Abstract
Recent evidence indicates that topical application of adenosine A2A receptor agonists, unlike growth factors, increases the rate at which wounds close in normal animals and promotes wound healing in diabetic animals as well as growth factors, yet neither the specific adenosine receptor involved nor the mechanism(s) by which adenosine receptor occupancy promotes wound healing have been fully established. To determine which adenosine receptor is involved and whether adenosine receptor-mediated stimulation of angiogenesis plays a role in promotion of wound closure we compared the effect of topical application of the adenosine receptor agonist CGS-21680 (2-p-[2-carboxyethyl]phenethyl-amino-5′-N-ethylcarboxamido-adenosine) on wound closure and angiogenesis in adenosine A2A receptor knockout mice and their wild-type littermates. There was no change in the rate of wound closure in the A2A receptor knockout mice compared to their wild-type littermates although granulation tissue formation was nonhomogeneous and there seemed to be greater inflammation at the base of the wound. Topical application of CGS-21680 increased the rate of wound closure and increased the number of microvessels in the wounds of wild-type mice but did not affect the rate of wound closure in A2A receptor knockout mice. Similarly, in a model of internal trauma and repair (murine air pouch model), endogenously produced adenosine released into areas of internal tissue injury stimulates angiogenesis because there was a marked reduction in blood vessels in the walls of healing air pouches of A2A receptor knockout mice compared to their wild-type controls. Inflammatory vascular leakage and leukocyte accumulation in the inflamed air pouch were similarly reduced in the A2A receptor knockout mice reflecting the reduced vascularity. Thus, targeting the adenosine A2A receptor is a novel approach to promoting wound healing and angiogenesis in normal individuals and those suffering from chronic wounds.
Adenosine, a potent endogenous physiological mediator, regulates a wide variety of physiological processes. Adenosine mediates its physiological effects via interaction with one or more of four known cell-surface receptors (A1, A2A, A2B, and A3). Previous studies have demonstrated that topical application of adenosine A1 or A2A receptor agonists promotes healing of full thickness dermal wounds although the adenosine receptors involved and the mechanism for this effect have not been fully established. 1,2 Among the pharmacological properties that may contribute to its effect in wound healing adenosine is reported to be angiogenic, based on in vitro studies. Adenosine, acting at A2 receptors, stimulates endothelial cell migration, proliferation, and secretion of vascular endothelial growth factor in vitro. 3-13 Adenosine is also angiogenic in the chorioallantoic membrane angiogenesis assay and it is found prominently at the edge of forming vasculature during retinal vasculogenesis and oxygen-induced retinopathy in vivo, where adenosine A2A and/or A2B receptors and 5′-nucleotidase are highly expressed. 13-17
In addition to the effects of adenosine on angiogenesis we and others have established that adenosine is a potent regulator of inflammation, the first stage of the wound-healing process. 18 The capacity of adenosine to suppress inflammation via occupancy of A2 receptors was first noted for neutrophils 19-21 and subsequently adenosine was shown to regulate the inflammatory functions of many other cell types including macrophages, endothelial cells, and lymphocytes. 22
The results reported here confirm the role of adenosine A2A receptors in promotion of wound healing after topical application of the selective A2A receptor agonist CGS-21680 and suggest a mechanism by which adenosine A2A receptor occupancy promotes wound healing. Evidence from experiments performed in adenosine A2A receptor knockout mice clearly demonstrate that the adenosine receptor involved in promotion of wound healing is the A2A receptor. We report further that interaction of exogenous adenosine A2A receptor agonists with their receptors increases angiogenesis in the healing wounds as compared to vehicle controls. Moreover, endogenously released adenosine interacts with A2A receptors to promote angiogenesis in an internal wound as well. These observations provide further evidence that agents that activate adenosine A2A receptors may be useful for promotion of wound healing and suggest that promotion of angiogenesis plays a major role in the mechanism by which adenosine A2A receptor agonists promote wound healing.
Materials and Methods
Materials
CGS-21680 (2-p-[2-carboxyethyl]phenethyl-amino-5′-N-ethylcarboxamido-adenosine), carrageenan (type I), thioglycollate medium (FTG), and Evans Blue were obtained from Sigma Chemical Co. (St. Louis, MO). All other materials were the highest quality that could be obtained.
Animal Subjects
BALB/c mice were purchased from Taconic Farms (Germantown, NY). Mice with a targeted disruption of the gene for the adenosine A2A receptor have been described in detail elsewhere. 23 The mice used in these experiments were derived from four original heterozygous breeding pairs. Mice were housed in the NYU animal facility, fed regular mouse chow, and given access to drinking water ad libitum. All procedures described below were reviewed and approved by the Institutional Animal Care and Use Committee of NYU Medical Center and performed under the supervision of the facility veterinary staff.
Polymerase Chain Reaction (PCR) Confirmation of Mouse Genotype
DNA was extracted from the tips of mouse tails using a standard protocol. Briefly, tail tips were lysed in 500 μl of lysis buffer (100 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 8.0, 10 mmol/L ethylenediaminetetraacetic acid, 0.5% sodium dodecyl sulfate, 400 μg/ml proteinase K) overnight at 55°C. To the lysed tips 300 μl of a saturated solution of NaCl was added and, after 10 minutes on ice, tubes were centrifuged (16,000 × g, 4°C for 10 minutes). Genomic DNA present in the supernatant was precipitated by the addition of 800 μl of isopropanol. Precipitates were washed once with 70% ethanol, vacuum-dried, and resuspended in 30 μl of TE buffer. Using a method originally worked out by the laboratory of Dr. M. Sitkovsky (personal communication) the genomic DNA was then subjected to PCR using the following primers 5′-AGCCAGGGGTTACATCTGTG-3′ (upstream) and 5′-TACAGACAGCCTCGACATGTG-3′ (downstream), which detect a 163-bp band for the wild-type A2A allele; and 5′-AGACAATCGGCTGCTCTGAT-3′ (upstream) and 5′-CAAGCTCTTCAGCAATATCACG-3′ (downstream), which detect a 618-bp band for the mutated A2A allele. To perform the PCR, 0.3 μg of genomic DNA was used in 30 μl of final reaction. The PCR was performed in a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer; Branchburg, NJ) under the following conditions: 95°C for 2 minutes followed by 40 cycles (94°C for 1 minute, 55°C for 20 seconds, and 72°C for 1 minute) and a final extension of 72°C for 10 minutes.
Excisional Wound Formation
Two sterile, full-thickness excisional wounds of 10 mm in diameter were created on the dorsum of anesthetized 12- to 15-week-old male and female mice using a template and scissors in a genotype-blind manner. Wounds were treated daily with topical application of 20 μl of either the adenosine agonist CGS-21680 (250 μg/ml) or vehicle [1.5% w/v carboxymethylcellulose in phosphate-buffered saline (PBS)]. Mice were kept individually caged to minimize licking of wounds. To determine rate of closure, wound outlines were traced onto clear plastic sheets and the area of the wounds was measured by digitization of the tracings with a WACOM artZ II graphics tablet (Wacom Co., Ltd., Taiwan) and SigmaScan Pro software (Jandel Scientific, San Rafael, CA). Wounds were considered completely healed when the wound area was entirely re-epithelialized, and the surface of the wound was smooth, homogenous in color, and without residual defects.
Histological Study of Wounds
Some animals were killed on the stated day by CO2 poisoning. Wounds were then excised and fixed in 10% neutral-buffered formalin for at least 3 days and then processed by usual histopathological tissue processing. Paraffin-embedded tissue sections (5-μm thick) were stained with hematoxylin and eosin (H&E) and reviewed.
Induction of Air Pouches and Carrageenan-Induced Inflammation
To induce an air pouch, 10- to 15-week-old mice were injected subcutaneously on the back with 3 ml of air. After 2 days the pouches were reinflated with 1.5 ml of air. On day 6, inflammation was induced by injection of 1 ml of a suspension of carrageenan (2% w/v in calcium- and magnesium-free PBS) into the air pouch, as we have previously described. 24 After 4 hours the mice were sacrificed by CO2 narcosis, the pouches were flushed with 2 ml of PBS, and the exudates harvested. Aliquots were diluted 1:1 with methylene blue (0.01% w/v in PBS) and cells were counted in a standard hemocytometer chamber (American Optical, Buffalo, NY).
Evans Blue Extravasation Index
Air pouches were induced as described above. On day 6, 0.1 ml of Evans Blue dye (6.25 mg/ml) was injected intraperitoneally 1 hour before induction of inflammation by injection of carrageenan. After 4 hours, animals were sacrificed, air pouch exudates were harvested, and blood obtained by cardiac puncture was subjected to centrifugation and 1:50 dilution in PBS. The extravasation index was calculated as the ratio of the absorbances of the cell-free exudates and diluted serum at 620 nm.
Histological Study of Air Pouches
Air pouches were induced as described above in a similar group of mice. On day 6, animals were sacrificed and air pouches harvested and fixed in 10% neutral-buffered formalin for at least 3 days and then processed by usual histopathological tissue processing. Paraffin-embedded tissue sections (5-μm thick) were stained with H&E and reviewed.
Peritoneal Inflammation
Thioglycollate peritonitis was induced by intraperitoneal injection of 0.5 ml of a sterile solution of thioglycollate medium (10% w/v in PBS). After 4 hours the animals were sacrificed by CO2 and their peritoneal cavities were lavaged with 3 ml of cold PBS. The peritoneal area was massaged before withdrawing the lavage fluid. Exudates were maintained at 4°C until aliquots were diluted 1:1 with methylene blue (0.01% w/v in PBS) and cells were counted in a standard hemocytometer chamber.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed paraffin-embedded tissues sections (5-μm thick) with appropriate positive and negative controls for Factor VIII and CD34. Antibodies, Factor VIII, and CD34 (Ventana Medical Systems, Tucson, AZ), were applied from prediluted dispensers on an automated immunostainer (NexES, Ventana Medical Systems) followed by washing and application of immunoperoxidase-labeled anti-goat antibodies. The positively stained cells were visualized by addition of immunoperoxidase substrate diaminobenzidine and the sections were counterstained with hematoxylin. The stained sections were reviewed for overall thickness of the dermal wound or induced air pouch, thickness of the granulation tissue layer, and a count of the blood vessels, as has been previously reported. 25-27 Tissue sections were reviewed at low magnification (×2) and counting of positive-stained endothelial cells was performed blindly on five random fields at a higher magnification (×40). The results are expressed as the mean (±SEM) of the values obtained from the areas studied.
Statistical Analysis
Differences between groups were analyzed by means of one-way and two-way analyses of variances using SigmaStat (SPSS, Inc., Chicago, IL).
Results
Topical Application of an Adenosine A2A Receptor Agonist Promotes Wound Healing in Wild-Type Mice but Not in A2A Knockout Mice
We have previously demonstrated, by use of receptor-specific antagonists and agonists, that occupancy of adenosine A2A receptors increases the rate at which wounds heal but others have reported that A1 receptors are involved in promotion of wound healing. 1,2 To test the hypothesis that occupancy of A2A receptors plays a role in the normal process of wound healing, we compared rates of dermal wound closure and final healing time in adenosine A2A receptor knockout mice and their wild-type littermates. The rate of wound healing was similar in A2A knockout mice and their wild-type littermates (50% of wounds closed between days 12 and 13 or between days 13 and 14, respectively; Figure 1 ▶ and Table 1 ▶ ) consistent with our previous observation that A2A receptor antagonists do not interfere with the rate of wound closure. 1 In contrast, application of the adenosine A2A receptor agonist CGS-21680 increased the rate of wound closure in wild-type mice (50% of wounds closed between days 11 and 12, P < 0.001, versus vehicle-treated; Figure 1 ▶ and Table 1 ▶ ) but not in A2A knockout mice (50% of wounds closed between days 12 and 13, NS versus vehicle-treated).
Figure 1.
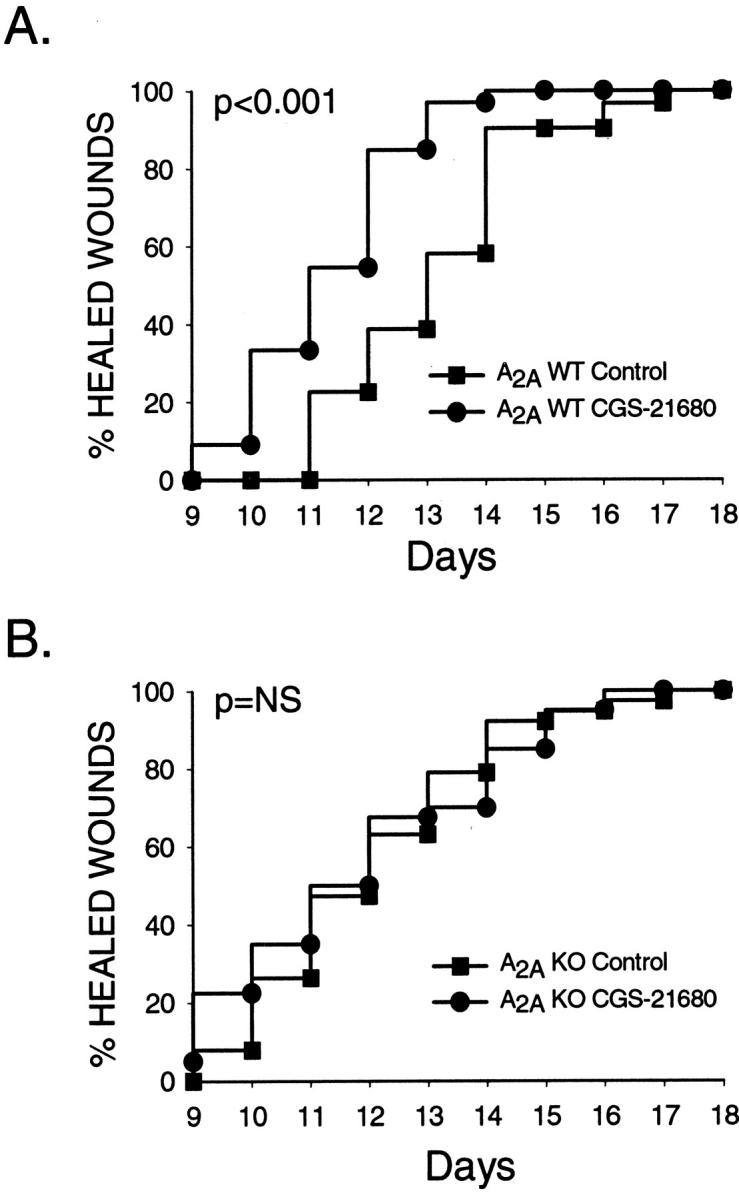
The effect of CGS-21680 treatment on wound healing in wild-type and A2A receptor knockout mice. Two excisional wounds (10 mm in diameter) were created on the dorsum of sex- and age-matched wild-type (A) and A2A knockout (B) mice on day 0 and treated daily with a topical application of vehicle or CGS-21680 (5 μg/wound) as described in Materials and Methods. Mice were kept individually caged to minimize licking of wounds. A: Results are presented as a Kaplan-Meier plot of cohorts of 30 control wounds and 32 CGS-21680-treated wounds on wild-type mice. B: Results are presented as a Kaplan-Meier plot of cohorts of 38 control wounds and 40 CGS-21680-treated wounds on A2A knockout mice.
Table 1.
Adenosine A2A Receptor Agonist Promotes Wound Healing
| 50% wound closure, day | 50% of wounds healed, day | |
|---|---|---|
| Wild type | 4.3 | 13.6 |
| Vehicle treated (n = 30) | ||
| Wild type | 2.9* | 11.8† |
| CGS-21680 treated (n = 32) | ||
| A2A receptor knockout | 3.3 | 12.2 |
| Vehicle treated (n = 38) | ||
| A2A receptor knockout | 3.2 | 12.0 |
| CGS-21680 treated (n = 40) |
Wounds (12-mm in diameter) were created on the dorsum of mice on day 0, the wound area determined, and expressed as the percentage of wound closure, as described in Materials and Methods. As a measure of the rate of wound closure the day at which 50% of the area of the original wound remained open is presented here (50% wound closure). A measure of the time at which complete wound closure occurred in 50% of the animals in any group was extrapolated from the plot in Figure 1 ▶ and is expressed as the time at which 50% of the wounds had completely healed.
*P < 0.05 versus vehicle-treated wild-type mice, 2-way analysis of variance (Tukey test).
†P < 0.001 versus vehicle-treated wild-type mice, 2-way analysis of variance (Tukey test).
A2A Knockout Mice Have a Defect in Granulation Tissue Formation in Dermal Excisional Wounds
Wound closure, as described above, represents primarily re-epithelialization and developing granulation tissue. In previous studies, we had observed that adenosine A2A receptor agonist treatment also affected granulation tissue formation. 1 We therefore compared the histological appearance of healing dermal wounds in the A2A knockout and wild-type control mice and observed a marked defect in granulation tissue formation in the A2A knockout mice. Three days after wound formation there was a thick layer of granulation tissue consisting of edematous matrix infiltrated by large numbers of fibroblasts, macrophages, and infiltrating neutrophils in the wild-type control mice. In contrast, 3 days after wound formation, granulation tissue was almost completely absent in the A2A receptor knockout mice (Figure 2A) ▶ . In the center of the healing wounds in knockout mice there was edematous, loose connective tissue and nonpolarized cells in addition to an infiltrate of inflammatory cells (neutrophils). A marked difference in granulation tissue persisted even by 6 days after wound formation (Figure 2B) ▶ . There was a uniform layer of granulation tissue at the base of the wounds in the wild-type control mice. In contrast, granulation tissue was spotty and nonuniform at the base of the wounds in A2A receptor knockout mice. In some areas granulation tissue was well organized and highly cellular whereas in other areas the granulation tissue consisted of fibroblasts and other cells lacking polarity and loose extracellular matrix. In other areas there appeared to be a relative increase in inflammatory cells as well. As expected from the macroscopic wound evaluations, the extent of re-epithelialization did not differ between wild-type controls and A2A knockout mice (Figure 2) ▶ . CGS-21680 treatment of wounds enhanced fibroblast infiltration, matrix density, and re-epithelialization in wild-type mice but it had no effect in knockout mice (Figure 2, A and B) ▶ .
Figure 2.

Histological examination of representative sections of untreated and CGS-21680-treated excisional wounds in wild-type and A2A receptor knockout mice. Excisional wounds were formed on the backs of wild-type and A2A knockout mice and treated as described. On the stated day, mice were killed and the wounds dissected out. The dissected wounds were fixed, embedded in paraffin, and stained with H&E using standard techniques. Original magnifications were ×100 (low power) or ×400 (high power). A: Wounds were harvested 3 days after wounding. 1: Wild-type mouse treated with vehicle, low power. 2: Wild-type mouse treated with CGS-21680, low power. 3: Wild-type mouse treated with vehicle, high power. 4: Wild-type mouse treated with CGS-21680, high power. 5: A2A receptor knockout mouse treated with vehicle, low power. 6: A2A receptor knockout mouse treated with CGS-21680, high power. 7: A2A receptor knockout mouse treated with vehicle, high power. 8: A2A knockout mouse treated with CGS-21680, high power. B: Wounds were harvested 6 days after wounding. 1: Wild-type mouse treated with vehicle, low power. 2: Wild-type mouse treated with CGS-21680, low power. 3: Wild-type mouse treated with vehicle, high power. 4: Wild-type mouse treated with CGS-21680, high power. 5: A2A receptor knockout mouse treated with vehicle, low power. 6: A2A receptor knockout mouse treated with CGS-21680, high power. 7: A2A receptor knockout mouse treated with vehicle, high power. 8: A2A knockout mouse treated with CGS-21680, high power.
Topical Application of an Adenosine A2A Receptor Agonist Increases the Number of Factor VIII-Positive Endothelial Cells in the Granulation Tissue of Wild-Type Mice
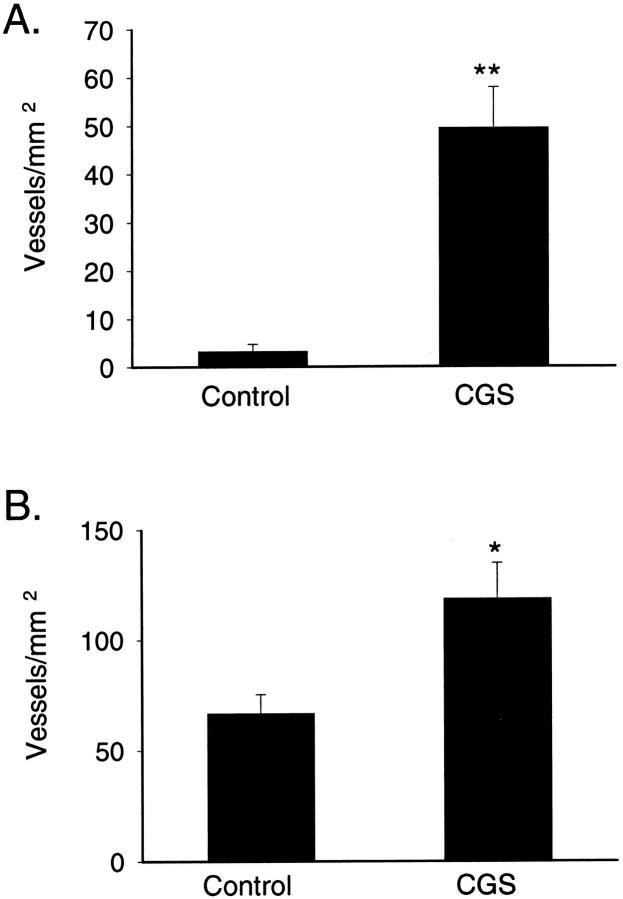
Previous studies have indicated that adenosine, acting at A2A receptors, promotes angiogenesis in in vitro models, 3-7,9-11,28 a critical component of granulation tissue. To determine whether angiogenesis was similarly compromised in the wounds of the A2A receptor knockout mice and whether application of an A2A receptor agonist promotes angiogenesis in vivo we examined the vascularity of wounds in knockout and wild-type control mice. In all of the sections of wounds stained for Factor VIII there was high background staining for Factor VIII because of presumed leakage of plasma into the tissue at the site of the wound. Nonetheless, individual cells and blood vessels were clearly differentiated from the background staining by the immunostaining for Factor VIII. In the 3-day-old wounds of wild-type mice there was a 15-fold increase in the number of Factor VIII-positive endothelial cells in the CGS-21680-treated wounds (50 ± 8 endothelial cells/mm2 versus 3 ± 2 endothelial cells/mm2, n = 19 and 14, respectively, P < 0.001; Figures 3 and 4A ▶ ▶ ). The CGS-21680-induced increase in vascularity persisted in the 6-day-old wounds although the disparity was not as great as at 3 days (119 ± 8 endothelial cells/mm2 versus 67 ± 9 endothelial cells/mm2, n = 18, P < 0.005; Figures 3 and 4B ▶ ▶ ). In the knockout mice, Factor VIII-positive endothelial cells were hardly found in the edematous 3-day-old wounds, and were not distributed uniformly in the 6-day-old wounds; most vessels were found in groups or aligned in the middle of the granulation tissue along a band of well-organized granulation tissue and no vessels were observed in the areas where granulation tissue was unorganized or where there was marked edema. Consequently, we were unable to adequately assess uniformly the number of vessels in the wounds of the knockout mice (Figure 3) ▶ .
Figure 3.

Immunohistological examination of representative sections of untreated and CGS-21680-treated excisional wounds in wild-type and A2A receptor knockout mice. Excisional wounds were formed on the backs of wild-type and A2A knockout mice and treated as described. On the stated day, mice were killed and the wounds dissected out. The dissected wounds were fixed, embedded in paraffin, and stained immunohistologically with a goat anti-Factor VIII antibody, visualized by immunoperoxidase-mediated conversion of diaminobenzidine, and counterstained with hematoxylin. Original magnification, ×400.
Figure 4.
Number of Factor VIII-positive endothelial cells in the granulation tissue of untreated and CGS-21680-treated excisional wounds in wild-type mice. The number of Factor VIII-positive endothelial cells was enumerated in the immunohistologically labeled sections, as described. A: Shown are the means (±SEM) of five different fields counted on six control (untreated) and six CGS-21680-treated 3-day-old wounds. B: Shown are the means (±SEM) of five different fields counted on six control (untreated) and six CGS-21680-treated 6-day-old wounds. **, P < 0.001 versus control mice, Student’s t-test; *, P < 0.005 versus control mice, Student’s t-test.
A2A Knockout Mice Have a Defect in Granulation Tissue Formation and Fewer Microvessels in the Air Pouch Wall
To better study the endogenous effect of adenosine A2A receptor on angiogenesis, we examined vessel formation and vascular function in a different model of wound healing, the air pouch model. In this model a mechanical injury is induced in the subcutaneous tissue by injection of air forming a pouch, which is lined by newly formed granulation tissue. Microscopic examination of the air pouch walls revealed a difference between the granulation tissue formed in the wild-type and A2A knockout mice that was similar to that observed in the dermal wounds (Figure 5) ▶ . In the pouches of the knockout mice the extracellular matrix was less dense with fewer infiltrating cells than in the pouches of wild-type control mice. Moreover, in the 6-day-old pouches we observed a 47% reduction in Factor VIII-positive endothelial cells in the knockout mice as compared to the wild-type controls (Figures 5 and 6) ▶ ▶ . Interestingly, as compared to excisional wounds in the dermis, there were approximately three times as many Factor VIII-positive endothelial cells present in the walls of the air pouches in both the wild-type and knockout mice at day 6, as reflected in the increased density of these cells shown in Figure 5 ▶ .
Figure 5.

Histological and immunohistological examination of representative sections of air pouch wall in wild-type and A2A receptor knockout mice Subcutaneous vesicles were formed on the backs of wild-type and knockout mice as described. After 6 days, the mice were killed and the pouches dissected out. The dissected pouches were fixed, embedded in paraffin, and stained with H&E using standard techniques (A and B) or stained immunohistologically with a goat anti-Factor VIII antibody, visualized by immunoperoxidase-mediated conversion of diaminobenzidine, and counterstained with hematoxylin (C and D). A: Shown is a representative section from a pouch wall from a female wild-type mouse (original magnification, ×100). B: Shown is a representative section from a pouch wall from a female A2A receptor knockout mouse (original magnification, ×100). C: Shown is a representative section from a pouch wall from a female wild-type mouse (same block as in A; original magnification, ×400). D: Shown is a representative section from a pouch wall from a female knockout mouse (same block as in B; original magnification ×400).
Figure 6.
Number of Factor VIII-positive endothelial cells in the walls of air pouches of A2A receptor knockout mice The number of Factor VIII-positive endothelial cells was enumerated in the immunohistologically labeled sections as described. Shown are the means (±SEM) of five different fields counted on eight wild-type and 10 age- and sex-matched knockout mice. **, P < 0.001 versus wild-type mice, Student’s t-test.
A2A Knockout Mice Have Less Extravasation of Evans Blue and Less Leukocyte Accumulation in the Air Pouch Inflammatory Exudates
To confirm the immunohistochemical findings we assessed the vascularity of the air pouch walls by determining leakage of an intravascular dye into the lumen of the air pouch before and after an inflammatory stimulus. Little or no leakage of Evans Blue from the vasculature into the lumen of the air pouch could be detected in the pouches of either the wild-type or control mice when no inflammatory stimulus was injected into the air pouch. In contrast, there was a 27 ± 7% reduction in extravasation of Evans Blue dye into the lumen of the air pouches of A2A receptor knockout mice as compared to wild-type controls (P < 0.002, n = 6) after inducing inflammation with carrageenan. The reduction in dye extravasation into the inflamed air pouch lumen of A2A knockout mice was reflected by a 29% reduction in leukocyte accumulation (>95% polymorphonuclear cells in both wild-type and A2A knockout mice) in the air pouch exudates (Table 2) ▶ . The diminished leukocyte accumulation in the air pouch of knockout mice is not because of a generalized defect in leukocyte recruitment to inflamed sites, because leukocyte accumulation in the peritoneal cavity was similar in A2A receptor-deficient and wild-type mice (Table 2) ▶ and no corresponding alteration in peripheral leukocyte count was observed (data not shown).
Table 2.
Leukocyte Accumulation in Inflammatory Exudates
| A2A knockout (×106 cells/ml) (±SEM) | Wild-type (×106 cells/ml) (±SEM) | |
|---|---|---|
| Air pouch exudate | 1.84 ± 0.16* | 2.56 ± 0.15 |
| n = 26 | n = 25 | |
| Peritoneal exudate | 3.51 ± 0.32 | 2.95 ± 0.31 |
| n = 21 | n = 18 |
Inflammatory exudates were induced in either the air pouch or the peritoneum of knockout and wild-type mice, as described. After 4 hours the exudates were collected and the leukocytes quantitated. The wild-type control mice were derived from the same heterozygous breeding pairs and matched for age and sex. There was no difference in the number of leukocytes accumulating in the exudates of male versus female mice in either the knockout or wild-type control mice.
*P < 0.001 versus wild-type mice, Student’s t-test.
Discussion
Since the pioneering work of Drury and Szent-Gyorgi 29 70 years ago it has been clear that adenosine regulates many adaptive physiological processes. Adenosine modulates cellular and organ function via occupancy of specific cell-surface receptors, of which there are four known subtypes (A1, A2A, A2B, and A3), all of which are members of the large family of 7-transmembrane spanning, heterotrimeric G protein-associated family of receptors. 30 The results reported here demonstrate that activation of A2A receptors promotes wound healing and is required for uniform formation of granulation tissue and revascularization. Mice lacking A2A receptors form less dense granulation tissue and fewer blood vessels during wound repair and, surprisingly, accumulate fewer leukocytes in response to an inflammatory stimulus. However, as with all comparisons of knockout and wild-type mice, it is possible that the differences observed between the wild-type and knockout mice result from an adaptive change in the knockout mice although no other adaptive changes have previously been reported in A2A knockout mice.
Montesinos and colleagues 1 have recently reported that topical adenosine receptor agonists promote wound healing via occupancy of adenosine A2A receptors in healthy mice and diabetic rats. Other investigators suggested that A1 receptors may also be involved in the promotion of wound healing by adenosine, 2 however in that study antagonist data were not reported so that participation of A2A receptors at the high concentrations of the A1 agonist used could not be ruled out. The results presented here clearly confirm the involvement of A2A receptors in promoting wound healing, because mice lacking A2A receptors do not respond to topical application of selective adenosine A2A agonist.
The mechanism by which adenosine A2A receptor occupancy promotes wound healing has not been established and is likely to be multifactorial. The results of studies in knockout mice indicate that activation of A2A receptors by endogenous adenosine is essential for the proper formation of granulation tissue in two different models of injury, excisional wounds and air pouches. In the skin granulation tissue did not form properly in the A2A knockout mice for at least 3 days after creation of the wound and the deficit in granulation tissue was still obvious by day 6. Previous studies have demonstrated that adenosine and adenine nucleotides promote fibroblast and endothelial cell migration and proliferation 1,31-33 (most likely via activation of A2 receptors 34 ), critical steps in the formation of granulation tissue and consistent with the observed defect in granulation tissue formation. Moreover, the role of adenosine A2A receptors in granulation tissue formation probably accounts for the enhanced granulation tissue formation in the wounds of wild-type mice treated with CGS-21680. It has previously been demonstrated that adenosine A2A receptor occupancy inhibits keratinocyte proliferation and this may explain why re-epithelialization of dermal wounds was not compromised in the A2A knockout mice despite their defect in granulation tissue formation. 32,35
The angiogenic effects of adenosine, acting at A2 receptors, have previously been demonstrated in vitro. Adenosine A2A receptor occupancy promotes endothelial cell proliferation, migration, and synthesis of message for the important angiogenic growth factor vascular endothelial growth factor. 3-12 More recent studies have suggested that the A2 receptor involved is, however, the A2B receptor. 13,17 The results reported here confirm that adenosine A2 receptors are angiogenic and indicate, based on in vivo studies in A2A knockout mice and wild-type mice, that adenosine A2A receptors are involved, either directly or indirectly, in promotion of angiogenesis in healing wounds. Nonetheless, participation of A2B receptors in the enhanced angiogenesis observed in the CGS-21680-treated wounds studied here cannot be ruled out.
We were surprised to find fewer leukocytes in the inflammatory exudates in the air pouches of knockout mice, a finding inconsistent with the known anti-inflammatory role of adenosine A2A receptors in acute inflammation. Because there was no change in the number of circulating leukocytes and the inflammatory peritoneal exudates in the knockout mice did not differ from those in the wild-type mice it is unlikely that the induced deficiency in A2A receptors is associated with a defect in the acute inflammatory response or with a difference in the number or functional capacity of the A2A receptor-deficient leukocytes. 18,36-38 Thus, the reduction in the number of microvessels in the air pouch walls of the A2A knockout mice is the most likely factor leading to reduced leukocyte accumulation in the inflamed air pouch, a finding that correlates with the diminished extravasation of Evans Blue dye.
Adenosine is generated as a result of ATP catabolism and thus is well suited for the role of metabolic regulator of such processes as coronary vasodilation in response to ischemia; adenosine concentrations increase from nanomolar to micromolar during ischemia as a result of ATP utilization. 39-43 However, ischemia and increased work may not be the only stimuli for adenosine release; other types of cellular injuries can lead to release into the extracellular space of adenosine or adenine nucleotides that can be converted extracellularly to adenosine. 44-47 Moreover, necrosis of cells may occur after mechanical or inflammatory injury leading to release of intracellular contents and ATP is present intracellularly in millimolar concentrations. Adenine nucleotides are also released into the extracellular space by ischemic or injured cells and tissues where they are converted, ultimately, to adenosine by the action of ecto-5′nucleotidase. 44-47 After sublethal injury, loss of as little as 5% of cellular ATP to adenosine may lead to 10-fold increases in extracellular adenosine. 19,48 Pharmacological agents may also increase extracellular adenosine; we have found that treatment of animals with low-dose weekly methotrexate, sulfasalazine, salicylates, or an adenosine kinase inhibitor leads to increased adenosine concentrations and diminished leukocyte accumulation in inflammatory exudates. 24,49-51 Similarly, adenosine uptake inhibitors may also possess anti-inflammatory effects 52 by virtue of their capacity to increase extracellular adenosine concentrations.
The physiological and pharmacological effects of adenosine, acting at one or another of its receptors, are observed in nearly every tissue and organ. The studies reported here confirm that enhancement of wound healing and angiogenesis is mediated by adenosine A2A receptors. The observations reported here further suggest that targeting adenosine A2A receptors may lead to development of agents that are useful for stimulating wound healing.
Footnotes
Address reprint requests to Bruce N. Cronstein, NYU Medical Center, Department of Medicine, 550 First Ave., New York, NY 10016. E-mail: cronsb01@med.nyu.edu.
Supported by grants from the National Institutes of Health (AR41911, GM56268), King Pharmaceuticals, Inc., the General Clinical Research Center (M01RR00096), and the Kaplan Comprehensive Cancer Center.
References
- 1.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN: Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med 1997, 186:1615-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun LL, Xu LL, Nielsen TB, Rhee P, Burris D: Cyclopentyladenosine improves cell proliferation, wound healing, and hair growth. J Surg Res 1999, 87:14-24 [DOI] [PubMed] [Google Scholar]
- 3.Meininger CJ, Schelling ME, Granger HJ: Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am J Physiol 1988, 255:H554-H562 [DOI] [PubMed] [Google Scholar]
- 4.Des Rosiers C, Nees S: Functional evidence for the presence of adenosine A2-receptors in cultured coronary endothelial cells. NaunynSchmiedebergs Arch Pharmacol 1987, 336:94-98 [DOI] [PubMed] [Google Scholar]
- 5.Ethier MF, Chander V, Dobson JG, Jr: Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol 1993, 265:H131-H138 [DOI] [PubMed] [Google Scholar]
- 6.Schiele JO, Schwabe U: Characterization of the adenosine receptor in microvascular coronary endothelial cells. Eur J Pharmacol 1994, 269:51-58 [DOI] [PubMed] [Google Scholar]
- 7.Fischer S, Sharma HS, Karliczek GF, Schaper W: Expression of vascular permeability factor/vascular endothelial growth factor in pig cerebral microvascular endothelial cells and its upregulation by adenosine. Mol Brain Res 1995, 28:141-148 [DOI] [PubMed] [Google Scholar]
- 8.Sexl V, Mancusi G, Baumgartner-Parzer S, Schutz W, Freissmuth M: Stimulation of human umbilical vein endothelial cell proliferation by A2-adenosine and beta 2-adrenoceptors. Br J Pharmacol 1995, 114:1577-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP: Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci 1996, 37:2165-2176 [PubMed] [Google Scholar]
- 10.Takagi H, King GL, Ferrara N, Aiello LP: Hypoxia regulates vascular endothelial growth factor receptor KDR/Flk gene expression through adenosine A2 receptors in retinal capillary endothelial cells. Invest Ophthalmol Vis Sci 1996, 37:1311-1321 [PubMed] [Google Scholar]
- 11.Ethier MF, Dobson JG, Jr: Adenosine stimulation of DNA synthesis in human endothelial cells. Am J Physiol 1997, 272:H1470-H1479 [DOI] [PubMed] [Google Scholar]
- 12.Lutty GA, Mathews MK, Merges C, McLeod DS: Adenosine stimulates canine retinal microvascular endothelial cell migration and tube formation. Curr Eye Res 1998, 17:594-607 [PubMed] [Google Scholar]
- 13.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L: Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res 1999, 85:699-706 [DOI] [PubMed] [Google Scholar]
- 14.Dusseau JW, Hutchins PM: Hypoxia-induced angiogenesis in chick chorioallantoic membranes: a role for adenosine. Respir Physiol 1988, 71:33-44 [DOI] [PubMed] [Google Scholar]
- 15.Lutty GA, Merges C, McLeod DS: 5′ nucleotidase and adenosine during retinal vasculogenesis and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2000, 41:218-229 [PubMed] [Google Scholar]
- 16.Taomoto M, McLeod DS, Merges C, Lutty GA: Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2000, 41:230-243 [PubMed] [Google Scholar]
- 17.Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L: Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci 2001, 42:2068-2073 [PubMed] [Google Scholar]
- 18.Montesinos MC, Cronstein BN: Role of P1 receptors in inflammation. Williams M. P. A. M. eds. Handbook of Experimental Pharmacology, vol. 151/II. Purinergic and Pyrimidinergic Signalling II. Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Function. 2001, :pp 303-321 Springer, Berlin [Google Scholar]
- 19.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R: Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med 1983, 158:1160-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R: Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol 1985, 135:1366-1371 [PubMed] [Google Scholar]
- 21.Cronstein BN, Levin RI, Philips MR, Hirschhorn R, Abramson SB, Weissmann G: Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol 1992, 148:2201-2206 [PubMed] [Google Scholar]
- 22.Cronstein BN: Adenosine and its receptors during inflammation. Serhan CN Ward PA eds. Molecular and Cellular Basis of Inflammation. 1998, :pp 259-274 Humana Press, Totowa [Google Scholar]
- 23.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA: A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 1999, 19:9192-9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cronstein BN, Naime D, Ostad E: The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 1993, 92:2675-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH: Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology 1998, 55:575-581 [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen PB, Van den Eynden GG, Huget P, Goovaerts G, Weyler J, Lardon F, van Marck E, Hubens G, Dirix LY: Prospective study of intratumoral microvessel density, p53 expression. Br J Cancer 1999, 79:316-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokunaga T, Nakamura M, Oshika Y, Abe Y, Ozeki Y, Fukushima Y, Hatanaka H, Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Tamaoki N, Ueyama Y: Thrombospondin 2 expression is correlated with inhibition of angiogenesis and metastasis of colon cancer. Br J Cancer 1999, 79:354-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, Dobson JG, Jr: Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res 1998, 80:357-364 [DOI] [PubMed] [Google Scholar]
- 29.Drury AN, Szent-Gyorgi A: The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol 1929, 68:213-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khakh BS, Kennedy C: Adenosine and ATP: progress in their receptors’ structures and functions. Trends Pharmacol Sci 1998, 19:39-41 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed AH, Jacobson KA, Kim J, Heppel LA: Presence of both A1 and A2a adenosine receptors in human cells and their interaction. Biochem Biophys Res Commun 1995, 208:871-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dignass AU, Becker A, Spiegler S, Goebell H: Adenine nucleotides modulate epithelial wound healing in vitro. Eur J Clin Invest 1998, 28:554-561 [DOI] [PubMed] [Google Scholar]
- 33.Zhou LJ, Ono I: Stimulatory effects of dibutyryl cyclic adenosine monophosphate on cytokine production by keratinocytes and fibroblasts. Br J Dermatol 2000, 143:506-512 [DOI] [PubMed] [Google Scholar]
- 34.Thellung S, Florio T, Maragliano A, Cattarini G, Schettini G: Polydeoxy-ribonucleotides enhance the proliferation of human skin fibroblasts: involvement of A2 purinergic receptor subtypes. Life Sci 1999, 64:1661-1674 [DOI] [PubMed] [Google Scholar]
- 35.Cook PW, Ashton NM, Pittelkow MR: Adenosine and adenine nucleotides inhibit the autonomous and epidermal growth factor-mediated proliferation of cultured human keratinocytes. J Invest Dermatol 1995, 104:976-981 [DOI] [PubMed] [Google Scholar]
- 36.Salmon JE, Cronstein BN: Fcgamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy: A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol 1990, 145:2235-2240 [PubMed] [Google Scholar]
- 37.Cronstein BN, Duguma L, Nicholls D, Hutchison A, Williams M: The adenosine/neutrophil paradox resolved. Human neutrophils possess both A1 and A2 receptors which promote chemotaxis and inhibit O2- generation, respectively. J Clin Invest 1990, 85:1150-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett D, Liittschwager EB: Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol 2000, 35:56-59 [DOI] [PubMed] [Google Scholar]
- 39.Matherne GP, Headrick JP, Coleman SD, Berne RM: Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res 1990, 28:348-353 [DOI] [PubMed] [Google Scholar]
- 40.Schrader J: Adenosine. A homeostatic metabolite in cardiac energy metabolism. Circulation 1990, 81:389-391 [DOI] [PubMed] [Google Scholar]
- 41.Deussen A, Schrader J: Cardiac adenosine production is linked to myocardial pO2. J Mol Cell Cardiol 1991, 23:495-504 [DOI] [PubMed] [Google Scholar]
- 42.Borst MM, Schrader J: Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res 1991, 68:797-806 [DOI] [PubMed] [Google Scholar]
- 43.Moser GH, Schrader J, Deussen A: Turnover of adenosine in plasma of human and dog blood. Am J Physiol 1989, 256:C799-C806 [DOI] [PubMed] [Google Scholar]
- 44.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN: Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest 1998, 101:295-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitakaze M, Hori M, Morioka T, Takashima S, Minamino T, Sato H, Inoue M, Kamada T: Attenuation of ecto-5′-nucleotidase activity and adenosine release in activated human polymorphonuclear leukocytes. Circ Res 1993, 73:524-533 [DOI] [PubMed] [Google Scholar]
- 46.Kitakaze M, Node K, Minamino T, Komamura K, Funaya H, Shinozaki Y, Chujo M, Mori H, Inoue M, Hori M, Kamada T: Role of activation of protein kinase C in the infarct size-limiting effect of ischemic preconditioning through activation of ecto-5′-nucleotidase. Circulation 1996, 93:781-791 [DOI] [PubMed] [Google Scholar]
- 47.Node K, Kitakaze M, Minamino T, Tada M, Inoue M, Hori M, Kamada T: Activation of ecto-5′-nucleotidase by protein kinase C and its role in ischaemic tolerance in the canine heart. Br J Pharmacol 1997, 120:273-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newby AC, Holmquist CA, Illingworth J, Pearson JD: The control of adenosine concentration in polymorphonuclear leucocytes, cultured heart cells and isolated perfused heart from the rat. Biochem J 1983, 214:317-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cronstein BN, Naime D, Firestein G: The antiinflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthr Rheum 1995, 38:1040-1045 [DOI] [PubMed] [Google Scholar]
- 50.Gadangi P, Longaker M, Naime D, Levin RI, Recht PA, Montesinos MC, Buckley MT, Carlin G, Cronstein BN: The antiinflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol 1996, 156:1937-1941 [PubMed] [Google Scholar]
- 51.Cronstein BN, Montesinos MC, Weissmann G: Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci USA 1999, 96:6377-6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colli S, Tremoli E: Multiple effects of dipyridamole on neutrophils and mononuclear leukocytes: adenosine-dependent and adenosine-independent mechanisms. J Lab Clin Med 1991, 118:136-145 [PubMed] [Google Scholar]