Abstract
Acquired abdominal aortic aneurysms are usually associated with a mural thrombus through which blood continues to flow. Some early data suggest that aneurysmal evolution correlates with the biological activity of the thrombus. Our hypothesis was therefore that the thrombus could adsorb blood components and store, release, and participate in the activation of proteases involved in aneurysmal evolution. For this purpose, we have explored both the metalloproteinase and fibrinolytic systems in the thrombus and the wall of human aneurysms. We have first investigated blood clot formation and lysis in vitro. Spontaneous clotting induces a release of promatrix metalloproteinase (pro-MMP)-9 into the serum that was fourfold higher than in paired control plasma (P < 0.001). Fibrinolysis progressively released more MMP-9 in a time-dependent manner (P < 0.01). After selective isolation, we demonstrated that polymorphonuclear leukocytes are the main source of MMP-9 release during clot formation. Protease content was then analyzed in 35 mural thrombi and walls of human abdominal aortic aneurysms sampled during surgical repair. In 15 aneurysms, the liquid phase at the interface between the thrombus and the wall was sampled separately. Both thrombus and wall contained MMP-2 and MMP-9 but the ratio MMP-9/MMP-2 was higher in the thrombus than in the wall. The liquid interface also contained active MMP-9. Immunohistochemistry of the thrombus confirmed these findings, showing the presence of polymorphonuclear leukocytes at the luminal pole of the thrombus, co-localizing with MMP-9 storage. In contrast, MMP-3 and MMP-7 were only present in the aneurysmal wall. Plasminogen was present in the mural thrombus but plasmin activity was present in both thrombus and wall. In the liquid interface, plasmin-α2-anti-plasmin complexes were detected demonstrating in vivo the activation of plasminogen. In contrast, u-PA and t-PA were detectable only in the wall, suggesting that plasminogen present in the thrombus could be activated by factors secreted by the arterial wall. This was demonstrated in vitro, in which co-incubation of thrombus and wall extracts generated plasmin in the presence of a fibrin matrix and activated MMPs. In conclusion, our study strongly suggests that the mural thrombus, by trapping polymorphonuclear leukocytes and adsorbing plasma components could act as a source of proteases in aneurysms that may play a critical role in enlargement and rupture.
In contrast with atherosclerotic stenosis, which predominantly involves accumulation of cells and extracellular matrix within the intima, aneurysm formation predominantly involves degradation of the medial layer. 1 Since the early 1980s, 2 new molecular and cellular concepts of the pathophysiology of aneurysm evolution toward rupture have emerged, mainly involving the ability of proteinases to hydrolyze insoluble extracellular matrix components. 3 In parallel, the plasminogen (Pg) activation cascade has also been implicated in aneurysm evolution. 4
The development of acquired abdominal aortic aneurysms of atheromatous origin is usually characterized by the presence of a mural thrombus. Despite this observation, previous studies have mainly focused on the localization of proteinases within the aneurysmal wall, 5-8 and only a few have explored the involvement of the thrombus in aneurysmal evolution. 9 In contrast with arterial occlusive diseases, blood flow is maintained through the aneurysmal thrombus resulting in a persistent remodeling activity of its components. 10 Evolution of aneurysmal diameter has been reported to correlate with plasma markers of fibrin formation and degradation, 11 and circulating plasmin (Pn)-α2-anti-Pn 12 corresponding to thrombus turnover. Furthermore, the presence of a crescent sign on computed tomography scan, corresponding to a hemorrhage within the thrombus, is considered as a predictive marker of imminent rupture. 13
The purpose of this study was to determine the differential localization of proteinases and their activators within the thrombus and the aneurysmal wall and their release into the liquid phase that is present at the interface between thrombus and wall. First, we explored the ability of blood clots to store and release matrix metalloproteinases (MMPs) during their formation and lysis in vitro. MMPs and the Pg activation cascade were then investigated in aneurysmal thrombus, wall, and their liquid interface. Our data strongly suggest that the thrombus represents a site of storage and release of leukocyte and plasma proteinases, and its parietal surface, a target for activators present in the wall.
Materials and Methods
Blood Samples from Eight Healthy Volunteers
Sera were obtained after incubation of blood at 37°C for 30 minutes; 9, 32, and 56 hours; and 3, 4, and 7 days. Citrated plasma samples were used directly or recalcified (30 mmol/L CaCl2) to induce fibrin formation in the absence of blood cells. To analyze the cellular source of proteases, polymorphonuclear leukocytes (PMNs), monocytes, lymphocytes, and platelets were isolated as previously described (n = 5, 5 ml per volunteer). 14,15 MMPs were extracted in 0.05 mol/L of Tris-HCl, pH 7.5, containing 0.01 mol/L of CaCl2 and 0.2% Triton X-100. Homogenates were sonicated and centrifuged and supernatants were collected. Protein concentration was determined by the Bradford assay (Bio-Rad, Ivry-sur-Seine, France).
Tissue Samples
Infrarenal aortic specimens were obtained from 35 patients who provided informed consent before surgical abdominal aortic aneurysm repair. The largest diameter of the aneurysm was measured on computed tomography scan (mean diameter, 57.9 ± 17.7 mm; range, 36 to 120 mm). Thrombi (n = 35) and aneurysmal walls (n = 35) were harvested separately during surgery and quickly frozen in liquid nitrogen. In 15 patients, a liquid phase was present at the interface between thrombus and wall, which we assume to be a consequence of the fibrinolysis at the abluminal pole of the thrombus, and was sampled separately.
Protein Extraction
Tissue samples were homogenized in 0.05 mol/L of Tris-HCl, pH 7.5, containing 0.01 mol/L of CaCl2, 2 mol/L of guanidinium chloride, and 0.2% Triton X-100 using a Polytron as previously described. 16 Protein concentration was determined by the Bradford assay (Bio-Rad).
Gelatin and α-Casein Zymographies
Extracts (10 or 30 μg of proteins) were analyzed by electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels containing 1 mg/ml of gelatin (Sigma, St. Quentin-Fallavier, France) or α-casein (Fluka-St. Quentin-Fallavier, France) under nonreducing conditions as previously described. 16 Activities were quantified by densitometry using the NIH Image 1.60 ppc software. A standard sample was run on each gel and served as reference.
Reverse Gelatin Zymography
Extracts (50 μg of proteins) were submitted to 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis on gels that contained 1 mg/ml of gelatin and 160 ng/ml of pro-MMP-2 (Euromedex, Souffelweyersheim, France) under nonreducing conditions as previously described. 17 A solution of TIMP-1 and -2 (Euromedex) was used as reference.
Immunohistochemistry for MMP-9, Platelets, and PMN Staining
Seven-μm cryostat sections obtained of aneurysmal thrombi were mounted on poly-l-lysine-coated slides and fixed in acetone. Some sections were then treated with 3% H2O2 for 10 minutes at room temperature and incubated overnight at 4°C with anti-MMP-9 monoclonal (Ab-1; Calbiochem, Meudon, France), and anti-GPIIb/IIIa monoclonal (7E3; Dr. JP Rosa, INSERM U348, Paris) 18 primary antibodies (10 μg/ml). Sections were then processed using biotin-streptavidin-peroxidase complexes. Peroxidase activity was revealed with diaminobenzidine/cobalt substrate. Negative controls included omission of the primary antibody or replacement of the primary antibody with naïve mouse IgG1κ (10 μg/ml). Other serial sections were stained with hematoxylin, phloxin, and safran to evaluate general topography and cell distribution.
Pg and Pn Activities
Pg quantity in each extract was determined on tPA-fibrin-coated wells. 19 For tPA (Biopool, American Diagnostica, Andresy, France) coating, 50 μl of t-PA [20 IU/ml in phosphate-buffered saline buffer and 2 mg/ml of bovine serum albumin (BSA)] were incubated in fibrin-coated wells for 1 hour at 37°C. After washing, 25 μl of each sample and 25 μl of 1.5 mmol/L CBS 0065 (Diagnostica Stago, Asnières, France) were added and incubated at 37°C. The Pg activation is detected by measuring the change in absorbance at 405 nm as a function of time (0 to 2 hours) using a microplate reader (Dynex, Issy-les-Moulineaux, France). Results were expressed as nmol of Pg/mg soluble proteins by reference to a standard curve constructed with purified Pg. Plasmin (Pn) activity was determined in the same samples on 4% BSA-coated wells. Initial rates of p-nitro-aniline release from the chromogenic substrate were transformed into pmol of Pn/mg soluble proteins as previously described. 19
Pg Activator Zymography
Fifty μg of proteins were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described. 20 The gel was then carefully overlaid on a Pg-fibrin-agar gel, incubated at 37°C for 24 to 96 hours in a moist chamber and photographed at regular intervals using dark-ground illumination. Fibrinolytic activities were quantified by densitometry using the NIH Image 1.60 ppc software. Purified t-PA (Biopool) and u-PA (Choay, Gentilly, France) were used as reference.
Analysis of Pg and MMP Activation by Thrombus/Wall Interactions in Vitro
Extracts of thrombus and wall (100 μl) were first depleted in Pn by adsorption on aprotinin-Sepharose. Thirty μl of Pn-free wall extract was then preincubated with or without a fibrin matrix for 2 hours at 37°C. After washing with phosphate-BSA buffer, 20 μl of Pn-free thrombus extract and chromogenic substrate selective for Pn (CBS 0065) were added. The activation of Pg present in the extract was monitored in a microplate reader. In parallel experiments, wall and thrombus extracts were co-incubated with or without a fibrin matrix for 24 hours at 37°C and further analyzed by gelatin zymography to visualize MMP activation.
Western Blot
Electrophoresis was performed as described by Laemmli on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under reducing conditions. 16 Antibodies to kringle 1 of Pn/Pg as previously described, 21 α2-anti-Pn (Biopool); MMP-2, -3, -7, and -9; and TIMP-1 and -2 (Chemicon, Euromedex, Souffelweyersheim, France), were used at the concentration of 1 μg/ml and horseradish peroxidase IgG (DAKO, Trappes, France) was used after dilution (1/1000). Peroxidase activity was detected using chemiluminescence reagent (NEN, Courtaboeuf, France).
Statistical Analysis
Data are expressed as means ± SD. The paired t-test and one-way factorial analysis (analysis of variance) followed by the Scheffé F-test were used to compare groups. Differences were considered significant when P < 0.05.
Results
Clot Formation, Retraction, and Lysis Result in Release of MMP-9 in Vitro
To demonstrate that the thrombus can indeed be a source of storage and release of MMPs, gelatinase activities were measured in plasma versus serum and in blood cell pellet versus blood clot sampled from healthy volunteers.
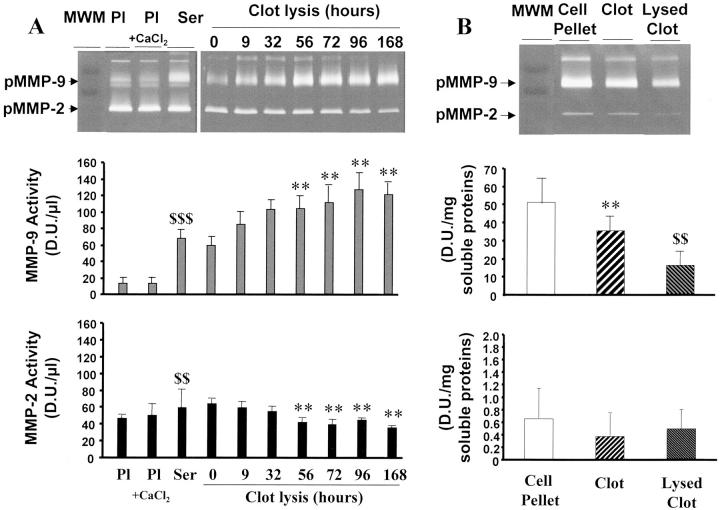
Pro-MMP-9 and pro-MMP-2 activities were similar in plasma and in serum-derived plasma, ie, respectively, before and after addition of 30 mmol/L CaCl2, respectively, which results in fibrin formation (Figure 1A) ▶ . Thus, there was no adsorption of pro-MMP-9 and pro-MMP-2 activities during fibrinogenesis in the absence of blood cells, including platelets.
Figure 1.
Gelatinase activities in plasma, serum, blood cell pellet, and clot. A: MMP-2 and MMP-9 activities, detected on zymograms (top), were measured in plasma (Pl), recalcified plasma (Pl + CaCl2), and in serum as a function of time (MMP-9, middle; MMP-2, bottom). **, P < 0.01 as compared to zero point time; $$, P < 0.01; $$$, P < 0.001 as compared to plasma. B: Gelatinase activities were measured in extracts of blood cells, clot, and 7-day-lysed clot (top) and quantified by densitometry (MMP-9, middle; MMP-2, bottom). **, P < 0.01 as compared to cell pellet; $$, P < 0.01 as compared to nonlysed clot.
Pro-MMP-9 activity was fourfold higher in serum than in plasma (P < 0.001) (Figure 1A) ▶ . Pro-MMP-9 further increased in serum during clot retraction and lysis (P < 0.01) (Figure 1A) ▶ . Conversely, pro-MMP-9 content was lower in the clot than in the blood cell pellet (P < 0.01) (Figure 1B) ▶ . Pro-MMP-9 activity further decreased in the clot during thrombus lysis (P < 0.01). Pro-MMP-9 was thus released during clot formation, retraction, and lysis in vitro.
Pro-MMP-2 activity in serum was slightly higher than in plasma (P < 0.01, Figure 1A ▶ ). Pro-MMP-2 activity was not modified during clot retraction and decreased during clot lysis (P < 0.01). In cell pellet and blood clots, pro-MMP-2 activities were very low (Figure 1B) ▶ .
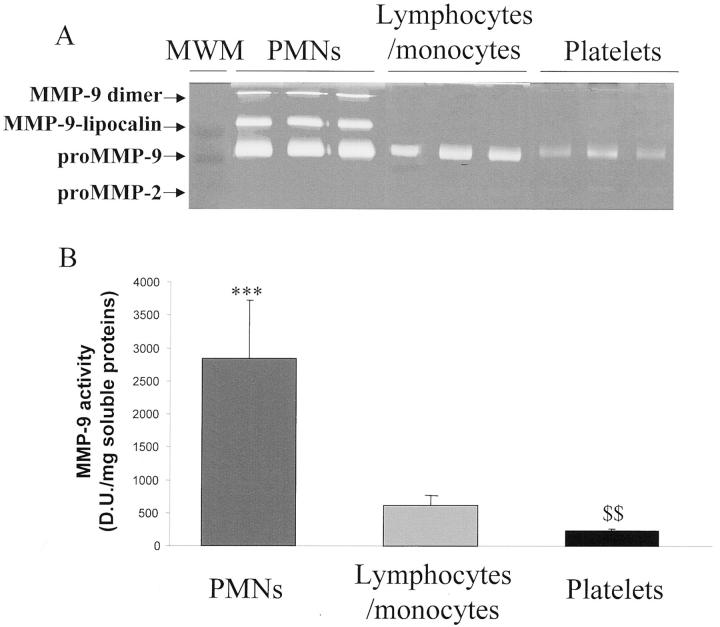
To analyze the cellular source of MMPs during clot formation, PMNs, monocytes/lymphocytes, and platelets were isolated and MMPs were extracted from cell pellets. Pro-MMP-9 activity was significantly higher in PMNs than in platelets (P < 0.001) and significantly lower in platelets than in monocytes/lymphocytes (P < 0.01). Low pro-MMP-2 activity was found only in platelet extracts (Figure 2A) ▶ .
Figure 2.
Gelatinase activities in extracts of PMNs, monocytes/lymphocytes, and platelets. A: MMP-2 and MMP-9 activities, detected on zymograms. B: MMP-9 activity was quantified by densitometry. ***, P < 0.001 as compared to platelets;$$, P < 0.01 as compared to monocytes/lymphocytes.
MMP-9/MMP-2 Ratio Is Higher in the Thrombus than in the Aneurysmal Wall
There was no significant difference between the soluble protein contents of mural thrombus and aneurysmal wall (23 ± 6 and 20 ± 7 mg/ml, respectively) but a significantly higher concentration was detected in the liquid interface (124 ± 14 mg/ml).
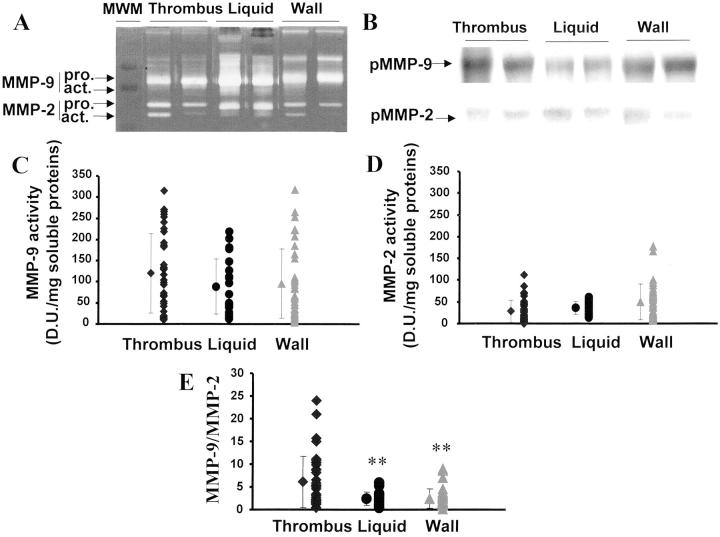
Gelatinolytic activities detected at apparent molecular weight of 96 kd correspond to pro-MMP-9 (Figure 3A) ▶ . This identity was confirmed by Western blot (Figure 3B) ▶ . The proform of MMP-9 was detected in thrombus, liquid, and wall whereas the active form was only detected in the liquid interface between thrombus and wall (Figure 3A) ▶ .
Figure 3.
Gelatinase activities in mural thrombus, liquid interface, and aneurysmal wall. MMP-2 and MMP-9 activities detected on zymograms (A) were further characterized by Western blot (B). MMP-9 (C) and MMP-2 (D) activities were quantified by densitometry and MMP-9/MMP-2 ratios (E) were calculated. **, P < 0.01 as compared to thrombus extracts.
Gelatinolytic activities at apparent molecular weights of 72 and 64 kd correspond to latent and active forms of MMP-2 (Figure 3A) ▶ . This identity was again confirmed on Western blot (Figure 3B) ▶ . Latent and active MMP-2 were similarly detected in thrombus, wall, and liquid (Figure 3D) ▶ .
Mean MMP-9 and MMP-2 activities were similar in the three compartments (Figure 3, C and D) ▶ ; however, the MMP-9/MMP-2 ratio was 2.5-fold higher in the thrombus than in the wall and liquid interface (P < 0.01) (Figure 3E) ▶ .
MMP-9 and PMN Leukocytes Are Present in the Thrombus
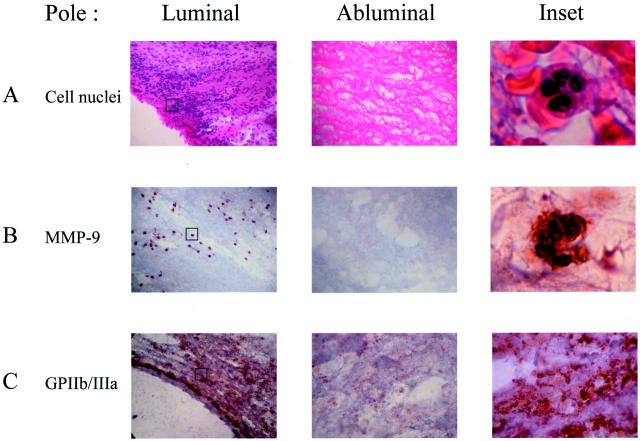
Hematoxylin, phloxin, and safran staining of sections showed a marked enrichment of polymorphonuclear cells in the most inner layer of the mural thrombus. In contrast the abluminal thrombus is devoid of cellular components (Figure 4A) ▶ . PMN smears were used as a positive control for MMP-9 immunostaining (data not shown). MMP-9 immunostaining co-localized exactly with PMNs. There was no MMP-9 immunostaining in the abluminal part of the thrombus (Figure 4B) ▶ . Platelet smears were used as a positive control for GPIIb/IIIa immunostaining and were strictly negative for MMP-9 (data not shown). The GPIIb/IIIa immunostaining showed an intense staining in the luminal thrombus (Figure 4C) ▶ .
Figure 4.
Immunochemistry of MMP-9 in mural thrombus. A and inset: Hematoxylin, phloxin, and safran staining showing the presence of PMNs in the luminal pole of the thrombus. B: MMP-9 staining in the luminal and abluminal pole of the thrombus and inset showing the co-localization of MMP-9 and PMNs in luminal pole. C: GPIIb/IIIa staining in the luminal and abluminal pole of the thrombus and inset showing a diffuse marking of GPIIb/IIIa corresponding to platelets in the luminal pole. PMN and platelet smears were used as positive controls for MMP-9 and GPIIb/IIIa immunostaining, respectively (data not shown). Original magnifications, ×100 (insets in A–C).
MMP-3 and MMP-7 Are Detected Only in Aneurysmal Wall
Casein zymography showed lytic activities at 50 kd and 28 kd (data not shown). By Western blotting, these activities were characterized as MMP-3 and MMP-7, respectively. MMP-3 and MMP-7 activities and proteins were detected only in the aneurysmal wall (data not shown).
TIMP-1 and -2 Are Significantly Lower in the Liquid Interface
MMP-2 inhibitory activities were detectable at apparent molecular weights of 27 kd and 21 kd in tissue extracts and were shown to correspond to TIMP-1 and -2 on Western blot (data not shown). TIMP-1 activity was similar in thrombus and wall (55,930 ± 28,860 and 57,607 ± 30,141 D.U./mg soluble proteins, respectively) and significantly lower in the liquid interface (25,890 ± 19,810 D.U./mg soluble proteins, P < 0.001). TIMP-2 activity was significantly higher in the wall than in the thrombus (67,810 ± 32,250 and 46,130 ± 25,790 D.U./mg soluble proteins, respectively, P < 0.01) and in the liquid interface (31,180 ± 14,500 D.U./mg soluble proteins, P < 0.01).
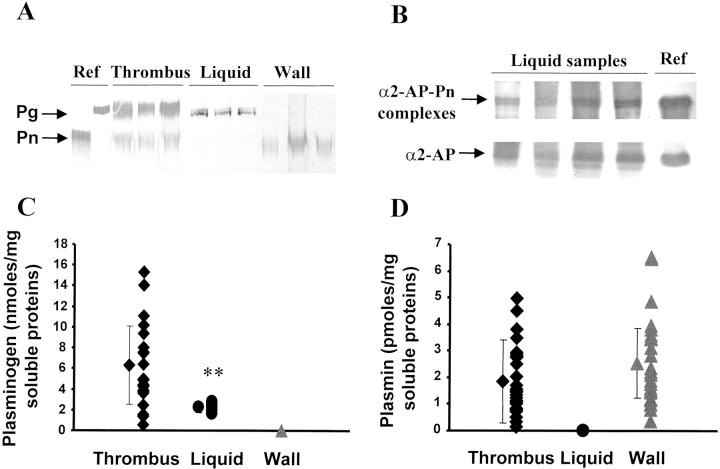
Pg Is Localized in the Mural Thrombus
Pn, as a possible activator of MMPs, and its precursor Pg were detected by Western blotting, and their activities were measured on a synthetic substrate. Pg was significantly higher in the mural thrombus than in the liquid interface and was not detected in the wall (Figure 5, A and C) ▶ (P < 0.01). Pn activity was similar in thrombus and wall extracts and was not detected in the liquid interface (Figure 5, A and D) ▶ . The absence of Pn activity in the liquid interface may be because of the presence of inhibitors. Indeed, α2-anti-Pn and Pn-α2-anti-Pn complexes were detected in the liquid interface as shown on Western blot (Figure 5B) ▶ . Pn-α2-anti-Pn complexes were also detected in extracts of thrombus and wall (data not shown).
Figure 5.
Pg and Pn in the three compartments. Pg and Pn (A) and free α2-anti-Pn (α2-AP) and α2-anti-Pn-Pn complexes (α2-AP-Pn) (B) were detected on Western blots. Pg (C) and Pn quantities (D) were determined through their activity on a chromogenic substrate. **, P < 0.01 as compared to thrombus.
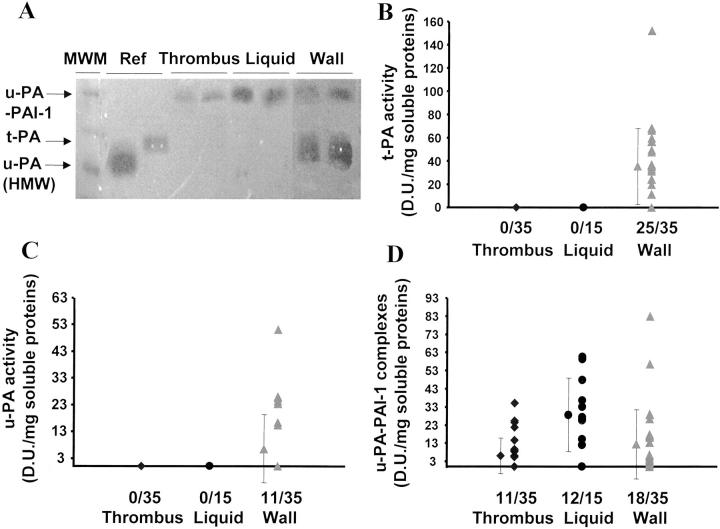
Free t-PA and u-PA Activities Are Present Only in the Aneurysmal Wall
Fibrin-Pg zymography showed activities at apparent molecular weights of 120, 70, and 54 kd (Figure 6A) ▶ . Enzyme activity at 70 kd was inhibited by polyclonal antibodies to t-PA and activities at 120 and 54 kd were inhibited by polyclonal antibodies to u-PA (data not shown). The enzyme activities at 120 and 54 kd correspond to u-PA-PAI-1 complexes and free u-PA, respectively; the enzyme activity at 70 kd corresponds to free t-PA. The u-PA-PAI-1 complexes were detected in thrombus, liquid interface, and wall (Figure 6, A and D) ▶ . Free t-PA and u-PA activities were only measurable in the arterial wall (Figure 6;A, B, and C) ▶ . t-PA-PAI-1 complexes were not detected.
Figure 6.
Free Pg activators were localized in the wall. Pg activators were detected through the activation of Pg in fibrin-agar gels (A). t-PA (B) and u-PA (C) activities and uPA-PAI-1 complexes (D) were quantified by densitometry.
Fibrin Solid Phase-Dependent Activation of Pg and MMPs in Vitro
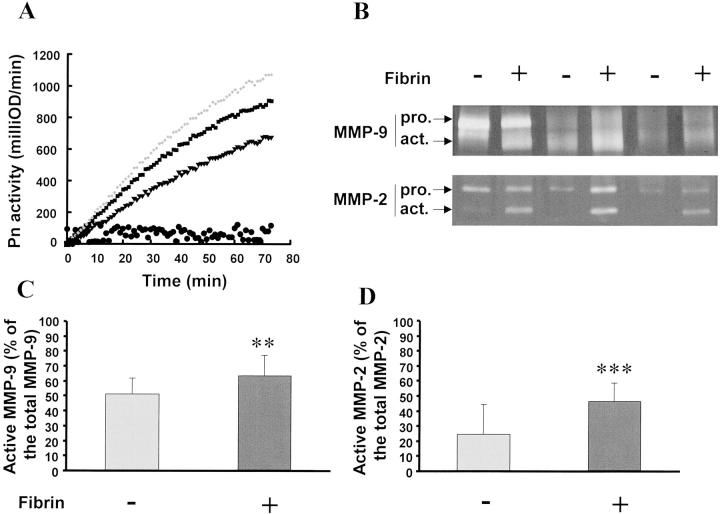
To explain the role of the interaction of the thrombus with wall components, extracts of thrombus and wall were co-incubated in absence or presence of a fibrin matrix in vitro. When the two extracts were co-incubated for 2 hours at 37°C in BSA-coated wells, there was no generation of Pn. In contrast, in fibrin-coated wells, incubation of thrombus extracts, which contain Pg (Figure 5C) ▶ , with wall extracts, which contain activators (Figure 6, B and C) ▶ , induced formation of Pn (Figure 7A) ▶ .
Figure 7.
In vitro interaction between thrombus and wall extracts. Thrombus/wall extract interactions were monitored in vitro in the absence (−) or presence (+) of a fibrin matrix. Pg from thrombus extracts was activated by the Pg activators contained in the wall extract on fibrin-coated wells (A, ♦, ▪, ▾: three representative experiments, initial rates: 364, 306, 226 fmol/min, respectively, and •: control experiments in BSA-coated wells). Co-incubated extracts were analyzed on gelatin zymography (B). Percentage of active MMP-9 (C) and MMP-2 (D) were calculated in the presence or absence of fibrin matrix. **, P < 0.01; ***, P < 0.001.
Co-incubation during 24 hours of the two extracts in the absence of fibrin increased the percentage of the active form of MMP-9 and MMP-2 (51 ± 10% and 24 ± 20%, respectively, n = 15) as compared to isolated thrombus extracts (0% and 10 ± 5%, respectively; P < 0.01). Similar co-incubation in the presence of a fibrin solid phase significantly increased further these percentages (63 ± 13%, P < 0.01; 46 ± 12%, P < 0.001, respectively) (Figure 7, B and C) ▶ . These data suggest an interaction between the substrates present in the thrombus and the activators secreted by the wall and a catalyzing role of the solid-phase fibrin in the generation of Pn at the interface between thrombus and wall.
Discussion
In this study, we have explored in vitro the ability of blood clots to store and release MMPs to better understand the role of the biological activity of the mural aneurysmal thrombus in this respect. Sera obtained after spontaneous clot formation, retraction, and lysis, always contain more MMP-9 than plasma. Because fibrin polymer formation obtained from recalcified citrated plasma, devoid of any cellular components, did not induce release of MMP-9, we can conclude that MMP-9 released from the thrombus originates from trapped cells and probably from PMNs and platelets. Therefore, we compared the MMP-9 content in PMN leukocytes and platelets and have shown that the role of PMNs was predominant in MMP-9 release in vitro. Pro-MMP-9 is stored in tertiary granules of PMNs that are the first to degranulate. 22 Therefore our data strongly suggest that PMNs, trapped in the fibrin mesh during clotting, partially degranulate and release pro-MMP-9. These data fit well with the results of a previous study, suggesting that serum cannot be used to determine the circulating level of MMP-9. 23 On the other hand, the blood clot also contains pro-MMP-9, suggesting its entrapment and storage during fibrinogenesis. The calcium-phosphate-dependent binding of MMP-9 to fibrin can participate in this storage. 24
Ex vivo analysis of the mural thrombus gave parallel results showing a higher MMP-9/MMP-2 ratio compared to the wall. Histology demonstrated the accumulation of PMNs in the inner layer of the thrombus and their co-localization with MMP-9 staining. By analogy to our in vitro observations, these data suggest that MMP-9 could be stored and released by PMNs entrapped in the thrombus on its luminal side. Adolph and co-workers 10 have shown that the aneurysmal thrombus is active. This activity is associated with a leukocyte gradient from the luminal to the abluminal pole of the thrombus. 10 Monocyte/macrophages 25 and PMNs 26 easily adhere to the co-polymer of fibrin-fibronectin, and PMNs release latent MMP-9 when they adhere to fibronectin-collagen co-polymer. 27 Similarly, Sakalihasan and co-workers 9 reported an enrichment of MMP-9 in aneurysms that was associated with a negative gradient of latent MMP-9, and a positive one for MMP-9 activation, from the luminal to the parietal pole of the thrombus. Our ex vivo evidence of PMN trapping and MMP-9 storage within the aneurysmal thrombus associated with the in vitro demonstration of the role of leukocyte trapping in MMP-9 storage and release during thrombus formation strongly suggest that the active luminal pole of the thrombus could trap circulating leukocytes that then release pro-MMP-9. Pro-MMP-9 could be both stored within the aneurysmal thrombus and released into the circulating blood. Therefore MMP-9 release by the luminal thrombus could be also the main source of high levels of plasma MMP-9 reported in patients with aortic aneurysms. 28 The reported normalization of plasma pro-MMP-9 levels by aneurysm repair may predominantly reflect exclusion of the thrombus; 29 and conversely, the absence of regression of these levels would suggest the persistence of a thrombus activity, providing evidence of endoleaks. 30
Nevertheless, pro-MMP-9 was also present in the aneurysmal wall, 31 probably mainly synthesized by infiltrated inflammatory cells or diffusing from the thrombus. 32 The important role of MMP-9 in aneurysm development and evolution has been recently stressed by experimental approaches showing that MMP-9 gene disruption in mice prevented the development of elastase-induced aneurysms 33 and that smooth muscle endoluminal seeding prevented both experimental aneurysm development and MMP-9 expression. 17
In contrast with pro-MMP-9, pro-MMP-2 activity in vitro was only slightly higher in serum than in plasma, corresponding to a small release during clot formation. This release of pro-MMP-2 probably provides evidence of platelet aggregation associated with thrombus formation. 34 Latent MMP-2 is constitutively expressed and secreted by vascular cells. 35,36 and diffuses easily into the culture medium when tissue is incubated ex vivo. 35
MMP-3 and -7, revealed by their caseinolytic activity, are only present in the arterial wall. MMP-3 (stromelysin 1) is expressed in human aneurysmal wall 37 and mainly secreted by inflammatory cells. 38 MMP-3 is also involved in the activation of MMP-9. 39 Because MMP-3 possesses a hemopexin-like domain 40 it binds to heparan sulfate 41-43 and could be stored in tissue.
MMP-7 (matrilysin) is also a MMP tightly bound to heparan sulfate. 41 Pro-MMP-7 is mainly synthesized and secreted by activated fibroblasts. 44 In response to proteolytic medial injury, adventitial fibroblasts activate, proliferate, and secrete collagen limiting the outward expansion of aneurysm. 45 The presence of MMP-7 in the aneurysmal wall probably provides evidence of the activity of adventitial fibroblasts in aneurysmal remodeling.
We explored also the localization of Pg activation able to form active MMPs. Pg was present in the thrombus and liquid interface, and absent in the wall. Circulating Pg binds to fibrin and is adsorbed from the plasma onto the aneurysmal thrombus, serving as a substrate for Pn generation. Pn is detectable in both thrombus and wall but, in the thrombus, the quantity of Pn is 3000-fold lower than that of Pg. In contrast, free Pg activators were only detectable in the wall. u-PA is mainly produced by inflammatory cells in the wall and its role in aneurysm development has been recently pointed out through experimental approaches. 46,47 For the first time we observe here the complementary localization of the substrate Pg in the thrombus and its activators in the aneurysmal wall suggesting a cooperation between thrombus and wall in generating active Pn which probably plays a major role in MMP activation on the parietal surface of the thrombus. To demonstrate these points, thrombus and wall extracts were co-incubated in vitro and our results showed the ability of this interaction to activate MMPs and the fibrin-dependency of Pn generation. The presence of a fibrinolytic liquid phase containing active MMPs at the interface between aneurysmal thrombus and wall also provides evidence of such activation in vivo. The absence of detectable free Pn in this liquid interface and the presence of Pn-α2-anti-Pn complexes, contrasting with the finding of active Pn in the thrombus and the wall, also suggest that a solid phase is critical for Pn activity, thrombus lysis, and MMP activation. These data fit well with the reported predominant localization on in situ zymography of MMP activities in the inner wall of human aneurysms. 48
In conclusion, our study strongly suggests that the mural thrombus, by aggregating platelets, trapping circulating cells, and adsorbing plasma components, could act as a source of secreted proteases within the aneurysm. In contrast, the arterial wall contains mainly tissue proteases, including MMPs and Pg activators of inflammatory and mesenchymal cell origin. Finally, the abluminal part of the thrombus could serve as a solid phase catalyst between the thrombus and the wall. The formation of a liquid fibrinolytic phase at the interface between thrombus and wall, devoid of Pn activity but containing active forms of MMPs, provides evidence of these topological interactions between substrates vehiculed by the thrombus and activators originating from the wall. These proteases linked to thrombus activities could play a critical role in the evolution of abdominal aortic aneurysms toward enlargement and rupture.
Acknowledgments
We thank Dr. Mary Osborne-Pellegrin for her constructive criticism of this manuscript, Dr. Jean-Philippe Rosa (INSERM U348 Hôpital Lariboisière) for providing the monoclonal antibody anti-GPIIb/IIIa (7E3), and Isabelle Prevost and Aïda Debieu (Service d’Anatomie Pathologique, Chu Bichat Claude Bernard, Paris) for their skilful technical assistance.
Footnotes
Address reprint requests to Jean-Baptiste Michel, M.D., Ph.D., INSERM Unit 460, Hôpital Xavier Bichat, 46 rue Henri Huchard, 75870 Paris Cedex 18, France. E-mail: jpmichel@bichat.inserm.fr.
Supported by a Progres grant from INSERM, the Fondation de France, and by a grant from the Société de Chirurgie Vasculaire (to V. F.).
References
- 1.Michel JB: Contrasting outcomes of atheroma evolution: intimal accumulation versus medial destruction. Arterioscler Thromb Vasc Biol 2001, 21:1389-1392 [PubMed] [Google Scholar]
- 2.Busutill R, Rinderbreich H, Flesher A, Camarck C: Elastase activity, the role of elastase in aortic aneurysm formation. J Surg Res 1982, 32:214-217 [DOI] [PubMed] [Google Scholar]
- 3.Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB: Elastase-induced experimental aneurysms in rats. Circulation 1990, 82:973-981 [DOI] [PubMed] [Google Scholar]
- 4.Shireman P, McCarthy W, Pearce W, Shively V, Cipollone M, Kwaan H: Elevation of tissue-type plasminogen activator and differential expression of urokinase-type plasminogen activator in diseased aorta. J Vasc Surg 1997, 25:157-164 [DOI] [PubMed] [Google Scholar]
- 5.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW: Expression and localization of macrophage elastase (matrix metalloprotease-12) in abdominal aortic aneurysms. J Clin Invest 1998, 102:1900-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herron GS, Unemori E, Wong M, Rapp JH, Hibbs MH, Stoney RJ: Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb 1991, 11:1667-1677 [DOI] [PubMed] [Google Scholar]
- 7.Irizarry E, Newman KM, Gandhi RH, Nackman GB, Halpern V, Wishner S, Scholes JV, Tilson MD: Demonstration of interstitial collagenase in abdominal aortic aneurysm disease. J Surg Res 1993, 54:571-574 [DOI] [PubMed] [Google Scholar]
- 8.Vine N, Powell JT: Metalloproteinases in degenerative aortic disease. Clin Sci 1991, 81:233-239 [DOI] [PubMed] [Google Scholar]
- 9.Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapiere CM: Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J Vasc Surg 1996, 24:127-133 [DOI] [PubMed] [Google Scholar]
- 10.Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC: Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg 1997, 25:916-926 [DOI] [PubMed] [Google Scholar]
- 11.Yamazumi K, Ojiro M, Okumura H, Aikou T: An activated state of blood coagulation and fibrinolysis in patients with abdominal aortic aneurysm. Am J Surg 1998, 175:297-301 [DOI] [PubMed] [Google Scholar]
- 12.Lindholt JS, Jorgensen B, Fasting H, Henneberg EW: Plasma levels of plasmin-antiplasmin-complexes are predictive for small abdominal aortic aneurysms expanding to operation-recommendable sizes. J Vasc Surg 2001, 34:611-615 [DOI] [PubMed] [Google Scholar]
- 13.Siegel CL, Cohan RH, Korobkin M, Alpern MB, Courneya DL, Leder RA: Abdominal aortic aneurysm morphology: CT features in patients with ruptured and nonruptured aneurysms. Am J Roentgenol 1994, 163:1123-1129 [DOI] [PubMed] [Google Scholar]
- 14.Mtairag EM, Abdelghaffar H, Douhet C, Labro MT: Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob Agents Chemother 1995, 39:1676-1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jandrot-Perrus M, Lagrue AH, Okuma M, Bon C: Adhesion and activation of human platelets induced by convulxin involve glycoprotein VI and integrin alpha2beta1. J Biol Chem 1997, 272:27035-27041 [DOI] [PubMed] [Google Scholar]
- 16.Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP: Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol 2000, 192:105-112 [DOI] [PubMed] [Google Scholar]
- 17.Allaire E, Forough R, Clowes M, Starcher B, Clowes A: Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest 1998, 102:1413-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coller BS: A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest 1985, 76:101-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleury V, Angles-Cano E: Characterization of the binding of plasminogen to fibrin surfaces: the role of carboxy-terminal lysines. Biochemistry 1991, 30:7630-7638 [DOI] [PubMed] [Google Scholar]
- 20.Gaussem P, Grailhe P, Angles-Cano E: Sodium dodecyl sulfate-induced dissociation of complexes between human tissue plasminogen activator and its specific inhibitor. J Biol Chem 1993, 268:12150-12155 [PubMed] [Google Scholar]
- 21.Montes R, Paramo JA, Angles-Cano E, Rocha E: Development and clinical application of a new ELISA assay to determine plasmin-alpha2-antiplasmin complexes in plasma. Br J Haematol 1996, 92:979-985 [DOI] [PubMed] [Google Scholar]
- 22.Borregaard N, Cowland J: Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89:3505-3521 [PubMed] [Google Scholar]
- 23.Jung K, Nowak L, Lein M, Henke W, Schnorr D, Loening S: Role of specimen collection in preanalytical variation of metalloproteinases and their inhibitors in blood. Clin Chem 1996, 42:2043-2045 [PubMed] [Google Scholar]
- 24.Makowski GS, Ramsby ML: Binding of latent matrix metalloprotease 9 to fibrin: activation via a plasmin-dependent pathway. Inflammation 1998, 22:287-305 [DOI] [PubMed] [Google Scholar]
- 25.Blystone SD, Kaplan JE: The role of fibronectin in macrophage-fibrin binding: a potential mechanism for high affinity, high capacity clearance of circulating fibrin. Blood Coag Fibrinol 1993, 4:769-781 [PubMed] [Google Scholar]
- 26.Kuijper PHM, Torres HIG, Linden JAMVD, Lammers JWJ, Sixma JJ, Zwaginga JJ, Koenderman L: Neutrophil adhesion to fibrinogen and fibrin under flow conditions is diminished by activation and L-selectin shedding. Blood 1997, 89:2131-2138 [PubMed] [Google Scholar]
- 27.Lindsey M, Wedin K, Brown M, Keller C, Evans A, Smolen J, Burns A, Rossen R, Michael L, Entman M: Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase-9 in myocardial ischemia/reperfusion. Circulation 2001, 103:2181-2187 [DOI] [PubMed] [Google Scholar]
- 28.McMillan W, Pearce W: Increased plasma levels of metalloproteinase-9 are associated with abdominal aortic aneurysms. J Vasc Surg 1999, 29:122-127 [DOI] [PubMed] [Google Scholar]
- 29.Hoysepian D, Ziporin S, Sakurai M, Lee J, Curci J, Thompson R: Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: a circulating marker of degenerative aneurysm disease. J Vasc Interv Radiol 2000, 11:1345-1352 [DOI] [PubMed] [Google Scholar]
- 30.Sangiorgi G, D’Averio R, Mauriello A, Bondio M, Pontillo M, Castelvecchio S, Trimarchi S, Tolva V, Nano G, Rampoldi V, Spagnoli LG, Inglese L: Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation 2001, 104:I288-I295 [DOI] [PubMed] [Google Scholar]
- 31.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, Tilson MD: Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb 1994, 1:1315-1320 [DOI] [PubMed] [Google Scholar]
- 32.McMillan WD, Patterson BK, Keen RR, Shively VP, Cipollone M, Pearce WH: In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol 1995, 15:1139-1144 [DOI] [PubMed] [Google Scholar]
- 33.Pyo R, Lee J, Shipley J, Curci J, Mao D, Ziporin S, Ennis T, Shapiro S, Senior R, Thompson R: Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysm. J Clin Invest 2000, 105:1641-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazes I, Elalamy I, Sraer JD, Hatmi M, Nguyen G: Platelet release of trimolecular complex components MT1-MMP/TIMP2/MMP2: involvement in MMP2 activation and platelet aggregation. Blood 2000, 96:3064-3069 [PubMed] [Google Scholar]
- 35.Feldman LJ, Mazighi M, Scheuble A, Deux JF, De Benedetti E, Badier-Commander C, Brambilla E, Henin D, Steg PG, Jacob MP: Differential expression of matrix metalloproteinases after stent implantation and balloon angioplasty in the hypercholesterolemic rabbit. Circulation 2001, 103:3117-3122 [DOI] [PubMed] [Google Scholar]
- 36.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT: Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 1998, 18:1625-1633 [DOI] [PubMed] [Google Scholar]
- 37.Carrell TW, Burnand KG, Wells GM, Clements JM, Smith A: Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms. Circulation 2002, 105:477-482 [DOI] [PubMed] [Google Scholar]
- 38.Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, Humphries S: Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 1991, 88:8154-8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro S, Fliszar C, Broekelmann T, Mecham R, Senior R, Welgus H: Activation of 92-kDa gelatinase by stromelysin and 4-aminophenylmercuric acetate. Differential processing and stabilization of the carboxyl-terminal domain by tissue inhibitor of metalloproteinases (TIMP). J Biol Chem 1995, 270:6351-6356 [DOI] [PubMed] [Google Scholar]
- 40.Nagase H, Woessner J: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 41.Yu WH, Woessner JF: Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J Biol Chem 2000, 275:4183-4191 [DOI] [PubMed] [Google Scholar]
- 42.Gadher S, Eyre D, Wotton S, Schmid T, Wooley D: Degradation of cartilage collagens type II, IX, X, and XI by enzymes derived from human articular chondrocytes. Matrix 1990, 10:154-163 [DOI] [PubMed] [Google Scholar]
- 43.Silence J, Lupu F, Collen D, Lijnen H: Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol 2001, 21:1440-1445 [DOI] [PubMed] [Google Scholar]
- 44.Woessner J: Matrilysin. Methods Enzymol 1995, 248:485-495 [DOI] [PubMed] [Google Scholar]
- 45.Lindholt J, Heickendorff L, Vammen S, Fasting H, Henneberg E: Five-year results of elastin and collagen markers as predictive tools in the management of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001, 21:235-240 [DOI] [PubMed] [Google Scholar]
- 46.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D: Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 1997, 17:439-444 [DOI] [PubMed] [Google Scholar]
- 47.Wang YX, Martin-McNulty B, Freay AD, Sukovich DA, Halks-Miller M, Li WW, Vergona R, Sullivan ME, Morser J, Dole WP, Deng GG: Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol 2001, 159:1455-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knox JB, Sukhova GK, Whittemore AD, Libby P: Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 1997, 95:205-212 [DOI] [PubMed] [Google Scholar]