Abstract
Inflammatory bladder disorders such as interstitial cystitis (IC) deserve attention since a major problem of the disease is diagnosis. IC affects millions of women and is characterized by severe pain, increased frequency of micturition, and chronic inflammation. Characterizing the molecular fingerprint (gene profile) of IC will help elucidate the mechanisms involved and suggest further approaches for therapeutic intervention. Therefore, in the present study we used established animal models of cystitis to determine the time course of bladder inflammatory responses to antigen, Escherichia coli lipopolysaccharide (LPS), and substance P (SP) by morphological analysis and cDNA microarrays. The specific aim of the present study was to compare bladder inflammatory responses to antigen, LPS, and SP by morphological analysis and cDNA microarray profiling to determine whether bladder responses to inflammation elicit a specific universal gene expression response regardless of the stimulating agent. During acute bladder inflammation, there was a predominant infiltrate of polymorphonuclear neutrophils into the bladder. Time-course studies identified early, intermediate, and late genes that were commonly up-regulated by all three stimuli. These genes included: phosphodiesterase 1C, cAMP-dependent protein kinase, iNOS, β-NGF, proenkephalin B and orphanin, corticotrophin-releasing factor (CRF) R, estrogen R, PAI2, and protease inhibitor 17, NFkB p105, c-fos, fos-B, basic transcription factors, and cytoskeleton and motility proteins. Another cluster indicated genes that were commonly down-regulated by all three stimuli and included HSF2, NF-κB p65, ICE, IGF-II and FGF-7, MMP2, MMP14, and presenilin 2. Furthermore, we determined gene profiles that identify the transition between acute and chronic inflammation. During chronic inflammation, the urinary bladder presented a predominance of monocyte/macrophage infiltrate and a concomitant increase in the expression of the following genes: 5-HT 1c, 5-HTR7, β2 adrenergic receptor, c-Fgr, collagen 10α1, mast cell factor, melanocyte-specific gene 2, neural cell adhesion molecule 2, potassium inwardly-rectifying channel, prostaglandin F receptor, and RXR-β cis-11-retinoic acid receptor. We conclude that microarray analysis of genes expressed in the bladder during experimental inflammation may be predictive of outcome. Further characterization of the inflammation-induced gene expression profiles obtained here may identify novel biomarkers and shed light into the etiology of cystitis.
Clinical and animal models of acute and chronic urinary bladder inflammation have provided several lines of evidence suggesting a central role of mast cells, sensory nerves, and neurokinin (NK)-1 receptors. Fundamental work regarding the participation of sensory nerves and mast cells in cystitis provides indirect evidence, such as increased numbers of mast cells in the detrusor and submucosa, and morphological evidence of mast cell activation and degranulation. 1-4 In addition, the extensive tissue remodeling seen in some clinical forms of bladder inflammation such as interstitial cystitis, 4 along with increased urinary levels of histamine and tryptase 5 suggest a role for mast cells. Because both mast cells 6-8 and NK-1 receptors are increased in bladder biopsies of interstitial cystitis (IC) patients, 9,10 it is tempting to propose a role for sensory peptides-mast cell communication in the pathogenesis of this disorder.
Experimentally, we have used classical morphometric analysis and microarray technology to determine the role of mast cells, NK-1 receptors, and bacterial toxins in animal models of cystitis. Time-course experiments indicated early and late genes involved in bladder inflammatory responses. 11 The availability of mice genetically deficient in neurokinin-1 receptor (NK-1R−/−) allowed us to propose a mandatory role of SP receptors in cystitis. 12 We also determined genes that depend on the presence of tissue mast cells for their expression by comparing inflammatory responses in mast cell-deficient (KitW/KitW−v), congenic normal (+/+), and KitW/KitW−v mice that were reconstituted with +/+ bone marrow stem cells (BMR) to restore mast cells. 13 Bladder inflammation occurred in +/+ and BMR, but not in KitW/KitW−v mice. These results demonstrate an important role for mast cells in allergic cystitis and indicate that mast cells can alter their environment by regulating tissue gene expression. 13 An interesting hypothesis for increased pain observed in cystitis is that bacterial products can exacerbate the activity of sensory peptides. Among the possible mechanisms of LPS-peptide interaction, we found that LPS induced a time-dependent gene up-regulation in the bladder. 11 We also reported that intravesical inoculation of mice with LPS induced up-regulation of peptide receptors such as bradykinin-1 (BK1) 14 and NK1. 15 Moreover, we observed that LPS-induced cystitis was associated with activation of nuclear transcription factor κ B (NF-κB). 15 Inhibition of NF-κB with lactacystin blocked LPS-induced inflammation and NK1 receptor up-regulation. 15
It is not clear from available data which responses of the urinary bladder are inflammatory stimulus-specific, and/or whether there are some central universal patterns of response. Defining the universal and stimulus-specific patterns of response to inflammatory stimuli is critical to begin to understand why some acute inflammatory responses resolve and some transition to the chronic inflammatory process. New methods of cDNA microarray analysis of gene expression provide fresh tools to address these issues definitively.
Our overall hypothesis is that bladder responses to inflammation elicit a specific universal gene expression response regardless of the stimulating agent. Therefore, we sought to compare bladder inflammatory responses to antigen, LPS, and SP by morphological analysis and cDNA microarray profiling. In addition, we determined gene profiles that identify the transition between acute and chronic inflammation. Such studies may provide important insight into human bladder disorders such as IC, as obtaining large-scale gene expression profiles of inflammation may allow for the future identification of subsets of genes that function as prognostic disease markers or biological predictors of a therapeutic response.
Materials and Methods
Animals
All animal experimentation described here was performed in conformity with the “Guiding Principles for Research Involving Animals and Human Beings” (OUHSC Animal Care and Use Committee protocol #00–109).
Three groups of 10- to 12-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used in these experiments. Animals were maintained in housing facilities and allowed food and water ad libitum.
Antigen Sensitization Protocol
All mice in this study were sensitized intraperitoneally (i.p.) with 1 μg DNP4-human serum albumin (HSA; Molecular Probes, Eugene, OR) in 1 mg alum on days 0, 7, 14, and 21. This protocol induces sustained levels of IgE antibodies up to 56 days postsensitization. 16 One week after the last sensitization, cystitis was induced.
Induction of Cystitis
Acute cystitis was induced as we described previously. 11-13,17 Briefly, sensitized female C57BL/6J mice were anesthetized (ketamine 40 mg/kg and xylazine 2.5 mg/kg, i.p.), then transurethrally catheterized (24-gauge; 3/4 inch; Angiocath, Becton Dickinson, Sandy, UT), and the urine was drained by applying slight digital pressure to the lower abdomen. The urinary bladders were instilled with 150 μl of one of the following substances: pyrogen-free saline, SP (10 μmol/L), Escherichia coli LPS strain 055:B5 (Sigma, St. Louis, MO; 100 μg/ml), or antigen DNP4-OVA (1 μg/ml). Substances were infused at a slow rate to avoid trauma and vesicoureteral reflux. 18 To ensure consistent contact of substances with the bladder, infusion was repeated twice within a 30-minute interval and a 1-ml Tb syringe was maintained on the catheter end retained intravesical solution for at least for 1 hour. After that the catheter was removed and mice were allowed to void normally. One, four, and twenty-four hours after instillation, mice were sacrificed with pentobarbital (20 mg/kg, i.p.) and bladders were removed rapidly.
Chronic cystitis was induced by LPS (100 μg/ml) instillations performed every 24 hours for 4 days. Mice were sacrificed 24 hours after the last instillation. Control mice for this group received the same volume of pyrogen-free saline at the same time points and will be denoted as saline chronic hereafter. Bladders from all experimental treatments were randomly distributed into the following groups: RNA extraction (n = 3), replicate of RNA extraction (n = 3), and morphological analysis (n = 6).
Alterations at Histological Level
The urinary bladder was evaluated for inflammatory cell infiltrates, mast cell numbers, and the presence of interstitial edema. A semiquantitative score using defined criteria of inflammation severity was used to evaluate cystitis (11–13,17). A cross-section of bladder wall was fixed in formalin, dehydrated in graded alcohol and xylene, embedded in paraffin, and cut serially into four 5-μm sections (8 μm apart) to be stained with hematoxylin and eosin (H&E) and Giemsa. Tissues that received chronic stimulation with saline or LPS underwent immunohistochemistry with rat anti-mouse macrophage monocyte antibody (MCA519G, Serotec LTD, Oxford, UK) and secondary antibody anti-mouse Fab-HRP. This antibody reacts with the 110-kd Mac-3 antigen expressed on the mouse mononuclear phagocytes. 19 Histology slides were scanned using a Nikon digital camera (DXM1200; Nikon, Japan) mounted on a Nikon microscope (Eclipse E600, Nikon). Image analysis was performed using a MetaMorph Imaging System (Universal Imaging Corporation, West Chester, PA). The severity of lesions in the urinary bladder was graded 11-13,17 as follows: 1+, mild (infiltration of a 0–10 neutrophils/cross-section in the lamina propria, and little or no interstitial edema); 2+, moderate (infiltration of 10–20 neutrophils/cross-section in the lamina propria, and moderate interstitial edema); 3+, severe (diffuse infiltration of >20 neutrophils/cross-section in the lamina propria and severe interstitial edema). Identification of mast cells and quantification of their degree of degranulation was performed in Giemsa-stained sections. Degree of degranulation is presented as the percentage of mast cells per cross-section that exhibited degranulation.
Sample Preparation for cDNA Expression Arrays
We used the same sample preparation technology as described previously. 11,13 Briefly, three bladders from each group were homogenized together in Ultraspec RNA solution (Biotecx, Houston, TX) for isolation and purification of total RNA. Mouse bladders were pooled to ensure enough RNA for gene array analysis. The justification for this approach is that there is not enough RNA in a single mouse bladder for performing cDNA array experiments and the purification step reduces the yield of total RNA. RNA was DNase-treated according to manufacturer’s instructions (Clontech Laboratories, Palo Alto, CA), and 10 μg of RNA was evaluated by denaturing formaldehyde/agarose gel electrophoresis. This procedure was repeated using an additional three bladders in each experimental group. Therefore, two pools of RNA were generated per experimental group for a total of six mice and two separate hybridizations per group.
Mouse cDNA Expression Arrays
cDNA probes prepared from DNase-treated RNAs obtained from each of the experimental groups were hybridized simultaneously to membranes containing Atlas Mouse 1.2 Arrays (Clontech, Cat. #7853–1). A complete list of genes present in this array can be found at http://www.clontech.com/atlas/genelists/index.html. Briefly, 5 μg of DNase-treated RNA was reverse-transcribed and labeled with [α-32P]dATP, according to the manufacturer’s protocol (Clontech). The radioactively labeled complex cDNA probes were hybridized overnight to mouse cDNA expression arrays (Clontech) using ExpressHyb hybridization solution with continuous agitation at 68°C. After high- and low-stringency washes, the hybridized membranes were exposed overnight at room temperature to a ST Cyclone phosphor screen (Packard BioScience Company, Downers Grove, IL).
Quantification of Gene Expression
The phosphorimaging screen contains phosphor crystals that absorb the energy emitted by the radioactivity of the sample and re-emits that energy as a blue light when excited by a red laser. Results are presented as digital light units (DLU). Spots on the arrays were quantified using grid analysis provided by OptiQuant Image Analysis Software (Packard BioScience Company, Downers Grove, IL). Quantification of each detectable spot was performed by measuring the digital light units generated by OptiQuant. The results were placed into an Excel spreadsheet (Microsoft Corporation) and the background was subtracted. Previous experiments indicate that inflammation did not alter β-actin expression. 11,13 As described before, 13 within each membrane, expression was calculated as percentage of β-actin. Genes whose expression was lower than 0.2% of β-actin were considered too close to the background and discarded.
To determine the reproducibility of the arrays, we performed regression analysis using an Excel spreadsheet. This test compared the results obtained with two pools of RNA isolated from mice that were challenged with saline. cDNA probes were prepared from DNase-treated RNAs and two separate hybridizations were performed. The same analysis was performed using RNA isolated from mice that were challenged with antigen, LPS, and SP.
Cluster Analysis
Genes presenting a similar expression profile were identified based on a Euclidean distance metric, and normalized within experiments to a mean of zero and a standard deviation (SD) of 1. Cluster analysis was performed using self-organizing maps (SOMs) as described by Tamayo et al. 20 SOM is an unsupervised network learning algorithm which has been successfully used for the analysis and organization of large data files. 21 We applied SOM to analyze the time course of gene regulation during LPS-induced cystitis 11 and others have shown that SOM is an excellent tool for the analysis and visualization of gene expression profiles. 20-22 SOMs are a type of mathematical cluster analysis that is particularly well suited for recognizing and classifying features in complex, multidimensional data. 20 The method has been implemented in the GeneCluster software, which performs the analytical calculations and provides easy data visualization(http://www_genome.wi.mit.edu/cancer/software/software.html).This focuses attention on the “shape” of expression patterns rather than on absolute levels of expression. The advantage of this approach is that large data sets can be clustered much faster than by using hierarchical clustering because a lower number of clusters are assigned.
Bladder Genes Regulated in Common by LPS-, SP-, and Ag-Induced Inflammation
To determine which genes had their expression altered during inflammation regardless of the initiating stimulus, we set the arbitrary criteria that the gene should be up-regulated at least threefold in response to antigen-, LPS-, or substance P-challenge when compared to saline-treated bladder tissue. For this purpose, Venn diagram analysis was performed using GeneSpring software (Silicon Genetics, Redwood City, CA) using raw data and filtering genes that were at least threefold up-regulated when comparisons were made within each animal group between two conditions, antigen- and saline-challenge. Another set of analyses was performed on the same group of mice by comparing gene up-regulation in response to SP- and saline-challenge and between LPS- and saline-challenge. Finally, we determined genes that satisfied all three conditions in response to antigen, LPS, and substance P. To determine gene down-regulation, we determined the ratio of expression between saline-treated and the respective stimulus.
Bladder Genes Expression Specifically Regulated by Each Stimulus
To determine which genes had their expression specifically regulated by each stimulus, we set the arbitrary criteria that the gene should be up-regulated at least threefold in response to one stimulus and the same gene should not be up-regulated in response to the other two stimuli. For this purpose, Venn diagram analysis was performed using GeneSpring software (Silicon Genetics) using raw data and filtering genes that were at least threefold up-regulated in a single group.
LPS-Induced Acute and Chronic Alteration in Gene Expression
Results obtained with acute LPS stimulation (1, 4, and 24 hours) were compared to chronic LPS stimulation. For this purpose, genes that were up- or down-regulated at least threefold regarding their respective control were sorted by SOM as described above.
Statistical Analysis
For each group, 1200 genes were analyzed by two different hybridizations using two different pools of RNA isolated from sensitized mice that were challenged with saline, antigen, LPS, and SP. Mice were sacrificed following three different time points (1, 4, and 24 hours after acute stimulation). Therefore, a total of 28,800 data points were analyzed using GeneCluster software. Comparison between acute (1, 4, and 24 hours) and chronic LPS- and saline-stimulation were performed using 19,200 data points and were also analyzed by GeneCluster software. For analysis of acute and chronic results, SOMs were constructed by choosing a 6 × 4 grid that generated 24 clusters. As this type of analysis assumes that the data can be divided into a certain number of clusters and that they are well separated, it was also our concern to present the fewest clusters possible that would still give a clear picture of antigen- and substance P-induced gene expression. Increasing the number of clusters by increasing the grid did not produce additional correlation between genes. The GeneCluster software provides a list of genes present in each cluster and a centroid. However, to permit comparisons, in each cluster, gene expression was averaged and the SEM calculated. In each cluster, comparisons between gene regulation in response to antigen-, LPS, and SP-challenge were obtained by the ratio of gene expression in relation to the correspondent saline group. Significant differences were obtained by unpaired Student’s t-test. 23
The statistical analysis of histological data were performed using Wilcoxon’s rank sum test. Results are expressed as mean ± SEM. The n values reported refer to the number of animals used for each experiment. In all cases, a value of P < 0.05 was considered indicative of significant difference. 23
Reagents
Dinitrophenyl (DNP) was conjugated to ovalbumin (OVA) and human serum albumin (HSA) (Sigma) as previously described. 24 Alum adjuvant was purchased from Intergen (Purchase, NY). E. coli LPS strain 055:B5 was purchased from Sigma. All drugs were prepared in pyrogen-free saline immediately before use.
Results
Morphological Consequences of Acute Antigen-, SP-, and LPS-Challenge
We examined whether the mouse bladder mounts an inflammatory response to all three stimuli by morphological analysis of tissue sections (Table 1) ▶ . LPS, SP, and Ag induced slightly different degrees of acute inflammation characterized by vasodilation, edema, and intense polymorphonuclear neutrophil (PMN) infiltration in the mucosa and submucosal layers (Table 1) ▶ . Confirming our results obtained with LPS, 11 substance P, 17 and antigen stimulation, 12 both edema formation and PMN migration were observed as early as 4 hours and were stabilized 24 hours after stimulation (data not shown). In a separate study, we also examined additional time points at 48 and 72 hours after LPS stimulation to make sure the maximal PMN infiltration and edema was obtained at 24 hours. 11 In conclusion, our results indicate that both edema and PMN migration were observed as early as 4 hours and peaked at 24 hours. In contrast, animals catheterized and instilled with saline did not develop an inflammatory response (data not shown).
Table 1.
Histological Severity of Antigen-, SP-, and LPS-Induced Cystitis
| Stimulus | Edema | PMNs | Mast cells* |
|---|---|---|---|
| Saline | 0.10 ± 0.14 | 0.18 ± 0.14 | 8.0 ± 0.7 |
| LPS | 2.70 ± 0.10† | 2.30 ± 0.10† | 10.1 ± 1.7 |
| SP | 2.10 ± 0.20† | 2.20 ± 0.20† | 8.4 ± 2.6 |
| Antigen | 2.88 ± 0.06† | 2.42 ± 0.11† | 7.5 ± 0.4‡ |
The histologic severity of cystitis was graded by a score of 0–3.
*Number of mast cells per bladder cross-section. Bladder tissue was fixed in formalin and then paraffin-embedded. Four 5-μm serial sections (8 μm apart) were stained with hematoxylin and eosin for histological analysis. Additional sections were stained with Giemsa for evaluation of mast cell numbers. Values are means ± SEM. P values were obtained using Wilcoxon’s rank sum test.
†P < 0.0001 vs. the respective control (instilled with saline).
‡Not significant when compared with the respective control (instilled with saline).
Morphological Alterations Secondary to Chronic LPS Stimulation
Chronic inflammation was provoked by repeated bladder instillation with LPS. During chronic inflammation, cross-sections of the mouse bladder presented a mixed inflammatory cell infiltrate containing predominantly macrophages, lymphocytes, and plasma cells, with some polymorphs as minor components (Figure 1) ▶ . The most dramatic alteration of chronic bladder stimulation with LPS was the presence of macrophages/monocytes in the suburothelial and submucosa layers as determined by immunohistochemistry using a rat anti-mouse macrophage/monocyte antibody (MCA519G) and a decrease in the numbers of PMNs.
Figure 1.
A-F: Acute and chronic inflammation. A: H&E stains of cross-sections of mouse urinary bladder responding to acute (A and D) and chronic (B, C, E, F) intravesical stimulation with LPS. Notice intense leukocyte infiltrate in the acute response (A, D) and intense perivascular infiltrate of plasma cells, lymphocytes, and monocytes in the chronic model (B, C, E, F). D–F: Immunohistochemistry of cross-sections obtained from chronic inflamed bladders stained with rat anti-mouse macrophage monocyte antibody and secondary antibody anti-mouse Fab-HRP. Black arrow and circle indicate a plasma cell, red arrows indicate PMNs, white arrows indicate lymphocytes; and green arrows indicate macrophages/monocytes.
Reproducibility of Array Hybridization
We previously presented evidence of the reproducibility of gene-array methodology for the analysis of bladder inflammatory genes 11 and verified the results using RNase protection assay. 13 In the present work, we determined the reproducibility of our hybridization technique by performing regression analysis from values obtained with two different pools of RNA. Table 2 ▶ represents regression analysis of RNA isolated from the bladder of sensitized mice that were challenged with saline, LPS, antigen, and SP and sacrificed 24 hours later. All results indicate good correlations between samples resulting in correlation coefficients and slopes of the regression lines not different from 1 (Table 2) ▶ .
Table 2.
Reproducibility of Arrays
| Treatment | Regression | Correlation coefficient |
|---|---|---|
| Saline | 1.1271x–1.280 | 0.9484 |
| LPS | 0.9459x–1.040 | 0.9715 |
| SP | 1.1363x–0.126 | 0.9528 |
| Antigen | 0.9245x–0.620 | 0.9840 |
Mice were treated with SP, LPS, antigen, and saline and sacrificed 24 hours after stimulation. Bladders from all experimental treatments were randomly distributed into the following groups: A) RNA extraction (n = 3); B) replicate of RNA extraction (see Materials and Methods). Regression lines were obtained by plotting gene expression results of group A versus B.
Bladder Genes Commonly Regulated by LPS-, SP-, and Ag-Induced Inflammation
We profiled gene expression across multiple time points in the bladder following stimulation with antigen, LPS, SP, or saline. Organs were pooled from at least three mice for each time point of the study, to provide enough RNA for the study and to minimize variation between animals. In all, 24 1.2K mouse cDNA microarrays were used to assess two replications of gene expression in response to four different stimuli (saline, LPS, antigen, SP) at three time points (1, 4, and 24 hours).
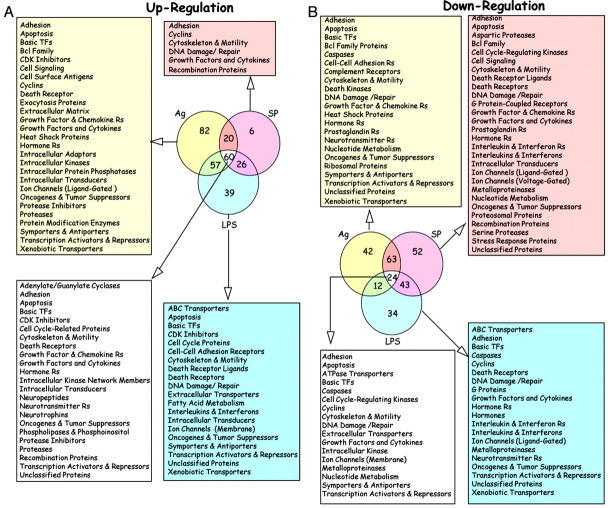
To determine which genes had their expression altered during inflammation regardless of the initiating stimulus, we set the arbitrary criteria that the gene should be up-regulated at least threefold in response to antigen-, LPS-, or SP-challenge when compared to saline-treated bladder tissue. Venn diagram analysis indicates that 60 genes were up-regulated in response to antigen, SP, and LPS (Figure 2A) ▶ . In contrast, 24 genes were down-regulated by all three treatments (Figure 2B) ▶ .
Figure 2.
A–B: Venn diagram of ratio of gene expression obtained in bladder isolated from sensitized C57BL/6J mice that were instilled with 150 μl of one of the following substances: pyrogen-free saline, SP (10 μmol/L), LPS (100 μg/ml), or antigen DNP4-OVA (1 μg/ml). Twenty-four hours after, bladders were removed, RNA was extracted, reverse-transcribed to cDNA, and hybridized to 1.2K mouse membranes (Clontech). Venn diagrams were obtained using raw data and filtering genes that were at least threefold up-regulated when comparisons were made within each animal group between the two conditions, antigen and saline, SP and saline, and LPS and saline. Results in A represent number of genes that were up-regulated and in B genes that were down-regulated. For each group 1200 genes were analyzed by two different hybridizations using two different pools of RNA isolated from sensitized mice that were challenged with saline, antigen, LPS, and SP. Mice were sacrificed following three different time points (1, 4, and 24 hours after acute stimulation). Therefore, a total of 28,800 data points were analyzed.
Genes that were up-regulated in response to all three stimuli were further sorted according to peak expression at 1, 4, and 24 hours in early (Table 3A) ▶ , intermediate (Table 3B) ▶ , and late genes (Table 3C) ▶ and with few exceptions, commonly up-regulated genes exhibited the same time-to-peak of expression in response to all three stimuli. This finding further indicates a consistency of this response to inflammatory stimulus. Those genes included several cell surface and cytoplasmic receptors (neuropeptides, neurotransmitter, hormones, and growth factors), adhesion proteins, transcription factors, and signal transducers. In addition, genes involved in cell survival and proliferation such as nerve growth factor (NGF) and proto-oncogenes (iNOS) where co-expressed with death receptor proteins, proteases, and protease inhibitors. Moreover, inflammation also altered the expression of cytoskeleton and motility proteins.
Table 3A.
Early Genes Up-Regulated by SP, LPS, and Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | semaphorin IIIB | Semaphorin IIIB | X85990 | 3.0 | 1.0 | 1.0 | 3.0 | 1.0 | 1.0 | 3.0 | 1.5 | 1.3 |
| Basic Transcription Factors | CUTL2 | Cut-related homeobox-2 | U45665 | 5.5 | 2.2 | 2.7 | 4.0 | 0.5 | 1.1 | 3.4 | 0.8 | 1.0 |
| Basic Transcription Factors | SOX13 | HMG-box transcription factor | AJ000740 | 3.0 | 1.0 | 0.3 | 3.0 | 0.8 | 1.0 | 3.0 | 1.0 | 1.0 |
| Cytoskeleton & Motility Proteins | vitronectin | Vitronectin precursor | X72091 | 3.8 | 1.0 | 1.0 | 3.8 | 1.0 | 1.0 | 3.8 | 1.2 | 1.0 |
| Growth Factors and Cytokines | FGF 9 | Fibroblast growth factor 9 | D38258 | 3.6 | 1.6 | 0.5 | 3.6 | 1.6 | 1.0 | 3.6 | 1.6 | 1.0 |
| Growth Factors and Cytokines | MIP2-alpha | Macrophage inflamatory protein 2 alpha | X53798 | 3.7 | 1.0 | 1.0 | 3.7 | 1.0 | 1.0 | 3.7 | 1.0 | 1.0 |
| Growth Factors and Cytokines | VEGF | Vascular endothelial growth factor | M95200 | 3.7 | 1.0 | 0.4 | 3.7 | 1.0 | 1.0 | 8.6 | 1.7 | 1.0 |
| Growth Factors and Cytokines | WNT5B | Wingless-related MMTV integration | M89799 | 3.7 | 1.0 | 1.2 | 3.7 | 1.0 | 1.0 | 3.7 | 0.9 | 1.0 |
| Hormone Receptors | CRF R | Corticotropin releasing factor receptor | X72305 | 3.8 | 1.0 | 1.0 | 3.8 | 1.0 | 1.0 | 3.8 | 1.0 | 1.0 |
| Hormone Receptors | estrogen R | Estrogen receptor | M38651 | 3.1 | 1.0 | 1.0 | 3.1 | 1.0 | 1.0 | 3.1 | 0.4 | 1.0 |
| Hormone Receptors | galanin R 1 | Galanin receptor 1 | U90657 | 3.1 | 1.0 | 1.0 | 3.1 | 1.0 | 0.3 | 3.1 | 1.9 | 1.0 |
| Hormone Receptors | melanocortin 5 R | Melanocortin 5 receptor | X76295 | 3.4 | 1.0 | 1.0 | 3.4 | 1.0 | 1.0 | 3.4 | 0.5 | 1.0 |
| Intracellular Transducers | TAK1 | TGF-beta-activated kinase 1 | D76446 | 3.1 | 1.0 | 0.3 | 3.1 | 1.0 | 1.0 | 3.1 | 1.0 | 1.0 |
| Neuropeptides | proenkephalin B | Proenkephalin B precursor | AF026537 | 3.1 | 0.5 | 3.6 | 4.1 | 1.1 | 1.8 | 3.1 | 0.9 | 1.7 |
| Neurotransmitter Receptors | ACH R | Acetylcholine receptor alpha | M17640 | 3.1 | 1.0 | 1.0 | 3.1 | 1.0 | 1.0 | 3.1 | 1.0 | 1.0 |
| Neurotransmitter Receptors | GABRA1 | Gamma-aminobutyric-acid R alpha-1 | M86566 | 4.6 | 1.0 | 0.6 | 3.0 | 1.0 | 1.0 | 3.0 | 1.0 | 1.0 |
| Neurotrophins | beta-NGF | Nerve growth factor beta precursor | K01759 | 3.6 | 1.0 | 1.0 | 3.6 | 1.0 | 1.0 | 3.6 | 0.5 | 1.0 |
| Oncogenes | c-fos | c-fos | V00727 | 3.0 | 0.4 | 0.3 | 3.9 | 0.4 | 1.4 | 5.7 | 2.3 | 2.7 |
| Oncogenes | fos-B | Fos-B | X14897 | 0.9 | 0.9 | 4.4 | 3.0 | 1.0 | 1.0 | 3.0 | 0.8 | 1.0 |
| Phospholipases & Phosphoinositol | PLC gamma | Phospholipase C gamma | X95346 | 1.1 | 0.3 | 3.0 | 5.5 | 1.0 | 1.3 | 17.0 | 3.6 | 2.6 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 3B.
Intermediate Genes Up-Regulated by SP, LPS, and Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adenylate/Guanylate Cyclases | PDE 1C | Phosphodiesterase 1C | L76946 | 1.5 | 3.9 | 3.9 | 1.5 | 3.9 | 3.9 | 0.6 | 3.1 | 1.0 |
| Apoptosis | INOS | Inducible nitric oxide synthase | M87039 | 1.2 | 3.0 | 1.1 | 1.2 | 3.0 | 1.0 | 1.2 | 3.0 | 1.0 |
| Basic Transcription Factors | FOG | Friend of GATA 1 | AF006492 | 1.0 | 3.8 | 0.3 | 1.0 | 3.8 | 1.0 | 1.0 | 3.8 | 1.0 |
| Basic Transcription Factors | FREAC7 | Forkhead-related TF7 | X92498 | 1.7 | 3.4 | 0.5 | 0.5 | 3.4 | 0.4 | 1.7 | 3.4 | 1.0 |
| Basic Transcription Factors | GTX | Homeobox protein GTX | L08074 | 1.0 | 3.5 | 0.3 | 1.0 | 3.5 | 1.0 | 1.0 | 3.5 | 1.0 |
| Basic Transcription Factors | maf1 | Transcription factor | L36435 | 1.0 | 3.0 | 0.7 | 1.0 | 3.0 | 0.3 | 1.0 | 3.5 | 1.0 |
| Basic Transcription Factors | NKX-3.2 | Drosophila NK3 TF | U87957 | 0.4 | 3.4 | 2.4 | 0.5 | 3.4 | 0.5 | 1.5 | 3.4 | 1.0 |
| CDK Inhibitors | p57kip2 | Cdk-inhibitor kip2 | U20553 | 1.0 | 3.9 | 1.0 | 1.0 | 3.9 | 1.0 | 1.0 | 4.7 | 1.5 |
| Cell Cycle-Related Proteins | PK cAMP | Protein kinase, cAMP dependent | X61434 | 4.8 | 2.1 | 4.1 | 1.3 | 0.9 | 3.9 | 3.6 | 1.1 | 1.0 |
| Cytoskeleton & Motility Proteins | NEFM | Neurofilament triplet M protein | X05640 | 1.2 | 3.1 | 1.0 | 1.2 | 3.1 | 0.3 | 1.2 | 4.1 | 1.0 |
| Intracellular Kinase Network Members | WBP6 | WW domain binding protein 6 serine K | U92456 | 1.0 | 3.2 | 1.0 | 1.0 | 3.2 | 1.0 | 1.0 | 3.2 | 1.0 |
| Intracellular Transducers | DIDFF | DNase inhibited by DNA fragmentation | AB009377 | 1.0 | 3.9 | 1.0 | 1.0 | 3.9 | 1.0 | 1.0 | 3.4 | 1.0 |
| Intracellular Transducers | PAR4 | Protease-activated receptor 4 | AF080215 | 1.0 | 3.9 | 1.0 | 1.0 | 3.9 | 1.0 | 1.0 | 3.7 | 1.0 |
| Neuropeptides | orphanin | Nociceptin precursor FQ | D82866 | 0.6 | 3.4 | 2.3 | 0.6 | 3.4 | 0.9 | 0.6 | 2.6 | 3.0 |
| Oncogenes & Tumor Suppressors | mybL2 | Myb-related protein B | X70472 | 1.0 | 3.8 | 1.4 | 1.0 | 3.8 | 0.3 | 1.0 | 4.1 | 1.0 |
| Transcription Activators & Repressors | EKLF | Erythroid Kruppel-like | M97200 | 1.0 | 3.0 | 1.0 | 0.4 | 3.0 | 1.0 | 1.0 | 4.2 | 3.9 |
| Transcription Activators & Repressors | IRF1 | Interferon regulatory factor 1 | M21065 | 2.9 | 4.4 | 2.7 | 0.8 | 3.1 | 1.5 | 10.1 | 10.8 | 1.0 |
| Transcription Activators & Repressors | NF-kB p105 | Nuclear factor kappaB p105 | M57999 | 1.0 | 3.8 | 0.7 | 1.0 | 3.8 | 1.0 | 1.0 | 26.7 | 2.4 |
| Transcription Activators & Repressors | PGDH | D-3-phosphoglycerate dehydrogenase | L21027 | 0.7 | 3.2 | 0.7 | 1.4 | 3.2 | 0.3 | 1.4 | 4.2 | 1.0 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 3C.
Late Genes Up-Regulated by SP, LPS, and Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | occludin | Occludin | U49185 | 3.3 | 1.7 | 4.1 | 0.5 | 3.0 | 3.1 | 1.8 | 2.2 | 3.8 |
| Basic Transcription Factors | CLIM1B | Lim homeobox | U89487 | 1.0 | 1.0 | 3.4 | 1.0 | 1.0 | 3.6 | 1.0 | 1.0 | 3.3 |
| Basic Transcription Factors | PTC1 | Patched homolog 1 | U46155 | 0.3 | 1.0 | 3.4 | 1.0 | 1.0 | 3.4 | 1.0 | 1.0 | 3.8 |
| Death Receptors | CD 30L | CD 30L receptor | U25416 | 0.1 | 0.5 | 6.1 | 0.4 | 0.9 | 3.6 | 1.0 | 1.0 | 3.6 |
| Growth Factor & Chemokine Receptors | CD 40L R | CD 40L receptor | M83312 | 0.4 | 1.0 | 3.0 | 1.0 | 1.0 | 3.9 | 1.0 | 1.2 | 3.4 |
| Growth Factors and Cytokines | Lymphotoxin | Tumor necrosis factor beta | M16819 | 1.0 | 1.0 | 3.8 | 0.3 | 1.0 | 3.8 | 1.0 | 1.2 | 3.8 |
| Growth Factors and Cytokines | prepro-ET-3 | Prepro-endothelin-3 | U32330 | 2.5 | 1.4 | 3.9 | 2.5 | 1.4 | 3.9 | 2.5 | 0.7 | 3.3 |
| Growth Factors and Cytokines | THPO | Thrombopoietin precursor | L34169 | 1.0 | 1.0 | 3.8 | 1.0 | 1.0 | 3.8 | 1.0 | 0.5 | 3.7 |
| Intracellular Transducers | FZD9 | Frizzled homolog 9 | AF033585 | 1.0 | 1.0 | 4.0 | 1.0 | 1.0 | 4.0 | 1.0 | 1.0 | 3.5 |
| Intracellular Transducers | IFNgR2 | Interferon-gamma receptor | S69336 | 1.0 | 1.2 | 3.5 | 1.0 | 3.3 | 1.5 | 1.0 | 3.3 | 3.9 |
| Oncogenes & Tumor Suppressors | TSG101 | Tumor susceptibility protein 101 | U52945 | 2.5 | 1.3 | 7.9 | 0.7 | 0.8 | 3.0 | 1.7 | 2.1 | 4.0 |
| Protease Inhibitors | PAI2 | plasminogen activator inhibitor 2 | X16490 | 0.8 | 1.2 | 3.8 | 0.8 | 0.9 | 3.8 | 0.8 | 1.3 | 4.0 |
| Protease Inhibitors | PI 17 | Protease inhibitor 17 | AJ001700 | 1.7 | 0.4 | 3.8 | 1.7 | 1.0 | 3.8 | 1.7 | 1.0 | 2.7 |
| Proteases | Kininogen | Plasma kallikrein | M58588 | 1.0 | 1.0 | 4.0 | 1.0 | 0.6 | 4.0 | 1.0 | 0.7 | 3.9 |
| Recombination Proteins | DMC1 | Meiotic recombination protein | D64107 | 2.4 | 0.4 | 3.7 | 2.4 | 1.0 | 3.7 | 6.5 | 0.5 | 3.6 |
| Unclassified Proteins | formin 4 | Formin 4 | X62379 | 0.8 | 1.3 | 3.1 | 0.8 | 2.2 | 3.1 | 2.6 | 4.0 | 3.6 |
| Unclassified Proteins | huntingtin-AP | Huntingtin-associated | AJ000262 | 1.1 | 0.8 | 3.5 | 1.4 | 2.2 | 3.5 | 7.4 | 5.1 | 3.1 |
| Unclassified Proteins | PRESENILIN-1 | Presenilin-1 | AF007560 | 1.1 | 0.5 | 3.6 | 1.1 | 1.6 | 3.6 | 6.5 | 1.9 | 3.8 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Supplemental Table 1 ▶ (at www.amjpathol.org) lists genes that were down-regulated by all three stimuli. They include cell adhesion, transcription factors and activators, apoptosis-associated proteins, caspases, cyclins, cell cycle-regulating kinases, nucleotide metabolism, and DNA damage and repair. Together with genes involved in apoptosis and cell survival, several genes encoding growth factors, cytokines and chemokines, and metalloproteinases were also down-regulated. Moreover, inflammation also down-regulated the expression of cytoskeleton and motility proteins. The groups down-regulated by each individual stimulus are presented in Figure 2B ▶ and listed on Supplemental Table 2 ▶ ▶ ▶ that can be found on the AJP website: http://www.amjpathol.org.
Table 5.
Supplemental Table 1 ▶ .
Genes Down-Regulated by SP, LPS, and Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | DSG3 | Desmoglein 3 | U86016 | 1.0 | 3.0 | 0.3 | 2.2 | 3.2 | 0.9 | 1.0 | 3.2 | 0.3 |
| Apoptosis | presenilin 2 | Presenilin 2 | U57324 | 3.4 | 1.5 | 0.4 | 7.2 | 1.5 | 1.1 | 5.7 | 2.8 | 5.5 |
| ATPase Transporters | PMCA | Calcium-transporting ATPase | AF053471 | 3.1 | 0.5 | 2.1 | 1.9 | 0.3 | 3.3 | 4.3 | 0.1 | 1.0 |
| Basic Transcription Factors | EBF2 | Early B-cell factor 2 | U71189 | 3.5 | 1.0 | 0.5 | 3.2 | 1.0 | 0.3 | 3.2 | 1.8 | 0.3 |
| Basic Transcription Factors | PITX3 | Pituitary homeobox 3 protein | AF005772 | 4.3 | 7.8 | 1.6 | 4.6 | 1.9 | 0.5 | 3.1 | 2.9 | 0.4 |
| Caspases | ICE | Interleukin-converting enzyme | L28095 | 3.3 | 1.0 | 0.9 | 3.3 | 1.3 | 0.7 | 10.3 | 2.9 | 0.5 |
| Cell Cycle-Regulating Kinases | CKS-2 | Cyclin-dependent K regulatory subunit 2 | AA289122 | 0.9 | 3.6 | 0.7 | 0.9 | 3.6 | 0.7 | 0.8 | 4.7 | 0.7 |
| Cyclins | CCNA1 | G2/mitotic-specific cyclin A1 | X84311 | 8.2 | 29.9 | 3.0 | 1.7 | 5.5 | 0.5 | 1.9 | 2.0 | 4.1 |
| Cytoskeleton & Motility Proteins | COL6A1 | Collagen 6 alpha 1 subunit | X66405 | 8.3 | 7.2 | 1.5 | 3.1 | 2.0 | 1.0 | 1.5 | 3.4 | 3.6 |
| Cytoskeleton & Motility Proteins | CFL1 | Non-muscle cofilin 1 | D00472 | 2.9 | 12.5 | 1.8 | 2.1 | 3.2 | 1.2 | 1.6 | 3.4 | 6.2 |
| DNA Damage/Repair | UBE2B | Ubiquitin-conjugating enzyme E2 17-kDa | X96859 | 4.3 | 5.0 | 1.4 | 1.9 | 3.7 | 0.8 | 0.8 | 3.4 | 1.4 |
| DNA Damage/Repair | RAD23 | Excision repair protein homolog A | X92410 | 3.8 | 1.9 | 2.4 | 3.3 | 2.3 | 1.3 | 1.9 | 0.8 | 3.2 |
| Extracellular Transporters | LCAT | Lecithin-cholesterol acyltransferase | J05154 | 2.3 | 3.0 | 0.3 | 3.4 | 2.5 | 0.7 | 1.0 | 0.9 | 8.0 |
| Growth Factors and Cytokines | FGF-7 | Keratinocyte growth factor | Z22703 | 3.8 | 2.7 | 0.6 | 4.4 | 2.6 | 0.4 | 3.8 | 1.1 | 0.6 |
| Growth Factors and Cytokines | IGF-II | Insulin-like growth factor II precursor | M14951 | 3.8 | 4.4 | 2.6 | 1.9 | 3.5 | 0.5 | 2.7 | 3.5 | 1.0 |
| Intracellular Kinase | LIM | Domain kinase 1 | U15159 | 0.4 | 4.1 | 0.4 | 0.7 | 4.5 | 0.7 | 0.4 | 5.0 | 0.4 |
| Ion Channels (Membrane) | K 2 | Potassium channel, subfamily K | U73488 | 11.4 | 1.1 | 1.0 | 3.1 | 0.9 | 1.0 | 3.1 | 0.2 | 1.0 |
| Metalloproteinases | MMP14 | Matrix metalloproteinase 14 | X83536 | 3.9 | 2.6 | 0.8 | 4.2 | 2.2 | 0.8 | 2.5 | 3.7 | 0.8 |
| Metalloproteinases | MMP2 | Matrix metalloproteinase 2 | M84324 | 4.2 | 1.9 | 1.4 | 7.7 | 2.3 | 0.6 | 3.1 | 1.2 | 3.8 |
| Nucleotide Metabolism | PNMTase | Phenylethanolamine N-methyltransferase | L12687 | 3.2 | 0.7 | 0.6 | 2.6 | 0.4 | 3.4 | 4.2 | 0.3 | 1.0 |
| Symporters & Antiporters | VAT-1 | Synaptic vesicle membrane | X95562 | 4.5 | 1.4 | 1.4 | 6.3 | 3.8 | 2.4 | 10.0 | 0.7 | 0.7 |
| Transcription Activators & Repressors | HSF 2 | Heat shock transcription factor 2 | X61754 | 0.5 | 3.4 | 3.0 | 0.9 | 12.4 | 3.1 | 0.6 | 5.5 | 1.0 |
| Transcription Activators & Repressors | NF-kB p65 | NF-kappa-B transcription factor p65 | M61909 | 2.4 | 8.6 | 1.3 | 1.0 | 3.0 | 0.7 | 2.4 | 1.8 | 3.0 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 6A.
Supplemental Table 2A ▶ .
Genes Down-Regulated by Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | AG | SP | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | EPOR | erythropoietin receptor precursor | J04843 | 3 | 2 | 1 | 2 | 3 | 2 | 1 | 1 | 1 |
| Apoptosis | nur77 | early response protein; nuclear hormone receptor | J04113 | 4 | 1 | 1 | 2 | 1 | 2 | 2 | 0 | 1 |
| Basic Transcription Factors | HOX-4.5 | Homeobox Protein 4.5 | S94664 | 2 | 8 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| Basic Transcription Factors | HOX10 | homeobox protein 10 | L34808 | 1 | 4 | 1 | 2 | 1 | 2 | 1 | 2 | 1 |
| Basic Transcription Factors | NAB1 | transcription repressor | U47008 | 1 | 3 | 0 | 2 | 2 | 1 | 2 | 1 | 1 |
| Basic Transcription Factors | HHEX | hematopoietically expressed homeobox protein | Z21524 | 3 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| Bcl Family Proteins | BAK1 | bcl-2 homologous antagonist/killer | Y13231 | 3 | 2 | 1 | 2 | 1 | 1 | 2 | 0 | 1 |
| Caspases | ICE | interleukin-converting enzyme | L28095 | 4 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 |
| Cell-Cell Adhesion Receptors | N-cadherin | neural cadherin precursor | M31131 | 3 | 1 | 1 | 3 | 1 | 1 | 2 | 2 | 1 |
| Complement Receptors | C5A receptor R | C5A receptor | L05630 | 3 | 1 | 1 | 2 | 1 | 1 | 3 | 0 | 1 |
| Cytoskeleton & Motility | COL10A1 | collagen 10 alpha 1 | X67348 | 3 | 4 | 1 | 2 | 2 | 1 | 1 | 2 | 1 |
| Cytoskeleton & Motility | MLC3NM | non-muscle myosin light chain 3 | U04443 | 6 | 5 | 2 | 3 | 2 | 1 | 1 | 2 | 2 |
| Cytoskeleton & Motility | unconventional myosin | unconventional myosin VI | U49739 | 3 | 4 | 1 | 2 | 3 | 1 | 1 | 1 | 2 |
| Death Kinases | RAC-PK-alpha | serine/threonine kinase | M94335 | 4 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| DNA Damage/Repair | XPAC | xeroderma pigmentosum group A correcting protein | X74351 | 3 | 3 | 1 | 2 | 2 | 1 | 2 | 2 | 1 |
| DNA Damage/Repair | Ung1 | uracil-DNA glycosylase | X99018 | 3 | 3 | 1 | 2 | 1 | 1 | 2 | 2 | 1 |
| DNA Damage/Repair | HR21spA | protein involved in DNA double-strand break repair | D49429 | 3 | 5 | 0 | 1 | 2 | 1 | 1 | 2 | 1 |
| DNA Damage/Repair | ERCC1 | DNA excision repair protein | X07414 | 5 | 5 | 1 | 2 | 2 | 1 | 2 | 2 | 2 |
| Growth Factor & Chemokine Receptors | C-C chemokine R 1 | monocyte chemoattractant protein 1 receptor | U56819 | 3 | 1 | 1 | 2 | 1 | 2 | 1 | 0 | 1 |
| Heat Shock Proteins | MTJ1 | DNAJ-like heat-shock protein from mouse tumor | L16953 | 1 | 3 | 1 | 0 | 2 | 2 | 0 | 0 | 1 |
| Heat Shock Proteins | HSP84 | 84-kDa heat shock protein | M36829 | 1 | 3 | 0 | 1 | 2 | 1 | 0 | 1 | 2 |
| Hormone Receptors | somatostatin R 2 | somatostatin receptor 2 | M81832 | 3 | 1 | 1 | 3 | 2 | 1 | 3 | 2 | 1 |
| Prostaglandin Receptors | prostaglandin I R | prostaglandin I receptor (IP) | D26157 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Neurotransmitter Receptors | acetylcholine R alpha 7 | acetylcholine receptor alpha 7 neural | L37663 | 2 | 3 | 1 | 2 | 2 | 1 | 2 | 3 | 1 |
| Nucleotide Metabolism | HDC | histidine decarboxylase | X57437 | 3 | 3 | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Oncogenes & Tumor Suppressors | Gli | Gli oncogene; zinc finger transcription factor | S65038 | 1 | 4 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Oncogenes & Tumor Suppressors | fos-L2 | fos-related antigen 2 | X83971 | 1 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Oncogenes & Tumor Suppressors | NDK B | nucleoside diphosphate kinase B | X68193 | 1 | 4 | 1 | 1 | 2 | 1 | 0 | 0 | 1 |
| Oncogenes & Tumor Suppressors | c-Cbl | c-Cbl proto-oncogene | X57111 | 2 | 5 | 1 | 2 | 3 | 1 | 1 | 2 | 1 |
| Oncogenes & Tumor Suppressors | c-Fgr | c-Fgr proto-oncogene | X52191 | 2 | 5 | 0 | 1 | 3 | 1 | 1 | 1 | 2 |
| Ribosomal Proteins | LAMR1 | lamimin receptor 1 | J02870 | 1 | 4 | 1 | 1 | 3 | 1 | 1 | 2 | 1 |
| Symporters & Antiporters | VAT-1 | Synaptic membrane protein | X95562 | 1 | 5 | 0 | 1 | 3 | 1 | 1 | 1 | 1 |
| Transcription Activators & Repressors | BARX1 | homeodian transcription factor | Y07960 | 5 | 10 | 1 | 1 | 2 | 1 | 1 | 2 | 1 |
| Transcription Activators & Repressors | OCT6 | octamer-binding transcription factor 6 | X56959 | 4 | 6 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Transcription Activators & Repressors | RXR gamma | retinoic acid receptor gamma | M84819 | 1 | 3 | 1 | 1 | 3 | 0 | 1 | 1 | 1 |
| Transcription Activators & Repressors | Stat6 | signal transducer and activator of transcription 6 | L47650 | 5 | 3 | 1 | 1 | 2 | 0 | 1 | 2 | 1 |
| Transcription Activators & Repressors | EED | embryonic ectoderm development protein | U78103 | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| Unclassified Proteins | FMN | formin | X53599 | 2 | 4 | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Unclassified Proteins | KL | klotho protein | AB005141 | 1 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Unclassified Proteins | COBL | cordon-bleu protein | U26967 | 2 | 3 | 1 | 2 | 2 | 1 | 1 | 2 | 1 |
| Unclassified Proteins | FBN1 | fibrillin 1 precursor | L29454 | 3 | 3 | 1 | 2 | 2 | 1 | 1 | 2 | 2 |
| Xenobiotic Transporters | GPX3 | plasma glutathione peroxidase precursor | U13705 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 1 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 6B.
Supplemental Table 2B ▶ .
Genes Down-Regulated by Substance P-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | AG | SP | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | semaphorin N | semaphorin N | AF036585 | 0 | 1 | 0 | 3 | 2 | 0 | 2 | 2 | 1 |
| Apoptosis | mPIN | protein inhibitor of neuronal nitric oxide synthase | AF020185 | 1 | 1 | 0 | 5 | 4 | 1 | 1 | 1 | 2 |
| Apoptosis | DAD1 | defender against cell death 1 | U83628 | 2 | 1 | 0 | 5 | 2 | 1 | 1 | 0 | 1 |
| Aspartic Proteases | cathepsin D | cathepsin D | X53337 | 1 | 1 | 1 | 4 | 2 | 0 | 2 | 2 | 1 |
| Bcl Family | BAD | BCL2 binding component 6 | L37296 | 1 | 1 | 1 | 4 | 2 | 1 | 2 | 1 | 3 |
| Bcl Family | Mcl-1 | induced myeloid leukemia cell differentiation | U35623 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 1 | 1 |
| Bcl Family | BCLW | B-cell lymphoma protein W | U59746 | 1 | 1 | 0 | 4 | 2 | 1 | 2 | 1 | 1 |
| Bcl Family | BID | BH3 interacting domain death agonist | U75506 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 0 | 1 |
| Cell Cycle-Regulating Kinases | BUB1B | mitotic checkpoint protein kinase | AF107296 | 1 | 3 | 1 | 1 | 4 | 1 | 2 | 1 | 1 |
| Cell Signaling & Extracellular Communication | AMPA 1 | glutamate receptor; ionotropic | X57497 | 1 | 1 | 0 | 3 | 1 | 1 | 2 | 1 | 1 |
| Cytoskeleton & Motility | THB53 | thrombospondin 3 precursor | L04302 | 1 | 1 | 0 | 3 | 2 | 1 | 2 | 2 | 2 |
| Cytoskeleton & Motility | CK13 | type I cytoskeletal keratin 13 | U13921 | 0 | 0 | 0 | 3 | 1 | 1 | 2 | 0 | 1 |
| Cytoskeleton & Motility | LAMB3 | laminin beta 3 subunit precursor | U43298 | 1 | 1 | 1 | 3 | 2 | 1 | 2 | 1 | 0 |
| Death Receptor Ligands | TRAIL | TNF-related apoptosis inducing ligand | U37522 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 1 | 2 |
| Death Receptors | adenosine A1M | adenosine A1M receptor | U05671 | 1 | 1 | 0 | 3 | 2 | 1 | 2 | 1 | 1 |
| Death Receptors | RXR-beta | RXR-beta cis-11-retinoic acid receptor | X66224 | 2 | 2 | 1 | 4 | 1 | 1 | 1 | 1 | 1 |
| DNA Damage/Repair | MSH2 | DNA mismatch repair protein | U21011 | 1 | 1 | 1 | 4 | 3 | 1 | 2 | 1 | 2 |
| DNA Damage/Repair | Atm | ataxia telongiectasia murine homolog | U43678 | 1 | 0 | 0 | 3 | 1 | 1 | 2 | 1 | 1 |
| DNA Synthesis, Recombination & Repair Protein | MECP2 | methyl-CpG-binding protein 2 | AF072251 | 1 | 2 | 1 | 3 | 2 | 1 | 2 | 2 | 1 |
| G Protein-Coupled Receptors | adenosine A2b R | adenosine A2b receptor | U05673 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 2 | 2 |
| Growth Factor & Chemokine Receptors | CD28 precursor | T-cell-specific surface glycoprotein | M34563 | 2 | 1 | 0 | 4 | 2 | 1 | 1 | 1 | 2 |
| Growth Factor & Chemokine Receptors | GCSF | granulocyte colony—stimulating factor receptor | M58288 | 2 | 2 | 1 | 6 | 4 | 1 | 1 | 0 | 1 |
| Growth Factor & Chemokine Receptors | IGFR II | insulin-like growth factor receptor II | U04710 | 2 | 1 | 0 | 4 | 2 | 1 | 2 | 1 | 1 |
| Growth Factor & Chemokine Receptors | activin type I R | activin type I receptor | Z31663 | 2 | 2 | 0 | 5 | 3 | 1 | 1 | 1 | 1 |
| Growth Factors and Cytokines | FGF15 | fibroblast growth factor 15 | AF007268 | 1 | 0 | 0 | 4 | 1 | 1 | 2 | 1 | 3 |
| Growth Factors and Cytokines | WNT7A | wingless-related MMTV integration site 7A | M89801 | 2 | 1 | 2 | 3 | 1 | 1 | 2 | 1 | 1 |
| Growth Factors and Cytokines | WNT10A | wingless-related MMTV integration site 10A | U61969 | 1 | 0 | 1 | 4 | 1 | 1 | 2 | 1 | 3 |
| Growth Factors and Cytokines | FGF-7 | keratinocyte growth factor | Z22703 | 2 | 1 | 0 | 4 | 1 | 1 | 2 | 1 | 1 |
| Prostaglandin Receptors | prostaglandin F R | prostaglandin F receptor | D17433 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 1 | 1 |
| Hormone Receptors | insulin R | insulin receptor | J05149 | 2 | 2 | 2 | 3 | 3 | 1 | 2 | 3 | 1 |
| Hormone Receptors | PRLR2 | prolactin receptor | M22959 | 1 | 1 | 0 | 3 | 1 | 1 | 2 | 1 | 1 |
| Interleukin & Interferon Receptors | interleukin-8 R | interleukin-8 receptor | D17630 | 2 | 2 | 0 | 4 | 3 | 1 | 3 | 2 | 1 |
| Interleukin & Interferon Receptors | IL-5R alpha | interleukin-5 receptor alpha subunit | D90205 | 3 | 1 | 1 | 4 | 1 | 2 | 3 | 1 | 1 |
| Interleukins & Interferons | IL-10 | interleukin 10 | M37897 | 1 | 0 | 0 | 5 | 1 | 0 | 2 | 1 | 2 |
| Intracellular Transducers | RHOD | aplysia ras-related homolog D | D89821 | 0 | 0 | 0 | 4 | 2 | 1 | 1 | 1 | 1 |
| Ion Channels (Ligand-Gated) | nicotinic R | nicotinic acetylcholine receptor | M14537 | 1 | 2 | 1 | 2 | 3 | 1 | 1 | 1 | 1 |
| Ion Channels (Membrane & Transporters) | potassium M alpha | potassium large conductance calcium-activated | L16912 | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 1 |
| Ion Channels (Voltage-Gated) | CCHB3 | calcium channel (dihydropyridine-sensitive; L-type) | U20372 | 1 | 1 | 0 | 3 | 2 | 1 | 1 | 1 | 2 |
| Metalloproteinases | MMP14 | matrix metalloproteinase 14 precursor | X83536 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 1 | 1 |
| Nucleotide Metabolism | PAH | phenylalanine-4-hydroxylase | X51942 | 2 | 1 | 0 | 4 | 1 | 1 | 1 | 1 | 2 |
| Oncogenes & Tumor Suppressors | abl | abl proto-oncogene | L10656 | 1 | 2 | 0 | 2 | 3 | 0 | 1 | 1 | 1 |
| Oncogenes & Tumor Suppressors | R-ras | R-ras protein | M21019 | 1 | 1 | 1 | 6 | 2 | 0 | 1 | 0 | 2 |
| Oncogenes & Tumor Suppressors | Lfc | Lfc proto-oncogene | U28495 | 1 | 1 | 0 | 3 | 1 | 0 | 1 | 0 | 1 |
| Oncogenes & Tumor Suppressors | EB1 | EB1 APC-binding protein | U51196 | 1 | 1 | 0 | 3 | 2 | 1 | 1 | 1 | 1 |
| Oncogenes & Tumor Suppressors | Met | Met proto-oncogene | Y00671 | 1 | 1 | 1 | 5 | 2 | 0 | 2 | 1 | 2 |
| Oncogenes & Tumor Suppressors | c-Mpl | thrombopoietin receptor | Z22649 | 2 | 3 | 1 | 4 | 2 | 1 | 2 | 2 | 2 |
| Proteosomal Proteins | Macropain | Proeasome Component C8 | AF055983 | 1 | 1 | 1 | 5 | 2 | 1 | 2 | 1 | 2 |
| Recombination Proteins | RAG2 | V(D)J recombination activating protein | M64796 | 1 | 1 | 0 | 3 | 3 | 1 | 2 | 1 | 2 |
| Serine Proteases | EC 3.4.21.7 | Plasminogen Precursor (EC 3.4.21.7) | J04766 | 1 | 0 | 0 | 4 | 1 | 1 | 2 | 2 | 2 |
| Stress Response Proteins | EI24 | etoposide induced p53 responsive | U41751 | 0 | 2 | 1 | 1 | 3 | 1 | 1 | 1 | 2 |
| Unclassified Proteins | NGF-inducible protein | NGF-inducible protein TIS21 | M64292 | 3 | 2 | 1 | 3 | 2 | 1 | 2 | 1 | 1 |
| Unclassified Proteins | EC 3.1.3.48 | Putative tyrosine phosphatase | U92437 | 1 | 1 | 1 | 4 | 2 | 1 | 2 | 0 | 1 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 6C.
Supplemental Table 2C ▶ .
Genes Down-Regulated by LPS-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | AG | SP | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| ABC Transporters | ABC8 | ATP-binding casette 8 | U34920 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 4 |
| Adhesion | semophorin J | semaphorin J | U69535 | 2 | 2 | 1 | 3 | 1 | 0 | 7 | 2 | 1 |
| Basic Transcription Factors | JUMONJI | Jumonji protein | D31967 | 1 | 2 | 0 | 1 | 2 | 0 | 4 | 7 | 1 |
| Basic Transcription Factors | PMX2 | paired mesoderm homeobox protein 2 | X52875 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 |
| Basic Transcription Factors | RPX | anterior-restricted homeobox | X80040 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 5 | 1 |
| Caspases | CASP2 | caspase-2 precursor | D28492 | 2 | 1 | 1 | 2 | 1 | 2 | 4 | 0 | 1 |
| Cyclins | CCNB2 | G2/M-specific cyclin B2 | X66032 | 1 | 3 | 1 | 2 | 2 | 1 | 2 | 4 | 3 |
| Death Receptors | adenosine A3 R | adenosine A3 receptor | L20331 | 1 | 1 | 0 | 3 | 2 | 1 | 3 | 1 | 1 |
| DNA Damage/Repair | MmRad52 | DNA repair protein Rad52 homolog | Z32767 | 2 | 2 | 1 | 2 | 1 | 1 | 0 | 3 | 2 |
| DNA Damage/Repair | MmMre11a | putative endo/exonuclease | U58987 | 2 | 2 | 1 | 2 | 1 | 1 | 3 | 4 | 1 |
| G Proteins | E13g | guanine nucleotide binding protein | M36777 | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 6 | 3 |
| Growth Factors and Cytokines | GPI | glucose-6-phosphate isomerase | M14220 | 3 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 |
| Growth Factors and Cytokines | FGF14 | fibroblast growth factor 14 | U66204 | 1 | 1 | 0 | 2 | 1 | 1 | 3 | 1 | 1 |
| Hormone Receptors | VIP R 2 | vasoactive intestinal peptide receptor 2 | D28132 | 0 | 2 | 0 | 1 | 3 | 1 | 4 | 3 | 1 |
| Hormone Receptors | oxytocin R | oxytocin receptor | D86599 | 1 | 2 | 0 | 2 | 2 | 1 | 4 | 2 | 3 |
| Hormone Receptors | androgen R | androgen receptor | X53779 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 0 | 1 |
| Hormones | CRH binding protein | corticotropin releasing hormone binding protein | U33323 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 6 | 1 |
| Interleukin & Interferon Receptors | IL-7R alpha | interleukin-7 receptor alpha | M29697 | 2 | 2 | 0 | 3 | 2 | 0 | 3 | 1 | 1 |
| Interleukins & Interferons | IFN-BETA | Interferon beta precursor | K00020 | 2 | 3 | 1 | 2 | 2 | 0 | 3 | 1 | 1 |
| Ion Channels (Ligand-Gated) | KVLQT1 | voltage-gated potassium channel protein KQT-like 1 | U70068 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 5 |
| Metalloproteinases | MMENDO | membrane metallo endopeptidase | M81591 | 2 | 2 | 1 | 3 | 2 | 1 | 1 | 4 | 1 |
| Neurotransmitter Receptors | glutamate R delta 1 | glutamate receptor, ionotropic, delta 1 | D10171 | 1 | 2 | 0 | 2 | 3 | 1 | 4 | 3 | 3 |
| Neurotransmitter Receptors | GRP R | gastrin releasing peptide receptor | M57922 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 3 | 2 |
| Neurotransmitter Receptors | 5-HT 2c | 5-hydroxytryptamine receptor 2c | Z15119 | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 1 | 1 |
| Oncogenes & Tumor Suppressors | RB1 | retinoblastoma-associated protein 1 | M26391 | 1 | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 3 |
| Oncogenes & Tumor Suppressors | HRA | thyroid hormone receptor alpha 1 | X51983 | 1 | 2 | 1 | 2 | 2 | 1 | 3 | 5 | 2 |
| Transcription Activators & Repressors | DLK | delta-like protein precursor | L12721 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 3 | 2 |
| Transcription Activators & Repressors | DBP | D-binding protein | U29762 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 4 |
| Transcription Activators & Repressors | CTCF | transcription factor CTCF (11 zinc fingers) | U51037 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 3 | 1 |
| Transcription Activators & Repressors | Hox-3.1 | homeobox protein 3.1 | X07439 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 8 | 1 |
| Transcription Activators & Repressors | Hox-8 | homeobox protein 8 | X59252 | 0 | 0 | 0 | 2 | 2 | 0 | 4 | 1 | 3 |
| Unclassified Proteins | DGCR6 | DiGeorge syndrome chromosome region 6 protein | AF021031 | 0 | 1 | 1 | 2 | 3 | 1 | 1 | 2 | 3 |
| Unclassified Proteins | LEP | leptin precursor | U22421 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | 4 | 2 |
| Xenobiotic Transporters | glutathione reductase | glutathione reductase | X76341 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Bladder Genes Uniquely Regulated by LPS-, SP-, and Ag-Induced Inflammation
Venn diagram analysis (Figure 2) ▶ also indicates that 82 genes were up-regulated in response to antigen alone, six genes were up-regulated by SP alone, and 39 genes were up-regulated specifically by LPS. (Supplemental Table 3A ▶ ▶ and Tables 4A and 4B ▶ ▶ )
Table 7.
Supplemental Table 3A ▶ .
Genes Up-Regulated by Antigen-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | cadherin 5 | vascular epithelial cadherin precursor | X83930 | 2.5 | 2.8 | 4.1 | 0.8 | 0.8 | 1.7 | 1.1 | 1.7 | 2.1 |
| Adhesion | semaphorin III | semaphorin IIIC | X85994 | 1.8 | 6.5 | 5.7 | 0.2 | 0.5 | 1.4 | 0.4 | 1.4 | 1.3 |
| Apoptosis | PCD2 | programmed cell death 2 | U10903 | 1.4 | 1.7 | 6.5 | 1.0 | 2.5 | 1.0 | 1.0 | 1.5 | 1.0 |
| Apoptosis | Flt3/Flk2 | fms-related tyrosine kinase 3 | U04807 | 0.7 | 0.6 | 4.1 | 0.4 | 0.7 | 1.0 | 0.9 | 1.2 | 0.5 |
| Apoptosis | interleukin-1 R | interleukin-1 receptor | M20658 | 0.7 | 1.0 | 4.0 | 0.7 | 0.7 | 1.9 | 0.6 | 0.3 | 0.4 |
| Basic Transcription Factors | HES1 | helix-loop-helix factor hairy & enhancer of | D16464 | 3.2 | 3.7 | 6.8 | 0.6 | 0.5 | 1.9 | 0.7 | 0.5 | 0.5 |
| Basic Transcription Factors | TCF8 | transcription factor 8 | D76432 | 1.3 | 7.1 | 4.3 | 0.6 | 0.4 | 1.5 | 1.1 | 0.6 | 0.9 |
| Basic Transcription Factors | OCT1 | octamer-binding transcription factor 1 | X56230 | 1.8 | 3.9 | 5.1 | 0.2 | 0.5 | 1.5 | 0.3 | 1.5 | 1.3 |
| Basic Transcription Factors | BKLF | CACCC Box-binding protein | U36340 | 2.9 | 3.4 | 5.3 | 1.0 | 1.0 | 2.6 | 1.0 | 1.0 | 2.6 |
| Basic Transcription Factors | EYA2 | eyes absent homolog 2 | U71208 | 2.0 | 3.1 | 4.2 | 0.5 | 2.1 | 1.8 | 0.8 | 2.0 | 1.7 |
| Basic Transcription Factors | HNF3A | hepatocyte nuclear factor 3 alpha | X74936 | 1.6 | 1.4 | 4.6 | 0.6 | 0.9 | 1.2 | 1.2 | 2.3 | 1.2 |
| Basic Transcription Factors | NAB2 | NGFI-A binding protein-2 | U47543 | 0.2 | 0.2 | 4.4 | 0.4 | 0.7 | 0.9 | 1.0 | 1.8 | 0.2 |
| Basic Transcription Factors | LDB1 | LIM domain-binding protein 1 | U69270 | 2.3 | 1.0 | 3.2 | 1.5 | 1.0 | 2.4 | 1.5 | 1.0 | 2.3 |
| Bcl Family Proteins | BCLW | B-cell lymphoma protein W | U59746 | 0.6 | 0.3 | 5.2 | 0.3 | 0.5 | 1.5 | 0.5 | 1.6 | 0.4 |
| Bcl Family Proteins | NIP3 | adenoviral E1B-interacting protein | AF041054 | 2.2 | 0.5 | 4.8 | 0.2 | 1.2 | 1.5 | 0.3 | 1.1 | 1.9 |
| Bcl Family Proteins | Mcl-1 | myeloid leukemia cell | U35623 | 1.3 | 1.2 | 4.1 | 1.1 | 0.6 | 1.2 | 0.4 | 0.4 | 0.9 |
| CDK Inhibitors | SATB1 | special AT-rich sequence-binding | U05252 | 2.3 | 2.2 | 4.2 | 0.3 | 0.7 | 2.7 | 0.8 | 1.1 | 2.0 |
| Cell Signaling | EPIM | epimorphin | D10475 | 4.1 | 1.5 | 4.7 | 0.9 | 0.6 | 1.0 | 2.5 | 1.8 | 1.2 |
| Cell Surface Antigens | OSF2 | osteoblast-specific factor 2 | D13664 | 1.1 | 0.6 | 4.6 | 0.4 | 0.4 | 1.3 | 0.9 | 1.4 | 0.8 |
| Cell Surface Antigens | m-numb | m-numb | U70674 | 2.4 | 0.9 | 4.4 | 1.0 | 0.3 | 1.3 | 1.0 | 1.0 | 2.0 |
| Cell Surface Antigens | PCP-4 | brain specific poly peptide | X17320 | 0.8 | 1.6 | 4.0 | 0.4 | 0.7 | 0.9 | 1.3 | 1.0 | 0.5 |
| Cyclins | CCNG | G2/M-specific cyclin G | Z37110 | 10.1 | 3.9 | 4.1 | 0.6 | 0.6 | 1.8 | 1.3 | 2.2 | 1.3 |
| Death Receptor | RIP | receptor interacting protein | U25995 | 1.3 | 1.9 | 4.6 | 0.2 | 0.6 | 1.5 | 0.6 | 1.9 | 0.8 |
| Death Receptor | FAF1 | fas-associated factor 1 | U39643 | 0.6 | 0.7 | 4.3 | 0.3 | 1.5 | 1.1 | 0.5 | 1.8 | 0.4 |
| Death Receptor | STAM | signal transducing adaptor molecule | U43900 | 0.6 | 0.9 | 4.1 | 1.2 | 0.5 | 1.1 | 1.2 | 0.8 | 0.3 |
| Death Receptors | TNFR1 | tumor necrosis factor receptor 1 | X57796 | 0.3 | 0.3 | 4.4 | 0.2 | 0.6 | 1.1 | 0.5 | 1.5 | 0.2 |
| Exocytosis Proteins | MDR1 | multidrug resistance protein 1 | M14757 | 0.7 | 1.1 | 7.5 | 0.5 | 0.7 | 0.9 | 1.6 | 0.8 | 0.4 |
| Extracellular Matrix Proteins | MAOBP | myelin-associated oligodendrocytic | U81317 | 0.6 | 1.2 | 6.7 | 0.3 | 0.7 | 1.0 | 0.5 | 1.7 | 0.4 |
| Extracellular Matrix Proteins | myelin protein | myelin protein zero | M62860 | 0.2 | 1.7 | 4.2 | 0.2 | 1.1 | 1.1 | 0.5 | 2.4 | 0.2 |
| Growth Factor & Chemokine Receptors | Ednrb | endothelin b receptor | U32329 | 1.9 | 7.2 | 3.4 | 0.1 | 0.2 | 0.5 | 1.1 | 1.3 | 1.6 |
| Growth Factors and Cytokines | TGFB2 | transforming growth factor beta 2 | X57413 | 6.1 | 3.5 | 3.2 | 2.5 | 0.4 | 1.5 | 2.5 | 1.3 | 0.8 |
| Growth Factors and Cytokines | MADR2 | Mad related protein 2 | U60530 | 5.7 | 3.9 | 3.0 | 0.5 | 0.6 | 2.3 | 0.3 | 1.4 | 0.4 |
| Growth Factors and Cytokines | IGFIA | insulin-like growth factor-IA | X04480 | 0.9 | 6.0 | 2.4 | 0.4 | 0.6 | 1.2 | 0.4 | 0.7 | 0.6 |
| Growth Factors and Cytokines | IGFBP3 | IGFBP 3 | X81581 | 1.6 | 4.7 | 4.8 | 0.9 | 0.6 | 1.9 | 1.6 | 1.7 | 1.0 |
| Growth Factors and Cytokines | GLYCAM 1 | endothelial ligand for L-selectin | M93428 | 0.4 | 0.6 | 4.3 | 0.4 | 0.6 | 1.7 | 0.5 | 2.0 | 0.3 |
| Heat Shock Proteins | HSP60 | heat shock protein 60-kDa | X53584 | 2.1 | 1.5 | 6.4 | 0.5 | 0.5 | 1.1 | 0.5 | 2.7 | 1.9 |
| Heat Shock Proteins | HSP27 | heat shock 27-kDa protein | U03560 | 1.9 | 0.8 | 6.1 | 0.2 | 0.5 | 1.2 | 0.6 | 2.2 | 1.6 |
| Heat Shock Proteins | MTJ1 | DNAJ-like heat-shock | L16953 | 2.7 | 1.5 | 4.4 | 0.6 | 0.2 | 2.3 | 2.5 | 0.6 | 2.3 |
| Heat Shock Proteins | HSP105 | 105-kDa heat shock protein | L40406 | 0.4 | 1.0 | 3.1 | 2.3 | 0.5 | 1.4 | 1.7 | 2.7 | 2.2 |
| Hormone Receptors | CGRP-R | CGRP-R | AF028242 | 1.3 | 1.7 | 4.6 | 0.5 | 1.1 | 0.9 | 0.5 | 0.9 | 0.9 |
| Intracellular Adaptors | Crk | Crk adaptor protein | S72408 | 6.2 | 4.7 | 2.3 | 1.0 | 1.6 | 0.5 | 2.1 | 1.8 | 0.9 |
| Intracellular Kinases | CSNK2A1-RS4 | casein kinase II alpha 1 | U51866 | 3.2 | 5.8 | 2.0 | 0.1 | 1.0 | 0.7 | 0.1 | 1.0 | 0.6 |
| Intracellular Protein Phosphatases | PTH | protein tyrosine phosphatase | D83966 | 3.7 | 4.9 | 2.8 | 1.2 | 1.7 | 0.7 | 1.8 | 2.6 | 1.0 |
| Intracellular Transducers | Frizzled-3 | frizzled homolog 3 | U43205 | 8.2 | 1.6 | 2.9 | 0.3 | 1.5 | 2.5 | 0.9 | 1.2 | 1.1 |
| Intracellular Transducers | RXRA | retinoid X R alpha | M84817 | 4.3 | 2.3 | 2.2 | 0.6 | 1.3 | 1.1 | 0.2 | 1.3 | 1.6 |
| Ion Channels (Ligand-Gated) | NMDA2B | glutamate receptor | D10651 | 2.0 | 2.4 | 4.8 | 0.4 | 0.4 | 2.2 | 0.9 | 1.1 | 1.7 |
| Ion Channels (Ligand-Gated) | ACh R | acetylcholine receptor delta | K02582 | 2.4 | 1.4 | 4.4 | 0.1 | 0.5 | 1.6 | 0.4 | 1.0 | 2.1 |
| Ion Channels (Ligand-Gated) | KVLQT1 | potassium channel-KQT-like 1 | U70068 | 1.7 | 1.2 | 4.2 | 0.2 | 0.5 | 1.0 | 1.2 | 2.7 | 1.2 |
| Ion Channels (Ligand-Gated) | nicotinic R | nicotinic acetylcholine receptor | M14537 | 1.8 | 1.9 | 4.1 | 0.6 | 0.5 | 2.3 | 0.9 | 2.0 | 1.3 |
| Oncogenes & Tumor Suppressors | ezrin | NF-2 (merlin) related filament | X60671 | 5.4 | 1.5 | 4.1 | 0.6 | 0.4 | 1.4 | 0.6 | 2.0 | 0.2 |
| Oncogenes & Tumor Suppressors | TJP1 | tight junction protein ZO1 | D14340 | 4.9 | 3.8 | 3.0 | 0.3 | 0.9 | 1.8 | 1.0 | 2.3 | 2.5 |
| Oncogenes & Tumor Suppressors | HRA | thyroid hormone R alpha 1 | X51983 | 4.4 | 2.2 | 2.6 | 2.2 | 0.5 | 1.7 | 1.8 | 2.8 | 2.1 |
| Oncogenes & Tumor Suppressors | VEGFR1 | vascular endothelial growth factor R1 | L07297 | 3.8 | 0.7 | 6.5 | 1.5 | 0.7 | 1.0 | 2.2 | 0.6 | 1.1 |
| Oncogenes & Tumor Suppressors | Fli-1 | Fli-1 ets-related | X59421 | 3.2 | 0.8 | 6.0 | 0.9 | 0.7 | 1.8 | 0.8 | 0.9 | 0.5 |
| Oncogenes & Tumor Suppressors | MCSF | macrophage colony stimulating F 1 | X05010 | 1.5 | 0.5 | 5.4 | 0.2 | 0.4 | 2.7 | 0.3 | 0.7 | 1.0 |
| Oncogenes & Tumor Suppressors | Elk-1 | Elk-1 ets-related | X87257 | 1.0 | 0.3 | 4.6 | 1.2 | 0.3 | 0.8 | 1.1 | 0.8 | 0.6 |
| Oncogenes & Tumor Suppressors | c-ret | ret proto-oncogene precursor | X67812 | 1.4 | 0.5 | 4.4 | 1.0 | 0.9 | 1.0 | 1.0 | 2.2 | 1.0 |
| Oncogenes & Tumor Suppressors | Cot | Cot p | D13759 | 1.3 | 0.5 | 4.3 | 0.6 | 0.7 | 1.4 | 0.3 | 2.1 | 0.8 |
| Oncogenes & Tumor Suppressors | snoN | snoN; ski-related oncogene | U36203 | 2.6 | 0.9 | 4.2 | 0.2 | 0.5 | 1.9 | 0.9 | 1.6 | 2.2 |
| Oncogenes & Tumor Suppressors | H-ras | H-ras proto-oncogene | Z50013 | 2.8 | 1.4 | 4.0 | 1.1 | 0.5 | 1.7 | 2.0 | 1.9 | 2.4 |
| Protease Inhibitors | SPI2-2 | serine protease inhibitor 2-2 | M64086 | 9.1 | 0.6 | 1.4 | 0.3 | 0.6 | 1.7 | 0.6 | 2.2 | 1.1 |
| Proteases | CATHEPSIN C | DIPEPTIDYL-PEPTIDASE I | U89269 | 1.9 | 0.8 | 4.3 | 0.1 | 0.4 | 1.4 | 0.3 | 1.3 | 1.6 |
| Protein Modification Enzymes | PRP | major prion protein precursor | M13685 | 0.6 | 0.7 | 4.6 | 0.3 | 1.0 | 1.7 | 0.2 | 0.7 | 0.4 |
| Symporters & Antiporters | GABT1 | GABA transporter 1 | M92378 | 1.8 | 2.0 | 5.2 | 0.5 | 0.5 | 2.7 | 0.8 | 2.0 | 1.4 |
| Symporters & Antiporters | GABT3 | sodGABA transporter 3 | L04662 | 1.0 | 2.2 | 5.2 | 0.9 | 0.9 | 0.8 | 0.7 | 0.5 | 0.6 |
| Symporters & Antiporters | GABA-A T 3 | GABA-A transporter 3 | L04663 | 1.7 | 2.1 | 4.9 | 0.7 | 0.5 | 1.3 | 1.3 | 1.1 | 1.2 |
| Symporters & Antiporters | EAAC1 | High affinity glutamate transporter | U73521 | 0.9 | 2.0 | 4.6 | 0.4 | 0.9 | 1.3 | 0.4 | 1.8 | 0.6 |
Table 7A.
Supplemental Table 3A ▶ .
Continued
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Transcription Activators & Repressors | S-II | transcription factor S-II | D00926 | 6.1 | 2.8 | 3.5 | 0.2 | 1.0 | 1.0 | 1.0 | 1.0 | 0.4 |
| Transcription Activators & Repressors | NF-1B | NF-1B protein | D90176 | 5.2 | 1.3 | 6.3 | 0.5 | 0.9 | 1.8 | 0.7 | 0.1 | 0.2 |
| Transcription Activators & Repressors | YB1 | YB1 DNA binding protein | X57621 | 4.2 | 1.9 | 4.3 | 0.2 | 0.8 | 2.3 | 0.6 | 1.3 | 1.6 |
| Transcription Activators & Repressors | STAT1 | STAT 1 | U06924 | 3.3 | 7.1 | 7.2 | 0.3 | 0.5 | 2.3 | 0.1 | 0.5 | 0.8 |
| Transcription Activators & Repressors | U2AF1-RS1 | U2 small nuclear ribonucleoprotein | D17407 | 3.3 | 1.6 | 5.2 | 0.5 | 0.5 | 1.5 | 1.2 | 1.1 | 0.7 |
| Transcription Activators & Repressors | nyk-R | tyrosine-protein kinase ryk | M98547 | 2.4 | 6.4 | 2.1 | 0.2 | 0.5 | 2.5 | 0.7 | 2.4 | 2.0 |
| Transcription Activators & Repressors | RNF2 | ring finger protein 2 | Y12880 | 3.0 | 4.6 | 5.2 | 0.3 | 0.5 | 1.5 | 0.4 | 0.7 | 0.2 |
| Transcription Activators & Repressors | RXR gamma | retinoic acid receptor gamma | M84819 | 1.0 | 1.0 | 16.0 | 0.6 | 0.5 | 1.3 | 1.1 | 0.8 | 0.7 |
| Transcription Activators & Repressors | CRABP-II | cellular retinoic acid-binding protein II | M35523 | 1.0 | 1.0 | 8.7 | 0.3 | 0.4 | 1.5 | 0.9 | 1.5 | 0.7 |
| Transcription Activators & Repressors | RIP 15 | retinoid X receptor interacting protein | U09419 | 2.6 | 0.4 | 7.0 | 0.1 | 0.2 | 1.1 | 0.5 | 0.5 | 2.2 |
| Transcription Activators & Repressors | TF 1 HSP | transcription factor 1 for heat shock | X61753 | 2.0 | 0.8 | 4.9 | 0.7 | 0.4 | 1.4 | 2.3 | 0.8 | 1.8 |
| Transcription Activators & Repressors | EED | embryonic ectoderm development | U78103 | 1.0 | 0.8 | 4.4 | 0.6 | 0.6 | 1.2 | 1.0 | 1.9 | 0.7 |
| Transcription Activators & Repressors | MF 5 | myogenic factor 5 | X56182 | 1.0 | 1.0 | 4.4 | 0.6 | 0.6 | 1.4 | 0.5 | 1.2 | 0.7 |
| Xenobiotic Transporters | GST5-5 | glutathione S-transferase 5 mu | J04696 | 5.1 | 5.4 | 2.9 | 0.4 | 0.3 | 0.9 | 0.6 | 0.3 | 0.1 |
| Xenobiotic Transporters | GSTT1 | gluthathione S-transferase theta 1 | X98055 | 1.2 | 2.6 | 5.1 | 0.4 | 0.4 | 1.4 | 1.1 | 0.8 | 0.8 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Table 4A.
Genes Up-Regulated by Chronic LPS-Challenge
| Group | Abbreviation | Name | GenBank | LPS/Saline |
|---|---|---|---|---|
| Adhesion | NCAM2 | neural cell adhesion molecule 2 precursor | AF001287 | 3.1 |
| Adhesion | semaphorin F | semaphorin F | X97817 | 3.0 |
| Basic Transcription Factors | DE 1 | dermis expressed 1 | U36384 | 4.3 |
| Basic Transcription Factors | MRG1 | MSG-related protein 1 | Y15163 | 3.8 |
| Basic Transcription Factors | MYF-6 | myogenic factor | M30499 | 3.3 |
| Basic Transcription Factors | PBX2 | pre-B-cell leukemia transcription factor 2 | AF020198 | 3.0 |
| Cell Cycle-Regulating Kinases | p58/GTA | p58/GTA | M58633 | 3.9 |
| Channels (Membrane) | k J12 | potassium inwardly-rectifying channel | X80417 | 3.3 |
| Cytoskeleton & Motility | CK4 | basic keratin complex 2 gene 4 | X03491 | 3.5 |
| Cytoskeleton & Motility | COL10A1 | collagen 10 alpha 1 | X67348 | 3.3 |
| Death Receptors | RXR-beta c | RXR-beta cis-11-retinoic acid R | X66224 | 4.2 |
| Growth Factors and Cytokines | HGF | hepatocyte growth factor | X72307 | 5.4 |
| Growth Factors and Cytokines | mast cell factor | mast cell factor | U44725 | 3.1 |
| Growth Factors and Cytokines | SARP1 | secreted apoptosis-related protein 1 | AF017989 | 4.0 |
| GTP/GDP Exchangers | Rab GDI alpha | Rab GDI alpha | U07950 | 4.6 |
| Hormone Receptors | adrenergic Rb2 | adrenergic receptor, beta 2 | X15643 | 4.7 |
| Hormone Receptors | prostaglandin F R | prostaglandin F R | D17433 | 4.7 |
| Intracellular Transducers | TSC2 | Tuberin | U37775 | 3.0 |
| Intracellular Transducers | CISH7 | cytokine inducible SH2-containing protein 7 | U88325 | 4.4 |
| Intracellular Transducers | Eph3 | tyrosine-protein kinase receptor | L25890 | 3.9 |
| Intracellular Transducers | notch3 | neurogenic locus notch homolog 3 precursor | X74760 | 3.0 |
| Kinase Activators & Inhibitors | Mac-Marcks | Marcks-related protein | X61399 | 3.3 |
| Neurotransmitter Receptors | 5-HT1c | 5-hydroxytryptamine R 1c | X72230 | 3.1 |
| Neurotransmitter Receptors | 5-HT7 | 5-hydroxytryptamine R 7 | Z23107 | 3.5 |
| Neurotransmitter Receptors | GABA-A beta 2 | gamma-aminobutyric acid | U14419 | 4.7 |
| Oncogenes & Tumor Suppressors | c-Fgr | c-Fgr | X52191 | 3.6 |
| Other Cell Cycle Proteins | myeloblastin | trypsin-chymotrypsin serine protease | U43525 | 3.7 |
| Protease Inhibitors | ALP | antileukoproteinase 1 precursor | U73004 | 3.1 |
| Receptors (by Ligands) | LDLR | low-density lipoprotein receptor precursor | Z19521 | 3.2 |
| Transcription Activators & Repressors | PAX-8 | paired box protein PAX 8 | X57487 | 4.0 |
Table 4B.
Genes Down-Regulated by Chronic LPS-Challenge
| Group | Abbreviation | Name | GenBank | Saline/LPS |
|---|---|---|---|---|
| Apoptosis | CLU | clusterin precursor | L08235 | 5.0 |
| Apoptosis | CPR | NADPH-cytochrome P450 reductase | D17571 | 3.7 |
| Basic Transcription Factors | NKX-2.6 | Drosophila NK2 transcription factor-related locus 6 | AF030113 | 4.3 |
| Basic Transcription Factors | HFH-4 | hepatocyte nuclear factor 3 | L13204 | 3.4 |
| Basic Transcription Factors | SOX13 | HMG-box transcription factor | AJ000740 | 4.0 |
| Basic Transcription Factors | TF II D | transcription initiation factor TF II D | D01034 | 3.0 |
| Basic Transcription Factors | GLI2 | zinc finger protein | X99104 | 4.6 |
| Cell-Cell Adhesion Receptors | ADAM8 | cell surface antigen MS2 | D10911 | 4.1 |
| Cell-Cell Adhesion Receptors | EPHA6 | ephrin type A R 6 | U58332 | 5.3 |
| Cell-Cell Adhesion Receptors | CD14 | CD14 | M34510 | 4.3 |
| Cell-Cell Adhesion Receptors | integrin alpha 7 | integrin alpha 7 | L23423 | 4.4 |
| Cyclins | cyclin T1 | cyclin T1 | AF109179 | 3.7 |
| Cyclins | CCNE2 | G1/S-specific cyclin E2 | AF091432 | 3.1 |
| Cytoskeleton & Motility | LAMC2 | laminin gamma 2 subunit precursor | U43327 | 4.4 |
| Cytoskeleton & Motility | talin | talin | X56123 | 3.5 |
| DNA Damage/Repair | CTCBF | ATP-dependent DNA helicase II 70-kDa subunit | M38700 | 3.1 |
| Growth Factor & Chemokine Receptors | PDGF | pre-platelet-derived growth factor R | X04367 | 6.0 |
| Growth Factors and Cytokines | VGR1 | bone morphogenetic protein 6 precursor | X80992 | 4.0 |
| Growth Factors and Cytokines | CERR1 | cerberus-related protein1 | AF031896 | 4.1 |
| Growth Factors and Cytokines | Mdkk1 | dickkopf homolog 1 | AF030433 | 3.8 |
| Growth Factors and Cytokines | GCSF | granulocyte colony-stimulating factor | M13926 | 5.1 |
| Growth Factors and Cytokines | GDF9 | growth differentiation factor 9 | X77113 | 3.0 |
| Growth Factors and Cytokines | HBGF5 | heparin-binding growth factor 5 precursor | M30643 | 4.1 |
| Growth Factors and Cytokines | LIF | leukemia inhibitory factor | X06381 | 3.7 |
| Growth Factors and Cytokines | SHH | sonic hedgehog homolog | X76290 | 3.4 |
| Intracellular Adaptors | PTPN11 | non-receptor type 11 protein tyrosine phosphatase | D84372 | 4.2 |
| Intracellular Adaptors | Sik | Src-related intestinal kinase | U16805 | 4.6 |
| Intracellular Kinase | S6KII-alpha | ribosomal protein S6 kinase II alpha 1 | M28489 | 3.4 |
| Intracellular Transducers | B-50 | neuromodulin | J02809 | 3.7 |
| Intracellular Transducers | TLL | tolloid-like protein | U34042 | 3.6 |
| Ion Channels (Voltage-Gated) | SODIUM CH | voltage-gated sodium channel | L36179 | 4.6 |
| Oncogenes & Tumor Suppressors | c-fos | c-fos | V00727 | 5.5 |
| Oncogenes & Tumor Suppressors | c-Src | c-Src | M17031 | 3.4 |
| Oncogenes & Tumor Suppressors | ERP | ets-domain protein elk3 | Z32815 | 4.8 |
| Oncogenes & Tumor Suppressors | G-protein R 27 | G-protein coupled receptor 27 | U79525 | 3.9 |
| Oncogenes & Tumor Suppressors | R-ras | R-ras protein | M21019 | 4.8 |
| Oncogenes & Tumor Suppressors | MMP11 | matrix metalloproteinase 11 | Z12604 | 3.5 |
| Oncogenes & Tumor Suppressors | TSG101 | tumor susceptibility protein 101 | U52945 | 3.4 |
| Oncogenes & Tumor Suppressors | Vav | GDP-GTP exchange factor | X64361 | 7.0 |
| Recombination Proteins | RAG2 | V(D)J recombination activating protein | M64796 | 3.6 |
| Transcription Activators & Repressors | ATOH2 | atonal protein homolog 2 | U29086 | 3.4 |
| Transcription Activators & Repressors | IRF1 | interferon regulatory factor 1 | M21065 | 3.0 |
| Transcription Activators & Repressors | mf 5 | myogenic factor 5 | X56182 | 3.9 |
| Transcription Activators & Repressors | PSD-95 | PSD-95/SAP90A | D50621 | 3.0 |
| Transcription Activators & Repressors | STAT1 | STAT1 | U06924 | 4.2 |
| Unclassified Proteins | FMR2 | fragile X mental retardation syndrome | AJ001549 | 3.5 |
| Unclassified Proteins | HUD | Huntington disease homolog | U24233 | 7.6 |
| Unclassified Proteins | HAP1 | huntingtin-associated protein 1 | AJ000262 | 4.6 |
| Unclassified Proteins | petaxin-related | petaxin-related C-reactive protein | X13588 | 3.4 |
| Unclassified Proteins | PS1 | presenilin-1 | AF007560 | 4.6 |
| Unclassified Proteins | tubby | tubby | U54643 | 3.6 |
| Unclassified Proteins | WSB1 | WSB1 protein | AF033186 | 4.2 |
Antigen-Induced Gene Regulation
In addition to genes that were commonly up-regulated by all three stimuli, we selected a group of genes that were up-regulated specifically by antigen challenge (Supplemental Table 3A ▶ ▶ at www.amjpathol.org). Those genes include several receptors for neurotransmitters and sensory peptides such as endothelin B, glutamate, CGRP, GABA-A transporter, and NMDA2B. Interestingly, antigen stimulation also up-regulated cytokines and growth factors, including insulin-like growth factor. In addition, genes with complementary functions were simultaneously up-regulated; this was the case of genes involved in inflammatory cell migration through theendothelium. These included GLYCAM 1, VEGFR1, VE-cadherin, and cadherin 5. Moreover, several genes related to the expression of heat shock proteins (HSP) were concomitantly up-regulated. These included HSP transcription factor 1, HSP27, and HSP105. Finally, antigen also altered the expression of proteases inhibitors, as well as glutathione S-transferase GST μ and θ 1.
SP-Induced Gene Regulation
In addition to NGF up-regulation that occurred in response to all stimuli, SP also induced the expression of glial cell line-derived neurotrophic factor (Supplemental Table 3A ▶ ). Another gene uniquely altered by SP was ICAM1. It remains to be determined whether the differential expression of ICAM1 by SP and GLYCAM 1, VEGFR1, VE-cadherin, and cadherin 5 by antigen may be responsible for a differential recruitment of mast cells and immune cells to the site of inflammation.
Table 7B.
Supplemental Table 3B ▶ .
Genes Up-Regulated by Substance P-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| Adhesion | ICAM1 | intercellular adhesion molecule 1 | X52264 | 1.7 | 0.6 | 2.9 | 0.4 | 0.3 | 3.4 | 1.6 | 1.4 | 0.4 |
| Cyclins | CCND1 | G1/S-specific cyclin D1 | S78355 | 0.6 | 0.5 | 1.9 | 0.4 | 0.8 | 3.1 | 0.1 | 0.5 | 0.3 |
| Cytoskeleton & Motility Proteins | MLC1A | myosin light subunit 1 | M19436 | 0.5 | 0.4 | 1.0 | 0.4 | 0.6 | 3.2 | 0.5 | 0.5 | 1.2 |
| DNA Damage/Repair | Nibrin | cell cycle regulatory protein p95 | AF092840 | 0.9 | 0.4 | 0.7 | 1.0 | 0.5 | 3.2 | 0.9 | 0.2 | 1.2 |
| Growth Factors and Cytokines | GDNF | glial cell line-derived neurotrophic F | D49921 | 0.3 | 0.5 | 1.6 | 0.3 | 1.3 | 3.2 | 0.6 | 1.3 | 2.4 |
| Recombination Proteins | RAG2 | V(D)J recombination activating | M64796 | 1.0 | 1.0 | 1.0 | 1.0 | 3.6 | 1.0 | 1.8 | 1.6 | 1.0 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
LPS-Induced Gene Regulation
LPS specifically up-regulated several genes listed on Supplemental Table 3C ▶ . These genes included integrin-α 7 and CD14, a cell surface protein involved in LPS binding. Interestingly, in addition to the up-regulation of NGF, LPS was the only stimulus that also up-regulated NGF-inducible protein. Moreover, in addition to NF-κB that was up-regulated by all three stimuli, LPS was the only stimulus to induce c-rel, transcription factor AP-1, and transcription factor E2F3.
Table 7C.
Supplemental Table 3C ▶ .
Genes Up-Regulated by LPS-Induced Bladder Inflammation
| Group | Abbreviation | Gene | GenBank | Antigen | Substance P | LPS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 24 | 1 | 4 | 24 | 1 | 4 | 24 | ||||
| ABC Transporters | ABC8 | ATP-binding casette 8 | U34920 | 0.5 | 1.2 | 0.5 | 0.5 | 2.8 | 1.0 | 0.4 | 6.1 | 1.0 |
| Apoptosis | GADD45 | GADD45 | L28177 | 0.2 | 1.1 | 1.8 | 0.2 | 0.6 | 1.3 | 0.7 | 4.1 | 1.4 |
| Basic Transcription Factors | Act 2 | macrophage inflammatory protein 1 beta | M35590 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 4.8 | 5.2 | 1.0 |
| CDK Inhibitors | p19ink4 | cdk4 and cdk6 inhibitor | U19597 | 0.3 | 2.4 | 1.0 | 0.4 | 0.6 | 1.0 | 1.0 | 4.1 | 1.0 |
| Cell Cycle Proteins | Cdc25a | M-phase inducer phosphatase 1 | U27323 | 1.0 | 1.9 | 2.0 | 0.3 | 0.7 | 2.4 | 1.0 | 1.3 | 4.0 |
| Cell-Cell Adhesion Receptors | CD14 | LPS receptor | M34510 | 1.0 | 0.4 | 1.6 | 0.8 | 0.7 | 1.4 | 3.2 | 1.0 | 0.4 |
| Cell-Cell Adhesion Receptors | desmocollin 2 | desmocollin 2 | L33779 | 0.9 | 0.9 | 2.5 | 1.0 | 0.8 | 0.3 | 0.3 | 6.9 | 1.0 |
| Cell-Cell Adhesion Receptors | integrin alpha 7 | integrin alpha 7 | L23423 | 1.9 | 1.9 | 1.3 | 0.6 | 0.8 | 1.8 | 2.3 | 4.1 | 3.7 |
| Cytoskeleton & Motility | TUBB4 | tubulin beta 4 | M28730 | 1.5 | 2.3 | 1.1 | 0.5 | 2.3 | 1.1 | 1.5 | 4.7 | 2.7 |
| Death Receptor Ligands | FASL | fas antigen ligand; TNFSF6 | U06948 | 0.3 | 1.0 | 0.3 | 0.5 | 1.3 | 1.0 | 0.4 | 4.8 | 1.0 |
| Death Receptors | beta2-RAR | retinoic acid receptor beta-2 | S56660 | 0.7 | 1.2 | 0.3 | 0.6 | 2.6 | 1.0 | 1.0 | 4.0 | 1.0 |
| DNA Damage/Repair | FACC | Fanconi anemia group C | L08266 | 2.0 | 0.8 | 1.0 | 0.6 | 1.4 | 0.8 | 5.1 | 1.7 | 2.6 |
| DNA Damage/Repair | POLA | DNA polymerase alpha | D17384 | 1.8 | 0.8 | 2.3 | 0.5 | 1.1 | 0.6 | 4.8 | 1.3 | 2.3 |
| DNA Damage/Repair | RAD51 | DNA repair protein RAD51 homolog 1 | D13473 | 1.0 | 0.8 | 2.0 | 0.4 | 0.4 | 0.8 | 5.1 | 2.9 | 1.6 |
| Extracellular Transporters | TBPA | transthyretin precursor | D89076 | 0.5 | 0.7 | 1.1 | 0.3 | 0.9 | 0.5 | 0.8 | 5.1 | 1.0 |
| Fatty Acid Metabolism | FAAH | fatty acid amide hydrolase | U82536 | 0.3 | 1.2 | 1.1 | 0.5 | 1.8 | 1.5 | 0.6 | 5.1 | 2.8 |
| Interleukins & Interferons | IL-6 | Interleukin-6 precursor | X06203 | 1.0 | 1.0 | 1.0 | 2.4 | 1.0 | 1.0 | 11.7 | 3.7 | 1.0 |
| Intracellular Transducers | SOCS2 | cytokine inducible SH2 | U88327 | 1.9 | 0.7 | 2.0 | 0.9 | 0.5 | 1.2 | 4.2 | 1.0 | 2.3 |
| Ion Channels (Membrane) | SCF 1-7 | solute carrier family 1, member 7 | L42115 | 0.3 | 2.8 | 1.0 | 0.5 | 2.8 | 1.0 | 0.5 | 5.4 | 1.0 |
| Ion Channels (Membrane) | QT | QT potassium channel | U04294 | 0.4 | 0.9 | 0.6 | 0.8 | 1.5 | 1.0 | 0.3 | 4.9 | 1.0 |
| Ion Channels (Membrane) | SCF 1-6 | solute carrier family 1, member 6 | D83262 | 0.3 | 2.5 | 1.0 | 0.7 | 2.5 | 1.0 | 0.7 | 4.6 | 1.0 |
| Oncogenes & Tumor Suppressors | c-jun | transcription factor AP-1 | J04115 | 0.8 | 0.3 | 1.0 | 1.8 | 0.7 | 1.1 | 6.8 | 2.6 | 0.4 |
| Oncogenes & Tumor Suppressors | INI1A | integrase interactor 1A protein | AJ011739 | 1.0 | 2.2 | 0.9 | 0.3 | 0.9 | 0.4 | 1.0 | 4.8 | 1.0 |
| Oncogenes & Tumor Suppressors | c-rel | c-rel | X15842 | 0.5 | 2.7 | 1.0 | 0.3 | 2.7 | 1.0 | 1.0 | 4.7 | 1.0 |
| Oncogenes & Tumor Suppressors | abl | abl | L10656 | 0.6 | 1.2 | 1.5 | 0.3 | 2.1 | 1.9 | 0.8 | 4.0 | 2.1 |
| Symporters & Antiporters | SYTIII | synaptotagmin III | D45858 | 0.5 | 1.1 | 1.0 | 0.6 | 1.0 | 1.0 | 0.6 | 5.4 | 1.0 |
| Transcription Activators & Repressors | Pml | murine homolog of the leukemia | U33626 | 0.5 | 2.7 | 1.0 | 1.0 | 2.7 | 0.3 | 1.0 | 5.6 | 1.0 |
| Transcription Activators & Repressors | E2F-3 | transcription factor E\ | AF015948 | 2.0 | 1.3 | 2.2 | 2.4 | 0.6 | 1.7 | 2.4 | 2.3 | 4.0 |
| Unclassified Proteins | WSB1 | WSB1 protein | AF033186 | 2.1 | 1.5 | 1.5 | 2.3 | 2.5 | 1.1 | 14.9 | 13.4 | 1.5 |
| Unclassified Proteins | frataxin | Friedreich ataxia protein | U95736 | 1.6 | 0.4 | 1.8 | 0.9 | 0.5 | 1.1 | 13.9 | 2.2 | 1.8 |
| Unclassified Proteins | huntingtin | Huntington disease homolog | U24233 | 1.8 | 0.9 | 0.5 | 0.5 | 1.2 | 0.7 | 12.5 | 5.2 | 1.9 |
| Unclassified Proteins | NGF-I | NGF-inducible protein TIS21 | M64292 | 0.8 | 0.7 | 1.2 | 2.2 | 0.6 | 1.1 | 11.3 | 1.6 | 0.3 |
| Unclassified Proteins | LEP | leptin precursor | U22421 | 1.2 | 0.7 | 2.0 | 1.8 | 0.3 | 2.6 | 9.4 | 0.7 | 2.6 |
| Unclassified Proteins | tubby | tubby | U54643 | 1.6 | 0.9 | 2.3 | 1.0 | 0.4 | 1.1 | 8.0 | 2.0 | 1.1 |
| Unclassified Proteins | COBL | cordon-bleu protein | U26967 | 0.9 | 0.5 | 1.8 | 0.5 | 0.5 | 0.8 | 7.0 | 0.5 | 1.8 |
| Unclassified Proteins | petaxin | petaxin-related C-reactive protein | X13588 | 0.9 | 0.7 | 1.8 | 0.4 | 0.6 | 1.3 | 5.9 | 1.6 | 1.7 |
| Unclassified Proteins | KL | klotho protein | AB005141 | 0.3 | 0.8 | 2.0 | 0.8 | 0.4 | 1.2 | 5.8 | 1.2 | 2.0 |
| Unclassified Proteins | PPEF2 | protein phosphatase with EF-hands 2 | AF023458 | 1.4 | 0.7 | 1.3 | 1.7 | 0.4 | 0.9 | 5.4 | 1.3 | 2.9 |
| Unclassified Proteins | ajuba | ajuba protein | U79776 | 0.8 | 0.7 | 1.5 | 0.4 | 0.3 | 1.3 | 4.2 | 0.9 | 1.3 |
| Xenobiotic Transporters | HO-2 | Heme oxygenase | AF029874 | 0.9 | 0.4 | 1.8 | 0.9 | 0.5 | 2.0 | 0.9 | 2.1 | 4.0 |
Ratio of Gene Expression (Treatment/Saline) was calculated from individual expressions as % of beta-actin.
Numbers in bold indicate peak of gene expression.
Gene Up-Regulation by Chronic LPS Stimulation
Chronic stimulation with LPS led to a predominant increase of macrophages and monocytes in the bladder submucosa. Bladders obtained after chronic LPS stimulation presented a different profile of both up-regulated and down-regulated genes (Table 4, A and B ▶ ▶ , respectively).
Genes that were uniquely up-regulated during chronic inflammation included those involved in tissue remodeling such as mast cell factor, melanocyte-specific gene 2, neural cell adhesion molecule 2, and collagen 10 α1. Moreover, chronic stimulation with LPS up-regulated cellular and cytoplasmatic receptors such as serotoninergic 1c and 7, β adrenergic 2, prostaglandin F receptor, and RXR-β cis-11-retinoic acid.
Chronic stimulation with LPS resulted in down-regulation of several genes (Table 4B) ▶ . Interestingly, some of genes down-regulated during chronic inflammation had their expression specifically up-regulated by acute LPS administration. Those include CD14, integrin α 7, and matrix metalloproteinase 11. It remains to be determined whether this down-regulation is part of a compensatory mechanism or a consequence of LPS-induced death and shedding of urothelial cells. 17 As the urothelium works with underlying afferent nerves to detect the presence of irritant stimuli, 25 it will be of value to determine whether CD14, integrin α 7, and MMP11 are part of the unique set of urothelial genes contrasting with those found in the bladder detrusor muscle. Ongoing experiments in this laboratory are determining which genes are uniquely expressed by each of the urinary bladder layers.
Discussion
Response to Injury
The urinary bladder responds to injury with an acute inflammatory response that may be followed by chronic inflammation, repair, fibrosis, 26 or loss of function as indicated by a reduced bladder capacity in patients with chronic cystitis. 27 This study expands understanding of two elements of this process: it characterizes both the sets of genes which respond uniformly as well as uniquely to diverse stimuli, and the differences between acute and chronic inflammatory responses.
To facilitate these studies of bladder inflammation, we used our previously developed multiple animal models of cystitis. We have also provided evidence of LPS-induced cystitis 17 and the time-dependent gene expression in response to LPS, 11 but could not define common responses from a single model system. Morphological changes confirm the presence of the cells pathognomonic of acute and chronic inflammation in these models. Immunohistochemistry indicates that during the transition between acute and chronic stimulation, the predominant cell type that infiltrates into the bladder changed from polymorphonuclear leukocytes to monocytes/macrophages.
Genes Up-Regulated by All Stimuli
In the present work, we sought to define common pathways involved in acute forms of bladder inflammation. For this purpose, we investigated the time course of morphological and genomic alterations in responses to antigen-, SP-, and LPS-stimulation. We selected genes that were up-regulated in response to all stimuli (SP, antigen, or LPS) and therefore, represent a relatively universal bladder response to inflammation. One of the most interesting aspects of this study was the finding that genes commonly up-regulated by any given stimulus have virtually identical time courses of expression (Table 3, A–C) ▶ ▶ ▶ . Together these results strongly suggest the existence of recursive molecular pathways supporting bladder inflammatory responses. This pathway should protect the bladder from noxious stimulus, and once the stimulus ceases, gene expression should down-regulate to avoid tissue damage and chronic inflammation. The latter hypothesis is under investigation by our laboratory and preliminary results indicate that the responses of wild-type and mice deficient in anti-inflammatory cytokines (interleukin (IL)-10 knockout mice) to LPS present a different time course of gene regulation (Nguyen N-B, Saban MR, Hammond TG, Saban R. Time course of LPS-induced gene regulation in the bladder of wild-type and IL-10 knockout mice. Genomics and Proteomics in Kidney and Urologic Diseases Conference, National Institutes of Health, July, 2001).
In addition, we found genes that are up-regulated uniquely by a single stimulus. The reason to present these results is that some readers may be interested in a particular gene or pathway, and therefore, this information indicates which stimulus should be used to alter that particular gene expression. Except for the completeness of the Venn diagrams themselves, we arenot sure about the relevance of gene changes common to two stimuli when comparing unique gene alterations in response to one stimulus alone or to all three stimuli.
In terms of time response, morphological results indicate absence of inflammatory cells at early time points and therefore, gene regulation observed at 1 hour may reflect the effect of the stimulus on resident cells. In contrast, at late time points such as 24 hours, a considerable number of migrating inflammatory cells were observed and gene regulation has the potential to be a combined response from of both resident and migrating cells.
Experimental bladder inflammations resulted in up-regulation of some gene families regardless of the initiating stimulus. Therefore, we focused this discussion on the commonly expressed gene families that also have been described in the urinary system, urine infection, interstitial cystitis, bladder or prostate cancer, and that participate in the inflammatory response. Those families included several cell surface and cytoplasmic receptors (neuropeptides, neurotransmitter, hormones, and growth factors), adhesion proteins, transcription factors, and signal transducers. In addition, genes involved in cell survival and proliferation such as NGF and proto-oncogenes (iNOS) were co-expressed with death receptor proteins, proteases, and protease inhibitors. Moreover, inflammation also altered the expression of cytoskeleton and motility proteins.
Genes Involved in Bladder Physiology
Several genes that were commonly up-regulated have already been described to participate in mechanisms controlling micturition. These include enkephalins, nociceptin which resembles endogenous enkephalins, GABA A receptor, and entothelins (ET). These proteins and receptors may be involved in the regulation of nociceptive processing. Indeed, systemic or central administration of nociceptin, 28 enkephalins (intrathecal naloxone, 29 ), and GABA, 30 inhibits the micturition reflex. Interestingly, peripheral inflammation induces nociceptin in primary sensory neurons 31 and increases pro-dynorphin mRNA in dorsal horn neurons. 32 Except for ET receptors which have been described in the bladder and initiate detrusor contractile activity in animals and human detrusor 33 with a potential role in bladder outlet obstruction, 34 the information is scanty regarding the presence of these proteins in the urinary bladder. Therefore, our results indicate the need for tissue bath experiments to elucidate whether the local expression of these receptors also participates in the altered bladder function observed during inflammation. 35
Genes Encoding Proteins Already Described to Be Involved in Interstitial Cystitis
Some of the gene products found up-regulated by all three stimuli have already been associated with interstitial cystitis. These include tryptase receptors (PAR), NGF, iNOS, fibroblast growth factor (FGF), and insulin-like growth factors (IGFs). In addition, heat shock proteins were found among the genes commonly down-regulated. The latter coincides with the description that IC urine alters the production of a 72-kd stress protein by urothelial cells. 36
PAR and Tryptase
We presented evidence indicating that mast cell tryptase is elevated in the urine of IC patients 5 and others presented immunohistological findings of mast cell containing tryptase in the bladder of IC patients. 37 Tryptase responses are modulated by proteinase-activated receptors (PAR) coupled to a G-protein. These receptors are present in bladder afferent neurons 38 and mediate plasma extravasation induced by tryptase and thrombin. 38 PA receptors are also present in normal human kidneys, 39 and mediate the response of human proximal tubular cells to thrombin. 39 Others have reported that activation of PAR induced detectable levels of IL-6 production in a dose-dependent fashion. 40 Our results indicated that PAR4 mRNA was up-regulated in response to all three stimuli. As mast cells and tryptase are common features of IC, it is tempting to suggest that tryptase generated during the mast cell degranulation along with an up-regulation of PAR4 may contribute to abnormal neurotransmission and motility in the inflamed bladder. It remains to be determined whether PAR4 are up-regulated in the bladder of IC patients.
NGF
Clinically, NGF levels are higher in samples from patients with painful bladder conditions than in samples from controls 41 and NGF levels are elevated in the urine of patients with IC and bladder cancer. 5 In addition, overexpression of NGF suggested a potential treatment for diabetic neuropathy 42 indicating a local control of NGF in bladder motility. Furthermore, recent evidence supports the hypothesis that p75NTR is a mediator of neurotrophin dependence during the critical phase of developmental cell death and during the progression of carcinogenesis in prostate cancer. 43 Experimentally, we have shown that NGF is one of the early genes up-regulated by LPS-induced cystitis. 11 Others have shown a rapid increase in both NGF mRNA 44 and protein 45 following experimental bladder inflammation. Such neuroplasticity may be a possible explanation for the association of bladder inflammation with long-term symptoms and pain after the inflammation subsides. 46 More recently, an intriguing hypothesis proposed that alteration in MMP activity may modify the influence of NGF on apoptosis and cell survival. The explanation for such hypothesis is that NGF is secreted in a proform (proNGF) that has high-affinity for p75NT receptor which mediates apoptosis. MMPs and plasmin cleave proNGF generating mature NGF. Mature NGF has high affinity for TrkA receptors which mediates cell differentiation and survival. 47 In this context it is interesting to notice that along with the up-regulation of NGF, all three stimuli down-regulated both MMP2 and MMP14. Further research is needed to determine the consequences of altered expression of proteinases and tissues inhibitors of proteinases on the balance of pro-NGF and NGF and its consequence on the overall inflammatory response during bladder inflammation.
iNOS
iNOS is expressed in bladder smooth muscle cells and is up-regulated during obstruction, 48 after treatment with cyclophosphamide, 49 following E. coli infection, 50 and LPS instillation. 51 In spinal cord injury patients with a chronic indwelling bladder catheter, iNOS is found overexpressed in bladder mucosa macrophages. 52 In interstitial cystitis patients the results are contradictory since both an increase 53 as well a decrease 54 in NO levels has been reported.
Insulin-Like Growth Factors
Insulin-like growth factors (IGFs), including IGF-1 and IGF-2, are mitogenic peptides for smooth muscle cells and are involved in the regulation of cell proliferation, differentiation, and apoptosis. These factors have been implicated in chronic inflammatory diseases, 55 prostate, 56 and bladder cancer. 57 Overexpression of IGF-I and underexpression of IGFBP 3 and 5 may lead to hyperplasia of the bladder smooth muscle. 58 Interestingly, IGF is decreased in the urine of IC patients 59 and an anti-proliferative factor purified from urothelial cells of IC patients has been shown to stimulate the production of IGF binding protein 3. 60
Heat Shock Transcription Factor 2
Down-regulation of this transcription factor by all three stimuli is noticeable because of the role of heat shock proteins in the balance of cell proliferation and apoptosis. Heat shock factor 1 is overexpressed in human prostate cancer cells. 61 Although there is no previous information on the expression of HSF-2 in the bladder, a recent report indicates that urothelial cells can respond to urine of IC patients with a modest alteration in a 72-kd stress protein. 36 It has been shown that heat shock proteins and stress can reduce the inflammatory response. The proposed mechanism involves induction of NF-κB’s endogenous inhibitor, IkBα, a putative heat shock protein. 62 Therefore, the interplay between NFkB and heat shock proteins in cystitis should be pursued.
Cytokines and Cystitis
IL-6 was described as the major cytokine found in human with interstitial cystitis. 63 Experimentally however, only LPS was capable of up-regulating this cytokine. An interaction between IL-6 and M-CSF switches monocyte differentiation to macrophages rather than dentritic cells. 64 It remains to be determined whether or not the increased macrophage accumulation by chronic LPS stimulation depends on such interplay. In addition to IL-6, in experimental cystitis, all stimuli resulted in up-regulation of interferon regulatory factor-1 (IRF-1), interferon-gamma receptor, tumor necrosis factor β, and macrophage inflammatory protein (MIP). In other systems, it has been shown that LPS-induces IRF-1 function synergistically with NFkB. 65 Recent evidences indicated that LPS/IFNγ not only induced the production of cytotoxic NO through IRF-1 but also initiated the NO-independent apoptotic pathway through the induction of caspase-11 expression. 66 In addition, MIP-2 is a major CXC chemokine involved in the migration of PMNs to sites of inflammation. Following intravesical E. coli infection, several C-X-C chemokines are secreted into the urine, but only MIP-2 concentrations correlate to neutrophil numbers that cross the epithelial barrier of the infected urinary tract. 67
Hormone Receptors
The group of hormone receptors (CRF R, melanocortin 5R, estrogen R, galanin R 1) deserves special attention because more than 90% of those affected with IC are women and their pain scores correlate with hormonal variations. 27 Indeed, in all models of acute experimental cystitis, up-regulation of several hormone receptors, including estrogen receptor β (ERβ), galanin R1, and CRF-R1, was observed.
ERβ
ERβ is found in the rat urinary tract 68 and estrogen receptor-α is present in parasympathetic preganglionic neurons innervating the cat bladder. 69 Estrogen receptors are also expressed in the human prostate. 70 It is known that estrogen affects autonomic functions such as micturition. In addition, cross-talk between ER and NFkB does occur in vivo and may indeed contribute significantly to effects of estrogen in inflammation. 71 Estrogen receptors have been described on mast cells 72 and may influence the outcome of cystitis. 73 Estrogen can enhance NGF-stimulated neurite sprouting in an ER α-dependent manner. 74 In addition, short-term estrogen replacement increases β-preprotachykinin mRNA levels in uninjured dorsal root ganglion neurons. 74 Together these observations emphasize the need of basic research toward the role of estrogen receptor regulation on the bladder responses to inflammation.
Galanin R1
Galanin-immunoreactive nerve fibers have been described in the human detrusor muscle. 75 Galanin is widely distributed in enteric nerve terminals and acts to modulate intestinal motility by altering smooth muscle contraction and in the human colon, alterations in Gal1-R expression may have an important role in colitis. 76 Indeed, inflammation up-regulates this receptor by an NF-κB-dependent pathway. 77 Interestingly, pathogenic E. coli, but not the nonpathogenic strain, activate NF-κB, and increase Gal-R1 mRNA synthesis. 78
CRF
CRF is the principal hypothalamic factor governing the pituitary-adrenal axis, but the wide extra-pituitary distribution of CRF and its receptors suggest a major role for this neuropeptide in the integration of the overall physiological and behavioral responses of an organism to stress. CRF receptors have not yet been described in the bladder. However, it has been suggested that CRF from Barrington’s nucleus may serve to inhibit micturition. 79 In addition, CRF receptor type 2 mRNA expression seems to be modulated by LPS and glucocorticoids. 80 Activation of mast cells seems to be a possible explanation for the pro-inflammatory actions of CRF. 81 Therefore, CRF receptor-mediated effects may contribute to the link between stress and the pathogenesis of cystitis.
Signal Transduction Pathways
We have described an important role for NF-κB in mediating experimental inflammation in response to LPS. 15 The present results indicate that NF-κB is a pathway commonly up-regulated as the bladder responds to other pro-inflammatory stimuli such as antigen and SP stimulation. In contrast to NF-κB that was up-regulated by all three stimuli, LPS was the only stimulus to induce the transcription factor AP-1 and transcription factor E2F3. Among the signal transduction pathway, all three stimuli induced up-regulation of phosphodiestarases (PDE), TGF β-activated kinase 1 (TAK1), and cAMP-dependent protein kinase (PKA).
c-fos
Up-regulation of c-fos is part of human urothelial cell response to arsenic-induced urothelial cell proliferation. 82 Experimental c-fos and fos-b are part of the early bladder response to LPS. 11 Others have shown that c-fos expression in the spinal cord is part of the immediate response of acute irritation or inflammation of the lower urinary tract 83 and prostate. 84 Indeed, following irritation of the lower urinary tract, c-fos gene expression is up-regulated in lumbo-sacral spinal cord neurons by a mechanism involving activation of tachykinins and NK2 receptors. 85 As an early responder, c-fos up-regulation can be used to detect how fast a peripheral injury is relayed to the central nerve system.
PDE
Phosphodiestarase 1C was up-regulated by all stimuli. Interestingly, selective inhibitors of the phosphodiestarases have been proposed for the symptomatic treatment of detrusor instability. 86 Other have presented evidence that both the selective PDE 4 inhibitor, rolipram, and the non-selective PDE inhibitor, theophylline, markedly reduced LPS-induced pulmonary inflammation. 87 Our preliminary results using rolipram indicate that this compound reduces LPS-induced bladder inflammation (R. Saban, unpublished observations). It remains to be determined whether rolipram can block all forms of experimental cystitis. The continued investigation of PDEs and the development of potent and selective inhibitors should provide more therapeutic agents to decrease the unwanted effects of cystitis.
TGF β activated kinase 1 (TAK1), a mitogen-activated protein kinase (MAPK) is also associated with inflammation and regulates multiple protein kinase cascades activated by LPS. 88 TAK1 is implicated in TGFβ, bone morphogenetic protein (BMP), and IL-1 signaling. 89 In addition, TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-κB in LPS-stimulated macrophages. 90 Thus TAK1 must be considered as an important component of intracellular pathways in cells involved in host responses to physiological and/or environmental stress signals during inflammation.
Acute and Chronic Inflammation
Chronic inflammation was characterized by an increased infiltration of monocytes and macrophages into the bladder wall. A distinct group of genes which characterized the transition from acute to chronic inflammation are drawn from pathways already implicated in this process. Despite the observation that the inflammatory process favors tissue degradation, during chronic inflammation, there is also evidence for an evolution toward tissue repair. The release of growth factors and cytokines capable of stimulating the proliferation and synthetic capacity of smooth muscle cells is the main driving force to this end. This process results in the deposition of extracellular matrix components, such as proteoglycans and collagens. The latter could be modulated by genes such as collagen 10 α1 subunit precursor (COL10A1) and cytokeratin 4 that were found to be up-regulated here specifically during chronic inflammation. In addition, the increased inflammatory infiltrate may be responsible for up-regulation of mast cell factor, melanocyte-specific gene 2, cytokine inducible SH2-containing protein 7 (CISH7), MSG-related protein 1 (MRG1), and prostaglandin F receptor.
In contrast, several genes were down-regulated during LPS-induced chronic inflammation (Table 5B). Interestingly, some of the genes down-regulated during chronic inflammation had their expression up-regulated specifically by acute LPS administration. Those include CD14, integrin α7, and MMP 11. Both CD14 and integrin α7 91 are found in the human bladder mucosa. Integrins (α4β7 integrin) are involved in tissue homing of mast cells 92 and CD14 plays a major role in the inflammatory response induced by LPS. 93 Furthermore, LPS significantly induced CD14 mRNA expression. 94
A possible explanation for the down-regulation is that during chronic inflammation, inhibitory feedback mechanisms would decrease the expression of CD14, integrin, and MMP11. Indeed, CD14 was found to be inversely correlated with severity of disease. 95 An alternative explanation is that the down-regulation is a consequence of death and shed of the urothelial cells. In support for this hypothesis, we previously described that the major morphological alteration induced by acute LPS administration in the mouse bladder was the vacuolization of urothelial cells. 17 In addition, others have presented evidence that in response to infection, superficial bladder cells exfoliate and are removed with the flow of urine. 96 It remains to be determined whether the urothelium presents a unique set of genes in contrast with other regions of the bladder such as the detrusor muscle. Ongoing experiments in this laboratory are determining which genes are uniquely expressed by the each of the layers of the urinary bladder.
Proteomic Correlation
To fairly interpret gene cluster analysis we must be aware of a growing body of evidence that gene and protein changes can be dissociated. For instance, increased abundance of urinary bladder nerve growth factor mRNA is not always associated with increased total urinary bladder nerve growth factor. 45 The discrepancy between two measures (mRNA and protein) may reflect retrograde axonal transport of nerve growth factor to the dorsal root ganglia. 45 Future proteomic correlation must determine how directly mRNA changes reflect translated protein levels and the physiological consequence of these proteins. 97 Despite all of the downstream regulatory steps, understanding of the pattern and timing of gene expression changes is pivotal if we are to interpret and eventually learn to intervene in the pathophysiology of cystitis.
Summary
In conclusion, the cDNA array experimental approach characterized the common pathways of inflammatory response to diverse stimuli, as well as the gene expression profiles which characterize the differences between acute and chronic inflammation. These responses in gene expression may represent a balance between the cytoprotective and degenerative processes that accompany bladder response to injury. This sets the stage for future research using gene cluster analysis techniques to begin to understand clinically relevant issues, such as how and why the transition from acute to chronic inflammation occurs only in selected circumstances, and which pathophysiological and therapeutic strategies change the normal balance of apoptosis and necrosis in populations of bladder cells. Further characterization of the inflammation-induced gene expression profiles obtained here may identify novel biomarkers and shed light into the etiology of cystitis. By profiling the time-dependent gene and protein expression in animal model of bladder inflammation, it may soon be possible to determine potential anti-inflammatory effects of emerging therapeutics.
Footnotes
Address reprint requests to Ricardo Saban, D.V.M., Ph.D., Associate Professor, Department of Physiology, College of Medicine, Oklahoma University Health Sciences Center (OUHSC), 940 SL Young Blvd., Room 605, Oklahoma City, OK 73104. E-mail: ricardo-saban@ouhsc.edu.
Supported by National Institutes of Health grants DK 55828–01 (to R.S.), DK51392 (to T.G.H.), and OCAST HR01–127 (to R.S.).
References
- 1.Lavelle JP, Apodaca G, Meyers SA, Ruiz WG, Zeidel ML: Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. Am J Physiol 1998, 274:F205-F214 [DOI] [PubMed] [Google Scholar]
- 2.Bullock AD, Becich MJ, Klutke CG, Ratliff TL: Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. J Urol 1992, 148:1951-1956 [DOI] [PubMed] [Google Scholar]
- 3.Elbadawi A: Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 1997, 49:14-40 [DOI] [PubMed] [Google Scholar]
- 4.Granum RS, Theoharides TC: The role of mast cells in cystitis. Urol Clin North Am 1994, 21:41-50 [PubMed] [Google Scholar]
- 5.Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M: Elevated tryptase, NGF, NT-3, and GDNF levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 1999, 161:438-441 [PubMed] [Google Scholar]
- 6.Jasmin L, Janni G, Ohara PT, Rabkin SD: CNS-induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J Urol 2000, 164:852-855 [DOI] [PubMed] [Google Scholar]
- 7.Peeker R, Enerback L, Fall M, Aldenborg F: Recruitment, distribution, and phenotypes of mast cells in interstitial cystitis. J Urol 2000, 163:1009-1015 [PubMed] [Google Scholar]
- 8.Bjorling DE, Jerde TJ, Zine MJ, Busser BW, Saban MR, Saban R: Mast cells mediate the severity of experimental cystitis in mice. J Urol 1999, 162:231-236 [DOI] [PubMed] [Google Scholar]
- 9.Marchand JE, Sant GR, Kream RM: Increased expression of substance P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br J Urol 1998, 81:224-228 [DOI] [PubMed] [Google Scholar]
- 10.Pang X, Marchand J, Sant GR, Theoharides TC: Increased number of SP-positive fibres in interstitial cystitis. Br J Urol 1995, 75:744-750 [DOI] [PubMed] [Google Scholar]
- 11.Saban MR, Hellmich H, Nguyen N-B, Winston J, Hammond TG, Saban R: Time course of LPS-induced gene-expression in a mouse model of genitourinary inflammation. Physiol Genomics 2001, 5:147-160 [DOI] [PubMed] [Google Scholar]
- 12.Saban R, Saban MR, Nguyen N-B, Lu B, Gerard C, Gerard NP, Hammond TG: Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am J Pathol 2000, 156:775-780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saban R, Saban MR, Nguyen N-B, Hammond TG, Wershil BK: Mast cell regulation of inflammation and gene-expression during antigen-induced bladder inflammation in the mouse. Physiol Genomics 2001, 7:35-43 [DOI] [PubMed] [Google Scholar]
- 14.Busser BW, Hammond TG, Bjorling DE, Saban R: Lipopolysaccharide (LPS) up-regulates bradykinin 1 (B1) receptors in the isolated mouse bladder. J Urol 1998, 160:2267-2273 [DOI] [PubMed] [Google Scholar]
- 15.Wang X-C, Saban R, Kaysen JH, Saban MR, Allen PL, Benes EN, Hammond TG: Nuclear factor κ B mediates lipopolysaccharide-induced inflammation in the urinary bladder. J Urol 2000, 163:993-998 [PubMed] [Google Scholar]
- 16.Haak-Frendscho M, Saban R, Shields R, Jardieu P: Blockade of histamine release and tissue contraction by antibodies to IgE in an established IgE response. Immunology 1998, 94:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saban MR, Saban R, Hammond TG, Haak-Frendscho M, Steinberg H, Tengowski MW, Bjorling DE: LPS-sensory peptide communication in experimental cystitis. Am J Physiol 2002, 282:F202-F210 [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Brown JJ: Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J Infect Dis 1996, 173:746-749 [DOI] [PubMed] [Google Scholar]
- 19.Ho M-K, Springer TA: Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem 1983, 258:636-642 [PubMed] [Google Scholar]
- 20.Tamayo P, Slonim D, Mesirov J, Zhu Q, Kitareewan S, Dmitrovsky E, Lander ES, Golub TR: Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc Natl Acad Sci USA 1999, 96:2907-2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt BS, Landau S, Leese M: Cluster Analysis, ed 4 2001. Arnold and Oxford University Inc, New York
- 22.Toronen P, Kolehmainen M, Wong G, Castren E: Analysis of gene expression data using self-organizing maps. FEBS Lett 1999, 451:142-146 [DOI] [PubMed] [Google Scholar]
- 23.Bernard R: Hypothesis testing for correlation coefficients. Bernard R eds. Fundamentals of Biostatistics. 1990:pp 443-455 PWS Publishers, Boston
- 24.Little SR, Eisen AN: Preparation of immunogenic 2, 4-dinitrophenyl and 2, 4, 6-trinitrophenyl proteins. Methods Immunol Immunochem 1967, 1:128-133 [Google Scholar]
- 25.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ: Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 2001, 98:13396-13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemack GE, Zimmern PE: Interstitial cystitis: reevaluation of patients who do not respond to standard treatments. Prog Urol 2001, 11:239-244 [PubMed] [Google Scholar]
- 27.Messing E: Walsh PC Retik AB Stamey TA Vaughan ED, Jr eds. Interstitial cystitis and related syndromes. Campbell’s Urology. 1992:982-1005 WB Saunders, Philadelphia
- 28.Lecci A, Giuliani S, Meini S, Maggi CA: Nociceptin and the micturition reflex. Peptides 2000, 21:1007-1021 [DOI] [PubMed] [Google Scholar]
- 29.Kameoka H, Shiraiwa Y, Fukaya Y, Yokota T, Shishido K, Yamaguchi O: Effect of naloxone on the bladder activity of rabbits with acute spinal injury. Int J Urol 1998, 5:588-594 [DOI] [PubMed] [Google Scholar]
- 30.Kanie S, Yokoyama O, Komatsu K, Kodama K, Yotsuyanagi S, Niikura S, Nagasaka Y, Miyamoto KI, Namiki M: GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol 2000, 279:1230-1238 [DOI] [PubMed] [Google Scholar]
- 31.Itoh M, Takasaki I, Andoh T, Nojima H, Tominaga M, Kuraishi Y: Induction by carrageenan inflammation of prepronociceptin mRNA in VR1-immunoreactive neurons in rat dorsal root ganglia. Neuroscience Res 2001, 40(Suppl):227-233 [DOI] [PubMed] [Google Scholar]
- 32.MacArthur L, Ren K, Pfaffenroth E, Franklin E, Ruda MA: Descending modulation of opioid-containing nociceptive neurons in rats with peripheral inflammation and hyperalgesia. Neuroscience 1999, 88:499-506 [DOI] [PubMed] [Google Scholar]
- 33.Okamoto-Koizumi T, Takeda M, Komeyama T, Hatano A, Tamaki M, Mizusawa T, Tsutsui T, Obara K, Tomita Y, Arai K, Takahashi K: Pharmacological and molecular biological evidence for ETA endothelin receptor subtype mediating mechanical responses in the detrusor smooth muscle of the human urinary bladder. Clin Sci 1999, 96:397-402 [PubMed] [Google Scholar]
- 34.Khan MA, Dashwood MR, Thompson CS, Mumtaz FH, Mikhailidis DP, Morgan RJ: Up-regulation of endothelin-B (ETB) receptors and ETB receptor-mediated rabbit detrusor contraction in partial bladder outlet obstruction. Br J Urol Int 1999, 84:714-719 [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Longhurst PA, Wein AJ, Levin RM: Effects of sensitization on female guinea pig urinary bladder function: in vivo and in vitro studies. J Urol 1991, 146:454-457 [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Stein PC, Parsons CL, Schmidt JD: Elevated stress protein in transitional cells exposed to urine from interstitial cystitis patients. Int J Urol 1998, 5:444-448 [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Murayama T, Mita H, Akiyama K: Subtypes of bladder mast cells in interstitial cystitis. Int J Urol 2000, 7:292-297 [DOI] [PubMed] [Google Scholar]
- 38.de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, D’Andrea MR, Mayer EA, Wallace JL, Hollenberg MD, Andrade-Gordon P, Bunnett NW: Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol 2001, 133:975-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandaliano G, Monno R, Ranieri E, Gesualdo L, Schena FP, Martino C, Ursi M: Regenerative and proinflammatory effects of thrombin on human proximal tubular cells. J Am Soc Nephrol 2000, 11:1016-1025 [DOI] [PubMed] [Google Scholar]
- 40.Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, Dileepan KN: Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-α. J Interferon Cytokine Res 2001, 21:231-240 [DOI] [PubMed] [Google Scholar]
- 41.Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL: Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 1997, 79:572-577 [DOI] [PubMed] [Google Scholar]
- 42.Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, Bennett NJR, de Groat WC, Glorioso JC, Chancellor MB: Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol 2001, 165:1748-1754 [PubMed] [Google Scholar]
- 43.Rabizadeh S, Rabizadeh S, Ye X, Wang JJ, Bredesen DE: Neurotrophin dependence mediated by p75NTR: contrast between rescue by BDNF and NGF. Cell Death Differ 1999, 6:1222-1227 [DOI] [PubMed] [Google Scholar]
- 44.Oddiah D, Anand P, McMahon SB, Rattray M: Rapid increase of NGF, BDNF, and NT-3 mRNAs in inflamed bladder. Neuroreport 1998, 9:1455-1458 [DOI] [PubMed] [Google Scholar]
- 45.Vizzard MA: Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 2000, 161:273-284 [DOI] [PubMed] [Google Scholar]
- 46.Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD: Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol 2001, 166:1111-1118 [PubMed] [Google Scholar]
- 47.Lee R, Kermani P, Teng KK, Hempstead BL: Regulation of cell survival by secreted proneurotrophins. Science 2001, 294:1945-1948 [DOI] [PubMed] [Google Scholar]
- 48.Lemack GE, Burkhard F, Zimmern PE, McConnell JD, Lin VK: Physiologic sequelae of partial infravesical obstruction in the mouse: role of inducible nitric oxide synthase. J Urol 1999, 161:1015-1022 [PubMed] [Google Scholar]
- 49.Xu X, Cubeddu LX, Malave A: Expression of inducible nitric oxide synthase in primary culture of rat bladder smooth muscle cells by plasma from cyclophosphamide-treated rats. Eur J Pharmacol 2001, 416:1-9 [DOI] [PubMed] [Google Scholar]
- 50.Poljakovic M, Svensson ML, Svanborg C, Johansson K, Larsson B, Persson K: Escherichia coli-induced inducible nitric oxide synthase and cyclooxygenase expression in the mouse bladder and kidney. Kidney Int 2001, 59:893-904 [DOI] [PubMed] [Google Scholar]
- 51.Wheeler MA, Yoon JH, Olsson LE, Weiss RM: Cyclooxygenase-2 protein and prostaglandin E(2) production are up-regulated in a rat bladder inflammation model. Eur J Pharmacol 2001, 417:239-248 [DOI] [PubMed] [Google Scholar]
- 52.Wall BM, Dmochowski RR, Malecha M, Mangold T, Bobal MA, Cooke CR: Inducible nitric oxide synthase in the bladder of spinal cord injured patients with a chronic indwelling urinary catheter. J Urol 2001, 165:1457-1461 [PubMed] [Google Scholar]
- 53.Ehren I, Hosseini A, Lundberg JO, Wiklund NP: Nitric oxide: a useful gas in the detection of lower urinary tract inflammation. J Urol 1999, 162:327-329 [DOI] [PubMed] [Google Scholar]
- 54.Korting GE, Smith SD, Wheeler MA, Weiss RM, Foster HE, Jr: A randomized double-blind trial of oral L-arginine for treatment of interstitial cystitis. J Urol 1999, 161:558-565 [PubMed] [Google Scholar]
- 55.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB: Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn’s disease. Am J Physiol 2001, 280:G1022-G1029 [DOI] [PubMed] [Google Scholar]
- 56.Djavan B, Waldert M, Seitz C, Marberger M: Insulin-like growth factors and prostate cancer. World J Urol 2001, 19:225-233 [DOI] [PubMed] [Google Scholar]
- 57.Sun HZ, Wu SF, Tu ZH: Blockage of IGF-1R signaling sensitizes urinary bladder cancer cells to mitomycin-mediated cytotoxicity. Cell Res 2001, 11:107-115 [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Gawad M, Elhilali MM, Huynh H: Alteration of the insulin-like growth factor system of mitogens in hyperplastic bladders of paraplegic rats. J Urol 1999, 161:699-705 [PubMed] [Google Scholar]
- 59.Keay S, Zhang C-O, Kagen DI: Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol 1997, 158:1983-1988 [DOI] [PubMed] [Google Scholar]
- 60.Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW: Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol 2000, 164:2112-2118 [PubMed] [Google Scholar]
- 61.Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P: A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol 2000, 156:857-864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeMeester SL, Buchman TG, Cobb JP: The heat shock paradox: does NF-κB determine cell fate? EMBO J 2001, 15:270-274 [DOI] [PubMed] [Google Scholar]
- 63.Erickson DR, Belchis DA, Dabbs DJ: Inflammatory cell types and clinical features of interstitial cystitis. J Urol 1997, 158:790-793 [DOI] [PubMed] [Google Scholar]
- 64.Chomarat P, Banchereau J, Davoust J, Palucka AK: IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 2000, 1:510-514 [DOI] [PubMed] [Google Scholar]
- 65.Akishita M, Horiuchi M, Yamada H, Zhang L, Shirakami G, Tamura K, Ouchi Y, Dzau VJ: Inflammation influences vascular remodeling through AT2 receptor expression and signaling. Physiol Genomics 2000, 2:13-20 [DOI] [PubMed] [Google Scholar]
- 66.Lee J, Hur J, Lee P, Kim JY, Cho N, Kim SY, Kim H, Lee MS, Suk K: Dual role of inflammatory stimuli in activation-induced cell death of mouse microglial cells: initiation of two separate apoptotic pathways via induction of interferon regulatory factor-1 and caspase-11. J Biol Chem 2001, 276:32956-32965 [DOI] [PubMed] [Google Scholar]
- 67.Hang L, Haraoka M, Agace WW, Leffler H, Burdick M, Strieter R, Svanborg C: Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol 1999, 162:3037-3044 [PubMed] [Google Scholar]
- 68.Salmi S, Santti R, Gustafsson JA, Makela S: Co-localization of androgen receptor with estrogen receptor β in the lower urinary tract of the male rat. J Urol 2001, 166:674-677 [PubMed] [Google Scholar]
- 69.VanderHorst VG, Meijer E, Holstege G: Estrogen receptor-α immunoreactivity in parasympathetic preganglionic neurons innervating the bladder in the adult ovariectomized cat. Neuroscience Lett 2001, 298:147-150 [DOI] [PubMed] [Google Scholar]
- 70.Pasquali D, Staibano S, Prezioso D, Franco R, Esposito D, Notaro A, De Rosa G, Bellastella A, Sinisi AA: Estrogen receptor β expression in human prostate tissue. Mol Cell Endocrinol 2001, 178:47-50 [DOI] [PubMed] [Google Scholar]
- 71.McKay LI, Cidlowski JA: Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr Rev 1999, 20:435-459 [DOI] [PubMed] [Google Scholar]
- 72.Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, Hannigan BM: Expression of oestrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages, or other immune cells in human upper airways. Thorax 2001, 56:205-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pang X, Cotreau-Bibbo MM, Sant GR, Theoharides TC: Bladder mast cell expression of high affinity oestrogen receptors in patients with interstitial cystitis. Br J Urol 1995, 75:154-161 [DOI] [PubMed] [Google Scholar]
- 74.Gollapudi L, Oblinger MM: Estrogen effects on neurite outgrowth and cytoskeletal gene expression in ER α-transfected pc12 cell lines. Exp Neurol 2001, 171:308-316 [DOI] [PubMed] [Google Scholar]
- 75.Drake MJ, Hedlund P, Mills IW, McCoy R, McMurray G, Gardner BP, Andersson KE, Brading AF: Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest 2000, 80:1491-1499 [DOI] [PubMed] [Google Scholar]
- 76.Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV: Dextran sulfate sodium-induced murine colitis activates NF-κB and increases galanin-1 receptor expression. Am J Physiol 2000, 278:G797-G804 [DOI] [PubMed] [Google Scholar]
- 77.Benya RV, Matkowskyj KA, Danilkovich A, Hecht G: Galanin causes Cl-secretion in the human colon: potential significance of inflammation-associated NF-κ B activation on galanin-1 receptor expression and function. Ann NY Acad Sci 19998, 863:64-77 [DOI] [PubMed] [Google Scholar]
- 78.Hecht G, Marrero JA, Danilkovich A, Matkowskyj KA, Savkovic SD, Koutsouris A, Benya RV: Pathogenic Escherichia coli increase Cl-secretion from intestinal epithelia by up-regulating galanin-1 receptor expression. J Clin Invest 1999, 104:253-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavcovich LA, Valentino RJ: Central regulation of micturition in the rat the corticotropin-releasing hormone from Barrington’s nucleus. Neurosci Lett 1995, 196:185-188 [DOI] [PubMed] [Google Scholar]
- 80.Kageyama K, Gaudriault GE, Bradbury MJ, Vale WW: Regulation of corticotropin-releasing factor receptor type 2 β messenger ribonucleic acid in the rat cardiovascular system by urocortin, glucocorticoids, and cytokines. Endocrinology 2000, 141:2285-2293 [DOI] [PubMed] [Google Scholar]
- 81.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G: Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139:403-413 [DOI] [PubMed] [Google Scholar]
- 82.Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI: Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res 2000, 60:3445-3453 [PubMed] [Google Scholar]
- 83.Vizzard MA: Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol 2000, 278:R1027-R1039 [DOI] [PubMed] [Google Scholar]
- 84.Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA: Similarity of distributions of spinal c-Fos and plasma extravasation after acute chemical irritation of the bladder and the prostate. J Urol 2000, 164:1751-1756 [PubMed] [Google Scholar]
- 85.Kiss S, Yoshiyama M, Cao YQ, Basbaum AI, de Groat WC, Lecci A, Maggi CA, Birder LA: Impaired response to chemical irritation of the urinary tract in mice with disruption of the preprotachykinin gene. Neuroscience Lett 2001, 313:57-60 [DOI] [PubMed] [Google Scholar]
- 86.Truss MC, Stief CG, Uckert S, Becker AJ, Schultheiss D, Machtens S, Jonas U: Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J Urol 2000, 18:439-443 [DOI] [PubMed] [Google Scholar]
- 87.Escofier N, Boichot E, Germain N, Silva PM, Martins MA, Lagente V: Effects of interleukin-10 and modulators of cyclic AMP formation on endotoxin-induced inflammation in rat lung. Fundam Clin Pharmacol 1999, 13:96-101 [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Mira-Arbibe L, Ulevitch RJ: TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J Leukoc Biol 2000, 68:909-915 [PubMed] [Google Scholar]
- 89.Eggen BJ, Benus GF, Folkertsma S, Jonk LJ, Kruijer W: TAK1 activation of the mouse JunB promoter is mediated through a CCAAT box and NF-Y. FEBS Lett 2001, 506:267-271 [DOI] [PubMed] [Google Scholar]
- 90.Irie T, Muta T, Takeshige K: TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-κB in lipopolysaccharide-stimulated macrophages. FEBS Lett 2000, 467:160-164 [DOI] [PubMed] [Google Scholar]
- 91.Grossman HB, Lee C, Bromberg J, Liebert M: Expression of the α6β4 integrin provides prognostic information in bladder cancer. Oncol Rep 2000, 7:13-16 [PubMed] [Google Scholar]
- 92.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF: Intestinal mast cell progenitors require CD49dβ7 (α4β7 integrin) for tissue-specific homing. J Exp Med 2001, 194:1243-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfeiffer A, Bottcher A, Orso E, Kapinsky M, Nagy P, Bodnar A, Spreitzer I, Liebisch G, Drobnik W, Gempel K, Horn M, Holmer S, Hartung T, Multhoff G, Schutz G, Schindler H, Ulmer AJ, Heine H, Stelter F, Schutt C, Rothe G, Szollosi J, Damjanovich S, Schmitz G: Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol 2001, 31:3153-3164 [DOI] [PubMed] [Google Scholar]
- 94.Yang T, Sun D, Huang YG, Smart A, Briggs JP, Schnermann JB: Differential regulation of COX-2 expression in the kidney by lipopolysaccharide: role of CD14. Am J Physiol 1999, 277:F10-F16 [DOI] [PubMed] [Google Scholar]
- 95.Gluck T, Silver J, Epstein M, Cao P, Farber B, Goyert SM: Parameters influencing membrane CD14 expression and soluble CD14 levels in sepsis. Eur J Med Res 2001, 6:351-358 [PubMed] [Google Scholar]
- 96.Mulvey MA, Schilling JD, Hultgren SJ: Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 2001, 69:4572-4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Debouk C, Goodfellow PN: DNA microarrays in drug discovery. Nature Med 1999, 21:48-50 [DOI] [PubMed] [Google Scholar]