Abstract
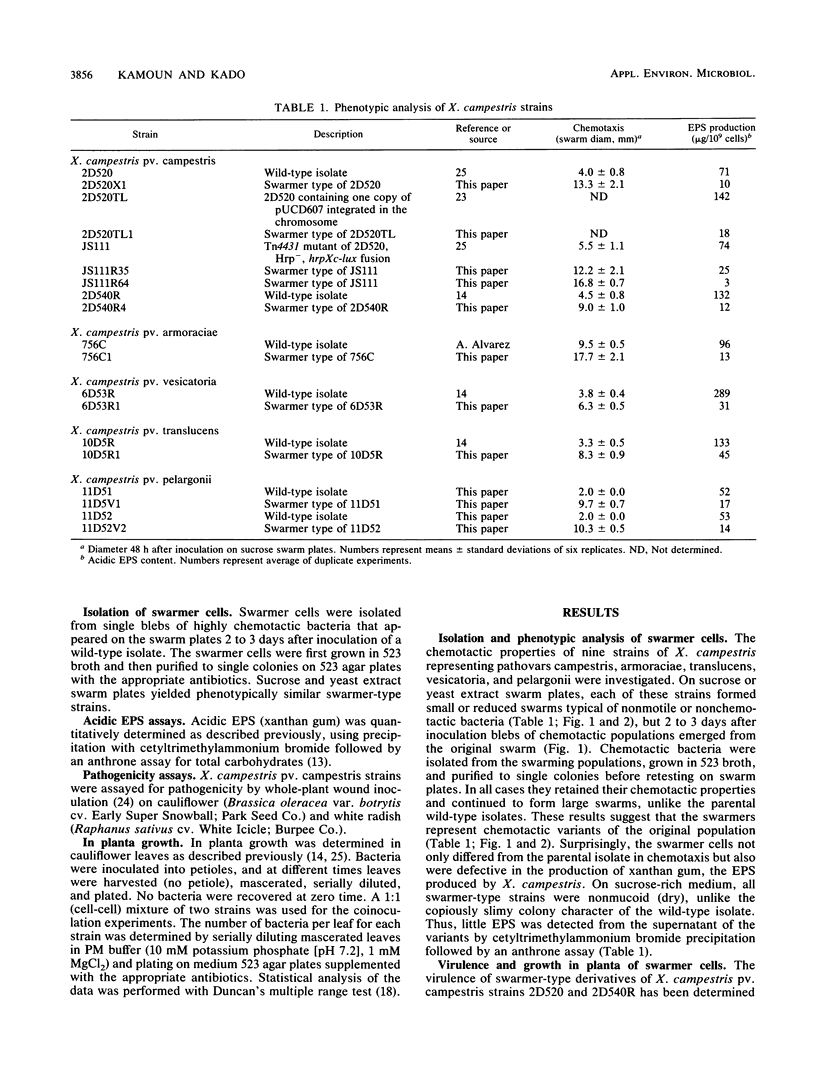
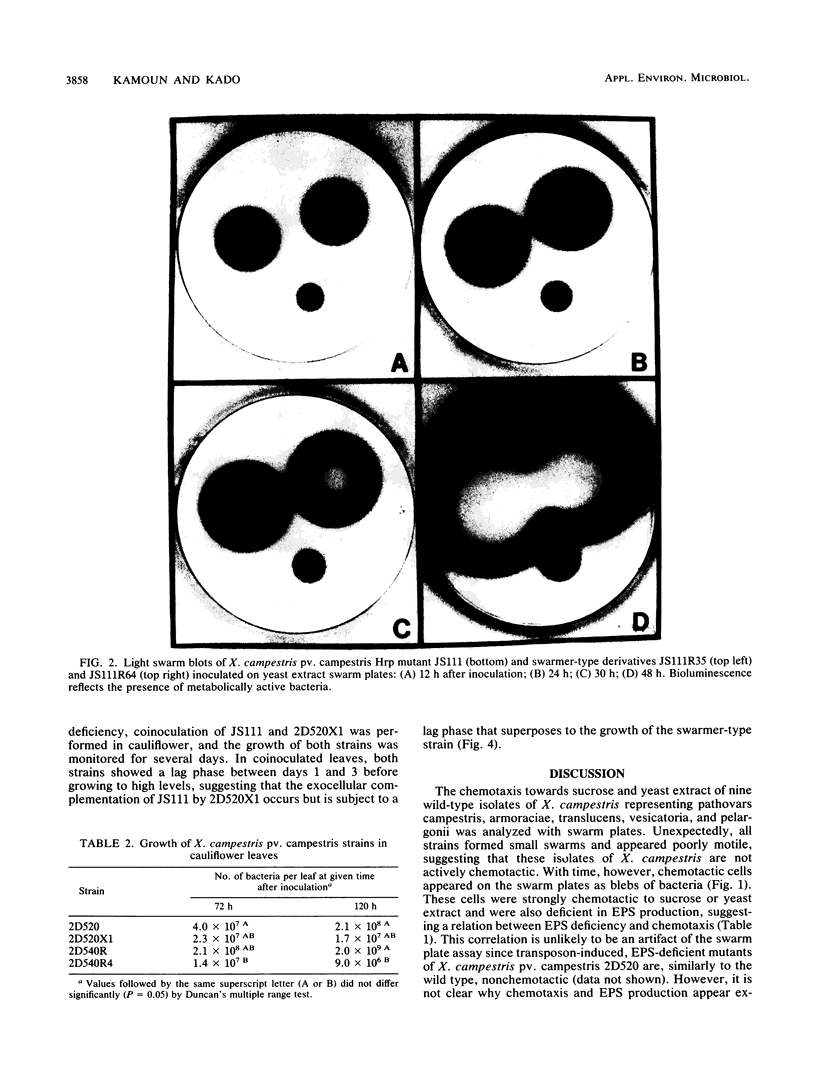
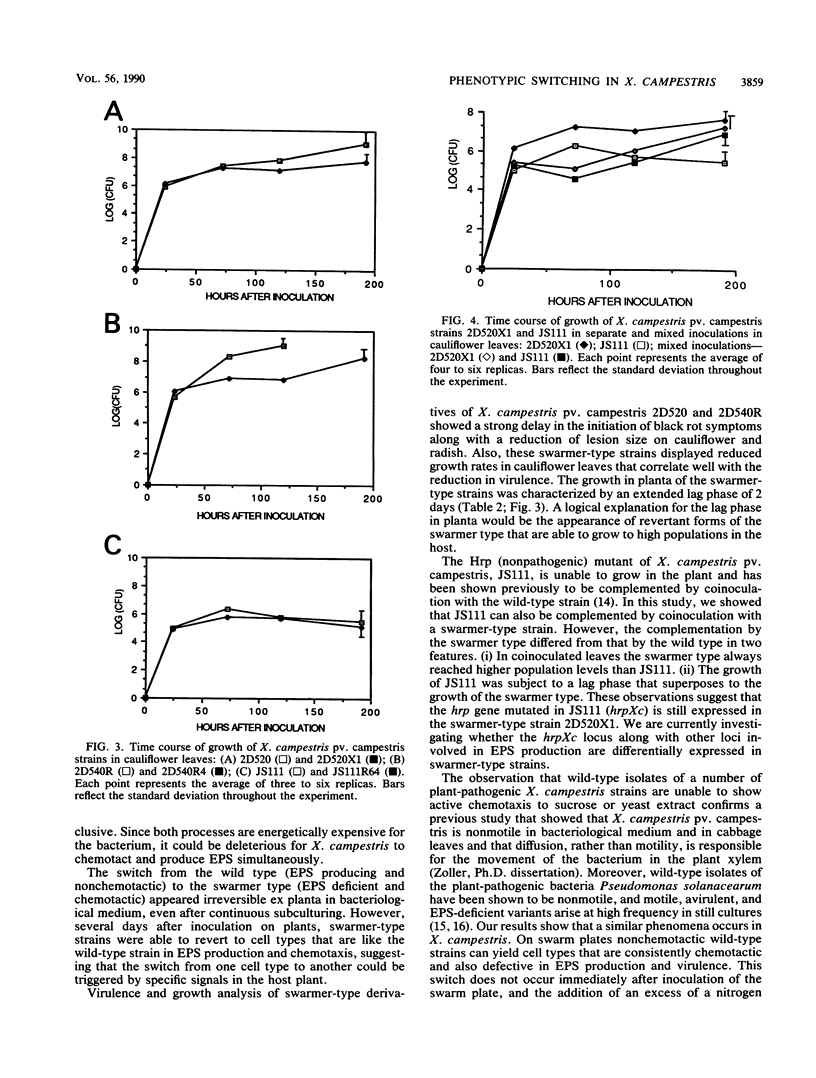
The chemotaxis towards sucrose and yeast extract of nine strains of Xanthomonas campestris representing pathovars campestris, armoraciae, translucens, vesicatoria, and pelargonii was analyzed by using swarm plates. Unexpectedly, each of these strains formed small or reduced swarms typical of nonmotile or nonchemotactic bacteria. With time, however, chemotactic cells appeared on the swarm plates as blebs of bacteria. These cells were strongly chemotactic and were concomitantly deficient in exopolysaccharide production. The switch from the wild type (exopolysaccharide producing and nonchemotactic) to the swarmer type (exopolysaccharide deficient and chemotactic) appeared irreversible ex planta in bacteriological medium. However, in radish leaves swarmer-type strains of X. campestris pv. campestris were able to revert to the wild type. Swarmer-type derivatives of two X. campestris pv. campestris wild-type isolates showed reduced virulence and growth in the host plants cauliflower and radish. However, exocellular complementation of X. campestris pv. campestris Hrp (nonpathogenic) mutant was achieved by coinoculation with a swarmer-type strain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COREY R. R., STARR M. P. Colony types of Xanthomonas phaseoli. J Bacteriol. 1957 Aug;74(2):137–140. doi: 10.1128/jb.74.2.137-140.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadmus M. C., Rogovin S. P., Burton K. A., Pittsley J. E., Knutson C. A., Jeanes A. Colonial variation in Xanthomonas campestris NRRL B-1459 and characterization of the polysaccharide from a variant strain. Can J Microbiol. 1976 Jul;22(7):942–948. doi: 10.1139/m76-136. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- Goto M. Interrelationship between colony type, phage susceptibility and virulence in Xanthomonas oryzae. J Appl Bacteriol. 1972 Sep;35(3):505–515. doi: 10.1111/j.1365-2672.1972.tb03729.x. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Cleary J. M., Cabañas D. K., Rosen I. G., Kang K. S. Genetic and physical analyses of a cluster of genes essential for xanthan gum biosynthesis in Xanthomonas campestris. J Bacteriol. 1987 Jun;169(6):2854–2861. doi: 10.1128/jb.169.6.2854-2861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B. Structure of extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975 Dec;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- Kamoun S., Cooley M. B., Rogowsky P. M., Kado C. I. Two chromosomal loci involved in production of exopolysaccharide in Agrobacterium tumefaciens. J Bacteriol. 1989 Mar;171(3):1755–1759. doi: 10.1128/jb.171.3.1755-1759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S., Kado C. I. A plant-inducible gene of Xanthomonas campestris pv. campestris encodes an exocellular component required for growth in the host and hypersensitivity on nonhosts. J Bacteriol. 1990 Sep;172(9):5165–5172. doi: 10.1128/jb.172.9.5165-5172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- Melton L. D., Mindt L., Rees D. A., Sanderson G. R. Covalent structure of the extracellular polysaccharide from Xanthomonas campestris: evidence from partial hydrolysis studies. Carbohydr Res. 1976 Feb;46(2):245–257. doi: 10.1016/s0008-6215(00)84296-2. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988 Sep;52(3):327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne L., Tansey L., Pollock T. J. Clustering of mutations blocking synthesis of xanthan gum by Xanthomonas campestris. J Bacteriol. 1987 Aug;169(8):3593–3600. doi: 10.1128/jb.169.8.3593-3600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




