of PAR-2 agonists induced resolution of TNBS-induced colitis. 13 It has been established that the induction of hypotension can be responsible for a decreased inflammation in models such as carrageenan-induced paw inflammation, 48 and systemic administration of PAR-2-AP is known to cause hypotension, 8,49,50 it is thus possible that hypotension induced by a chronic and systemic treatment with PAR-2-AP is responsible for the anti-inflammatory effects observed in the model of TNBS-induced colitis. Systemic versus local administration of PAR-2-AP activates different target cells expressing PAR-2 and these cells might be implicated differently in the inflammatory cascade. For example, it has been shown that endothelial activation of PAR-2, which might occur after systemic injection of PAR-2-AP, reduced tissue damage induced by ischemia-reperfusion injuries 14 and cardiac inflammation, 51 whereas PAR-2 activation on epithelial cells has been shown to induce the release of proinflammatory cytokines such as IL-8 and IL-6 52 or the release of arachidonic acid, the precursor of lipid inflammatory mediators, from enterocytes. 26
In summary, our study shows that a direct local activation of PAR-2 in the mouse colon leads to intestinal inflammation and a severe impairment of intestinal permeability. Trypsin and tryptase, through the activation of PAR-2, also causes colonic inflammation. Considering the large presence of those two proteinases in the gut, and the increased luminal tryptic activity associated with inflammatory bowel disease, it is likely that trypsin and/or tryptase can locally activate PAR-2, and might then participate in the intestinal pathophysiological changes observed in inflammatory bowel disease patients.
Footnotes
Address reprint requests to Dr. Nathalie Vergnolle, Dept. of Pharmacology and Therapeutics, 3330 Hospital Dr. NW, Calgary, Alberta, T2N4N1 Canada. E-mail: nvergnol@ucalgary.ca.
Supported by grants from the Canadian Institute of Health Research (to N. V., J. L. W., M. D. H.), NicOx S.A. (to N. V.), the Canadian Association of Gastroenterology (to N. V., C. N.), Johnson & Johnson Focused Giving (to J. L. W., M. D. H., N. W. B.), the UPSA Foundation (to N. C., R. G. V., L. B.), the French Institut National de la Recherche Agronomique (to N. C., R. G. V., L. B.), Pfizer Laboratories Fresnes, France (to A. C.), and the National Institutes of Health (grants DK 43207, 57480, 39957 to N. W. B.).
N. C. is a Ministère de la Recherche (France) studentship recipient, and N. V. is a Canadian Institute of Health Research Scholar.
References
- 1.Brass LF, Molino M: Protease-activated G protein-coupled receptors on human platelets and endothelial cells. Thromb Haemost 1997, 78:234-241 [PubMed] [Google Scholar]
- 2.Vergnolle N: Review article: proteinase-activated receptors-novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 2000, 14:257-266 [DOI] [PubMed] [Google Scholar]
- 3.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD: Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001, 22:146-152 [DOI] [PubMed] [Google Scholar]
- 4.Vu T, Hung D, Wheaton V, Coughlin S: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR: PAR3 is a cofactor for PAR4 activation by thrombin. Nature 2000, 404:609-613 [DOI] [PubMed] [Google Scholar]
- 6.Vergnolle N, Hollenberg MD, Sharkey K, Wallace JL: Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR-2)-activating peptides in the rat paw. Br J Pharmacol 1999, 127:1083-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhoff M, Vergnolle N, Young S, Tognetto M, Amadesi S, Ennes H, Trevisani M, Hollenberg MD, Wallace JL, Caughey G, Mitchell S, Williams L, Geppetti P, Mayer E, Bunnett N: Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 2000, 6:151-158 [DOI] [PubMed] [Google Scholar]
- 8.Damiano B, Cheung W, Santulli R, Fung-Leung P, Ngo K, Ye R, Darrow A, Derian C, De Garavilla L, Andrade-Gordon P: Cardiovascular responses mediated by proteinase-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J Pharmacol Exp Ther 1999, 288:671-678 [PubMed] [Google Scholar]
- 9.Hwa J, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E: Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ Res 1996, 78:581-588 [DOI] [PubMed] [Google Scholar]
- 10.Vergnolle N: Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol 1999, 163:5064-5069 [PubMed] [Google Scholar]
- 11.Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, Dileepan KN: Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res 2001, 21:231-240 [DOI] [PubMed] [Google Scholar]
- 12.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD: A protective role for protease-activated receptors in the airways. Nature 1999, 398:156-160 [DOI] [PubMed] [Google Scholar]
- 13.Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G: Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA 2001, 98:13936-13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napoli C, Cicala C, Wallace JL, de Nigris F, Santagada V, Caliendo G, Franconi F, Ignarro LJ, Cirino G: Protease-activated receptor-2 modulates myocardial ischemia-reperfusion injury in the rat heart. Proc Natl Acad Sci USA 2000, 97:3678-3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohm S, Kong W, Bromme D, Smeekens S, Anderson D, Connolly A, Kahn M, Nelken N, Coughlin S, Payan D, Bunnett N: Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J 1996, 314:1009-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J: Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem 1995, 232:84-89 [DOI] [PubMed] [Google Scholar]
- 17.Hollenberg MD, Saifeddine M, Al-Ani B, Kawabata A: Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol 1997, 75:832-841 [PubMed] [Google Scholar]
- 18.Compton S, Cairns J, Holgate S, Walls A: The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1b and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol 1998, 161:1939-1946 [PubMed] [Google Scholar]
- 19.McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P: Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol 2000, 279:G90-G99 [DOI] [PubMed] [Google Scholar]
- 20.Bradley PP, Priebat DA, Christensen RD, Rothstein G: Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982, 78:206-209 [DOI] [PubMed] [Google Scholar]
- 21.Vergnolle N, Comera C, Bueno L: Annexin 1 is overexpressed and specifically secreted during experimentally induced colitis in rats. Eur J Biochem 1995, 232:603-610 [DOI] [PubMed] [Google Scholar]
- 22.Zou W, Durand-Gasselin I, Dulioust A, Maillot MC, Galanaud P, Emilie D: Quantification of cytokine gene expression by competitive PCR using a colorimetric assay. Eur Cytokine Netw 1995, 6:257-264 [PubMed] [Google Scholar]
- 23.Legoux P, Minty C, Delpech B, Minty AJ, Shire D: Simultaneous quantitation of cytokine mRNAs in interleukin-1 beta stimulated U373 human astrocytoma cells by a polymerisation chain reaction method involving co-amplification with an internal multi-specific control. Eur Cytokine Netw 1992, 3:553-563 [PubMed] [Google Scholar]
- 24.Deitch EA, Kemper AC, Specian RD, Berg RD: A study of the relationships among survival, gut-origin sepsis, and bacterial translocation in a model of systemic inflammation. J Trauma 1992, 32:141-147 [PubMed] [Google Scholar]
- 25.Cuffe JE, Bertog M, Velazquez-Rocha S, Dery O, Bunnett N, Korbmacher C: Basolateral PAR-2 receptors mediate KCl secretion and inhibition of Na(+) absorption in the mouse distal colon. J Physiol 2002, 539:209-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong W, McConalogue K, Khitin L, Hollenberg MD, Payan D, Bohm S, Bunnett N: Luminal trypsin may regulate enterocytes through proteinase-activated receptor-2. Proc Natl Acad Sci USA 1997, 94:8884-8889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos J, Saperas E, Nogueiras C, Mourelle M, Antolin M, Cadahia A, Malagelada JR: Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998, 114:640-648 [DOI] [PubMed] [Google Scholar]
- 28.Santos J, Bayarri C, Saperas E, Nogueiras C, Antolin M, Mourelle M, Cadahia A, Malagelada JR: Characterisation of immune mediator release during the immediate response to segmental mucosal challenge in the jejunum of patients with food allergy. Gut 1999, 45:553-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scudamore C, Pennington A, Thornton E, McMillan L, Newlands G, Miller H: Basal secretion and anaphylactic release of rat mast cell protease-II (RMCP-II) from ex vivo perfused rat jejunum: translocation of RMCP-II into the gut lumen and its relation to mucosal histology. Gut 1995, 37:235-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RY, Schwartz LB, Curry A, Pesola GR, Knight RJ, Lee HS, Bakalchuk L, Tenenbaum C, Westfal RE: Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J Allergy Clin Immunol 2000, 106:65-71 [DOI] [PubMed] [Google Scholar]
- 31.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J, Schechter N, Woolkalis MJ, Brass LF: Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem 1997, 272:4043-4049 [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K: Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun 1999, 67:1471-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youakim A, Ahdieh M: Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999, 276:G1279-G1288 [DOI] [PubMed] [Google Scholar]
- 34.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD: Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116:301-309 [DOI] [PubMed] [Google Scholar]
- 35.Ma TY: Intestinal epithelial barrier dysfunction in Crohn’s disease. Proc Soc Exp Biol Med 1997, 214:318-327 [DOI] [PubMed] [Google Scholar]
- 36.Gassler N, Autschbach F, Heuschen G, Witzgall R, Otto HF, Obermuller N: Expression of clusterin in Crohn’s disease of the terminal ileum. Histol Histopathol 2001, 16:755-762 [DOI] [PubMed] [Google Scholar]
- 37.Playford RJ, Hanby AM, Patel K, Calam J: Influence of inflammatory bowel disease on the distribution and concentration of pancreatic secretory trypsin inhibitor within the colon. Am J Pathol 1995, 146:310-316 [PMC free article] [PubMed] [Google Scholar]
- 38.Bustos D, Negri G, De Paula JA, Di Carlo M, Yapur V, Facente A, De Paula A: Colonic proteinases: increased activity in patients with ulcerative colitis. Medicina(B Aires) 1998, 58:262-264 [PubMed] [Google Scholar]
- 39.Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Reimann J, Claesson MH, Bland PW: The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol 2000, 157:1927-1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raithel M, Winterkamp S, Pacurar A, Ulrich P, Hochberger J, Hahn EG: Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand J Gastroenterol 2001, 36:174-179 [DOI] [PubMed] [Google Scholar]
- 41.Shimizu H, Nagakui Y, Tsuchiya K, Horii Y: Demonstration of chymotryptic and tryptic activities in mast cells of rodents: comparison of 17 species of the family Muridae. J Comp Path 2001, 125:76-79 [DOI] [PubMed] [Google Scholar]
- 42.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, Stevens RL: Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol 1998, 160:5537-5545 [PubMed] [Google Scholar]
- 43.Tremaine WJ, Brzezinski A, Katz JA, Wolf DC, Fleming TJ, Mordenti J, Strenkoski-Nix LC, Kurth MC: Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Aliment Pharmacol Ther 2002, 16:407-413 [DOI] [PubMed] [Google Scholar]
- 44.Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L: Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 2002, 122:1035-1047 [DOI] [PubMed] [Google Scholar]
- 45.Peskar BM, Maricic N, Gretzera B, Schuligoi R, Schmassmann A: Role of cyclooxygenase-2 in gastric mucosal defense. Life Sci 2001, 69:2993-3003 [DOI] [PubMed] [Google Scholar]
- 46.Mazelin L, Theodorou V, Fioramonti J, Bueno L: Vagally dependent protective action of calcitonin gene-related peptide on colitis. Peptides 1999, 20:1367-1374 [DOI] [PubMed] [Google Scholar]
- 47.Granger DN, Kubes P: Nitric oxide as antiinflammatory agent. Methods Enzymol 1996, 269:434-442 [DOI] [PubMed] [Google Scholar]
- 48.Torpy DJ, Webster EL, Zachman EK, Aguilera G, Chrousos GP: Urocortin and inflammation: confounding effects of hypotension on measures of inflammation. Neuroimmunomodulation 1999, 6:182-186 [DOI] [PubMed] [Google Scholar]
- 49.Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, Cruchley A, Kapas S, Howells GL, Cirino G: Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation 1999, 99:2590-2597 [DOI] [PubMed] [Google Scholar]
- 50.Emilsson K, Wahlestedt C, Sun MK, Nystedt S, Owman C, Sundelin J: Vascular effects of proteinase-activated receptor 2 agonist peptide. J Vasc Res 1997, 34:267-272 [DOI] [PubMed] [Google Scholar]
- 51.Napoli C, de Nigris F, Cicala C, Wallace JL, Caliendo G, Condorelli M, Santagada V, Cirino G: Protease-activated receptor-2 activation improves efficiency of experimental ischemic preconditioning. Am J Physiol 2002, 282:H2004-H2010 [DOI] [PubMed] [Google Scholar]
- 52.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA: Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol 2002, 168:3577-3585 [DOI] [PubMed] [Google Scholar]