Abstract
Podocyte injury or podocyte loss in the renal glomerulus has been proposed as the crucial mechanism in the development of focal segmental glomerulosclerosis. However, it is poorly understood how podocytes respond to injury. In this study, glomerular expression of connexin43 (Cx43) gap junction protein was examined at both protein and transcript levels in an experimental model of podocyte injury, puromycin aminonucleoside (PAN) nephrosis. A striking increase in the number of immunoreactive dots with anti-Cx43 antibody was demonstrated along the glomerular capillary wall in the early to nephrotic stage of PAN nephrosis. The conspicuous change was not detected in the other areas including the mesangium and Bowman’s capsule. Immunoelectron microscopy showed that the immunogold particles for Cx43 along the capillary wall were localized predominantly at the cell-cell contact sites of podocytes. Consistently, Western blotting and ribonuclease protection assay revealed a distinct increase of Cx43 protein, phosphorylation, and transcript in glomeruli during PAN nephrosis. The changes were detected by 6 hours after PAN injection. These findings indicate that the increase of Cx43 expression is one of the earliest responses that have ever been reported in podocyte injury. To show the presence of functional gap junctional intercellular communication (GJIC) in podocytes, GJIC was assessed in podocytes in the primary culture by transfer of fluorescent dye, Lucifer yellow, after a single-cell microinjection. Diffusion of the dye into adjacent cells was observed frequently in the cultured podocytes, but scarcely in cultured parietal epithelial cells of Bowman’s capsule, which was compatible with their Cx43 staining. Thus, it is concluded that Cx43-mediated GJIC is present between podocytes, suggesting that podocytes may respond to injury as an integrated epithelium on a glomerulus rather than individually as a separate cell.
Visceral epithelial cells of renal glomeruli, referred to as podocytes, are a highly specialized epithelium structurally adapted to facilitate bulk flow of the glomerular filtrate through the intercellular spaces or filtration slits. They are situated on the glomerular basement membrane as the terminal element in the ultrafiltration barrier. In adult rats, these cells rarely undergo cell division even after subtotal nephrectomy. 1-3 Regardless of the nature of the initial insult, once podocytes are injured and lost, they cannot be replaced by new replicated cells. A considerable number of clinical and experimental studies have presented evidence that podocyte injury is the starting point to focal segmental glomerulosclerosis and eventual glomerular tuft destruction, which are common histological findings in the progression of chronic renal diseases. 4 Thus, podocytes are one of the critical components to maintain the glomerular structure.
Understanding the behavior of podocytes in response to injury is indispensable to elucidate the mechanism leading to the glomerular diseases. Podocytes exhibit common morphological changes in glomerular diseases caused by direct intoxication, hemodynamic stress, inflammation, or immune complex deposition. 5,6 Their fine interdigitated foot processes are retracted and flattened. The main intercellular junctions, slit diaphragms, are extensively dislocated and rearranged, whereas typical tight junctions are frequently formed in lieu of the slit diaphragms. The molecular bases underlying the morphological alterations are coming to light by the discovery of many novel proteins and the characterization of their physical and functional interactions. 7 However, it remains entirely unknown how podocytes interact with adjacent cells under pathological conditions, and which molecules mediate their interactions.
Puromycin aminonucleoside (PAN) nephrosis is widely used as a model of nephrotic syndrome progressing to focal segmental glomerulosclerosis. 8,9 Although the pathogenesis of proteinuria is not clearly explained, it has been reported that oxygen radicals, which are produced during the metabolism of PAN, cause podocyte injury, resulting in nephrotic syndrome. 10,11 In recent immunofluorescence staining studies, we have discovered a striking change of a gap junctional protein, connexin43, in podocytes in PAN nephrosis. Gap junctions mediate cell-to-cell communication in various tissues. 12-15 They contain channels that connect neighboring cells, allowing the movement of molecules smaller than 1000 d such as ions, nutrients, metabolites, and second messengers. This type of intercellular communication permits coordinated cellular activity and has been implicated in diverse biological processes, such as development, cellular metabolism, and cellular growth control. Gap junctions are created across the intervening extracellular space by docking of two hemichannels contributed by each adjacent cell. Each hemichannel is an oligomer of six connexin (Cx) molecules. These proteins are encoded by a multigene family and are named according to their predicted molecular weight. Nine connexins have been known to be expressed in the kidney namely, Cx26, Cx30.3, Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, and Cx46. 15 However, knowledge about their localization in the renal tissues is very limited, although some of them have been shown immunohistochemically in tubules, glomeruli, in the juxtaglomerular apparatus, and in the vasculature. 16-20 Among them, Cx43 is expressed most abundantly by a variety of cell types. 14,15
The crucial functions of gap junctions for tissue homeostasis led us to hypothesize that Cx43 may play an important role in the integration of podocyte function of maintaining glomerular filtration and in coordinated responses of podocytes to injury. In the present study, we examined the expression of glomerular Cx43 during development of PAN nephrosis in an attempt to test our hypothesis, and confirmed the presence of gap junctional intercellular communication (GJIC) by using podocytes in the primary culture.
Materials and Methods
Animals and Induction of PAN Nephrosis
Female WKY rats were purchased from Charles River Japan (Ataugi, Japan) and used in these experiments at the ages of 8 to 12 weeks. PAN nephrosis was induced by a single intravenous injection of PAN (Sigma, St. Louis, MO) at a dose of 5 mg/100 g of body weight in phosphate-buffered saline (PBS). Control animals received an identical volume of PBS. The rats were housed in individual metabolic cages and their 24-hour urine specimens were collected before injection and 2, 4, 6, and 10 days after injection of PAN. Rats were sacrificed under ether anesthesia 6 hours, and 2, 4, 6, and 10 days after PAN injection, and kidneys were removed and processed for immunohistochemical analysis, Western blotting, or ribonuclease protection assay. Glomeruli isolated from four or six kidneys at each time point were mixed and used as one sample of glomerular protein or RNA.
Immunofluorescence Microscopy
The indirect immunofluorescence technique was applied to frozen kidney sections and outgrowths from glomeruli as described previously. 21 In brief, the rat kidneys were snap-frozen at −70°C, sectioned at a thickness of 3 μm in a cryostat, fixed in 2% paraformaldehyde in PBS for 5 minutes, and processed for double-label immunostaining. Outgrowths from explants cultured on eight-well glass chamber slides were fixed in methanol for 5 minutes, or fixed in 2% paraformaldehyde in PBS for 5 minutes, permeabilized with 0.3% Triton X-100 in PBS for 3 minutes, and stained with antibodies. For double-label immunofluorescence microscopy, rabbit anti-Cx43 antibody (Sigma) and murine monoclonal antibody against ZO-1 (Zymed Laboratories, South San Francisco, CA) were mixed and applied as primary antibodies simultaneously. After washing with PBS, the sections were stained with fluorescein isothiocyanate-conjugated anti-rabbit IgG, rewashed with PBS, and subsequently reacted with tetramethylrhodamine isothiocyanate-conjugated anti-mouse IgG. PBS, normal rabbit serum, or murine IgG1 monoclonal antibody (against rotavirus), shown not to react with rat glomeruli, were used as negative controls for the primary antibodies. Immunofluorescence of the sections and cultured cells were observed with an Olympus microscope (BX50) equipped with epi-illumination optics and appropriate filters, or with a laser-scanning confocal microscope (MRC-1024; Bio-Rad Laboratories, Hercules, CA).
Immunoelectron Microscopy
Immunoelectron microscopic observations of kidneys from rats with PAN nephrosis and control rats were performed as reported previously. 9,22 In brief, 1-mm3 tissue blocks from paraformaldehyde-lysine-periodate-perfused kidneys were placed in the PLP fixative for 4 hours at 4°C, hydrated, and then embedded in hydrophilic methacrylate resin. The ultrathin sections collected on nickel grids were stained with the immunogold technique.
Western Blotting
Glomeruli were isolated from renal cortices by a sieving method. The isolated glomeruli and renal cortices were homogenized in lysis buffer (8 mol/L urea, 1 mmol/L dithiothreitol, 1 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L Tris-HCl, pH 8.0) on ice. The homogenates were centrifuged at 20,000 × g to remove insoluble debris. The protein in samples was quantified by Lowry’s method, as modified by Peterson, 23 after precipitation by trichloroacetate with sodium deoxycholate. Western blotting was performed as described previously, 24 except that aliquots (20 μg proteins) of the supernatants were separated by electrophoresis on a 15% sodium dodecyl sulfate-polyacrylamide gel, and that detection of immunoactivity was performed with the enhanced chemiluminescence plus Western blotting detection system (Amersham Pharmacia Biotech). Densitometric analysis was performed with NIH image software (version 1.62), a public domain image-processing and analysis program, according to the instruction manual provided on-line by the National Institutes of Health (Bethesda, MD).
For the treatment of the samples with alkaline phosphatase, glomeruli were isolated from nephrotic rats 9 days after PAN injection, and sonicated for 10 seconds three times on ice in buffer containing 100 mmol/L Tris-HCl, pH 8.0, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin. The sonicated samples were centrifuged for 5 minutes at 200 × g at 4°C to remove debris. Supernatants were collected and centrifuged for 30 minutes at 19,000 × g at 4°C. Preliminary experiments showed that the major part of Cx43 was present in the pellets. Aliquots of the pellet (70 μg total protein) were solubilized in 50 μl of the above buffer containing 1% sodium dodecyl sulfate, incubated with or without 30 U alkaline phosphatase (calf intestine phosphatase; Boehringer Mannheim, Mannheim, Germany) for 4 hours at 37°C, and then subjected to Western blot analysis.
Ribonuclease Protection Assay
Ribonuclease protection assay was performed as described previously. 25,26 Isolated glomeruli were homogenized in TRIzol (Life Technologies, Inc., Grand Island, NY) with a sonicator, and total cellular RNA was extracted from these samples. Plasmids containing the cDNA corresponding to 498 to 1085 (588 bp) of rat Cx43 (X06656) were amplified by polymerase chain reaction, 27 inserted in pGEM-T Easy vector (Promega, Madison, WI), cloned, and sequenced by a DNA sequencer (ABI PRISM 310; Applied Biosystems Japan, Tokyo, Japan). Detection and analysis of bands were performed by phosphor-imaging techniques using the Molecular Imager FX (Bio-Rad Laboratories, Hercules, CA). The results of ribonuclease protection assay were represented as the ratio of Cx43 to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene.
Primary Culture of Podocytes and Parietal Epithelial Cells of Bowman’s Capsule
Glomeruli were isolated from rat kidneys by the gentle method as described previously. 21 Decapsulated glomeruli or encapsulated glomeruli were selected under an inverted tissue culture microscope with phase-contrast optics, and were cultured on type I collagen-coated culture dishes in Dulbecco’s modified Eagle’s medium nutrient mixture F-12 HAM (Sigma), supplemented with Insulin-transferrin-selenium liquid media supplement (Sigma), 5% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cell outgrowths from decapsulated or encapsulated glomeruli were used as cultured podocytes or parietal epithelial cells (PECs) of Bowman’s capsule, respectively.
Measurement of GJIC
GJIC was assessed by transfer of the membrane-impermeant fluorescent dye, Lucifer yellow, after a single-cell microinjection with an automated microinjection system as described previously. 28
Statistics
All data are expressed as means ± SD, and are considered statistically significant for P < 0.05. For comparison between control animals and PAN-treated animals, the unpaired t-test or the Mann-Whitney U-test was used as appropriate.
Results
Urinary Protein in PAN Nephrosis
Measurement of 24-hour urinary protein levels revealed that a single intravenous injection of PAN induced massive proteinuria at day 6 (142.8 ± 49.6 mg/day, n = 12) and day 10 (223.9 ± 86.2 mg/day, n = 19) after the injection. No or a slight increase in urinary protein was detected at day 2 (2.0 ± 0.5 mg/day, n = 20) or day 4 (3.7 ± 1.7 mg/day, n = 37) in comparison with control rats (1.7 ± 0.7 mg/day, n = 77).
Immunolocalization of Cx43 in the Rat Glomerulus
Cx43 distribution in the glomerulus was examined by double-label immunofluorescence microscopy using rabbit anti-Cx43 antibody in combination with murine monoclonal anti-ZO-1 antibody (Figure 1) ▶ . Because the tight junction protein ZO-1 is concentrated in the intercellular junctions of podocytes under both physiological and pathological conditions, 29,30 ZO-1 staining was used to locate the glomerular capillary wall. In the normal kidney, immunofluorescent dots for Cx43 were observed mainly in the extraglomerular mesangium and the neighboring intraglomerular mesangium in accord with previous studies (Figure 1, a and a’) ▶ . 16,17,27 Sparse but significant dots were also detected within glomeruli. Most of them were located along the glomerular capillary wall. By day 2 of PAN nephrosis, Cx43 dots along the capillary wall increased strikingly in number (Figure 1, b and b’) ▶ . The increase was significant but not so distinct 1 day after PAN injection. At day 4, numerous dots were crowded along the capillary wall, which exceeded the extraglomerular mesangium in the intensity of the fluorescent signal (Figure 1, c and c’) ▶ . At day 10, each fluorescent dot became bigger and brighter, but the dots appeared to decrease in number in comparison with those at day 4 (Figure 1, d and d’) ▶ . Such dramatic changes were not observed in the mesangium. In addition, PECs of Bowman’s capsule and proximal tubular cells scarcely exhibited Cx43 immunofluorescence in the course of PAN nephrosis.
Figure 1.

Double-labeled immunofluorescence photomicrographs of frozen sections of rat kidneys incubated with antibodies against Cx43 and ZO-1. Rabbit anti-Cx43 antiserum was detected with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG; mouse monoclonal anti-ZO-1 antibody was detected with tetramethylrhodamine isothiocyanate-conjugated goat anti-mouse IgG. a to d show only Cx43 staining, and a’ to d’ are merged images for Cx43 and ZO-1. a and a’ show a glomerulus of a normal rat kidney. b and b’, c and c’, and d and d’ are glomeruli of PAN-treated rats at day 2, day 4, and day 10, respectively. In the normal glomerulus, punctate Cx43 immunofluorescence is prominent in the extraglomerular mesangium (arrows), and is sparse along the glomerular capillary wall, which is indicated by ZO-1 staining (a and a’). Note that signals for Cx43 increase dramatically along the glomerular capillary wall in the time course of PAN nephrosis (b–d and b’–d’). Extraglomerular mesangium (arrows) does not show significant changes in Cx43 staining. PECs scarcely exhibit Cx43 immunofluorescence (small arrows). Scale bars, 20 μm.
The precise localization of Cx43 molecules in the glomerular capillary wall was examined by immunoelectron microscopy. The glomerular capillary wall is comprised of three layers: a fenestrated endothelium, an interposed glomerular basement membrane, and the slit pore formed at the intercellular junction of interdigitated foot processes of podocytes. In the normal kidney, plasma membranes of adjacent podocytes are widely spaced at the slit pore, and are bridged by the slit diaphragm. On occasion, this wide space appears completely obliterated with focal regions of contact between apposing plasma membranes in or near the plane of the slit diaphragm. 31 Significant accumulation of immunogold particles for Cx43 was restricted to the focal regions of contact (Figure 2, a and b) ▶ . There were no significant gold particles in the slit diaphragm. Cx43 labeling in glomerular endothelial cells was very rare in comparison with podocytes. Foot processes are retarded and flattened in PAN nephrosis. In parallel, the extracellular space at the level of the slit pore disappears and close intercellular junctions including typical tight junctions are newly formed in podocytes. 32,33 Immunogold particles for Cx43 were frequently found in the close intercellular junctions (Figure 2, c and d) ▶ . Splendid decoration of such junctions with the gold particles was occasionally observed at day 10 of PAN nephrosis (Figure 2e) ▶ . Significant increase in Cx43 gold particles was not detected in the intercellular junctions of endothelial cells or mesangial cells; no gold labeling was found in those of PECs (Figure 2f) ▶ .
Figure 2.

Immunogold localization of Cx43 in the glomerular capillary wall. a and b: Vertical and tangential sections of the glomerular basement membrane (asterisks) in the normal kidney, respectively. Cx43 immunogold particles (arrows) are localized at cell-cell contact sites of podocytes but not in slit diaphragms. At days 2 (c), 4 (d), and 10 (e) of PAN nephrosis, accumulation of gold particles is frequently observed at close cell-cell contact sites of podocytes. No labeling for Cx43 is seen in the intercellular junction of PECs (arrowhead in f). Scale bar, 0.5 μm.
Cx43 Protein in PAN Nephrosis
Western blotting was performed to quantify Cx43 protein in the glomerulus and to examine its electrophoretic mobility, because Cx43 resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis has been reported to migrate as a faster, nonphosphorylated form and slower, phosphorylated isoforms. 12,34,35 At least three bands were seen in all samples on Western blotting. The band with the highest electrophoretic mobility was dominant in the normal glomerulus (Figure 3a ▶ , lane 1). Glomerular Cx43 changed distinctly at day 10 of PAN nephrosis. Signals of the fastest band decreased and those of the slowest one were conspicuously enhanced (Figure 3a ▶ , lane 2). Densitometric analysis revealed a 3.3-fold increase of total Cx43 protein (884 ± 201 in five samples of nephrotic rats versus 270 ± 55 in five samples of normal rats, P < 0.05), and a 27.7-fold increase of the ratio of the slowest band to the fastest one (6.64 ± 2.65 in the nephrotic samples versus 0.24 ± 0.12 in the normal samples, P < 0.05). Cx43 in the cortex did not show significant changes (Figure 3a ▶ , lanes 3 and 4). Alkaline phosphatase treatment increased the density of the fastest band of the protein at the expense of the other slower bands that were totally eliminated, indicating that the slower bands are attributable to phosphorylated products of the fastest nonphosphorylated form (Figure 3b) ▶ . The shift of the dominant band from the fastest to the slowest in glomerular Cx43 occurred steadily in the time course of PAN nephrosis, and had already begun at day 2 (Figure 3c ▶ , lanes 1 to 5). We examined glomeruli isolated at 6 hours after PAN injection, and found that the signals of the slowest bands increased significantly (3.6-fold) in comparison with the normal control (P < 0.05), although those of the fastest did not show any significant difference (Figure 3c ▶ , lanes 5 and 6).
Figure 3.
Immunoblot analysis of Cx43 in glomeruli and cortices separated from untreated and PAN-treated rat kidneys. a: Representative immunoblot comparing untreated control rats (lanes 1 and 3) and nephrotic ones at day 10 of PAN nephrosis (lanes 2 and 4). Cx43 in glomeruli (lanes 1 and 2) and cortices (lanes 3 and 4) migrates as three bands. The fastest band is dominant in the normal glomeruli, and the slowest is extremely enhanced in the nephrotic rats. Cx43 in the cortices does not show significant change in the nephrotic rats. b: Alkaline phosphatase treatment of protein samples from the nephrotic glomeruli. The slower bands of Cx43 are prominent before the treatment (lane 1) or after the incubation without phosphatase (lane 2). Alkaline phosphatase treatment collapsed these bands into the fastest one (lane 3). c: Immunoblot showing Cx43 changes in isolated glomeruli from the control (lane 1), at days 2 (lane 2), 4 (lane 3), 6 (lane 4), and 10 (lane 5) of PAN nephrosis, and from the control (lane 6), and at 6 hours after PAN injection (lane 7). The increase of the slowest band is detected from 6 hours after PAN injection. Twenty μg of total protein from isolated glomeruli or cortices was separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and blotted with anti-Cx43 antibody.
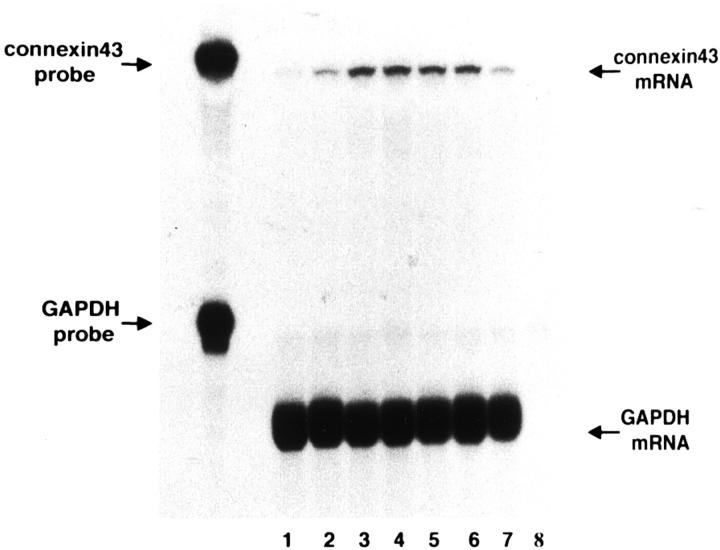
Cx43 mRNA in PAN Nephrosis
An analysis of Cx43 transcripts in glomeruli during different stages of PAN nephrosis is shown in Figure 4 ▶ . Ribonuclease protection assay detected a prompt increase of Cx43 transcripts after PAN injection. Relative to the transcript level (the ratio of Cx43 to GAPDH) in normal glomeruli, which was defined as “1,” the transcript abundance rose to 1.4 after 6 hours, 2.8 at day 1, 3.0 at day 2, and 2.9 at day 4, and, thereafter, turned to decline to 2.4 at day 6 and 1.6 at day 10.
Figure 4.
Expression of Cx43 mRNA in glomeruli in PAN nephrosis detected by ribonuclease protection assay. Total RNA samples (5 μg each) were obtained from isolated glomeruli during different stages of PAN nephrosis. Lane 1, normal control; lane 2, 6 hours after PAN injection; lane 3, day 1; lane 4, day 2; lane 5, day 4; lane 6, day 6; lane 7, day 10; lane 8, tRNA as negative control. Increase of Cx43 mRNA is seen in the very early stages such as 6 hours and 1 day after PAN injection. Abbreviation: GAPDH, glycealdehyde-3-phosphate dehydrogenase.
GJIC in Cultured Podocytes
GJIC was assessed in podocytes in the primary culture. Cells growing out from glomeruli devoid of Bowman’s capsule were used as cultured podocytes (Figure 5a) ▶ . They share more characteristics with in vivo podocytes under the pathological condition than ones under the physiological condition, because they lose foot processes and show intense staining for desmin. 21,36,37 Cobblestone-like polygonal cells from encapsulated glomeruli are regarded as PECs because of their phenotypic similarities to the in vivo counterpart. 21,36,38 They were used as a control in this experiment (Figure 5b) ▶ .
Figure 5.

GJIC of podocytes and PECs in culture. Cells growing out from decapsulated and encapsulated glomeruli were used as cultured podocytes (a, c, e, e’) and PECs (b, d, f, f’), respectively. a and b: Phase-contrast micrographs showing the unique morphology of outgrowing podocytes (a) and PECs (b) after 5 days of culture. c and d: Confocal immunofluorescence microscopy using anti-Cx43 antibody detecting punctate Cx43 staining at the cell-cell contact sites of podocytes (c) and no or feeble staining at those of PECs (d). e, e’, f, f’: Lucifer yellow diffusion from a microinjected podocyte (arrows in e and e’) and PEC (arrows in f and f’). After Lucifer yellow was pressure injected into a single cell, the dye diffusion into adjacent cells was monitored throughout a 3-minute time period. e and f: Phase-contrast micrographs. e’ and f’: Lucifer yellow fluorescence. At least three cells adjacent to the microinjected podocytes are positive for the fluorescence (e’), whereas no fluorescent cells are observed around the microinjected PECs (f’). Asterisks, remaining glomeruli. Scale bars, 100 μm.
Consistent with the in vivo findings, podocytes showed a pattern of discrete punctate immunoreactivity for Cx43 at cell-cell interfaces (Figure 5c) ▶ . In contrast, PECs were not stained with the antibody except for occasional faint but significant labeling at points of intercellular contact (Figure 5d) ▶ .
To examine the presence of GJIC, the transfer of Lucifer yellow was studied. Microinjection of the fluorescent dye into a single cell yielded apparent transfer of the dye between podocytes (Figure 5, e and e’) ▶ . There was a significant difference in podocytes and PECs in the rates of the dye transfer (P < 0.0001) (Figure 5, f and f’) ▶ . The number of dye-transferred cells per microinjected cell was 5.17 ± 2.82 in podocytes (n = 12), and 0.27 ± 0.64 in PECs (n = 11).
Discussion
In this study, the distribution and expression of Cx43 was examined in the rat kidney in PAN nephrosis. Several relevant observations resulted from this analysis. First, Cx43 was observed at the cell-cell contact sites of podocytes under the physiological and pathological conditions. Second, Cx43 expression changed dramatically in PAN nephrosis, which was specific to podocytes. Third, the Cx43 changes in PAN nephrosis occurred in a short time after PAN injection. Finally, GJIC was present between cultured podocytes that showed distinct anti-Cx43 labeling.
The localization of Cx43 in the kidney has been studied the best in the rat among mammals. 16,17,27,39,40 Abundant punctate immunofluorescence for Cx43 is observed in collecting ducts 16,17,27 and renal arterial and arteriolar vasculature including the extraglomerular mesangium. 17 Only some immunofluorescent dots for Cx43 have been reported within rat glomeruli. 17,27,39 Podocytes lose the typical intercellular junctions of epithelial cells such as adherens junctions and desmosomes in the development, which is compatible with their highly differentiated phenotypes adapted to the glomerular filtration. 41-43 Likewise, gap junctions have not been found in podocytes under the normal condition, although the presence of gap junctions has been suggested by the freeze-fracture technique in nephrotic rats 32,33 and in kidneys perfused with the polycation protamine sulfate. 44
Morphologically, intercellular junctions of podocytes are divided into at least two types: slit diaphragms and junctions with close contact. 31 Immunoelectron microscopy in this study revealed that Cx43 was restricted in the latter junctions. The former are major junctions in normal podocytes, and their molecular composition and roles in the glomerular filtration have been elucidated through the studies of congenital nephrotic syndrome. 7,45 In contrast, the latter junctions remain obscure in their functions and components. The close junctions are rarely encountered in the normal glomerulus, but increase under nephrotic conditions or in protamine sulfate-perfused kidneys where slit diaphragms are dislocated or disappear. 32,33,44 ZO-1, a protein normally associated with tight junctions, is only one component reported in the junctions. 30 The freeze-fracture studies have shown that they consist of assembly of globular intramembranous particles to form strands indicating tight junctions. 32,33,44 Interestingly, all of the studies have revealed clusters of tightly packed particles in close association with the tight junctions, which are reminiscent of gap junctions. Based on the immunohistological findings for Cx43, it is proper to assume that the latter junctions contain Cx43-positive gap junctions not only under the pathological conditions but also under the physiological condition.
Cx43 in glomeruli increased strikingly at both the protein and mRNA levels in PAN nephrosis, which is consistent with an important role for transcriptional regulation in the synthesis of Cx43 gap junctions. The changes occurred mainly in podocytes as shown by the immunolocalization study. We did not detect significant changes in the mesangium or endothelial cells. PAN administration also induces vacuolation and detachment from the basement membrane in PECs, which is similar to the cytotoxicity observed in podocytes, 46 but PECs did not show any Cx43 staining during PAN nephrosis. The up-regulation of Cx43, therefore, is specific to podocytes. We have examined the other experimental models including anti-glomerular basement membrane-type and circulating immune complex-type nephritis by immunostaining using anti-Cx43 antibody. All of the models showed conspicuous Cx43 staining along the glomerular capillary wall (unpublished observation). Thus, it is likely that Cx43 up-regulation is one of common responses of podocytes to injury, but not a specific response to PAN administration.
The behavior of podocytes under the pathological condition has been investigated by using rats with PAN nephrosis. In addition to the morphological changes of foot processes and slit diaphragms, the up-regulation of various proteins in podocytes has been noted, eg, α-actinin, 47 heparin-binding epidermal growth factor-like growth factor, 48 desmin, 37 and HSP27. 49 Among them, desmin staining has been used frequently as a marker of podocyte injury because of its notable changes. 50,51 In comparison with desmin, the changes of Cx43 occurred in the very early stage of PAN nephrosis. Significant increase of fluorescent dots for Cx43 was detected by day 2, whereas enhanced staining for desmin is recognizable after day 7. 37 Western blotting and ribonuclease protection assay demonstrated that Cx43 changes at the protein and mRNA levels occurred by 6 hours after PAN injection. Moreover, it has been reported that Cx43 has a half-time in the range of 1 to 3 hours, much faster than average turnover times for integral membrane proteins. 35 These findings indicate that Cx43 is one of the earliest markers appearing in response to podocyte injury.
One of the notable findings in this study is the shift of the dominant band of Cx43 from the fastest to the slowest in the time course of PAN nephrosis as revealed by Western blotting, indicating the remarkable enhancement of Cx43 phosphorylation. In PAN nephrosis, foot processes are retracted, tight junctions are formed, and massive proteinuria appears. All of the structural changes and the disruption of the glomerular filtration barrier are closely correlated with tyrosine phosphorylation of proteins in podocytes. 52-55 Cx43 phosphorylation seems involved in the dynamic regulation of tyrosine phosphorylation, although phosphorylation of Cx43 occurs not only on tyrosine but also on serine residues. Phosphorylation is an important mechanism regulating gating properties of gap junction channels, but the effects have been reported in both a positive and negative manner. 35,56 It remains to be determined whether the enhanced phosphorylation of Cx43 results in an increase of GJIC in podocytes.
The presence of connexin molecules is not necessarily indicative of GJIC, because GJIC is dependent on several factors including extracellular calcium and cell adhesion molecules in addition to phosphorylation. 35 To evaluate the GJIC in podocytes, we examined the primary culture of podocytes outgrowing from decapsulated glomeruli. 21 The cultured podocytes retain in vivo differentiated phenotypes such as intense staining for podocalyxin, synaptopodin, and WT-1. On the grounds of their distinct desmin staining, disappearance of foot processes and loss of reactivity with monoclonal antibody 5-1-6, their characters are very similar to those under the pathological condition rather than under the normal condition. 21 The transfer assay using Lucifer yellow clearly demonstrated frequent GJIC of podocytes in culture. The result strongly suggests GJIC of podocytes in vivo especially under the pathological condition. In contrast, polygonal cells from encapsulated glomeruli showed no or scarce GJIC. The functional property denotes the difference of the polygonal cells from podocytes. Besides, polygonal cells were scarcely labeled with anti-Cx43 antibody. These findings corroborate the notion that they originate from PECs, which remains controversial. 57
GJIC has been demonstrated in vitro to have opposite effects on cell injury. Exposure of cells to irradiation or treatment with ganciclovir of herpes simplex virus thymidine kinase-transduced cells induces injury or death in not only the irradiated cells or the herpes simplex virus thymidine kinase-transduced cells but also adjacent nonirradiated cells or nontransduced cells. 58,59 These adjacent normal cells falling victim have been termed “bystander cells.” Substantial evidence indicates that the bystander effect is mediated chiefly by GJIC. Oppositely, it has been shown that the bystander cells, particularly those that are capable of GJIC, can protect herpes simplex virus thymidine kinase-transduced cells from ganciclovir-induced cytotoxicity. 59 These studies indicate that GJIC mediates mutual cell injury or rescue by coupling cells. Based on the same notion, podocytes are likely to behave themselves interacting with each other via GJIC under pathological conditions. They may respond to injury as a whole epithelium on a glomerulus rather than an individual cell. Whether the effect of GJIC ameliorates or deteriorates PAN nephrosis is unanswered now. However, there are some reports that facilitation of GJIC inhibits epithelial injury caused by free radicals, eg, alveolar epithelial injury in exposure to nitrogen dioxide and ischemia-reperfusion injury of gastric mucosa. 60,61 It is fascinating to speculate that GJIC may contribute to decreased susceptibility to injury and increased podocyte survival.
Acknowledgments
We thank Dr. H. Pavenstädt and Dr. R. Nitschke (Freiburg) for helpful discussion.
Footnotes
Address reprint requests to Eishin Yaoita, Department of Structural Pathology, Institute of Nephrology, Graduate School of Medical and Dental Sciences, Niigata University, Asahimachi-dori 1, Niigata, 951-8510, Japan. E-mail: ren-path@med.niigata-u.ac.jp.
Supported by a Grant-in-Aid for Scientific Research (C) from the Japanese Ministry for Education, Science, and Culture (no. 14571017).
References
- 1.Pabst R, Sterzel RB: Cell renewal of glomerular cell types in normal rats: an autoradiographic analysis. Kidney Int 1983, 24:626-631 [DOI] [PubMed] [Google Scholar]
- 2.Rasch R, Nørgaard JOR: Renal enlargement: comparative autographic studies of [3H]thymidine uptake in diabetic and uninephrectomized rats. Diabetologia 1983, 25:280-287 [DOI] [PubMed] [Google Scholar]
- 3.Fries JWU, Sandstrom DJ, Meyer TW, Rennke HG: Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab Invest 1989, 60:205-218 [PubMed] [Google Scholar]
- 4.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998, 54:687-697 [DOI] [PubMed] [Google Scholar]
- 5.Farquhar MG, Vernier RL, Good RA: An electron microscopic study of the glomerulus in nephrosis, glomerulonephritis and lupus erythematosus. J Exp Med 1957, 106:649-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerjaschki D: Dysfunctions of cell biological mechanisms of visceral epithelial cell (podocytes) in glomerular diseases. Kidney Int 1994, 45:300-313 [DOI] [PubMed] [Google Scholar]
- 7.Kerjaschki D: Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001, 108:1583-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond JR, Karnovsky MJ: Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol 1986, 122:481-487 [PMC free article] [PubMed] [Google Scholar]
- 9.Kihara I, Yaoita E, Kawasaki K, Yamamoto T: Limitation of podocyte adaptation for glomerular injury in puromycin aminonucleoside nephrosis. Pathol Int 1995, 45:625-634 [DOI] [PubMed] [Google Scholar]
- 10.Diamond JR, Bonventre JV, Karnovsky MJ: A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int 1986, 29:478-483 [DOI] [PubMed] [Google Scholar]
- 11.Nosaka K, Takahashi T, Nishi T, Imaki H, Suzuki T, Suzuki K, Kurosawa K, Endou H: An adenosine deaminase inhibitor prevents puromycin aminonucleoside nephrotoxicity. Free Radic Biol Med 1997, 22:597-605 [DOI] [PubMed] [Google Scholar]
- 12.Beyer E: Gap junctions. Int Rev Cytol 1993, 137:1-37 [PubMed] [Google Scholar]
- 13.Kumar NM, Gilula NB: The gap junctional communication channel. Cell 1996, 84:381-388 [DOI] [PubMed] [Google Scholar]
- 14.Goodenough DA, Goliger JA, Paul DL: Connexins, connexons, and intercellular communication. Annu Rev Biochem 1996, 65:475-502 [DOI] [PubMed] [Google Scholar]
- 15.White TW, Bruzzone R, Paul DL: The connexin family of intercellular channel forming proteins. Kidney Int 1995, 48:1148-1157 [DOI] [PubMed] [Google Scholar]
- 16.Beyer EC, Kistler J, Paul DL, Goodenough DA: Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 1989, 108:595-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barajas L, Liu L, Tucker M: Localization of connexin43 in the rat kidney. Kidney Int 1994, 46:621-626 [DOI] [PubMed] [Google Scholar]
- 18.Butterweck A, Gergs U, Elfgang C, Willecke K, Traub O: Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membr Biol 1994, 141:247-256 [DOI] [PubMed] [Google Scholar]
- 19.Hillis GS, Duthie LA, Mlynski R, McKay NG, Mistry S, MacLeod AM, Simpson JG, Haites NE: The expression of connexin 43 in human kidney and cultured renal cells. Nephron 1997, 75:458-463 [DOI] [PubMed] [Google Scholar]
- 20.Seul K, Beyer EC: Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res 2000, 59:140-148 [DOI] [PubMed] [Google Scholar]
- 21.Yaoita E, Kurihara H, Sakai T, Ohshiro K, Yamamoto T: Phenotypic modulation of parietal epithelial cells of Bowman’s capsule in culture. Cell Tissue Res 2001, 304:339-349 [DOI] [PubMed] [Google Scholar]
- 22.Kamiie J, Nameta M, Ma M, Takata T, Fujinaka H, Yoshida Y, Yaoita E, Yamamoto T: Localization and expression of the aquaporin-1 water channel in mesangial cells in the human glomerulus. Arch Histol Cytol 2002, 65:83-90 [DOI] [PubMed] [Google Scholar]
- 23.Peterson GL: A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 1977, 83:346-356 [DOI] [PubMed] [Google Scholar]
- 24.Funaki H, Yamamoto T, Koyama Y, Kondo D, Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H, Kihara I: Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol 1998, 275:C1151-C1157 [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Fujinaka H, Marumo F, Kihara I: Expression of AQP family in rat kidneys during development and maturation. Am J Physiol 1997, 272:F198-F204 [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Yamamoto T: FAT is a component of glomerular slit diaphragms. Kidney Int 2001, 59:1003-1012 [DOI] [PubMed] [Google Scholar]
- 27.Guo R, Liu L, Barajas L: RT-PCR study of the distribution of connexin 43 mRNA in the glomerulus and renal tubular segments. Am J Physiol 1998, 275:R439-R447 [DOI] [PubMed] [Google Scholar]
- 28.Yao J, Morioka T, Oite T: PDGF regulates gap junction communication and connexin43 phosphorylation by PI 3-kinase in mesangial cells. Kidney Int 2000, 57:1915-1926 [DOI] [PubMed] [Google Scholar]
- 29.Schnabel E, Anderson JM, Farquhar MG: The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990, 111:1255-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 1992, 141:805-816 [PMC free article] [PubMed] [Google Scholar]
- 31.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974, 60:423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pricam C, Humbert F, Perrelet A, Amherdt M, Orci L: Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest 1975, 33:209-218 [PubMed] [Google Scholar]
- 33.Caulfield JP, Reid JJ, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 1976, 34:43-59 [PubMed] [Google Scholar]
- 34.Musil LS, Goodenough DA: Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 1991, 115:1357-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampe PD, Lau AF: Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 2000, 384:205-215 [DOI] [PubMed] [Google Scholar]
- 36.Yaoita E, Yamamoto Y, Takashima N, Kawasaki K, Kawachi H, Shimizu F, Kihara I: Visceral epithelial cells in rat glomerular cell culture. Eur J Cell Biol 1995, 67:136-144 [PubMed] [Google Scholar]
- 37.Yaoita E, Kawasaki K, Yamamoto T, Kihara I: Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol 1990, 136:899-908 [PMC free article] [PubMed] [Google Scholar]
- 38.Yaoita E, Yamamoto Y, Saito M, Kawasaki K, Kihara I: Desmin-positive epithelial cells outgrowing from rat encapsulated glomeruli. Eur J Cell Biol 1991, 54:140-149 [PubMed] [Google Scholar]
- 39.Haefliger JA, Demotz S, Brassant O, Suter E, Waeber B, Nicod P, Meda P: Connexin 40 and 43 differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int 2001, 60:190-201 [DOI] [PubMed] [Google Scholar]
- 40.Arensbak B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH: Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol 2001, 115:479-487 [DOI] [PubMed] [Google Scholar]
- 41.Garrod DR, Fleming S: Early expression of desmosomal components during kidney tubule morphogenesis in human and murine embryos. Development 1990, 108:313-321 [DOI] [PubMed] [Google Scholar]
- 42.Yaoita E, Goto S: Transient expression of desmoplakins in murine glomerulogenesis. Oite T Shimizu F Kihara I eds. Cell-Cell Interaction in Kidney. 1997:pp 29-40 Nihon-Igakukan, Tokyo
- 43.Goto S, Yaoita E, Matsunami H, Kondo H, Yamamoto T, Kawasaki K, Arakawa M, Kihara I: Involvement of R-cadherin in the early stage of glomerulogenesis. J Am Soc Nephrol 1998, 9:1234-1241 [DOI] [PubMed] [Google Scholar]
- 44.Kerjaschki D: Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli. The effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest 1978, 39:430-440 [PubMed] [Google Scholar]
- 45.Miner JH: Focusing on the glomerular slit diaphragm. Podocin enters the picture. Am J Pathol 2002, 160:3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein T, Cameron R, Katz A, Silverman M: Rat glomerular epithelial cells in culture express characteristics of parietal, not visceral, epithelium. J Am Soc Nephrol 1992, 3:1279-1287 [DOI] [PubMed] [Google Scholar]
- 47.Smoyer WE, Mundel P, Gupta A, Welsh MJ: Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol 1997, 273:F158-F169 [DOI] [PubMed] [Google Scholar]
- 48.Paizis K, Kirkland G, Polihronis M, Katerelos M, Kanellis J, Power DA: Heparin-binding epidermal growth factor-like growth factor in experimental models of membranous and minimal change nephropathy. Kidney Int 1998, 53:1162-1171 [DOI] [PubMed] [Google Scholar]
- 49.Smoyer WE, Gupta A, Mundel P, Ballew JD, Welsh MJ: Altered expression of glomerular heat shock protein 27 in experimental nephrotic syndrome. J Clin Invest 1996, 97:2697-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, Couser WG: Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo: expression of antiadhesive proteins and cytoskeletal changes. Lab Invest 1992, 67:486-497 [PubMed] [Google Scholar]
- 51.Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, Nagata M: Podocyte injury promotes progressive nephropathy in Zucker diabetic fatty rats. Lab Invest 2002, 82:25-35 [DOI] [PubMed] [Google Scholar]
- 52.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of tight junctions between glomerular foot processes. Am J Physiol 1995, 268:F514-F524 [DOI] [PubMed] [Google Scholar]
- 53.Sharma R, Sharma M, Li JZ, McCarthy ET, Savin VJ: Direct effects of platelet-activating factor on glomerular capillary permeability. Kidney Blood Press Res 1997, 20:25-30 [DOI] [PubMed] [Google Scholar]
- 54.Reiser J, Pixley FJ, Hug A, Kriz W, Smoyer WE, Stanley ER, Mundel P: Regulation of mouse podocyte process dynamics by protein tyrosine phosphatase. Kidney Int 2000, 57:2035-2042 [DOI] [PubMed] [Google Scholar]
- 55.Li B, Yao J, Morioka T, Oite T: Nitric oxide increases albumin permeability of isolated rat glomeruli via a phosphorylation-dependent mechanism. J Am Soc Nephrol 2001, 12:2616-2624 [DOI] [PubMed] [Google Scholar]
- 56.Musil LS, Cunningham BA, Edelman GM, Goodenough DA: Differential phosphorylation of the gap junction protein connexin-43 in junctional communication-competent and -deficient cell lines. J Cell Biol 1990, 111:2077-2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yaoita E, Yoshida Y: Polygonal epithelial cells in glomerular cell culture: podocyte or parietal epithelial origin? Microsc Res Tech 2002, 57:212-216 [DOI] [PubMed] [Google Scholar]
- 58.Azzam EI, de Toledo SM, Little JB: Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from a-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 2001, 98:473-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wygoda MR, Wilson MR, Davis MA, Trosko JE, Rehemtulla A, Lawrence TS: Protection of herpes simplex virus thymidine kinase-transduced cells from ganciclovir-mediated cytotoxicity by bystander cells: the good Samaritan effect. Cancer Res 1997, 57:1699-1703 [PubMed] [Google Scholar]
- 60.Gordon RE, Heller FH, Del Valle JR, Heller RF: Membrane perturbations and mediation of gap junction formation in response to taurine treatment in normal and injured alveolar epithelia. Exp Lung Res 1989, 15:895-908 [DOI] [PubMed] [Google Scholar]
- 61.Iwata F, Joh T, Ueda F, Yokoyama Y, Itoh M: Role of gap junctions in inhibiting ischemia-reperfusion injury of rat gastric mucosa. Am J Pathol 1998, 275:G883-G888 [DOI] [PubMed] [Google Scholar]