Abstract
Previous studies using metallothionein (MT)-overexpressing transgenic mice have demonstrated that MT protects the liver from oxidative injury induced by alcohol. The mechanism of action of MT is unknown. Because MT primarily binds to zinc under physiological conditions and releases zinc under oxidative stress and zinc is an antioxidant element, it is likely that zinc mediates the protective action of MT. The present study was undertaken to determine the distinct role of zinc in hepatic protection from alcoholic injury. MT I/II-knockout (MT-KO) mice along with their wild-type controls were treated with three gastric doses of ethanol at 5 g/kg at 12-hour intervals. Zinc sulfate was injected intraperitoneally in a dosage of 5 mg/kg/day for 3 days before ethanol treatment. MT concentrations in MT-KO mice were very low and zinc concentrations in MT-KO mice were lower than in wild-type mice. Zinc treatment significantly elevated hepatic MT concentrations only in wild-type mice and increased zinc concentrations in both MT-KO and wild-type mice. Ethanol treatment caused degenerative morphological changes and necrotic appearance in the livers of MT-KO mice. Microvesicular steatosis was the only ethanol-induced change in the liver of wild-type mice. Ethanol treatment decreased hepatic glutathione concentrations and increased hepatic lipid peroxidation, and the concentrations of lipid peroxide products in the wild-type mice were lower than in the MT-KO mice. All of these alcohol-induced toxic responses were significantly suppressed by zinc treatment in both MT-KO and wild-type mouse livers. These results demonstrate that zinc, independent of MT, plays an important role in protection from alcoholic liver injury. However, MT is required to maintain high levels of zinc in the liver, suggesting that the protective action of MT in the liver is likely mediated by zinc.
Many investigations have demonstrated that alcohol administration induces generation of reactive oxygen species in the liver leading to oxidative injury. 1,2 After the pioneering report of Di Luzio 3 showing that supplementation of antioxidant inhibited acute alcohol-induced fatty liver and lipid peroxidation, antioxidant supplementation has been widely applied to prevent alcoholic liver injury in both animal models and human clinical trials. Most studies suggest that alcohol-induced liver injury could be reduced by supplementation with antioxidants, including glutathione (GSH) precursor, vitamins E and C, and some trace elements. 4,5
Metallothionein (MT) is a highly conserved, low molecular weight, cysteine-rich protein. MT shares important features with GSH. The sulfhydryl groups of GSH and MT are the major thiolate pool in the cell. However, the thiolate groups in MT are preferential targets for H2O2 compared to GSH. 6 Kinetic studies demonstrated that MT was 38.5-fold more potent than GSH on a molar basis in preventing HO·-induced DNA degradation. 7 Recent studies in this laboratory have demonstrated that MT provides effective protection from ethanol-induced oxidative hepatotoxicity in vivo. 8
The mechanisms by which MT functions as a potent antioxidant are poorly understood. MT is the only known protein that is implicated in zinc distribution. 9 Many studies have demonstrated that zinc-induced MT expression provides effective protection against hepatotoxicity induced by acetaminophen, 10 carbon tetrachloride, 11,12 doxorubicin, 13 and GSH depletors. 14 However, zinc-induced protection is not always correlated with MT elevation. 15 On the other hand, increasing evidence indicates that zinc has antioxidant properties by inhibition of HO· formation through antagonism of redox-transition metals. 16 Most importantly, zinc is released from MT under oxidative stress. 17,18 Therefore, it is of great interest to determine whether zinc mediates the antioxidant action of MT. This has to be determined unequivocally by using unique experimental approaches such as MT-transgenic and MT-null mouse models. MT-null mice (MT-KO) lacking MT-I and MT-II, the only mouse hepatic MT isoforms, have been produced by a gene-targeting technique. 19 This model thus serves as a valuable tool to reveal the link between zinc and MT in cytoprotection from oxidative injury.
This study was undertaken to determine the role of zinc in MT protection against ethanol-induced hepatotoxicity and the possible mechanisms. MT-KO mice and wild-type controls were treated with three doses of alcohol with or without zinc pretreatment. Hepatic MT, zinc concentrations, lipid peroxidation, and reduced glutathione concentrations were determined by biochemical methods. Hepatic histopathological and ultrastructural changes were observed by light and electron microscopy.
Materials and Methods
Animals
Homozygous MT-KO mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were produced on the 129/Sv genetic background. 19 Both MT-KO and 129/Sv wild-type (WT) controls were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/dark cycle and had free access to rodent chow and tap water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Ethanol Administration
A binge drinking mouse model developed by Carson and Pruett 20 was followed for ethanol challenge. This model was designed to achieve blood alcohol levels, behavioral effects, and physiological changes comparable to human binge drinking. MT-KO as well as WT mice at the age of 9 weeks were divided into four groups in a 2 × 2 factorial design (+/− zinc, +/− ethanol): control, ethanol treatment, zinc treatment and zinc/ethanol treatment. Zinc sulfate (ZnSO4) was administrated by intraperitoneally injection at a dosage of 5 mg Zn/kg once a day for 3 days before ethanol administration. Three doses of ethanol (25%, w/v) (Aldrich, Milwaukee, WI) at of 5 g/kg were administrated by gavage at 12-hour intervals. Control mice received isocaloric maltose solution. At 4 hours after the last ethanol dosing, the mice were anesthetized with sodium pentobarbital (0.05 mg/g body weight) (Abbott Laboratories, North Chicago, IL). Blood was drawn from the dorsal vena cava and sera were obtained by centrifugation using a serum separator tube. The liver samples were frozen in liquid nitrogen and maintained at −80°C. A portion of each liver was taken and fixed for light microscopic examination. For electron microscopy, the liver was perfused in situ with saline and fixative (see below).
MT Assay
Tissue MT concentrations were determined by a cadmium-hemoglobin affinity assay. Briefly, liver tissues were homogenized in 4 volumes of 10 mmol/L Tris-HCl buffer, pH 7.4 at 4°C. After centrifugation of the homogenate at 10,000 × g for 15 minutes, 200 μl of supernatant was transferred to microtubes for MT analysis as described previously. 21
Determination of Zinc
Hepatic zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy (Jarrel-Ash, Model 1140, Waltham, MA) after lyophilization and digestion of the tissues with nitric acid and hydrogen peroxide. 22 Zinc concentrations in the liver were expressed as μg/g dry tissue.
Light Microscopic Examination
Liver tissues were cut into ∼3-mm-thick slices and fixed with 10% neutral formalin. The tissue slices were embedded in paraplast. Tissue sections of 5 μm were stained by hematoxylin and eosin (H&E) and observed with a Nikon Eclipse E400 light microscope.
Electron Microscopic Examination
To observe ethanol-induced ultrastructural changes by conventional electron microscopy, livers were fixed in situ by vascular perfusion with Karnovsky’s fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4) and post-fixed in 1% osmium tetraoxide. Ultra-thin sections were stained by uranyl acetate and lead citrate and observed with a Philips transmission electron microscope.
Determination of GSH Concentrations
Liver GSH concentrations were assayed by the glutathione-disulfide reductase and 5′5-dithiobis (2-nitrobenzoic acid) recycling assay. 23 Briefly, liver tissue was homogenized with 5% sulfosalicylic acid and centrifuged at 10,000 × g for 5 minutes. The supernatant was divided into two aliquots. One aliquot was directly used to measure total glutathione and the other aliquot was first treated with 2-vinylpyridine to block the GSH, followed by measuring the oxidized glutathione (GSSG). The GSH value was determined by subtracting GSSG from total glutathione.
Lipid Peroxidation Assay
Hepatic lipid peroxidation was quantified by measuring thiobarbituric acid-reactive substance (TBARS) as described previously. 24 Liver tissue was homogenized in 9 volumes of 50 mmol/L Tris-HCl buffer (pH 7.4) containing 180 mmol/L KCl, 10 mmol/L EDTA, and 0.02% butylated hydroxytoluene. To 0.2 ml of the tissue homogenate, 0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid, 1.5 ml of 0.9% thiobarbituric acid, and 0.6 ml of distilled water were added and vortexed. The reaction mixture was placed in a water bath at 95°C for 1 hour. After cooling on ice, 1.0 ml of distilled water and 5.0 ml of butanol/pyridine mixture (15:1, v/v) were added and vortexed. After centrifugation at 10,000 × g for 10 minutes, absorbance of the resulting lower phase was determined at 532 nm. The TBARS concentration was calculated using 1,1,5,5-tetraethoxypropane as standard.
Statistics
All measurements are expressed as mean ± SD (n = 5–8). The data were analyzed by analysis of variance (analysis of variance) and Tukey’s Honestly Significant Difference (HSD). Differences between groups were considered significant at P < 0.05.
Results
Hepatic MT Concentrations
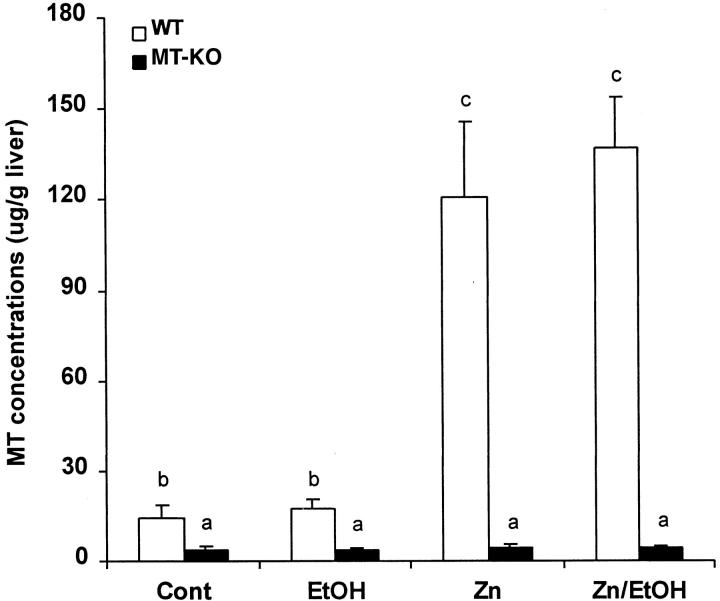
As shown in Figure 1 ▶ , MT concentrations in the liver of WT mice were elevated about 7-fold by zinc treatment or zinc plus ethanol treatment. However, ethanol per se did not induce a significant increase in hepatic MT concentrations. In the MT-KO mice, hepatic MT concentrations were very low and were not affected by ethanol, zinc, or zinc plus ethanol treatments. The MT level in the MT-KO mice has been reported to represent the assay background but not MT protein content in the liver. 25
Figure 1.
Hepatic MT concentrations in WT and MT-KO mice (Cont) and the effect of ethanol (EtOH), zinc (Zn), and zinc/ethanol (Zn/EtOH). MT concentrations were determined by a cadmium-hemoglobin affinity assay. Results are means ± SD (n = 5–8). The data were analyzed by analysis of variance and Tukey’s HSD. Significant difference (P < 0.05) is identified by letter superscripts.
Hepatic Zinc Concentrations
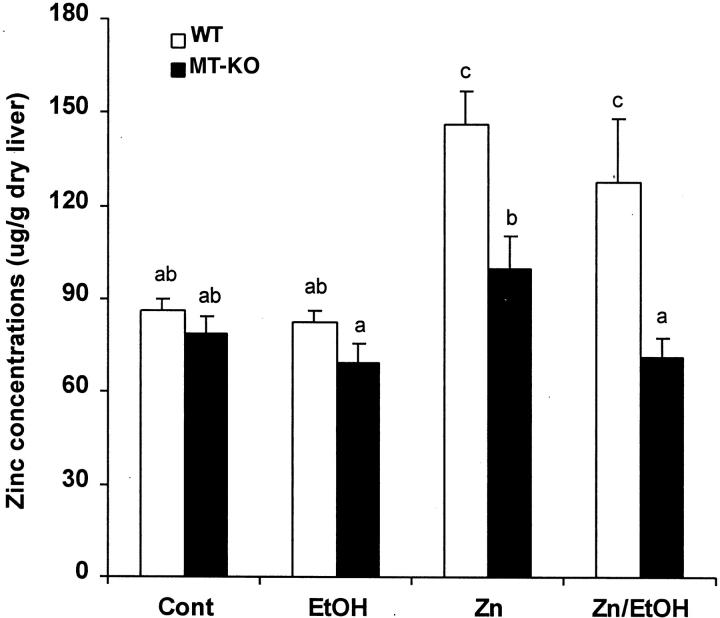
Zinc concentrations in the liver of MT-KO mice were lower than in WT mice before and after ethanol treatment, although the differences were not significant (Figure 2) ▶ . Zinc treatment led to increases in hepatic zinc concentrations in both WT and MT-KO mice, but this elevation was significantly higher in the WT mice. In mice treated with zinc plus ethanol, hepatic zinc concentrations were lower in both WT and MT-KO mice compared to their zinc-treated counterparts.
Figure 2.
Hepatic zinc concentrations in WT and MT-KO mice (Cont) and the effect of ethanol (EtOH), zinc (Zn), and zinc/ethanol (Zn/EtOH). Zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy. Results are means ± SD (n = 5–8). The data were analyzed by analysis of variance and Tukey’s HSD. Significant difference (P < 0.05) is identified by letter superscripts.
Prevention of Ethanol-Induced Histopathological Changes by Zinc Pretreatment
The prominent histopathological change induced by ethanol in the WT mouse liver was microvesicular steatosis (Figure 3) ▶ . However in MT-KO mice, ethanol caused apparent necrosis as suggested by cell enlargement, vacuolization, and nuclear dissolution. Pretreatment with zinc apparently inhibited ethanol-induced hepatic damage in both WT and MT-KO mice to the same extent; only minor steatosis was observed in livers pretreated with zinc.
Figure 3.
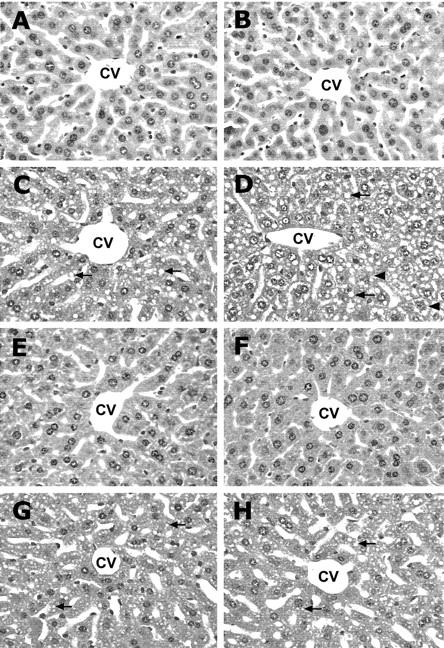
Ethanol-induced histopathological changes in the liver of WT and MT-KO mice. WT mice: control (A), ethanol (C), zinc (E), and zinc/ethanol (G). MT-KO mice: control (B), ethanol (D), zinc (F), and zinc/ethanol (H). The prominent histopathological change induced by ethanol in the WT mouse liver was microvesicular steatosis (arrows). Ethanol administration caused more severe damage in the liver of MT-KO mice that included cell enlargement, cytoplasmic vacuolization and nuclear dissolution (suggestive of necrosis, arrowheads), in addition to microvesicular steatosis. However, these changes were all suppressed by zinc pretreatment. CV, central vein. H&E staining; magnification, ×260.
Prevention of Ethanol-Induced Ultrastructural Changes in Hepatocytes by Zinc Pretreatment
In comparison to the hepatocytes of normal controls (Figure 4,A and B) ▶ , ethanol administration caused lipid droplet accumulation and mild disorganization of rough endoplasmic reticulum and condensation of chromatin condensation in the WT mice (Figure 4C) ▶ . However, the same ethanol treatment caused much more severe ultrastructural changes in MT-KO mice (Figure 4D) ▶ . The prominent abnormalities of ethanol-treated MT-KO mouse hepatocyte include condensation and degeneration of mitochondria and rough endoplasmic reticulum, focal degeneration of cytoplasm, and condensation of chromatin. The ultrastructure of zinc-treated livers was similar to the normal controls (Figure 4, E and F) ▶ . Pretreatment with zinc dramatically inhibited ethanol-induced abnormalities in both WT and MT-KO mice (Figure 4, G and H) ▶ . The organelles and nuclei of hepatocytes were basically normal and the size and number of lipid droplets were decreased, although rough endoplasmic reticulum was scanty in the MT-KO mice.
Figure 4.
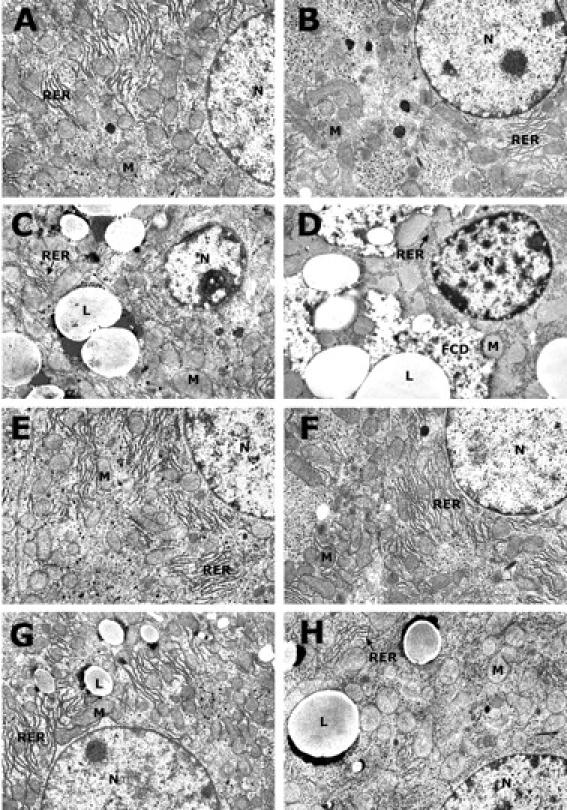
Ethanol-induced ultrastructural changes in the hepatocytes of WT and MT-KO mice. WT mice: control (A), ethanol (C), zinc (E), and zinc/ethanol (G). MT-KO mice: control (B), ethanol (D), zinc (F), and zinc/ethanol (H). In comparison with WT mice, ethanol-induced ultrastructural damages were more severe in the liver of MT-KO mice. In particular, focal cytoplasmic degeneration and organelle condensation were observed in the MT-KO mice. These ultrastructural changes were improved by zinc pretreatment, although rough endoplasmic reticulum was scanty in the MT-KO mice. N, nucleus; M, mitochondria; RER, rough endoplasmic reticulum; L, lipid droplets; FCD, focal cytoplasmic degeneration. Magnification, ×7500.
Inhibition of Ethanol-Induced Hepatic GSH Depletion by Zinc Pretreatment
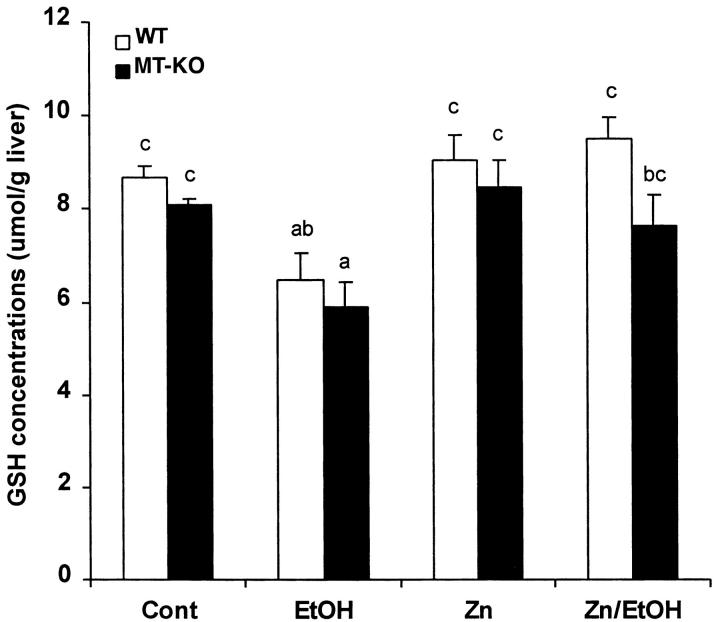
There was no significant difference in hepatic GSH concentrations between MT-KO and WT mice, though MT-KO mice had relatively lower GSH concentrations (Figure 5) ▶ . In response to ethanol exposure, hepatic GSH concentrations significantly decreased in both WT and MT-KO mice. Zinc treatment significantly inhibited ethanol-induced decreases in hepatic GSH concentrations in both WT and MT-KO mice, but the inhibition was more profound in the WT mouse liver.
Figure 5.
Hepatic GSH concentrations in the WT and MT-KO mice and the effect of ethanol treatment. Cont, control; EtOH, ethanol. Results are means ± SD (n = 5–8). The data were analyzed by analysis of variance and Tukey’s HSD. Significant difference (P < 0.05) is identified by letter superscripts.
Inhibition of Ethanol-Induced Hepatic Lipid Peroxidation by Zinc Pretreatment
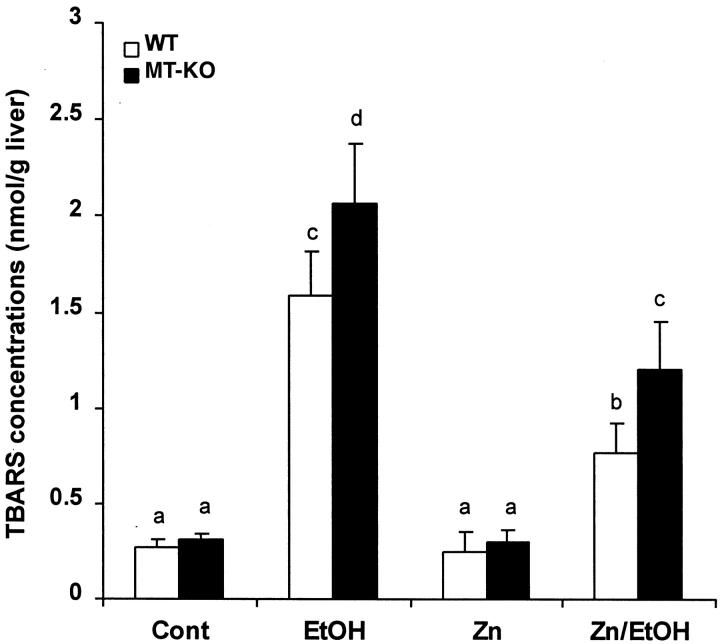
Ethanol-induced hepatic lipid peroxidation was assessed by measuring TBARS concentrations. As shown in Figure 6 ▶ , ethanol administration caused a significant increase in lipid peroxidation in the livers of both WT and MT-KO mice. The extent of ethanol-induced lipid peroxidation was significantly greater in the MT-KO mice. Pretreatment with zinc significantly suppressed ethanol-induced lipid peroxidation in both WT and MT-KO mice, although the hepatic TBARS content in the MT-KO mice was higher than in the WT mice.
Figure 6.
Hepatic lipid peroxidation in the WT and MT-KO mice. Lipid peroxidation was quantified by measuring thiobarbituric acid-reactive substance (TBARS). Cont, control; EtOH, ethanol. Results are means ± SD (n = 5–8). The data were analyzed by analysis of variance and Tukey’s HSD. Significant difference (P < 0.05) is identified by different letter superscripts.
Discussion
The data obtained from this study demonstrate that MT-KO mice are more sensitive than WT mice to ethanol-induced hepatotoxicity. Ethanol administration induced fat droplet accumulation in both MT-KO and WT mice. However, necrotic degeneration and organelle abnormalities were observed only in MT-KO mice. In association with hepatic pathological changes, ethanol administration induced marked decreases in hepatic GSH concentrations and increased hepatic lipid peroxidation. The ethanol-induced hepatotoxic responses were significantly suppressed in both MT-KO and WT mice by zinc pretreatment. These findings indicate that zinc functions in protection from alcoholic liver injury. The protective action of zinc is independent of MT, but MT is important in maintaining high levels of zinc in the liver.
MT has been repeatedly shown to provide protection from oxidative injury in multiple organ systems. 26,27 Investigations using MT-overexpressing transgenic mice have demonstrated the role of MT in protection from oxidative cardiac injury induced by doxorubicin, 21,28-32 hydrogen peroxide, 33 ischemia/reperfusion, 34,35 and mineral malnutritional effects such as dietary copper deficiency. 36 In contrast, MT-KO mice were found to be more sensitive to cadmium-, acetaminophen-, and cisplatin-induced hepatotoxicity, 10,37,38 caerulein-induced pancreatic toxicity, 39 and ethanol-induced gastrointestinal toxicity. 40 Several studies have also shown that zinc administration induces MT expression which provides protection from hepatotoxicity induced by a variety of toxic chemicals. 10-14 Although the protective action of zinc has been often ascribed to MT induction, there are also evidence showing that zinc-induced tolerance to carbon tetrachloride hepatotoxicity is not due to the trichloromethyl radical-scavenging action of MT. It thus remains to be determined whether zinc functions as a protective agent independent of MT or through the production of MT.
The use of MT-KO mice in the present study provided an unequivocal approach to address the question above. In the MT-KO mice, MT concentrations in the liver were very low and were not elevated by zinc treatment. Zinc concentrations in the liver of MT-KO mice were lower than in the WT mice, indicating a lack of MT-bound zinc in the MT-KO mouse liver. In response to zinc supplementation, zinc concentrations in the liver of both WT and MT-KO mice were elevated, however more profound elevation was observed in the WT mouse liver, correlating with MT elevation. Ethanol treatment decreased zinc concentrations in the liver of MT-KO mice, but much less in the WT mice. These changes likely reflect a decrease in the non-MT-bound zinc pool in the MT-KO mice and the buffering role of MT in preventing total loss of zinc in the livers of WT mice. Interestingly, the elevated zinc concentration in the zinc supplemented MT-KO mouse livers was significantly decreased in response to ethanol treatment, but slightly decreased in the WT mouse livers. This change indicates that MT plays a critical role in maintaining high levels of zinc in the liver especially under stress conditions.
The changes in zinc concentrations in the liver apparently affected pathological changes induced by ethanol treatment. However, it appeared that there might be a cytoprotective threshold level, above which varying concentrations of zinc would produce the same protection. As demonstrated by the results, in comparison with WT mice, MT-KO mice were more sensitive to alcoholic hepatotoxicity, which correlated with lower concentrations of zinc in the MT-KO mouse liver. Zinc supplementation significantly suppressed the cellular and subcellular damages induced by ethanol in both WT and MT-KO mice. It is specifically noteworthy that the MT concentrations were not increased, but zinc concentrations were elevated in the MT-KO mouse livers. Therefore, the protection was related to zinc per se, but not dependent on MT.
The question then is: what is the role of high levels of MT in the liver? MT is the only known protein responsible for cellular zinc distribution, and each MT molecule can bind seven zinc atoms by its thiolate ligands. The MT-bound zinc can be released as a consequence of stress-induced changes in cellular redox state. 17,18 Zinc released from MT may function as a mediator of MT cytoprotection because zinc itself has antioxidant properties. Therefore, high levels of MT in the liver at least maintain high levels of liver zinc.
Oxidative stress has been shown to mediate the pathogenesis of alcoholic liver disease. 1,2 We have observed that MT provides cytoprotection through inhibition of ethanol-induced oxidative stress. 8 If zinc mediates MT antioxidant action, hepatic protection by zinc from ethanol-induced oxidative stress should be observed. Therefore, the effect of zinc on ethanol-induced lipid peroxidation and GSH depletion were measured. The results demonstrate that zinc significantly suppressed ethanol-induced lipid peroxidation and GSH depletion in both WT and MT-KO mice. These observations agree with many previous studies. For example, zinc supplementation significantly suppressed hepatic lipid peroxidation induced by a variety of chemicals, including ethanol, 41 carbon tetrachloride, 11,12 and dimethylnitrosamine. 42 Hepatic GSH depletion by carbon tetrachloride and dimethylnitrosamine was also attenuated by zinc administration. 12,42 In these studies, however, zinc was used as an inducer for MT production and hepatic protection was ascribed to MT induction. The present study aimed to separate the role of zinc and MT by using the MT-KO mouse model. It clearly showed that zinc itself inhibited lipid peroxidation and GSH depletion induced by ethanol in the liver.
Although the mechanisms are largely unclear, the antioxidant actions of zinc are thought to involve the antagonism of redox-active transition metals such as iron and copper. Many studies have demonstrated that trace amounts of redox-active metals are required to catalyze the formation of HO· and other radicals. Metal-catalyzed formation of HO· can result in the abstraction of hydrogen from unsaturated fatty acid or protein leading to lipid peroxidation or protein oxidation. 16 Zinc has been shown to antagonize the catalytic properties of the redox-active metals and inhibit HO· and O2· formation in different systems. In a chemical system, zinc was found to reduce the HO· formation from iron and cysteine. 43 A study using a biochemical system has demonstrated that zinc suppresses iron mediated, xanthine/xanthine oxidase-induced membrane peroxidation of erythrocytes. 44 In a cardiac perfusion system, zinc was shown to reduce post-ischemic formation of HO· in association with decreased copper content in the heart. 45 In the pathogenesis of alcoholic liver injury, iron has been a major determinant. Many investigations have demonstrated ethanol administration increases the redox-active iron concentration in the liver, leading to lipid peroxidation. 46 The present study demonstrated that zinc itself indeed has antioxidant action. However, further investigations are required to determine whether the antioxidant action of zinc is related to antagonism of redox-active iron overloaded by ethanol administration.
In conclusion, this study demonstrates that zinc is directly involved in the protection of liver from alcoholic injury. However, MT is critical to maintain high levels of zinc in the liver. The coordination between zinc and MT is thus that MT retains high levels of zinc in the liver under physiological conditions and releases zinc under oxidative stress conditions, leading to potent antioxidant action.
Acknowledgments
We thank Donald Mosley, Cathie Caple, and Gwen Dahlen for technical assistance.
Footnotes
Address reprint requests to Dr. Y. James Kang or Dr. Zhanxiang Zhou, University of Louisville School of Medicine, Department of Medicine, 511 South Floyd Street, MDR 530, Louisville, KY 40202. E-mail: yjkang01@athena.louisville.edu.
Supported in part by the University of Louisville Hospital and by a grant from the Jewish Hospital Foundation, Louisville, Kentucky. Y.J.K. is a University scholar of the University of Louisville.
References
- 1.Noedmann R, Ribiere C, Rouach H: Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med 1992, 12:219-240 [DOI] [PubMed] [Google Scholar]
- 2.Kurose I, Higuchi H, Kato S, Miura S, Isshii H: Ethanol-induced oxidative stress in the liver. Alcohol Clin Exp Res 1996, 20:77A-85A [DOI] [PubMed] [Google Scholar]
- 3.Di Luzio NR: Prevention of the acute ethanol-induced fatty liver by the simultaneous administration of anti-oxidants. Life Sci 1994, 3:113-119 [DOI] [PubMed] [Google Scholar]
- 4.Noedmann R: Alcohol and antioxidant system. Alcohol Alcohol 1994, 29:513-522 [PubMed] [Google Scholar]
- 5.Mullen KD, Dasarathy S: Potential new therapies for alcoholic liver disease. Clin Liver Dis 1998, 2:851-881 [Google Scholar]
- 6.Quesada AR, Byrnes RW, Krezoski SO, Pettering DH: Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites. Arch Biochem Biophys 1996, 334:241-250 [DOI] [PubMed] [Google Scholar]
- 7.Abel J, de Ruiter N: Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett 1989, 47:191-196 [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z, Sun X, Kang YJ: Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med 2002, 227:214-222 [DOI] [PubMed] [Google Scholar]
- 9.Ye B, Maret W, Vallee B: Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci USA 2001, 98:2317-2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Liu Y, Hartley D, Klaassen CD, Shehin-Johnson SE, Lucas A, Cohen SD: Metallothionein-I/II knockout mice are sensitive to acetaminophen-induced hepatotoxicity. J Pharmacol Exp Ther 1999, 289:580-586 [PubMed] [Google Scholar]
- 11.Cagen SZ, Klaassen CD: Protection of carbon tetrachloride-induced hepatotoxicity by zinc: role of metallothionein. Toxicol Appl Pharmacol 1979, 51:107-116 [DOI] [PubMed] [Google Scholar]
- 12.Dhawan D, Goel A: Further evidence for zinc as a hepatoprotective agent in rat liver toxicity. Exp Mol Pathol 1995, 63:110-117 [DOI] [PubMed] [Google Scholar]
- 13.Kimura T, Fujita I, Itoh N, Muto N, Nakanishi T, Takahashi K, Azuma J, Tanaka K: Metallothionein acts as a cytoprotection against doxorubicin toxicity. J Pharmacol Exp Ther 2000, :299-302 [PubMed] [Google Scholar]
- 14.Haidara K, Moffatt P, Denizeau F: Metallothionein induction attenuates the effects of glutathione depletors in rat hepatocytes. Toxicol Sci 1999, 49:297-305 [DOI] [PubMed] [Google Scholar]
- 15.Hanna PM, Kadiiska MB, Jordan SJ, Mason RP: Role of metallothionein in zinc(II) and chromium(III) mediated tolerance to carbon tetrachloride hepatotoxicity: evidence against a trichloromethyl radical-scavenging mechanism. Chem Res Toxicol 1993, 6:711-717 [DOI] [PubMed] [Google Scholar]
- 16.Powell SR: The antioxidant properties of zinc. J Nutr 2000, 130:1447S-1454S [DOI] [PubMed] [Google Scholar]
- 17.Maret W: Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci USA 1994, 91:237-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang LJ, Maret W, Vallee BL: The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci USA 1998, 95:3483-3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD: Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA 1994, 91:584-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson EJ, Pruett SB: Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res 1996, 20:132-138 [DOI] [PubMed] [Google Scholar]
- 21.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN: Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 1997, 100:1501-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen FH, Zimmerman TJ, Shuler TR: Interactions among nickel, copper, and iron in rats: liver and plasma contents of lipid and trace elements. Biol Trace Elem Res 1982, 4:1225-1243 [DOI] [PubMed] [Google Scholar]
- 23.Tietze F: Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969, 27:502-522 [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979, 44:276-278 [DOI] [PubMed] [Google Scholar]
- 25.Davis SR, McMahon RJ, Cousins RJ: Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 (ZnT-1) expression. J Nutr 1998, 128:825-831 [DOI] [PubMed] [Google Scholar]
- 26.Kang YJ: The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med 1999, 222:263-273 [DOI] [PubMed] [Google Scholar]
- 27.Klaassen CD, Liu J, Choudhuri S: Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 1999, 39:267-294 [DOI] [PubMed] [Google Scholar]
- 28.Wu HY, Kang YJ: Inhibition of buthionine sulfoximine-enhanced doxorubicin toxicity in metallothionein-overexpressing transgenic mouse heart. J Pharmacol Exp Ther 1998, 287:515-520 [PubMed] [Google Scholar]
- 29.Wang GW, Kang YJ: Inhibition of doxorubicin toxicity in cultured neonatal mouse cardiomyocytes with elevated metallothionein levels. J Pharmacol Exp Ther 1999, 288:938-994 [PubMed] [Google Scholar]
- 30.Kang YJ, Zhou Z, Wang GW, Buridi A, Klein JB: Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. J Biol Chem 2000, 275:13690-13698 [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z, Kang YJ: Immunocytochemical localization of metallothionein and its relation to doxorubicin toxicity in transgenic mouse heart. Am J Pathol 2000, 156:1653-1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun XH, Zhou Z, Kang Y: Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res 2001, 61:3382-3387 [PubMed] [Google Scholar]
- 33.Wang GW, Schuschke DA, Kang YJ: Metallothionein-overexpressing neonatal mouse cardiomyocytes are resistant to hydrogen peroxide toxicity. Am J Physiol 1999, 276:H167-H175 [DOI] [PubMed] [Google Scholar]
- 34.Wang GW, Zhou Z, Klein JB, Kang YJ: Inhibition of hypoxia/reoxygen-induced apoptosis in metallothionein-overexpressing cardiomyocytes. Am J Physiol 2001, 280:H2292-H2299 [DOI] [PubMed] [Google Scholar]
- 35.Kang YJ, Li G, Saari JT: Metallothionein inhibits ischemia-reperfusion injury in mouse heart. Am J Physiol 1999, 276:H993-H997 [DOI] [PubMed] [Google Scholar]
- 36.Kang YJ, Zhou Z, Wu H, Wang G, Saari JT, Klein JB: Metallothionein inhibits myocardial apoptosis in copper-deficient mice: role of atrial natriuretic peptide. Lab Invest 2000, 80:745-757 [DOI] [PubMed] [Google Scholar]
- 37.Habeebu SS, Liu J, Liu Y, Klaassen CD: Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci 2000, 55:223-232 [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Liu Y, Habeebu SS, Klaassen CD: Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol 1998, 55:223-232 [DOI] [PubMed] [Google Scholar]
- 39.Fu K, Tomita T, Sarras MP, Jr, De Lisle RC, Andrews GK: Metallothionein protects against caerulein-induced acute pancreatitis: analysis using transgenic mice. Pancreas 1998, 17:238-246 [DOI] [PubMed] [Google Scholar]
- 40.Takano H, Satoh M, Shimada A, Sagai M, Yoshikawa T, Tohyama C: Cytoprotection by metallothionein against gastroduodenal mucosal injury caused by ethanol in mice. Lab Invest 2000, 80:371-377 [DOI] [PubMed] [Google Scholar]
- 41.Cabre M, Folch J, Gimenez A, Matas C, Pares A, Caballeria AJ, Paternain JL, Rodes J, Joven J, Camps J: Influence of zinc intake on hepatic lipid peroxidation and metallothioneins in alcoholic rats: relationship to collagen synthesis. Int J Vitam Nutr Res 1995, 65:45-50 [PubMed] [Google Scholar]
- 42.Rana SV, Kumar A: Metallothionein induced by cadmium or zinc inhibits lipid peroxidation in rats exposed to dimethynitrosamine. Arh Hig Rada Toksikol 2000, 51:279-286 [PubMed] [Google Scholar]
- 43.Searle AJF, Tomasi A: Hydroxyl free radical production in iron-cysteine solutions and protection by zinc. J Inorg Biochem 1982, 17:161-167 [Google Scholar]
- 44.Girrotti AW, Thomas JP, Jordan JE: Inhibitory effect of zinc(II) on free radical lipid peroxidation in erythrocyte membrane. J Free Radic Biol Med 1984, 1:395-401 [DOI] [PubMed] [Google Scholar]
- 45.Powell SR: Salicylate trapping of OH as a tool for studying post-ischemic oxidative injury in the isolated heart. Free Radic Res Commun 1994, 21:355-370 [DOI] [PubMed] [Google Scholar]
- 46.Britton RS, Bacon BR: Role of free radicals in liver diseases and hepatic fibrosis. Hepatogastroenterology 1994, 41:343-348 [PubMed] [Google Scholar]