Abstract
The mammalian subtilisin/kexin-like proprotein convertase (PC) family has been implicated in the activation of a wide spectrum of proteins. These proteins are usually synthesized as inactive precursors before their conversion to fully mature bioactive forms. A large majority of these active proteins such as matrix metalloproteases, growth factors, and adhesion molecules are crucial in the processes of cellular transformation, acquisition of the tumorigenic phenotype, and metastases formation. Inhibition of PCs significantly affects the malignant phenotype of various tumor cells. In addition to direct tumor cell proliferation and migration blockade, PC inhibitors can also be used to target tumor angiogenesis. In this Review article we discuss a number of recent findings on the clinical relevance of PCs in cancer patients, their implication in the regulation of multiple cellular functions that impact on the invasive/metastatic potential of cancer cells. Thus, PC inhibitors may constitute new promising agents for the treatment of multiple tumors and/or in adjuvant therapy to prevent recurrence.
To regulate biological activity, a wide variety of proteins are synthesized as inactive precursors that are subsequently converted to their mature active forms by proteolytic enzymes known as proprotein convertases (PCs). The PCs are usually activating proteases and have not been reported to inactivate polypeptides, a process usually performed by degradative enzymes. To date, eight mammalian members of subtilisin-related PCs have been identified including, furin, PC1/PC3, PC2, PC4, PACE4, PC5/PC6, PC7/LPC/PC8, and SKI-1/S1P. 1-7 PCs are multidomain serine proteinases consisting of a signal peptide followed by pro, catalytic, middle, and cytoplasmic domains. Homology is highest in the catalytic domains and lowest in the carboxyl-terminal domains. Furin, PC1, PC2, PC4, PACE4, PC5, and PC7 cleave precursor proteins at basic residues within the general motif (K/R)-(X)n-(K/R)↓, where n = 0, 2, 4 or 6 and X is usually not Cys. 1-5 In contrast, the subtilisin kexin isozyme-1 (SKI-1) processes precursors at non-basic amino acids within the motif (R/K)-X-(L,V)-(L,T,K,F)↓. 1,6-9 Furin, PC5-B, PC7, and SKI-1 are the only members of the mammalian PCs with a transmembrane domain and cycle between the trans-Golgi network and the cell surface. These enzymes as well as PC5, PACE4, and PC4 are involved in the processing of proteins secreted via the constitutive pathway. In contrast, PC1 and PC2 are found within dense core secretory granules and process proteins secreted by the regulated secretory pathway. 1-9 Like many other proteases, PCs are synthesized as inactive zymogens with an N-terminal prosegment extension. This conserved region is autocatalytically removed during PCs/SKI-1 maturation, 1-7 by cleavage either at RXKR↓ (for PCs) or for SKI-1 at the motif RX(V,L)(K,F,L)↓. So far the only known substrates of the novel enzyme SKI-1/S1P 6,7 are: probrain-derived neurotrophic factor, 6 sterol regulatory element-binding proteins, 7 the endoplasmic reticulum-stress response transcription factor ATF6, 8 and the surface glycoprotein GP-C of Lassa virus. 9 Thus, PCs are responsible for processing of neuropeptides, receptors, growth factors (GFs), cell surface glycoproteins, and enzymes, whereas SKI-1 cleaves proproteins that control cholesterol and lipid metabolism, and are involved in neural protection and growth and in the endoplasmic reticulum-stress response pathway. After proteolysis by the convertases, usually the mature proteins/peptides are subject to several other modifications necessary to achieve full bioactivity. The most common being the removal of carboxy-terminal basic residues by carboxypeptidase E or D (CPE, CPD). 10
PCs in Cancers and Clinical Relevance
Multiple approaches, eg, suppression of gene expression or enzyme inhibition, support the hypothesis that PCs play a role in the genesis and progression of different proliferative disorders, including cancer. 11-22 Although elevated expression of different PCs was reported for different human cancers and tumor cell lines, 23-37 the relative importance of various PCs in these cancers has not yet been clarified. Tumor expression of PCs can be studied at the protein level by techniques such as immunohistochemical staining and Western blot analysis, and at the mRNA level by reverse transcriptase-polymerase chain reaction, Northern blot analysis, RNase protection, or in situ hybridization. Table 1 ▶ summarizes the results of studies on the expression of various PCs in human cancers and tumor cell lines. Early studies revealed a high furin expression in advanced lung tumors. 23 Such an association has subsequently been confirmed in other malignancies such as breast, 24 head, and neck 25 cancers. Based on these studies furin expression in tumors may constitute a significant prognostic factor independent of other conventional clinicopathological ones. Other studies described a significant association between high expression of PC1 and PC2 in neuroendocrine tumors, suggesting their involvement in the malignancy of tumor cells with a neural and/or endocrine phenotype. 26-28 Although these studies showed a positive association between PC1 or PC2 expression and the extent of tumors, further research is required to elucidate the importance and the prognostic role of these enzymes in endocrine-related cancers.
Table 1.
Proprotein Convertase Expression in Human Cancers and Tumor Cell Lines
| Expressed PCs | Co-localized substrates | Techniques used | Ref. | |
|---|---|---|---|---|
| Human tumors | ||||
| Pituitary adenomas | PC1, PC2 | ACTH, chromogranin A | RT-PCR, in situ hybridization, immunostaining | 2, 26 |
| Head and neck tumors | Furin, PACE4 | MT1-MMP | RT-PCR, Western blot | 25 |
| Breast tumors | PC1, Furin, PAECE4, PC7 | – | RT-PCR, in situ hybridization | 24 |
| Lung tumors | Northern blot | 23 | ||
| Adenocarcinoma | Furin, PACE4 | – | ||
| Squamous cell lung carcinoma | Furin | – | ||
| Small-cell lung carcinoma | Furin PC2 | – | ||
| Carcinoids | In situ hybridization, immunostaining | 28 | ||
| Bronchial carcinoids | PC1, PC2 | – | ||
| Rectal carcinoids | PC1 | – | ||
| Bile duct carcinoids | PC1 | – | ||
| Thyroid medullary carcinoma | PC1 | – | ||
| Tumor cell lines | ||||
| Pituitary adenomas | ||||
| HP75, Att-20 | Furin, PC1, PC2, PACE4 | Chromogranin A | RT-PCR, in situ hybridization, immunostaining | 5, 27, 32 |
| Medullary thyroid carcinoma | ||||
| rMTC 6–23 | Furin, PC2 | Neurotensin | Northern blot, Western blot | 32 |
| Head and neck carcinomas | ||||
| SCC9, SCC12, SCC13, SCC15 | Furin, PACE4 | MT1-MMP | RT-PCR, immunostaining, Western blot | 25 |
| SCC71, A253, det.262, FaDu | ||||
| Spindle cell carcinoma | ||||
| CC4B, CH72 | PACE4 | Stromelysin 3 | RT-PCR, Western blot analysis, immunostaining | 17 |
| Glioblastoma | ||||
| Lln-18, U138MG, U87MG | Furin | TGF-β1,2 | Northern blot, Western blot | 22 |
| D247MG, T98G, LN-229 | ||||
| U373MG, LN-308, LN-428, U251MG | ||||
| Lung cancer cells | ||||
| H82, H345, H520, H209, H354, H510, H69, H146 | PC1, PC2, furin | Neurotensin, GRP | Northern blot, Rnase protection assay | 23, 37 |
| Pancreas | ||||
| RIN5F, 027B2 | PC1, PC2 furin | Cholecystokinin, proinsulin | Western blot, immunostaining | 30, 34 |
| Somatostatin, proglucagon | ||||
| Breast cancer | ||||
| MCF-7, ZR-75-1, T-470, BT-20 | PC1, furin, PAECE4, PC7 | Enkephalin, vasopressin | RT-PCR, Western blot | 24, 35, 36 |
| MDA-MB-157, MDA-MB-468 | ||||
| MDA-MB-231 | ||||
| Intestine | ||||
| STC-1 | PC1, PC2 | Cholecystokinin | Western blot | 30 |
| Colon cancer | ||||
| HCT8, LoVo, HT29 | PC5 | Neurotensin | RT-PCR, Western blot | 29 |
| LS174T, coloDM320, C119A | ||||
| Gonadal cancer | ||||
| H-500 rat Leydig tumor cells | Furin | PTHRP | Immunostaining | 13 |
Studies on the prognostic impact of PACE4 expression in tumors are less conclusive. We previously reported that PACE4 expression is significantly higher in breast tumors. 24 In studies reported by Bassi and colleagues 25 PACE4 expression was found to be up-regulated in human head and neck tumors and tumor cell lines. Using an animal model of human squamous cell carcinoma, the same group demonstrated that PACE4 expression is implicated in the process of tumor progression and invasiveness. 17 However, this may be tissue-dependent because analysis of lung solid tumors revealed that only half expressed PACE4 and therein its mRNA level was lower than that of furin. 23
PC5 and PC7 have been examined for their prognostic relevance in only a few human cancers (Table 1) ▶ . 24,29 These studies showed a positive association between PC7 expression and the extent of breast tumors of which PC5 was undetectable. 24 In contrast, analysis of various human colon cancer cells, revealed the expression of PC5. 29 The significance of these findings is at present not known.
In conclusion, what seems clear is that furin up-regulation is correlated with tumor progression and invasiveness. Further research is required to elucidate the prognostic role of the other PCs in various types of cancer.
PC Substrates in Tumor Growth and Metastasis
No report has yet appeared to indicate that the PCs could inactivate protein substrates or peptides in trans, even at high concentrations. The only exceptions are the inactivation of their inhibitory prosegment by an autocatalytic mechanism and the inactivation of the inhibitors pro-SAAS and 7B2 by their cognate enzymes PC1 and PC2, respectively. The exact role of the modulation of PC expression and/or activity in tumor development and metastasis remains unclear. Nevertheless, because PCs are directly responsible for the activation of critical proteins implicated in neoplasia, they may be targets of cancer therapy. Among these substrates, matrix metalloproteases (MMPs), GFs, and adhesion molecules through degradation of the extracellular matrix (ECM), modulation of cell growth, and/or migration are involved in tumor progression and metastasis (Table 2) ▶ .
Table 2.
Sequence of the Cleavage Sites of Precursor Proteins
| Site(s) of processing | P6 | P5 | P4 | P3 | P2 | P1 ↓ | P′1 | P′2 | NCBI, accession | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinases | |||||||||||
| STR-1: | V | M | R | K | P | R | C | G | XM 058067 | ||
| STR-2: | V | M | R | K | P | R | C | G | AAH02591 | ||
| STR-3: | 1- | S | L | R | P | P | R | C | G | P24347 | |
| 2- | R | N | R | Q | K | R | F | V | P24347 | ||
| MT-1MMP | 1- | A | M | R | R | P | R | C | G | P50281 | |
| 2- | N | V | R | R | K | R | Y | A | P50281 | ||
| MT-2 MMP | 1- | W | M | K | R | P | R | C | G | P51511 | |
| 2- | R | R | R | R | K | R | Y | A | P51511 | ||
| MT-3 MMP | 1- | W | M | K | K | P | R | C | G | P51512 | |
| 2- | H | I | R | R | K | R | Y | A | P51512 | ||
| MT-4 MMP | 1- | L | M | K | T | P | R | C | S | Q9ULZ9 | |
| 2- | Q | A | R | R | R | R | Q | A | Q9ULZ9 | ||
| MT-5 MMP | 1- | W | M | K | K | P | R | C | G | Q9Y5R2 | |
| 2- | R | R | R | N | K | R | Y | A | Q9Y5R2 | ||
| MMP-1: | V | M | K | Q | P | R | C | G | P03956 | ||
| MMP-2: | T | M | R | K | P | R | C | G | P08253 | ||
| MMP-8: | M | M | K | K | P | R | C | G | XP006273 | ||
| MMP-9: | A | M | R | T | P | R | C | G | XP029934 | ||
| MMP-13: | V | M | K | K | P | R | C | G | XP040746 | ||
| ADAM1 | P | P | R | S | R | K | P | D | AAA74920 | ||
| ADAM8 | P | S | R | E | T | R | Y | V | XP005675 | ||
| ADAM9 | L | L | R | R | R | R | A | V | NP003807 | ||
| ADAM10 | L | L | R | K | K | R | T | T | XP007741 | ||
| ADAM12 | A | R | R | H | K | R | E | T | XP005838 | ||
| ADAM15 | H | I | R | R | R | R | D | V | Q13444 | ||
| ADAM17 | V | H | R | V | K | R | R | A | P78536 | ||
| ADAMTS-1 | S | I | R | K | K | R | F | V | Q9UHI8 | ||
| ADAMTS-2 | 1- | G | V | R | T | R | R | A | A | P79331 | |
| 2- | R | R | R | M | R | R | H | A | P79331 | ||
| ADAMTS-3 | T | M | R | R | R | R | H | A | O15072 | ||
| ADAMTS-4 | P | R | R | A | K | R | F | A | XP042446 | ||
| ADAMTS-5/11 | W | R | R | R | R | R | S | I | Q9UNA0 | ||
| ADAMTS-13 | R | Q | R | Q | R | R | A | A | CAC83682 | ||
| Integrins | |||||||||||
| Integrin αIIb | H | K | R | D | R | R | Q | I | P08514 | ||
| Integrin α3 | P | Q | R | R | R | R | Q | L | XP008432 | ||
| Integrin α4 | H | V | I | S | K | R | S | T | XP039011 | ||
| Integrin α5 | H | H | Q | Q | K | R | E | A | AAH08786 | ||
| Integrin α6 | N | S | R | K | K | R | E | I | NP000201 | ||
| Integrin α7 | R | D | R | R | R | R | E | L | Q13683 | ||
| Integrin α8 | H | L | V | R | K | R | D | V | AAA93514 | ||
| Integrin αE | T | A | R | Q | R | R | A | L | XP008508 | ||
| Integrin αv | H | L | I | T | K | R | D | L | XP002379 | ||
| Growth factor | |||||||||||
| TGF-β1 | S | S | R | H | R | R | A | L | XP_008912 | ||
| Lefty protein | R | S | R | G | K | R | F | S | O00292 | ||
| Pancreatic polypeptide | P | R | Y | G | K | R | H | K | P01298 | ||
| Gastrin | A | S | H | H | R | R | Q | L | P01350 | ||
| Insulin | 1- | T | P | K | T | R | R | E | A | XP028180 | |
| 2- | G | S | L | Q | K | R | G | I | XP028180 | ||
| IGF-1 | P | A | K | S | A | R | S | V | P01343 | ||
| IGF-2 | P | A | K | S | E | R | D | V | XP028189 | ||
| PDGF-A | P | I | R | R | K | R | S | I | NP002598 | ||
| PDGF-B | L | A | R | G | R | R | S | L | NP148937 | ||
| PDGF-C | F | G | R | K | S | R | V | V | NP057289 | ||
| PDGF-D | H | D | R | K | S | K | V | D | AAK56136 | ||
| VEGF-C | H | S | I | I | R | R | S | L | P49767 | ||
| VEGF-D | Y | S | I | I | R | R | S | I | NP_004460 | ||
| FGF-23 | P | R | R | H | T | R | S | A | Q9GZV9 | ||
| EGF | 1- | H | H | Y | S | V | R | N | S | ||
| 2- | K | W | W | E | L | R | H | A | P01133 | ||
| Endothelin-1 | L | R | R | S | K | R | C | S | P05305 | ||
| PTHRP | S | R | R | L | K | R | A | V | P12272 | ||
| Parathyroid hormone | K | S | V | K | K | R | S | V | XP031173 | ||
| Neurotrophin-3 | T | S | R | R | K | R | Y | A | P20783 | ||
| Neurotrophin-4 | N | R | S | R | R | G | V | S | A42687 | ||
| β-NGF | T | H | R | S | K | R | S | S | XP002122 | ||
| BDNF | S | M | R | V | R | R | H | S | XP006027 | ||
| APRIL | R | S | R | K | R | R | A | V | O75888 | ||
| BAFF | N | S | R | N | K | R | A | V | Q9Y275 | ||
| HB-EGF | R | D | R | K | V | R | D | L | Q99075 | ||
| HGF | K | T | K | Q | L | R | V | V | XP052260 | ||
| Growth factor receptors | |||||||||||
| Insulin receptor | P | S | R | K | R | R | S | L | XP048347 | ||
| IGF-1 receptor | P | E | R | K | R | R | D | V | IGHUR1 | ||
| HGF receptor | E | K | R | K | K | R | S | T | P08581 | ||
| Others | |||||||||||
| Ldl-related protein | S | N | R | H | R | R | Q | I | Q07954 | ||
| Leptin receptor | Q | V | R | G | K | R | L | D | P48357 | ||
| Notch-1-receptor | S | R | K | R | R | R | Q | H | AAG33848 | ||
Most of the indicated PC-like sites have been proven in cellular and/or in vitro experiments. However, others are only predicted.
Metalloproteinases
The destruction of the basement membrane and ECM is associated with tumor cell invasion and metastasis. ECM is a complex structure that consists of collagen, proteoglycans, and other protein such as fibronectin, vitronectin, and laminin. Secreted proteinases from malignant and stromal cells degrade many ECM components, facilitating the detachment of these cells and their invasiveness. The level of expression of these proteinases in tumor cells is associated with advanced-stage tumorigenesis and poor prognosis. ECM degradation is a complex process involving a cascade of proteolytic events in which the primary step likely implicates enzyme activation by the PCs. The latter were reported to process the following metalloproteinases: stromelysin-3 (str-3), 38,39 membrane-type MMPs (MT-MMPs), 40 the adamalysin metalloproteinases (ADAMs), 41 and the adamalysin metalloproteinases with thrombospondin motifs (ADAM-TS). 42,43 The expression of these metalloproteinases has been correlated with increased local aggressiveness, metastasis, and poor clinical outcome. 44-49
Stromelysin-3 (Str-3)
Str-3 is a member of the MMP family of which expression has been correlated with both increased local aggressiveness and poor clinical outcome. 50 Activation of Str-3 by furin occurred intracellularly before secretion (Table 2) ▶ . 38,39
MT-MMPs
MT-MMPs, a new family of MMPs, are overexpressed in a wide variety of carcinomas, especially in the colon 44,45 and brain 51 and were reported to be involved in metastasis. Expression of these MMPs, particularly MT1-MMP was shown to process pro-MMP-2 thereby enhancing invasiveness both in vitro and in vivo. 40,52 MMP-2 is the enzyme that degrades collagen IV, a major type of collagen in basement membranes. MT1-MMP possesses two typical recognition motifs for PCs, namely ArgArgProArg 92 and ArgArgLysArg 111 that were recently reported to be cleaved by furin-like enzymes at both sites (Table 2) ▶ . 40
Adamalysin Metalloproteinases
ADAMs are a family of membrane-associated multidomain zinc-dependent metalloproteinases with high sequence homology and domain organization, similar to the snake venom metalloproteases of the adamalysin subfamily. 46 Sixteen of the 30 ADAM proteins identified to date are predicted to be catalytically active, based on the presence of a conserved zinc-binding sequence (HEXXH) in the protease domain, whereas the other members are not likely to be active proteases. The prodomains of several ADAM proteins such as ADAM12 are constitutively cleaved by a furin-type PCs as they progress through the secretory pathway (Table 2) ▶ . 41 This family was reported to play a role in diverse biological processes such as fertilization, myogenesis, neurogenesis, and cell surface proteolysis and shedding of different proteins. 41,47,53-59 Of the proteins shed by the ADAMs, are cytokines and GFs such as transforming growth factor-α (TGF-α), epidermal growth factor (EGF), heparin-binding EGF, tumor necrosis factor-α (TNF-α), c-Kit-ligand-1 (KL-1), colony-stimulating factor-1 (CSF-1), and Fas-ligand (Fas-L), receptors such as TNF receptor-I (TNFR1, p60 TNFR), TNF receptor-II (TNFR2, p80 TNFR), p75 nerve growth factor receptor (p75NGFR), interleukin-6 receptor (IL-6R), thyroid-stimulating hormone receptor, adhesion molecules such as L-selectin, and others proteins such as protein tyrosine phosphatase σ (PTPσ), protein tyrosine phosphatase LAR (LAR), amyloid precursor protein, and angiotensin-converting enzyme. 53,56 Certain released molecules can be cleaved by more than one enzyme, and some enzymes can cleave more than one substrate. For example, cleavage of TNF-α can be mediated by ADAM17 (TACE) and ADAM10, and α-secretase activity for amyloid precursor protein has been attributed also to ADAM17, 54 ADAM9, 57 and ADAM10. 59 It is not known how these protease(s) select their substrate because reliable consensus cleavage sites have not been identified. In addition to their proteolytic function, some members of the ADAM family such as ADAM15 and ADAM2 can support integrin binding via their disintegrin domain. 58,60,61 It is increasingly recognized that ADAMs represent a novel group of membrane proteases that are important for cellular interactions under physiological and pathophysiological conditions including cancer. Recently, several ADAM family members were described to be dramatically up-regulated in many tumor cells. This includes cells derived from a range of hematological malignancies 47 and breast, prostate, lung, and colon cancer. 42,48,62 Therefore, it is of particular interest that the member of the ADAM family reported to shed cell-associated neural adhesion molecules such as L1 may be relevant to promote cell migration and invasion. 62,63 Interestingly, in these cells the putative tumor suppressor gene MDC (ADAM11) was expressed at a very low level. 62,63
Adamalysin Metalloproteinases with Thrombospondin Motifs
ADAM-TSs are a new member of the ADAM family containing thrombospondin-type motifs. They consist of multiple domains of proteins common to the ADAM family, including pro-, metalloprotease-like, and disintegrin-like. The first member of this family, called ADAM-TS1, was originally cloned from a colon adenocarcinoma cell line. 42 Based on its capacity to form a covalent complex with α2-macroglobulin, recent studies demonstrated that ADAM-TS1 protein is proteolytically active (Table 2) ▶ . 43 In addition, the maturation of ADAM-TS1 precursor is impaired in the furin-deficient cell line, LoVo, and the processing ability of the cells is restored by the co-expression of the furin cDNA. 64 The only members of the ADAM-TS family with established substrates are ADAM-TS2 (procollagen-N-proteinase) and ADAM-TS4 and -TS11 (aggrecanases-1 and -2, respectively). 42,64,65 These proteinases cleave aggrecan at one or more of five specific sites in the aggrecan core protein. 42,64,65 Recently, brain-enriched hyaluronan binding (BEHAB)/brevican, a brain-specific ECM protein was reported to be processed by ADAM-TS4. The processed form of BEHAB/brevican is dramatically increased in human gliomas, a notoriously invasive tumor. 49 The rat 9L gliosarcoma cell line, which does not express BEHAB/brevican and forms noninvasive tumors when grown as intracranial grafts, can form invasive tumors when it is transfected with a 5′ cDNA fragment of BEHAB/brevican, but not when transfected with the full-length cDNA. 49 Interestingly, the expression of ADAM-TS4 is induced during endothelial cells undergoing differentiation into tube-like structures suggesting its implication in angiogenesis. 66 These observations link ADAM-TS family members to invasion and the blocking the activation of ADAM-TSs by PC inhibitors may provide a novel therapeutic strategy.
Adhesion Molecules
Cell adhesion molecules (CAMs) are cell-surface proteins that mediate cell-cell and/or cell-ECM interactions. They control cellular traffic, transmigration through the endothelium, homing in and localization to various target organs during inflammation, and tumor cell colonization. 67,68 Most of the CAMs characterized so far fall into three categories of proteins: the immunoglobulin (Ig), integrin, or selectin families.
The Immunoglobulin Family
The Ig family includes intercellular adhesion molecules ICAM-1, ICAM-2, and ICAM-3, vascular CAM-1 (VCAM-1), and mucosal addressin CAM-1 (MadCAM-1), none of which are believed to be substrates for PC processing. However, the convertases seem to be required for their expression and probably function. Indeed, the expression of ICAM-1 and VCAM-1 on endothelial cells is induced by various cytokines and GFs such as interferon-γ, interleukin-1 (IL-1), TNF-α, insulin-like growth factor (IGF-1), and endothelins. 69-76 Some of these precursors were reported to be directly processed by PCs such as IGF-1 and endothelins (Table 2) ▶ . 77-79 Others are indirectly regulated by the PCs through the cleavage of their cognate enzymes, for example TNF-α converting enzyme (TACE) 80 and ADAM10 56 that process pro-TNF-α are themselves activated by PCs. 59,80,81
The Integrin Family
Its now well established that integrins are implicated in tumor progression and metastasis. 82,83 Integrins are heterodimeric αβ transmembrane receptors that bind ligands such as the ICAMs, VCAMs, and several ECM components. The extracellular domains of both subunits are required for ligand binding whereas the cytoplasmic tails interact with the cytoskeleton, induce changes in cell shape and motility, and transduce growth and survival signals. 84,85 In addition, activation of integrins was reported to mediate MMPs and urokinase activity of many tumor cells, including melanoma and colon carcinoma. 86-88 Although β-subunits are not cleaved by PCs, a total of 9 of 18 known α-subunits possess a potential PC-cleavage site, with α3, α4, α5, α6, and αv proven to be PC5 and furin substrates (Table 2) ▶ . 89,90 Recently, Berthet and colleagues 91 reported that the endoproteolytic cleavage of αv integrin is important for the signal transduction pathway mediating cell adhesion. Blockade of αvβ5 cleavage resulted in a decreased phosphorylation of focal adhesion kinase (FAK) and paxillin, two important molecules involved in cell adhesion.
The Selectin Family
The selectin family members, L-selectin, P-selectin, and E-selectin are involved in the adhesion of leukocytes to activate the endothelium. Adhesion is initiated by weak interactions that produce a characteristic rolling motion of leukocytes on the endothelial surface. This rapid on-off attachment is necessary for activation and engagement of integrins and their counterreceptors on the leukocytes and endothelial cells, respectively. The integrin-mediated, high-affinity binding is in turn required for leukocyte arrest and extravasation. 67 On the basis of in vitro studies it is postulated that similar cell-cell interactions may also occur between circulating malignant cells and the vascular endothelium during tumor dissemination. 92-94 In general, the selectins bind sialylated, glycosylated, or sulfated glycans on glycoproteins, glycolipids, or proteoglycans. 95 The tetrasaccharides sialyl-Lewisx (sLewx) and sialyl-Lewisa (sLewa) are recognized by all three selectins. In vitro adhesion studies showed that human colorectal, pancreatic, and gastric carcinoma cells use sLewx and related carbohydrate determinants to adhere to TNF-α-inducible E-selectin on cultured vascular endothelial cells. 93,96-98 In vivo studies in turn showed that inhibition of liver metastasis of the highly metastatic human pancreas adenocarcinoma (PCI) cells could be blocked with antibodies to sLewa and that lung colonization by colon carcinoma HT29 cells could be blocked by a soluble E-selectin fusion protein. 97,98 Under normal physiological conditions, vascular endothelial cells express low constitutive levels of E-selectin. Several cytokines and GFs such as IL-1, TNF-α, vascular endothelial growth factor, IGF-1, and endothelins induce the expression of E-selectin. 75,77,99,100 Many of these molecules directly or indirectly require PC activity (Table 2) ▶ . Thus, although like VCAMs and ICAMs, selectins are not processed by PCs, the latter may indirectly control their expression via the activation of some of the above inducers. Recently, we demonstrated that E-selectin expression on sinusoids was described to be one of the early molecular events involved in metastasis. 101 The arrest of tumor cells in the hepatic circulation causes a cascade of events, which start with a rapid release of IL-1 and TNF-α (and other mediators). In turn, these stimulate the expression of E-selectin on hepatic endothelial cells, resulting in an enhanced tumor cell adhesion and subsequent metastases. 101
GFs and Their Receptors (GFRs)
The availability of GFs is critical for malignant transformation and metastasis. These molecules mediate cell entry into, and progression through, the cell cycle. They are divided into two main categories namely, competence factors such as platelet-derived growth factor, vascular endothelial growth factor, and basic fibroblast growth factor that enable cells to enter the G1 phase and progression factors such as IGF-1 that are required for progression from G1 into S phase and ultimately resulting in cell division. 102-105 Many of these proteins as synthesized as proproteins that are processed and activated by PC-like enzymes (Table 2) ▶ . Parallel increased expression of both GFs and PCs may result in tumor growth. Of the GFs shown to be processed by PCs include IGF-1, endothelins, nerve growth factor, PTH, and TGF-β (Table 2) ▶ . 77,78,106-108 Others, are suspected to be PC substrates based on the presence of the RXXR↓ motif, eg, FGF23 (Table 2) ▶ 109 that is reported to be involved in tumor-induced osteomalacia. Many GFs mediate their effects through receptor tyrosine kinases (RTKs) that transmit signals to the nucleus through an intricate network of adapter and signaling molecules. 104 Receptor activation through ligand binding generally induces receptor dimerization and autophosphorylation through a trans mechanism. 104,110 Transphosphorylation at specific tyrosine residues in turn generates binding sites that are recognized by proteins involved in cellular signaling. Several proteins that are phosphorylated through GF-associated kinase activity were identified as downstream mediators of receptor-associated signal transduction include: phospholipase Grb, 111 Cγ (PLCγ), 112 GTPase-activating protein, 113 phosphatidylinositol 3-kinase (PI-3 k), 114 insulin receptor substrate (IRS), and homology and collagen (Src). 115,116 RTKs are implicated in malignant transformation and tumor progression. 117,118 Overexpression or mutations/insertions in GF receptors (GFRs) that result in constitutively high levels of proteins, or active kinases are documented in many tumors. 119,120 Frequently this is accompanied by constitutive high expression of the respective ligands providing an autocrine mechanism for growth autonomy. 121 Several GFRs contain a consensus PC-cleavage site, and are critical for tumor growth and metastases, eg, the HGF receptor (HGF-R), 122 insulin receptor, 123-125 and IGF-1 receptor. 20,126 Hwang and colleagues, 125 reported that cleavage of insulin receptor is essential in the signal transduction of insulin. In agreement with this observation, we recently showed that the uncleaved IGF-1 receptor is unable to undergo the critical steps for IGF-1-mediated growth and survival of tumor cells, ie, IGF-1-sensitive autophosphorylation and IRS-1 phosphorylation. 20 However, Komada and colleagues 122 previously reported that both cleaved and uncleaved HGF receptors can bind HGF and mediate their autophosphorylation and cell growth. This unusual result indicates that proteolytic processing of this receptor may not be essential for some of the tested signal transduction pathways of HGF. However, to better define the role of PC-mediated cleavage of this receptor, more extensive analysis is required.
PC Inhibitors
The potential clinical and pharmacological role of the convertases has fostered the development of both peptide- and protein-based PC inhibitors. 1-5 The most promising protein-based specific inhibitors of PCs are a α1-anti-trypsin variant known as α1-anti-trypsin Portland or α1-PDX 127,128 and the individual PC prosegment-based inhibitors. 129,130
The α1-Anti-Trypsin Variant, α1-Anti-Trypsin Portland (α1-PDX)
Like furin substrates, inhibitors of furin also require the interaction of enzyme subsites with the basic residues of the substrate. Previously, the trypsin inhibitor and the third domain of turkey ovomucoid have been reported to be inhibitors for furin. 131,132 However, their equilibrium constant was representative of a moderate, rather than a potent, inhibitor. Inhibition of furin in the subnanomolar range was accomplished by bioengineering the α1-anti-trypsin variant, α1-PDX (α1-anti-trypsin Portland). 127 Kinetic analysis shows that a portion of bound α1-PDX operates as a suicide inhibitor. 128 Once bound to furin’s active site, α1-PDX can either undergo proteolysis by furin or form a kinetically trapped sodium dodecyl sulfate-stable complex with the enzyme. 128,133 In an attempt to produce a specific furin inhibitor others researcher mutated the bait region of the general protease inhibitor α2-macroglobulin (RVGFYESDVM690 into RVRSKRSLVM690). 134 This variant was reported to inhibit processing of furin substrates, eg, human immunodeficiency virus type-1 glycoprotein gp160, von Willebrand factor, and TGF-β1 was inhibited in COS-1 cells. 134 The ovalbumin-type serpin human proteinase inhibitor-8, containing two instances of the minimal furin recognition sequence (VVRNSRCSRM343), has also been shown to inhibit furin in a rapid, tight binding manner that is characteristic of physiological serpin-proteinase interactions. 135 However, the cytosolic localization of inhibition inhibitor-8 requires the addition of a signal peptide before it could inhibit furin in vivo, and even then its ex vivo inhibitory property is yet to be proven. More recently Cameron and colleagues 136 reported that although the hexa-d-arginine is a potent and relatively specific furin inhibitor, the reduced ability of this highly charged peptide to cross the cell membrane for in vivo therapeutics is problematic. Based on these studies, to date the most promising protein-based specific inhibitor of PCs is the α1-anti-trypsin variant, α1-PDX. This serpin was first showed to be a potent inhibitor of furin-mediated cleavage of HIV gp160, 127,137 but subsequently demonstrated to also inhibit all PCs involved in processing within the constitutive secretory pathway. 138-140 Recent studies showed that endogenous inhibition of precursor convertases by α1-PDX reduces the production of the amyloid precursor α-secretase product amyloid precursor protein-α 59-141 and blocks the activation of the pore-forming toxin proaerolysin, 142 the maturation of the surface glycoproteins of infectious pathogens, 127,128,137 the proteolytic activation of bone morphogenetic protein-4 (BMP-4), 143 the cleavage of Notch, 144 insulin-like growth factor-1 receptor (IGF-1R), 20 and TGF-β. 145,146 The potential therapeutic value of PC inhibitors was recently reinforced by a report showing that exogenous addition of α1-PDX potentially inhibits the furin-dependent processing of human cytomegalovirus envelope glycoprotein gB, thus reducing the titer of infectious hCMV more effectively than currently used anti-herpetic agents. 128 As uptake of α1-PDX into the cell could not be detected in cell lines lacking the enzyme, it was suggested that the reported furin inhibition by the external application of α1-PDX occurs because furin is localized to the trans-Golgi network and cycles to the cell surface, where it could meet α1-PDX, and back via endosomal compartments. 128 A similar mechanism was attributed for the prevention of Pseudomonas exotoxin A activation when extracellular α1-PDX was applied to A7 melanoma cells. 128 These studies collectively suggest that inhibition of PCs localized in the interior of the cell can be initiated at the extracellular cell surface by PC binding to α1-PDX and uptake, resulting in inhibition of pathological disease processes including cancer.
α1-PDX and Tumor Cell Malignant Phenotypes
The in vitro and in vivo studies recently reported by our group and others demonstrated that the serpin α1-PDX could be a powerful tool for tumorigenesis inhibition. 21,20 In ex vivo studies, using human leukemia Jurkat T and colon carcinoma HT29 cell lines and mouse pituitary tumor AtT20 cells, we found that stable expression of α1-PDX provoked dramatic changes in several malignant phenotypes and metastatic potential. This includes cellular growth, survival, invasiveness, and tumorigenesis.
The Role of PCs in Cell Growth
The availability of GFs is critical for malignant transformation and metastasis. The anti-proliferate effect of α1-PDX on tumor cells is probably because of the inhibition of the processing of various proteins, including GFs and/or their corresponding receptors. 77,147-150 Previously, it was postulated that the critical nature of the furin processing of various precursors may explain the anti-proliferative effect of furin blockade on H-500 rat Leydig tumor cells, 12 the pancreatic β-cell line MIN6, 16 and the gastric mucus cells. 15 Kayo and colleagues 16 showed that conditioned medium derived from furin-overexpressing MIN6 cells stimulated the growth of their parental control cells, whereas the medium from cells expressing α1-PDX resulted in a lower growth rate. These results suggest that high furin expression stimulates growth through an autocrine/paracrine mechanism. In agreement with this hypothesis our group recently demonstrated the importance of the processing of IGF-1 receptor in the mediation of IGF-1-mediated tumor cell proliferation. 20 A functional IGF-1R is known to be required for cell growth of various transformed cells. Overexpression and/or constitutive activation of IGF-1R in a variety of cell types leads to ligand-dependent growth in serum-free medium and to the establishment of a transformed phenotype such as the ability to form colonies in soft agar and tumors in mice. 118,151 Similarly, studies reported on TGF-β1 processing by PCs revealed that inhibition of TGF-β1 in glioblastoma and in head neck squamous cell carcinoma (HNSCC) cell lines by α1-PDX could be a promising tool in modulating tumor growth and immunotherapy. 21,22
The Role of PCs in Cell Survival
Similarly to PCs role in growth, PC cell-survival function is also substantial. Thus, the expression of α1-PDX in tumor cells exaggerates the apoptotic phenotype (DNA degradation and propidium iodide-positive cells) after serum deprivation, as we reported for HT-29 and Jurkat leukemia cells. 20 The role of PC in cellular survival can be explained by their role in the maturation of various proteins known to be anti-apoptotic mediators participating in various autocrine/paracrine mechanisms. A likely scenario may involve one or more secreted ligands and/or their receptors, all of which require processing by PC-like enzymes. Examples include IGF-1 and IGF-2 that are synthesized and secreted by these cells 152 and processed by furin. 148,149 Because overexpression of α1-PDX in tumor cells inhibited the processing of IGF-1R and its furin-processed ligands, IGF-1 and IGF-2, it is liable to abrogate their autocrine/paracrine protective effects. The protective effect of many proteins is apparently dependent on their ability to induce a cascade of events leading to downstream effector pathway phosphorylation, including FAK, 153,154 PI-3K1, 155 and IRS-1. 156 After phosphorylation these molecules mediated their anti-apoptotic effect through the activation of several negative death regulators such as Bcl-2 or inhibition of IL-converting enzyme-like caspases. 153-157 Interestingly, expression of α1-PDX in tumor cells resulted in a reduction of basal tyrosine phosphorylation of FAK, PI-3K, and IRS-1. 20 Exogenous addition of FAK, PI-3K, and IRS-1 activators such as IGF-1, failed to increase tyrosine phosphorylation of these molecules in tumor cells and to rescue the cells from apoptosis. This suggests a blockade in the transmission of the autocrine/paracrine anti-apoptotic signals in these cells.
The Role of PCs in Cell Invasion
PCs role in in vitro invasion is now well documented. Inhibition of PCs in different tumor cells resulted in a significant reduction in their invasiveness. 20,25 This reduction is because of processing blockade of proteins directly involved in the mechanism of invasion such as MMPs or to proteins such as GFs and/or integrins reported to induce the expression of ECM-degrading protein including urokinase and MMPs. 20,25,38,39,40-43 Recently, the inhibitory effect of α1-PDX on in vivo invasion was reported by Bassi and colleagues. 21 They used an in vivo invasion assay based on the penetration of tumor cells into the tracheal wall. When transplanted in the tracheas the control-transfected cells penetrated deeply into the tracheal wall and reached the adventitia and the surrounding peritracheal tissues. Whereas, the penetration of α1-PDX-transfected cells in the tracheal wall was markedly decreased and never reached the outer surface of the trachea. They estimated this in vivo invasion reduction to be ∼70 to 80%. We also found that α1-PDX decreased the invasiveness of colon carcinoma HT-29 20 in which the processing of MT1-MMP was inhibited (Figure 1) ▶ . In these cells the mRNA level of plasminogen activator urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA), the urokinase-type plasminogen activator receptor (uPAR), and the uPA inhibitor plasminogen activator inhibitor-1 (PAI-1), molecules involved in invasion processes and believed not to be processed by PCs, was significantly reduced. This data demonstrated both the direct and the indirect involvement of PCs in tumor cell invasion.
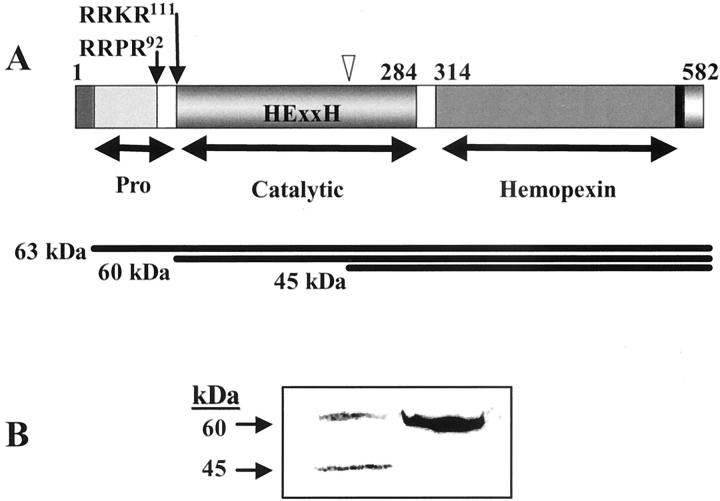
Figure 1.
Pro-MT1-MMP processing. A: Schematic representation of the structural aspects of the human pro-MT1-MMP (63 kd) and its PC-processing sites at the RRPR92↓ and RRKR111↓ The mature enzyme (63 kd) can be further autocatalytically cleaved (itrio) into a 45-kd C-terminal form. Shown are the pro-, catalytic (with the HExxH signature), and hemopexin-like domains. B: Western blots of the cell lysates obtained from the colon carcinoma cell line HT-29 transfected with pIRES2-EGFP vector alone (HT-29) or a recombinant vector containing cDNA of α1-PDX (HT-29/PDX) using mouse anti-human MT1-MMP monoclonal antibody (mAb 3319) recognizing the catalytic domain.
The Role PCs in Tumorigenesis and Angiogenesis
The anti-tumor effects of PC inhibition was initially reported by our group. Expression of α1-PDX in colon carcinoma HT-29 tumor cells delayed the appearance, the incidence, and the vascularization of palpable tumors. 20 Subsequently, the study reported by Bassi and colleagues 21 have confirmed the inhibition of tumor growth by PC inhibition and found that the levels of furin mRNA and protein expression correlate with the aggressiveness of tumor cell lines derived from head and neck and lung cancers. These studies demonstrated that the inhibitory effect of α1-PDX on tumor growth and invasiveness in in vivo systems may be underestimated because of the significant loss in α1-PDX expression in the subcutaneous tumors during their growth. However, it is not clear whether the observed progressively lower levels of α1-PDX mRNA in tumors 21 is because of a specific loss of the cDNA from tumor cells. Future studies should address this issue by comparing the levels of α1-PDX to those of a specific marker co-expressed with this serpin, eg, green fluorescent protein. This may eliminate the trivial explanation of a dilution effect of the original tumor cells by host cells. However, similar observations were reported by Leitlein and colleagues, 22 on the loss of α1-PDX expression ex vivo. These authors claim that α1-PDX expression was not very stable in glioma cell lines and was lost within a few passages in vitro. Angiogenesis is a significant prognostic factor in various cancers, but the factors that control angiogenesis in vivo are not well defined. Usually tumor angiogenesis is mediated by tumor-secreted angiogenic GFs that interact with their surface receptors expressed on endothelial cells. Multiple angiogenic proteins are known, including vascular endothelial growth factor and its four isoforms (121, 165, 189, and 206 amino acids), TGF-β1, pleiotrophin, acidic and basic fibroblast growth factor. Immunohistochemical analysis of CD31 antigen expression, a marker of endothelial cells revealed a reduced tumor vascularization of tumors developed from tumor cells expressing α1-PDX (Figure 2) ▶ . 20 This suggests the importance of the PCs in tumor vessel formation through direct/indirect activation of various angiogenic proteins.
Figure 2.
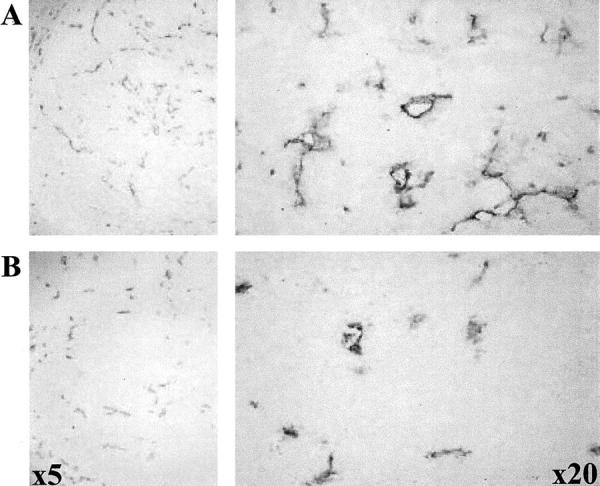
Extent of angiogenesis determined by immunostaining for CD31. The immunohistochemical analysis were performed on tissue obtained from control tumors (HT-29) or tumors developed from HT-29 cells expressing α1-PDX c-DNA (HT-29/PDX).
PC Prosegment-Based Inhibitors
To date the only naturally occurring intracellular PC inhibitor found in the constitutive secretory pathway are PC’s own propeptides or prosegments, 2,6,158 and in the case of PC1 its C-terminal domain. 159 The activities of the regulated secretory pathway convertases, PC1 and PC2, are however regulated by selective and specific inhibitors known as pro-SAAS 160,161 and 7B2, 162 respectively. For many proteins, the prosegment serves as an intramolecular chaperone that is essential for their correct folding, 163 or/and transport and secretion. 164 In the case of many proteolytic enzymes such as cathepsins, carboxypeptidases, papain, and subtilases, this prosegment was reported to be a very potent inhibitor that is highly specific for its associated protease. In these enzyme systems the prosegment is still an effective inhibitor even after initial proteolysis and in most cases an additional cleavage within the prosegment to release it and thereby to fully activate the enzyme. 158,165 In accordance with the notion that PCs are initially produced as zymogens and proteolytically activated, Anderson and colleagues 129 demonstrated that the prosegment of furin when used as a fusion protein to glutathione S-transferase exhibits a potent in vitro inhibitory activity on furin. 165 We have also shown that the prosegment of furin and PC7 purified from bacterial culture media, are very potent inhibitors of their respective enzymes. Additionally, in vitro studies demonstrated the synthetic peptides derived from the prosegments of PC1, PC7, and furin are potent inhibitors of their enzymes. 129,130,166,167 Based on these data, we surmised that, aside from α1-PDX, the PC prosegments may be applied in cancer therapy, as they were in Alzheimer’s disease β-secretase analysis. 168 Indeed these prosegments were shown to inhibit intracellularly the processing of GF precursors such as nerve growth factor and brain-derived neurotrophic factor, 129 both of which have been linked to neuronal cancer and metastasis. 169 In addition, we recently demonstrated that the cellular processing of IGF-1R, known to be crucial for the malignant phenotype of tumor cells, 20,118,151 is effectively blocked by α1-PDX 20 as well as by the prosegments of furin and PC5 but not by that of PC7 (Figure 3) ▶ . Although some data exist to show that α1-PDX does not significantly inhibit thrombin, 127 these are not exhaustive and the ability of α1-PDX to inhibit other classes of proteases is yet to be fully elucidated. In addition, because prosegment inhibitors are not always completely selective for their cognate enzymes and can inhibit other PCs, 129 it will be necessary to modify their structure to improve their selectivity without compromising their potency (N. Nour and N. G. Seidah, in preparation).
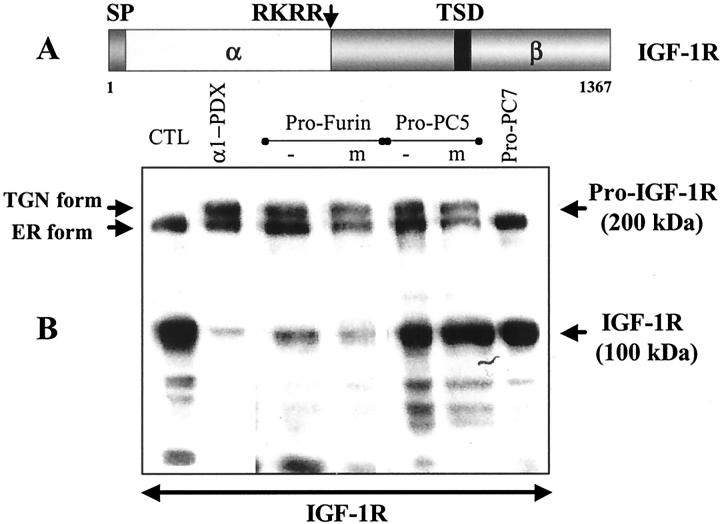
Figure 3.
Inhibition of the proteolytic processing of IGF-1R by PC prosegments. A: Schematic representation of the primary structure of human IGF-1R. The positions of its signal peptide (SP), PC-processing site (RKRR), and transmembrane domain (TMD) as well as the α- and β-subunits are shown. B: IGF-1R processing was analyzed by Western blotting of cell lysates obtained from transiently transfected HK293 cells with IGR-1R cDNA together with either the pIRES2-EGFP vector alone (control, CTL) or a recombinant vector containing either profurin, pro-PC5, or pro-PC7 cDNA. Cells expressing α1-PDX are shown for comparison. Note that the inhibitors specifically inhibit the processing of the trans Golgi network form of pro-IGF-1R (210 kd) and not the endoplasmic reticulum-form (200 kd) into the 100-kd β-chain. Also note that the prosegment inhibitor mutated at its secondary cleavage site (m) (148) is as effective as the wild-type inhibitor and that pro-PC7 does not inhibit processing.
Concluding Remarks
Regulation of cellular proliferation, differentiation, and adhesion are complex processes in which different biological systems interact. Disruption of these processes is a hallmark of malignant transformation resulting in tumor progression and acquisition of a metastatic potential. The key to successfully developing efficient clinical strategies against cancers is to target a protein known to be essential in the growth of tumors and to validate that the disruption of this target selectively blocks the growth of the transformed cells. In this Review we have described recent progress made in establishing a novel approach to inhibiting tumor growth and tumor cell malignant phenotypes. We have outlined PC inhibition as a strategy to simultaneously disrupt the function of numerous proteins involved in the acquisition of the invasive/metastatic potential of cancer cells (Figure 4) ▶ . The potential of this approach has been firmly established by the use of PC inhibitors (α1-PDX and PC-prosegments) targeting the activity of the PCs. The cumulative data available established that α1-PDX is not only able to block the interaction of GFs and their receptors such as IGF-1/IGF-1R, and the activation of several MMPs but also to inhibit tumor cell proliferation and invasion (Figure 4) ▶ . This potent effect further translates into an ability to slow down the growth of human tumors in a nude mouse model through an anti-proliferative and anti-angiogenic mechanism (Figure 2) ▶ . 20,21
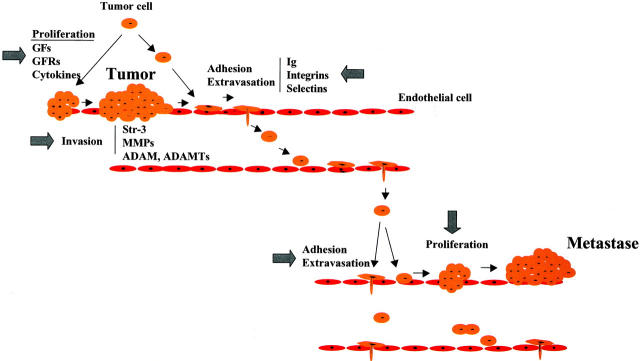
Figure 4.
Schematic representation of cascade events implicating PCs for tumor growth and metastasis. By activating GFs, cytokines, and their receptors (GFRs), PCs control tumor cell proliferation and growth. By activating or inducing adhesion molecules convertases modulate adhesion, invasion, and migration of tumor cells and subsequently metastases formation. Arrows indicate the potential sites for PCs inhibitors.
These studies represent the first example of a potentially new approach to controlling tumor cell growth and behavior through the inhibition of precursor processing. Although the use of general PC inhibitors may be advantageous, in some cases it may be necessary to target only one member of the PC family. Therefore, one of the important future developments would be to find and express PC inhibitors specific for each member of the family. This is feasible, as was demonstrated for PC1 (pro-SAAS) 160,161,170 and PC2 (7B2). 162,171 These could be used alone or in combination to target specific tumors. In the long term, these inhibitors may provide a rationale for testing this family of compounds as anti-metastatic agents or in conjunction with standard therapy in clinical settings. Thus, the anticipated results will improve our knowledge of the role of convertases in proliferative diseases, and lead to the design of potent and selective convertase-inhibiting reagents and novel pharmacological strategies.
Acknowledgments
We thank Mrs. Edwige Marcinkiewicz for her assistance during the immunohistochemistry analysis.
Footnotes
Address reprint requests to Abdel-Majid Khatib, Laboratory of Biochemical Neuroendocrinology, Clinical Research Institute of Montreal, 110 Pine Ave. West, Montreal, Quebec, Canada H2W 1R7. E-mail: khatiba@ircm.qc.ca.
References
- 1.Seidah NG, Chrétien M: Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res 1999, 848:45-62 [DOI] [PubMed] [Google Scholar]
- 2.Seidah NG, Chrétien M: Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol 1997, 8:602-607 [DOI] [PubMed] [Google Scholar]
- 3.Nakayama K: Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J 1997, 327:625-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou A, Webb G, Zhu X, Steiner DF: Proteolytic processing in the secretory pathway. J Biol Chem 1999, 274:20745-20748 [DOI] [PubMed] [Google Scholar]
- 5.Seidah NG, Chretien M, Day R: The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie 1994, 76:197-209 [DOI] [PubMed] [Google Scholar]
- 6.Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, Benjannet S, Toure BB, Basak A, Munzer JS, Marcinkiewicz J, Zhong M, Barale JC, Lazure C, Murphy RA, Chretien M, Marcinkiewicz M: Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci USA 1999, 96:1321-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai J, Rawson RB, Espenshade PJ, Cheng D, Seegmiller AC, Goldstein JL, Brown MS: Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol Cell 1998, 2:505-514 [DOI] [PubMed] [Google Scholar]
- 8.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL: ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 2000, 6:1355-1364 [DOI] [PubMed] [Google Scholar]
- 9.Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W: The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci USA 2001, 98:12701-12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reznik SE, Fricker LD: Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cell Mol Life Sci 2001, 58:1790-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbikay M, Seidah NG, Chrétien M: From proopiomelanocortin to cancer: possible role of convertases in neoplasia. Ann NY Acad Sci 1993, 680:13-19 [DOI] [PubMed] [Google Scholar]
- 12.Vieau D, Seidah NG, Mbikay M, Chrétien M, Bertagna X: Expression of the prohormone convertase PC2 correlates with the presence of corticotropin-like intermediate lobe peptide in human adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab 1994, 79:1503-1506 [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Amizuka N, Goltzman D, Rabbani SA: Inhibition of processing of parathyroid hormone-related peptide by anti-sense furin: effect in vitro and in vivo on rat Leydig (H-500) tumor cells. Int J Cancer 1995, 63:276-281 [DOI] [PubMed] [Google Scholar]
- 14.Chrétien M, Mbikay M, Gaspar L, Seidah NG: Proprotein convertases and the pathophysiology of human diseases: prospective considerations. Proc Assoc Am Physicians 1995, 107:47-66 [PubMed] [Google Scholar]
- 15.Konda Y, Yokota H, Kayo T, Horiuchi T, Sugiyama N, Tanaka S, Takata K, Takeuchi T: Proprotein-processing endoprotease furin controls the growth and differentiation of gastric surface mucous cells. J Clin Invest 1997, 99:1842-1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayo T, Sawada Y, Suda M, Konda Y, Izumi T, Tanaka S, Shibata H, Takeuchi T: Proprotein-processing endoprotease furin controls growth of pancreatic beta-cells. Diabetes 1997, 46:1296-1304 [DOI] [PubMed] [Google Scholar]
- 17.Hubbard FC, Goodrow TL, Liu SC, Brilliant MH, Basset P, Mains RE, Klein-Szanto AJ: Expression of PACE4 in chemically induced carcinomas is associated with spindle cell tumor conversion and increased invasive ability. Cancer Res 1997, 57:5226-5231 [PubMed] [Google Scholar]
- 18.Bassi DE, Mahloogi H, Klein-Szanto AJ: The proprotein convertases furin and PACE4 play a significant role in tumor progression. Mol Carcinog 2000, 28:63-69 [PubMed] [Google Scholar]
- 19.Cheng M, Xu N, Iwasiow B, Seidah NG, Chrétien M, Shiu RPC: Elevated expression of proprotein convertases alters breast cancer cell growth in response to estrogen and tamoxifen. J Mol Endocrinol 2001, 26:95-105 [DOI] [PubMed] [Google Scholar]
- 20.Khatib AM, Siegfried G, Prat A, Luis J, Chretien M, Metrakos P, Seidah NG: Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J Biol Chem 2001, 276:30686-30693 [DOI] [PubMed] [Google Scholar]
- 21.Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ: Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci USA 2001, 98:10326-10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitlein J, Aulwurm S, Waltereit R: Processing of immunosuppressive pro-TGF-beta-1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases. J Immunol 2001, 166:7238-7243 [DOI] [PubMed] [Google Scholar]
- 23.Mbikay M, Sirois F, Yao J, Seidah NG, Chretien M: Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br J Cancer 1997, 75:1509-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng M, Watson PH, Paterson JA, Seidah NG, Chretien M, Shiu RP: Pro-protein convertase gene expression in human breast cancer. Int J Cancer 1997, 71:966-997 [DOI] [PubMed] [Google Scholar]
- 25.Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ: Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog 2001, 31:224-232 [DOI] [PubMed] [Google Scholar]
- 26.Takumi I, Steiner DF, Sanno N, Teramoto A, Osamura RY: Localization of prohormone convertases 1/3 and 2 in the human pituitary gland and pituitary adenomas: analysis by immunohistochemistry, immunoelectron microscopy, and laser scanning microscopy. Mod Pathol 1998, 11:232-238 [PubMed] [Google Scholar]
- 27.Jin L, Kulig E, Qian X, Scheithauer BW, Young WF, Jr, Davis DH, Seidah NG, Chretien M, Lloyd RV: Distribution and regulation of proconvertases PC1 and PC2 in human pituitary adenomas. Pituitary 1999, 1:187-195 [DOI] [PubMed] [Google Scholar]
- 28.Kajiwara H, Itoh Y, Itoh J, Yasuda M, Osamura RY: Immunohistochemical expressions of prohormone convertase (PC)1/3 and PC2 in carcinoids of various organs. Tokai J Exp Clin Med 1999, 24:13-20 [PubMed] [Google Scholar]
- 29.Rovere C, Barbero P, Maoret JJ, Laburthe M, Kitabgi P: Pro-neurotensin/neuromedin N expression and processing in human colon cancer cell lines. Biochem Biophys Res Commun 1998, 246:155-159 [DOI] [PubMed] [Google Scholar]
- 30.Yoon J, Beinfeld MC: Prohormone convertase 2 is necessary for the formation of cholecystokinin but not cholecystokinin-8, in RIN5F and STC-1 cells. Endocrinology 1997, 138:3620-3623 [DOI] [PubMed] [Google Scholar]
- 31.Patel YC, Galanopoulou AS, Rabbani SN, Liu JL, Ravazzola M, Amherdt M: Somatostatin-14, somatostatin-28, and prosomatostatin[1-10] are independently and efficiently processed from prosomatostatin in the constitutive secretory pathway in islet somatostatin tumor cells (1027B2). Mol Cell Endocrinol 1997, 131:183-194 [DOI] [PubMed] [Google Scholar]
- 32.Rovere C, Barbero P, Kitabgi P: Evidence that PC2 is the endogenous pro-neurotensin convertase in rMTC 6–23 cells and that PC1- and PC2-transfected PC12 cells differentially process pro-neurotensin. J Biol Chem 1996, 271:11368-11375 [DOI] [PubMed] [Google Scholar]
- 33.Lindberg I, Ahn SC, Breslin MB: Cellular distributions of the prohormone processing enzymes PC1 and PC2. Mol Cell Neurosci 1994, 5:614-622 [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y, Tanaka S, Takekoshi S, Itoh J, Osamura RY: Prohormone convertases (PC1/3 and PC2) in rat and human pancreas and islet cell tumors: subcellular immunohistochemical analysis. Pathol Int 1996, 46:726-737 [DOI] [PubMed] [Google Scholar]
- 35.Brar BK, Lowry PJ: The differential processing of proenkephalin A in mouse and human breast tumour cell lines. J Endocrinol 1999, 161:475-484 [DOI] [PubMed] [Google Scholar]
- 36.Du J, Keegan BP, North WG: Key peptide processing enzymes are expressed by breast cancer cells. Cancer Lett 2001, 165:211-218 [DOI] [PubMed] [Google Scholar]
- 37.Rounseville MP, Davis TP: Prohormone convertase and autocrine growth factor mRNAs are coexpressed in small cell lung carcinoma. J Mol Endocrinol 2000, 25:121-128 [DOI] [PubMed] [Google Scholar]
- 38.Santavicca M, Noel A, Angliker H, Stoll I, Segain JP, Anglard P, Chretien M, Seidah N, Basset P: Characterization of structural determinants and molecular mechanisms involved in pro-stromelysin-3 activation by 4-aminophenylmercuric acetate and furin-type convertases. Biochem J 1996, 315:953-958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei D, Weiss SJ: Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 1995, 375:244-247 [DOI] [PubMed] [Google Scholar]
- 40.Yana I, Weiss SJ: Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell 2000, 11:2387-2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loechel F, Gilpin BJ, Engvall E, Albrechtsen R, Wewer UM: Human ADAM 12 (meltrin α) is an active metalloprotease. J Biol Chem 1998, 273:16993-16997 [DOI] [PubMed] [Google Scholar]
- 42.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K: Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem 1997, 272:556-562 [DOI] [PubMed] [Google Scholar]
- 43.Kuno K, Terashima Y, Matsushima K: ADAMTS-1 is an active metalloproteinase associated with the extracellular matrix. J Biol Chem 1999, 274:18821-18826 [DOI] [PubMed] [Google Scholar]
- 44.Sardinha TC, Nogueras JJ, Xiong H, Weiss EG, Wexner SD, Abramson S: Membrane-type 1 matrix metalloproteinase mRNA expression in colorectal cancer. Dis Colon Rectum 2000, 43:389-395 [DOI] [PubMed] [Google Scholar]
- 45.Nabeshima K, Inoue T, Shimao Y, Okada Y, Itoh Y, Seiki M, Koono M: Front-cell-specific expression of membrane-type 1 matrix metalloproteinase and gelatinase A during cohort migration of colon carcinoma cells induced by hepatocyte growth factor/scatter factor. Cancer Res 2000, 60:3364-3369 [PubMed] [Google Scholar]
- 46.Primakoff P, Myles DG: The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet 2000, 16:83-87 [DOI] [PubMed] [Google Scholar]
- 47.Wu E, Croucher PI, McKie N: Expression of members of the novel membrane linked metalloproteinase family ADAM in cells derived from a range of haematological malignancies. Biochem Biophys Res Commun 1997, 235:437-442 [DOI] [PubMed] [Google Scholar]
- 48.McCulloch DR, Harvey M, Herington AC: The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol Cell Endocrinol 2000, 167:11-21 [DOI] [PubMed] [Google Scholar]
- 49.Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, Hockfield S: Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem 2000, 275:22695-22703 [DOI] [PubMed] [Google Scholar]
- 50.Tetu B, Brisson J, Lapointe H, Bernard P: Prognostic significance of stromelysin 3, gelatinase A, and urokinase expression in breast cancer. Hum Pathol 1998, 29:979-985 [DOI] [PubMed] [Google Scholar]
- 51.Lampert K, Machein U, Machein MR, Conca W, Peter HH, Volk B: Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol 1998, 153:429-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murakami K, Sakukawa R, Ikeda T, Matsuura T, Hasumura S, Nagamori S, Yamada Y, Saiki I: Invasiveness of hepatocellular carcinoma cell lines: contribution of membrane-type 1 matrix metalloproteinase. Neoplasia 1999, 1:424-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlöndorff J, Blobel CP: Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci 1999, 112:3603-3617 [DOI] [PubMed] [Google Scholar]
- 54.Black RA, White JM: ADAMs: focus on the protease domain. Curr Opin Cell Biol 1998, 10:564-569 [DOI] [PubMed] [Google Scholar]
- 55.Wolfsberg TG, White JM: ADAMs in fertilization and development. Dev Biol 1996, 180:389-401 [DOI] [PubMed] [Google Scholar]
- 56.Lunn CA, Fan X, Dalie B, Miller K, Zavodny PJ, Narula SK, Lundell D: Purification of ADAM 10 from bovine spleen as a TNFα convertase. FEBS Lett 1997, 400:333-335 [DOI] [PubMed] [Google Scholar]
- 57.Koike H, Tomioka S, Sorimachi H, Saido TC, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S: Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem J 1999, 343:371-375 [PMC free article] [PubMed] [Google Scholar]
- 58.Nath D, Slocombe PM, Stephens PE, Warn A, Hutchinson GR, Yamada KM, Docherty AJ, Murphy G: Interaction of metargidin (ADAM-15) with alphavbeta3 and alpha5beta1 integrins on different haemopoietic cells. J Cell Sci 1999, 112:579-587 [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Perez E, Zhang Y, Frank SJ, Creemers J, Seidah N, Checler F: Constitutive alpha-secretase cleavage of the beta-amyloid precursor protein in the furin-deficient LoVo cell line: involvement of the pro-hormone convertase 7 and the disintegrin metalloprotease ADAM10. J Neurochem 2001, 76:1532-1539 [DOI] [PubMed] [Google Scholar]
- 60.Chen MS, Almeida EA, Huovila AP, Takahashi Y, Shaw LM, Mercurio AM, White JM: Evidence that distinct states of the integrin alpha6beta1 interact with laminin and an ADAM. J Cell Biol 1999, 144:549-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y: RGD-independent binding of integrin alpha 9beta 1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem 2000, 275:34922-34930 [DOI] [PubMed] [Google Scholar]
- 62.Iba K, Albrechtsen R, Gilpin BJ, Loechel F, Wewer UM: Cysteine-rich domain of human ADAM 12 (meltrin) supports tumor cell adhesion. Am J Pathol 1999, 154:1489-1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutwein P, Oleszewski M, Mechtersheimer S, Agmon-Levin N, Krauss K, Altevogt P: Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J Biol Chem 2000, 275:15490-15497 [DOI] [PubMed] [Google Scholar]
- 64.Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E: The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem 2000, 275:25791-25797 [DOI] [PubMed] [Google Scholar]
- 65.Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Jr, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Burn TC: Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem 1999, 274:23443-23450 [DOI] [PubMed] [Google Scholar]
- 66.Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME: Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 2000, 156:1887-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Springer TA: Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol 1995, 57:827-872 [DOI] [PubMed] [Google Scholar]
- 68.Carlos TM, Harlan JM: Leukocyte-endothelial adhesion molecules. Blood 1994, 84:2101-2068 [PubMed] [Google Scholar]
- 69.Mantovani A, Garlanda C, Introna M, Vecchi A: Regulation of endothelial cell function by pro- and anti-inflammatory cytokines. Transplant Proc 1998, 30:4239-4243 [DOI] [PubMed] [Google Scholar]
- 70.Pober JS, Cotran RS: The role of endothelial cells in inflammation. Transplantation 1990, 50:537-544 [DOI] [PubMed] [Google Scholar]
- 71.Balaram SK, Agrawal DK, Edwards JD: Insulin like growth factor-1 activates nuclear factor-kappaB and increases transcription of the intercellular adhesion molecule-1 gene in endothelial cells. Cardiovasc Surg 1999, 7:91-97 [DOI] [PubMed] [Google Scholar]
- 72.Ishizuka T, Takamizawa-Matsumoto M, Suzuki K, Kurita A: Endothelin-1 enhances vascular cell adhesion molecule-1 expression in tumor necrosis factor α-stimulated vascular endothelial cells. Eur J Pharmacol 1999, 369:237-245 [DOI] [PubMed] [Google Scholar]
- 73.Hayasaki Y, Nakajima M, Kitano Y, Iwasaki T, Shimamura T, Iwaki K: ICAM-1 expression on cardiac myocytes and aortic endothelial cells via their specific endothelin receptor subtype 1. Biochem Biophys Res Commun 1996, 229:817-824 [DOI] [PubMed] [Google Scholar]
- 74.McCarron R, Wang L, Stanimirovic DB, Spatz M: Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci Lett 1993, 156:31-34 [DOI] [PubMed] [Google Scholar]
- 75.Morisaki N, Takahashi K, Shiina R, Zenibayashi M, Otabe M, Yoshida S, Saito Y: Platelet-derived growth factor is a potent stimulator of expression of intercellular adhesion molecule-1 in human arterial smooth muscle cells. Biochem Biophys Res Commun 1994, 200:612-618 [DOI] [PubMed] [Google Scholar]
- 76.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh G: VEGF stimulates expression of ICAM-1, VCAM-1 and E-selectin through nuclear factor-kappaB activation in endothelial cells. J Biol Chem 2001, 276:7614-7620 [DOI] [PubMed] [Google Scholar]
- 77.Duguay SJ, Lai-Zhang J, Steiner DF: Mutational analysis of the insulin-like growth factor I prohormone processing site. J Biol Chem 1995, 270:17566-17574 [DOI] [PubMed] [Google Scholar]
- 78.Denault JB, Claing A, D’Orleans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R: Processing of proendothelin-1 by human furin convertase. FEBS Lett 1995, 362:276-280 [DOI] [PubMed] [Google Scholar]
- 79.Schlöndorff J, Blobel CP: Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci 1999, 112:3603-3617 [DOI] [PubMed] [Google Scholar]
- 80.Black RA, White JM: ADAMs: focus on the protease domain. Curr Opin Cell Biol 1998, 10:564-569 [DOI] [PubMed] [Google Scholar]
- 81.Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F: Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J 2001, 15:1837-1839 [DOI] [PubMed] [Google Scholar]
- 82.Zetter BR: Adhesion molecules in tumor metastasis. Semin Cancer Biol 1993, 4:219-229 [PubMed] [Google Scholar]
- 83.Albelda SM: Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 1993, 68:4-17 [PubMed] [Google Scholar]
- 84.Juliano RL, Haskill S: Signal transduction from the extracellular matrix. J Cell Biol 1993, 120:577-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giancotti FG, Mainiero F: Integrin-mediated adhesion and signaling in tumorigenesis. Biochim Biophys Acta 1994, 198:47-64 [DOI] [PubMed] [Google Scholar]
- 86.Hempstead BL, Birge RB, Fajardo JE, Glassman R, Mahadeo D, Kraemer R, Hanafusa H: Expression of the v-crk oncogene product in PC12 cells results in rapid differentiation by both nerve growth factor- and epidermal growth factor-dependent pathways. Mol Cell Biol 1994, 4:1964-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daemi N, Thomasset N, Lissitzky JC, Dumortier J, Jacquier MF, Pourreyron C, Rousselle P, Chayvialle JA, Remy L: Anti-β4 integrin antibodies enhance migratory and invasive abilities of human colon adenocarcinoma cells and their MMP-2 expression. Int J Cancer 2000, 85:850-856 [DOI] [PubMed] [Google Scholar]
- 88.Khatib AM, Nip J, Fallavollita L, Lehmann M, Jensen G, Brodt P: Regulation of urokinase plasminogen activator/plasmin-mediated invasion of melanoma cells by the integrin vitronectin receptor αvβ3. Int J Cancer 2001, 91:300-308 [DOI] [PubMed] [Google Scholar]
- 89.Lehmann M, Rigot V, Seidah NG, Marvaldi J, Lissitzky JC: Lack of integrin α-chain endoproteolytic cleavage in furin-deficient human colon adenocarcinoma cells LoVo. Biochem J 1996, 317:803-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lissitzky JC, Luis J, Munzer JS, Benjannet S, Parat F, Chretien M, Marvaldi J, Seidah NG: Endoproteolytic processing of integrin pro-α subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7. Biochem J 2000, 346:133-138 [PMC free article] [PubMed] [Google Scholar]
- 91.Berthet V, Rigot V, Champion S, Secchi J, Fouchier F, Marvaldi J, Luis J: Role of endoproteolytic processing in the adhesive and signaling functions of αvβ5 integrin. J Biol Chem 2000, 275:33308-33313 [DOI] [PubMed] [Google Scholar]
- 92.Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, Irimura T: Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res 1995, 216:215-221 [DOI] [PubMed] [Google Scholar]
- 93.Tozeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW: E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer 1995, 60:426-431 [DOI] [PubMed] [Google Scholar]
- 94.Bevilacqua MP, Nelson RM: Selectins: selectins. J Clin Invest 1993, 91:379-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, Fennie C, Gillett N, Watson SR, Rosen SD: An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell 1992, 69:927-938 [DOI] [PubMed] [Google Scholar]
- 96.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R: Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res 1993, 53:354-361 [PubMed] [Google Scholar]
- 97.Iwai K, Ishikura H, Kaji M, Sugiura H, Ishizu A, Takahashi C, Kato H, Tanabe T, Yoshiki T: Importance of E-selectin (ELAM-1) and sialyl Lewis(a) in the adhesion of pancreatic carcinoma cells to activated endothelium. Int J Cancer 1993, 54:972-977 [DOI] [PubMed] [Google Scholar]
- 98.Mannori G, Santoro D, Carter L, Corless C, Nelson RM, Bevilacqua MP: Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol 1997, 151:233-243 [PMC free article] [PubMed] [Google Scholar]
- 99.Kuijpers TW, Raleigh M, Kavanagh T, Janssen H, Calafat J, Roos D, Harlan JM: Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J Immunol 1994, 152:5060-5069 [PubMed] [Google Scholar]
- 100.Balaram SK, Agrawal DK, Allen RT, Kuszynski CA, Edwards JD: Cell adhesion molecules and insulin-like growth factor-1 in vascular disease. J Vasc Surg 1997, 25:866-876 [DOI] [PubMed] [Google Scholar]
- 101.Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P: Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res 1999, 9:1356-1361 [PubMed] [Google Scholar]
- 102.Pardee AB: G1 events and regulation of cell proliferation. Science 1989, 246:603-608 [DOI] [PubMed] [Google Scholar]
- 103.Baserga R, Rubin R: Cell cycle and growth control. Crit Rev Eukaryot Gene Expr 1993, 3:47-61 [PubMed] [Google Scholar]
- 104.Rodrigues GA, Park M: Oncogenic activation of tyrosine kinases. Curr Opin Genet 1994, 4:15-24 [DOI] [PubMed] [Google Scholar]
- 105.Kimura I, Honda R, Okai H, Okabe M: Vascular endothelial growth factor promotes cell-cycle transition from G0 to G1 phase in subcultured endothelial cells of diabetic rat thoracic aorta. Jpn J Pharmacol 2000, 83:47-55 [DOI] [PubMed] [Google Scholar]
- 106.Bresnahan PA, Leduc R, Thomas L, Thorner J, Gibson HL, Brake AJ, Barr PJ, Thomas G: Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-β-NGF in vivo. J Cell Biol 1990, 111:2851-2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberté J, Lazure C, Chrétien M, Murphy RA: Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J 1996, 314:951-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hendy GN, Bennett HPJ, Gibbs BF, Lazure C, Day R, Seidah NG: Proparathyroid hormone is preferentially cleaved to parathyroid hormone by the prohormone convertase furin: a mass spectrometric study. J Biol Chem 1995, 270:9517-9525 [DOI] [PubMed] [Google Scholar]
- 109.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 2001, 98:6500-6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yarden Y, Ullrich A: Growth factor receptor tyrosine kinases. Annu Rev Biochem 1988, 57:443-478 [DOI] [PubMed] [Google Scholar]
- 111.Nguyen L, Holgado-Madruga M, Maroun C, Fixman ED, Kamikura D, Fournier T, Charest A, Tremblay ML, Wong AJ, Park M: Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem 1997, 272:20811-20819 [DOI] [PubMed] [Google Scholar]
- 112.Meisenhelder J, Suh PG, Rhee SG, Hunter T: Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell 1989, 57:1109-1122 [DOI] [PubMed] [Google Scholar]
- 113.Molloy CJ, Bottaro DP, Fleming TP, Marshall MS, Gibbs JB, Aaronson SA: PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature 1989, 342:711-714 [DOI] [PubMed] [Google Scholar]
- 114.Kaplan DR, Whitman M, Schaffhausen B, Pallas DC, White M, Cantley L, Roberts TM: Common elements in growth factor stimulation and oncogenic transformation: 85 kda phosphoprotein and phosphatidylinositol kinase activity. Cell 1987, 50:1021-1029 [DOI] [PubMed] [Google Scholar]
- 115.White MF, Yenush L: The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol 1998, 228:179-208 [DOI] [PubMed] [Google Scholar]
- 116.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG: A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 1992, 70:93-104 [DOI] [PubMed] [Google Scholar]
- 117.LeRiche VK, Asa SL, Ezzat S: Epidermal growth factor and its receptor (EGF-R) in human pituitary adenomas: EGF-R correlates with tumor aggressiveness. J Clin Endocrinol Metab 1996, 81:656-662 [DOI] [PubMed] [Google Scholar]
- 118.Kimura G, Kasuya J, Giannini S, Honda Y, Mohan S, Kawachi M, Akimoto M, Fujita-Yamaguchi Y: Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Int J Urol 1996, 3:39-46 [DOI] [PubMed] [Google Scholar]
- 119.Rodrigues GA, Park M: Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene 1994, 9:2019-2027 [PubMed] [Google Scholar]
- 120.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH: Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 1989, 109:877-889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hiscox SE, Hallett MB, Puntis MC, Nakamura T, Jiang WG: Expression of the HGF/SF cancers. Cancer Invest 1997, 15:513-521 [DOI] [PubMed] [Google Scholar]
- 122.Komada M, Hatsuzawa K, Shibamoto S, Ito F, Nakayama K, Kitamura N: Proteolytic processing of the hepatocyte growth factor/scatter factor receptor by furin. FEBS Lett 1993, 328:25-29 [DOI] [PubMed] [Google Scholar]
- 123.Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, Vannelli GB, Brand R, Goldfine ID, Vigneri R: Elevated insulin receptor content in human breast cancer. J Clin Invest 1990, 86:1503-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robertson BJ, Moehring JM, Moehring TJ: Defective processing of the insulin receptor in an endoprotease-deficient Chinese hamster cell strain is corrected by expression of mouse furin. J Biol Chem 1993, 268:24274-24277 [PubMed] [Google Scholar]
- 125.Hwang JB, Hernandez J, Leduc R, Frost SC: Alternative glycosylation of the insulin receptor prevents oligomerization and acquisition of insulin-dependent tyrosine kinase activity. Biochim Biophys Acta 2000, 1499:74-84 [DOI] [PubMed] [Google Scholar]
- 126.Brodt P, Fallavollita L, Khatib AM, Samani AA, Zhang D: Cooperative regulation of the invasive and metastatic phenotypes by different domains of the type I insulin-like growth factor receptor beta subunit. J Biol Chem 2001, 276:33608-33615 [DOI] [PubMed] [Google Scholar]
- 127.Anderson ED, Thomas L, Hayflick JS, Thomas G: Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α 1-antitrypsin variant. J Biol Chem 1993, 268:24887-24891 [PubMed] [Google Scholar]
- 128.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G: α1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA 1998, 23:7293-7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong M, Munzer JS, Basak A, Benjannet S, Mowla SJ, Decroly E, Chrétien M, Seidah NG: The prosegments of furin and PC7 as potent inhibitors of proprotein convertases: in vitro and ex vivo assessment of their specificity and selectivity. J Biol Chem 1999, 274:33913-33920 [DOI] [PubMed] [Google Scholar]
- 130.Bhattacharjya S, Xu P, Zhong M, Chretien M, Seidah NG, Ni F: Inhibitory activity and structural characterization of a C-terminal peptide fragment derived from the prosegment of the proprotein convertase PC7. Biochemistry 2000, 39:2868-2877 [DOI] [PubMed] [Google Scholar]
- 131.Lu W, Zhang W, Molloy SS, Thomas G, Ryan K, Chiang Y, Anderson S, Laskowski M, Jr: Crystal structure of the complex of subtilisin BPN′ with its protein inhibitor Streptomyces subtilisin inhibitor. The structure at 4.3 Angstroms resolution. J Mol Biol 1979, 131:855-869 [DOI] [PubMed] [Google Scholar]
- 132.Lu W, Zhang W, Molloy SS, Thomas G, Ryan K, Chiang Y, Anderson S, Laskowski M, Jr: Arg15-Lys17-Arg18 turkey ovomucoid third domain inhibits human furin. J Biol Chem 1993, 268:14583-14585 [PubMed] [Google Scholar]
- 133.Dufour EK, Denault JB, Hopkins PC, Leduc R: Serpin-like properties of alpha1-antitrypsin Portland towards furin convertase. FEBS Lett 1998, 426:41-46 [DOI] [PubMed] [Google Scholar]
- 134.Van Rompaey L, Ayoubi T, Van De Ven W, Marynen P: Inhibition of intracellular proteolytic processing of soluble proproteins by an engineered alpha 2-macroglobulin containing a furin recognition sequence in the bait region. Biochem J 1997, 326:507-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dahlen JR, Jean F, Thomas G, Foster C, Kisiel W: Inhibition of soluble recombinant furin by human proteinase inhibitor 8. J Biol Chem 1998, 273:1851-1854 [DOI] [PubMed] [Google Scholar]
- 136.Cameron A, Appel J, Houghten RA, Lindberg I: Polyarginines are potent furin inhibitors. J Biol Chem 2000, 275:36741-36749 [DOI] [PubMed] [Google Scholar]
- 137.Vollenweider F, Benjannet S, Decroly E, Savaria D, Lazure C, Thomas G, Chrétien M, Seidah NG: Comparative cellular processing of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp160 by the mammalian subtilisin/kexin-like convertases. Biochem J 1996, 314:521-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benjannet S, Savaria D, Laslop A, Munzer JC, Chretien M, Marcinkiewicz M, Seidah NG: α1-antitrypsin Portland inhibits processing of precursors mediated by pro-protein convertases primarily within the constitutive secretory pathway. J Biol Chem 1997, 272:26210-26218 [DOI] [PubMed] [Google Scholar]
- 139.Bahbouhi B, Bendjennat M, Guétard D, Seidah NG, Bahraoui E: Effect of alpha-1 antitrypsin Portland variant (α1-PDX) on HIV-1 replication. Biochem J 2000, 352:91-98 [PMC free article] [PubMed] [Google Scholar]
- 140.Tsuji A, Hashimoto E, Ikoma T, Taniguchi T, Mori K, Nagahama M, Matsuda Y: Inactivation of proprotein convertase, PACE4, by α1-antitrypsin Portland (α1-PDX), a blocker of proteolytic activation of bone morphogenetic protein during embryogenesis: evidence that PACE4 is able to form an SDS-stable acyl intermediate with α1-PDX. J Biochem (Tokyo) 1999, 126:591-603 [DOI] [PubMed] [Google Scholar]
- 141.Lopez-Perez E, Seidah NG, Checler F: Proprotein convertase activity contributes to the processing of the Alzheimer’s β-amyloid precursor protein in human cells: evidence for a role of the prohormone convertase PC7 in the constitutive α-secretase pathway. J Neurochem 1999, 73:2056-2062 [PubMed] [Google Scholar]
- 142.Abrami L, Fivaz M, Decroly E, Seidah NG, Jean F, Thomas G, Leppla SH, Buckley JT, van der Goot FG: The pore-forming toxin proaerolysin is activated by furin. J Biol Chem 1998, 273:32656-32661 [DOI] [PubMed] [Google Scholar]
- 143.Cui Y, Jean F, Thomas G, Christian JL: BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J 1998, 17:4735-4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A: The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA 1998, 95:8108-8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dubois CM, Blanchette F, Laprise M-H, Leduc R, Grondin F, Seidah NG: Evidence that furin is an authentic transforming growth factor β1-converting enzyme. J Am Pathol 2001, 58:305-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R: Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem 1995, 270:10618-10624 [DOI] [PubMed] [Google Scholar]
- 147.Duguay SJ, Jin Y, Stein J, Duguay AN, Gardner P, Steiner DF: Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J Biol Chem 1998, 273:18443-18451 [DOI] [PubMed] [Google Scholar]
- 148.Duguay SJ: Post-translational processing of insulin-like growth factor. Horm Metab Res 1999, 31:43-49 [DOI] [PubMed] [Google Scholar]
- 149.Alarcon C, Cheatham B, Lincoln B, Kahn CR, Siddle K, Rhodes CJ: A Kex2-related endopeptidase activity present in rat liver specifically processes the insulin proreceptor. Biochem J 1994, 301:257-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Campan M, Yoshizumi M, Seidah NG, Lee ME, Bianchi C, Haber E: Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry 1996, 35:3797-3802 [DOI] [PubMed] [Google Scholar]
- 151.Baserga R: The IGF-I receptor in cancer research. Exp Cell Res 1999, 253:1-6 [DOI] [PubMed] [Google Scholar]
- 152.Lahm H, Amstad P, Wyniger J, Yilmaz A, Fischer JR, Schreyer M, Givel JC: Blockade of the insulin-like growth-factor-I receptor inhibits growth of human colorectal cancer cells: evidence of a functional IGF-II-mediated autocrine loop. Int J Cancer 1994, 58:452-459 [DOI] [PubMed] [Google Scholar]
- 153.Ruoslahti E: Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999, 76:1-20 [DOI] [PubMed] [Google Scholar]
- 154.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH: Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol 1998, 143:547-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Franke TF, Kaplan DR, Cantley LC: PI3K: downstream AKTion blocks apoptosis. Cell February1997, 88:435-437 [DOI] [PubMed] [Google Scholar]
- 156.Ueno H, Kondo E, Yamamoto-Honda R, Tobe K, Nakamoto T, Sasaki K, Mitani K, Furusaka A, Tanaka T, Tsujimoto Y, Kadowaki T, Hirai H: Association of insulin receptor substrate proteins with Bcl-2 and their effects on its phosphorylation and antiapoptotic function. Mol Biol Cell 2000, 11:735-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jarpe MB, Widmann C, Knall C, Schlesinger TK, Gibson S, Yujiri T, Fanger GR, Gelfand EW, Johnson GL: Anti-apoptotic versus pro-apoptotic signal transduction: checkpoints and stop signs along the road to death. Oncogene 1998, 17:1475-1482 [DOI] [PubMed] [Google Scholar]
- 158.Seidah NG: Cellular limited proteolysis of precursor proteins and peptides. Dalbey RE Sigman DS eds. The Enzymes: Co- and Posttranslational Proteolysis of Proteins. 2001, :pp 237-258 Academic Press, San Diego [Google Scholar]
- 159.Jutras I, Seidah NG, Reudelhuber TL, Brechler V: Two activation states of the prohormone convertase PC1 in the secretory pathway. J Biol Chem 1997, 272:15184-15188 [DOI] [PubMed] [Google Scholar]
- 160.Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J: Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci 2000, 20:639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chretien M, Seidah NG: Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J Biol Chem 2001, 276:32720-32728 [DOI] [PubMed] [Google Scholar]
- 162.Mbikay M, Seidah NG, Chretien M: Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J 2001, 357:329-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shinde U, Inouye M: Intramolecular chaperones and protein folding. Trends Biochem Sci 1993, 18:442-446 [DOI] [PubMed] [Google Scholar]
- 164.Stoller TJ, Shields D: The propeptide of preprosomatostatin mediates intracellular transport and secretion of alpha-globin from mammalian cells. J Cell Biol 1989, 108:1647-1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G: Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO J 1997, 16:1508-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boudreault A, Gauthier D, Lazure C: Proprotein convertase PC1/3-related peptides are potent slow tight-binding inhibitors of murine PC1/3 and Hfurin. J Biol Chem 1998, 273:31574-31580 [DOI] [PubMed] [Google Scholar]
- 167.Lazure C, Gauthier D, Jean F, Boudreault A, Seidah NG, Bennett HP, Hendy GN: In vitro cleavage of internally quenched fluorogenic human proparathyroid hormone and proparathyroid-related peptide substrates by furin. Generation of a potent inhibitor. J Biol Chem 1998, 273:8572-8580 [DOI] [PubMed] [Google Scholar]
- 168.Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chretien M, Seidah NG: Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem 2001, 276:10879-10887 [DOI] [PubMed] [Google Scholar]
- 169.Nicolson GL, Menter DG: Trophic factors and central nervous system metastasis. Cancer Metastasis Rev 1995, 14:303-321 [DOI] [PubMed] [Google Scholar]
- 170.Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chretien M, Seidah NG: Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J Biol Chem 2001, 276:32720-32728 [DOI] [PubMed] [Google Scholar]
- 171.Mbikay M, Seidah NG, Chretien M: Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J 2001, 357:329-342 [DOI] [PMC free article] [PubMed] [Google Scholar]