Abstract
Germ-free interleukin-10 knockout (IL-10 KO) mice developed inflammatory bowel disease (IBD) after they were colonized with a pure culture of Enterococcus faecalis. E. faecalis not only induced IBD (primarily in colon and rectum) but rectal dysplasia and adenocarcinoma was also found in the IL-10 KO mice. Conventional (complex-intestinal flora) IL-10 KO mice developed IBD within 10 to 15 weeks of age and showed more pathology in the cecum (typhlitis) than we observed with E. faecalis-induced IBD in gnotobiotic IL-10 KO mice. Conversely, neither germ-free IL-10 mice nor IL-10 KO mice colonized as adults, with a pure culture of Candida albicans, Escherichia coli, Lactobacillus casei, L. reuteri, L. acidophilus, a Bifidobacterium sp., Lactococcus lactis, or a Bacillus sp. developed IBD during the 25- to 30-week study. E. faecalis is a common intestinal microbe of man and animals that can trigger IBD, dysplasia, and carcinoma in a genetically susceptible murine host.
Inflammatory bowel diseases (IBD), which affects several million patients, 1 has two main clinical manifestations: 1) ulcerative colitis, an inflammatory disease that occurs in the colonic and rectal mucosa; and 2) Crohn’s disease that affects both the small and large intestine and is associated with transmural granulomatous inflammation of the bowel.
Genetic, autoimmune, infectious, and epithelial cell function(s) and metabolism have all been proposed as factors involved in the etiology and pathogenesis of IBD. 2 Recent clinical and research data point to an infectious cause of IBD but the etiological agent(s) is not known. 3
Spontaneous and genetically engineered animal models of IBD have been described. 2,4,5 IBD can also be induced in experimental animals with chemicals. 6-9 IBD occurs in conventional flora and specific pathogen-free rodents that have been genetically altered [knockout (KO) or transgenic] to impair their capacity to control T-cell-mediated immune responses to intestinal antigens. 10-16 Recent studies have demonstrated the induction of IBD in genetically susceptible, conventional flora mice by adoptive transfer of specific T cells suggesting that dysfunctional, unregulated, T-cell-mediated immune responses play an important role in IBD pathogenesis. 15,17,18
Several recent studies have focused attention on an important role for intestinal microbes in the etiology of IBD. For example, antibiotics can ameliorate IBD. 2,19 Also, IBD-susceptible, genetically engineered rodents are protected from IBD by maintaining them under germ-free conditions. 10-16,20 Certain rodents (Tgε26 mice) when treated with bone marrow develop IBD if they have a complex intestinal flora. However, germ-free Tgε26 mice are protected from IBD by maintaining them germ-free after treatment with bone marrow. 21
Several studies have attempted to induce IBD in genetically susceptible germ-free rodents by colonizing them with a pure culture of an intestinal microbe 22 or with various combinations of intestinal microbes; 20,23 however, to date no common intestinal microbe in pure culture has been able to induce IBD in a genetically susceptible germ-free host.
In this study, we demonstrate that a clinical isolate of E. faecalis, a common component of the intestinal flora of man and animals, 20,24,25 can induce IBD, dysplasia, and carcinoma in interleukin (IL)-10−/− KO mice.
Materials and Methods
Mice
Germ-free IL-10 × 129SEV mice were caesarian derived into the germ-free state and breeding colonies were established at the University of Wisconsin Gnotobiotic Research Laboratory (http://www.medsch.wisc.edu/gnotolab/). Adult IL-10 KO mice (6 to 8 weeks of age) were transferred into germ-free isolators and orally inoculated with a viable, pure culture of E. faecalis (kindly supplied by Dr. M. Huycke, Oklahoma University Health Science Center, Oklahoma City, OK). Verification of the microbial integrity of the experiments was performed weekly by culturing fecal samples on aerobic and anaerobic growth media. E. faecalis, in pure cultures, was isolated throughout the entire study.
Microbiology
Mice were sacrificed at various time intervals after colonization with E. faecalis. Animals were euthanized (CO2) and dissected under sterile conditions. Contents were collected from stomach, small intestine, cecum, and colon. The numbers of viable microbes present in the alimentary tracts were quantified by culturing diluted samples of intestinal contents onto nutrient agar plates. The inoculated plates were incubated at 37°C. Colonies that developed in 24 hours were counted and results are expressed as the number of viable microbes per gram of intestinal contents (dry weight).
Histopathology
The small and large intestine were fixed in 10% neutral-buffered formalin. Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin or a gram stain. Tissues were scored (blinded) by a clinical pathologist (Dr. Thomas Warner, Dept of Surgical Pathology at the University of Wisconsin-Madison, Madison, WI) for IBD.
Grade I
Thickness of intestine three times normal with hyperplastic glands, a reduction in mucus-secreting goblet cells, pseudostratification of glandular lining epithelium, or stratification (two to three cells in thickness). Increased mucosal lymphoid cells including plasma cells with or without infiltration through the muscularis mucosae. Mucosal lymphoid tissue was excluded.
Grade II
The above features (ie, grade I) and scattered crypt abscesses—usually five or more polymorphonuclear leukocytes per crypt. The mucosal infiltrate included a progressive increase in the number of polymorphonuclear leukocytes from grade II to grade IV.
Grade III
Features of grade I and multiple crypt abscesses, at least three per ×10 field.
Grade IV
The above features or grade I, II, or III with ulceration often accompanied by glandular distortion.
Results
In this study, we ascertained whether E. faecalis could colonize the alimentary tract of germ-free mice and induce chronic inflammation in an IBD-susceptible rodent model, the IL-10 KO mouse.
Our laboratory has associated, in pure culture, germ-free IL-10 KO mice with several intestinal microbes. Among these were C. albicans, E. coli, several Lactobacillus spp. (L. acidophilus, L. reuteri, L. casei), a Bifidobacterium sp., and a Bacillus sp. None of the latter microbes was able to induce IBD in IL-10 KO mice.
When the germ-free IL-10 KO mice were monoassociated with E. faecalis, they developed a chronic progressive IBD. Table 1 ▶ shows that the gnotobiotic mice were chronically colonized with E. faecalis throughout the study.
Table 1.
Viable E. faecalis Present in the Alimentary Tract of Gnotobiotic IL-10−/− KO Mice
| Weeks after colonization | No. E. faecalis/g intestinal contents | |||
|---|---|---|---|---|
| Stomach | Small intestine | Cecum | Colon | |
| 5 | 107–108 | 108–109 | 108–109 | 108–109 |
| 10 | 107–108 | 107–108 | 108–109 | 108–109 |
| 15 | 107–108 | 108–109 | 108–109 | 108–109 |
| 20 | 107–108 | 108–109 | 108–109 | 108–109 |
| 25 | 107–108 | 108–109 | 108–109 | 108–109 |
Intestinal contents from three mice at each time point, were homogenized, diluted, and plated onto nutrient agar plates. Colonies were counted at 72 hours of incubation at 37°C.
Histopathology in E. faecalis Colonized IL-10 KO Mice
In IL-10 KO mice, histological changes were found mainly in the distal colon and rectum.
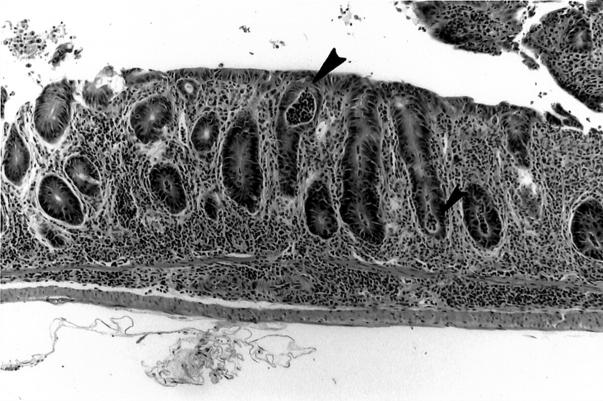
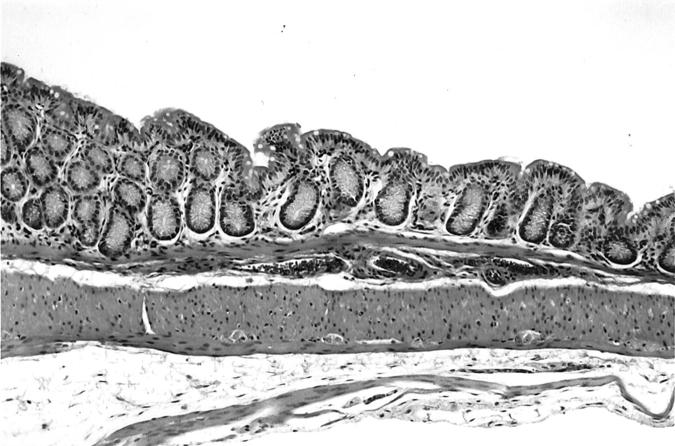
Table 2 ▶ shows the number of E. faecalis colonized mice that manifested IBD early (5 to 15 weeks) and late (20 to 28 weeks) after colonization. Cecal lesions were more common in the late group (15 of 16 mice) and ulceration of the cecum was found in 3 of 16 mice in this group (Table 3) ▶ . The cecal ulcers were not superficial and gaping but were deep fissures in a hyperplastic mucosa (Figure 1) ▶ . Colonic lesions (Tables 2 and 3) ▶ ▶ were found in the left colon, especially as it joined the rectum. Grade IV colonic lesions were present only in the late group (20 to 28 weeks, Table 3 ▶ ). The degree of rectal inflammation (Figure 2) ▶ was comparable in both early and late groups (Table 3) ▶ . Normal rectum is shown in Figure 3 ▶ for comparison.
Table 2.
Induction of IBD in IL-10-KO Mice by E. faecalis
| Group | Weeks colonized* | No. of mice† | No. of mice with IBD histopathology‡ | ||
|---|---|---|---|---|---|
| Cecum | Colon | Rectum | |||
| I Early (5 to 15 weeks) | 5 | 3 | 0 | 0 | 1 |
| 7 | 1 | 1 | 0 | 1 | |
| 10 | 10 | 0 | 8 | 9 | |
| 12.5 | 1 | 1 | 1 | 1 | |
| 15 | 4 | 1 | 2 | 4 | |
| II Late (20 to 28 weeks) | 20 | 5 | 4 | 4 | 5 |
| 21 | 4 | 4 | 4 | 4 | |
| 24 | 3 | 3 | 3 | 3 | |
| 28 | 4 | 3 | 4 | 3 | |
| Colonized with E. faecalis from birth | 25 | 5 | 1 | 0 | 5 |
*Indicates length of time mice were colonized by E. faecalis. Mice were colonized at 7 to 17 weeks of age, except those colonized at birth.
†Number of mice colonized with E. faecalis.
‡Number of mice with IBD.
Table 3.
Histopathology Scores for IL-10 KO Mice Colonized with E. faecalis
| Group | No. of mice | Tissue affected | Histopathology scores* | Total | |||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||
| I Early† 5 to 15 weeks after colonization | 19‡ | Cecum | 2/19 | 1/19 | 0/19 | 0/19 | 3/19 |
| Colon | 1/19 | 3/19 | 7/19 | 0/19 | 11/19 | ||
| Rectum | 2/19 | 1/19 | 10/19 | 3/19 | 16/19 | ||
| II Late 20 to 28 weeks after colonization | 16 | Cecum | 10/16 | 2/16 | 0/16 | 3/16 | 15/16 |
| Colon | 1/16 | 0/16 | 11/16 | 3/16 | 15/16 | ||
| Rectum | 0/16 | 1/16 | 8/16 | 7/16 | 16/16 | ||
| Late colonized from birth to 25 weeks | 5 | Cecum | 0/5 | 1/5 | 0/5 | 0/5 | 1/5 |
| Colon | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | ||
| Rectum | 0/5 | 0/5 | 2/5 | 3/5 | 5/5 | ||
*Grade I, Thickness of intestine three times normal with hyperplastic glands, a reduction in mucus-secreting goblet cells, pseudostratification of glandular lining epithelium, or stratification (two to three cells in thickness). Increased mucosal lymphoid cells including plasma cells with or without infiltration through the muscularis mucosae. Mucosal lymphoid tissue was excluded. Grade II, The above features (i.e., grade I) and scattered crypt abscesses—usually five or more PMN/crypt. The mucosal infiltrate included a progressive increase in the number of PMN from grade II to grade IV. Grade III, Features of grade I and multiple crypt abscesses, at least three per ×10 field. Grade IV, The above features or grade I, II, or III with ulceration often accompanied by glandular distortion.
†Indicates length of time mice were colonized by E. faecalis. Mice were colonized at 7 to 17 weeks of age, except those colonized at birth.
‡Number of mice colonized with E. faecalis.
Figure 1.
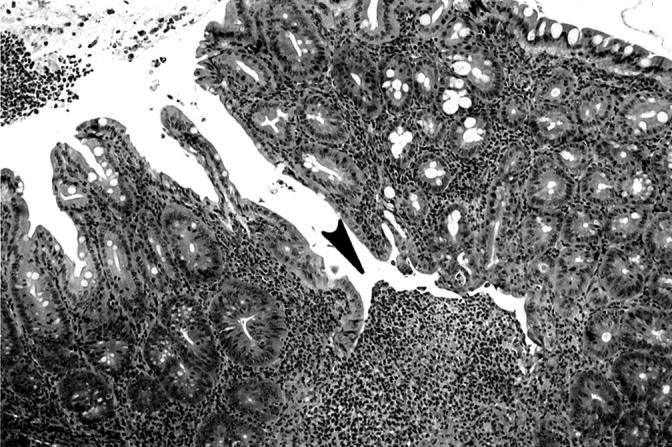
Fissure (arrowhead) in cecum of IL-10−/− mouse infected with E. faecalis for 20 weeks. H&E; original magnification, ×100.
Figure 2.
Cryptabscesses (arrowheads) and loss of mucosal goblet cells in rectal mucosa of IL-10−/− mouse infected with E. faecalis for 10 weeks. H&E; original magnification, ×100.
Figure 3.
Normal rectal mucosa from an IL-10−/− germ-free mouse at age 21 weeks. H&E; original magnification, ×100.
Five mice were colonized with E. faecalis at birth (to 25 weeks of age); cultured fecal samples showed 108 to 109 viable E. faecalis (per gram) throughout the 25 weeks. The latter mice were not included in the early and late groups (Table 3) ▶ . They had no colonic lesions but severe rectal involvement was seen (grade III: two of five; grade IV: three of five). This was a different disease spectrum than we observed in IL-10 KO mice that were colonized for a similar period as adult germ-free mice (8 to 10 weeks of age).
Table 4 ▶ summarizes the rectal dysplasia and carcinoma we observed in this study. Focal rectal dysplasia was found in four IL-10 KO mice (4 of 16) in the late group and in one mouse colonized at birth (one of five). Dysplasia consisted of cribriform or branching glands in the mid- or deep mucosa, pseudostratification of nuclei, or several layers of enlarged nuclei; it was accompanied by mucosal fibrosis. Carcinoma was present in two of these mice; one tumor was invasive (Figure 4 ▶ ; colonized 24 weeks with E. faecalis) and one was intramucosal (colonized 21 weeks).
Table 4.
Dysplasia and Rectal Carcinoma in IL-10 KO Mice Colonized with E. faecalis
| Time colonized (weeks)* | Grade of proctitis | Dysplasia | Carcinoma |
|---|---|---|---|
| 20 | III | + | − |
| 20 | III | + | − |
| 21 | IV | + | + (Mucosal) |
| 27 | IV | + | + (Invasive) |
*Sixteen mice were colonized with E. faecalis at 7 weeks of age for 20 to 27 weeks.
Figure 4.
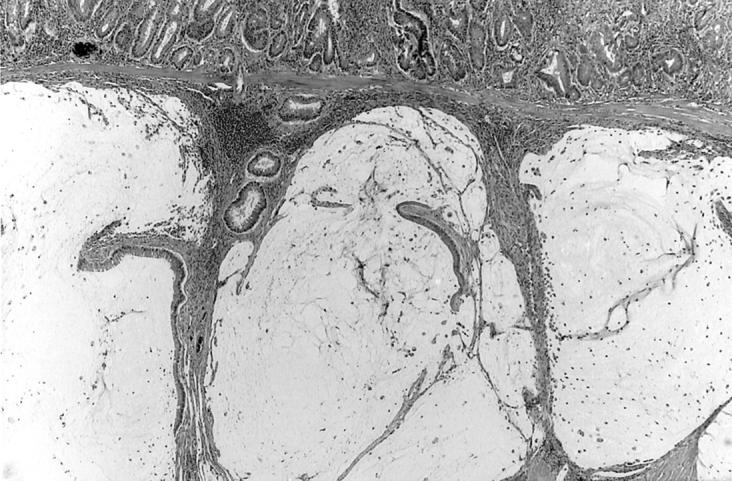
Extra-rectal extension of invasive adenocarcinoma showing dilated mucin-filled acini. IL-10−/− mouse infected with E. faecalis for 24 weeks. H&E; original magnification, ×100.
Nine IL-10 KO mice that were colonized with Lactococcus lactis for 10 (three mice), 27 (three mice), and 28 (three mice) weeks did not develop IBD. However, two of three mice colonized for 28 weeks developed a focal proctitis in a 1-mm length of mucosa just inside the anorectal junction. Six control gnotobiotic C57/BL6 (IL-10-sufficient) mice that were colonized with L. lactis for 24 weeks were free of intestinal inflammation.
In this study, we also examined conventional flora IL-10 KO mice. These mice were colonized with a complex intestinal flora by inoculating them (orally and as adults) with cecal contents from conventional C57BL/6 mice. Conventional flora IL-10 KO mice showed grade IV cecal lesions in five of six mice colonized for 5 and 15 weeks. IL-10 KO mice colonized with E. faecalis for 4 to 5 weeks (eight mice) did not show any signs of IBD. Overall, cecal and rectal lesions were observed earlier and were often more severe in conventional IL-10 KO mice than in IL-10 mice colonized with E. faecalis. Colonic and rectal dysplasia and carcinoma of the cecum was present in one of these conventional flora mice at 15 weeks; however, dysplasia and carcinoma was not detected in the E. faecalis colonized mice until weeks 20 to 24 of the study (Table 4) ▶ .
Discussion
Many microbes have been implicated as the etiological agent of Crohn’s disease and ulcerative colitis, but the precise etiological agent(s) has not been identified. In this study, we demonstrate that E. faecalis, a common component of the microbial flora of man and animals, is able to induce IBD, dysplasia, and carcinoma in IL-10-deficient mice.
Current research with genetically engineered rodents has provided new opportunities to clarify the role of microbes in the etiology of IBD. Rodents with gene deletions that cause specific impairment of their immune response have been shown to spontaneously develop IBD when housed in conventional (complex microbial flora in the intestinal tract) environment. 10,12,16 If these IBD susceptible rodents are raised germ-free, they show little 10,12,16,26 or no 20 spontaneous IBD.
Several investigators have attempted to colonize IBD-susceptible rodents with known intestinal bacteria. For example, it has been demonstrated that a mixture of six common colitis-associated microbes (Bacteroides vulgatus, Streptococcus faecium [Group D], E. coli, Peptostreptococcus productus, Eubacterium contartum, and Streptococcus avium) induced colitis in HLA-B27+ transgenic rats, 23 however, IL-10 KO mice colonized with these same microbes manifested a delayed onset and very mild colitis. 20 Bacteroides vulgatus, isolated from guinea pigs with carrageenan-induced colitis only induced a mild inflammation in IL-10 KO mice. 20,27 Cryptosporidium parvum (a protozoan) induced an IBD-like disease in gnotobiotic T-cell receptor α-KO mice. 22 Helicobacter hepaticus, in pure culture, does not seem to induce IBD in IL-10 KO mice, 28 but it can apparently induce IBD when gavaged or injected (intraperitoneally) into SPF IL-10 KO mice. 29
The induction of IBD in IL-10−/− KO mice by intestinal microbes seems to be dependent on an uncontrolled T-helper cell (TH-1) response in the host. 18,21,29,30 Likewise, in cell-transfer studies T cells apparently can induce IBD in SCID mice or Tgε26 transgenic mice treated with bone marrow cells; 15 however, it is necessary for a microbial flora to be present for IBD to be induced and perpetuated in bone marrow-treated Tgε26 mice. 21
We have colonized IL-10−/− KO mice with a variety of microbes (in pure culture) but the IL-10 KO mice did not manifest IBD (eg, C. albicans, E. coli, Lactobacillus spp. [L. casei, L. reuteri, L. acidophilus] a Bifidobacterium sp., a Bacillus sp., and Lactococcus lactis). C. albicans can stimulate AMI and CMI in IL-10 KO mice but we observed no IBD in the IL-10−/− KO mice that were colonized with C. albicans for 35 weeks. 31 Thus, a variety of host responses take place when IBD-susceptible rodents are colonized with a pure culture or a mixture of intestinal bacteria. Some microbes cause IBD only when other intestinal microbes are in the gut. 20,21,23,26 Various rodent modelsof IBD respond differently to a similar microbial flora. For example, a mixture of intestinal bacteria that produced IBD in HLA-B27 rats 26 was unable to induce IBD in IL-10−/− KO mice. 20
Our study demonstrated that a pure culture of E. faecalis can induce IBD, dysplasia, and rectal carcinoma in IL-10 KO mice. E. faecalis is an opportunistic pathogen that is found in the alimentary tract of both man and animals. It is a microbe that is notorious for its capacity to acquire virulence factors (and) antibiotic resistance genes (eg, vancomycin resistance) that have made this opportunistic microbe a major problem for patients and clinicians. 24,25,32 Strains of E. faecalis are also used in the food industry and it has recently been demonstrated that virulence factors can be transferred by these food industry strains. 25
How E. faecalis is able to induce and perpetuate IBD in IL-10−/− KO mice is unknown at this time. This model of IBD certainly lends itself to answering important questions about the immune mechanisms involved in the induction of the disease and to exploring methods that can be used for the prophylaxis and therapy of IBD.
We also colonized IL-10 KO mice with Lactococcus lactis. L. lactis, although it did not induce IBD in IL-10 KO mice (in pure culture), it did cause focal proctitis in two gnotobiotic mice. L. lactis is currently being researched as a microbe that can be genetically manipulated to provide immune factors (eg, IL-10) to a host after it colonized the alimentary tract. 33 We have pointed out previously that so-called probiotic or genetically manipulated microbes may pose a threat to immunodeficient hosts, especially neonates. 34 The use of microbes to deliver therapeutic factors, or to act as probiotics for enhancement of factors that can promote the health and well being of a host must be pursued with caution in immunodeficient hosts.
Footnotes
Address reprint requests to Edward Balish, Ph.D., Medical University of South Carolina, Department of Medical Microbiology/Immunology, 173 Ashley Ave., Room 535 BSB, Charleston, SC 29425. E-mail: balish@musc.edu.
Supported by the Crohn’s and Colitis Foundation of America and a grant from the National Institutes of Health (RO1 DE13968).
References
- 1.Karlinger K, Gyorke T, Mako E, Mester A, Tarjan Z: The epidemiology and the pathogenesis of inflammatory bowel disease. Eur J Radiol 2000, 35:154-167 [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C: Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998, 115:182-205 [DOI] [PubMed] [Google Scholar]
- 3.Prantera C, Scribano ML: Crohn’s disease: the case for bacteria. Ital J Gastroenterol Hepatol 1999, 31:244-246 [PubMed] [Google Scholar]
- 4.Boismenu R, Chen Y: Insights from mouse models of colitis. J Leukoc Biol 2000, 67:267-278 [DOI] [PubMed] [Google Scholar]
- 5.MacDonald TT: Cytokine gene deleted mice in the study of gastrointestinal inflammation. Eur J Gastroenterol Hepatol 1997, 9:1051-1055 [DOI] [PubMed] [Google Scholar]
- 6.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R: A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98:694-702 [DOI] [PubMed] [Google Scholar]
- 7.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL: Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96:795-803 [PubMed] [Google Scholar]
- 8.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W: Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 1995, 182:1281-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boirivant M, Fuss IJ, Chu A, Strober W: Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med 1998, 188:1929-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I: Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993, 75:253-261 [DOI] [PubMed] [Google Scholar]
- 11.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W: Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75:263-274 [DOI] [PubMed] [Google Scholar]
- 12.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S: Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 1993, 75:274-282 [DOI] [PubMed] [Google Scholar]
- 13.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M: Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 1994, 8:2964-2973 [DOI] [PubMed] [Google Scholar]
- 14.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L: Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet 1995, 10:143-150 [DOI] [PubMed] [Google Scholar]
- 15.Hollander GA, Simpson SJ, Mizoguchi E, Nichogiannopoulou A, She J, Gutierrez-Ramos JC, Bhan AK, Burakoff SJ, Wang B, Terhorst C: Severe colitis in mice with aberrant thymic selection. Immunity 1995, 3:27-38 [DOI] [PubMed] [Google Scholar]
- 16.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE: The germ-free state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994, 180:2359-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermiston ML, Gordon JI: Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 1995, 270:1203-1207 [DOI] [PubMed] [Google Scholar]
- 18.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL: Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B17 scid mice. Int Immunol 1993, 5:1461-1471 [DOI] [PubMed] [Google Scholar]
- 19.Sartor RB: Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol Clin North Am 1995, 24:475-507 [PubMed] [Google Scholar]
- 20.Sellon RK, Tonkonogy SL, Schultz M, Dieleman LA, Grenther WB, Balish E, Rennick D, Sartor RB: Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998, 66:5224-5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veltkamp C, Tonkonogy SL, De Jong YP, Albright C, Grenther WB, Balish E, Terhorst C, Sartor RB: Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tgε26 mice. Gastroenterology 2001, 120:900-913 [DOI] [PubMed] [Google Scholar]
- 22.Sacco RE, Haynes JS, Harp JA, Waters WR, Wannemuehler MJ: Cryptosporidium parvum initiates inflammatory bowel disease in germ-free T cell receptor-alpha-deficient mice. Am J Pathol 1998, 153:1717-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB: Normal luminal bacteria, especially bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta 2 microglobulin transgenic rats. J Clin Invest 1996, 98:945-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jett BD, Huycke MM, Gilbore MS: Virulence of Enterococci. Clin Microbiol Rev 1994, 7:462-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton TJ, Gasson MJ: Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol 2001, 67:1628-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, Kwon J, Grenther WB, Balish E, Horak I, Sartor RB: IL-2-deficient mice raised under germ-free conditions develop delayed mild focal intestinal inflammation. Am J Physiol 1999, 39:G1461-G1472 [DOI] [PubMed] [Google Scholar]
- 27.Onderdonk AB, Franklin ML, Cisneros RL: Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect Immun 1981, 32:225-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieleman LA, Arends A, Tonkonogy SL, Goerres MA, Craft DW, Grenther WB, Sellon RK, Balish E, Sartor RB: Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun 1999, 68:5107-5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A: Helicobacter hepaticus-inducedcolitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun 2001, 69:4232-4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobel JD, Muller G, McCormick JF: Experimental chronic vaginal candidiasis in rats. Sabouraudia 1985, 23:199-206 [DOI] [PubMed] [Google Scholar]
- 31.Vazquez-Torres A, Jones-Carson J, Wagner RD, Warner T, Balish E: Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun 1999, 67:670-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emori TG, Gaynes RP: An overview of nosocomial infections including the role of the microbiology laboratory. Clin Microbiol Rev 1993, 6:428-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steidler L, Hans W, Schutte L, Neirnck S, Obermeier F, Falk W, Fiers W, Remaut F: Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289:1352-1355 [DOI] [PubMed] [Google Scholar]
- 34.Wagner RD, Balish E: Potential hazards of probiotic bacteria for immunodeficient patients. Bull Inst Pasteur 1998, 96:165-170 [Google Scholar]