Abstract
Immature dendritic cells (DCs) are scattered throughout peripheral tissues and act as sentinels that sample the antigenic environment. After activation, they modify their chemokine receptor profile and migrate toward lymphoid tissues. On arrival, they have matured into chemokine-producing DCs that express co-stimulatory molecules and can prime naive T cells. Normal temporal arteries contain immature DCs that are located at the media-adventitia border. In temporal arteries affected by giant cell arteritis, DCs are highly enriched and activated and have matured into fully differentiated cells producing the chemokines, CCL18, CCL19, and CCL21. In keeping with their advanced maturation, DCs in the granulomatous lesions possess the chemokine receptor, CCR7. CCR7 binds CCL19 and CCL21, causing the highly activated DCs to be trapped in the peripheral tissue site. The co-stimulatory molecule, CD86, which is critical for DC/T-cell interaction, is expressed by a subset of DCs captured in the arterial wall. DC/T-cell interaction does not involve interleukin-12; transcripts for interleukin-12 p40 are absent in the vasculitic infiltrates. We propose that differentiation of DCs and the autocrine and paracrine actions of chemokines in granulomatous lesions misdirect DCs away from their usual journey to lymphoid organs and are critical in maintaining T-cell activation and granuloma formation in giant cell arteritis.
Giant cell arteritis (GCA) is a granulomatous vasculitis that preferentially targets medium- and large-size arteries. Activated T cells and macrophages accumulate in the arterial wall and induce a response-to-injury program that causes lumen-occlusive intimal hyperplasia. 1 Granulomatous microarrangements often include multinucleated giant cells that align along the media-intima border. Granulomas preferentially form in the tunica media and correlate with profound, spatially restricted oxidative tissue damage. 2,3 Smooth muscle cells and infiltrating macrophages and T cells in the media are targeted by lipid peroxidation. Combined oxidative and nitrosative stress leads to protein nitration in endothelial cells of medial microvessels. 4
T cells are mandatory for the generation of granulomatous lymphoid microstructures. In GCA, selected CD4 T cells undergo clonal expansion in the vascular lesions; 5,6 T-cell depletion disrupts vasculitis in an experimental model of the disease. 7 The T-cell product, interferon (IFN)-γ, is a key cytokine in GCA. 8 The anti-inflammatory activity of aspirin is closely linked to its ability to suppress the transcription factor, activating protein-1, which regulates IFN-γ transcription, 9 and the chronicity of GCA in corticosteroid-treated patients is because of the relative sparing of IFN-γ production by steroids. 10 Concentrations of tissue IFN-γ correlate with the degree of intimal thickening, the extent of neovascularization, and the formation of multinucleated giant cells. 11,12 It is not known how T-cell activation in the arterial wall is induced and maintained nor has it been resolved which antigen-presenting cells support the expansion of selected CD4 clonotypes in the vascular infiltrates.
Dendritic cells (DCs) are believed to be the principal initiators of T-cell-mediated immune responses. 13,14 They possess numerous qualities that place them at the interface of the innate and adaptive immune systems and in a critical position to regulate T-cell function. Although many cells can function as antigen-presenting cells, only DCs can trigger naive T cells. Besides presenting exogenous antigens, DCs also endocytose self-antigens and determine the fate of self-reactive T cells. 15,16 Central tolerance of T cells in the thymus is mediated by thymic DCs that facilitate negative selection of autoreactive T cells. Continuous presentation of self-antigens by DCs in lymphoid organs is thought to underlie peripheral tolerance. 15 Two models have been forwarded to explain the dual role of DCs in stimulating T-cell immunity and providing tolerogenic signals. 17,18 One model suggests that distinct DC subtypes specialize in activating antigen-specific T cells or removing self-reactive T cells from the repertoire. The alternative model assumes that all DCs have the capacity to induce immunity or tolerance and that the outcome of DC/T-cell interaction depends on the stage of DC maturation. Immature or partially mature, yet quiescent, DCs have been implicated in inducing tolerance, whereas fully mature DCs have the capability to initiate T-cell growth and effector functions. 19,20
DCs pass through distinct stages in their life cycle. 14 In their immature form, they are scattered throughout peripheral tissues, where they function as sentinels to capture and process environmental antigens. If activated by danger signals such as cytokines, heat shock proteins, or pathogen-associated molecular patterns, DCs migrate out of the damaged tissue and move toward lymphoid organs. While traveling in lymph vessels, they undergo phenotypic and functional changes. They lose CCR6, gain expression of CCR7, up-regulate adhesion molecules, and express co-stimulatory molecules on their surface. 21 On arrival at the lymphoid organ, they have partially matured. Their endocytic activity has ceased; instead, they have markedly increased MHC class II expression. By preventing further antigen uptake and peptide loading, surface MHC class II molecules are stabilized and DCs are now optimized to present antigen.
Here, we report that DCs participate in granuloma formation in GCA. They are highly activated and synthesize chemokines typically produced by DCs that have migrated to the T-cell zones of lymph nodes. Instead of transporting antigens to lymphoid organs, DCs are recruited to and trapped in the peripheral vascular lesions. A subset of DCs in the vessel wall expresses the co-stimulatory molecules necessary for T-cell activation. We propose that the local maturation and immobilization of DCs are critical factors in stabilizing T-cell activation and granuloma formation in GCA.
Materials and Methods
Patients and Tissues
After approval of this study by the Mayo Clinic Institutional Review Board and obtaining appropriate informed consent, we collected temporal artery specimens from patients undergoing diagnostic biopsy for suspected GCA. Forty-two patients (29 female, 13 male) with typical histomorphological findings of GCA were enrolled. The average age at GCA onset for the patients was 71.3 years. At the time of the temporal artery biopsy, 23% of the patients were on corticosteroids. Twenty-three patients (13 female, 10 male) lacking histomorphological findings of vasculitis served as controls. Patients with polymyalgia rheumatica were excluded from this protocol. Tissues were either immediately shock-frozen or, if used for immunohistochemistry, were embedded in OCT compound (Sikura Fine-Tek, Torrence, CA) and stored at −80°C or were embedded in paraffin following routine procedures.
Immunohistochemical Analysis
The antibodies (Abs) used in the study and their optimal working dilutions are listed in Table 1 ▶ . Paraffin-embedded samples were cut into 5-μm sections and deparaffinized in 100% xylene. Endogenous peroxidase activity was blocked with methanol/3% hydrogen peroxide and the sections were incubated in 5% goat or rabbit serum (Invitrogen Life Technologies, Carlsbad, CA). Slides were stained with primary Ab (S-100, anti-CD68) at room temperature for 1 hour or overnight at 4°C in a humidified chamber. The sections were then washed with tap water and incubated at room temperature with biotin-conjugated goat anti-rabbit Ig or rabbit anti-mouse Ig Ab for 30 minutes. After washing with tap water, avidin-biotinylated enzyme solution (ABC-IP kit; Vector Laboratories, Burlingame, CA) was added for 30 minutes at room temperature. Red staining was produced using 3-amino-9-ethylcarbazole (AEC) as the chromagen (Vector Laboratories) for 20 to 30 minutes. Sections were counterstained with hematoxylin solution (Surgipath, Richmond, IL) for ∼5 minutes.
Table 1.
Antibodies Used for Immunohistochemistry
| Specificity | Species | Dilution | Vendor |
|---|---|---|---|
| S-100 | Rabbit anti-cow | 1:1600 | DAKO, Carpinteria, CA |
| CD83 | Mouse anti-human | 1:1000 | Research Diagnostics, Flanders, NJ |
| CD86 | Goat anti-human | 1:100 | Santa Cruz Biotechnology, Santa Cruz, CA |
| CD68 | Mouse anti-human | 1:100 | DAKO |
| CCL19 (MIP-3β) | Mouse anti-human | 1:10,000 | R&D Systems, Minneapolis, MN |
| CCL21 (6cKine) | Goat anti-human | 1:5000 | R&D Systems |
| CCR7 | Mouse anti-human | 1:1000 | BD-Pharmingen, San Diego, CA |
| CD1a | Mouse anti-human | 1:100 | DAKO |
| CD3 | Mouse anti-human | 1:100 | DAKO |
| Rabbit Ig | Goat anti-Rabbit | 1:300 | DAKO |
| Mouse Ig | Rabbit anti-mouse | 1:300 | DAKO |
| Goat Ig | Rabbit anti-goat | 1 :300 | DAKO |
OCT-embedded sections of temporal arteries were double-labeled as previously described. 3,12 Tissue sections (5 μm) were fixed with acetone for 10 minutes, dried for 30 minutes, and soaked in 1% paraformaldehyde solution, pH 7.4, for 5 minutes. After blocking endogenous peroxidase and incubating with 5% normal rabbit serum (Invitrogen Life Technologies), the sections were stained for 1 hour with unconjugated primary Ab (anti-CCR7, anti-CCL19, anti-CCL21, anti-CD1a, anti-CD83, or anti-CD86), followed by biotin-conjugated rabbit anti-mouse or rabbit anti-goat Ig Ab (30 minutes at room temperature). The sections were developed with avidin/biotinylated alkaline phosphatase (ABC-AP, Vector Laboratories) and Vector Blue chromagen (Vector Laboratories). Tissue sections were washed with tap water and blocked with 5% rabbit serum. Each section was then incubated for 1 hour at room temperature with the second primary Ab (anti-CD83, anti-CD86, anti-CCR7, or anti-CCL19) diluted in 1% rabbit serum, followed by biotin-conjugated secondary rabbit anti-mouse Ig or rabbit anti-goat Ig Ab and the ABC-AP complex (Vector Laboratories) using Vector Red as a chromagen. Slides were counterstained with hematoxylin (Surgipath) for 5 minutes. To control for nonspecific binding in the single and the double-Ab labeling, control stains with isotype-matched primary Abs were included. Results from two-color immunohistochemistry were confirmed by single-color staining on serial sections and by switching the sequence of the primary Abs. Tissue sections were viewed by light or fluorescent microscopy and were photographed using an LSM-510 microscopic system (Carl Zeiss Instruments, Thornwood, NY).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Sequences of primers used in the study are listed in Table 2 ▶ . Optimal conditions for all primers were established by amplifying cDNA samples from human tonsil or activated peripheral blood mononuclear cells. Total cellular RNA from the arteries was isolated using TRIzol reagent (Invitrogen Life Technologies), reverse-transcribed into single-stranded cDNA using AMV reverse transcriptase (Roche Molecular Biochemicals, Indianapolis, IN), and amplified by PCR on a Perkin-Elmer 9600 (Perkin-Elmer, Emeryville, CA) using specific primer pairs. The amplification protocol involved 30 cycles of denaturation at 95°C for 60 seconds, primer annealing at 55°C for 60 seconds, and primer extension at 72°C for 90 seconds. The reaction products were visualized on ethidium bromide-stained 1% agarose gels and digitally documented for further analysis (GelDoc 2000; Bio-Rad, Richmond, CA).
Table 2.
PCR Primers
| CD83 | 5′-GTTATTGGAGGGTGGTGAAGAGAGG-3′ |
| 5′-GTGAGGAGTCACTAGCCCTAAATGC-3′ | |
| CCL18 | 5′-GGTGTCATCCTCCTAACCAAG-3′ |
| 5′-GGAAAGGGGAAAGGATGATA-3′ | |
| DC-SIGN | 5′-GATTCCGACAGACTCGAGGA-3′ |
| 5′-CCTACAGCTTGGATTTCTCT-3′ | |
| CD1a | 5′-CAATGCAGACGGGCTCAAG-3′ |
| 5′-GAATTGCAGTTTGAATATCCTTTTG-3′ | |
| IL-12p40 | 5′-TCACAAAGGAGGCGAGGTTCTA-3′ |
| 5′-CATGACCTCAATGGGCAGACTC-3′ | |
| CCL21 | 5′-CCCCAGGACCCAAGGCAGTGATGGA-3′ |
| 5′-TGCAAGAGGACTGAGCGGTCACA-3′ | |
| CCL19 | 5′-CCAATGATGCTGAAGACTGCT-3′ |
| 5′-GCCAAGATGAAGCGCCGCA-3′ | |
| β-actin | 5′-ATGGCCACGGCTGCTTCCAGC-3′ |
| 5′-CATGGTGGTGCCGCCAGACAG-3′ |
Transcripts for the chemokines, CCL19, CCL21, and CCL18 were semiquantified using the LightCycler PCR instrument (Roche Molecular Diagnostics). cDNA (1 μl) was diluted in a total volume of 20 μl of SYBR green master mix (Roche Molecular Diagnostics). The PCR thermal cycling was as follows: initial denaturation at 95°C for 30 seconds, followed by 40 cycles of amplification at 95°C for 0 seconds, 57°C for 7 seconds, and 72°C for 16 seconds. Melting curve analysis was accomplished by 95°C for 0 seconds, 60°C for 30 seconds, and 95°C for 0 seconds.
Statistical Analysis
Quantitative cytokine transcript data from GCA and control samples were compared using the Wilcoxon signed-rank test (SigmaStat; SPSS, Chicago, IL).
Results
DCs Are in Normal and Inflamed Temporal Arteries
Tissue-resident DCs are a heterogeneous group of cells. In human tonsils, only the S-100 marker (fascin, p55) is commonly expressed by all DC subsets. 22 We stained temporal artery sections from patients with GCA and those without vasculitis with S-100-specific Abs. All arteries, including those negative for GCA, had S-100+ cells (Figure 1) ▶ . In noninflamed arteries, S-100+ cells were typically located on the adventitial side of the lamina elastica externa and were distributed in a ring-like configuration (Figure 1D) ▶ . Staining with anti-CD68 Ab confirmed that the S-100+ cells at the adventitia-media border of noninflamed arteries were phagocytic and clearly distinct from neurons, which are S-100+ and present in the adventitia. In tissue sections from 27 patients with mononuclear cell infiltrates and a diagnosis of GCA, frequencies of S-100+ DC were increased 5- to 10-fold with cross-sections containing 50 to 100 DCs. Typically, the DCs were associated with granulomatous formations in the media (Figures 1 and 2) ▶ ▶ . Adventitial DCs were maintained in arteries affected by GCA and accounted for 10 to 25 cells per section.
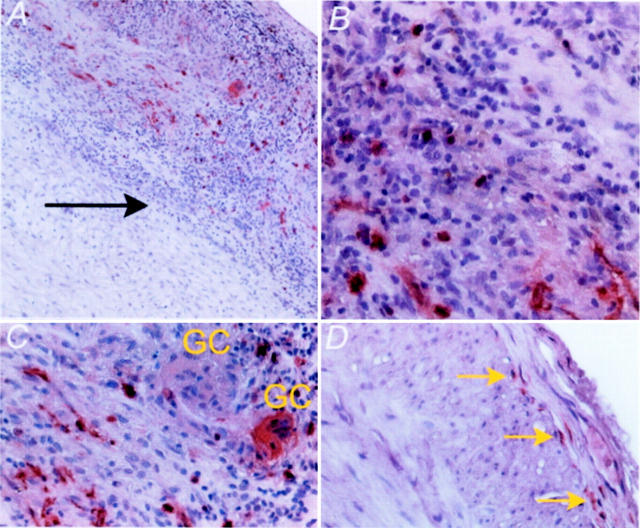
Figure 1.
Topography of DCs in normal and inflamed arteries. Paraffin-embedded temporal arteries that were either normal or affected by GCA were stained with anti-S-100 Ab and developed with ABC-IP and AEC (Vector Laboratories). In arteries with GCA, S-100+ DCs were found in the media and the adventitia (black arrow, media-adventitia border), often tracking with the mononuclear infiltrates (A and B). Selected multinucleated giant cells (GCs) were positive for the S-100 marker (C). Normal arteries contained a distinct population of S-100+ DCs arranged in the proximal adventitia along the lamina elastica externa (yellow arrows, D). Original magnifications: ×100 (A); ×400 (B–D).
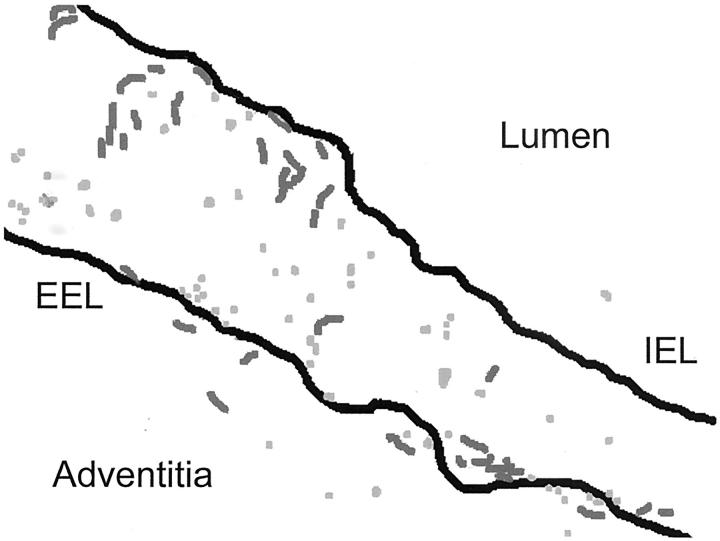
Figure 2.
Preferential localization of S-100+ DCs in the granulomatous microstructures. A diagram of the topography of DCs in tissue lesions is shown. S-100+ cells were traced and landmarks in the arterial circumference were marked (EEL, external elastic lamina; IEL, internal elastic lamina). Two types of S-100+ DC could be distinguished, round lymphoid-like cells (light gray) and spindle-shaped cells with dendrites (dark gray). Both DC types displayed preference for the media and were in close association with granulomatous reactions.
No unique relationship between the fragmented internal elastic lamina and DCs was found. Also, there was no preference for DCs to co-localize with lymphocytes. However, we commonly found that selected multinucleated giant cells were positive for S-100 (Figure 1C) ▶ . Such giant cells were intermingled with giant cells that remained negative for the marker.
In negative arteries, the morphology of all DCs was that of spindle-shaped cells with long membrane extensions. In the arteries with GCA, two distinct DC morphologies were present (Figure 1B and 2) ▶ ▶ . About one-half of the S-100+ cells were large, contained vacuoles in the cytoplasm, and had dendrites. The remaining cells were smaller and round, resembling lymphocytes. Topographic analysis suggested that the spindle-shaped DCs had a tendency to be aligned in linear configurations, whereas the lymphoid DCs had no particular arrangement.
Phenotyping of Arterial Wall DCs
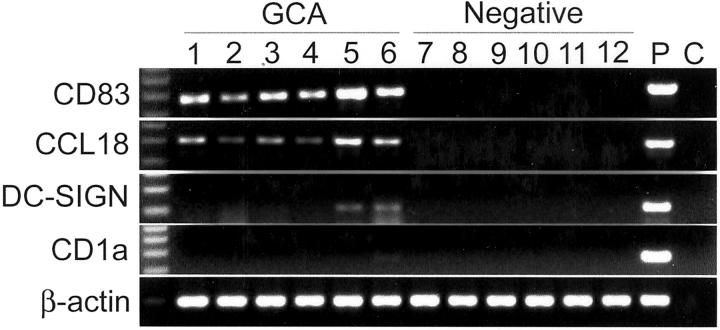
To assign functional properties to the DCs, we used a panel of markers for phenotypic analysis. Tissue extracts were generated from 11 arteries with GCA and 10 arteries negative for vasculitis. The extracts were analyzed by reverse transcriptase (RT)-PCR for the following DC markers: the activation marker, CD83; 23 the C-type lectin receptor, DC-SIGN; 24 the chemokine, CCL18; and the nonpolymorphic MHC class I molecule, CD1a. 25 Results are shown in Figure 3 ▶ . All of the arteries with GCA and none of the noninflamed control arteries contained CD83 transcripts. Immunohistochemical staining confirmed that almost all DCs in the GCA arteries reacted with anti-CD83 Ab and, thus, have undergone activation. None of the DCs in negative arteries expressed the CD83 molecule, identifying them as being immature and nonactivated. Unlike Langerhans cells, DCs in normal and inflamed arteries lacked expression of the CD1a molecule.
Figure 3.
Characterization of tissue-infiltrating DCs. cDNA was prepared from temporal arteries that were either noninflamed (negative) or affected by GCA. Gene expression of molecules typically found in tissue DCs was examined by RT-PCR. Quality of the cDNA was assessed by amplifying β-actin transcripts. All arteries from patients with GCA contained sequences specific for CD83 and CCL18. Weak bands for DC-SIGN were found in the minority of samples and CD1a was not detected. P, positive control; C, negative control.
In only 4 of the 11 positive arteries and in none of the negative arteries did we find transcripts specific for DC-SIGN. When present, the signals were weak, indicating that this receptor was not part of the typical markers of arterial DCs.
Possible Antigen-Presenting Functions of DCs in Granulomatous Vasculitis
DC-SIGN is a C-type lectin receptor that has been implicated in antigen uptake and in facilitating DC/T-cell communication. 26 Lack of DC-SIGN expression raised the question whether the DC network in normal arteries and the DC populations accumulated in the granulomatous lesions had the capacity to communicate with and present antigens to T cells. All DCs, including resting DCs in negative arteries, expressed the lysosomal marker, CD68, indicating their ability for endocytosis. When interacting with T lymphocytes, DCs release interleukin (IL)-12, a cytokine considered to be crucial in polarizing T cells toward the TH1 pathway, 27,28 which is characterized by IFN-γ production. The secretion of the bioactive p70 form of IL-12 by DCs is tightly regulated, involving separate control of the p40 and p35 components. 29 Immunohistochemistry with anti-IL-12 Ab did not reveal any cellular stores of this cytokine (data not shown). We, therefore, proceeded with RT-PCR analysis for the IL-12p40 component. As shown in Figure 4 ▶ , no IL-12p40-specific transcripts were produced in arteries with GCA. As expected, negative arteries did not contain mRNA for IL-12.
Figure 4.
Arteries with GCA lack transcripts for IL-12. cDNA samples were prepared from temporal artery specimens and analyzed by RT-PCR for the expression of IL-12p40. Neither negative nor positive (GCA) arteries contained sequences specific for IL-12. Amplification of β-actin was performed to assess the quality of the cDNA. P, positive control; C, negative control.
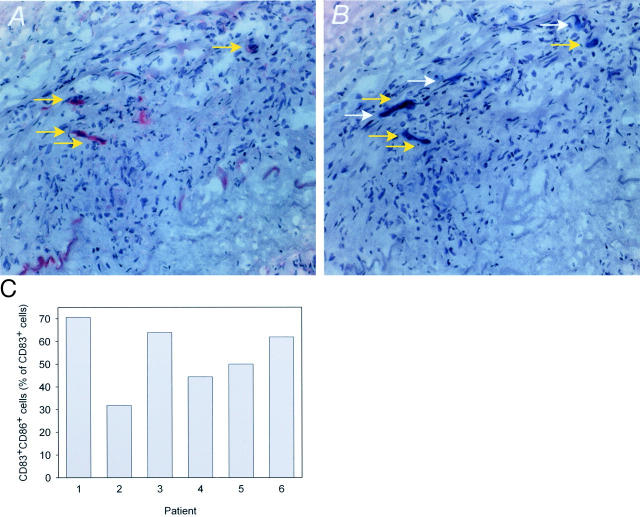
The absence of DC-SIGN and IL-12 reiterated the question whether DCs in the arterial wall were able to communicate with T cells and to act as antigen-presenting cells. To address this issue from another direction, we evaluated tissue sections for the expression of the co-stimulatory molecule, CD86. CD86 was undetectable in control arteries. In inflamed arteries, CD86 was expressed on a distinct population of cells, often in the tunica media. Two-color analysis identified CD86 on a subset of CD83+ DCs (Figure 5, A and B) ▶ . As shown in Figure 5C ▶ , ∼30 to 70% of all DCs expressed this co-stimulatory molecule. CD83−CD86+ cells were infrequent and were, in most cases, endothelial cells.
Figure 5.
Expression of co-stimulatory CD86 by tissue-infiltrating DCs in GCA. Frozen sections from temporal artery specimens were stained with Abs against CD86 (A) and CD83 (B). A subset of CD83+ DCs expressed CD86 (yellow arrows; CD83+CD86−, white arrows). To determine the frequency of CD86-expressing DCs, frozen sections were either double-labeled with anti-CD83 and anti-CD86 Abs or serial sections were individually stained with the two Abs. Results of both experimental approaches were identical. Frequencies of CD86+CD83+ DCs were determined. Results from six patients are shown (C). Original magnifications, ×200 (A and B).
The demonstration of CD86 established that DCs in the granulomatous lesions are equipped to provide a stimulatory and not a tolerizing signal to T cells. This interaction does not involve the release of IL-12.
Chemokine Production by DCs in Arteritic Infiltrates
A major functional activity of DCs is the production of chemokines, through which they regulate the traffic of T cells and other DCs to and within the T-cell zones of lymph nodes. 30 CCL18 is a chemokine that is exclusively expressed by activated mature DCs in lymphoid tissues and has been implicated in the recruitment of naive T cells. Activated, mature DCs also produce CCL19 and CCL21 that guide the migration of immature DCs to the lymphoid tissue and attract T cells to the T-cell zones. Tissue extracts from inflamed and control arteries were examined for the production of CCL18, CCL19, and CCL21. Sequence-specific transcripts for all three chemokines were quantified by quantitative PCR in cDNA samples that were adjusted to contain 2 × 105 copies of β-actin mRNA. All three chemokines were transcribed at high levels in the arteries from patients with GCA (Figure 6) ▶ . CCL18 was not detected in negative arteries; without exception, positive arteries contained transcripts for this chemokine (median, 997 copies). Of the three chemokines that were analyzed, CCL19 was most abundant with low levels being detectable in noninflamed arteries. Control arteries contained a median of 172 CCL19 transcripts whereas arteries with GCA had a median of 7963 copies. Although tissue production of CCL21 was greatly increased in arteries with vasculitis (median, 1433 copies), baseline transcription was also found in tissues free of inflammation (median, 223 copies). The numbers of CCL18- and CCL19-specific transcripts were closely correlated, whereas the level of CCL21-specific sequences could not be predicted from the production of the two other chemokines.
Figure 6.

Tissue production of CCL18, CCL19, and CCL21 in temporal arteries affected by GCA. cDNA was prepared from tissue extracts of temporal artery specimens. Transcripts for β-actin, CCL18, CCL19, and CCL21 were determined using quantitative PCR. Concentrations of chemokine transcripts were adjusted to 2 × 105 β-actin copies and are shown as box plots (median and 25th and 75th percentiles) with whiskers marking the 10th and 90th percentiles. Transcripts of all three chemokines were abundant in arteries with vasculitis. Control arteries were negative for CCL18 and CCL19 but contained low numbers of CCL21 mRNA.
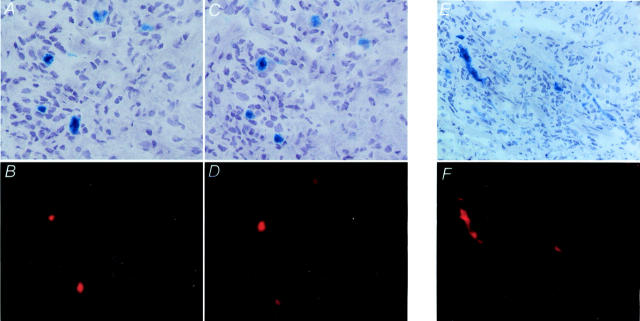
To identify the cellular sources for these chemokines, we proceeded with staining for CD83 and either CCL19 or CCL21. Representative results from staining of temporal artery cryosections are shown in Figure 7; A to D ▶ . The vast majority of cells positive for the activation marker, CD83, also expressed CCL19 and CCL21 protein. By combining two-color staining with serial section analysis, we were able to demonstrate that activated DCs in the media produced both CCL19 and CCL21. Synthesis of CCL19 and CCL21 protein was not bound to the morphology of the DC, both types of DCs were stained by CCL19- and CCL21-specific Abs. DCs in the adventitia were indistinguishable from those in the media in terms of CD83 expression and chemokine production.
Figure 7.
Production of CCL19 and CCL21 and expression of CCR7 by DCs in the granulomatous lesions. Two-color immunohistochemistry of frozen sections of temporal arteries from patients with GCA was used to identify the cellular source of CCL19 and CCL21. Top: Tissue sections were stained with anti-CCL21 (A), anti-CCL19 (C), and anti-CCR7 (E) Abs. Antibody binding was detected with Vector Blue. Bottom: DCs were identified with anti-CD83 Ab and Vector Red. Serial tissue sections were used to stain for CCL21 (A)- and CCL19 (C)-producing cells, which demonstrated that both chemokines derived from the same cell population. CCR7 (E) was exclusively found on CD83+ DCs (F), whereas tissue-infiltrating T cells were negative for this chemokine receptor. Original magnifications, ×400.
Although CD83+ DCs were the major source for CCL19 and CCL21, they were not the only cells producing these chemokines. Weaker staining with anti-CCL19 Ab was occasionally found in macrophages in the media. Also, in some patients, endothelial cells of the vasa vasorum were positive for CCL19. In GCA, new capillaries are formed in the media and the hyperplastic intima. These microvessels remained negative for CCL19. CCL21 had a more restricted expression pattern. Macrophages did not express CCL21, but occasionally CCL21-specific Ab bound to endothelial cell layers of adventitial vessels.
Expression of CCR7 on Dendritic Cells
CCL19 and CCL21 are instrumental in the migration of DCs to the T-cell zones of secondary lymphoid organs. Maturing DCs switch their chemokine receptor profile and become responsive to CCL19 and CCL21, 31 are recruited to the T-cell zones, and begin to produce these two chemokines, themselves. Receptors that can be triggered by CCL19 and CCL21 are expressed on naive T cells. DCs producing CCL19 and CCL21 can, therefore, attract naive T cells to optimize the chance of finding an antigen-specific T cell for priming. 30
In the vascular lesions of GCA, almost all T cells are memory T cells. To determine whether the CCL19 and CCL21 synthesized in the arterial wall contributed to the disease process, we identified the cells expressing the appropriate receptor, CCR7. Lymphocytes in the vascular infiltrates were negative for CCR7, but CD83+ DCs consistently expressed the CCR7 protein. Representative results from the analysis of 12 different temporal artery samples are shown in Figure 7, E and F ▶ . Expression of CCR7 on DCs was consistently linked to CD83 positivity. DCs in different locations (Figures 1 and 2) ▶ ▶ possessed CCR7. There was no other cell in the arterial wall that expressed CCR7.
Discussion
GCA is a T-cell-dependent disease with the formation of tissue-damaging granulomas and the clonal expansion of CD4 T cells in the lesion. 32 Here, we report that DCs participate in the granulomatous reactions and that at least some of them have the molecular equipment to interact with T cells and to trigger T-cell immunity. DCs have mostly been regarded as migratory cells that capture antigen in the periphery and transport it to secondary lymphoid tissues to find antigen-specific T cells. 13 Recently, DCs have also been recognized as critical regulators of tolerance. 16 Their roles in priming naive T cells and transmitting tolerogenic signals to self-reactive T cells are linked to their unique migratory capabilities. 33 Indeed, antigen ingestion and processing is best performed by immature DCs, whereas T-cell stimulation requires fully mature DCs that have passed through developmental stages and have switched their program from endocytic cells to antigen-presenting cells. 34 The DCs accumulating in the vascular lesions of GCA break with this paradigm. Localized in peripheral tissue, they have lost the ability to migrate to lymphoid organs. Instead, they are captured in the peripheral lesion through an autocrine or paracrine mechanism, releasing chemokines that react to receptors on their own surface. Essentially all of them have reached full maturation, as indicated by the surface expression of CD83 and the production of CCL18, CCL19, and CCL21. Half of them possess the co-stimulatory molecule, CD86, and are able to function in antigen-specific T-cell stimulation. Despite the key role of IFN-γ in GCA, DC/T-cell interaction does not involve the secretion of IL-12; IL-12p40 transcripts are not present in the lesions. In essence, granulomas in GCA include highly active DCs that are trapped in the peripheral tissue and, because of their nonmigratory behavior, may directly contribute to the breakdown of tolerance in this tissue site.
An important finding of the current study is the presence of DCs as natural residents in the arterial wall. These DCs, sitting on the outside of the lamina elastica externa, may have a role as sentinels, raising the question as to the type of antigens that could be encountered in this unique microenvironment. In medium- and large-size arteries, the intima and tunica media are avascular. Capillaries, deriving from vasa vasorum, are restricted to the adventitia. The wall of the artery is partially supplied by diffusion from the macrolumen. Antigens may penetrate through the wall, en route from the lumen to the draining vessels in the adventitia, where they are sampled by DCs at the media-adventitia border. It will be important to understand the functional role of DCs in normal arteries because they may be a key in the establishment of inflammatory lesions in this territory. Our studies demonstrate that DCs in noninflamed arteries are immature and resting. Possibly, they represent tolerogenic DCs that protect this microenvironment from inflammation.
Arteries with GCA contain large numbers of DCs that have undergone full maturation and release chemokines. Such DCs were encountered in two microenvironments, the granulomas in the media and in the adventitia. Granulomas are formed by the immune system to deal with intracellular microbes and antigens that are difficult to digest. Perhaps, DCs may participate in the granuloma reaction by sampling the antigenic content at this tissue site. We have previously reported that the media is under oxidative stress, not unexpected in areas of granuloma formation. 2,3 Membranes of medial smooth muscle cells and mononuclear cells are attacked by lipid peroxidation, and the cells respond to oxidative stress with the induction of genes such as aldose reductase. It is unlikely that this microenvironment would be suitable for the establishment of DC/T-cell clusters, which could induce the response of antigen-specific T cells. Accordingly, we have found that activated T cells have a preference for the adventitia. 7 How DCs maneuver in the lesion, and how they find T cells and establish contact to them is not known. Morphological analysis demonstrated that DCs in the media were not surrounded by T cells.
DCs are prone to leave the peripheral tissue and migrate to lymphoid organs unless they have reached a developmental stage that is associated with the expression of CCR7. CCR7 is bound by CCL19 and CCL21, two chemokines readily available in the lesion. 35 Production of CCL19 and CCL21 and expression of the complementary receptor by the same cell prevents migration of DCs and instead secures their capture at the site. Also, CCR7-expressing DCs could be recruited from outside and, instead of homing to lymphoid organs, could contribute to peripheral inflammatory lesions. In preliminary experiments, we have seen low frequencies of CD83+CCR7+ cells circulating in the blood. Such cells could gain disease relevance by supplying the vascular lesions with DCs and possibly ferrying in antigens from outside.
If DCs in granulomatous lesions are in an antigen collection mode, they will inevitably endocytose self-antigens. Tolerance induction by DCs has been assigned to only partially matured DCs, which is certainly not the case for arterial wall DCs. Alternatively, it has been suggested that the dual role of initiating T-cell immunity and inducing tolerance may reflect the involvement of distinct lineages of DCs. Morphological analysis of S-100+ DCs in GCA arteries demonstrated two distinct types, small round cells, possibly representing plasmacytoid DCs, and spindle-shaped DCs representing the myeloid subtype. It is almost impossible to assign a unique surface phenotype to these plasmacytoid or lymphoid-like DCs in situ. 22 They reportedly express CD68, CD4, and CD123, an IL-3 receptor chain. This panel of markers is also expressed on macrophages. Thus, a clear distinction between this DC subset and macrophages in the tissue cannot be achieved. Alternatively, small lymphoid-like DCs may not represent a distinct lineage but could instead be precursors of large DCs with dendrites. However, we have not seen any differences in markers of DC differentiation, such as chemokine production or CD83 expression, between these types of cells. Based on their cell surface profile, there is no evidence that the two cell types serve different functions. In particular, neither cell type in the active GCA lesion appears to be tolerogenic. The mere presence of sufficient numbers of activated DCs in a peripheral lesion may create the condition for autoreactivity and, as such, is a risk factor for chronic disease.
CCR7 was exclusively detected on DCs and not on T cells. Recent reports have stated that CCR7 can be rapidly down-regulated after ligand binding. 36 This mechanism obviously does not apply to DCs because they remained positive for the receptor despite being surrounded by CCL19- and CCL21-producing cells. One possibility is that these cytokines and their receptors guide DCs within the lesion. Particularly, the spindle-shaped DCs were often arranged in chains, raising the possibility that such cells are polarized in the tissue and follow a pathway created by a local chemokine gradient.
A surprising finding was the absence of IL-12, a quintessential DC product involved in regulating the differentiation of T cells into functionally distinct subsets. 27 DCs have been implicated in inducing both TH1 and TH2 responses, depending on the presence or absence of IL-12. T-cell populations in the inflamed temporal artery fulfill all criteria of TH1 cells. IL-4 is essentially absent, whereas IFN-γ has been closely linked to T-cell and macrophage activation. 37-39 IL-12 production by DCs is known to require several factors, including microbial products, CD154, contact with activated T cells, and the appropriate cytokine milieu. 28 On the other hand, IL-12 release by DCs has been found to be under tight temporal control. DCs have been described to enter a stage of exhaustion after producing an IL-12 pulse. 40 Nevertheless, it is highly unlikely that all DCs in the vascular lesions would be exhausted if IL-12 is critical in directing T-cell responses. Previous studies have demonstrated that T-cell priming does not occur in the vessel wall. 5,6 Rather, identical CD4 T-cell clonotypes have been isolated from the right and the left temporal arteries of the same patient. This finding suggests that T cells are primed outside and then seed the arterial wall. For such primed T cells, IL-12 may no longer be a necessary regulator in IFN-γ production. We have evidence that IL-18, a cytokine important in enhancing IFN-γ transcription, is abundantly produced in GCA arteries (CMW and JJG, unpublished observations).
DCs are critical in the activation of naive T cells. The findings of this study demonstrate that DCs remain an important partner for T cells in established lesions that are composed of memory T cells. Based on the mandatory expression of CD86 to function as antigen-presenting cells, T cells have surprisingly few options to be stimulated in the arterial tissue. The vast majority of CD86-expressing cells were DCs, making this cell type central in the granulomatous reaction. Several other cell types participating in the vascular infiltrates, particularly macrophages, endothelial cells, and smooth muscles cells, have been suspected to acquire antigen-presenting capabilities. This seems not to be case, although a small number of endothelial cells were positive for CD86 and, thus, may serve as antigen-presenting cells. With the expression of CCR7 and the secretion of CCR7-binding chemokines, DCs in the vessel wall lesion are literally trapped, and, instead of traveling to lymphoid organs, they create a microenvironment for effective T-cell stimulation in the tissue. The capturing of DCs in the tissue site may represent a key event in breaking tolerance and allowing for the establishment of adaptive immune responses in the artery. This model proposes that molecular pathways regulating DC trafficking predisposes individuals to develop GCA and that trapping of DCs by paracrine mechanisms contributes to the self-sustaining nature of granulomatous reactions and the chronicity of this vasculitis. Indeed, it is clinical experience that the vascular inflammation in GCA is not associated with any regional lymphadenopathy. If DCs function by sampling antigens in the center of the tissue lesion and process them for T-cell recognition, self-antigens would inevitably be represented in the antigen pool. It is difficult to envision how DCs at a tissue site could distinguish between exogenous antigens that need to be recognized and those that result from damage to host cells. Misdirected, mature DCs, unable to leave the tissue site yet loaded with self-antigens, and equipped with T-cell-stimulatory properties, can jeopardize peripheral tolerance and maintain a disease lesion.
Acknowledgments
We thank Linda H. Arneson for secretarial support and James W. Fulbright for assistance with the figures and with editing.
Footnotes
Address reprint requests to Cornelia M. Weyand, M.D., Guggenheim 401, 200 First St. SW, Mayo Clinic, Rochester, MN 55905. E-mail: weyand.cornelia@mayo.edu.
Supported by grants from the National Institutes of Health (R01 EY11916) and the Mayo Foundation.
References
- 1.Weyand CM, Goronzy JJ: Arterial wall injury in giant cell arteritis. Arthritis Rheum 1999, 42:844-853 [DOI] [PubMed] [Google Scholar]
- 2.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM: Tissue-destructive macrophages in giant cell arteritis. Circ Res 1999, 84:1050-1058 [DOI] [PubMed] [Google Scholar]
- 3.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM: Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest 1999, 103:1007-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borkowski A, Younge BR, Szweda LI, Mock B, Bjornsson J, Moeller K, Goronzy JJ, Weyand CM: Reactive nitrogen intermediates in giant cell arteritis: selective targeting of neocapillaries. Am J Pathol 2002, 161:115-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weyand CM, Schonberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ: Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med 1994, 179:951-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Taboada V, Hunder NN, Hunder GG, Weyand CM, Goronzy JJ: Recognition of tissue residing antigen by T cells in vasculitic lesions of giant cell arteritis. J Mol Med 1996, 74:695-703 [DOI] [PubMed] [Google Scholar]
- 7.Brack A, Geisler A, Martinez-Tabaoda VM, Younge BR, Goronzy JJ, Weyand CM: Giant cell vasculitis is a T cell-dependent disease. Mol Med 1997, 3:530-543 [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner AD, Bjornsson J, Bartley GB, Goronzy JJ, Weyand CM: Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol 1996, 148:1925-1933 [PMC free article] [PubMed] [Google Scholar]
- 9.Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ: Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 2002, 46:457-466 [DOI] [PubMed] [Google Scholar]
- 10.Brack A, Rittner HL, Younge BR, Kaltschmidt C, Weyand CM, Goronzy JJ: Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest 1997, 99:2842-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyand CM, Tetzlaff N, Bjornsson J, Brack A, Younge B, Goronzy JJ: Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum 1997, 40:19-26 [DOI] [PubMed] [Google Scholar]
- 12.Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM: Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol 1999, 155:765-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM: The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991, 9:271-296 [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1998, 392:245-252 [DOI] [PubMed] [Google Scholar]
- 15.Steinman RM, Pack M, Inaba K: Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev 1997, 156:25-37 [DOI] [PubMed] [Google Scholar]
- 16.Steinman RM, Nussenzweig MC: Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA 2002, 99:351-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert ML, Jegathesan M, Darnell RB: Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol 2001, 2:1010-1017 [DOI] [PubMed] [Google Scholar]
- 18.Shortman K, Heath WR: Immunity or tolerance? That is the question for dendritic cells. Nat Immunol 2001, 2:988-989 [DOI] [PubMed] [Google Scholar]
- 19.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC: Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 2001, 194:769-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N: Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 2001, 193:233-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, Massacrier C, Vanbervliet B, Zlotnik A, Vicari A: Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol 2000, 22:345-369 [DOI] [PubMed] [Google Scholar]
- 22.Summers KL, Hock BD, McKenzie JL, Hart DN: Phenotypic characterization of five dendritic cell subsets in human tonsils. Am J Pathol 2001, 159:285-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou LJ, Tedder TF: CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA 1996, 93:2588-2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y: The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol 2002, 168:2118-2126 [DOI] [PubMed] [Google Scholar]
- 25.Ardavin C, Martinez DH, Martin P, Anjuere F, Arias CF, Marin AR, Ruiz S, Parrillas V, Hernandez H: Origin and differentiation of dendritic cells. Trends Immunol 2001, 22:691-700 [DOI] [PubMed] [Google Scholar]
- 26.Figdor CG, van Kooyk Y, Adema GJ: C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev Immunol 2002, 2:77-84 [DOI] [PubMed] [Google Scholar]
- 27.Moser M, Murphy KM: Dendritic cell regulation of TH1-TH2 development. Nat Immunol 2000, 1:199-205 [DOI] [PubMed] [Google Scholar]
- 28.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Sousa C: CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 2000, 13:453-462 [DOI] [PubMed] [Google Scholar]
- 29.Shortman K, Liu YJ: Mouse and human dendritic cell subtypes. Nature Rev Immunol 2002, 2:151-161 [DOI] [PubMed] [Google Scholar]
- 30.Luster AD: The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol 2002, 14:129-135 [DOI] [PubMed] [Google Scholar]
- 31.Randolph GJ: Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol 2001, 13:267-274 [DOI] [PubMed] [Google Scholar]
- 32.Weyand CM: The Dunlop-Dottridge Lecture: the pathogenesis of giant cell arteritis. J Rheumatol 2000, 27:517-522 [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A: Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev 2000, 177:134-140 [DOI] [PubMed] [Google Scholar]
- 34.Watts C, Amigorena S: Antigen traffic pathways in dendritic cells. Traffic 2000, 1:312-317 [DOI] [PubMed] [Google Scholar]
- 35.Rossi D, Zlotnik A: The biology of chemokines and their receptors. Annu Rev Immunol 2000, 18:217-242 [DOI] [PubMed] [Google Scholar]
- 36.Bardi G, Lipp M, Baggiolini M, Loetscher P: The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol 2001, 31:3291-3297 [DOI] [PubMed] [Google Scholar]
- 37.Weyand CM, Hicok KC, Hunder GG, Goronzy JJ: Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med 1994, 121:484-491 [DOI] [PubMed] [Google Scholar]
- 38.Weyand CM, Goronzy JJ: Giant cell arteritis: pathogenesis. Hoffman GS Weyand CM eds. Inflammatory Diseases of Blood Vessels. 2002:pp 413-424 Marcel Dekker, Inc., New York
- 39.Weyand CM, Goronzy JJ: Pathogenic principles in giant cell arteritis. Int J Cardiol 2000, 75(Suppl 1):S9-S15 [DOI] [PubMed] [Google Scholar]
- 40.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F: Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol 2000, 1:311-316 [DOI] [PubMed] [Google Scholar]