Abstract
The expression of CD97, a member of the EGF-TM7 family with adhesive properties, is proportional to the aggressiveness and lymph node involvement in thyroid tumors. CD97 has never been systematically investigated in other tumors. First, we examined colorectal carcinoma cell lines (n = 18) for CD97 expression and regulation. All cell lines were CD97-positive. The level of CD97 in each line correlated with migration and invasion in vitro. This result was confirmed in CD97-inducible Tet-off HT1080 cells. Transforming growth factor-β, which inhibits proliferation in transforming growth factor-β-sensitive LS513 and LS1034 cells, down-regulated CD97 in these cell lines. Examining CD97 during sodium butyrate-induced cell differentiation of Caco-2 cells, we could demonstrate a CD97-decreasing effect. Second, we screened 81 colorectal adenocarcinomas by immunohistology for expression of CD97. Normal colorectal epithelium is CD97-negative. Seventy-five of 81 of the carcinomas expressed CD97. The strongest staining for CD97 occurred in scattered tumor cells at the invasion front compared to cells located within solid tumor formations of the same tumor. Carcinomas with more strongly CD97-stained scattered tumor cells showed a poorer clinical stage as well as increased lymph vessel invasion compared to cases with uniform CD97 staining. In summary, CD97 expression correlates with dedifferentiation, migration, and invasion in colorectal tumor cell lines. Moreover, more strongly CD97-stained tumor cells at the invasion front of colorectal carcinomas indicate the involvement of the molecule in tumor migration and invasion.
CD97 is a member of a subfamily of class II G-protein-coupled receptors referred to as EGF-TM7, which is expressed predominantly in leukocytes. 1 EGF-TM7 proteins possess a unique hybrid structure that consists of varying numbers of N-terminal EGF-like domains coupled to a seven-span transmembrane (TM7) domain by a mucin-like spacer. 1 To date, six EGF-TM7 molecules have been described, namely CD97, 2,3 EMR1, 4 EMR2, 5 EMR3, 6 EMR4, 7,8 and ETL. 9 The varying numbers of EGF-like domains in CD97 result from alternative splicing of the precursor transcript. Various CD97 isoforms have been detected possessing three (EGF 1,2,5), four (EGF 1,2,3,5), or five (EGF 1,2,3,4,5) EGF-like domains. Co-expression and co-regulation of these three isoforms occurs in normal lymphoid cells. 10
Based on its structure and expression pattern, CD97 has been implicated in cellular adhesion by interacting with other cell surface proteins or extracellular matrix proteins. Adhesion studies demonstrated that both lymphocytes and erythrocytes specifically bind to CD97 transfectants through interactions with CD55, 11 a membrane-bound molecule acting as a regulatory protein of the complement cascade. We previously found that CD97 is present at low levels on resting lymphocytes, but is strongly up-regulated within a few hours after lymphocyte activation, 12 which is consistent with CD97 playing a role in adhesion. CD97 is constitutively expressed by monocytes and granulocytes. 12 In normal tissues, abundant expression of CD97 is only detected in macrophages and dendritic cells except for glial cells, and in some T and B cells. 13
CD97 is strongly expressed in dedifferentiated thyroid carcinomas. 14,15 Immunostaining for CD97 in thyroid cancer and adjacent thyroid tissue suggests that CD97 expression parallels the aggressiveness and lymph node involvement of thyroid tumors. Epidermal growth factor (EGF), which stimulates the growth and invasion of differentiated thyroid carcinoma cells, 16 up-regulated CD97 in these cells. All-trans retinoic acid treatment redifferentiates thyroid carcinomas 17 and leads to a decrease in total cell number and in CD97-positive thyroid carcinoma cells. 15 These data suggest that CD97 expression may play an important role in the dedifferentiation of thyroid tumors.
CD97 has never been systematically investigated in other types of tumors. In this study, we first examined CD97 in colorectal carcinoma cell lines by flow cytometry and investigated whether the expression level of CD97 correlates with the migratory and invasive capacity of these cell lines. To confirm the results obtained, stable transfected, CD97 inducible Tet-off HT1080 cells were generated and inserted into the same tests. We further studied whether induction of differentiation influences CD97 expression in the cell line Caco-2 that undergoes enterocytic differentiation in culture after treatment with sodium-butyrate (NaBT) 18,19 or by blocking phosphatidylinositol 3-kinase (PI3K), an important mediator of intracellular signal transduction. 20 We assessed the relationship of the proliferating inhibiting effect of transforming growth factor-β (TGF-β) to CD97 expression in both TGF-β-sensitive and -insensitive cell lines. In a second part, colorectal carcinomas and the corresponding normal tissue of the patients were screened for CD97 expression by immunohistology to gain an insight into the distribution pattern of the molecule.
Materials and Methods
Cell Lines and Cells
The human colorectal cancer cell lines Caco-2, Co115, Colo 201, Colo 205, DLD-1, HCT 116, HCT-15, HT-29, Lisp-1, LoVo, LS1034, LS174T, LS411N, LS513, SW 1116, SW 480, SW 837, and WiDr were obtained from the American Type Culture Collection (ATCC, Rockville, MD) or the European Collection of Cell Cultures (ECACC, Salisbury, UK). The cells were maintained in Dulbecco’s modified minimal essential medium or Dulbecco’s modified minimal essential medium/Ham’s F12 (Life Technologies GmbH, Karlsruhe, Germany) containing 10% fetal calf serum except for Colo 201, Colo 205, and HCT-15 that were grown in RPMI 1640/10% fetal calf serum.
Generation of Stable CD97 (EGF 1,2,5)-Inducible HT1080 Cells
CD97(EGF 1,2,5) cDNA was amplified by polymerase chain reaction (PCR) from the vector pcDNA3.1/Zeo+ containing the desired cDNA. 2 Primers generating MluI and NheI sites during amplification were used (PrCD97 MluI: 5′-cga cgc gtg cca cca tgg gag gcc gcg-3′; PrCD97NheI: 5′-aac get agc cct tga tat gcc gga-3′). The cDNA was inserted into the MluI and NheI sites of pBI-EGFP (BD Clontech, Heidelberg, Germany).
HT1080 Tet-off cells (Clontech) were grown in Dulbecco’s modified minimal essential medium, supplemented with 10% Tet system-approved fetal calf serum (Life Technologies), 4 mmol/L l-glutamine, and 100 μg/ml G418 (Roche Diagnostics GmbH, Mannheim, Germany). The cells were co-transfected with 5 μg of pBI-EGFP-CD97 (EGF 1,2,5) and 250 ng pWE-4 (Clontech) by electroporation. Selection of stable cell lines was initiated 2 days after transfection using 100 μg/ml of hygromycin (Clontech). Fourteen days after transfection, several colonies were isolated and maintained in medium supplemented with 1 μg/ml of doxycycline (Clontech) in 24-well plates. To induce the expression of CD97, cells were cultured without doxycycline. Experiments were performed on cells cultured for 72 hours with or without the antibiotic. Expression of CD97 was verified by flow cytometry.
Migration and Invasion Assay
The migration and invasion capacity of colon carcinoma cell lines were evaluated in 24-well transwell chambers (Costar, Bodenheim, Germany) or 6-well Biocoat Matrigel invasion chambers (BD Biosciences, Heidelberg, Germany) containing Matrigel-precoated filters, respectively. In both assays, the upper and lower culture compartments were separated by polycarbonate filters with a pore size of 8 μm. Five μg/ml of collagen I (Sigma-Aldrich Chemie GmbH; Taufkirchen, Germany) was used as a chemoattractant in the lower chamber compartments. Before starting the invasion assay, the Matrigel matrix of the chambers was reconstituted by adding serum-free RPMI 1640 for 2 hours.
Tumor cells were radiolabeled for 12 hours using (methyl-3H)-thymidine (0.1 μCi/ml, 7.4 MBq/mmol) and detached with 0.02% ethylenediaminetetraacetic acid. For the invasion assay, 8 × 105 cells per well were incubated on the reconstituted membrane for 72 hours. For the migration assay, 1 × 105 cells per well were seeded onto the filter for 24 hours. Cells passing the filters and attaching to the lower sites of uncoated or Matrigel-coated membranes were harvested using trypsin/ethylenediaminetetraacetic acid. Cell-bound radioactivity was measured using a liquid scintillation counter. The percentages of migration and invasion were calculated on the number of migrating cells compared to the total number of cells. To specify our results, the highly malignant osteosarcoma cell line MG-63 (ATCC) was used as a positive standard cell line in both assays. All quantifications were done in triplicate. The correlation between percent of migration and invasion to mean fluorescence intensity of CD97 was calculated using Spearman’s method.
Monoclonal Antibodies (mAbs)
MEM-180 and CLB-CD97/3 21 bind to the stalk region of CD97 and do not show reactivity to EMR2, another member of the EGF-TM7 family. CLB-CD97/1 11 binds to the N-terminal EGF-domain of CD97 but also detects EMR2. Thus, the mAb CLB-CD97/1 was used in combination with MEM-180 to achieve specificity in the enzyme-linked immunosorbent assay (ELISA) system. The CD55 mAb was purchased from BD Biosciences.
Flow Cytometry
Carcinoma cells were phenotyped with the desired mAb by an indirect immunofluorescence method using a F(ab′)2 fragment of goat anti-mouse immunoglobulin (FITC-GAM; DAKO, Hamburg, Germany) with a FACSscan (Becton Dickinson, Mountain View, CA). The levels of CD97 and CD55 were determined as mean fluorescence intensity of stained cells in comparison to cells stained with an isotype-matched but irrelevant mAb. To determine the level of CD97 in transfected Tet-off HT1080 cells, the CD97 mAb (clone MEM-180) was coupled to N-hydroxysuccinimidobiotin (Sigma). After incubation with the biotin-labeled CD97 mAb and washing, the HT1080 cells were stained with streptavidin-phycoerythrin (DAKO).
Regulation of CD97 by TGF-β, Wortmannin, and NaBT
The effect on proliferation was evaluated in parallel using the CellTiter96AQueous one-solution cell proliferation assay (Promega GmbH, Mannheim, Germany) at day 9. Colorectal carcinoma cells were seeded at an initial density of 3 × 103 cells per well in 96-well plates. Wortmannin (0.01 to 1 μmol/L, Sigma), TGF-β (0.01 to 1 ng/ml, R & D systems), and/or NaBT (0.5 to 50 mmol/L, Sigma) were added at day 0. Control cells received the same amount of the solvent of the agents. Fivefold cultures were analyzed in four experiments. To detect apoptosis, cells were treated with the required concentration of TGF-β, wortmannin, and/or NaBT for 48 hours; after that, they were trypsinized, double-stained with propidium iodine and annexin V-FITC using the annexin V-Fluos staining kit (Roche) according to the manufacturer’s instructions, and analyzed by flow cytometry. Annexin V binds to phosphatidylserine residues expressed on the outer leaflet of the cell membrane as an early event in apoptosis. Cells that stain for annexin V-FITC but not propidium iodine are considered apoptotic. The loss of cell viability in the detached cells was confirmed by the loss of the ability of the cells to exclude trypan blue.
To determine the effect of the different agents on CD97 expression, 1 × 105 cells/well were cultured on 24-well plates for 24 hours. The medium was aspirated and replaced with 500 μl of Opti-Mem (Life Technologies) without fetal calf serum containing the desired concentration of TGF-β, wortmannin, and/or NaBT. The cells were trypsinized and stained with CD97 mAbs for fluorescence-activated cell sorting analysis after 12, 24, 48, 72, and 96 hours.
Detection of the CD97 mRNA Isoforms
Total cellular RNA was isolated from tissues and cell cultures with the Qiagen total RNA isolation kit (Qiagen) in accordance with the manufacturer’s instructions. Five μg of total RNA was taken to synthesize cDNA using a first-strand cDNA synthesis kit from Amersham Pharmacia Biotech (Freiburg, Germany) in a reaction volume of 15 μl. The primer pair PhCD97EGFs1 (5′-tcc tgc cgg cag ctc caa ml-3′)/r1 (5′-ggc agc ggc agc tgt atg aac-3′) spans the alternatively spliced EGF-like domain coding region resulting in a 452 by (EGF 1,2,5), 584 by (EGF 1,2,3,5), or 731 by (EGF 1,2,3,4,5) PCR product. The primer pair PhCD97TM7s1 (5′-ctg gcc gcc ttc tgc tgg atg ag-3′)/r1 (5′-ctg cgc gat ggc cgt gat gg-3′) partly amplifies the transmembrane coding region of CD97 resulting in a 356 by-product in all CD97 isoforms. To correct for variations across different cDNA preparations, all samples were first adjusted to contain equal input of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA concentrations. 14 Each 25-μl amplification reaction contained 2.5 μl 10× concentrated PCR buffer (15 mmol/L MgCl2), 0.3 U TaqDNA polymerase, 100 μmol/L dNTPs (all from Roche), 0.1 μmol/L of each primer, and 1 μl cDNA in adjusted dilution.
Patients
The study comprised a series of 81 sporadic colorectal adenocarcinomas obtained from patients undergoing surgery at the Department of Surgery, University of Leipzig, between October 1999 and July 2001. Fourteen were from the proximal colon (cecum, ascending, and transverse colon), 27 were from the distal colon (descending and sigmoid colon) and 40 were from the rectum. Normal mucosal specimens from at least 5 cm away from colorectal carcinoma lesions were obtained from all patients. Samples were cryopreserved in liquid nitrogen.
The study was approved by the local Committees of Medical Ethics; all patients gave written consent. No patient had received chemotherapy or radiotherapy before surgery. Histological diagnosis and staging were established following the TNM classification (stage I, n = 10; II, n = 21; III, n = 27; IV, n = 23) proposed by the International Union Against Cancer as evaluated in the Institute of Pathology, University of Leipzig, according to standard criteria. Every tumor was further examined histologically 1) for differentiation, according to the predominant growth pattern (well differentiated, grade I, n = 3; moderately differentiated, grade II, n = 59; poorly differentiated, grade III, n = 19); 2) for the presence of blood and lymph vessel invasion; 22 and 3) for the evidence of tumor budding defined as small clusters of undifferentiated cancer cells ahead of the invasive front of the lesion as described. 23 Tumor budding was divided into two groups according to degree: none or mild (BD-1) and moderate or severe (BD-2). Classification of lesions as BD-1 or BD-2 was based on predominant pattern of tumor budding in the specimen. Sera were obtained from all patients on the day before operation and stored in aliquots at −80°C. Age- and sex-matched healthy blood donors were used as controls (n = 26).
CD97 Immunostaining of Tissues
Tissues were snap-frozen in liquid nitrogen. Serial frozen sections were cut at 5 μm, fixed in ice-cold methanol for 10 minutes followed by a short rinse in 0.2 mol/L phosphate-buffered saline, and then incubated with the anti-CD97 mAb (clone CLB-CD97/3). The anti-leukocyte common antigen mAb (DAKO) was used to reliably discriminate between tumor cells and tumor-infiltrating leukocytes that are partly CD97+. In negative control sections, an irrelevant mAb of the same isotype was applied at the same concentrations as the primary antibody. After incubation with the mAb (4°C, overnight), bound antibody was detected with a supersensitive detection kit (BioGenex, San Ramon, CA) including biotinylated anti-mouse Ig and horseradish peroxidase-conjugated streptavidin.
Scoring and Computerized Nuclear Morphometry
Immunoreactivity was scored semiquantitatively by two independent investigators who were unaware of the histological results using a light microscope (Axioskop; Carl Zeiss Jena GmbH, Jena, Germany). Tissue from a patient with a dedifferentiated thyroid tumor was used for each staining procedure as a positive control. The percentage of positive cells was judged ranging from 1 to 4 (1, < 10%; 2, 10 to 50%; 3, 51 to 80%; 4, >80% positive tumor cells) and the level of staining intensity was estimated to grades between 0 and 3 (0, negative; 1, weak; 2, moderate; 3, strong staining). The product of positive cells and staining intensity gave the score (0 to 12). We considered samples positive if the score was higher than 2. A score of 3 to 6 was regarded as moderate, and >6 as strong. CD97-positive carcinomas were further examined for the presence of more strongly stained scattered cells or scattered cell groups near or on the invasion front. Staining intensity of these stronger CD97-positive scattered tumor cells was compared to the staining intensity of the tumor cells located within solid tumor formations. The association of CD97 expression and clinicopathological features was assessed with the Fisher’s exact test. A level of P < 0.05 was considered significant.
The mean nuclear area of more strongly CD97-positive scattered tumor cells was compared to the mean nuclear area of tumor cells located within solid tumor formations by computerized nuclear morphometry 24 using a color video camera module and color image freezer (HC300Z; Fuji Photo Film Ltd. Co., Japan). Two hundred cancer nuclei of more strongly CD97-positive scattered tumor cells or tumor cells located within solid tumor formations with complete and clearly identifiable outlines were registered from each patient, and cancer cell nucleus areas were calculated using the Axiovision software (Carl Zeiss Vision GmbH, Hallbergmoos, Germany).
Assays for sCD97 and Soluble Tumor Markers
The ELISA for CD97 has been described elsewhere. 25 Briefly, ELISA plates were coated with 5 μg/ml of the mAb MEM-180 at 4°C overnight. After washing the plates, undiluted serum was applied to the plates for 60 minutes at room temperature. After washing, the wells were incubated with 1 μg/ml of biotinylated CLB-CD97/1. Thereafter, streptavidin-peroxidase, and subsequently tetramethylbenzidine were added to the plates and the optical densities were measured in an ELISA reader. Results are expressed as arbitrary units (U/ml) of a standard amount of CD97 (supernatant of COS-7 cells expressing a soluble CD97 construct) in duplicate measurements. CEA (IRMA-coatCEA; Byk-Sangtec Diagnostica, Dietzenbach, Germany), CA15-3 IRMA-matCA15-3; Byk-Sangtec), CA19-9 (IRMA-matCA19-9; Byk-Sangtec), and CA72-4 (ELSA-CA72-4, CISbio international, Gis-Sur-Yvet, France) were determined preoperatively according to the manufacturer’s protocol.
Results
Expression of CD97 on Tumor Cell Lines
All colon cell lines examined in this study expressed CD97 with varying intensity (Table 1) ▶ . CD55, the cellular ligand for CD97, was present on all cell lines except SW480. In Colo 205 cells CD55 expression is rather low. There was no correlation between CD55 and CD97 (r = −0.39, P = 0.11) staining intensity.
Table 1.
Expression of CD97 and CD55 in Colorectal Tumor Cell Lines Determined by Flow Cytometry (Percentage of Cells Expressing the Antigen and Mean Fluorescence Intensity)
| Cell line | CD97 | CD55 | % Migration | % Invasion | ||
|---|---|---|---|---|---|---|
| % | Intensity | % | Intensity | |||
| Caco-2 | 90–100 | 10.0 ± 4.2 | 90–100 | 45.8 ± 14.3 | 7.1 ± 2.3 | 1.1 ± 0.4 |
| Co115 | 90–100 | 15.2 ± 0.4 | 90–100 | 40.4 ± 15.4 | 6.5 ± 1.9 | 1.9 ± 0.3 |
| Colo 201 | 90–100 | 46.3 ± 2.3 | 90–100 | 6.1 ± 0.5 | 55.4 ± 5.3 | 20.3 ± 4.2 |
| Colo 205 | 90–100 | 56.8 ± 7.0 | 5–50 | 0.7 ± 0.4 | 42.1 ± 3.9 | 19.6 ± 2.1 |
| DLD-1 | 90–100 | 40.6 ± 1.3 | 90–100 | 21.6 ± 2.8 | 32.0 ± 2.5 | 16.3 ± 2.4 |
| HCT 116 | 90–100 | 31.4 ± 3.4 | 90–100 | 30.4 ± 3.1 | 27.1 ± 3.8 | 11.2 ± 1.7 |
| HCT-15 | 90–100 | 15.9 ± 0.8 | 90–100 | 11.2 ± 1.2 | 9.4 ± 2.1 | 4.9 ± 0.6 |
| HT-29 | 90–100 | 20.9 ± 4.9 | 90–100 | 22.1 ± 5.1 | 9.4 ± 1.9 | 3.8 ± 0.5 |
| Lisp-1 | 90–100 | 16.4 ± 2.4 | 50–90 | 7.8 ± 3.7 | 9.7 ± 2.3 | 5.2 ± 1.4 |
| LoVo | 90–100 | 13.0 ± 0.8 | 90–100 | 41.9 ± 3.2 | 8.3 ± 1.7 | 4.9 ± 0.7 |
| LS1034 | 90–100 | 29.1 ± 9.3 | 90–100 | 20.1 ± 8.4 | 26.1 ± 2.5 | 14.8 ± 2.4 |
| LS174T | 90–100 | 26.4 ± 5.1 | 90–100 | 8.5 ± 2.3 | 15.8 ± 4.6 | 7.2 ± 2.3 |
| LS411N | 90–100 | 14.0 ± 3.1 | 90–100 | 25.0 ± 4.9 | 7.2 ± 0.4 | 4.2 ± 0.4 |
| LS513 | 90–100 | 41.9 ± 5.6 | 90–100 | 20.1 ± 8.4 | 55.2 ± 7.1 | 21.7 ± 3.3 |
| SW 1116 | 90–100 | 45.2 ± 5.6 | 90–100 | 17.5 ± 0.5 | 33.2 ± 4.3 | 19.5 ± 3.4 |
| SW 480 | 90–100 | 7.4 ± 0.1 | 0–5 | 0 | 0.7 ± 0.2 | 0.2 ± 0.0 |
| SW 837 | 90–100 | 25.1 ± 0.3 | 90–100 | 12.8 ± 2.1 | 12.3 ± 2.0 | 7.1 ± 0.9 |
| WiDr | 90–100 | 12.4 ± 0.8 | 90–100 | 33.3 ± 4.1 | 0.4 ± 0.1 | 2.2 ± 0.2 |
The percentage of migration and invasion of the tumor cell lines were calculated from the number of migrating cells compared to the total number of cells in chamber assays. mean ± SD, n = 3.
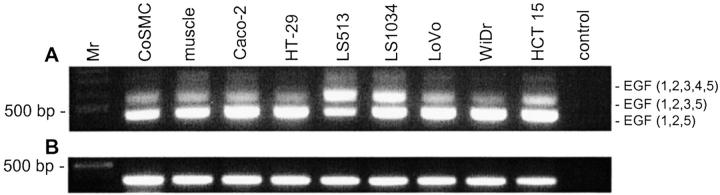
CD97 mRNA analysis by reverse transcriptase-PCR with primers that flank the exons coding the EGF-like domains indicated that all three CD97 isoforms are present in the cell lines (examples are shown in Figure 1 ▶ ). In most cell lines, the shortest CD97 isoform was expressed at the highest level as demonstrated for leukocytes. The relative expression of CD97 (EGF 1,2,3,5) and CD97 (EGF 1,2,3,4,5) mRNA varied between the cell lines. Higher expression of CD97 (EGF 1,2,3,5) mRNA was characteristic for LS513 cells.
Figure 1.
Detection of CD97 isoform mRNA by reverse transcriptase-PCR. A: The primer pair PhCD97EGFs1/r1 overspans the alternatively spliced EGF-like domain-coding region resulting in a 452-bp (EGF 1,2,5), 584-bp (EGF 1,2,3,5), or 731-bp (EGF 1,2,3,4,5) PCR product. B: Amplification of a part of the transmembrane-coding region of CD97 results in a 356-bp product. Positive control, peripheral blood lymphocytes; muscle, muscular coat of the colon; Mr, 100-bp DNA ladder.
Migration and Invasion Assay
We found a strong correlation between the expression level of CD97 measured by flow cytometry (Table 1) ▶ and migration as well as the invasion capacity of the cell lines (Figure 2) ▶ . The highly malignant osteosarcoma cell line MG-63, used as a positive control, showed 69% migration and 33% invasiveness.
Figure 2.
Colorectal carcinoma cell lines (n = 18): correlation between CD97 expression level determined as mean fluorescence intensity in flow cytometry and percent migration (A) or percent invasion (B) determined using in vitro assays (see Table 1 ▶ ).
To verify these results, we established stable CD97 (EGF 1,2,5) cDNA-transfected Tet-off HT1080 cells. The expression level of CD97 can be down-regulated in these cells by adding antibiotics. Stable CD97 (EGF 1,2,5) cDNA-transfected cells cultured without doxycycline (CD97on) revealed levels 300 times higher than the cells cultured with doxycycline (CD97off) and a CD97 level up to 500 times higher than in wild-type Tet-off HT1080 cells, showing only a faint CD97 signal. All three cell types (CD97on, CD97off, and wild-type HT-1080 cells) were inserted in the in vitro migration and invasion assays. CD97on cells showed a significantly higher migration and invasion capacity than CD97off or wild-type Tet-off HT1080 cells (Table 2) ▶ .
Table 2.
Correlation between CD97 Expression in CD97 (EGF 1,2,5) cDNA-Transfected Tet-off HT1080 Cells and Migration or Invasion Determined Using In Vitro Assays (n = 4)
| Tet-off HT1080 cells | % Migration | % Invasion |
|---|---|---|
| Wild-type | 25.1 ± 2.7 | 10.7 ± 1.1 |
| CD97 transfected, with doxycyclin: CD97off | 34.6 ± 5.0* | 23.3 ± 1.2* |
| CD97 transfected, without doxycyclin: CD97on | 80.3 ± 10.1*† | 56.2 ± 7.6*† |
*P < 0.01 compared to the wild type,
†P < 0.01 compared CD97off and CD97on.
Effect of NaBT and Wortmannin on CD97 Expression
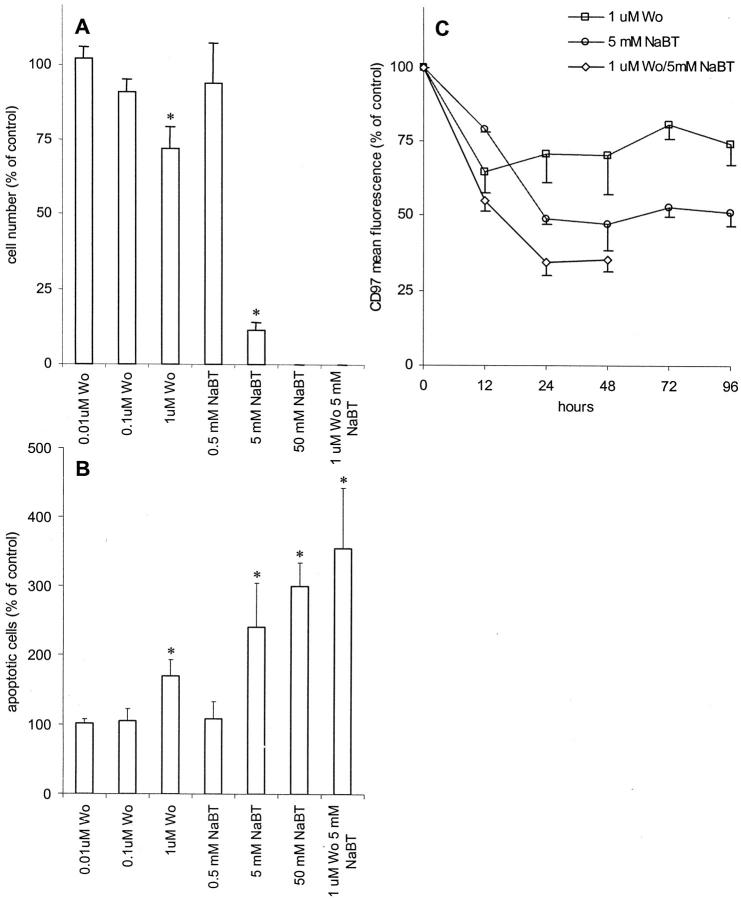
Induction of differentiation with NaBT or by blocking of the PI3K in Caco-2 cells is associated with inhibition of proliferation (Figure 3A) ▶ . NaBT showed a strong effect at 5 mmol/L whereas wortmannin, a specific inhibitor of PI3K, significantly down-regulated proliferation in the Caco-2 cells only at a concentration of 1 μmol/L. Adding both NaBT and wortmannin simultaneously, proliferation is completely abrogated. We observed microscopically that treated Caco-2 cells detached from the dishes floating in the media with increasing time of treatment with NaBT. Thus, we examined apoptosis (Figure 3B) ▶ . Forty-eight hours after treatment with 5 mmol/L of NaBT, a twofold to threefold increase in the percentage of apoptotic cells compared to untreated cells (percent apoptotic cells, mean ± SD, n = 4: 17.5 ± 8.4%) was detected among the cells that were still attached. The rate of apoptosis increased up to fourfold compared to controls within 72 hours (not shown). The detached cells were dead as shown by trypan blue exclusion. Wortmannin caused an increase in the percentage of apoptotic cells at higher concentrations only.
Figure 3.
Effect of induction of differentiation in Caco-2 cells by blocking of PI3K with wortmannin or by treatment with NaBT. Results were normalized and expressed as means ± SE (n = 4) compared to the control (untreated cells). *, P ≤ 0.05 versus control. A: Effect on cell proliferation. The cells were treated with various concentrations of wortmannin (wo) and/or NaBT for 9 days. The anti-proliferative effect was evaluated in parallel using the CellTiter96AQueous one solution cell proliferation assay. B: Induction of apoptosis measured by flow cytometry. The cells were treated with various concentrations of wortmannin and/or NaBT for 48 hours. Treated and untreated cells (control) were stained with annexinV-FITC and propidium iodine. The figure demonstrates the percentage of annexin V-positive apoptotic cells. C: Effect of wortmannin and/or NaBT on the expression of CD97. Cells were treated with 1 μmol/L of wortmannin and/or 5 mmol/L of NaBT and the expression of CD97 was examined in flow cytometry.
In the second part of the experiment, we investigated the effect of NaBT and wortmannin on the CD97 expression levels measured by flow cytometry in the same cell lines. Treatment with NaBT and/or wortmannin resulted in a time-dependent decrease of CD97 expression in Caco-2 cells that were still attached (Figure 3C) ▶ . The CD97-down-regulating effect was observed after 3 hours (not shown) and continued up to 96 hours. Cells cultured with NaBT and wortmannin could not be further analyzed after 48 hours because the cells detached completely after this time.
Effect of TGF-β on CD97 Expression
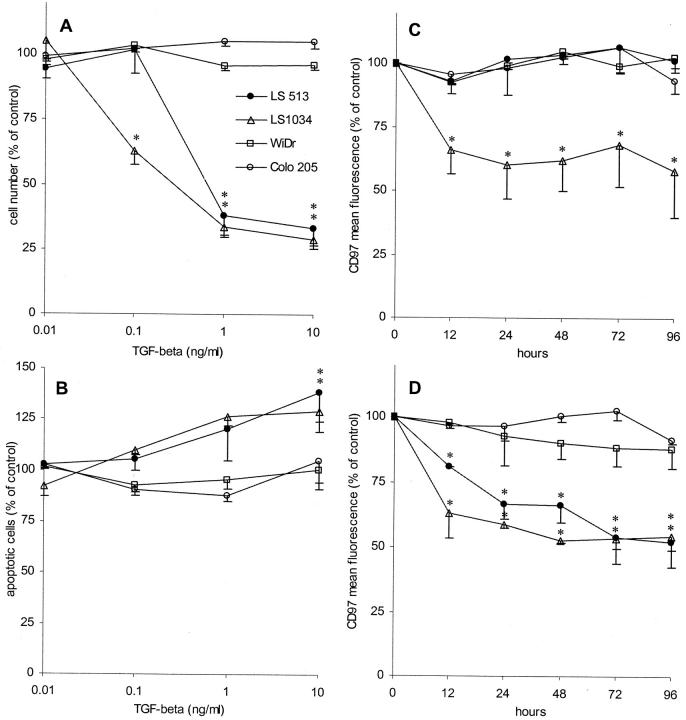
We did not observe any inhibition in proliferation and signs of apoptosis induced by TGF-β in either WiDr, Colo215 (Figure 4, A and B) ▶ , HT-29, or Caco-2 cells (not shown). In contrast, LS513 and LS1034 cells, known to be sensitive for the inhibitory effects of TGF-β, were both responsive to the cytokine. In the proliferation assay, LS1034 cells were even sensitive to 0.1 ng/ml of TGF-β, whereas LS513 cells responded only to 1 ng/ml. TGF-β induced a slight increase in apoptotic cell numbers in both carcinoma cell lines at higher concentrations compared to untreated cells (percent apoptotic cells, mean ± SD, n = 4: LS 513: 26.9 ± 10.1%; LS1034: 18.6 ± 7.3%) (Figure 4B) ▶ .
Figure 4.
TGF-β sensitivity of colorectal carcinoma cell lines. Results were normalized and expressed as means ± SE (n = 4) compared to the control (untreated cells). *, P ≤ 0.05 versus control. A: Effect of TGF-β on cell proliferation. The cells were treated with various concentrations of TGF-β for 9 days (for further details see Figure 3A ▶ ). B: Induction of apoptosis measured by flow cytometry. The cells were treated with various concentrations of TGF-β for 48 hours (for further details see Figure 3B ▶ ). C and D: Effect of TGF-β on the expression of CD97. Cells were treated with 0.1 (C) and 1 ng/ml of TGF-β (D) and the expression of CD97 was examined in flow cytometry.
In the second part of the experiments, we investigated the effect of 0.01, 0.1, and 1 ng/ml of TGF-β on the CD97 expression level measured by flow cytometry in the same cell lines. TGF-β treatment resulted in a dose-dependent decrease of CD97 expression in TGF-β-sensitive LS1034 and LS513 cells, whereas WiDr and Colo215 cells were unaffected (Figure 4, B and C) ▶ . Both LS1034 and LS513 showed a down-regulation of CD97 that continued throughout 96 hours using 1 ng/ml of TGF-β (Figure 4C) ▶ . In LS1034 cells, even 0.1 ng/ml of TGF-β decreased CD97 (Figure 4B) ▶ . No effect was observed using 0.01 ng/ml of TGF-β in any cell line (not shown).
CD97 Expression in Colorectal Tissues
The epithelium of normal colon mucosa is virtually CD97-negative (score ≤2; Figure 5A ▶ ), regardless of the location in the large intestine. Only 20 of 81 normal samples showed very weak staining for CD97 (score, mean ± SEM, 1.8 ± 0.2) on epithelial cells. In contrast, 75 of 81 of the colorectal carcinomas expressed CD97 (6.1 ± 2.3).
Figure 5.
Immunohistological expression pattern of CD97 on cryosections of colorectal normal tissue and carcinoma. A: Normal epithelial cells are CD97-negative (open arrow), whereas tumor cells express the antigen (arrow). Note the strong staining of smooth muscle cells in the lamina muscularis mucosae and the muscular coat (asterisk). B: Stronger cytoplasmic and membranous expression of CD97 in scattered tumor cells or tumor cells groups (arrow) surrounded by stroma compared to tumor cells located in tumor glands or solid tumor trabecula (open arrow). C: Stronger membranous CD97 staining of the outer parts of tumor cells at the tumor margin or invasion front (arrow) and weaker or no membrane expression on tumor cells within the center of the carcinoma. Moreover, there is a group of tumor cells seeming to detach from the tumor formation with stronger CD97 expression (open arrow). Scale bars: 100 μm (A); 50 μm (B and C).
CD97 expression was heterogeneous in carcinomas within 46.7% (35 of 75) of all CD97-positive tumors having areas with varying levels of this molecule. Two typical locations of more strongly CD97-positive tumor cells could be observed. First, very strong staining with CD97 was found in scattered tumor cell clusters or single tumor cells completely surrounded by stroma (n = 25, 10.4 ± 1.8) compared to cells in glands or trabecula of the same tumor (5.6 ± 2.4, P < 0.0001) (Figure 5B) ▶ . The mean nuclear area of stronger CD97-positive scattered tumor cells was significantly higher (79.4 ± 15.9 μm2) than that of tumor cells located within solid tumor formations in CD97-positive carcinomas with nonuniform staining (36.4 ± 10.5 μm2) as determined by the Mann-Whitney test (P < 0.0001).
Second, strong expression of CD97 was observed at the margin of solid tumor glands or trabecula (n = 10). Depending on the distance to the margin or invasion front, CD97 expression became weaker. Larger homogeneous tumor areas showed weaker CD97 expression (Figure 5C) ▶ . In these areas, tumor cells even tended to lose CD97 membrane staining (Figure 5, B and C) ▶ .
The accumulation of CD97 in scattered tumor cells or tumor cells at the tumor margin may be only transient. In three cases we could additionally examine liver metastases of the primary tumors containing strong CD97-positive scattered tumor cells. Tumor cells in growing metastases showed again a phenotype lacking or with lower CD97 expression in the center of tumor glands (not shown). Apart from tumor cells, two other cell types were CD97-positive. First, smooth muscle cells strongly expressed CD97 in normal and carcinoma tissue samples (Figure 5A) ▶ . The CD97 mAb labeled smooth muscle cells of the lamina muscularis mucosae and the muscular coat of the colon partly infiltrated by tumor cells (Figure 5, A and B) ▶ . Second, leukocytes located in the lamina propria mucosae of normal colorectal tissues were CD97-positive. The CD97-positive leukocytes seem to represent mostly monocytes/macrophages, not lymphocytes, based on their morphology. Lymphocytes located in follicles in the mucosae appeared to be CD97-negative or only slightly positive (not shown).
Clinicopathological Features
CD97 expression was compared between tumors with more strongly CD97-positive cells at the invasion front or tumor margin (n = 35) and with uniform CD97 staining (n = 40, Table 3 ▶ ). The following parameters showed no relation to the pattern of CD97 expression: age, sex, pT, pN, M, differentiation, as well as histological blood vessel infiltration. Carcinomas with more strongly stained CD97 cells at the invasion front showed a higher clinical stage and tumor budding compared to carcinomas with homogenous CD97 staining (Table 3) ▶ . Seventy-five percent of the carcinomas containing more strongly CD97-positive cells were graded as clinical stages 3 and 4, whereas 54% of the tumors with homogenous CD97 staining belonged to these stages. Not in every carcinoma scattered tumor cells observed in hematoxylin and eosin-stained sections examined as tumor cell budding were more strongly CD97-positive compared to the tumor cells within solid formations. The percentage of tumors containing tumor cells that were more strongly CD97-positive increased in relation to tumor cell budding (Table 3) ▶ .
Table 3.
CD97 Expression and Clinicopathological Features
| CD97hom | CD97scatt | P | |
|---|---|---|---|
| Number of cases | 40 | 35 | |
| Age (mean ± SD) | 65.0 ± 10.7 | 65.6 ± 12.0 | |
| Sex | |||
| Male | 23 | 22 | 0.813 |
| Female | 17 | 13 | |
| Tumor location | |||
| Colon | 17 | 17 | 0.647 |
| Rectum | 23 | 18 | |
| Tumor size (pT) | |||
| 1 or 2 | 11 | 5 | 0.258 |
| 3 or 4 | 29 | 30 | |
| Lymph node metastasis (pN) | |||
| 0 | 21 | 11 | 0.801 |
| 1 or 2 | 29 | 24 | |
| Distant metastasis (M) | |||
| 0 | 27 | 20 | 0.473 |
| 1 | 13 | 15 | |
| Tumor stage | |||
| 1 or 2 | 20 | 9 | 0.036* |
| 3 or 4 | 20 | 26 | |
| Differentiation (grade) | |||
| I or II (well, moderate) | 31 | 26 | 0.791 |
| III (poor) | 9 | 9 | |
| Blood vessel invasion | |||
| No (V0) | 26 | 19 | 0.358 |
| Yes (V1) | 14 | 16 | |
| Lymph vessel invasion | |||
| No (L0) | 13 | 4 | 0.050* |
| Yes (L1) | 27 | 31 | |
| Tumor budding | |||
| BD-1 (non/mild) | 16 | 3 | 0.003* |
| BD-2 (moderate/severe) | 24 | 32 |
The CD97-positive carcinomas were subdivided in two groups: carcinomas with homogenous CD97 staining (CD97hom) and carcinomas with stronger stained scattered tumor cells (CD97scatt).
*P ≤ 0.05.
CEA, CA15-3, CA19-9, and CA72-4 serum levels did not correlate with the expression of CD97. The mean levels (±SEM) of sCD97 in serum were not higher in patients with colorectal carcinomas (0.18 ± 0.02 U/ml) compared to the control group (0.16 ± 0.01 U/ml). There was no correlation between sCD97 serum levels and CD97 expression in tumor tissues that did not support a release of sCD97 from the tumor cells themselves.
Discussion
Our observation that in nearly half of the examined colorectal carcinomas CD97 is strongly localized in isolated tumor cells or small tumor cell clusters at the invasion front and that in central parts of the tumor the opposite is the case (ie, low or even absent expression of CD97), support the recently obtained data showing that CD97 is involved in invasion. 14,15 Carcinomas containing strongly CD97-positive tumor cells at the invasion front showed significantly more often lymph vessel invasion and higher clinical stage, that are strong prognostic factors in colorectal carcinomas, 26 compared to carcinomas with homogenous CD97 staining. However, the accumulation of CD97 in scattered tumor cells or tumor cells at the tumor margin can be transient as demonstrated for changes in β-catenin localization 27 because tumor cells in growing metastases show again a phenotype with lower or lacking CD97 expression in the center of tumor glands.
Other studies confirmed that a temporary changed expression of adhesive, proteolytic, or extracellular matrix molecules like components of the E-cadherin/catenin complex, integrins, and angiomodulin, components of the tissue destructive plasminogen activation system, or certain laminin-5 subunits at the invasion front of colorectal carcinomas result in migration and invasion of the single tumor cells or cell clusters. 28-30 β-catenin shows a membrane-bound distribution pattern within compact tumor formations, but nuclear and cytoplasmic overexpression in tumor cells localized predominantly at the invasion front. 31,32 The changed cellular localization causes changes in function. Normally, membrane-bound β-catenin hold cells together, whereas nuclear or cytoplasmic β-catenin transmits Wnt-ligand signals to the nucleus initiating the amplification of oncogenes. 33 Collectively, these studies suggest that in colorectal cancer, molecules such as CD97 contributing to cell-matrix and cell-cell interactions may identify a subset of carcinoma cells prone to invade focally, spread, and metastasize. Tumor border configuration is a factor that has been shown by multiple studies to be promising in its possible prognostic values for outcome in colorectal carcinomas. 23,26 We found a strong correlation between the appearance of moderate or severe tumor budding and accumulation of CD97 in these scattered tumor cells.
The idea that CD97 participates in the migration and invasion of tumor cells has been strengthened by the observation that CD55, the ligand for CD97, is overexpressed in the tumor environment of colorectal carcinomas. 34 Here, at the tumor-stroma interface, CD97 is preferentially overexpressed in tumor cells. By direct receptor-ligand interactions, CD97 may be involved in the remodeling of adhesive contacts of the invading tumor cells with the extracellular matrix. In vitro colorectal tumor cell lines expressing CD55, as confirmed in our study, and endothelial cells deposit CD55 into the extracellular matrix. 34 Perhaps such exogenic signals from the specific tumor environment regulate the cellular distribution of CD97 and consequently activation of its unknown target genes. Although the mechanisms for CD97 and CD55 overexpression at the invasion front remain unclear, both molecules may be good targets for tumor therapy. In the case of CD55 this has been already demonstrated. 791Tgp72, an antigen that was successfully used as a target for both colorectal tumor imaging and cancer vaccines, has been recently identified as CD55. 35
Our in vivo data indicate that the cancer cells acquire or enhance the ability to recognize the environment by displaying CD97 as an adhesive receptor. We have confirmed the linear correlation between CD97 and increased migratory and invasive potential in vitro on colorectal carcinoma cell lines. Stable transfection of CD97 (EGF 1,2,5) in Tet-off HT1080 cells also results in a significant increase of migratory and invasive capacity. However, with higher nonphysiological CD97 expression as present in the transfected Tet-off HT-1080 cells showing an increase in CD97 mean fluorescence intensity of up to 500 times that of the wild-type cells, the increase in the rate of invasion and migration slowed down, indicating a saturation or plateau effect. Factors responsible for this observation that limit the increase of the migratory and invasive capacity of the cells lie in the physical properties of the test system and biological characteristics of the cells, such as time-limited rearrangement of the cytoskeleton. 36-38
To examine the relation of differentiation and CD97 expression in vitro, we used the Caco-2 cell line as a suitable model to delineate potential pathways leading to an enterocyte-like phenotype that includes induction of alkaline phosphatase and cell polarization. 39 One possibility for achieving differentiation of Caco-2 cells is treatment with NaBT. 18 Butyrate, a short-chain fatty acid, is a natural fermentation product of dietary fibers of the colon microflora that contributes to the apparent protective action exerted by dietary fibers against colon cancer. 19 Inhibition of PI3K with wortmannin is another possibility for enhancing enterocyte-like differentiation of these cells. 20 Increased PI3K activity can convert differentiated colon and gastric cancer cells to a less-differentiated and more malignant phenotype. 40 Our results indicate that the induction of a differentiated phenotype in colon cancer cells by NaBT or by blocking of PI3K is associated with a marked reduction in CD97 expression. We demonstrated that treatment with NaBT caused growth arrest and induction of apoptosis as confirmed in other studies. 41,42 NaBT influenced the β-catenin-dependent Wnt signaling pathway by modulation of gene expression through specific promotor regions in colorectal cell lines. 18,41,42 In our study, blocking with wortmannin also up-regulated CD97 expression and proliferation, but only slightly induced apoptosis in Caco-2 cells. However, wortmannin in combination with NaBT synergistically induced apoptosis in all cells.
In the second part of our in vitro experiments, we evaluated the correlation of the proliferation inhibitory effect of TGF-β and CD97. TGF-β inhibits growth in normal colon enterocytes 43 and in TGF-β-sensitive colorectal carcinoma cell lines such as LS513 and LS1034, 44 which were used in our study. The mechanisms for resistance to TGF-β growth inhibition in the other cell lines examined here has been attributed to deficient intracellular pathways and/or to expression of aberrant or absent TGF-β receptors. 43 The disruption of the TGF-β signaling cascade is considered an important mechanism by which tumor cells can escape from growth suppression. 43 In our study, TGF-β decreased CD97 only in the sensitive LS513 and LS1034 cells. The sensitivity to growth inhibition correlated well with the extent of CD97 down-regulation. These findings indicate that the anti-tumorigenic role of TGF-β in the gastrointestinal tract may be associated with CD97 down-regulation. Contradictory data have been published as to the effect of TGF-β on apoptosis induction in epithelial cells. 45-47 Perhaps several pathways such as Smad or MAP kinase signaling are involved. Here we show that TGF-β has only minor apoptotic effects on TGF-β-sensitive colorectal cell lines.
Taken together, our findings indicate that CD97 is aberrantly expressed in colorectal carcinoma cell lines and tissues. The in vitro and in vivo data suggest that CD97 enables cancer cells to invade the surrounding matrix and survive through foreign microenvironments. CD97 seems to be involved in cell-cell and cell-extracellular matrix interactions that control tumor cell migration. Thus, CD97 may be a potential therapeutic target in cancer treatment.
Acknowledgments
We thank D. Sittig for performing the in vitro experiments; V. Horejsi, Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Prague, for providing mAb MEM-180; and R. Witzgall, Institute of Anatomy and Cell Biology, University of Heidelberg, for his help in establishing the CD97 Tet-off HT1080 cells.
Footnotes
Address reprint requests to Dr. Gabriela Aust, University of Leipzig, Institute of Anatomy, Ph.-Rosenthal-Str. 55, Leipzig, Germany D-04103. E-mail: ausg@medizin.uni-leipzig.de.
Supported by the Deutsche Krebshilfe (project 10-1530-Au I).
References
- 1.McKnight AJ, Gordon S: The EGF-TM7 family: unusual structures at the leukocyte surface. J Leukoc Biol 1998, 63:271-280 [DOI] [PubMed] [Google Scholar]
- 2.Hamann J, Eichler W, Hamann D, Kerstens HM, Poddighe PJ, Hoovers JM, Hartmann E, Strauss M, van Lier RA: Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven-span transmembrane molecule of the secretion receptor superfamily with an unusual extracellular domain. J Immunol 1995, 155:1942-1950 [PubMed] [Google Scholar]
- 3.Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, Yarme A, Stetler-Stevenson MA, Siebenlist U, Kelly K: CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol 1996, 157:5438-5447 [PubMed] [Google Scholar]
- 4.Baud V, Chissoe SL, Viegas-Pequignot E, Diriong S, N’Guyen VC, Roe BA, Lipinski M: EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics 1995, 26:334-344 [DOI] [PubMed] [Google Scholar]
- 5.Lin HH, Stacey M, Hamann J, Gordon S, McKnight AJ: Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics 2000, 67:188-200 [DOI] [PubMed] [Google Scholar]
- 6.Stacey M, Lin HH, Hilyard KL, Gordon S, McKnight AJ: Human epidermal growth factor (EGF) module-containing mucin-like hormone receptor 3 is a new member of the EGF-TM7 family that recognizes a ligand on human macrophages and activated neutrophils. J Biol Chem 2001, 276:18863-18870 [DOI] [PubMed] [Google Scholar]
- 7.Caminschi I, Lucas KM, O’Keeffe MA, Hochrein H, Laabi Y, Kontgen F, Lew AM, Shortman K, Wright MD: Molecular cloning of F4/80-like-receptor, a seven-span membrane protein expressed differentially by dendritic cell and monocyte-macrophage subpopulations. J Immunol 2001, 167:3570-3576 [DOI] [PubMed] [Google Scholar]
- 8.Stacey M, Chang GW, Sanos SL, Chittenden LR, Stubbs L, Gordon S, Lin HH: EMR4, a novel EGF-TM7 molecule up-regulated in activated mouse macrophages, binds to a putative cellular ligand on B lymphoma cell line, A20. J Biol Chem 2002, 277:29283-29293 [DOI] [PubMed] [Google Scholar]
- 9.Nechiporuk T, Urness LD, Keating MT: ETL, a novel seven transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the EGF-TM7 subfamily. J Biol Chem 2001, 276:4150-4157 [DOI] [PubMed] [Google Scholar]
- 10.Eichler W: CD97 isoform expression in leukocytes. J Leukoc Biol 2000, 68:561-567 [PubMed] [Google Scholar]
- 11.Hamann J, Vogel B, van Schijndel GM, van Lier RA: The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 1996, 184:1185-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichler W, Aust G, Hamann D: Characterization of the early activation-dependent antigen on lymphocytes defined by the monoclonal antibody BL-Ac(F2). Scand J Immunol 1994, 39:111-115 [DOI] [PubMed] [Google Scholar]
- 13.Jaspars LH, Vos W, Aust G, van Lier RA, Hamann J: Tissue distribution of the human CD97 EGF-TM7 receptor. Tissue Antigens 2001, 57:325-331 [DOI] [PubMed] [Google Scholar]
- 14.Aust G, Eichler W, Laue S, Lehmann I, Heldin N-E, Lotz O, Scherbaum WA, Dralle H, Hoang-Vu C: CD97: a dedifferentiation marker in human thyroid carcinomas. Cancer Res 1997, 57:1798-1806 [PubMed] [Google Scholar]
- 15.Hoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, Köhrle J, Dralle H: Regulation of CD97 protein in thyroid carcinoma. J Clin Endocrinol Metab 1999, 84:1104-1109 [DOI] [PubMed] [Google Scholar]
- 16.Holting T, Siperstein AE, Clark OH, Duh QY: Epidermal growth factor (EGF)- and transforming growth factor alpha-stimulated invasion and growth of follicular thyroid cancer cells can be blocked by antagonism to the EGF receptor and tyrosine kinase in vitro. Eur J Endocrinol 1995, 132:229-235 [DOI] [PubMed] [Google Scholar]
- 17.Schmutzler C, Kohrle J: Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid 2000, 10:393-406 [DOI] [PubMed] [Google Scholar]
- 18.Souleimani A, Asselin C: Regulation of C-fos expression by sodium butyrate in the human colon carcinoma cell line Caco-2. Biochem Biophys Res Commun 1993, 193:330-336 [DOI] [PubMed] [Google Scholar]
- 19.Basson MD, Turowski GA, Rashid Z, Hong F, Madri JA: Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig Dis Sci 1996, 41:1989-1993 [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Wang X, Hernandez A, Kim S, Evers BM: Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology 2001, 120:1381-1392 [DOI] [PubMed] [Google Scholar]
- 21.Kwakkenbos MJ, van Lier RA, Hamann J: Mason D eds. Characterization of EGF-TM7 family members by novel monoclonal antibodies. Leucocyte Typing VII. White Cell Differentiation Antigens. 2002:381-383 Oxford University Press, Oxford, England
- 22.Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT: Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer 1988, 61:1417-1424 [DOI] [PubMed] [Google Scholar]
- 23.Hase K, Shatney C, Johnson D, Trollope M, Vierra M: Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 1993, 36:627-635 [DOI] [PubMed] [Google Scholar]
- 24.Ikeguchi M, Taniguchi T, Makino M, Kaibara N: Reduced E-cadherin expression and enlargement of cancer nuclei strongly correlate with hematogenic metastasis in colorectal adenocarcinoma. Scand J Gastroenterol 2000, 35:839-846 [DOI] [PubMed] [Google Scholar]
- 25.Hamann J, Wishaupt JO, van Lier RA, Smeets TJ, Breedveld FC, Tak PP: Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum 1999, 42:650-658 [DOI] [PubMed] [Google Scholar]
- 26.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C: Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000, 124:979-994 [DOI] [PubMed] [Google Scholar]
- 27.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T: Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA 2001, 98:10356-10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buo L, Meling GI, Karlsrud TS, Johansen HT, Aasen AO: Antigen levels of urokinase plasminogen activator and its receptor at the tumor-host interface of colorectal adenocarcinomas are related to tumor aggressiveness. Hum Pathol 1995, 26:1133-1138 [DOI] [PubMed] [Google Scholar]
- 29.Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B: Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 1998, 185:44-52 [DOI] [PubMed] [Google Scholar]
- 30.Adachi Y, Itoh F, Yamamoto H, Arimura Y, Kikkawa-Okabe Y, Miyazaki K, Carbone DP, Imai K: Expression of angiomodulin (tumor-derived adhesion factor/mac25) in invading tumor cells correlates with poor prognosis in human colorectal cancer. Int J Cancer 2001, 95:216-222 [DOI] [PubMed] [Google Scholar]
- 31.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T: Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract 1998, 194:701-704 [DOI] [PubMed] [Google Scholar]
- 32.Maruyama K, Ochiai A, Akimoto S, Nakamura S, Baba S, Moriya Y, Hirohashi S: Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology 2000, 59:302-309 [DOI] [PubMed] [Google Scholar]
- 33.Wong NA, Pignatelli M: Beta-catenin—a linchpin in colorectal carcinogenesis? Am J Pathol 2002, 160:389-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Spendlove I, Morgan J, Durrant LG: CD55 is over-expressed in the tumour environment. Br J Cancer 2001, 84:80-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spendlove I, Li L, Carmichael J, Durrant LG: Decay accelerating factor (CD55): a target for cancer vaccines? Cancer Res 1999, 59:2282-2286 [PubMed] [Google Scholar]
- 36.Dickinson RB, McCarthy JB, Tranquillo RT: Quantitative characterization of cell invasion in vitro: formulation and validation of a mathematical model of the collagen gel invasion assay. Ann Biomed Eng 1993, 21:679-697 [DOI] [PubMed] [Google Scholar]
- 37.Germain F, Doisy A, Ronot X, Tracqui P: Characterization of cell deformation and migration using a parametric estimation of image motion. IEEE Trans Biomed Eng 1999, 46:584-600 [DOI] [PubMed] [Google Scholar]
- 38.Dickinson RB, McCarthy JB, Tranquillo RT: Quantitative characterization of cell invasion in vitro: formulation and validation of a mathematical model of the collagen gel invasion assay. Ann Biomed Eng 1993, 21:679-697 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz B, Lamprecht SA, Polak-Charcon S, Niv Y, Kim YS: Induction of the differentiated phenotype in human colon cancer cell is associated with the attenuation of subcellular tyrosine phosphorylation. Oncol Res 1995, 7:277-287 [PubMed] [Google Scholar]
- 40.Kobayashi M, Nagata S, Iwasaki T, Yanagihara K, Saitoh I, Karouji Y, Ihara S, Fukui Y: Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 1999, 96:4874-4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordonaro M, Mariadason JM, Aslam F, Heerdt BG, Augenlicht LH: Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A but not curcumin. Cell Growth Differ 1999, 10:713-720 [PubMed] [Google Scholar]
- 42.Bordonaro M, Lazarova DL, Augenlicht LH, Sartorelli AC: Cell type- and promoter-dependent modulation of the Wnt signaling pathway by sodium butyrate. Int J Cancer 2002, 97:42-51 [DOI] [PubMed] [Google Scholar]
- 43.Gold LI: The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncol 1999, 10:303-360 [PubMed] [Google Scholar]
- 44.Suardet L, Gaide AC, Calmes JM, Sordat B, Givel JC, Eliason JF, Odartchenko N: Responsiveness of three newly established human colorectal cancer cell lines to transforming growth factors beta 1 and beta 2. Cancer Res 1992, 52:3705-3712 [PubMed] [Google Scholar]
- 45.Lin JK, Chou CK: In vitro apoptosis in the human hepatoma cell line induced by transforming growth factor beta 1. Cancer Res 1992, 52:385-388 [PubMed] [Google Scholar]
- 46.Yanagihara K, Tsumuraya M: Transforming growth factor beta 1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res 1992, 52:4042-4045 [PubMed] [Google Scholar]
- 47.Kim BC, Mamura M, Choi KS, Calabretta B, Kim SJ: Transforming growth factor beta 1 induces apoptosis through cleavage of BAD in a Smad3-dependent mechanism in FaO hepatoma cells. Mol Cell Biol 2002, 22:1369-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]