Abstract
The complement activation product, C5a, is a powerful phlogistic factor. Using antibodies to detect human or rat C5a, incubation at pH 7.4 of human blood neutrophils or rat alveolar macrophages (AMs) with C5 in the presence of phorbol 12-myristate 13-acetate (PMA) led to generation of C5a. Rat AMs activated with lipopolysaccharide also generated C5a from C5. With activated neutrophils, extensive cleavage of C5 occurred, whereas activated macrophages had much more selective proteolytic activity for C5. Peripheral blood human or rat mononuclear cells and rat alveolar epithelial cells when stimulated with phorbol ester all failed to demonstrate an ability to cleave C5, suggesting a specificity of C5 cleavage by phagocytic cells. With rat AMs, C5a generation was time-dependent and was blocked if AMs were pretreated with inhibitors of transcription or protein synthesis (actinomycin D or cycloheximide). Similar treatment of activated human polymorphonuclear leukocytes only partially reduced C5a generation after addition of C5. C5a generated by activated AMs was biologically (chemotactically) active. This generation was sensitive to serine protease inhibitors but not to other classes of inhibitors. These data indicate that phagocytic cells, especially lung macrophages, can generate C5a from C5. In the context of the lung, this may represent an important C5a-generating pathway that is independent of the plasma complement system.
The complement system generating the complement activation products, C3a, C5a, and C5b-9 and the cellular defense system involving macrophages and neutrophils are known to form the first line of defense (innate immunity) against microorganisms and other tissue-damaging factors. 1,2 During acute lung inflammation, leukocytes are recruited from the vascular space into interstitial and distal airway compartments by complement activation products, especially C5a 3-5 and various chemotactic cytokines. 2,6 There is also evidence that C5a and C5b-9 enhance lung macrophage generation of cytokines and chemokines. 7 Systemic complement activation by intravenous infusion of purified cobra venom factor has been shown to cause pulmonary capillary injury and neutrophil accumulation in lungs, leading to acute lung injury. 8 Although the pathways of complement activation in plasma (alternate, classical, and lectin-binding) are well established, there is less definitive evidence about generation of complement components and complement activation products within the extravascular compartment. 7,9 In bronchoalveolar lavage (BAL) fluids, C5 fragments with C5a-like properties have been detected during acute 6,10 and chronic lung inflammation. 11 In these BAL fluids, a higher level of hemolytic C5 activity was found when compared to levels found in serum, suggesting that complement components may be formed in extravascular sites. 10 An extravascular cellular source of complement seems to be macrophages, which are ubiquitous in most tissues and are known to generate a variety of complement proteins, including many of the components required for activation of the alternative pathway. 12,13 Some in vitro studies have also suggested that noncomplement-derived convertases, namely, bacterially derived arginine-specific cysteine protease 14 and several serine proteases (eg, trypsin and elastase) have the ability to cleave complement components, such as C3 and C5, to produce biologically active anaphylatoxins. 15,16 Thus, C3a and C5a, which are powerful phlogistic peptides, can be generated by complement convertases as well as complement-independent convertases.
It has been shown that the co-presence of C5a or C5b-9, bacterial lipopolysaccharide (LPS), or immune complexes cause enhanced production and release of chemotactic cytokines by alveolar macrophages (AMs). 7 When C5a or C5b-9 were given into the airways of rats undergoing lung deposition of IgG immune complexes, there was enhanced pulmonary neutrophil accumulation and intensified inflammatory lung injury. 7 These data suggest that C5 activation products generated within lung in the presence of a co-stimulus can lead to the recruitment of neutrophils into the alveolar space. Relatively little is known about the extravascular generation of C5 activation products, the C5-cleaving enzyme(s) involved, and the biological functions of such products. In the current studies we have demonstrated that activated rat AMs and activated human neutrophils [but not rat alveolar epithelial cells (AECs) or human peripheral blood mononuclear cells (PBMCs)] can cleave human C5 to generate product(s) that in Western blots align with C5a immunoprecipitated from activated human serum. This C5a was chemotactically active for neutrophils and its functional activity could be blocked by antibody (Ab) to human C5a. Further, serine protease inhibitors [soybean trypsin inhibitor (SBTI) and secretory leukocyte protease inhibitor (SLPI)] were found to block the cleavage of C5 by activated macrophages. These studies imply that C5a can be directly generated by activated phagocytic cells in the presence of C5, extending the potential sources of the anaphylatoxin C5a- and C5-cleaving enzymes beyond proteins present in the plasma.
Materials and Methods
Reagents and Chemicals
Unless otherwise specified, chemicals and reagents and recombinant human C5a were purchased from Sigma Chemical Co. (St. Louis, MO). Purified human C5 was obtained from Quidel, Inc. (Mountain View, CA). Recombinant human SLPI and human tissue inhibitor of matrix metalloproteases inhibitor-2 (TIMP-2) were kindly provided by Drs. Thomas R. Ulich and Clifford D. Wright (Amgen, Inc., Thousand Oaks, CA).
Preparation and Characterization of Antibodies Against Human and Rat C5a and Human C5
Based on the recently published potent in vitro and in vivo effects of different anti-C5a Ab preparations, 17 polyclonal anti-C5a antibodies to the carboxyl-terminal peptide region of the rat C5a molecule (with the sequence CTIADKIRKESHHKGMLLGR, corresponding to amino acid residues 58 to 77) and to the carboxyl-terminal peptide domain of human C5a (CVVASQLRANISHKDMQLGR, corresponding to residues 55 to 74) were used in the present study. Peptide synthesis, Ab production, and affinity purification was done by Research Genetics, Inc. (Huntsville, AL). The anti-rat C5a polyclonal Ab does not cross-react with recombinant human C5a (data not shown). However, the anti-human C5a polyclonal Ab does cross-react with recombinant rat C5a but does not pick up human C5 on a Western blot (data not shown). We are unable to determine the crossreactivity of these antibodies with rat C5 because of the nonavailability of rat C5. Anti-human C5 Ab was obtained from Quidel.
Isolation and Culture Conditions for Rat AMs and AECs and Human Neutrophils and PBMCs
Rat AMs were isolated by repeatedly lavaging lungs of anesthetized, healthy animals (Long Evans rats; Harlan, Inc., Indianapolis, IN). After centrifugation of BAL fluids, cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM), pH 7.4 (Whittaker Bioproducts, Walkersville, MD). AMs (1 to 10 × 106 cells) were stimulated with phorbol ester (PMA) (100 ng/ml), while 10 mmol/L of ethylenediaminetetraacetic acid (EDTA) was added to nonstimulated AMs to avoid contact-mediated activation. To both groups, human C5, at concentrations ranging from 1 to 120 μg, was added and the cells incubated for the indicated time periods at 37°C. After an incubation period of 4 hours (or at other indicated time intervals), supernatant fluids were collected and remaining cell debris removed by a centrifugation step at 4°C. The supernatant fluids were then used for detection of C5-cleavage products as described below. AECs were isolated as primary cultures from specific pathogen-free male Long Evans rats (Harlan). The isolation was performed using a modified version of the elastase method. After anesthesia with ketamine (1 g/kg body weight), rats were bled out by cutting the abdominal aorta. An intratracheal catheter was then placed. After flushing of the pulmonary artery with 10 ml of Dulbecco’s phosphate-buffered saline (DPBS), the lungs were carefully removed together with the heart and subjected to BAL for 8 to 10 times with 10 ml of DPBS. This resulted in the removal of the majority of AMs. Next, the lungs were placed in a waterbath (37°C) for 25 minutes and 40 ml of DPBS containing ∼90 U of Elastase (Worthington, Lakewood, NJ) was slowly infused into the airways. The heart and any remaining tissue was removed and the lungs were minced for 3 minutes with scissors after adding 1000 U of deoxyribonuclease I (Sigma Chemical Co.). Enzyme activity was stopped by the addition of 5 ml of ultra low IgG fetal bovine serum (Life Technologies Inc., Rockville, MD). The suspension containing the AECs was incubated at room temperature and gently stirred for 20 minutes. The suspension was then filtered three times through a mesh (500 mm, 175 mm, 105 mm) (Spectrum Laboratories, Inc., Rancho Dominguez, CA) and suspended with 30 ml of DMEM. The cells were centrifuged at 1500 rpm for 10 minutes and then resuspended in 45 ml of DMEM. Next the cells were plated onto 100-mm Petri dishes precoated with 30 mg/ml of rabbit IgG, for 1 hour at 37°C to remove remaining AMs. Cells were than carefully collected by washing over the Petri dishes three to four times. After centrifugation, the cell pellet was carefully resuspended in 10 to 20 ml of DMEM containing 1% penicillin streptomycin mix, 1% l-glutamine (200 mmol/L), and 1% nonessential amino acids (10 mmol/L) purchased from Life Technologies, Inc. (Grand Island, NJ) and 10% heat-inactivated fetal bovine serum. Finally, cells were plated onto various tissue culture plates as needed, and cultured for 2 days before stimulation. All nonadherent cells were removed by washing the tissue culture dishes with DMEM at days 1 and 2 after isolation. Residual macrophages were <2% of total cells. Human blood neutrophils and PBMCs, were isolated as described below and elsewhere. 18
Western Blot Analysis for Human C5a
Supernatant fluids from the indicated cell suspensions, which were unstimulated or stimulated with PMA and to which human C5 (in the amounts indicated) was added, were electrophoresed under reducing conditions on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Schleicher & Schuell, Keene, NH) using a semidry electrophoresis apparatus (LkB Multiphor II; Pharmacia Biotech, Uppsala, Sweden). The blot was incubated with 5% fat-free milk in DPBS for 1 hour at room temperature and then probed with the indicated rabbit anti-human C5a IgG (at a final concentration of 3 μg/ml) overnight at 4°C. After washing, an alkaline phosphatase-conjugated goat anti-rabbit IgG Ab (Jackson ImmunoResearch, Inc., West Grove, PA) was used as a secondary Ab (1:1000) at room temperature for 1 hour, followed by an additional washing step (with DPBS containing 0.1% Tween 20), and alkaline phosphatase substrate color development (Bio-Rad Laboratories, Hercules, CA). When goat anti-human C5 IgG was used as the primary Ab, the secondary Ab was rabbit anti-goat IgG that was conjugated to alkaline phosphatase.
In some experiments C5a was generated in normal human serum by the addition of 10 mg of oyster glycogen/ml serum, followed by incubation at 37°C for 30 minutes. The serum was then diluted 1:5 with DPBS and C5a immunoprecipitated with protein A Sepharose beads and anti-human C5a rabbit IgG (Calbiochem, La Jolla, CA).
Effects of Protein Synthesis Inhibitors on Generation of C5a by Rat AMs
Rat AMs and human neutrophils were obtained in the usual way and were suspended in DMEM at 10 × 106 cells/ml. The cells were pretreated with cycloheximide or actinomycin D (20 μg/ml and 200 ng/ml, respectively) for 20 minutes at 37°C. After treatment, cells were incubated with the indicated amounts of C5 and PMA, as described above. The cells were then further incubated for 4 hours at 37°C. The cells were then pelleted and the supernatants analyzed by Western blotting. Cells were also incubated alone or with C5 and PMA treatment in the absence of exposure to actinomycin D or cycloheximide.
Protein Detection by Silver Stain
Some of the AM supernatant fluids samples were electrophoretically separated on a 15% polyacrylamide gel electrophoresis and the gels were silver-stained (Silver Stain Plus; Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s protocol. Briefly, after electrophoresis, gels were placed in a fixative enhancer solution for 20 minutes, followed by two washing steps in deionized distilled water. The final silver complex solution was added for 20 minutes in the presence of the image development reagent and the reaction stopped in 5% acetic acid solution. The gels were then washed again, dried, and photo-documented.
Hemolytic Complement Assay
The ability of protease inhibitors to interfere with the CH50 activity in whole human and rat serum was assessed by measurement of the hemolytic activity of fresh human and rat serum in the presence or absence of protease inhibitors. Briefly, sensitized sheep red blood cells (Colorado Serum Company, Denver, CO) were exposed at 37°C for 60 minutes to various dilutions of serum samples in triethanolamine-buffered saline (TBS), pH 7.35 (268 mmol/L NaCl, 20 mmol/L triethanolamine, 150 μmol/L CaCl2, and 500 μmol/L MgCl2) in presence or absence of SLPI or SBTI (each at 100 μg/ml). The complement reaction was stopped by ice-cold TBS (with 0.05% gelatin) followed by a centrifugation step (2500 × g, 5 minutes). Absorption values of the supernatant fluids were determined at 541 nm and the hemolytic activity of human and rat serum in the presence or absence of the indicated protease inhibitors was determined. 19
Neutrophil Chemotaxis Assay
Whole blood of healthy human volunteers was drawn from the antecubital vein into syringes containing the anticoagulant, anticoagulate citrate dextrose (ACD) (Baxter Health Care, Deerfield, IL), in a volume ratio of 1:10 (ACD:blood). Neutrophils were isolated using Ficoll-Paque gradient centrifugation (Pharmacia Biotech AB) followed by a dextran sedimentation step. After hypotonic lysis of residual red blood cells, neutrophils were resuspended in Hanks’ balanced salt solution and fluorescein-labeled with BCECF (2′, 7′-bis [2-carboxyethyl]-5-[and 6]-carboxy-fluorescein acetoxymethyl ester) (Molecular Probes, Inc., Eugene, OR) for 30 minutes at 37°C. After a washing step with DPBS, labeled neutrophils (5 × 106 cells/ml) were loaded into the upper chamber of a 96-well mini chamber (NeuroProbe, Inc., Gaithersburg, MD), separated by a polycarbonate filter with a porosity of 3 μm (NeuroProbe). The lower chambers were loaded with human C5a (0.1 to 1000 ng/ml) or different dilutions of supernatant fluids of the AM preparations in the presence or absence of anti-human C5a and/or anti-rat C5a antibodies (each at 10 μg/ml). Neutrophils were then incubated for 30 minutes at 37°C. The number of cells migrating through the polycarbonate membrane to the lower surface was determined by cytofluorometry (Cytofluor II; Per Septive Biosystems, Inc., Framingham, MA). For each measurement, samples were done at least in quadruplicates.
Statistical Analyses
All values were expressed as mean ± SEM. Data sets of chemotaxis assays and CH50 assays were analyzed with one-way analysis of variance; differences in the mean values among the experimental groups were then compared using the Tukey multiple comparison test. Results were considered statistically significant when P was <0.05.
Results
Ability of Different Phagocytic Cells and Lung Cells to Generate C5a
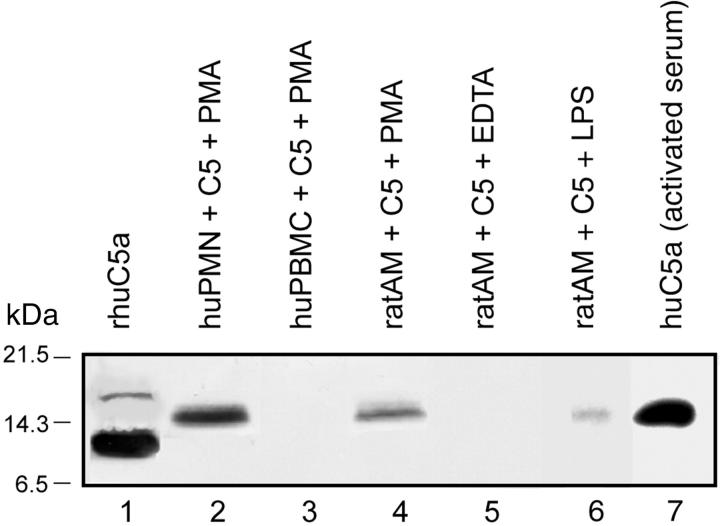
Various types of phagocytic cells (1 × 107) were incubated with PMA (100 ng/ml), 90 μg of human C5 at 37°C for 4 hours, and the supernatant fluids evaluated by Western blot analysis. Where indicated, 10 mmol/L of EDTA was also present. The data are shown in Figure 1 ▶ . Recombinant human C5a showed two bands, with the main one being between the 6.5- and 14.3-kDa markers and a less intense band between the 14.3- and 21.5-kDa markers (Figure 1 ▶ , lane 1). The approximate molecular weights of the α and β chains of human C5 are ∼118 kDa and 74 kDa, respectively. Molecular weight of glycosylated C5a is ∼12 to 14.5 kd. Bacterially expressed recombinant human C5a however has a molecular weight of ∼8 to 9 kd because it is not glycosylated. Activated human neutrophils [polymorphonuclear leukocytes (PMNs)] generated a C5a product aligning with the 14.3-kd marker (Figure 1 ▶ , lane 2), as well as with human C5a immunoprecipitated from activated human serum (Figure 1 ▶ , lane 7) the expected position of glycosylated C5a (because the added human C5 was isolated from human serum and the cleavage product would be glycosylated). Activated human PBMCs failed to demonstrate an ability to generate C5a (Figure 1 ▶ , lane 3). In data not shown, we also failed to detect evidence of C5a generation by activated rat PBMCs. Activated rat AMs whether activated with PMA (Figure 1 ▶ , lane 4) or LPS (Figure 1 ▶ , lane 6) generated a C5a product aligning with the 14.3-kd marker. As will be shown (below), this product was biologically active. The co-presence of EDTA prevented generation of C5a by activated rat AMs (Figure 1 ▶ , lane 5). Finally, C5a generation by rat neutrophils was observed only when >2 × 107 cells were incubated with C5 and PMA (data not shown).
Figure 1.
Generation of C5a by activated human PMNs, human PBMCs, or rat AMs. Cells (1 × 107) were incubated at pH 7.4 with 90 μg of human C5 and (where indicated) PMA (100 ng/ml) or LPS (0.5 μg/ml) for 4 hours at 37°C and then Western blot analysis with anti-human C5a was performed. Positions of the molecular weight markers are shown at the extreme left region of the figure. Data are representative of three or more separate and independent experiments.
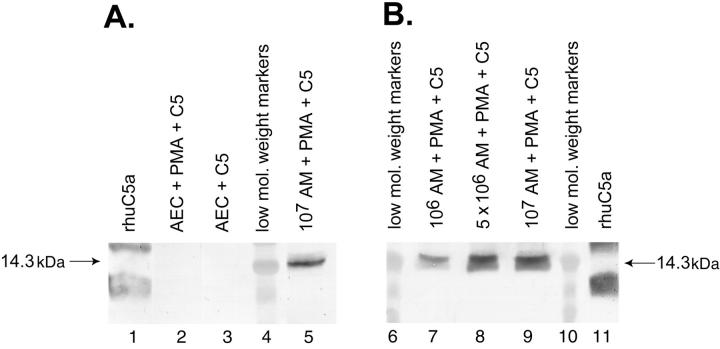
Primary cultures of rat AECs (type II cells) (<3% macrophages and >97% cells that were cytokeratin-positive) as well as freshly isolated rat AMs (≥97% macrophages) were incubated with PMA (100 ng/ml) and C5 (90 μg) for 4 hours at 37°C under the various conditions are shown in Figure 2 ▶ . The typical pattern for recombinant human C5a was found, with bands positioned slower or faster than the 14.3-kDa marker (Figure 2 ▶ , lanes 1 and 11). AECs (106) activated with PMA failed to produce any evidence of cleavage of C5 (Figure 2A ▶ , lane 2). AECs in the absence of PMA also showed no C5a product (Figure 2 ▶ , lane 3). In the same experiment, activated AMs (107) showed a cleavage product that was detected by anti-C5a and aligned with the 14.3-kDa marker (Figure 2 ▶ , lane 5). To assess the dose responses of PMA-stimulated AMs to generate C5a as a function of macrophage numbers, 1, 5, and 10 × 106 AMs were incubated with PMA and C5 under the conditions described above. The results (Figure 2 ▶ , lanes 7, 8, and 9) indicated a C5a product in each case. Accordingly, when equivalent numbers (106) of activated rat AMs (Figure 2 ▶ , lane 7) and AECs (Figure 2 ▶ , lane 2) were compared, only the former cells were capable of generating C5a, suggesting that C5a generation by various cells may be a selective cell response.
Figure 2.
Inability of PMA-stimulated rat AECs to generate C5a after incubation with 90 μg of C5 for 4 hours at 37°C. Conditions for Western blot analysis using anti-human C5a was similar to that described for Figure 1 ▶ . Data are representative of three or more separate and independent experiments.
C5 Cleavage Patterns by Activated Human Neutrophils and Rat AMs
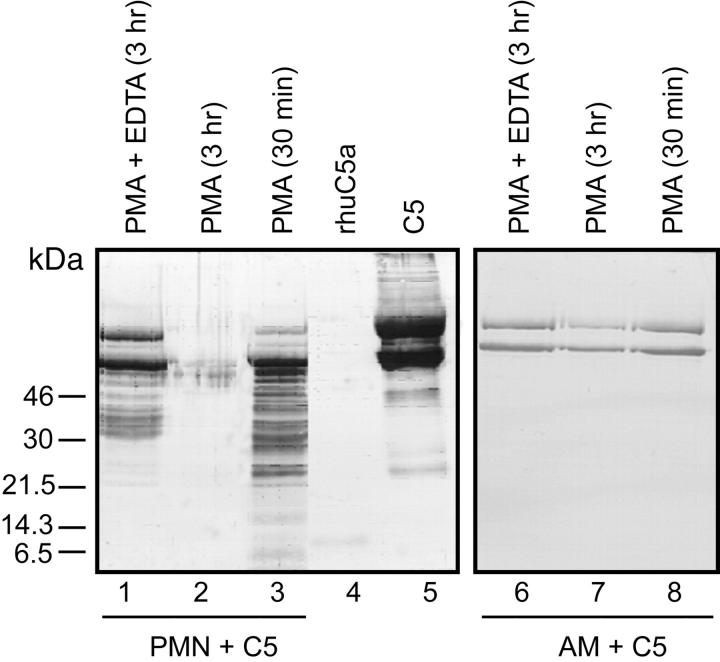
Two phagocytic cell types (human PMNs and rat AMs) with a robust ability to generate C5a from human C5 (as determined in Figures 1 and 2 ▶ ▶ ) were evaluated for the extent of C5 cleavage as a function of time using Ab to whole C5. The results are shown in Figure 3 ▶ . The α and β chains of otherwise unmanipulated C5 could be readily detected as the predominant proteins in the expected positions (Figure 3 ▶ , lane 5). Not surprisingly, the anti-human C5 Ab had a poor ability to detect human C5a (Figure 3 ▶ , lane 4). Incubation of C5 with PMNs for 30 minutes at 37°C in the presence of PMA led to extensive hydrolysis of both the α and β chains of human C5, resulting in many hydrolysis products (Figure 3 ▶ , lane 3). By 3 hours, virtually no detectable C5 cleavage products remained (Figure 3 ▶ , lane 2). The co-presence of EDTA, as might be expected, reduced the extent of hydrolysis of C5 by activated PMNs at 3 hours, with detectable α and β chains of C5 still present (Figure 3 ▶ , lane 1). When C5 was incubated for 30 minutes or 3 hours at 37°C with rat AMs stimulated with PMA, the α and β chains of C5 could still be detected and extensive hydrolysis products were not found (Figure 3 ▶ , lanes 7 and 8).
Figure 3.
Ability of activated human PMNs or rat AMs to hydrolyze C5 using conditions similar to those described in Figure 1 ▶ except that anti-human C5 Ab was used for immunoblotting. Data are representative of two separate and independent experiments.
Cleavage of Human C5 as a Function of Time and Dose
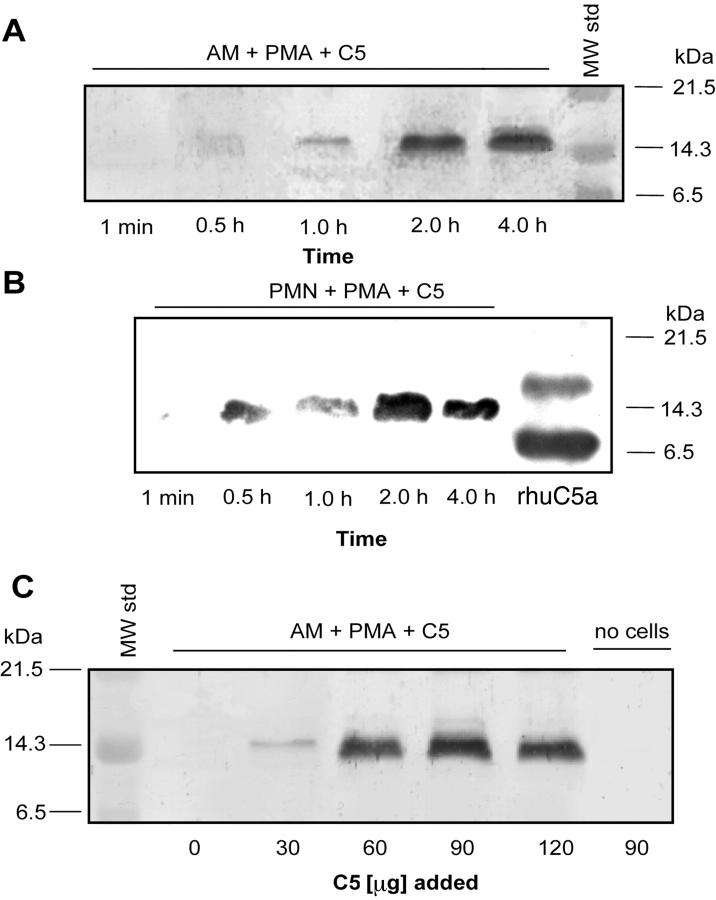
Supernatant fluids were evaluated from rat AMs and human PMNs (10 × 106 cells) that had been stimulated with 100 ng/ml of PMA in the presence of 90 μg of C5 at 37°C as a function of time (Figure 4) ▶ . Samples were subjected to electrophoresis and the products detected by Western blot analysis using anti-human C5a Ab. A C5 cleavage product that aligned with the 14.3-kDa marker and was reactive with anti-human C5a Ab was found, with increasing intensity as a function of time of incubation, faintly detectable at 1 hour and appearing to reach a maximum between 2 and 4 hours in activated macrophages (Figure 4A) ▶ . Similarly, in human PMNs, a C5a cleavage product was detectable as early as 30 minutes and appeared to peak at 2 hours. No other cleavage products from C5 were found when anti-C5 or anti-C5a Ab was used (Figures 1, 2, and 3) ▶ ▶ ▶ . In macrophages the intensity of the cleavage product as detected in Western blots increased when increasing doses of C5 was used (Figure 4C) ▶ . Thus, incubation of C5 with activated rat AMs and PMNs consistently yielded a C5 cleavage product that aligned with the 14.3-kDa marker. No evidence of this cleavage product from C5 was found when human C5 was omitted from or when C5 (90 μg) was present in the absence of cells (Figure 4C) ▶ .
Figure 4.
A and B: Western blot detection of human C5a in supernatant fluids obtained up to 4 hours after addition of 90 μg of human C5 to 10 × 106 AMs (A) or 10 × 106 PMNs (B) in the presence of PMA (100 ng/ml). Positions of molecular weight markers are shown in the extreme right lane. C: Western blot analysis of supernatant fluids from PMA-stimulated rat AMs in the presence of increasing amounts (30 to 120 μg) of C5. Western blots in A, B, and C were all probed with anti-human C5a. Data are representative of four or more separate and independent experiments.
Features of C5 Cleavage Products
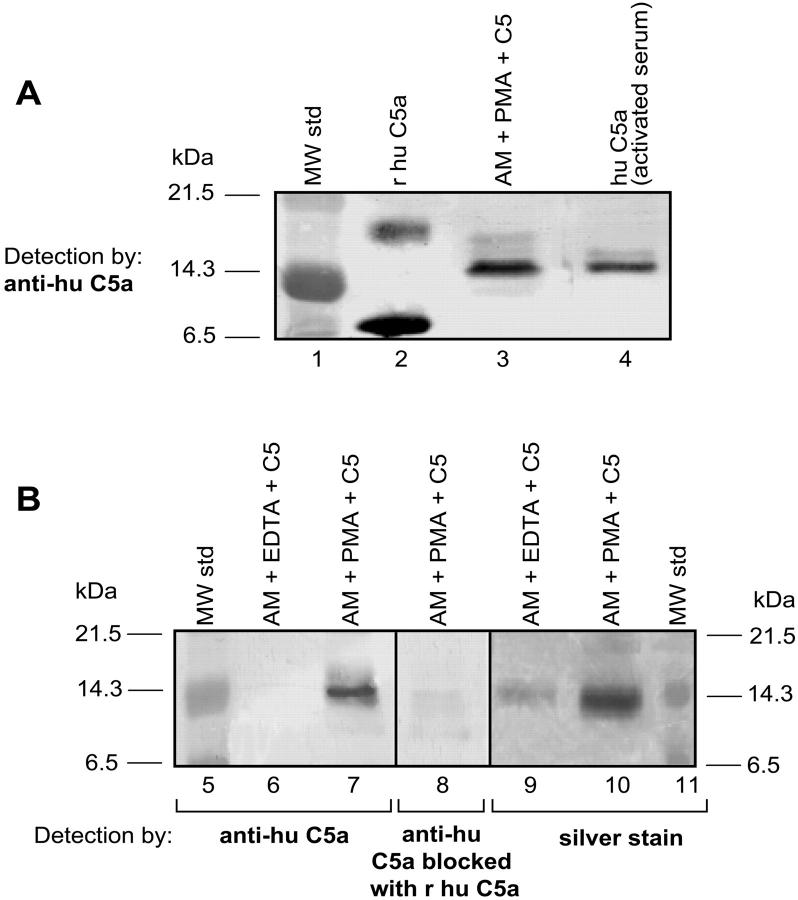
By Western blot analysis, recombinant human C5a showed the main band near the 6.5-kDa marker and a less intense band between the 14.3- and 21.5-kDa markers (Figure 5A ▶ , lanes 1 and 2). The C5 cleavage product generated by activated rat AMs is consistent with glycosylated C5a (Figure 5 ▶ , lane 3). C5a immunoprecipitated from glycogen-activated human serum also aligned near the 14.3-kDa position (Figure 5 ▶ , lane 4). No band could be detected in nonactivated human serum (data not shown). As shown in Figure 5B ▶ , the C5 cleavage product generated by PMA-activated AMs in the presence of C5 (Figure 5 ▶ , lane 7) was not found in the supernatant fluids that contained 10 mmol/L of EDTA (Figure 5 ▶ , lane 6). To further characterize the C5 cleavage products, the anti-C5a Ab was preabsorbed with recombinant human C5a, resulting in the disappearance of the ability to detect the expected cleavage product (Figure 5 ▶ , lane 8). The presence of a cleavage product was further confirmed by silver staining. The band produced by activated AMs in the presence of C5 (Figure 5 ▶ , lane 10) aligned with the 14.3-kDa marker, this band being barely detectable if EDTA had also been present in the mixture of C5 and rat AMs (Figure 5 ▶ , lane 9). These data indicate that the C5 cleavage product produced by activated AMs aligns with the 14.3-kDa marker and is likely C5a.
Figure 5.
A: Western blot analysis of various samples using anti-human C5a. Molecular weight markers are in lane 1 and recombinant human C5a in lane 2. The C5 cleavage product in supernatant fluids from the combination of PMAs, rat AMs, and C5 is shown in lane 3. C5a from activated human serum (lane 4) was first immunoprecipitated with anti-human C5a and then analyzed by Western blot analysis. B: Western blot analysis of AM-generated C5a. Molecular weight standards (lane 5); supernatant fluids from nonstimulated (10 mmol/L EDTA) rat AMs to which C5 was added (lane 6), and supernatant fluids from similarly treated rat AMs in the absence of EDTA (lane 7). Middle frame (lane 8): Antibody to human C5a was used as the immunodetecting Ab but preabsorbed with recombinant human C5a (10 μg/ml) and then used to detect the same products as shown in lane 7. Right frame: Silver stain of gel contained in lane 9 material similar to lane 6, whereas lane 10 contained material similar to that in lane 7. Reference molecular weight standards are in lane 11. Data are representative of two separate and independent experiments.
Generation by Rat AMs of Biological Activity from Human C5
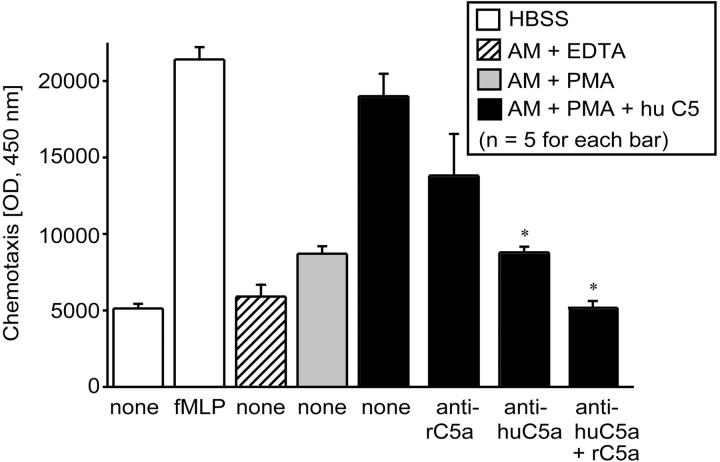
Because Western blot analysis indicated the presence of a product reactive with Ab to human C5a in supernatant fluids from activated rat AMs incubated with human C5 (described above), we performed functional analysis on these fluids by measuring the chemoattractant activity for human blood neutrophils and the ability of anti-human C5a to block this biological activity. The data are shown in Figure 6 ▶ in which cytofluorometric analysis of neutrophils that had migrated through the micropore filters was used. The chemotactic response of human neutrophils to 0.1 μmol/L of fMLP is shown in the second (from left) white bar in Figure 6 ▶ . The migratory response was very low in the presence of Hanks’ balanced salt solution alone (Figure 6 ▶ , first white bar) or in supernatant fluids to which either EDTA (Figure 6 ▶ , cross-hatched bar) or PMA (Figure 6 ▶ , gray bar) had been added to rat AMs (107) in the absence of C5. In contrast, supernatant fluids from PMA-stimulated rat AMs in the presence of human C5 showed a very robust migratory response (Figure 6 ▶ , first black bar), which was similar to the neutrophil chemotactic response to 100 nmol/L fMLP (Figure 6 ▶ , second white bar). Addition of anti-rat C5a Ab (10 μg/ml) to the supernatant fluids from activated AMs incubated with 90 μg of C5 showed only a slight and insignificant reduction of chemotactic activity (Figure 6 ▶ , second black bar). However, supernatant fluids from PMA-stimulated rat AMs incubated with human C5 and to which anti-human C5a Ab (10 μg/ml) was then added showed a dramatic drop in the chemotactic response (Figure 6 ▶ , third black bar). The combination of antibodies to rat and human C5a showed total suppression of the chemotactic response, resulting in cell migration at basal levels (Figure 6 ▶ , extreme right-hand black bar). Addition of nonspecific IgG to supernatant fluids from activated rat AMs incubated with human C5 was similar to the neutrophil response to fMLP (data not shown). These data demonstrate that cleavage of C5 by PMA-stimulated rat AMs generates a C5a-related functional (chemotactic) activity for neutrophils.
Figure 6.
Chemotactic activity for human PMNs using samples indicated in the inset. For these studies, 107 rat AMs were incubated with 90 μg of human C5a for 4 hours at 37°C. See text for details. The concentration of fMLP was 100 nmol/L. Statistical comparisons were to the supernatant fluids from PMA-stimulated AMs in the presence of C5 (black bar labeled “none”). IgG antibodies (10 μg/ml) to rat C5a or human C5a were added to supernatant fluids before chemotactic assays were performed. Data are representative of three separate and independent experiments.
Effects of Enzyme Inhibitors on Macrophage Generation of C5a
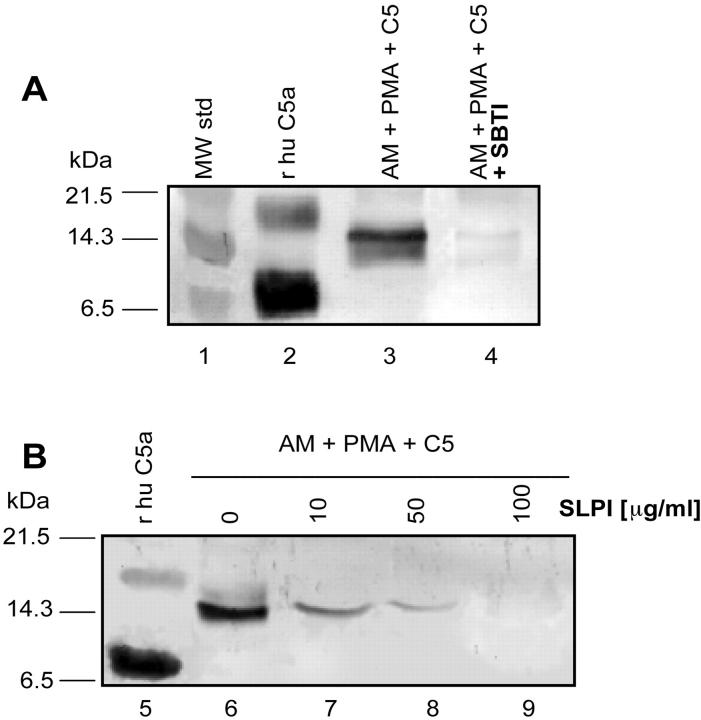
Various inhibitors were used in the rat AM/PMA/C5a-generating system (37°C for 4 hours) to assess the extent to which these compounds would affect C5 cleavage and the generation of C5a, based on Western blot analysis. In initial studies, the addition of a cocktail of enzyme inhibitors (containing SBTI, leupeptin, aprotinin, and pepstatin, each at 100 μg/ml) totally suppressed generation of C5a (Table 1) ▶ . However, two individual components of this cocktail, pepstatin and leupeptin, failed to show suppressive effects, whereas aprotinin partially and SBTI completely blocked generation of cleavage products (Table 1) ▶ . In the presence of tissue inhibitor of human metalloprotease-2 (TIMP-2), no inhibition of C5 cleavage was found. The C5 cleavage products were further evaluated by Western blot analysis in the presence or absence of SBTI or SLPI. For reference purposes, the molecular weight standards (Figure 7A ▶ , lane 1) and human C5a (Figure 7 ▶ , lane 2) are shown. The band near the 14.3-kDa marker was found in supernatant fluids from rat AMs after addition of 90 μg of C5 and PMA (100 ng/ml) (Figure 7 ▶ , lane 3), whereas in the presence of SBTI (100 μg/ml) this band could no longer be detected (Figure 7 ▶ , lane 4). In a dose-dependent manner, the addition of SLPI (10 to 100 μg/ml) also suppressed, proportional to the SLPI concentration, the appearance of the C5 cleavage product aligning with the 14.3-kDa marker (Figure 7B) ▶ . These data suggest that a serine protease, which is susceptible to SBTI and SLPI, is activated in rat AMs and is responsible (after addition of PMA) for cleavage of C5 and the appearance of C5a. Supernatant fluids obtained from PMA-stimulated AMs have consistently failed to demonstrate the presence of a C5-cleaving enzyme in cell supernatant fluids (data not shown).
Table 1.
Effects of Protease Inhibitors on C5a Generation by AM
| Inhibitor added* | Inhibition of C5a generation† |
|---|---|
| Protease inhibitor cocktail | Complete |
| Aprotinin | Partial |
| Pepstatin | None |
| Leupeptin | None |
| Soybean trypsin inhibitor | Complete |
| SLPI | Complete |
| TIMP-2 | None |
*Each inhibitor individually or together was used at a concentration of 100 μg/ml.
†Determined by Western blot analysis using anti-human C5a, as described in the text, after incubation of PMA-activated rat AMs with C5.
Figure 7.
A: Western blot analysis using anti-human C5a Ab: lane 1, molecular weight standards; lane 2, recombinant human C5a (100 ng); lane 3, supernatant fluids from PMA-stimulated rat AMs to which C5 (90 μg) was added and incubation was performed at 37°C for 4 hours; lane 4, similar to lane 3 with the exception that at the start of incubation SBTI (100 μg/ml) was added. B: Western blot using the same anti-human C5a Ab: lane 5, human C5a; lanes 6 to 9, supernatant fluids from PMA-stimulated AMs to which C5 (90 μg) had been added in the presence of 0 to 100 μg of SLPI/ml. Data are representative of two separate and independent experiments.
Effects of Inhibitors of Protein Synthesis on Generation of C5a from Activated Rat AMs and Human PMNs
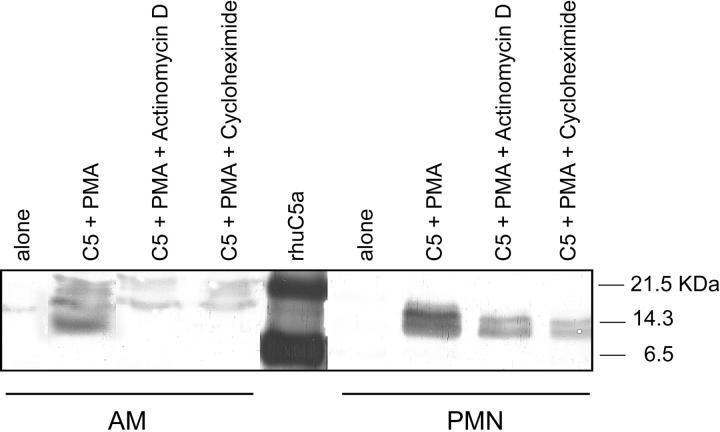
When rat AMs or human PMNs (107) were pretreated for 20 minutes at 37°C with actinomycin D (200 ng/ml) or cycloheximide (20 μg/ml) and then incubated with 90 μg of C5 and PMA (100 ng/ml) at 37°C for 4 hours, no evidence for C5a generation was found with activated AMs, in contrast to cells not exposed to either inhibitor (Figure 8) ▶ . When similar experiments were used with human PMNs, pretreatment with actinomycin D or cycloheximide caused some diminished intensity of the C5a bands, but they were clearly still distinguishable (Figure 8) ▶ . These results suggest that the C5-cleaving activity in AMs is inducible, whereas in PMNs the C5-cleaving activity appears to be related to significantly pre-existing C5-cleaving activity within PMNs.
Figure 8.
Ability of activated AMs and PMNs to generate C5a in the presence of actinomycin D and cycloheximide using conditions similar to those described in Figure 1 ▶ . Details are provided in the text. Western blots were probed with anti-human C5a Ab. Data are representative of two separate and independent experiments.
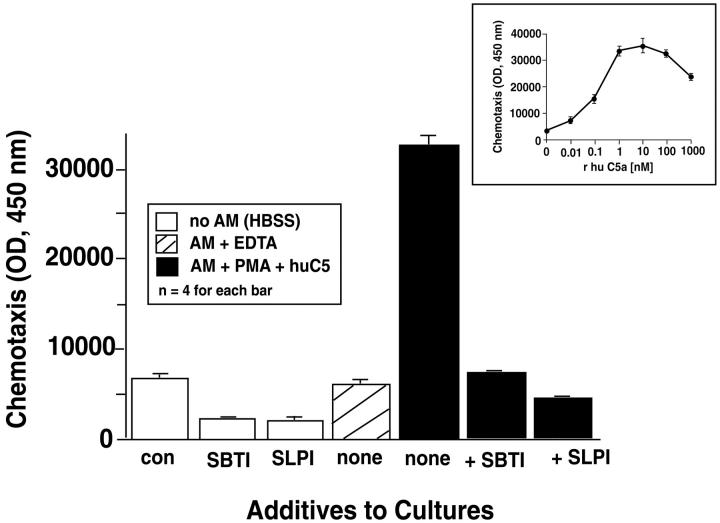
Inhibition by SBTI or SLPI in Macrophage Generation of Chemotactic Activity
Because the Western blots in Figure 7 ▶ indicated that the presence of either SBTI or SLPI with PMA-treated macrophages (in the presence of C5) substantially reduced or abolished the generation of C5a, we evaluated companion supernatant fluids for chemotactic activity using human neutrophils (Figure 9) ▶ . As expected, culture medium alone (ctrl), SBTI (100 μg/ml)alone, or SLPI (100 μg/ml) alone showed little evidence of inducing a migratory response of neutrophils (Figure 9 ▶ , white columns). The chemotactic activity in supernatant fluids from unstimulated AMs (Figure 9 ▶ , gray column) was at background levels. The combination of AMs (107), PMA (100 ng/ml), and C5 (90 μg) at 37°C for 4 hours yielded a very robust migratory response by neutrophils (Figure 9 ▶ , first black bar). In the presence of either SBTI or SLPI (each at 100 μg/ml), the generation of chemotactic activity fell back to background levels (Figure 9 ▶ , second and third black bars). Taking into account the amount of C5 added to the cell preparations and assuming nearly complete cleavage of C5a peptide from the α-chain of C5, it can be calculated that ∼50 nmol/L of C5a could theoretically be generated under such conditions. This is consistent with the dose response of human neutrophils to a range of doses of human C5a, as shown in the inset to Figure 9 ▶ . The fluorescence units of 32,000 in the tallest black bar in Figure 9 ▶ would extrapolate to a C5a concentration of ∼10 nmol/L.
Figure 9.
Chemotactic activity for human PMNs in samples indicated in the top left inset. Most details are similar to those described in the legend to Figure 6 ▶ . In some cases, SBTI (100 μg/ml) or SLPI (100 μg/ml) were added to the rat AM suspensions before addition of PMA and C5. A dose response for neutrophil chemotactic responses to recombinant human C5a (0.01 to 1000 nmol/L) is shown in the top right inset. Data are representative of three separate and independent experiments.
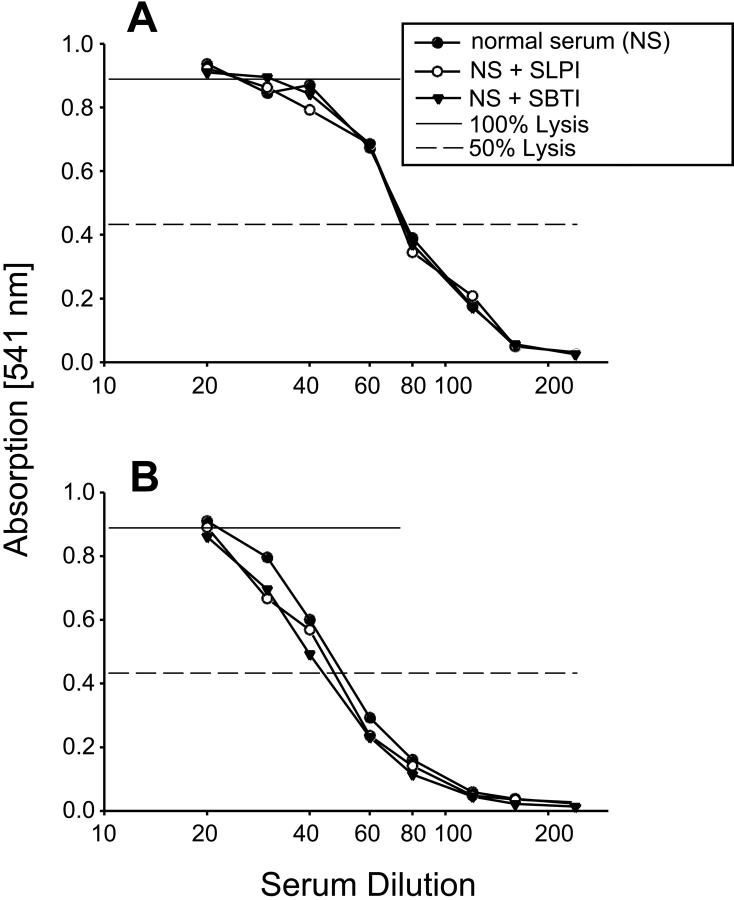
Lack of Inhibition of Serum CH50 by SBTI or SLPI
To gain further insight into the ability of SBTI or SLPI to interfere with C5 cleavage by activated rat AMs (Figure 7 ▶ , Table 1 ▶ ) and to address the question as to whether these inhibitors would interfere with serum CH50 (and by inference, the C3 and C5 convertases that are known to contain serine protease activity), SBTI or SLPI was added to various dilutions of whole human or rat serum at a constant concentration of 100 μg/ml each. CH50 levels were then determined. The results are shown in Figure 10 ▶ . As is evident by the data, the curves representative of CH50 activity were superimposable in the presence or absence of either protease inhibitor. Because these are primarily irreversible enzyme inhibitors, these findings suggest that, under the conditions used, these inhibitors do not block serum complement-derived convertases for C3 and C5. In turn, the data suggest that the C5-cleaving enzyme, which is associated with activated rat AMs and is blocked in the presence of SBTI or SLPI, is not a complement-derived C5 convertase.
Figure 10.
Hemolytic activity (CH50) of rat (A) or human (B) serum in the absence or presence of fixed concentrations of SBTI (100 μg/ml) or SLPI (100 μg/ml) added to various dilutions of serum. Dotted lines indicate the 50% hemolysis values. Data are representative of two separate and independent experiments.
Discussion
The complement activation product, C5a, is known to be involved in acute and chronic inflammatory injury of lungs. 2,4,5,7,8,20,21 Some clinical and experimental studies have described enhanced levels of C5a in BAL fluids obtained during bacterial 22 or aspiration pneumonia, 1 immune complex or LPS-induced acute lung injury, 2,4 and in chronic lung diseases. 11 When human C5a, C5adesarg, or bacterial chemotactic peptide were given intratracheally into rabbits or hamsters, extensive pulmonary accumulation of neutrophils was observed. 10,23 An intense pulmonary inflammatory reaction with extravasation of neutrophils also developed after intratracheal instillation of C5 into rabbits and hamsters, in which case 125I-C5 fragments were detectable in BAL fluids, 10,24 indicating a mechanism for C5 cleavage within the lung. In IgG immune complex-induced acute lung inflammatory injury, treatment with anti-C5a Ab greatly reduced the increase in lung vascular permeability and sharply diminished neutrophil accumulation. 4 It was suggested that the role of C5a during immune complex-induced inflammation was compartmentalized, because airway instillation of anti-C5a, but not intravenous infusion of anti-C5a, was protective. 4 Significant amounts of C5 activation products (C5a) may be generated in the airway compartments independent of components within the vascular compartment. 2,7 C5 activation products produced in the distal airway of lung may account for these observations. Potential local sources of complement components (especially C5) in the alveolar space may consist of several cell types, including AECs, 25,26 lung fibroblasts, 27 and lung interstitial macrophages. 9,12,13,28 The current studies indicate that activated human PMNs can generate C5a from C5 and that extensive hydrolysis of both α and β chains of C5 occurs. This is probably because of generalized protein degradation resulting in the generation of a C5a product by PMNs. Human PBMCs lack this activity, whereas rat AMs but not rat AECs have the ability to cleave C5 productive of C5a, but with rat AM hydrolysis of C5 (α and β chains) is very much more limited. In the current study we demonstrate that, in presence of C5, activated rat AMs can generate in a dose- and time-dependent manner a cleavage product that can be immunodetected by anti-human C5a Ab. In the presence of C5, cleavage products are detectable within 30 minutes. Pretreatment of AMs with actinomycin D or cycloheximide abolished the ability of those cells to generate C5a from C5, inferring that the C5-cleaving enzyme is inducible, as might be expected. No cleavage product of C5 could be detected if the anti-human C5a preparation had been preabsorbed with recombinant human C5a. The C5 cleavage product aligned with C5a that had been immunoprecipitated from activated human serum, consistent with the position of glycosylated C5a. Recombinant human C5a (generated in Escherichia coli) was invariably found in a position of ∼8 kDa, consistent with nonglycosylated C5a. C5a is known to be crucial for neutrophil influx into the alveolar space by mechanisms including: chemotactic activity for neutrophils, 2,15,21 enhancement of chemotactic cytokine release from macrophages, 2,7 and up-regulation of endothelial adhesion-cell molecules. 4,19 We focused on generation of neutrophil chemotactic activity. Supernatant fluids from stimulated rat AMs in the presence of C5 generated considerable chemotactic activity, almost equipotent to the activity of 100 nmol/L of fMLP or 10 nmol/L of human C5a. To further define the protein responsible for the functional activity, supernatant fluids were exposed to different anti-C5a Ab preparations. Whereas anti-rat C5a only slightly (but not significantly) reduced the chemotactic activity of supernatant fluids from activated rat AMs to which C5 had been added, anti-human C5a Ab completely inhibited the chemotactic activity, similar to the levels found in the absence of C5. These data indicate that AMs can generate biologically active C5a in the presence of added C5. Because macrophages have been reported to generate C5, 9,13 we speculate that the ability of anti-human C5a and anti-rat C5a together to totally block chemotactic activity of supernatant fluids from stimulated rat AMs may be because of not only human C5a but also because of rat C5a generated from endogenous rat C5, produced by AMs. 7
The well-established mechanisms of complement activation via its three different pathways involve formation and activation of C3 and C5 convertases. In complement-dependent convertases, the catalytically active center is known to reside in serine protease domains. 29 Beside these convertases, other proteases, such as cysteine protease gingipain-1 14 (an acid pH-required protease in macrophages), 30 and some proteins of the coagulation cascade (such as kallikrein and activated Hageman factor) have been described to exhibit C3 and C5 cleavage activity in vitro. 15 Because macrophages are known to produce and secrete many proteases, including matrix metallo-, cysteine-, serine-, and aspartic acid proteases, 9,22,31 we evaluated the macrophage-dependent C5-cleaving enzyme by using various protease inhibitors that block activities in the four major groups of proteases listed above. Although cysteine (leupeptin), aspartic acid (pepstatin), and matrix metalloproteinase inhibitors (TIMP-2) failed to interfere with the C5-cleaving enzyme, serine protease inhibitors partly (aprotinin) or completely (SBTI, SLPI) prevented C5a generation by AMs, thus implicating a serine protease as the C5-cleaving enzyme. SBTI and SLPI inhibited C5a generation by AMs in a dose-dependent manner and also completely suppressed the appearance of biological activity of cleavage products as determined by neutrophil chemotaxis assays. We have recently shown in IgG immune complex-induced injury of rat lung that SLPI protein is up-regulated in BAL fluids from the inflamed lung as early as 30 minutes after the onset of injury and remains up-regulated for up to 8 hours. 32 Jin and colleagues 33 have shown that LPS can induce SLPI mRNA in macrophages within 30 minutes of exposure, with peak expression at 24 hours. We have also shown that Ab-induced in vivo blockade of rat SLPI intensifies the degree of injury. 32 These data suggest a complex balance within the inflamed lung in which proinflammatory products (eg, C5-cleaving enzymes) and anti-inflammatory factors (eg, anti-proteases such as SLPI) determine the balance in the outcome of inflammation. Endogenous SLPI seems to play a regulatory role in the inflammatory response in lung. Intrapulmonary blockade of SLPI by Ab caused increased BAL levels of C5a, 32 suggesting that this endogenous inhibitor regulates C5a levels in lungs by blockade of the AM-derived serine protease. It has been suggested that a serine protease activity from human neutrophils can be acquired by macrophages that have phagocytized either fragmented or functionally intact neutrophil-derived serine elastase. 34 Other sources of serine proteases during lung inflammation include proteases liberated from damaged pulmonary tissues 31 and neutrophil-released elastase. 16,35 The C5-cleaving enzyme in lung may be chiefly derived from lung macrophages and seems to be inducible. Because SLPI and SBTI effectively blocked AM cleavage of C5, we investigated the effect of these inhibitors on the systemic complement activation as determined by hemolytic activity assays. Neither SBTI nor SLPI significantly interfered with CH50 activity of rat or human serum, suggesting that the rat AM-dependent C5-cleaving enzyme is not a complement-derived C5 convertase. 28 The present data underscore the diverse sources of C5a in biological systems, consistent with previous reports. 10,36
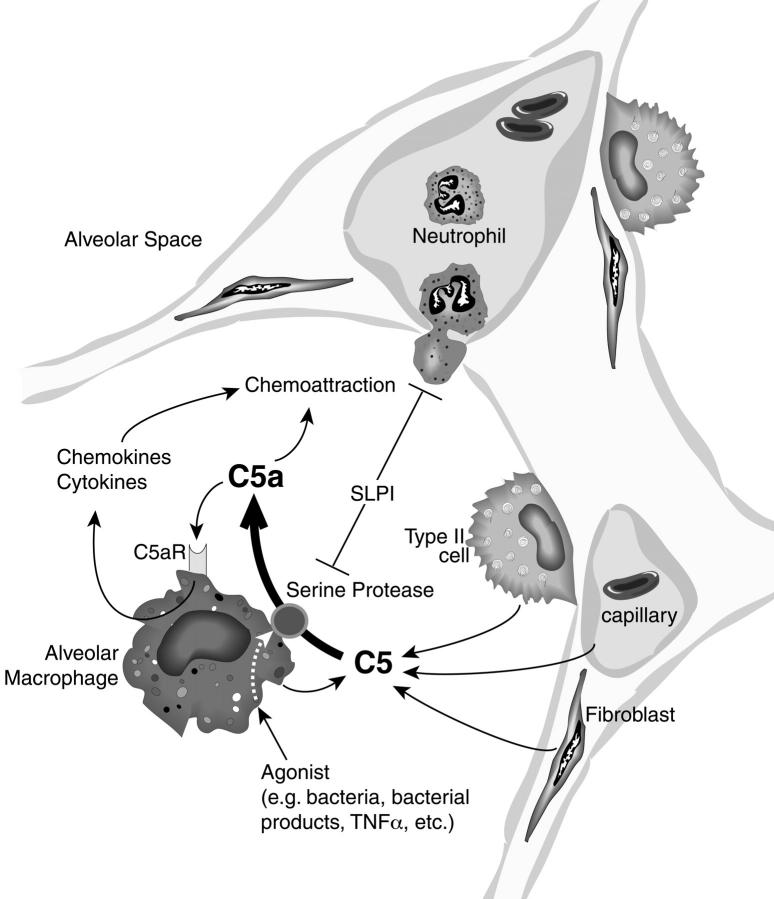
Figure 11 ▶ depicts a proposed model for generation of C5 activation products in the alveolar space independent of the plasma complement system. In this scheme, lung macrophages can be activated by a variety of agonists (bacteria, bacterial products, tumor necrosis factor-α, and so forth) to express, perhaps on their cell surfaces, a serine protease that has C5-cleaving activity productive of C5a. The source of C5 could be variable: it may derive from leakage of plasma C5 when vascular permeability has developed after injury of the vascular barrier, 2 or C5 may be produced by AECs, 25,26 by fibroblasts, 27 or by lung macrophages themselves. 9,12,13,28 The C5-cleaving enzyme then generates C5a, which could function as a chemoattractant for blood neutrophils and function also as an autocrine activator of lung macrophages. 7 C5a generation is inhibited by SLPI, which is known to be affected by oxidant stress 37 and has been recently shown to be up-regulated in inflamed lungs. 32 Obviously, the balance between these opposing pathways can determine the intensity of the lung inflammatory response.
Figure 11.
Proposed model of locally generated C5a in the alveolar compartment. C5 derived from the vascular compartment or produced locally from various types of lung cells is cleaved by activated lung macrophages to generate biologically active C5a. The cleavage enzyme from macrophages appears to be an inducible serine protease that can be blocked by naturally occurring SLPI. After C5 cleavage, C5a causes neutrophils to migrate into the alveolar space, resulting in release of inflammatory mediators, oxidative products, and other damaging proteases. C5a may also stimulate in an autocrine manner lung macrophages to enhance the inflammatory response.
Acknowledgments
We thank Beverly Schumann and Peggy Otto for excellent secretarial assistance in the preparation of the manuscript.
Footnotes
Address reprint requests to Peter A. Ward, M.D., Department of Pathology, The University of Michigan Medical School, 1301 Catherine Rd., Ann Arbor, Michigan 48109-0602. E-mail: pward@umich.edu.
Supported by the National Institutes of Health (grants GM-29507 and HL-31963).
References
- 1.Ishii Y, Kobayashi J, Kitamura S: Chemotactic factor generation and cell accumulation in acute lung injury induced by endotracheal acid instillation. Prostaglandins Leukot Essent Fatty Acids 1989, 37:65-70 [DOI] [PubMed] [Google Scholar]
- 2.Ward PA: Role of complement in lung inflammatory injury. Am J Pathol 1996, 149:1081-1086 [PMC free article] [PubMed] [Google Scholar]
- 3.Sibille Y, Reynolds HY: Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Med 1990, 141:471-501 [DOI] [PubMed] [Google Scholar]
- 4.Mulligan MS, Schmid E, Beck-Schimmer B, Till GO, Friedl HP, Brauer RB, Hugli TE, Miyasaka M, Warner RL, Johnson KJ, Ward PA: Requirement and role of C5a in acute lung injury in rats. J Clin Invest 1996, 98:503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomkin JS, Cotta LA, Satoh PS, Hurst JM, Nelson RD: Complement activation and clearance in acute illness and injury: evidence for C5a as a cell-directed mediator of the adult respiratory distress syndrome in man. Surgery 1984, 97:668-678 [PubMed] [Google Scholar]
- 6.Kazmierowski JA, Gallin JI, Reynolds HY: Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest 1977, 59:273-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czermak BJ, Sarma V, Bless NM, Schmal H, Friedl HP, Ward PA: In vitro and in vivo dependency of chemokine generation on C5a and TNFα. J Immunol 1999, 162:2321-2325 [PubMed] [Google Scholar]
- 8.Till GO, Morganroth ML, Kunkel R, Ward PA: Activation of C5 by cobra venom factor is required in neutrophil-mediated lung injury in the rat. Am J Pathol 1987, 129:44-53 [PMC free article] [PubMed] [Google Scholar]
- 9.Hetland G, Johnson E, Royset P, Eskeland T: Human alveolar macrophages and monocytes generate the functional classical pathway of complement in vitro. Acta Pathol Microbiol Immunol Scand 1987, 95:117-122 [DOI] [PubMed] [Google Scholar]
- 10.Henson PM, McCarthy K, Larsen GL, Webster RO, Giclas PC, Dreisin RB, King TE, Shaw JO: Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol 1979, 97:93-110 [PMC free article] [PubMed] [Google Scholar]
- 11.Groneck P, Oppermann M, Speer CP: Levels of complement anaphylatoxin C5a in pulmonary effluent fluids of infants at risk of chronic lung disease and effects of dexamethasone treatment. Pediatr Res 1993, 34:586-590 [DOI] [PubMed] [Google Scholar]
- 12.Ooi YM, Harris DE, Edelson PJ, Colten HR: Post-translational control of complement (C5) production by resident and stimulated mouse macrophages. J Immunol 1980, 124:2077-2081 [PubMed] [Google Scholar]
- 13.Hetland G, Johnson E, Aasebo U: Human alveolar macrophages synthesize the functional alternative pathway of complement and active C5 and C9 in vitro. Scand J Immunol 1986, 24:603-608 [DOI] [PubMed] [Google Scholar]
- 14.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE: Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from porphyromonas (bacteroides) gingivalis. J Biol Chem 1992, 276:18902-18907 [PubMed] [Google Scholar]
- 15.Wiggins RC, Giclas PC, Henson PM: Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med 1981, 153:1391-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward PA, Newman LJ: Neutrophil chemotactic factor from human C’5. J Immunol 1969, 102:93-99 [PubMed] [Google Scholar]
- 17.Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Younkin EM, Laudes IJ, Riedemann NC, Younger JG, Ward PA: Protective effects of anti-C5a peptide antibodies in experimental sepsis. EMBO J 2001, 15:568-570 [DOI] [PubMed] [Google Scholar]
- 18.Foreback JL, Remick DG, Crockett-Torabi E, Ward PA: Cytokine responses of human blood monocytes stimulated with Ig’s. Inflammation 1997, 21:501-517 [DOI] [PubMed] [Google Scholar]
- 19.Amsterdam EA, Stahl GL, Pa HL, Rendig SV, Fletcher MP, Longhur JC: Limitation of reperfusion injury by a monoclonal antibody to C5a during myocardial infarction in pigs. Am J Physiol 1995, 268:H448-H457 [DOI] [PubMed] [Google Scholar]
- 20.Mulligan MS, Schmid E, Till GO, Hugli TE, Friedl HP, Roth RA, Ward PA: C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol 1997, 158:1857-1861 [PubMed] [Google Scholar]
- 21.Robbins RA, Russ WD, Thomas KR, Rasmussen JK, Kay HD: Complement component C5 is required for release of alveolar macrophage-derived neutrophil chemotactic activity. Am Rev Respir Dis 1987, 135:659-664 [DOI] [PubMed] [Google Scholar]
- 22.Hopkins H, Stull T, von Essen SG, Robbins RA, Rennard SI: Neutrophil chemotactic factors in bacterial pneumonia. Chest 1989, 95:1021-1027 [DOI] [PubMed] [Google Scholar]
- 23.Desai U, Kreutzer DL, Showell HT, Arroyave CV, Ward PA: Acute lung inflammatory pulmonary reactions induced by chemotactic factor. Am J Pathol 1979, 96:71-83 [PMC free article] [PubMed] [Google Scholar]
- 24.Desai U, Dickey B, Varani J, Kreutzer DL: Demonstration of C5 cleaving activity in bronchoalveolar fluids and cells: a mechanism of acute and chronic alveolitis. J Exp Pathol 1984, 1:201-216 [PubMed] [Google Scholar]
- 25.Strunk RC, Eidlen DM, Mason RJ: Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest 1988, 81:1419-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman BL, Merrow M, Despins A, Kennedy T, Kreutzer DL: Effect of lipopolysaccharide on C3 and C5 production by human lung cells. J Immunol 1989, 143:196-202 [PubMed] [Google Scholar]
- 27.Rothman BL, Contrino J, Merrow M, Despins A, Kennedy T, Kreutzer DL: Silica induced suppression of the production of third and fifth components of the complement system by human lung. Immunopharmacol Immunotoxicol 1994, 16:525-551 [DOI] [PubMed] [Google Scholar]
- 28.Pettersen HB, Johnson E, Mollnes TE, Garred P, Hetland G, Osen SS: Synthesis of complement by alveolar macrophages from patients with sarcoidosis. Scand J Immunol 1990, 31:15-23 [DOI] [PubMed] [Google Scholar]
- 29.Narayana SVL, Babu YS, Volankis JE: Inhibition of complement serine proteases as a therapeutic strategy. Lambris JD Holers VM eds. Therapeutic Interventions in the Complement System. 2000:pp 113-153 Humana Press, Totowa
- 30.Murata A, Toda H, Uda KI, Nakagawa H: Novel protease inhibitory activities of the second domain of urinary trypsin inhibitor (R-20) and its effect on sepsis-induced organ injury in rat. Inflammation 1994, 18:337-347 [DOI] [PubMed] [Google Scholar]
- 31.McElvaney NG, Crystal RG: Proteases and lung injury. Crystal RG West JB Weilbel ER Barnes PJ eds. The Lung: Scientific Foundations. 1997:pp 2205-2218 Lippincott-Raven Publishers, Philadelphia
- 32.Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, Mueller WT, Johnson KJ, Ward PA: Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 1999, 162:3653-3662 [PubMed] [Google Scholar]
- 33.Jin F, Nathan CF, Radzioch D, Ding A: Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun 1998, 66:2447-2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGowan SE, Stone PJ, Calore JD, Snider GL, Franzblau C: The fate of neutrophil elastase incorporated by human alveolar macrophages. Am Rev Respir Dis 1983, 127:449-455 [DOI] [PubMed] [Google Scholar]
- 35.Doering G: The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med 1994, 150:114-117 [DOI] [PubMed] [Google Scholar]
- 36.Snyderman R, Shin HS, Dannenberg AM: Macrophage proteinase and inflammation: the production of chemotactic activity from the fifth component of complement by macrophage proteinase. J Immunol 1972, 109:896-898 [PubMed] [Google Scholar]
- 37.Vogelmeier C, Biedermann T, Maier K, Mazur G, Behr J, Krombach F, Buhl R: Comparative loss of activity of recombinant secretory leukoprotease inhibitor and α1-protease inhibitor caused by different forms of oxidative stress. Eur Respir J 1997, 10:2114-2119 [DOI] [PubMed] [Google Scholar]