Abstract
A multistep model of carcinogenesis has recently been proposed for pancreatic ductal adenocarcinomas. In this model, noninvasive precursor lesions in the pancreatic ductules accumulate genetic alterations in cancer-associated genes eventually leading to the development of an invasive cancer. The nomenclature for these precursor lesions has been standardized as pancreatic intraepithelial neoplasia or PanIN. Despite the substantial advances made in understanding the biology of invasive pancreatic adenocarcinomas, little is known about the initiating genetic events in the pancreatic ductal epithelium that facilitates its progression to cancer. Telomeres are distinctive structures at the ends of chromosomes that protect against chromosomal breakage-fusion-bridge cycles in dividing cells. Critically shortened telomeres can cause chromosomal instability, a sine qua non of most human epithelial cancers. Although evidence for telomeric dysfunction has been demonstrated in invasive pancreatic cancer, the onset of this phenomenon has not been elucidated in the context of noninvasive precursor lesions. We used a recently described in situ hybridization technique in archival samples (Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM: Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. American Journal of Pathology 2002, 160:1259–1268) for assessment of telomere length in tissue microarrays containing a variety of noninvasive pancreatic ductal lesions. These included 82 PanIN lesions of all histological grades (24 PanIN-1A, 23 PanIN-1B, 24 PanIN-2, and 11 PanIN-3) that were selected from pancreatectomy specimens for either adenocarcinoma or chronic pancreatitis. Telomere fluorescence intensities in PanIN lesions were compared with adjacent normal pancreatic ductal epithelium and acini (62 of 82 lesions, 76%), or with stromal fibroblasts and islets of Langerhans (20 of 82 lesions, 24%). Telomere signals were strikingly reduced in 79 (96%) of 82 PanINs compared to adjacent normal structures. Notably, even PanIN-1A, the earliest putative precursor lesion, demonstrated a dramatic reduction of telomere fluorescence intensity in 21 (91%) of 23 foci examined. In chronic pancreatitis, reduction of telomere signal was observed in all PanIN lesions, whereas atrophic and inflammatory ductal lesions retained normal telomere length. Telomere fluorescence intensity in PanIN lesions did not correlate with proliferation measured by quantitative Ki-67-labeling index or topoisomerase IIα expression. Thus, telomere shortening is by far the most common early genetic abnormality recognized to date in the progression model of pancreatic adenocarcinomas. Telomeres may be an essential gatekeeper for maintaining chromosomal integrity, and thus, normal cellular physiology in pancreatic ductal epithelium. A critical shortening of telomere length in PanINs may predispose these noninvasive ductal lesions to accumulate progressive chromosomal abnormalities and to develop toward the stage of invasive carcinoma.
The 5-year survival rate of patients with ductal adenocarcinoma of the pancreas is 4%, one of the lowest of any neoplasm. Each year, nearly 29,000 patients are diagnosed with pancreatic cancer in the United States, and almost all will succumb to their disease. 1 Currently, surgery, with or without adjuvant therapy, is the only acceptable therapeutic option, although most patients tend to present with advanced, unresectable disease. Thus, either primary prevention or early detection remains the best chance for cure from this lethal neoplasm. 2 However, to detect pancreas cancer at an early, potentially curative stage, it is critical that we understand the biology of precursors to pancreatic neoplasia.
Similar to the adenoma-carcinoma sequence in the colon, 3 there is histological and molecular evidence to suggest a multistep progression model for the development of pancreatic cancer. 4 In the pancreas, the noninvasive precursor lesions are called pancreatic intraepithelial neoplasia or PanIN. 5 PanINs are believed to progress from flat and papillary without dysplasia, to papillary with dysplasia, to carcinoma in situ (PanIN-1A to PanIN-1B to PanIN-2 toPanIN-3) (additional details are available at http://www.pathology.jhu.edu/pancreas_panin). This unification of terminology and diagnostic criteria is a critical first step toward a better understanding of the precursors to invasive pancreatic cancer, which have been studied for more than 100 years. 6
The strongest evidence in favor of the PanIN-ductal adenocarcinoma sequence in the pancreas comes from genetic analyses of these precursor lesions. PanINs share many of the molecular abnormalities seen in invasive cancer. For example, alterations in the KRAS2, CDKN2A/p16INK4A, BRCA2, TP53, and MADH4 (SMAD4, DPC4) genes have all been reported in PanINs. 7-9 However, except for activating mutations of the K-ras oncogene, most genetic abnormalities are observed in the histologically more advanced PanINs, ie, PanIN-2 and PanIN-3. Unlike the loss of an essential gatekeeper—the APC gene function—in the vast majority of colorectal adenomas, 3 no single overriding genetic abnormality has been detected in early PanINs. The initiating event(s) in neoplastic progression within the pancreatic ducts, therefore, remains primarily unknown.
Telomeres are structures present at the ends of linear chromosomes, comprising hexameric DNA repeat sequences (TTAGGG) in association with telomere-binding proteins. 10 Telomeric repeat sequences prevent fusion between ends of chromosomes, and telomeric dysfunction is a major mechanism for the generation of chromosomal instability (CIN). 11-16 Telomeric fusions between chromosomal arms may occur in the presence of critically shortened telomere repeat sequences; such fusions lead to ring and dicentric chromosomes that form so-called anaphase bridges during mitosis. 15 Breakage of anaphase bridges generates highly recombinogenic free DNA ends, with fusion of broken ends resulting in novel chromosomal rearrangements. Some of these abnormal chromosomes may, in turn, form bridges during the next cell division, setting in motion a self-perpetuating breakage-fusion-bridge cycle. 13,15,17 The presence of unbalanced chromosomal rearrangements is a sine qua non of most human epithelial cancers. Specifically, pancreatic adenocarcinomas, which are remarkable for their highly complex karyotypes, numerous chromosomal abnormalities, and multiple deletions on allelotyping, 18-20 demonstrate chromosome ends lacking telomeric repeat sequences in the majority of cases. 15 Thus, telomeric dysfunction with resultant CIN may be a key driving force in pancreatic carcinogenesis.
The timing of telomeric dysfunction has not been examined in the context of the multistage progression model of pancreatic adenocarcinomas. A fluorescence in situ hybridization protocol has recently been described for assessment of telomere repeat lengths in archival tissues. 21 We examined the telomere repeat lengths in a series of 82 PanIN lesions of all histological grades, using tissue microarrays (TMAs) containing a variety of noninvasive ductal lesions. Our results indicate that telomeric dysfunction is one of the most common early genetic aberrations observed in PanINs and may facilitate the progression of the pancreatic ductal epithelium toward cancer.
Materials and Methods
Archival Tissue Samples
Tissue samples were obtained from the surgical pathology archives of the Department of Pathology at the Johns Hopkins University School of Medicine. Formalin-fixed paraffin-embedded blocks were retrieved from 44 patients who underwent pancreaticoduodenectomy (Whipple resection) for pancreatic ductal adenocarcinoma, and from 32 patients who underwent surgery for chronic pancreatitis. Two TMAs were constructed, the first (TMA 1) containing PanIN lesions adjacent to pancreatic adenocarcinoma specimens, and the second (TMA 2), containing a mixture of PanIN, atrophic, and inflammatory duct lesions from the chronic pancreatitis specimens, respectively. PanIN lesions were selected by three authors (NTvH, RHH, and AM), and classified into PanIN-1A, PanIN-1B, PanIN-2, and PanIN-3 by using previously described criteria. 5 Inflammatory duct lesions in chronic pancreatitis were defined by extensive periductal and/or intraepithelial inflammation, usually mononuclear in nature. For the TMA construction, representative areas containing morphologically defined PanINs were circled on the glass slides and used as a template. TMAs were constructed using a manual Tissue Puncher/Arrayer (Beecher Instruments, Silver Spring, MD) as previously described. 22 For each selected lesion, a 1.4-mm core was punched from the donor block to ensure that the entire duct lesion and adequate surrounding tissue could be incorporated into the spot. A total of 99 cores (72 PanIN lesions and 27 tissue cores from a variety of extra-pancreatic tissues) were arrayed on the TMA 1 recipient block; similarly, 99 cores (63 pancreatic duct lesions and 36 miscellaneous extra-pancreatic tissue cores) were arrayed on the TMA 2 recipient block. Four serial sections were cut from both TMAs, of which one was stained with hematoxylin and eosin (H&E) as a reference. Overall, 66 PanIN foci of all histological grades (15 PanIN-1A, 18 PanIN-1B, 22 PanIN-2, and 11 PanIN-3) were adequate for evaluation using telomere-specific peptide nucleic acid fluorescence in situ hybridization (TEL-FISH) and immunohistochemistry in TMA 1; 16 PanIN lesions (8 PanIN-1A, 5 PanIN-1B, and 3 PanIN-2), 10 atrophic duct lesions, 6 inflammatory duct lesions, and 21 cores with normal pancreatic ducts were adequate for evaluation on TMA 2.
TEL-FISH
The protocol for combined staining of telomeres and DNA was performed without protease digestion, as previously described. 21 Briefly, the deparaffinized tissue array slides underwent steam heating in citrate buffer, followed by hybridization with a Cy3-labeled TEL-FISH probe, and processed for indirect immunofluorescence and counterstaining with DAPI (4′-6-diamidino-2-phenylindole).
Microscopy and Image Analysis
The TEL-FISH fluorescence microscopy was performed by three authors on the panel (NTvH, AKM, and AM). A serial H&E-stained reference slide was examined concurrently on a light microscope as a guide. Slides were imaged with a Zeiss Axioskop epifluorescence microscope (Carl Zeiss Inc, Thornwood, NY) equipped with appropriate fluorescence filter sets (Omega Optical, Brattleboro, VT). Telomere signals were visually evaluated in real-time by qualitative comparison of pixel intensity of the TEL-FISH probe in the PanINs with adjacent normal structures. Telomere lengths in PanIN lesions were compared with adjacent normal pancreatic ductal epithelium and acini (62 of 82 lesions, 76%), or with stromal fibroblasts and islets of Langerhans (20 of 82 lesions, 24%). Inflammatory and atrophic ducts in chronic pancreatitis were compared with residual acini, islets of Langerhans, or stromal fibroblasts. The telomere fluorescence results in the PanINs were classified as greater than, less than, or equal to the normal structures.
In addition, 10 nuclei each from one representative PanIN lesion from the four histological categories (PanIN-1A, PanIN-1B, PanIN-2, and PanIN-3) and corresponding 10 nuclei from normal ducts from the same case were quantified as described. 21 In this method, the sum of pixel intensities for the telomere signals in the Cy3 channel for a given cell nucleus is normalized to the total DAPI signal, such that the normalized telomere signals are linearly proportional to the mean telomere length as assessed independently by Southern blotting. 21 For each patient sample, comparisons of the mean ratios of telomeric signal to DAPI between the various cell types were done using the paired t-test with STATA 6.0 for Microsoft Windows (Stata Corp., College Station, TX); a P value of <0.05 was considered statistically significant.
Ki-67 and Topoisomerase IIα Immunolabeling
For detection of Ki-67 and topoisomerase IIα, immediately adjacent serial sections from TMA 1 were steamed for 20 minutes in sodium citrate buffer (diluted to 1× from 10× heat-induced epitope retrieval buffer; Ventana-Bio Tek solutions, Tucson, AZ). After cooling for 5 minutes, slides were labeled with a 1:100 dilution of mouse monoclonal antibody against Ki-67 (MIB-1; Immunotech, Westbrook, ME) or a 1:3200 dilution of mouse monoclonal antibody against topoisomerase II (clone TG100; Neomarkers, Freemont, CA) using the Bio Tek 1000 automated stainer (Ventana). Labeling was detected by addition of biotinylated secondary antibodies, avidin-biotin complex, and 3,3′-diaminobenzidine. All sections were counterstained with hematoxylin. Labeling was evaluated by two of the authors (NTvH, AM). Both Ki-67 and topoisomerase IIα are nuclear, and labeling of >10% of nuclei was considered positive and <10% cells was considered negative.
Results
Qualitative Assessment Versus Histology
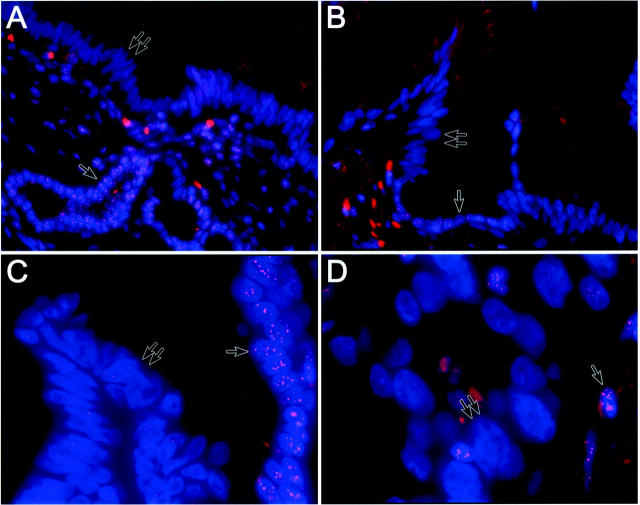
Microscopic examination demonstrated numerous intense fluorescence labeling spots in the nuclei of normal ductal epithelium, pancreatic acini, fibroblasts, and islets of Langerhans. In general, the intensities for TEL-FISH were comparable between the four reference control structures. Even atrophic ducts, which are characteristically abundant in the nonneoplastic pancreas adjacent to adenocarcinomas, contained intense TEL-FISH signals. No observable differences in telomere signal intensities were seen in the normal tissues obtained from patients with chronic pancreatitis versus those from patients harboring adenocarcinomas. The highest intensity of fluorescence, however, was seen in the lymphocytes, consistent with the long telomeric repeats that these cells are known to possess; 23 lymphocytes were therefore not used as a control for comparison of telomere lengths. In contrast to the reference normal cell types, telomeric repeat fluorescent signals were strikingly less intense with scattered weak to absent signals in the PanIN nuclei (Figure 1; A, B, and C) ▶ in 63 (95%) of 66 PanIN foci examined on TMA 1, and 16 (100%) of 16 PanIN foci on TMA 2. Thus, overall, 79 (96%) of 82 PanINs examined demonstrated reduction in telomere signal intensities, and no obvious difference in telomere intensities was seen between PanIN lesions adjacent to adenocarcinomas versus PanIN lesions arising in the context of chronic pancreatitis. When classified by histological subtype, 21 (91%) of 23 PanIN-1A, 23 (100%) of 23 PanIN-1B, 24 (96%) of 25 PanIN-2, and 11 (100%) of 11 PanIN-3 had unambiguous reduction in telomere repeat signals. There were no readily observable qualitative differences between telomere repeat lengths in PanINs of various histological grades, which were also borne out by the quantitative assessment (see below). In all but one PanIN lesion, signal reduction was seen in nuclei throughout the lesion; in a single PanIN-2, heterogeneity of signal intensities was observed with approximately half the lesional nuclei demonstrating signal reduction compared to normal. Often, a sharp demarcation associated with a dramatic reduction in telomere signal could be observed in the histological transition between PanINs and normal ductal epithelium, and this phenomenon was observed independent of the histological grade of the PanIN. In occasional cores, cancerization of ducts (replacement of ductal epithelium by infiltrating adenocarcinoma, simulating carcinoma-in situ) could be seen, and in all instances, the cancerized ducts demonstrated marked signal reduction, as did infiltrating adenocarcinoma (Figure 1D) ▶ . In contrast to unequivocal reduction in telomere fluorescence intensities in PanIN lesions, intense fluorescence labeling was observed in 10 atrophic and 6 inflammatory ducts in chronic pancreatitis.
Figure 1.
TEL-FISH for assessment of telomere repeat lengths performed on PanIN lesions adjacent to pancreatic adenocarcinoma. The telomeres are stained with Cy3-labeled anti-telomeric probe and are colored red; the DNA is counterstained with DAPI and colored blue. A: Low-grade PanIN (PanIN-1A) demonstrates weak telomeric signals in the nuclei (double arrows), while intense telomeric signals are retained in the subjacent normal epithelium (arrow) and the stromal fibroblasts. B: Low-grade PanIN (PanIN-1B) demonstrates weak telomeric signals in the papillary tufts (double arrows); in contrast, intense telomeric signals are retained in the strip of retained normal epithelium (arrow). C: High-grade PanIN (PanIN-3) with weak telomeric signals (double arrows); in contrast, there is a sharp transition with the normal epithelium demonstrating intense telomeric fluorescence (arrow). D: Weak telomeric fluorescence in cancerized ducts (double arrows); the interspersed bright signals (arrow) are lymphocytes within and surrounding the cancerized ducts. DAPI counterstain; original magnifications: ×40 (A, B); ×100 (C, D).
Quantitative Assessment Versus Histology
Ten nuclei each from one representative example of the four histological grades of PanIN were quantified for telomeric repeat length in conjunction with 10 nuclei from normal ductal epithelium for the same case, by TEL-FISH. The average telomeric repeat lengths in the four PanIN lesions and corresponding normal ducts are tabulated in Table 1 ▶ ; telomere repeat lengths were significantly shorter in all histological grades of PanINs compared to the normal pancreatic ducts. Quantification of the telomere signals, however, demonstrated no statistically significant differences in telomere repeat lengths between the PanIN-1A, the earliest precursor lesion, and histologically more advanced PanINs (PanIN-1B, PanIN-2, and PanIN-3).
Table 1.
Quantitative Assessment of Telomere Lengths in PanINs Compared with Normal Pancreatic Ducts from the PanIN TMA
| PanIN-lesion | PanIN nuclei (n) | PanIN mean | PanIN SD | Normal nuclei (n) | Normal mean | Normal SD | P (PanIN versus normal) |
|---|---|---|---|---|---|---|---|
| 1A | 10 | 57.5 | 32.8 | 10 | 134.8 | 37.0 | 0.002 |
| 1B | 10 | 32.6 | 24.0 | 10 | 156.9 | 53.8 | <0.001 |
| 2 | 10 | 90.7 | 51.8 | 10 | 233.1 | 74.1 | 0.002 |
| 3 | 10 | 89.7 | 57.7 | 10 | 247.2 | 61.7 | <0.001 |
Assessment of Proliferation in PanIN Lesions
Proliferation in PanIN lesions was assessed by Ki-67 and topoisomerase IIα nuclear immunolabeling on the TMA 1. In 11 of (100%) 11 PanIN-3 lesions, >10% of nuclei labeled for both Ki-67 and topoisomerase IIα, whereas 3 (14%) of 22 PanIN-2 were positive for Ki-67, but not topoisomerase IIα. In contrast, none of the PanIN-1A, PanIN-1B, or remaining 19 (86%) of 22 PanIN-2 lesions was positive for either protein.
Discussion
Functional telomeres protect chromosome ends from recombination and fusion and are therefore essential for maintaining chromosomal stability. 12-16 Telomere shortening has been suggested to be an important biological factor in aging, cellular senescence, cell immortality, and transformation to cancer; the last two are associated with reactivation of the enzyme telomerase in cells with critically shortened telomeres. 24 In the presence of shortened telomere repeat fragments, the ends of linear chromosomes may undergo so-called anomalous bridge-fusion-breakage events that result in both structural and numerical chromosomal abnormalities. 11,13,15,17 Thus, the genesis of unbalanced chromosomal translocations, a sine qua non of most human epithelial malignancies, may rely, at least in part, on the presence of shortened telomeres. The consequences of CIN are manifold, because breakage and fusion may disrupt critical genes involved in cell regulation, DNA repair, and apoptosis, setting in motion an autonomous proliferation of genetically disrupted cells that characterizes the essence of cancer. 25 The importance of CIN as an early driving force in carcinogenesis has been demonstrated in epithelial malignancies, such as colorectal cancers, in which anaphase bridges and allelic imbalance can be seen at the adenoma stage, which are the precursors to invasive cancer. 12,26 Although there are many potential causes for CIN, critical telomere shortening may be the most important mechanism for its occurrence, and therefore, it is postulated that CIN in colorectal adenomas is a consequence of telomere dysfunction. The demonstration of CIN in preneoplastic lesions has been greatly facilitated by the recent description of a fluorescence in situ hybridization methodology that is applicable to archival tissue samples. 21 Using this innovative technique, Meeker and colleagues 27 have reported the presence of strikingly shortened telomere repeat segments in the vast majority of high-grade prostatic intraepithelial neoplasia, the precursors to prostate cancer.
A growing body of morphological, clinical, and molecular evidence supports the hypothesis for a multistage progression model of pancreas cancer. 4,5,28 In this progression model, invasive pancreatic adenocarcinoma is preceded by the sequential appearance of morphologically recognizable noninvasive ductal lesions known as PanINs. 5 PanINs, especially the intermediate and higher grade lesions, share many of the genetic abnormalities observed in invasive cancers, which is compelling evidence for their neoplastic potential. Despite substantial progress made in understanding the molecular abnormalities during progression of pancreatic adenocarcinomas, the initiating genetic events remain primarily unknown. Invasive pancreatic adenocarcinomas have a remarkable degree of CIN, manifested as bizarre nonreciprocal translocations and numerical abnormalities. 18,19 It has been postulated that CIN in pancreas cancers, similar to other epithelial malignancies, is a manifestation of telomere dysfunction. 15 Although shortened telomere repeat lengths have been reported in the majority of cancer cases, 15 the onset of telomere dysfunction has not been studied in the context of the progression model of pancreas cancer. Were telomere shortening to occur as an early event, it would imply that the CIN observed in invasive cancers had its origins in the precursor ductal lesions that precede invasive adenocarcinoma. It would also suggest that CIN-induced abrogation of gene function might be a key mechanism driving the ductal epithelium toward neoplastic transformation. We therefore combined TMAs of noninvasive pancreatic ductal lesions with a recently described technique for telomere length assessment in archival tissues to study PanINs. We found reduction in telomere fluorescence intensity to be nearly universal in all histological grades of PanIN lesions [79 (96%) of 82], when compared to normal pancreatic ducts, acini, islets, or stromal fibroblasts. Even PanIN-1A, the lowest grade precursor lesion classified in the pancreatic cancer progression model, demonstrated strikingly reduced telomere signals in 21 (91%) of 23 foci compared to normal ductal epithelium. In almost all instances, an abrupt transition in telomere length was seen between normal ductal epithelium and the adjacent PanIN lesion, irrespective of the histological grade of the PanIN (Figure 1; A, B, and C) ▶ . In contrast, no readily observable differences were seen in telomere length between histologically low-grade and high-grade PanIN lesions or between PanIN and invasive adenocarcinoma (Figure 1D) ▶ .
Because it is well known that genetic similarities exist between invasive pancreatic adenocarcinomas and their adjacent noninvasive precursor lesions, we also determined the telomere signals in PanIN lesions from histologically documented benign pancreata with chronic pancreatitis only. All 16 PanIN lesions demonstrated a striking reduction, whereas the 10 atrophic and 6 inflammatory duct lesions retained normal telomere signal intensities (data not shown). There were no observable differences seen in the intensity of telomere signals between PanIN lesions adjacent to adenocarcinomas versus those arising in chronic pancreatitis. Therefore, on the basis of nearly universal telomere dysfunction, we believe that there are no benign PanIN lesions, and as implied by their nomenclature, PanINs are true noninvasive neoplasms of the pancreatic ductal epithelium. In contrast, based on currently available data, atrophic and inflammatory duct lesions in chronic pancreatitis are unlikely to harbor a significant neoplastic potential.
Thus, telomere dysfunction is by far the most common early genetic abnormality reported to date in the progression model of pancreatic adenocarcinoma, with the frequency of telomere length shortening (96%) exceeding the reported frequencies of K-ras oncogene activation (50%), loss of p16 expression (30%), and inactivation of DPC4, BRCA2, and TP53 (all 0%) in low-grade PanIN lesions (PanIN-1A and PanIN-1B). 7-9,29 In addition, unlike other genetic alterations previously described, the frequency of telomere length alterations seems to be independent of the histological grade of PanIN, because a readily observable progressive shortening of telomere length was not seen in the transition from PanIN-1A to PanIN-3. It may be conceivably argued that shortening of telomere length is a consequence of increased proliferation within PanIN lesions, a manifestation of the so-called “end replication” problem in telomerase-negative cells that are dividing. 10,30 However, early and intermediate PanINs have a relatively low proliferation rate, as demonstrated in this and other studies. 31 Thus, although we cannot rule out the possibility that telomere shortening occurred as a result of a remote proliferative burst in the ductal epithelium (perhaps during repeated episodes of injury and repair), it seems to be independent of ongoing proliferation. The genesis of telomere shortening in PanIN lesions remains a matter of speculation; postulated mechanisms include a role for reactive oxidant species, 32 or inactivation of one or more telomere-binding proteins that maintain telomere lengths, 33-35 and additional studies may be able to elucidate this issue further.
It is unlikely that shortening of telomeres is, in and of itself, sufficient to induce neoplasia. However, shortened telomeres could open the gates, setting the stage for acquiring progressive chromosomal abnormalities, even in the absence of excessive proliferation in early-grade PanINs. This model is also compatible with previous observations in the natural history of PanINs, including the presence of allelic imbalance (loss of heterozygosity) before intragenic mutation, 36 which is as expected were CIN an early phenomenon in the ductal epithelium in this neoplastic progression model.
In conclusion, we report that telomere shortening is one of the earliest demonstrable genetic aberrations in the precursor lesions of invasive pancreatic cancer and is nearly universal in all histological grades of PanINs. Telomere shortening is unlikely to provide any direct growth advantage to the ductal epithelial cells, but it presumably leads to the acquisition of chromosomal abnormalities that facilitates the progression of these cells toward invasive cancer. The demonstration of strikingly shortened telomeres in even the PanIN-1A lesions also provides compelling evidence that these earliest of noninvasive ductal lesions are integral to the progression model of pancreatic adenocarcinoma.
Acknowledgments
We thank Helen Fedor and Marcella Southerland for expertise in tissue microarray construction.
Footnotes
Address reprint requests to Anirban Maitra, M.D., Ross 632, Department of Pathology, Johns Hopkins University School of Medicine, 720 Rutland Ave., Baltimore, MD 21205. E-mail: amaitra1@jhmi.edu.
Supported by the National Cancer Institute (Gastrointestinal Specialized Program of Research Excellence grant CA62924).
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M: Cancer statistics, 2001. CA Cancer J Clin 2001, 51:15-36 [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Canto MI, Yeo CJ: Prevention of pancreatic cancer and strategies for management of familial pancreatic cancer. Dig Dis 2001, 19:76-84 [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Wilentz RE, Kern SE: Genetic progression in the pancreatic ducts. Am J Pathol 2000, 156:1821-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ: Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001, 25:579-586 [DOI] [PubMed] [Google Scholar]
- 6.Hulst SPL: Zur kenntnis der genese des adenokarzinoms und karzinoms des pankreas. Virchows Arch 1905, 180:288-316 [Google Scholar]
- 7.Goggins M, Hruban RH, Kern SE: BRCA2 is inactivated late in the development of pancreatic intraepithelial neoplasia: evidence and implications. Am J Pathol 2000, 156:1767-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, Yeo CJ, Kern SE, Hruban RH: Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998, 58:4740-4744 [PubMed] [Google Scholar]
- 9.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH: Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000, 60:2002-2006 [PubMed] [Google Scholar]
- 10.Harley CB, Villeponteau B: Telomeres and telomerase in aging and cancer. Curr Opin Genet Dev 1995, 5:249-255 [DOI] [PubMed] [Google Scholar]
- 11.O’Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, DePinho R: Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell 2002, 2:149-155 [DOI] [PubMed] [Google Scholar]
- 12.Rudolph KL, Millard M, Bosenberg MW, DePinho RA: Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 2001, 28:155-159 [DOI] [PubMed] [Google Scholar]
- 13.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA: Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406:641-645 [DOI] [PubMed] [Google Scholar]
- 14.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA: p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 1999, 97:527-538 [DOI] [PubMed] [Google Scholar]
- 15.Gisselsson D, Jonson T, Petersen A, Strombeck B, Dal Cin P, Hoglund M, Mitelman F, Mertens F, Mandahl N: Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci USA 2001, 98:12683-12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackett JA, Greider CW: Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 2002, 21:619-626 [DOI] [PubMed] [Google Scholar]
- 17.McClintock B: The stability of broken ends of chromosomes in Zea mays. Genetics 1941, 26:234-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin CA, Hruban RH, Morsberger LA, Ellingham T, Long PP, Jaffee EM, Hauda KM, Bohlander SK, Yeo CJ: Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res 1995, 55:2394-2399 [PubMed] [Google Scholar]
- 19.Brat DJ, Hahn SA, Griffin CA, Yeo CJ, Kern SE, Hruban RH: The structural basis of molecular genetic deletions. An integration of classical cytogenetic and molecular analyses in pancreatic adenocarcinoma. Am J Pathol 1997, 150:383-391 [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson B, Bardi G, Heim S, Mandahl N, Mertens F, Bak-Jensen E, Andren-Sandberg A, Mitelman F: Nonrandom chromosomal rearrangements in pancreatic carcinomas. Cancer 1992, 69:1674-1681 [DOI] [PubMed] [Google Scholar]
- 21.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM: Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol 2002, 160:1259-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manley S, Mucci NR, De Marzo AM, Rubin MA: Relational database structure to manage high-density tissue microarray data and images for pathology studies focusing on clinical outcome: the prostate specialized program of research excellence model. Am J Pathol 2001, 159:837-843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummendorf TH, Rufer N, Holyoake TL, Maciejewski J, Barnett MJ, Eaves CJ, Eaves AC, Young N, Lansdorp PM: Telomere length dynamics in normal individuals and in patients with hematopoietic stem cell-associated disorders. Ann NY Acad Sci 2001, 938:293-303 [DOI] [PubMed] [Google Scholar]
- 24.Shay JW: Molecular pathogenesis of aging and cancer: are telomeres and telomerase the connection? J Clin Pathol 1997, 50:799-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengauer C: How do tumors make ends meet? Proc Natl Acad Sci USA 2001, 98:12331-12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih IM, Zhou W, Goodman SN, Lengauer C, Kinzler KW, Vogelstein B: Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res 2001, 61:818-822 [PubMed] [Google Scholar]
- 27.Meeker AK, Hicks JE, Platz EA, March GE, Bennett CJ, DeMarzo AM: Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res 2002, in press [PubMed]
- 28.Luttges J, Kloppel G: Update on the pathology and genetics of exocrine pancreatic tumors with ductal phenotype: precursor lesions and new tumor entities. Dig Dis 2001, 19:15-23 [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Wilentz RE, Goggins M, Offerhaus GJ, Yeo CJ, Kern SE: Pathology of incipient pancreatic cancer. Ann Oncol 1999, 10:9-11 [PubMed] [Google Scholar]
- 30.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB: Telomere end-replication problem and cell aging. J Mol Biol 1992, 225:951-960 [DOI] [PubMed] [Google Scholar]
- 31.Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE: Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol 2002, 15:441-447 [DOI] [PubMed] [Google Scholar]
- 32.von Zglinicki T, Pilger R, Sitte N: Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med 2000, 28:64-74 [DOI] [PubMed] [Google Scholar]
- 33.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T: Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 2000, 20:1659-1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ancelin K, Brunori M, Bauwens S, Koering CE, Brun C, Ricoul M, Pommier JP, Sabatier L, Gilson E: Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol 2002, 22:3474-3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumann P, Cech TR: Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001, 292:1171-1175 [DOI] [PubMed] [Google Scholar]
- 36.Luttges J, Galehdari H, Brocker V, Schwarte-Waldhoff I, Henne-Bruns D, Kloppel G, Schmiegel W, Hahn SA: Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol 2001, 158:1677-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]