Abstract
The caveolin gene family consists of caveolins 1, 2, and 3. Caveolins 1 and 2 are co-expressed in many cell types, such as endothelial cells, fibroblasts, smooth muscle cells and adipocytes, where they form a heteroligomeric complex. In contrast, the expression of caveolin-3 is muscle-specific. Thus, the expression of caveolin-1 is required for caveolae formation in non-muscle cells, while the expression of caveolin-3 drives caveolae formation in striated muscle cell types (cardiac and skeletal). To create a truly caveolae-deficient mouse, we interbred Cav-1 null mice and Cav-3 null mice to generate Cav-1/Cav-3 double-knockout (Cav-1/3 dKO) mice. Here, we report that Cav-1/3 dKO mice are viable and fertile, despite the fact that they lack morphologically identifiable caveolae in endothelia, adipocytes, smooth muscle cells, skeletal muscle fibers, and cardiac myocytes. We also show that these mice are deficient in all three caveolin gene products, as caveolin-2 is unstable in the absence of caveolin-1. Interestingly, Cav-1/3 dKO mice develop a severe cardiomyopathy. At 2 months of age, analysis of Cav-1/3 dKO hearts via gated magnetic resonance imaging reveals a dramatic increase in left ventricular wall thickness, as compared with Cav-1-KO, Cav-3 KO, and wild-type mice. Further functional analysis of Cav-1/3 dKO hearts via transthoracic echocardiography demonstrates hypertrophy and dilation of the left ventricle, with a significant decrease in fractional shortening. As predicted, Northern analysis of RNA derived from the left ventricle of Cav-1/3 dKO mice shows a dramatic up-regulation of the atrial natriuretic factor message, a well-established biochemical marker of cardiac hypertrophy. Finally, histological analysis of Cav-1/3 dKO hearts reveals hypertrophy, disorganization, and degeneration of the cardiac myocytes, as well as chronic interstitial fibrosis and inflammation. Thus, dual ablation of both Cav-1 and Cav-3 genes in mice leads to a pleiotropic defect in caveolae formation and severe cardiomyopathy.
Caveolae are 50- to 100-nm omega-shaped invaginations of the plasma membrane found in various tissue types. The principal structural proteins of caveolar membranes are encoded by the caveolin gene family (caveolin-1, -2, and -3). Caveolin-1 and -2 are co-expressed in numerous tissue types, with particularly high expression in adipocytes, endothelial cells, fibroblasts, and epithelial cells. 1,2 Caveolin-3, on the other hand, is muscle-specific, being highly expressed in all muscle tissues, such as skeletal muscle, diaphragm, and heart. 3,4
Caveolin-1 and -3 form ∼350-kd homo-oligomers made up of ∼14–16 caveolin monomers. These homo-oligomers serve as the basic structural units that drive the formation of caveolae membranes. In contrast, Cav-2 either homodimerizes or forms high molecular mass hetero-oligomers with Cav-1. 5-7 Cav-1 and Cav-3 are both independently necessary and sufficient to drive caveolae formation in heterologous expression systems, while Cav-2 requires the presence of Cav-1 for proper membrane targeting and stabilization. In the absence of Cav-1, Cav-2 localizes to the Golgi complex where it is degraded by the proteasome. 8,9
Initially considered to be mere conduits for endocytosis, caveolae are now recognized to have pleiotropic effects on numerous cellular events. Caveolin family members have been proposed to participate in vesicular trafficking, 10 lipid metabolism, 11,12 and various signal transduction processes. The “caveolae signaling hypothesis” states that caveolae serve as an integrated platform to concentrate and modulate the activity of specific lipid-modified signaling molecules, including Src family tyrosine kinases, H-Ras, eNOS, and heterotrimeric G proteins. 13-16 With the centralized role that caveolins assume in multiple cellular processes, it is not surprising that mutations within the CAV1 and CAV3 loci have been identified in human breast cancers 17 and muscular dystrophy (limb girdle muscular dystrophy, type 1C; LGMD-1C), 18 respectively.
Gene deletion studies have confirmed and challenged our views of these crowded little caves. Targeted gene disruption of the Cav-1 locus in mice leads to a loss of caveolae in caveolin-1-expressing tissues, but a retention of caveolae in striated muscle tissues. The major tissue-specific defects include abnormalities in pulmonary structure and function, as characterized by hypercellularity, thickened alveolar septa, and exercise intolerance; decreased vascular tone, as assessed by aortic ring studies and determined to be secondary to eNOS activation; resistance to diet-induced obesity and fibrosis of fat pads with increasing age; and defects in caveolar endocytosis and marked hyperproliferation in mouse embryonic fibroblasts. 9,19,20
Interestingly, Cav-3 null mice show a loss of caveolae specifically within muscle tissues. Myopathic changes ranging from mild to moderate were noted in skeletal muscle and characterized by variability in muscle fiber size and the presence of necrotic fibers. Although no changes were noted in the expression levels of the members of the dystrophin-glycoprotein (DG) complex, the DG complex was no longer properly targeted to cholesterol-rich lipid rafts/caveolae. In addition, the T-tubule system appeared immature and was longitudinally oriented; diffuse mislocalization of T-tubule marker proteins was also noted. 21,22
Although much insight into caveolar function has been gained from the individual Cav-1 and Cav-3 knockouts, several questions remain unanswered. For example, (i) Does the persistence of tissue-specific caveolae in the single knock-outs (Cav-1 and Cav-3 null mice) allow for a functional compensation in certain tissues (ie, the heart, where ∼40% of the cells are non-muscle and therefore normally express Cav-1)? 23 and (ii) What are the developmental and physiological consequences of a caveolae-deficient animal? These remaining questions prompted us to generate a mouse model that lacks caveolae in both muscle and non-muscle cells; this was achieved by interbreeding Cav-1 null and Cav-3 null mice, to yield Cav-1/Cav-3 double-knockout (Cav-1/3 dKO) mice.
Surprisingly, we show here that Cav-1/3 dKO mice are viable and fertile. Loss of Cav-1 and Cav-3 protein expression was verified by Western blot analysis and the pleiotropic ablation of caveolae formation was demonstrated by transmission electron microscopy. Routine histopathological examination revealed that Cav-1/3 dKO mice exhibited lung, fat, and skeletal muscle defects of comparable severity to their single-knockout counterparts. However, Cav-1/3 dKO mice displayed grossly enlarged hearts, ie, cardiomegaly, greatly exceeding those of Cav-1 KO, Cav-3 KO, and wild-type mice. Gated MRI analysis of the Cav-1/3 dKO hearts revealed a significant increase of ∼40% in left ventricular wall thickness. Further functional analysis using transthoracic echocardiography demonstrated an ∼35% increase in left ventricular wall thickness, with left ventricular dilation. Moreover, systolic function, as determined by left ventricular fractional shortening, was significantly compromised in Cav-1/3 dKO mice. Histopathological examination of sections of cardiac tissue from Cav-1/3 dKO mice demonstrated cardiac myocyte hypertrophy, generalized myocyte disorganization and myocytolysis, accompanied by chronic inflammation and fibrosis. Finally, Northern blot analysis indicated that an embryonic marker, atrial natriuretic factor (ANF), was up-regulated in ventricular samples prepared from Cav-1/3 dKO mice. The cardiac defects observed in Cav-1/3 dKO mice emphasize the importance of both caveolin isoforms for normal cardiac structure and function. Future studies will be needed to define the diastolic and systolic phenotype and address the development of contractile failure in Cav-1/3 dKO mice, such as monitoring the generation of calcium sparks 24 and their exercise responses. 24
Materials and Methods
Materials
Caveolin-1, -2, and -3 mouse mAbs (used for immunoblotting; 3,25,26 ) were the generous gift of Dr. Roberto Campos-Gonzalez, BD-Transduction Laboratories, Inc. The ANF cDNA was the generous gift of Dr. Jil Tardiff, Albert Einstein College of Medicine. A variety of other reagents were of the highest purity grade and were obtained from the usual commercial sources.
Animal Studies
All animals were housed and maintained in a barrier facility at the Institute for Animal Studies, Albert Einstein College of Medicine. Cav-1(−/−)/Cav-3 (−/−)-deficient mice were generated by interbreeding Cav-1 (−/−) 9 and Cav-3 (−/−) mice, 21 that were in a C57Bl/6 background.
Immunoblot Analysis
Mice of various genotypes were sacrificed and fat, lung, heart, muscle, and aortic tissue samples were harvested. Approximately 100 mg of a given tissue sample was then homogenized in lysis buffer (10 mmol/L Tris, pH 7.5, 50 mmol/L NaCl, 1% Triton X-100, 60 mmol/L octyl glucoside), containing protease inhibitors (Boehringer Mannheim). Tissue lysates were then centrifuged at 12,000 × g for 10 minutes to remove insoluble debris. Protein concentrations were analyzed using the BCA reagent (Pierce) and the volume required for 10 μg of protein was determined. Samples were then separated by SDS-PAGE (12.5% acrylamide) and transferred to nitrocellulose. The nitrocellulose membranes were stained with Ponceau S (to visualize protein bands), followed by immunoblot analysis. All subsequent wash buffers contained 10 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 0.05% Tween-20, which was supplemented with 1% bovine serum albumin (BSA) and 2% nonfat dry milk (Carnation) for the blocking solution and 1% BSA for the antibody diluent. Primary antibodies were used at a 1: 500 dilution. Horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution, Pierce) were used to visualize bound primary antibodies with the Supersignal chemiluminescence substrate (Pierce).
Transmission Electron Microscopy
Fat, lung, heart, skeletal muscle, and aortic tissue samples were fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, postfixed with OsO4, and stained with uranyl acetate and lead citrate. Microtome sections were examined under a JEOL 1200 EX transmission electron microscope and photographed at a magnification of ×16,000. 9 Caveolae were identified by their characteristic flask shape, size (50–100 nm), and location at or near the plasma membrane. 27,28
Preparation of Heart Paraffin Sections
Mice were sacrificed and their hearts were removed and placed in buffered formalin (10%). The tissue was fixed for ∼24 hours, washed in PBS for 20 minutes, dehydrated through a graded series of ethanol washes, treated with xylene for 40 minutes, and then embedded in paraffin for 1 hour at 55°C. Paraffin-embedded 5-μm-thick sections were then prepared using a Microm (Baxter Scientific) microtome and placed on superfrost plus slides (Fisher). Slides were then stained with hematoxylin and eosin (H & E), according to standard laboratory protocols. Areas of the myocardium (left ventricle and intraventricular septum) were selected for imaging. Samples were examined in a blinded fashion by one of us (S.M.F.).
Northern Blot Analysis
Mice were sacrificed and the ventricular tissue was carefully separated from atrial tissue by dissection. Total RNA was extracted from 100 mg of left ventricular tissue from each sample using Trizol reagent protocol (Gibco). Twenty micrograms of total RNA for each sample was separated using a 1.2% agarose gel under RNase-free conditions and transferred to nitrocellulose. The filters were hybridized using the ExpressHyb solution (Clontech). The blots were probed with the radiolabeled ANF cDNA.
Non-Invasive Cardiac Imaging
Gated Cardiac Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) experiments were performed using a GE Omega 9.4T vertical bore magnetic resonance system equipped with a microimaging accessory and custom-built coils designed specifically for mice. Just before each image acquisition, the heart rate was determined from the ECG, and the spectrometer gating delay was set to acquire data in diastole and systole. Multislice spin-echo imaging with an echo time of 18 ms and a repetition time of approx. 100 to 200 ms was performed. A 35-mm field of view (with a 256 × 256 pixel image matrix) was used. Short and long axis images of the heart were acquired and MRI data were processed off-line with MATLAB-based custom-designed software.
Transthoracic Echocardiography
Transthoracic echocardiography was performed on 2- and 4-month-old mice, as we described previously. 29 Echocardiography was performed with mice in supine position on a heating pad set at 38°C. Light anesthesia was achieved using isoflurane inhalation. 29 Continuous, standard electrocardiograms were taken from electrodes placed on the extremities. Echocardiographic images were obtained using an annular array, broadband, 10/5 MHz transducer attached to an HDI 5000 CV ultrasound system (Advanced Technology Laboratories, Bothel, WA). A small gel standoff was placed between the probe and chest. Two-dimensional and M-mode images of the heart were obtained from the basal short axis view of the heart and stored on 3/4-inch SVHS video tapes for off-line measurements using the Nova-Microsonic (Kodak) Imagevue DCR workstation (Indianapolis, IN). All measurements were made in three to six consecutive cardiac cycles and the averaged values were used for analysis. Left ventricular end-diastolic and end-systolic diameters, as well as diastolic ventricular septal and posterior wall thickness were measured from M-mode tracings. Diastolic measurements were performed at the point of greatest cavity dimension, and systolic measurements were made at the point of minimal cavity dimension, using the leading edge method of the American Society of Echocardiography. 30 Additionally, the following parameters were calculated using the above-mentioned measurements: left ventricular diastolic wall thickness as the average of ventricular septal and left ventricular posterior wall thickness; left ventricular percent fractional shortening as {100 × [(end-diastolic diameter minus end-systolic diameter)/end-diastolic diameter]}; and relative wall thickness as (2 × left ventricular diastolic wall thickness)/end-diastolic diameter. Also, see this more recent review on the echocardiographic examination of the mouse. 31
Note that differences between the “absolute” wall thicknesses measured using MRI and echocardiography are commonly observed and are likely due to technical factors, such as differences in the time of gating; echocardiography may underestimate these values, while MRI may overestimate these values. Most importantly, however, the relative changes measured in left ventricular wall thickness using MRI and echocardiography are in agreement, ie, an increase of ∼34–41% for Cav-1/3 dKO mice (see below).
Results
Cav-1/3 dKO Mice Are Deficient in Cav-1, −2, and −3 Protein Expression and Lack Morphologically Identifiable Caveolae in Both Muscle and Non-Muscle Cells
We have previously shown the Cav-1 null mice lack caveolae in non-muscle cells (fibroblasts, adipocytes, endothelial cells), but continue to form caveolae in skeletal muscle fibers 9,20 (and data not shown). Conversely, Cav-3 null mice fail to form caveolae in skeletal muscle, but show abundant caveolae in non-muscle cells, such as endothelia. 21 These findings are consistent with the relatively ubiquitous expression of caveolin-1 in non-muscle cell types and the restricted tissue-specific expression of caveolin-3 in muscle cells. Thus, whole-body ablation of caveolae would require a complete loss of both caveolin-1 and caveolin-3 protein expression.
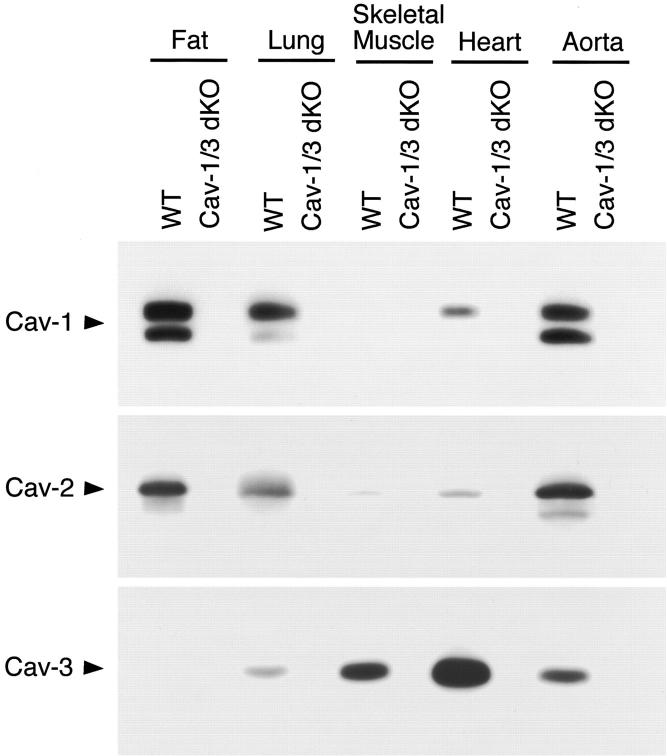
Here, we generated caveolin-1/caveolin-3 double-knockout mice by interbreeding Cav-1-null and Cav-3-null mice. Surprisingly, Cav-1/3 dKO mice are viable and fertile and exhibit no obvious external gross defects. As predicted based on their genotype, we show that Cav-1/3 dKO mice fail to express both the caveolin-1 and caveolin-3 protein products (Figure 1) ▶ . Note that in wild-type mice, fat and lung tissues abundantly co-express the Cav-1 and Cav-2 proteins, while the Cav-3 protein is highly expressed in striated muscle tissues (cardiac and skeletal); finally, all three caveolin family members are co-expressed in smooth muscle cells (such as in the aorta). 2,3,32 Western blot analysis using caveolin isoform-specific mAb probes clearly demonstrates a complete loss of Cav-1 and Cav-3 protein expression in all of the Cav-1/3 dKO tissues examined (Figure 1 ▶ , upper and lower panels). As Cav-2 requires Cav-1 for stabilization, oligomerization, and plasma membrane localization, Cav-2 protein expression is also dramatically down-regulated (by ∼95%) in Cav-1/3 dKO mice (Figure 1 ▶ , middle panel), as we previously reported for Cav-1 null mice. 8,9 Therefore, the Cav-1/3 dKO mice are deficient in the expression of all three caveolin gene products (Cav-1, −2, and −3).
Figure 1.
Caveolin-1/3 dKO mice are deficient in the expression of all three members of the caveolin gene family (Cav-1, -2, and -3). Protein expression of caveolin family members was examined in wild-type and Cav-1/3 dKO mice. Note that in wild-type mice, fat and lung tissues abundantly express Cav-1 and Cav-2 proteins, while Cav-3 is abundantly expressed in striated muscle tissues, and all three caveolin family members are expressed in the vasculature (as in the aorta). Immunoblot analysis using isoform-specific caveolin mAb probes demonstrates a loss of Cav-1 and Cav-3 protein expression in all of the Cav-1/3 dKO tissues examined. Note that as Cav-2 requires Cav-1 for stabilization, oligomerization, and plasma membrane localization, Cav-2 protein expression is also dramatically down-regulated (by ∼95%) in Cav-1/3 dKO mice.
We next assessed the status of caveolae formation in Cav-1/3 dKO mice by transmission electron microscopy. In both muscle and non-muscle cells, caveolae are classically identified as flask-shaped invaginations at the plasma membrane, but can also present as 50–100 nm vesicles or fuse to form rosettes and tubules. 33 As expected, wild-type adipocytes, endothelia, smooth muscle cells, skeletal muscle fibers, and cardiac myocytes all show an abundance of plasma membrane-associated caveolae (Figures 2 and 3) ▶ ▶ . In striking contrast, Cav-1/3 dKO mice show a distinct lack of morphologically identifiable caveolae in both muscle and non-muscle cells (Figures 2 and 3) ▶ ▶ . Thus, Cav-1/3 dKO mice are the first truly caveolae-deficient mouse model. As such, whole-body ablation of caveolae formation does not affect viability in mice. In Figure 3 ▶ , electron micrographs of cardiac myocytes and adjacent endothelia from Cav-1 null mice (upper right panel) and Cav-3 null mice (lower left panel) are also shown for comparison.
Figure 2.
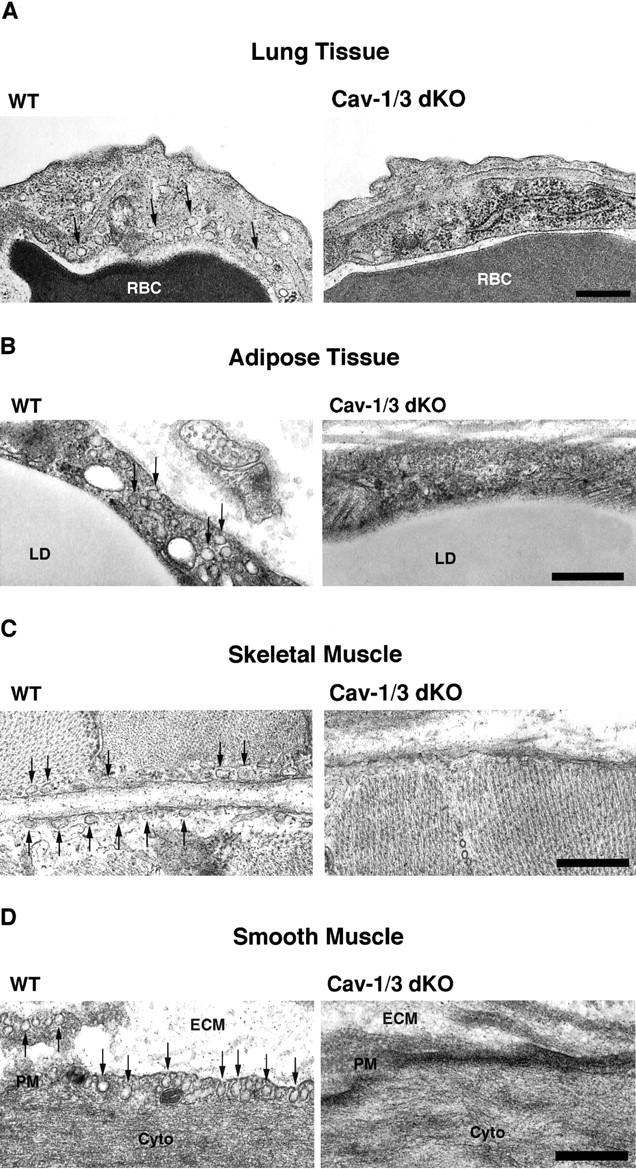
Cav-1/3 dKO mice exhibit a loss of morphologically identifiable caveolae in both muscle and non-muscle cell types. To assess the consequence of a loss of Cav-1, -2, and -3 protein expression, muscle and non-muscle cells that normally show abundant caveolae were examined by transmission electron microscopy. Note that wild-type lung endothelia (A), adipocytes (B), skeletal muscle fibers (C), and aortic smooth muscle cells (D) all contain numerous caveolae, located at or near the plasma membrane (arrows). In contrast, both muscle and non-muscle cells derived from Cav-1/3 dKO mice show a loss of caveolae. RBC, red blood cell; LD, lipid droplet; ECM, extracellular matrix; PM, plasma membrane; Cyto, cytoplasm. Scale bar, 500 nm.
Figure 3.
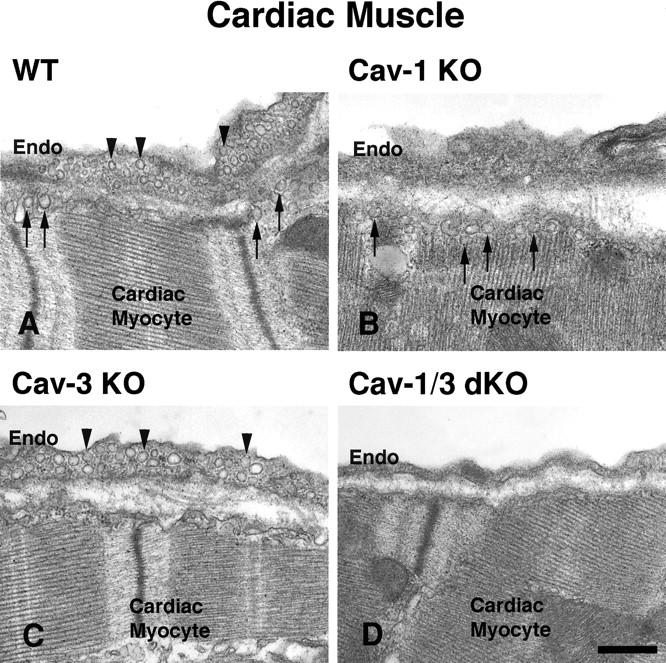
Dual ablation of both Cav-1 and Cav-3 genes is required to disrupt caveolae formation in both cardiac myocytes and their adjacent cardiac endothelial cells. Tissue-specific formation of caveolae is dictated by the caveolin isoform expressed. Arrowheads point to Cav-1-induced caveolae, while arrows point to caveolae induced by Cav-3 expression. Note that a Cav-1 deficiency (Cav-1 KO) leads to a selective loss of caveolae in cardiac endothelial cells, but does not affect caveolae formation in cardiac myocytes (B). Conversely, a Cav-3 deficiency (Cav-3 KO) leads to a loss of caveolae in cardiac myocytes, while caveolae in the cardiac endothelial cells remain unaffected (C). Therefore, a loss of both Cav-1 and Cav-3 expression is necessary to completely abolish caveolae formation in cardiac tissue (D). Endo, endothelial cell. Scale bar, 500 nm.
We next performed routine histopathology on Cav-1 KO, Cav-3 KO, Cav-1/3 dKO, and wild-type mice at 2 months of age. Interestingly, Cav-1/3 dKO mice exhibited the expected lung, fat, and skeletal muscle defects observed in their single knockout counterparts (Cav-1 KO and Cav-3 KO mice), but with no detectable increases in their severity (data not shown).
Cav-1/3 dKO Mice Exhibit Dramatic Cardiac Hypertrophy as Assessed by Gated Cardiac MRI and Gross Morphology
The heart consists of ∼60% cardiac myocytes (that express Cav-3), while the remaining ∼40% is made up of non-muscle cells, ie, cardiac fibroblasts, vascular endothelial cells, and endocardium (that express Cav-1). As such, we chose to focus on the heart in Cav-1/3 dKO mice, as almost half of the cells in the heart express caveolin-1, while the other half express caveolin-3. Thus, it might be expected that Cav-1/3 dKO mice would show an additive cardiac phenotype, as compared with Cav-1 and Cav-3 single-knockout mice.
The hearts of Cav-1/3 dKO mice were first assessed non-invasively using gated MRI. Non-invasive imaging techniques allow for accurate measurement of left ventricular wall thickness and chamber diameter in an intact functional heart. This is accomplished by timing image acquisition after ventricular contraction, as determined from the electrocardiogram.
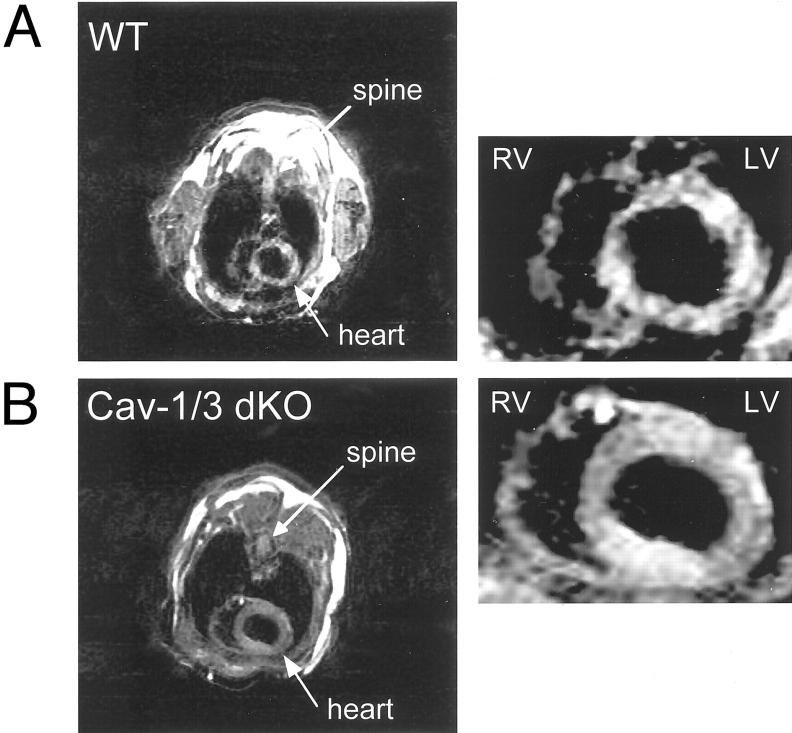
At 2 months of age, Cav-1/3 dKO mice show a significant increase in left ventricular wall thickness that exceeds wild-type mice by ∼41% (Table 1) ▶ ; a noticeable increase was also observed in right ventricular wall thickness (data not shown). Figure 4 ▶ shows a representative short axis (transverse) image of the hearts of Cav-1/3 dKO and wild-type mice taken at the mid-level. Note the marked increase in left ventricular wall thickness. Importantly, Cav-1 null mice and Cav-3 null mice only showed minor changes in left ventricular wall thickness (Table 1) ▶ , suggesting that the dual ablation of both Cav-1 and Cav-3 leads to a synergistic effect. Thus, we concentrated our efforts on the characterization of the cardiac phenotype of Cav-1/3 dKO mice.
Table 1.
MRI Analysis of the Hearts of WT and Cav-1/3 dKO Mice at 2 Months of Age
| Genotype | LV wall thickness (mm) | Mice |
|---|---|---|
| WT | 0.93 ± 0.06 | (n = 4) |
| Cav-1 KO | 1.09 ± 0.03 | (n = 4) |
| Cav-3 KO | 1.03 ± 0.01 | (n = 4) |
| Cav-1/3 dKO | 1.31 ± 0.11* | (n = 4) |
P < 0.05; Cav-1/3 dKO vs. WT; Cav-1/3 dKO vs. Cav-1 KO; Cav-1/3 dKO vs. Cav-3 KO.
Figure 4.
Cav-1/3 dKO mice exhibit cardiac hypertrophy as assessed by gated MRI. Representative short axis (transverse) images at the mid-level of the heart of WT (A) and Cav-1/3 dKO (B) during diastole. Note the concentric hypertrophy of the left ventricle (LV) in the Cav-1/3 dKO heart. RV, right ventricle; LV, left ventricle.
To assess these findings more directly, animals were next sacrificed and subjected to gross dissection. In accordance with the above findings, the hearts of 2-month-old Cav-1/3 dKO mice were grossly enlarged and exhibited increased heart to body weight ratios, exceeding wild-type animals by approximately 33% (Table 2) ▶ .
Table 2.
Total Body Weight and Heart Wet Weight at 2 Months
| Genotype | Weight (g) | Heart weight (g) | Mice |
|---|---|---|---|
| WT | 33.47 ± 4.43 | 0.176 ± 0.006 | (n = 4) |
| Cav-1/3 dKO | 32.87 ± 1.21 | 0.243 ± 0.025* | (n = 4) |
Indicates significant difference, P < 0.05.
Cav-1/3 dKO Mice Show Marked Left Ventricular Hypertrophy, Dilation, and Severe Reductions in Systolic Function, as Assessed by Transthoracic Echocardiography
Several parameters of cardiac function were next assessed using transthoracic echocardiography. Importantly, there was no significant difference in heart rate (beats per minute) between wild-type and Cav-1/3 dKO mice, at either 2 months or 4 months of age (Table 3) ▶ . Continuous electrocardiographic monitoring revealed no obvious conduction defects or cardiac arrhythmias in Cav-1/3 dKO mice. However, Cav-1/3 dKO hearts exhibited left ventricular hypertrophy and dilation, and showed decreased systolic function, as detailed below (Table 3) ▶ .
Table 3.
Heart Rate and Echocardiographic Data from WT and Cav-1/3 dKO Mice at 2 and 4 Months
| Group | Heart rate (bpm) | End diastolic diameter (mm) | End systolic diameter (mm) | Intraventricular septal thickness (mm) | Posterior wall thickness (mm) | Wall thickness diastole (mm) | Relative wall thickness (ratio) | Fractional shortening (%) |
|---|---|---|---|---|---|---|---|---|
| 2 months of age | ||||||||
| WT | 652 ± 69 | 2.71 ± 0.16 | 1.12 ± 0.13 | 0.74 ± 0.03 | 0.76 ± 0.04 | 0.75 ± 0.03 | 0.55 ± 0.03 | 58.65 ± 5.48 |
| dKO | 631 ± 61 | 3.27 ± 0.16† | 2.02 ± 0.07* | 0.99 ± 0.08* | 0.99 ± 0.06* | 0.99 ± 0.06* | 0.60 ± 0.04 | 38.33 ± 2.57† |
| 4 months of age | ||||||||
| WT | 612 ± 111 | 2.81 ± 0.11 | 1.28 ± 0.25 | 0.74 ± 0.07 | 0.75 ± 0.07 | 0.74 ± 0.07 | 0.53 ± 0.04 | 54.47 ± 7.68 |
| dKO | 674 ± 74 | 3.24 ± 0.13* | 1.92 ± 0.18† | 0.98 ± 0.07* | 0.97 ± 0.06* | 0.98 ± 0.06* | 0.60 ± 0.04‡ | 40.87 ± 4.50‡ |
n ≥ 4 for each experimental group.
P < 0.0007;
†P < 0.003;
‡P < 0.02.
Dilation
At 2 months of age, Cav-1/3 dKO hearts exhibited significant left ventricular dilation as compared with wild-type mice, as evidenced by increases in end-diastolic diameter (Cav-1/3 dKO, 3.27 ± 0.16 vs. WT, 2.71 ± 0.13 mm, P < 0.05) and end-systolic diameter (Cav-1/3 dKO, 2.02 ± 0.07 vs. WT, 1.12 ± 0.13 mm, P < 0.05).
Hypertrophy
At 2 months of age, Cav-1/3 dKO hearts showed clear hypertrophy, with increases of ∼34% in interventricular septal thickness, posterior wall thickness, and left ventricular wall thickness, consistent with concentric hypertrophy.
Decreased Systolic Function
A marked reduction in left ventricular systolic function was observed in Cav-1/3 dKO hearts, as evidenced by changes in left ventricular fractional shortening (Cav-1/3 dKO, 38.33 ± 2.57 vs. WT, 58.65 ± 5.48 mm, P < 0.05). Interestingly, no further deterioration of the above cardiac parameters was noted in 4-month-old Cav-1/3 dKO mice (Table 3) ▶ .
Histological Analysis of Cav-1/3 dKO Hearts Reveals Cardiac Myocyte Hypertrophy and Disorganization, with Inflammation and Interstitial Fibrosis
As Cav-1/3 dKO hearts show significant structural and functional changes as assessed by gated cardiac MRI and transthoracic echocardiography, Cav-1/3 dKO hearts were examined by routine histological analysis. Representative hematoxylin and eosin-stained sections of the myocardium from wild-type and Cav-1/3 dKO mice (at 2 months old) are shown in Figure 5 ▶ at various magnifications. Note that there is marked cardiac myocyte hypertrophy and disorganization, with significant chronic inflammation and interstitial fibrosis. Areas of cardiac myocyte “drop-out,” eg, myocytolysis, were also observed.
Figure 5.
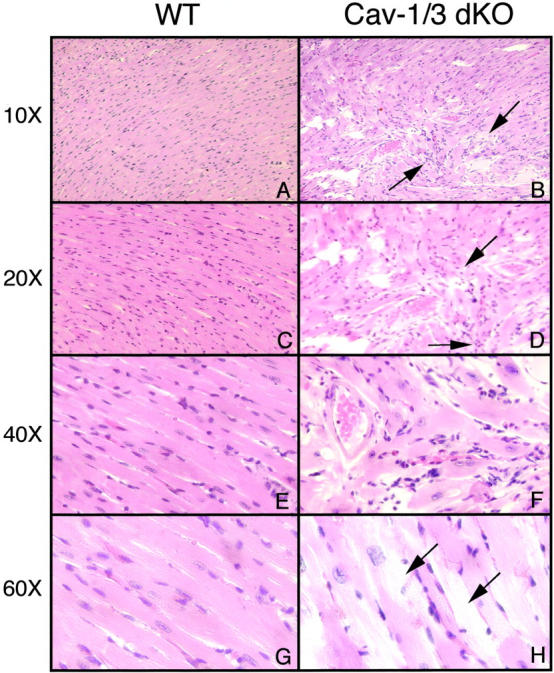
Cav-1/3 dKO hearts histologically exhibit cardiac myocyte hypertrophy and disorganization, with features of chronic inflammation and fibrosis. Representative H&E stained sections of the myocardium of wild-type mice (WT) and Cav-1/3 dKO mice (dKO) are shown at various magnifications. Micrographs were taken with different objectives (10×, 20×, 40×, or 60×), as indicated. A, C, E, and G represent wild-type cardiac histology. B, D, F, and H show Cav-1/3 dKO cardiac histology, with marked cardiac myocyte disorganization. In B and D there is chronic inflammation marked by increased cellular infiltrates and fibrosis (arrows). F demonstrates an area of marked cardiac myocyte hypertrophy and disarray. H shows cardiac myocyte hypertrophy, interspaced with areas of cardiac myocyte degeneration, ie, myocytolysis (arrows).
Caveolin-1/3 dKO Ventricular Tissue Exhibits a Switch to Fetal Programming, as Demonstrated by an Up-Regulation of ANF
Many forms of cardiac hypertrophy are accompanied by an increase in the expression of embryonic genes that are normally only expressed during fetal development. As such, cardiac hypertrophy leads to an activation of this fetal programming and the expression of natriuretic peptides and contractile proteins normally not expressed in adult ventricles. 34 Therefore, we examined the expression of ANF, a well-established biochemical marker of ventricular hypertrophy, in ventricular tissue derived from Cav-1/3 dKO mice.
As demonstrated in Figure 6 ▶ , the ANF transcript is highly up-regulated in the Cav-1/3 dKO ventricles, but absent in wild-type control ventricular tissue. Thus, these data provide further molecular evidence that Cav-1/3 dKO mice exhibit a cardiomyopathic phenotype with significant hypertrophy.
Figure 6.
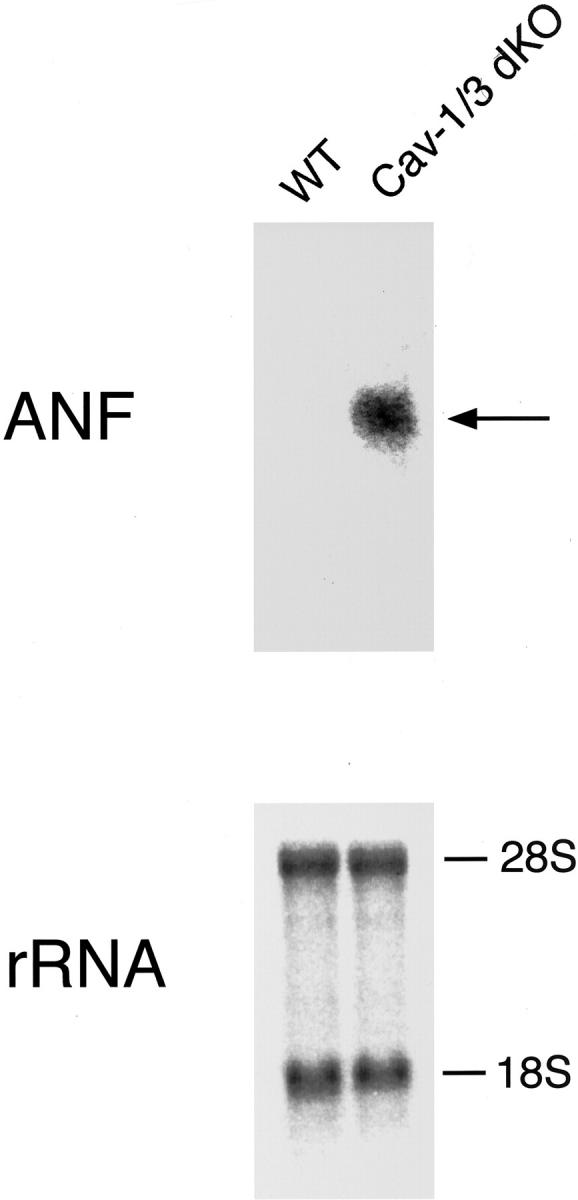
Caveolin-1/3 dKO ventricular tissue exhibits a switch to fetal programming, as demonstrated by an up-regulation of atrial natriuretic peptide (ANF). Cav-1/3 dKO and WT ventricular tissue was carefully separated from atrial tissue by dissection, and total RNA was extracted for Northern blot analysis. Note that the ANF transcript is highly up-regulated in the Cav-1/3 dKO ventricles, but absent in wild-type control ventricular tissue. Thus, these data provide further molecular evidence that Cav-1/3 dKO mice exhibit a hypertrophic cardiomyopathic phenotype.
Discussion
Caveolae have been shown to play an important role in the regulation of signal transduction, cell proliferation, differentiation, T-tubule development, and calcium regulation. 1 To better understand the functional roles played by these organelles in vivo, we generated mice that have null mutations in both the Cav-1 and Cav-3 genes. Since either Cav-1 or Cav-3 expression is sufficient to drive caveolae formation, 9,21 Cav-1/3 dKO mice are the first truly “caveolae-less” animal model. As these mice show a complete ablation of Cav-1 and Cav-3 protein expression, as well as near complete ablation of Cav-2 protein expression (reduced by ∼95%), Cav-1/3 dKO mice are deficient in all three caveolin gene family members, ie, the first “caveolin-less” mouse.
As mentioned previously, nearly 40% of the heart consists of cells expressing high levels of Cav-1 (vascular endothelium, endocardium, cardiac fibroblasts), while cardiac myocytes have abundant Cav-3 expression. 3 Thus, the heart is an ideal organ to study the function of both Cav-1- and Cav-3-generated caveolae within a single tissue. A joint role for Cav-1 and Cav-3 in the development of cardiac hypertrophy is suggested by the work of multiple groups. Chronic β-adrenergic infusion into mice induces cardiac hypertrophy with a concomitant decrease in both Cav-1 and Cav-3 expression exclusively in the heart. 35 Likewise, when hypertension is induced by surgical stenosis of the renal artery, this induces cardiac hypertrophy and leads to dramatic reductions in both Cav-1 and Cav-3 protein expression. 36 However, it remained unknown whether down-regulation of Cav-1 and Cav-3 expression was a cause or a consequence of cardiac hypertrophy. Here, we provide the first evidence that dual ablation of Cav-1 and Cav-3 gene expression produces left ventricular hypertrophy and dilation in Cav-1/3 dKO mouse hearts.
There are several possible mechanisms by which a complete loss of caveolae could result in cardiac hypertrophy. An important function of caveolae is to serve as a platform whereby preassembled signaling complexes are held in an inactive state through interactions with the resident caveolin proteins. 1 G-protein α subunits, H-Ras, and eNOS are concentrated in purified preparations of caveolae, harbor caveolin-binding motifs, and are in their inactive state when bound to caveolins. 37-40 All three of these proteins (G-protein α subunits, H-Ras, and eNOS) have been previously implicated in the development of cardiac hypertrophy.
A growing body of evidence has demonstrated that increased expression/activation of certain heterotrimeric GTP-binding proteins results in a cardiac hypertrophic response. For example, transgenic mice that overexpress either Gαs, Gαi, or Gαq show cardiac hypertrophy, with varying levels of interstitial fibrosis, hyperproliferation, hypercellularity, and apoptosis. 41-43 Similar studies have been performed by expressing an active mutant of Ras within the heart. 44 These mice manifest cardiac hypertrophy and myofibrillar disarray. Therefore, the complete loss of caveolins in the heart may lead to constitutive activation of G-proteins or Ras signaling, leading to cardiac hypertrophy.
The expression profiles of Cav-1 and Cav-3 indicate that they function as negative regulators of proliferation by modulating signal transduction. For example, Cav-1 expression levels are down-regulated in NIH 3T3 cells that are rapidly dividing and up-regulated when the cells become confluent. 45 Overexpression of Cav-1 inhibits the proliferation of human breast cancer cells. 46 Antisense-mediated down-regulation of caveolin-1 is sufficient to cause the hyperactivation of the p42/44 MAP kinase pathway in NIH 3T3 cells. Blocking caveolin-1 transcript using an RNA interference technique in Caenorhabditis elegans results in a hyperactivated Ras signaling phenotype. 47 Analogously, caveolin-3 is not expressed in proliferating myoblasts, but increases as myocyte differentiation ensues. 48 That caveolins play an important role in the proliferative response is most evident in the fact that in caveolin-1 KO mouse lung there is a marked hyperproliferative phenotype. In addition, embryonic fibroblasts isolated from these mice demonstrate an increased proportion of cells found in S-phase of the cell cycle, and this hyper-proliferation phenotype is rescued by re-expression of caveolin-1. 9 Thus, loss of caveolin may result in heightened activation of particular signaling pathways within the heart precipitating the formation of a hypertrophic response.
It is also possible that the cardiac hypertrophy observed in Cav-1/3 dKO mice may be the result an intrinsic defect within the cardiac myocyte itself, coupled with a secondary insult on the myocyte due to changes in the vasculature. Mutations within the Cav-3 gene (P104L; ΔTFT 63–65; A45T) result in LGMD-1C. 18,49 Given that there is a greater than 90% reduction in the expression of Cav-3 within the skeletal muscles of patients with LGMD-1C, the Cav-3 KO mouse has served as a model system for this disease process. Interestingly, the T-tubule system within both the Cav-3 KO mouse and human LGMD-1C skeletal muscles is structurally defective, thus suggesting possible functional derangements in this important membrane system. 21,50
Cav-1 KO mice exhibit significant functional alterations in their vasculature. When challenged with vasoconstrictive agents, the Cav-1 KO vessels exhibit blunted constrictive responses. 9,19 The Cav-1 KO vessels demonstrated markedly increased vasorelaxation responses compared to wild-type mice on exposure to acetylcholine. 9 These phenomena can be explained by the heightened basal nitric oxide levels from the vascular endothelium, as well as decreased calcium mobilization within the smooth muscle cells. The loss of Cav-1 results in the dysinhibition of eNOS and calcium coupling within the cells of the vasculature. 19 Therefore, a defective T-tubule system in cardiac myocytes (due to loss of Cav-3) coupled with vascular dysfunction (due to loss of Cav-1), may act together to elicit a cardiomyopathic phenotype in Cav-1/3 dKO mice.
Both endothelial cells and cardiac myocytes express eNOS. Caveolin-1 and caveolin-3 each serve to tonically inhibit eNOS activity within their respective cell types. Thus the combined loss of Cav-1 and Cav-3 may result in unregulated eNOS activity and nitric oxide production. Nitric oxide has been implicated in a variety of normal and pathological cardiac mechanisms. 51 Endothelial derived nitric oxide increases diastolic relaxation, 52 while decreasing cardiac oxygen consumption. 53 Myocardial derived nitric oxide has been shown to modulate pathways involved in autonomic regulation. 54,55 Thus, alterations in nitric oxide regulation may be a contributing factor in the development of Cav-1/3 dKO cardiac pathology.
In summary, in this study we have generated Cav-1/Cav-3 double-knock-out mice. Unlike mice that are null mutants for only one of these caveolin genes, Cav-1/3 dKO mice fail to form caveolae in both muscle and non-muscle cells, and are deficient in the expression of all three caveolin protein products (Cav-1, -2, and -3). Interestingly, Cav-1/3 dKO mice develop a severe cardiomyopathy with left ventricular hypertrophy and chamber dilation, as assessed by gross morphology, cardiac MRI, and transthoracic echocardiography. Consistent with these findings, histological examination of Cav-1/3 dKO heart tissue reveals marked cardiac myocyte hypertrophy, disarray, and cytolysis; interstitial fibrosis; and chronic inflammation.
Acknowledgments
We thank Dr. Roberto Campos-Gonzalez (BD Transduction Laboratories) for donating mAbs directed against caveolin-1, caveolin-2, and caveolin-3.
Footnotes
Address reprint requests to Michael P. Lisanti, Department of Molecular Pharmacology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461. E-mail: lisanti@aecom.yu.edu.
Supported by grants from the National Institutes of Health, the Muscular Dystrophy Association, and the American Heart Association, and a Hirschl/Weil-Caulier Career Scientist Award (all to M.P.L.). D.S.P. is supported by National Institutes of Health Graduate Training Program Grant TG-CA09475. S.E.W. and A.W.C. were supported by National Institutes of Health Medical Scientist Training Grant T32-GM07288. H.B.T. was supported by National Institutes of Health grant AI-12770.
D.S.P. and S.E.W. contributed equally to this work.
References
- 1.Razani B, Lisanti MP: Caveolin-deficient mice: insights into caveolar function and human disease. J Clin Invest 2001, 108:1553-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP: Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 1996, 93:131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP: Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins J Biol Chem 1996, 271:15160-15165 [DOI] [PubMed] [Google Scholar]
- 4.Galbiati F, Razani B, Lisanti MP: Role of caveolae and caveolin-3 in muscular dystrophy. Trends in Molecular Medicine 2001, 7:435-441 [DOI] [PubMed] [Google Scholar]
- 5.Galbiati F, Razani B, Lisanti MP: Emerging themes in lipid rafts and caveolae. Cell 2001, 106:403-411 [DOI] [PubMed] [Google Scholar]
- 6.Sargiacomo M, Scherer PE, Tang Z-L, Kubler E, Song KS, Sanders MC, Lisanti MP: Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA 1995, 92:9407-9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Z-L, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP: Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 1996, 271:2255-2261 [DOI] [PubMed] [Google Scholar]
- 8.Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP: Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane: retention of caveolin-2 at the level of the Golgi complex. J Biol Chem 1999, 274:25718-25725 [DOI] [PubMed] [Google Scholar]
- 9.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP: Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 2001, 276:38121-38138 [DOI] [PubMed] [Google Scholar]
- 10.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP: Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem 2001, 276:48619-48622 [DOI] [PubMed] [Google Scholar]
- 11.Smart E, Ying Y-S, Conrad P, Anderson RGW: Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol 1994, 127:1185-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Meer G: Caveolin, cholesterol, and lipid droplets? J Cell Biol 2001, 152:F29-F34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP: p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. J Biol Chem 1999, 274:32333-32341 [DOI] [PubMed] [Google Scholar]
- 14.Dessy C, Kelly RA, Balligand JL, Feron O: Dynamin mediates caveolar sequestration of muscarinic cholinergic receptors and alteration in NO signaling. EMBO J 2000, 19:4272-4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP: Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem 2001, 276:6727-6738 [DOI] [PubMed] [Google Scholar]
- 16.Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP: Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis J Biol Chem 1998, 273:20448-20455 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M: Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res 2001, 61:2361-2364 [PubMed] [Google Scholar]
- 18.Minetti C, Sotogia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Volonte’ D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F: Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 1998, 18:365-368 [DOI] [PubMed] [Google Scholar]
- 19.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV: Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293:2449-2452 [DOI] [PubMed] [Google Scholar]
- 20.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP: Caveolin-1 deficient mice are lean, resistant to diet-induced obesity, and show hyper-triglyceridemia with adipocyte abnormalities. J Biol Chem 2002, 277:8635-8647 [DOI] [PubMed] [Google Scholar]
- 21.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP: Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and T-tubule abnormalities. J Biol Chem 2001, 276:21425-21433 [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T: Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet 2000, 9:3047-3054 [DOI] [PubMed] [Google Scholar]
- 23.MacKenna D, Summerour SR, Villarreal FJ: Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res 2000, 46:257-263 [DOI] [PubMed] [Google Scholar]
- 24.Hoit BD, Walsh RA: Cardiovascular Physiology in the Genetically Engineered Mouse. 2002. Kluwer Academic Publishers, Dordrecht, The Netherlands
- 25.Scherer PE, Tang Z-L, Chun MC, Sargiacomo M, Lodish HF, Lisanti MP: Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution: identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem 1995, 270:16395-16401 [DOI] [PubMed] [Google Scholar]
- 26.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP: Cell-type and tissue-specific expression of caveolin-2: caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 1997, 272:29337-29346 [DOI] [PubMed] [Google Scholar]
- 27.Yamada E: The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol 1955, 1:445-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farquhar M, Palade G: Junctional complexes in various epithelia. J Cell Biol 1963, 17:375-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra M, Shirani J, Shtutin V, Weiss LM, Factor SM, Petkova SB, Rojkind M, Dominguez-Rosales JA, Jelicks LA, Morris SA, Wittner M, Tanowitz HB: Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. Int J Parasitol 2002, 32:207-215 [DOI] [PubMed] [Google Scholar]
- 30.Schiller NB: Two-dimensional echocardiographic determination of left ventricular volume, systolic function, and mass: summary and discussion of the 1989 recommendations of the American Society of Echocardiography. Circulation 1991, 84 (Suppl 3):I280-I287 [PubMed] [Google Scholar]
- 31.Hoit BD: New approaches to phenotypic analysis in adult mice. J Mol Cell Cardiol 2001, 33:27-35 [DOI] [PubMed] [Google Scholar]
- 32.Rothberg KG, Heuser JE, Donzell WC, Ying Y, Glenney JR, Anderson RGW: Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68:673-682 [DOI] [PubMed] [Google Scholar]
- 33.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP: Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 1999, 19:7289-7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter JJ, Chien KR: Signaling pathways for cardiac hypertrophy and failure. N Engl J Med 1999, 341:1276-1283 [DOI] [PubMed] [Google Scholar]
- 35.Oka N, Asai K, Kudej RK, Edwards JG, Toya Y, Schwencke C, Vatner DE, Vatner SF, Ishikawa Y: Down-regulation of caveolin by chronic β-adrenergic receptor stimulation in mice. Am J Physiol 1997, 273:C1957-C1962 [DOI] [PubMed] [Google Scholar]
- 36.Piech A, Massart PE, Dessy C, Feron O, Havaux X, Morel N, Vanoverschelde JL, Donckier J, Balligand JL: Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol 2002, 282:H219-H231 [DOI] [PubMed] [Google Scholar]
- 37.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP: Evidence for a regulated interaction between hetero-trimeric G proteins and caveolin. J Biol Chem 1995, 270:15693-15701 [DOI] [PubMed] [Google Scholar]
- 38.Li S, Couet J, Lisanti MP: Src tyrosine kinases, G α subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin: caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 1996, 271:29182-29190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song KS, Li S, Okamoto T, Quilliam L, Sargiacomo M, Lisanti MP: Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains: detergent-free purification of caveolae membranes J Biol Chem 1996, 271:9690-9697 [DOI] [PubMed] [Google Scholar]
- 40.Fujita T, Toya Y, Iwatsubo K, Onda T, Kimura K, Umemura S, Ishikawa Y: Accumulation of molecules involved in α1-adrenergic signal within caveolae: caveolin expression and the development of cardiac hypertrophy. Cardiovasc Res 2001, 51:709-716 [DOI] [PubMed] [Google Scholar]
- 41.Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF: Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS α overexpression. Circ Res 1996, 78:517-524 [DOI] [PubMed] [Google Scholar]
- 42.Geng YJ, Ishikawa Y, Vatner DE, Wagner TE, Bishop SP, Vatner SF, Homcy CJ: Apoptosis of cardiac myocytes in Gsα transgenic mice. Circ Res 1999, 84:34-42 [DOI] [PubMed] [Google Scholar]
- 43.Bohm M, Kirchmayr R, Erdmann E: Myocardial Gi α-protein levels in patients with hypertensive cardiac hypertrophy, ischemic heart disease, and cardiogenic shock. Cardiovasc Res 1995, 30:611-618 [PubMed] [Google Scholar]
- 44.Hunter JJ, Zhu H, Lee KJ, Kubalak S, Chien KR: Targeting gene expression to specific cardiovascular cell types in transgenic mice. Hypertension 1993, 22:608-617 [DOI] [PubMed] [Google Scholar]
- 45.Galbiati F, Volonté D, Engelman JA, Watanabe G, Burk R, Pestell R, Lisanti MP: Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 1998, 17:6633-6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP: Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 1997, 272:16374-16381 [DOI] [PubMed] [Google Scholar]
- 47.Scheel J, Srinivasan J, Honnert U, Henske A, Kurzchalia TV: Involvement of caveolin-1 in meiotic cell-cycle progression in Caenorhabditis elegans. Nat Cell Biol 1999, 1:127-129 [DOI] [PubMed] [Google Scholar]
- 48.Galbiati F, Volonte D, Engelman JA, Scherer PE, Lisanti MP: Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts: transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation J Biol Chem 1999, 274:30315-30321 [DOI] [PubMed] [Google Scholar]
- 49.Herrmann R, Straub V, Blank M, Kutzick C, Franke N, Jacob EN, Lenard HG, Kroger S, Voit T: Dissociation of the dystroglycan complex in caveolin-3-deficient limb girdle muscular dystrophy. Hum Mol Genet 2000, 9:2335-2340 [DOI] [PubMed] [Google Scholar]
- 50.Minetti C, Bado M, Broda P, Sotgia F, Bruno C, Galbiati F, Volonte D, Lucania G, Pavan A, Bonilla E, Lisanti MP, Cordone G: Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am J Pathol 2002, 160:265-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balligand JL, Cannon PJ: Nitric oxide synthases and cardiac muscle: autocrine and paracrine influences. Arterioscler Thromb Vasc Biol 1997, 17:1846-1858 [DOI] [PubMed] [Google Scholar]
- 52.Smith JA, Shah AM, Lewis MJ: Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol 1991, 439:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie YW, Shen W, Zhao G, Xu X, Wolin MS, Hintze TH: Role of endothelium-derived nitric oxide in the modulation of canine myocardial mitochondrial respiration in vitro: implications for the development of heart failure. Circ Res 1996, 79:381-387 [DOI] [PubMed] [Google Scholar]
- 54.Balligand JL, Kobzik L, Han X, Kaye DM, Belhassen L, O’Hara DS, Kelly RA, Smith TW, Michel T: Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J Biol Chem 1995, 270:14582-14586 [DOI] [PubMed] [Google Scholar]
- 55.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T: Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes: implications for the autonomic regulation of heart rate. J Biol Chem 1998, 273:30249-30254 [DOI] [PubMed] [Google Scholar]