Abstract
Ovarian cancer is characterized by rapid growth of solid intraperitoneal tumors and production of large volumes of ascites. Our previous studies of intraperitoneal ovarian carcinoma in an athymic mouse model demonstrated that a monoclonal antibody (mAb) to human vascular endothelial growth factor (VEGF) could prevent ascites formation. Although ascites was almost completely inhibited, tumor burden was variably reduced. To develop more effective therapy, we assessed the combination of a human VEGF mAb plus paclitaxel. Four groups of female athymic nude mice were inoculated intraperitoneally with OVCAR3 cells. Two weeks after inoculation, one group was treated with a human VEGF mAb intraperitoneally twice weekly plus paclitaxel intraperitoneally three times weekly for 6 weeks. The second group was treated with VEGF mAb alone. The third group was treated with paclitaxel alone. The remaining group was treated with vehicle only. Tumor burden in the VEGF mAb plus paclitaxel and paclitaxel alone groups was reduced by 83.3% and 85.7% and 58.5% and 59.5%, respectively, in two separate experiments, compared to controls. VEGF mAb alone caused no significant decrease in tumor burden, nor did treatment of mice inoculated intraperitoneally with HEY-A8 cells, a non-VEGF-secreting ovarian cell line. Virtually no ascites developed in the combined treatment group or the group treated with VEGF mAb alone. Paclitaxel alone reduced ascites slightly, but not significantly. Morphological studies demonstrated that VEGF immunoneutralization enhanced paclitaxel-induced apoptosis in these human ovarian cancers. Thus, combination therapy with inhibitors of VEGF plus paclitaxel may be an effective way to markedly reduce tumor growth and ascites in ovarian carcinoma.
Ovarian cancer is characterized by rapid growth and spread of solid intraperitoneal tumors and, in some patients, the formation of large volumes of ascites. It is the major cause of death from gynecological malignancy and is the fifth most common cause of death from cancer in American women. Despite improved methods of surgery and chemotherapy, the mortality rates in women with advanced, recurrent, or persistent ovarian cancer have remained largely unchanged for the last 4 decades. 1
Vascular endothelial growth factor (VEGF) is a dimeric glycoprotein, specific for endothelial cells, which stimulates angiogenesis. It also possesses potent vascular permeability-enhancing activity 2,3 and is also known as vascular permeability factor (VPF). VEGF/VPF induces ascites accumulation, at least in part, by increasing the permeability of diaphragmatic and tumor-associated vasculature. 4 In particular, VEGF/VPF plays an important role in ascites formation associated with ovarian cancer. 5-7 Our previous studies in a model of intraperitoneal ovarian carcinoma in athymic mice inoculated with SKOV3 cells demonstrated that a monoclonal antibody (mAb) to human VEGF can prevent ascites. 6 We also showed that administration of a VEGF mAb could reverse pre-existing ascites in mice inoculated with cells derived from an OVCAR3 cancer cell line, in which ascites develops earlier in the course of the disease than with the SKOV3 cell line. 8 Although ascites was almost completely inhibited, tumor burden was variably reduced.
In an effort to develop more effective forms of therapy for ovarian carcinoma, we sought to develop VEGF mAb-based combination therapy. In the past few years, several chemotherapeutic agents, including paclitaxel (Taxol), cis-platin, and etoposide have demonstrated some efficacy in the treatment of ovarian carcinoma. 9-11 However, resistance to these agents usually supervenes. Paclitaxel, a naturally-occurring diterpenoid originally isolated from the Pacific yew, is the first representative of a class of anti-tumor agents that is now widely used in the treatment of many forms of cancer, including those of the ovary, breast, and lung. 7,12,13 Paclitaxel promotes assembly of microtubules, inhibits tubulin disassembly, and blocks cell cycling at the G2/M stage. 14 Paclitaxel also inhibits DNA synthesis, 15 releases tumor necrosis factor-α, 15,16 and causes apoptotic cell death in a variety of cancer cell types. 9 Because of its unique mechanism of action and its wide spectrum of activity, 17,18 and because of recent studies in which paclitaxel was used in combination with other agents in other types of neoplasms, 19,20 we elected to test paclitaxel combined with the mAb to VEGF/VPF in our athymic mouse model 6 to assess the extent of inhibition of tumor burden as well as ascites. We explored the possible effects of the interaction between immunoneutralization of VEGF and paclitaxel by assessing tumor burden and ascites volume before, during, and after treatment. In addition, we assessed the effects of treatment on apoptosis. To further assess the central role of VEGF in these processes, we also studied the effects of the VEGF mAb plus paclitaxel in a non-VEGF-expressing ovarian cancer cell line.
Materials and Methods
Materials
Paclitaxel was obtained from Sigma Chemical Co. (St. Louis, MO). A mouse mAb (A4.6.1) directed against human VEGF was used to neutralize VEGF activity in vivo. Characterization of this antibody, including its specificity for human VEGF and its ability to inhibit VEGF activity in vitro and in vivo, as well as its ability to block binding of VEGF to its receptors in vivo, has been described previously. 21
All cell culture reagents were obtained from the Cell Culture Facility, University of California, San Francisco (UCSF).
Experimental Animals
Five- to seven-week-old female athymic immunodeficient mice (Simonsen Laboratories, Gilroy, CA) were delivered to the UCSF Laboratory Animal Resource Center, housed in isolated conditions, fed autoclaved standard pellets and water, and allowed to adapt to their new environment. All protocols involving immunodeficient mice were approved by the Committee on Animal Research, UCSF.
Experimental Design
Experiment 1
Four groups of female athymic nude mice (5 to 7 weeks of age) were inoculated intraperitoneally with OVCAR3 cells (n = 18). Two weeks after inoculation, one group (n = 5) was treated with the human VEGF mAb plus paclitaxel for 6 weeks. The second group of mice (n = 5) was treated with VEGF mAb alone. The third group (n = 4) was treated with paclitaxel alone. The remainder (n = 4) were treated with the same volume of vehicle (phosphate-buffered saline). The human VEGF mAb (5 μg/g body weight) was administered intraperitoneally twice weekly as in our previous studies. 5 The dose of paclitaxel (20 μg/g body weight), was based on previous studies. 22,23 Administration was twice weekly in the first week and increased to three times weekly for the last 5 weeks. There was no apparent toxicity.
Experiment 2
The design of experiment 2 was similar to that of experiment 1 except that paclitaxel was administrated three times weekly for 6 weeks, while paclitaxel was administrated twice weekly in the first week and increased to three times weekly for the last 5 weeks in experiment 1.
Four groups of female athymic nude mice (5 to 7 weeks of age) were inoculated intraperitoneally with OVCAR3 cells (n = 49). Two weeks after inoculation, one group of mice (n = 12) was treated with the human VEGF mAb (5 μg/g body weight) twice weekly plus paclitaxel (20 μg/g body weight) three times weekly for 6 weeks. The second group of mice (n = 13) was treated with VEGF mAb alone. The third group (n = 12) was treated with paclitaxel alone. The remainder (n = 12) was treated with the same volume of vehicle.
As an additional control, we inoculated nude mice (n = 20) with HEY-A8 ovarian cancer cells, which do not express VEGF mRNA and/or secrete the protein. 24 The protocol for HEY-A8-inoculated mice was the same as for OVCAR3 inoculated mice except that mice inoculated with HEY-A8 were treated for 4 weeks because they developed advanced disease earlier than the mice inoculated with OVCAR3, and did not survive longer than 4 weeks of treatment.
Methods
To prepare cells for inoculation, they were collected from the ascites fluid of athymic mice inoculated with the OVCAR3 line. Ascites fluid was collected and placed in a 4°C refrigerator for 1 to 2 hours. The supernatant was then discarded. The cells were diluted with medium RPMI 1640 supplemented with 2.0 g/L of glucose and 0.3 g/L of l-glutamine, which had been prewarmed in a 37°C incubator. Athymic nude mice (5 to 7 weeks of age) were inoculated intraperitoneally with OVCAR3 cells (n = 49; 2 × 106 cells per mouse in 500 μl of RPMI 1640). In addition, 20 athymic nude mice (5 to 7 weeks of age) were inoculated intraperitoneally with HEY-A8 cells (2 × 106 cells per mouse in 500 μl of RPMI 1640).
Abdominal circumference and body weight were measured twice weekly. At the end of the experiment, mice underwent euthanasia with CO2. The volume of ascites was measured, tumor tissue was excised, weighed, fixed in 4% paraformaldehyde, pH 7.4, at 4°C for 24 hours, and embedded in paraffin. Paraffin sections (5 μm) were used for histochemical analysis.
Light Microscopy and Analysis
Tumor tissue sections from OVCAR3-inoculated mice treated with VEGF mAb plus paclitaxel were examined with a Leica DMRB or Leica Ortholux II photomicroscope at low and high magnifications. Images were collected with a Photonics DEI-470 CCD camera and a RasterOps 24XLTV frame grabber, imported directly into Adobe Photoshop 4.0, and stored on a ZIP external 100 MB drive (Iomega). Photomicrographic plates were composed from the original data in Photoshop, without alteration or manipulation, and annotated with rub-on letters and symbols.
Assessment of Apoptosis
Paraffin sections (5 μm) of cancer tissue from OVCAR3 cell-inoculated mice treated with VEGF mAb plus paclitaxel were used to assess apoptosis. DNA labeling with digoxigenin deoxy-UTP and terminal transferase, followed by immunocytochemical staining with peroxidase-coupled anti-digoxigenin antibody and diaminobenzidine, was performed with the reagents supplied in the Apoptag kit (Intergen, Purchase, NY) according to the manufacturer’s instructions, except that Tris was substituted for phosphate in the wash buffer. After light counterstaining with hematoxylin, nuclei that stained brown were scored as positive for apoptosis and those that stained blue were scored as negative. At least five ×300 microscopic fields were scored, and the apoptotic index was calculated as the percentage of cells that were scored positive.
Statistics
Results are presented as means ± SE. Data were analyzed using one-way analysis of variance followed by unpaired Student’s t-test for comparison between groups. Differences between groups were considered statistically significant at P < 0.05. Experiments were performed in duplicate.
Results
We examined the potential interactions occurring between the angiogenesis inhibitor VEGF mAb (A.4.6.1) and paclitaxel, given singly and in combination, in the control of ovarian tumor growth and ascites formation, to assess whether these interactions increase the therapeutic effects of each agent individually.
At postmortem examination, tumors were found on the surface of the peritoneum, intestines, mesentery, and uterus in both treatment and control groups. Eighty-seven percent, 58%, and 20% of the mice in the control, VEGF antibody-treated and paclitaxel-treated groups, respectively, had tumors on the diaphragm and in the hilus of the liver. However, these tumors were not found in the combined VEGF mAb plus paclitaxel treatment groups. There were numerous small bead-like tumors in the VEGF mAb plus paclitaxel-treated mice, which are not commonly found in untreated control mice.
Experiment 1
The results of this initial study of treatment of VEGF mAb and paclitaxel, singly and in combination, are shown in Figure 1A ▶ . The mean tumor burden in the combined VEGF mAb plus paclitaxel-treated group was 1.47 ± 0.31 g (n = 4). (At the beginning of the experiment, there were five mice in this group. One mouse had leakage of ascites fluid because the peritoneum was accidentally torn with the injection needle 2 weeks before the end of the experiment; this mouse was not included in the analysis.)
Figure 1.
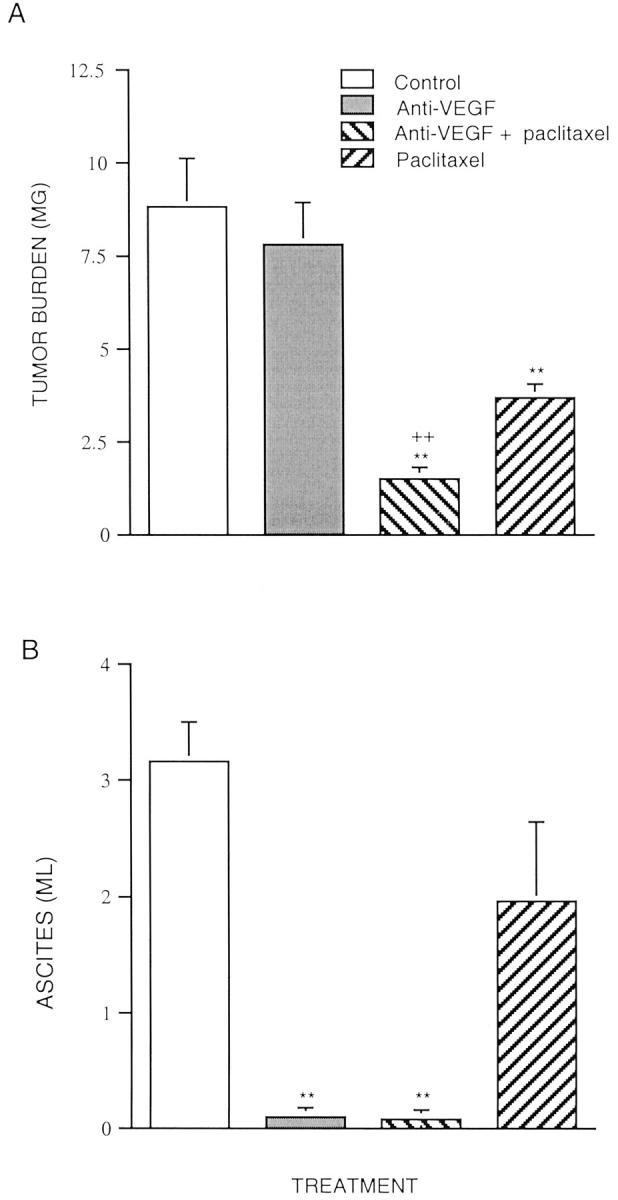
Effects of VEGF mAb plus paclitaxel on tumor burden (A) and ascites formation (B) in mice inoculated with OVCAR3 cells. Experiment 1. Four groups of athymic immunodeficient mice were used. OVCAR3 cells (2 × 106) were injected as a bolus intraperitoneally in 5- to 7-week-old athymic immunodeficient mice. Treatment was initiated 2 weeks after inoculation. Treatment groups consisted of control (vehicle alone), VEGF mAb alone, VEGF mAb plus paclitaxel, and paclitaxel alone. The VEGF mAb (5 μg/g body weight) was administered intraperitoneally twice weekly for 6 weeks. Administration of paclitaxel (20 μg/g body weight) was twice weekly in the first week and increased to three times weekly for the last 5 weeks. At autopsy, ascites fluid was quantified and tumors were excised and weighed (n = 15). Data are expressed as mean ± SE **. P < 0.01 versus control; ++, P < 0.01 versus paclitaxel.
The tumor burden in the group treated with paclitaxel alone (3.64 ± 0.42 g) was significantly reduced compared to that of the controls (8.78 ± 1.32 g) (n = 2). (At the beginning of the experiment, there were four mice in the control group. However, two died 10 and 22 days, respectively, before the end of the experiment, most likely as a result of the marked tumor burden and ascites.) The tumor burdens in the anti-VEGF plus paclitaxel and paclitaxel alone groups were reduced by 83.3% and 58.5%, respectively, compared to the control group. VEGF mAb alone (7.79 ± 1.33 g) (n = 4) had no significant effect on tumor burden compared to the control group.
Figure 1B ▶ shows the results of the initial study of the control of ascites formation by the VEGF mAb and paclitaxel treatment, singly and in combination. The mean volume of ascites in the control group was 3.15 ml. In contrast, virtually no ascites developed in the VEGF mAb plus paclitaxel-treated group nor in the group that was treated with VEGF mAb alone. Paclitaxel alone reduced ascites formation slightly, but not significantly.
Experiment 2
As in experiment 1, tumor burden in both the combined VEGF mAb plus paclitaxel (n = 12) and paclitaxel alone (n = 12) treated groups was significantly reduced compared to the control group (Figure 2A) ▶ . The tumor burden of the VEGF mAb plus paclitaxel group (n = 12) and the group treated with paclitaxel alone (n = 12) was reduced by 85.7% (0.529 ± 0.12 g) and 59.5% (150 ± 0.21 g), respectively, compared to the control group (3.71 ± 0.37 g) (n = 12). There was no significant difference in tumor burden between the group treated with VEGF mAb alone (n = 13) and the control group.
Figure 2.
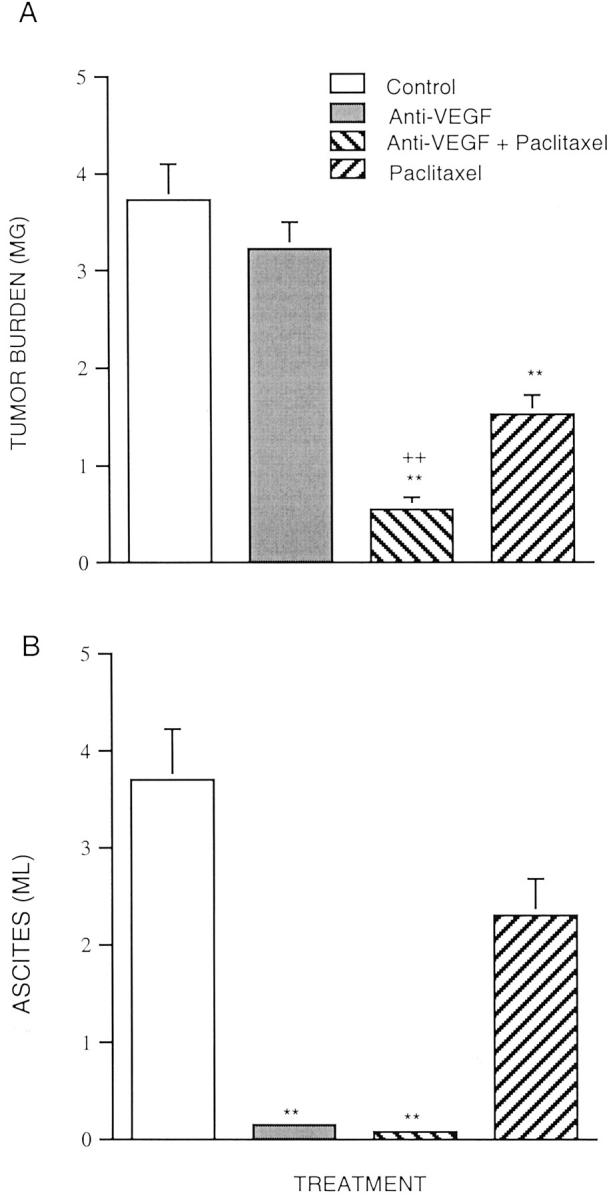
Effects of VEGF mAb plus paclitaxel on tumor burden (A) and ascites formation (B) in mice inoculated with OVCAR3 cells. Experiment 2. Four groups of athymic immunodeficient mice were used. The OVCAR3 cells (2 × 106) were injected as a bolus intraperitoneally in 5- to 7-week-old athymic immunodeficient mice. Treatment was initiated 2 weeks after inoculation. Treatment groups consist of control (vehicle alone), VEGF mAb alone, VEGF antibody plus paclitaxel, and paclitaxel alone. The VEGF mAb (5 μg/g body weight) was administered intraperitoneally twice weekly for 6 weeks. Administration of paclitaxel (20 μg/g body weight) was three times weekly for 6 weeks. At autopsy, ascites fluid was quantified and tumors were excised and weighed (n = 49). Data are expressed as mean ± SE. **, P < 0.01 versus control; ++, P < 0.01 versus paclitaxel.
Changes in ascites volume (Figure 2B) ▶ also were similar to the initial study. The volume of ascites in mice that did not receive VEGF mAb (control), was 3.6 ml, whereas ascites in the VEGF mAb alone or VEGF mAb plus paclitaxel-treated groups was barely detectable. Again, paclitaxel alone slightly, but not significantly, reduced ascites formation.
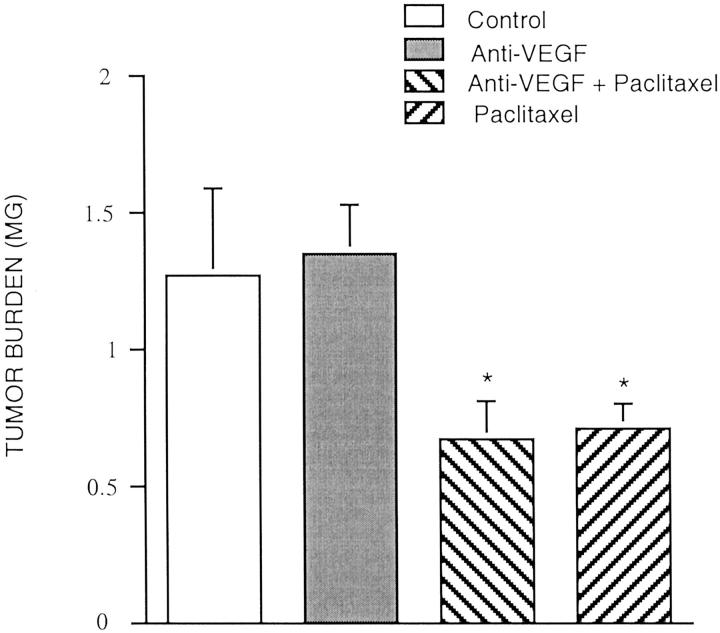
As an additional control we inoculated nude mice with HEY-A8 ovarian cancer cells, which do not express VEGF mRNA and do not secrete VEGF. 24 We found that the mice inoculated with HEY-A8 did not produce ascites, and the VEGF mAb did not enhance the inhibitory effect of paclitaxel alone on tumor growth (Figure 3) ▶ . The tumor burden in the group treated with paclitaxel alone (0.704 ± 0.09 g) (n = 5) was significantly reduced (44.3%) compared to the controls (1.26 ± 0.32 g) (n = 5). There were no significant differences in tumor burden between the group treated with paclitaxel alone and VEGF-mAb plus paclitaxel (0.678 ± 0.13 g) (n = 5) in the mice inoculated with HEY-A8. VEGF alone (1.346 ± 0.18 g) (n = 5) had no effect on tumor burden compared to the control group.
Figure 3.
Effects of VEGF mAb plus paclitaxel on tumor growth in mice inoculated with Hey-A8 cells. Four groups of athymic immunodeficient mice were used. Hey-A8 cells (2 × 106) were injected as a bolus intraperitoneally in 5- to 7-week-old athymic immunodeficient mice. Treatments were started 2 weeks after inoculation. Treatment groups consisted of control (vehicle alone), VEGF mAb alone, VEGF mAb plus paclitaxel, and paclitaxel alone. The VEGF mAb (5 μg/g body weight) was administered intraperitoneally twice weekly for 4 weeks. Paclitaxel (20 μg/g body weight) was administered three times weekly for 4 weeks. At autopsy, tumors were excised and weighed (n = 20). Data are expressed as mean ± SE. *, P < 0.05 versus control.
Apoptosis
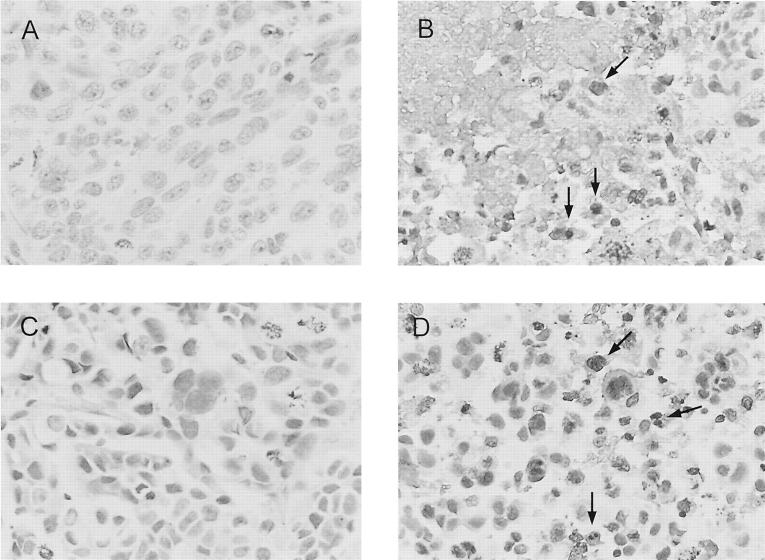
Figure 4 ▶ illustrates apoptotic cells in cancer tissue from OVCAR3-inoculated mice. The brown nuclei (indicated by arrows) indicate cells that underwent apoptosis. Approximately 30% of OVCAR3 cells in the paclitaxel-treated group were apoptotic, whereas more than 50% in the slides from the VEGF mAb plus paclitaxel group were necrotic and apoptotic, with cytoplasmic debris and calcification. There were no significant differences between the control group and the group treated with the VEGF mAb alone.
Figure 4.
Histological appearance and apoptosis in tumor tissue from OVCAR3-inoculated athymic mice with and without paclitaxel or VEGF mAb plus paclitaxel treatment. A: Representative section of tumor from control group. Tumor cells have large, atypical nuclei, prominent nucleoli, a moderate amount of cytoplasm, grow in sheets, and undergo mitosis. B: Section of tumor from VEGF mAb plus paclitaxel treatment group. Focus of necrosis and apoptosis with cytoplasmic debris and calcification. A few clusters of viable tumor cells surround this central focus (bottom right). Similar foci were scattered throughout tumors removed from the treated animals. The brown-stained cells (arrows) indicate apoptosis. C: Section of tumor from VEGF mAb-treated group shows tumor cells with large, atypical nuclei. Some cells are swollen, whereas in others the cytoplasm is dense and decreased in amount. No evidence of apoptosis was detected. D: Section of tumor from paclitaxel-treated group shows degenerative changes, including decreased nuclear size, hyperchromasia, and smudging of the nuclear chromatin. A few cells are swollen, whereas in others the cytoplasm is dense and decreased in amount. Arrows indicate apoptotic cells. Original magnifications, ×300.
Discussion
Recent studies indicate that the combination of anti-angiogenic agents and conventional chemotherapeutic agents can significantly inhibit tumor growth and metastasis. 25-28 The combination of TNP-470, an angiogenesis inhibitor, and microcyline resulted in marked inhibition of tumor growth in rats bearing implanted 9L-gliosarcomas. 25 Co-administration of TNP-470 and paclitaxel also produced a significant inhibition of growth in a non-small-cell lung cancer cell line. 26 Furthermore, the effects of TNP-470 on liver metastases of human pancreatic carcinoma in nude mice were enhanced by combination with cis-platin. 27,28 DC101, a mAb that targets VEGFR-2 (flk-1) in combination with paclitaxel, enhanced apoptosis and inhibited tumorigenesis, angiogenesis, and metastasis in human metastatic transitional cell carcinoma of the bladder in a murine model. 20 Continuous low-dose therapy with vinblastine and DC101 induces sustained xenografts of neuroblastoma regression without overt toxicity. 29 Tumor regression was more dramatic by combining DC101 with microtubule-modulating drugs than with those that damaged DNA. 29 The present data are consistent with these studies and demonstrate that the combination of the human VEGF mAb plus paclitaxel, which markedly inhibited tumor growth, was extremely effective in the treatment of ovarian carcinoma in that it virtually eliminated ascites and reduced tumor burden by >85.7%. Thus, the effect of paclitaxel on ovarian cancer was markedly enhanced by combination with the VEGF mAb. The anti-metastatic, anti-tumor, and anti-ascites effects of the VEGF mAb plus paclitaxel were markedly greater than those of paclitaxel alone.
The mechanism by which this combination therapy exerts its effect is not clear. A recent study demonstrates that survivin, an inhibitor of apoptosis, plays a role in the maintenance of microtubules within the mitotic spindle and appears to be overexpressed in common cancers but not in corresponding normal adult tissues. 30 VEGF-induced survivin expression is a major drug resistance mechanism downstream of the PI3K/PKB pathway; activation of this pathway may significantly hamper the ability of chemotherapeutic drugs to damage or kill activated endothelial cells. 31 The blockade of VEGF signaling can significantly enhance the efficacy of chemotherapeutic regimens. 29 We have demonstrated that the PI3K inhibitor, LY294002, enhances the effects of paclitaxel on tumor growth and ascites formation and decreases development of resistance to paclitaxel. 32 We also have demonstrated that OVCAR3 cells release high levels of VEGF, 33 which can result in overexpression of survivin. Our use of the VEGF mAb to neutralize VEGF could, in turn, reduce the expression of survivin and decrease the drug resistance that develops with paclitaxel alone.
Paclitaxel inhibits microtubule depolymerization and blockade of cell division in the G2/M phase of the cell cycle. 14 In addition, paclitaxel can inhibit angiogenesis by suppressing VEGF expression. 34 Thus, tumor growth might be affected not only by direct cytotoxicity but also by inhibition of new vessel formation, and the VEGF mAb could enhance the anti-angiogenic effects of paclitaxel, as well as decreasing development of drug resistance to paclitaxel. 32
On the other hand, the inhibition of angiogenesis might have led to the death of tumor cells most distal to the established vasculature, thereby decreasing tumor volume and facilitating access of the cytotoxic agents throughout the tumor tissue. 26 When we administered combination therapy to mice that had been inoculated with HEY-A8 ovarian cancer cells, which do not express VEGF mRNA and do not secrete VEGF, 24 we found that the mice did not produce ascites and that the VEGF mAb did not enhance the inhibitory effect of paclitaxel on tumor growth.
The present study indicates that, similar to our previous experiment, 6 the human VEGF mAb markedly inhibits the ascites that develops after intraperitoneal inoculation with OVCAR3 cells. The function-blocking human VEGF mAb, A.4.6.1, neutralized the tumor-derived VEGF activity, blocking access of VEGF to its receptors, specifically inhibiting the activity of tumor-derived VEGF, as it is specific for human VEGF. 21
The mechanisms by which the VEGF mAb inhibits ascites formation also are not completely understood. Ascites is linked to peritoneal, as well as tumor microvascular hyperpermeability, 22 and several studies have implicated VEGF in ascites formation by increasing vascular permeability. 5,6 Tumor secretion of VEGF is essential for ascites accumulation. 5,35,36 Anti-VEGF targeting agents block ascites formation 7,37,38 and immunoneutralization of VEGF alone in our nude mouse model inhibits ascites. 6 In contrast, inoculation with HEY-A8 cells, which do not express VEGF, was not associated with ascites formation. We have also demonstrated that VEGF release from OVCAR-3 cells modulates endothelial cell monolayer permeability in a dual-chamber permeability assay (L Hu, N Ferrara, and RB Jaffe, submitted). In that study, we used HEY-A8 and OCC1 cells as controls, as neither cell line expresses VEGF, and found that neither the HEY-A8 nor OCC1 cells induced significant increases in monolayer permeability.
Angiogenesis plays a pivotal role in the progression of cancer by permitting tumor growth and facilitating metastasis. The present studies, similar to our previous study using the SKOV3 cell line, 6 indicate that blocking tumor-derived VEGF activity with A.4.6.1 alone does not markedly inhibit intraperitoneal human ovarian tumor growth, although it did decrease subcutaneous SKOV3 cell growth during treatment.
Interestingly, in VEGF mAb-treated mice there were numerous small bead-like tumors, which are not commonly found in untreated control mice. This observation suggests that the inhibition of angiogenesis by the human VEGF antibody, which only inhibits tumor-derived VEGF, led to the inhibition of new vessel development, thereby limiting growth of tumors; cell death occurred in the cells most distant from the established vasculature. Because endogenous VEGF and/or other angiogenic factors may support some tumor growth and spread, tumors near established vasculature remain small and bead-like. Further, Xu and colleagues 39 have suggested that although VEGF/VPF may be the major substance involved in the ascites associated with ovarian carcinoma, interleukin-8, which also is angiogenic, may play a major role in solid tumor growth of ovarian and other 40,41 tumors. A recent report indicated that IL-8 reduced tumorigenicity of human ovarian cancer. 42 VEGF likely is not the only angiogenic factor that can be involved in the maintenance and growth of intraperitoneal carcinomatosis.
Our morphological observations, in agreement with previous studies, 43-45 indicate that paclitaxel induces nuclear pyknosis and fragmentation as well as reduced cytoplasmic volume in tumor cells, indicating apoptosis. Using digoxigenin-UTP and terminal transferase labeling with immunocytochemistry, we have also demonstrated that the VEGF mAb enhanced paclitaxel-induced OVCAR3 cell apoptosis in vivo. More than 50% of the cells from the VEGF mAb plus paclitaxel-treated groups underwent necrosis, perhaps because of apoptosis that subsequently can lead to necrosis. 46
In summary, our studies suggest that combination therapy with an anti-angiogenic agent, such as the VEGF mAb plus paclitaxel, administered intraperitoneally or via another route, may be an effective way to markedly inhibit tumor growth and ascites in ovarian cancer.
Footnotes
Address reprint requests to Robert B. Jaffe, M.D., Center for Reproductive Sciences, University of California, San Francisco, 505 Parnassus Ave., HSW 1695, San Francisco, CA 94143-0556. E-mail: jaffer@obgyn.ucsf.edu.
Supported in part by PO CA 646021 from the National Cancer Institute.
References
- 1.Cannistra SA: Cancer of the ovary. N Engl J Med 1993, 329:1550-1559 [DOI] [PubMed] [Google Scholar]
- 2.Connolly DT, Olander JV, Heuvelman D, Nelson R, Monsell R, Siegel N, Haymore BL, Leimgruber R, Feder J: Human vascular permeability factor. Isolation from U937 cells. J Biol Chem 1989, 264:20017-20024 [PubMed] [Google Scholar]
- 3.Nagy JA, Masse EM, Herzberg KT, Meyers MS, Yeo KT, Yeo TK, Sioussat TM, Dvorak HF: Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res 1995, 55:360-368 [PubMed] [Google Scholar]
- 4.Barton DP, Cai A, Wendt K, Young M, Gamero A, DeCesare S: Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res 1997, 3:1579-1586 [PubMed] [Google Scholar]
- 5.Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, Unger C, Marme D, Gastl G: Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999, 85:178-187 [PubMed] [Google Scholar]
- 6.Mesiano S, Ferrara N, Jaffe RB: Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol 1998, 153:1249-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Yoneda J, Herrera C, Wood J, Killion JJ, Fidler IJ: Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol 2000, 16:445-454 [DOI] [PubMed] [Google Scholar]
- 8.Hu L, Huang Z, Ferrara N, Jaffe RB: Reversal of pre-existing ovarian carcinoma-associated ascites with a human monoclonal antibody to vascular endothelial growth factor. Abstracts, 81st Annual Meeting Endocrine Society, San Diego, CA, June 12–15, 1999
- 9.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG: Paclitaxel: the first of the taxanes, an important new class of antitumor agents. Semin Oncol 1992, 19:646-662 [PubMed] [Google Scholar]
- 10.Cain JM, Collins C, Petersdorf S, Figge DC, Tamimi HK, Greer BE, Livingston RB: Phase II study of high-dose cisplatin, etoposide, and cyclophosphamide for refractory ovarian cancer. Am J Obstet Gynecol 1996, 1746:1688-1694 [DOI] [PubMed] [Google Scholar]
- 11.Williams S, Blessing JA, Liao SY, Ball H, Hanjani P: Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol 1994, 12:701-706 [DOI] [PubMed] [Google Scholar]
- 12.Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, Buzdar AU, Frye DK, Hortobagyi GN: Phase II trial of paclitaxel, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 1991, 83:1797-1805 [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Vermorken JB: The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs 1998, 55:5-30 [DOI] [PubMed] [Google Scholar]
- 14.Sorger PK, Dobles M, Tournebize R, Hyman AA: Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol 1997, 9:807-814 [DOI] [PubMed] [Google Scholar]
- 15.Slichenmyer WJ, Von Hoff DD: New natural products in cancer chemotherapy. J Clin Pharmacol 1990, 30:770-788 [DOI] [PubMed] [Google Scholar]
- 16.Manthey CL, Brandes ME, Perera PY, Vogel SN: Paclitaxel increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol 1992, 149:2459-2465 [PubMed] [Google Scholar]
- 17.Milas L, Hunter NR, Kurdoglu B, Mason KA, Meyn RE, Stephens LC, Peters LJ: Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with paclitaxel. Cancer Chemother Pharmacol 1995, 35:297-303 [DOI] [PubMed] [Google Scholar]
- 18.Danesi R, Figg WD, Reed E, Myers CE: Paclitaxel (Taxol) inhibits protein isoprenylation and induces apoptosis in PC-3 human prostate cancer cells. Mol Pharmacol 1995, 47:1106-1111 [PubMed] [Google Scholar]
- 19.Inoue K, Slaton JW, Perrotte P, Davis DW, Bruns CJ, Hicklin DJ, McConkey DJ, Sweeney P, Radinsky R, Dinney CP: Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res 2000, 6:4874-4884 [PubMed] [Google Scholar]
- 20.Inoue K, Slaton JW, Davis DW, Hicklin DJ, McConkey DJ, Karashima T, Radinsky R, Dinney CP: Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res 2000, 6:2635-2643 [PubMed] [Google Scholar]
- 21.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 22.Kubota T, Matsuzaki SW, Hoshiya Y, Watanabe M, Kitajima M, Asanuma F, Yamada Y, Koh JI: Antitumor activity of paclitaxel against human breast carcinoma xenografts serially transplanted into nude mice. J Surg Oncol 1997, 64:115-121 [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti MI, Lucchini V, Massazza G, Abbott BJ, D’Incalci M, Giavazzi R: Antitumor activity of paclitaxel (NSC-125973) in human ovarian carcinomas growing in the peritoneal cavity of nude mice. Ann Oncol 1993, 4:151-155 [DOI] [PubMed] [Google Scholar]
- 24.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ: Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst 1998, 90:447-454 [DOI] [PubMed] [Google Scholar]
- 25.Teicher BA, Holden SA, Ara G, Dupuis NP, Liu F, Yuan J, Ikebe M, Kakeji Y: Influence of an anti-angiogenic treatment on 9L gliosarcoma: oxygenation and response to cytotoxic therapy. Int J Cancer 1995, 61:732-737 [DOI] [PubMed] [Google Scholar]
- 26.Satoh H, Ishikawa H, Fujimoto M, Fujiwara M, Yamashita YT, Yazawa T, Ohtsuka M, Hasegawa S, Kamma H: Combined effects of TNP-470 and paclitaxel in human non-small cell lung cancer cell lines. Anticancer Res 1998, 18:1027-1030 [PubMed] [Google Scholar]
- 27.Placidi L, Scott EC, Eckoff D, Bynon S, Sommadossi JP: Metabolic drug interactions between angiogenic inhibitor, TNP-470 and anticancer agents in primary cultured hepatocytes and microsomes. Drug Metab Dispos 1999, 27:623-626 [PubMed] [Google Scholar]
- 28.Shishido T, Yasoshima T, Denno R, Mukaiya M, Sato N, Hirata K: Inhibition of liver metastasis of human pancreatic carcinoma by angiogenesis inhibitor TNP-470 in combination with cisplatin. Jpn J Cancer Res 1998, 89:963-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS: Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000, 105:R15-R24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao R, Connolly DC, Murphy M, Green J, Weinstein J, Pisarcik DA, Hamilton TC: Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst 2000, 294:522-528 [DOI] [PubMed] [Google Scholar]
- 31.Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS: A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA 2002, 99:4349-4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB: Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res 2002, 62:1087-1092 [PubMed] [Google Scholar]
- 33.Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, Jaffe RB: Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst 2001, 93:762-768 [DOI] [PubMed] [Google Scholar]
- 34.Lau DH, Xue L, Young LJ, Burke PA, Cheung AT: Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 1999, 14:31-36 [DOI] [PubMed] [Google Scholar]
- 35.Luo JC, Toyoda M, Shibuya M: Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res 1998, 58:2594-2600 [PubMed] [Google Scholar]
- 36.Shibuya M, Luo JC, Toyoda M, Yamaguchi S: Involvement of VEGF and its receptors in ascites tumor formation. Cancer Chemother Pharmacol 1999, 43:S72-S77 [DOI] [PubMed] [Google Scholar]
- 37.Luo JC, Toyoda M, Shibuya M: Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res 1998, 58:2594-2600 [PubMed] [Google Scholar]
- 38.Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM: Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist 2000, 1:S45-S50 [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Xie K, Mukaida N, Matsushima K, Fidler IJ: Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res 1999, 59:5822-5829 [PubMed] [Google Scholar]
- 40.Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E: Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer 1999, 81:647-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ: Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994, 54:3242-3247 [PubMed] [Google Scholar]
- 42.Lee F, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K, Ting JP: IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol 2000, 164:2769-2775 [DOI] [PubMed] [Google Scholar]
- 43.Costa A, Villa R, Zaffaroni N, Santi S, Bearzatto A, Silvestrini R: Paclitaxel induces apoptosis in various cancer cell lines: comparison among different methodological approaches. Eur J Histochem 1997, 2:69-70 [PubMed] [Google Scholar]
- 44.Al-alami O, Sammons J, Martin JH, Hassan HT: Divergent effect of paclitaxel on proliferation, apoptosis and nitric oxide production in MHH225 CD34 positive and U937 CD34 negative human leukaemia cells. Leuk Res 1998, 22:939-945 [DOI] [PubMed] [Google Scholar]
- 45.Frankel A, Buckman R, Kerbel RS: Abrogation of paclitaxel-induced G2-M arrest and apoptosis in human ovarian cancer cells grown as multicellular tumor spheroids. Cancer Res 1997, 57:2388-2393 [PubMed] [Google Scholar]
- 46.Sandoval D, Gukovskaya A, Reavey P, Gukovsky S, Sisk A, Braquet P: The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology 1996, 4:1081-1091 [DOI] [PubMed] [Google Scholar]