Abstract
Corticobasal degeneration (CBD) is an adult-onset progressive neurodegenerative disorder characterized by l-dopa-resistant rigidity, focal cortical deficits, and variable dementia. The neuropathological hallmark of CBD is the deposition of filamentous inclusions in neurons and glia composed of hyperphosphorylated tau with only four microtubule-binding repeats (4R-tau). To characterize the regional burden of tau pathology in CBD, we studied 12 brains with the neuropathological diagnosis of CBD using biochemical and histochemical techniques. Eleven brain regions were evaluated including gray and white matter from frontal, parietal, temporal, and occipital lobes and cerebellum as well as basal ganglia. Although the distribution of tau pathology was variable, neuropathological and biochemical data showed a similar burden of tau abnormalities in frontal, temporal, and parietal lobes and basal ganglia of both hemispheres. This included abundant, sarkosyl-insoluble 4R-tau in both gray and white matter of two or more of these cortical regions and basal ganglia, and to a lesser extent, cerebellar white matter. The insoluble tau pathology in gray and white matter showed overlapping but distinct phosphorylated epitopes suggesting cell-type and subcellular localization (ie, cell bodies versus cell processes)-specific differences in tau phosphorylation. In contrast, soluble tau was composed of normal 4R/3R-tau ratios indicating no gross abnormality in tau splicing. Thus, although clinically heterogeneous, CBD is a distinct lobar and basal ganglionic tauopathy with selective aggregation of 4R-tau.
Corticobasal degeneration (CBD) was first described in 1968 as a progressive neurological disorder clinically characterized by abnormalities in posture and motor function with relatively intact mental faculties. 1 Neuropathological examination revealed atrophy of the frontoparietal cortex with neuronal loss and gliosis in affected cortices and the substantia nigra as well as distinctive, swollen, pale neurons in the cortex. Hence, the term corticodentatonigral degeneration with neuronal achromasia for a disorder later known as CBD. Subsequent reports emphasized the distinct motor features of CBD with relatively intact cognition. 2-6 However, a number of reports described clinical heterogeneity in patients with pathologically confirmed CBD including presentations similar to Alzheimer’s disease (AD), frontotemporal dementia, primary progressive aphasia, and progressive supranuclear palsy (PSP). 7-17
The contemporary neuropathology of CBD includes prominent atrophy of parasagittal cortex particularly in peri-Rolandic regions, as well as depigmentation of the substantia nigra. 18 In affected regions, there is neuronal loss, gliosis, and prominent glial and neuronal intracytoplasmic filamentous tau-immunoreactive pathology. 19,20 Achromatic ballooned neurons, that are most numerous in cortical and limbic regions, are strongly immunoreactive for phosphorylated neurofilaments and αB-crystallin but variably positive for tau. 21,22 The glial tau pathology consists of characteristic astrocytic plaques as well as numerous tau-immunoreactive inclusions in gray and white matter in astrocytes and oligodendrocytes (coiled bodies). 23,24 Perhaps the most striking feature of CBD is the extensive accumulation of tau-immunoreactive cell processes throughout both the gray and white matter. 25 Unlike the neuropil threads of AD, the majority of threads do not stain with antibodies to neurofilament suggesting many are localized in glia. 25
The pathological filamentous inclusions in CBD are composed primarily of abnormally phosphorylated tau similar to many other sporadic and familial neurodegenerative diseases such as AD, PSP, Pick’s disease (PiD), and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), collectively referred to as tauopathies. 26-28 Tau is a phosphoprotein that regulates the assembly and stability of microtubules. 29,30 In the central nervous system (CNS), six tau isoforms are produced by alternative splicing. 31,32 Alternative splicing of exons 2 and 3 results in the insertion of 0, 29, or 58 amino acids near the amino-terminus. In the carboxy-terminal half of the molecule, alternative splicing of exon 10 results in either three or four microtubule-binding motifs (3R-tau and 4R-tau, respectively). 31,32 In AD, the filamentous tau aggregates are composed of all six tau isoforms, but in CBD and PSP, the aggregates are composed predominantly of 4R-tau whereas in PiD, they are composed of 3R-tau. 26-28
In this study, we analyzed tau pathology in the brains of 12 patients with pathologically confirmed CBD. We demonstrate extensive heterogeneity in tau abnormalities in CBD; however, there is a distinct phenotype characterized by the widespread aggregation of 4R-tau throughout the brain. The extent of tau pathology in gray and white matter detected by Western blot analysis correlated with that detected by immunohistochemistry (IHC).
Materials and Methods
Patients
All patients met histopathological criteria for the diagnosis of CBD assessed independently by at least two neuropathologists (MSF, SSMC, CB, and JQT). 18,19 Diagnostic criteria emphasized a cortical and subcortical distribution of tau pathology in both gray and white matter including neuronal and glial tau-positive inclusions and extensive thread pathology. 19 Although present in most cases, astrocytic plaques and ballooned neurons were not required for the diagnosis of CBD. The age, gender, and clinical diagnosis of these patients are given in Table 1 ▶ . Patients 2 and 4 have already been presented elsewhere. 9 Control AD brain tissue was obtained through the University of Pennsylvania Alzheimer’s Disease Center.
Table 1.
Clinical Characteristics of Patients with Pathologically Proven CBD
| Patient | Age at death, years | Gender | Duration, years | PMI, hours | Clinical presentation | Clinical diagnosis |
|---|---|---|---|---|---|---|
| 1 | 77 | M | 5 | 18 | Progressive memory loss associated with speech difficulties | AD |
| 2 | 56 | M | 3 | NA | Progressive dementia, depression, and paranoia | Unclassified |
| 3 | 64 | F | 3 | 14 | Asymmetric motor dysfunction | CBD |
| 4 | 68 | M | 6 | 13 | Hypersexuality, increasing gregariousness, and marked irritability | FTD |
| 5 | 76 | F | 3 | 12 | Memory loss, frequent falls | CBD |
| 6 | 61 | F | 7 | 15 | Loss of manual dexterity, right hand, and slurring of speech | CBD |
| 7 | 71 | M | 6 | 18 | Visual agnosia, memory loss | Atypical AD |
| 8 | 70 | F | 8 | 17 | Altered speech patterns, anhedonia, decreased energy levels with increased somnolence | FTD |
| 9 | 73 | F | 8 | NA | Left upper extremity stiffness, numbness and twisting | CBD |
| 10 | 71 | F | 6 | NA | Decreased manual dexterity of hands, progressive slowing of motor functions | CBD |
| 11 | 59 | F | 6 | NA | Shaking and pains in hands, parkinsonism | CBD |
| 12 | 71 | F | 7 | 6 | Parkinsonism, memory loss | PSP |
Histochemistry and IHC
Tissue obtained at the time of autopsy was fixed in either neutral-buffered formalin or 70% ethanol in 150 mmol/L NaCl, pH 7.4, paraffin-embedded, and cut into 6- to 10-μm-thick sections. Sections were stained with hematoxylin and eosin, Thioflavin S, and Gallyas silver stains. IHC was performed on sections of neocortex, basal ganglia, and cerebellum using a panel of antibodies to tau as well as α-synuclein and phosphorylated neurofilament (Table 2) ▶ . The avidin-biotin-peroxidase method with 3,3′-diaminobenzidine for color development was used for all immunostaining. Tau pathology in both gray and white matter was assessed as absent, mild, moderate, or severe.
Table 2.
Antibodies Used to Characterize Pathology
| Antibody | Source | Specificity | Recognition site | Reference |
|---|---|---|---|---|
| 17026 | Rabbit | PI tau* | Recombinant tau | 50 |
| Tau 14 | Mouse | PI tau | 141–178 | 51 |
| AT270 | Mouse | P tau† | Thr 181 | 52 |
| AT8 | Mouse | P tau | Ser 202/Thr 205 | 53 |
| PHF6 | Mouse | P tau | Thr 231 | 54 |
| 12E8 | Mouse | P tau | Ser 262 | 55 |
| PHF1 | Mouse | P tau | Ser 396/Ser 404 | 56 |
| Tau 46 | Mouse | PI tau | 404–441 | 51 |
| RMO24 | Mouse | P-NFH | Multiphosphorylation repeats in NFH | 57 |
| LB509 | Mouse | α-synuclein | 115–122 | 58 |
*PI, phosphorylation independent.
†P, phosphorylated.
Tau Preparations and Western Blot Analysis
Fresh, frozen brain tissue from four neocortical brain regions (frontal, parietal, temporal, and occipital lobes), basal ganglia, and cerebellum including deep white matter from each case (where available) were used for biochemical analysis. Tissue was obtained from the contralateral hemisphere from that used for histochemical analysis. Gray matter and white matter were dissected from each brain region (except basal ganglia) and soluble and insoluble tau proteins were extracted as described previously. 33 Soluble and insoluble tau fractions were resuspended in 10 mmol/L Tris, 1 mmol/L ethylenediaminetetraacetic acid, pH 7.6, at concentrations of 0.5 and 0.1 ml/g of tissue, respectively. Where indicated, tau was dephosphorylated by treatment with Escherichia coli alkaline phosphatase (Sigma, St. Louis, MO) at 67°C for 1 hour. For Western blot analysis nitrocellulose replicas were prepared from 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) slab gels containing either the soluble or insoluble protein samples and probed with antibodies for tau as indicated. Monoclonal and polyclonal antibodies were detected with horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG, respectively (Santa Cruz Biotechnologies, Santa Cruz, CA). Immunoreactive proteins were revealed using the enhanced chemiluminescence (NEN Life Science, Boston, MA) and/or 3,3′-diaminobenzidine detection systems. Relative quantities of insoluble tau pathology were assessed as absent, mild, moderate, or severe.
Results
Topographic Distribution of Tau Pathology in CBD Patients
The neuropathological diagnosis of CBD was confirmed in all 12 cases using criteria that emphasize a cortical and subcortical distribution of tau pathology in both gray and white matter. 18,19 This includes neuronal and glial tau-positive inclusions including extensive thread pathology and astrocytic plaques as well as phosphorylated neurofilament-positive ballooned neurons (Figures 1 and 2) ▶ ▶ . In two cases (patients 1 and 2), there was hippocampal sclerosis consistent with previous ischemic/hypoxic injury. None of the 12 cases fulfilled criteria for other neurodegenerative disorders including AD, Lewy body disorders, PSP, and PiD. The main findings on immunohistochemical analysis for tau are summarized in Table 3 ▶ . In grading the severity of regional involvement, emphasis was placed on the thread pathology in both the gray and white matter using at least two antibodies specific for tau as well as Gallyas silver stains because this is the most prominent abnormality in CBD (Figure 2) ▶ . There was extensive, but highly variable, tau pathology in both the gray and white matter throughout all brain regions of each patient except for occipital lobes and cerebellar gray matter (Figure 2 ▶ and Table 3 ▶ ). The basal ganglia and the frontal lobes were moderately to severely affected in all patients except patients 1 (basal ganglia) and 5 (frontal lobe). Both the gray and white matter of the temporal and parietal lobes as well as the cerebellar white matter showed variable involvement, ranging from mild to severe pathology, but the extent of white matter tau pathology was at least equivalent to that in gray matter. The topographic distribution of tau-immunoreactive pathology only loosely correlated with the clinical presentation and/or diagnosis of the patient (Tables 1 and 3) ▶ ▶ . These results are not surprising because focal involvement of the neocortex may account for specific clinical phenotypes and in this analysis, we sampled specific cortical regions to standardize comparison between patients. 6,9,17,34,35
Figure 1.
Characteristic brain lesions in CBD. A: Astrocytic plaque in neocortex (tau immunostain, PHF1). B: Balloon neuron in neocortex (neurofilament immunostain, RMO24). C: Neuronal cytoplasmic inclusions in nucleus basalis (tau immunostain, PHF1). D: Astrocytic cytoplasmic inclusion in neocortex (tau immunostain, PHF1). E: Oligodendroglial inclusion (coiled body) in subcortical white matter (tau immunostain, PHF1). Scale bars: 30 μm (A); 10 μm (B–E).
Figure 2.
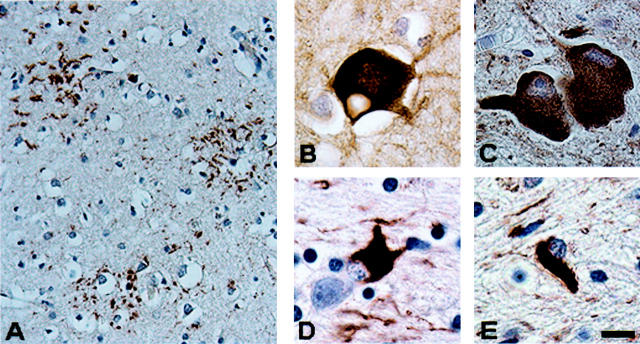
Topographic distribution of tau-immunoreactive pathology shows regional variability. A: Marked thread pathology in the basal ganglia of patient 10. B: In contrast, the occipital lobe of patient 10 is virtually devoid of tau pathology. C and D: Thread pathology in both the frontal lobe and occipital lobe of patient 7 who presented with a visual agnosia. Tau immunostains, PHF1. Scale bar, 80 μm.
Table 3.
Tau-Immunoreactive Pathology in CBD, IHC
| Patient | Frontal lobe | Temporal lobe | Parietal lobe | Occipital lobe | Basal ganglia | Cerebellum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grey matter | White matter | Grey matter | White matter | Grey matter | White matter | Grey matter | White matter | Striatum | Globus pallidus | Folia | White matter | |
| 1 | 2 | 3 | NA | NA | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| 2 | 1 | 3 | 1 | 1 | NA | NA | NA | NA | 3 | 3 | 0 | 2 |
| 3 | 3 | 3 | 1 | 0 | 2 | 2 | NA | NA | 2 | 3 | 0 | 2 |
| 4 | 3 | 3 | 2 | 2 | 2 | 2 | NA | NA | 3 | 3 | 0 | 2 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | NA |
| 6 | 3 | 3 | 2 | 2 | 2 | 2 | 0 | 0 | 3 | 3 | 0 | NA |
| 7 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 1 | 3 | NA | 0 | NA |
| 8 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | NA | 3 | 0 | 1 |
| 9 | 3 | 3 | 2 | 2 | 1 | 2 | 0 | 1 | 3 | 3 | 0 | 2 |
| 10 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 3 | 3 | 0 | 1 |
| 11 | 2 | 3 | 1 | 1 | 1 | 2 | 1 | 1 | 3 | 3 | 0 | 2 |
| 12 | 3 | 3 | 2 | 2 | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 2 |
Frontal lobe, mid-frontal gyrus; temporal lobe, superior temporal gyrus; parietal lobe, inferior parietal lobule; occipital lobe, calcarine cortex.
0, absent; 1, mild density; 2, moderate density; 3, high density; NA, not available.
Biochemical Characterization and Topographic Distribution of Sarkosyl-Insoluble Tau in CBD
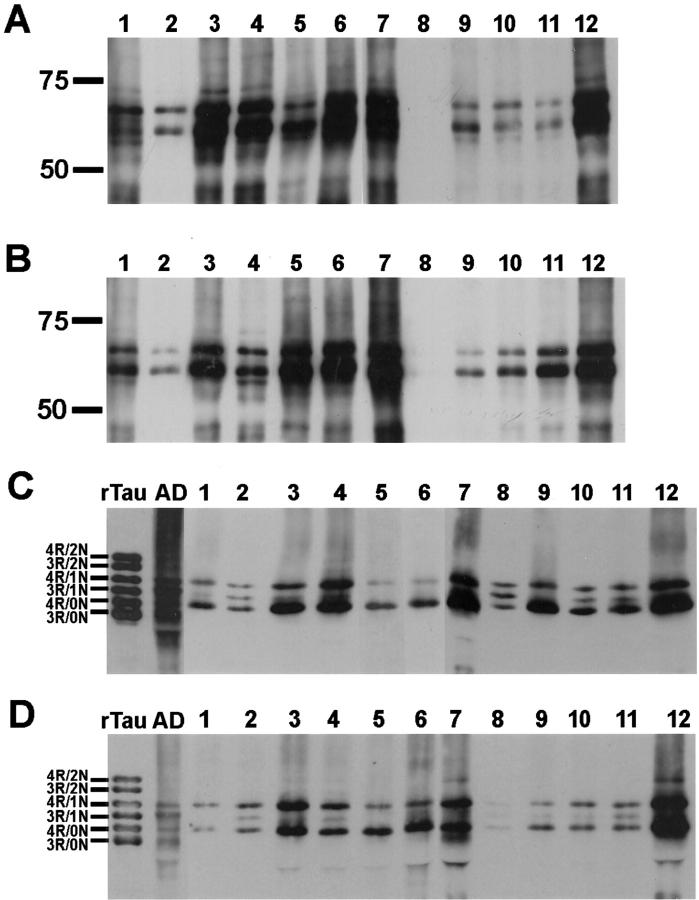
To further analyze tau pathology in CBD, we performed Western blot analysis of sarkosyl-insoluble tau from multiple neocortical regions as well as basal ganglia and cerebellum from the hemisphere contralateral to that used for histochemical and IHC analysis. In all patients, insoluble tau in both gray and white matter was composed of two major protein bands of ∼64 and 68 kd (Figures 3 and 4) ▶ ▶ . On dephosphorylation, these major protein bands co-migrated with 4R-tau isoforms but 3R-tau with one amino terminal insert could occasionally be detected in some of the cases (Figure 3, C and D) ▶ . The presence of 4R-tau was confirmed with an antibody specific for exon 10 that encodes for the alternatively spliced microtubule-binding domain that distinguishes 4R-tau from 3R-tau (data not shown). In contrast, AD tau pathology is localized predominantly within gray matter and is composed of both 4R- and 3R-tau isoforms (Figure 3, C and D) ▶ .
Figure 3.
Western blot analysis of insoluble tau in gray and white matter of the parietal lobe of CBD brains shows marked interpatient variability. Insoluble tau fractions extracted from gray (A) and white (B) matter of the parietal lobe of 12 CBD brains were resolved by SDS-PAGE and immunoblotted with PHF1. The insoluble tau protein is composed predominantly of two major proteins of 64 and 68 kd. Aliquots of the insoluble fractions were dephosphorylated with E. coli alkaline phosphatase, resolved by SDS-PAGE, and immunoblotted with T14 and T46 that recognize phosphorylation-independent tau epitopes on the amino- and carboxy terminus, respectively. On dephosphorylation, the insoluble tau fractions from gray (C) and white (D) matter co-migrate with 4R-tau; 3R-tau with one amino terminal insert could occasionally be detected in some of the cases. In contrast, in AD, dephosphorylated, insoluble tau fractions co-migrate with both 4R-tau and 3R-tau isoforms. Prolonged exposure of the immunoblots revealed at least mild tau pathology in all samples as reflected in Table 4 ▶ . Recombinant tau isoforms (rTau) are indicated to the left of C and D. Molecular weight standards are as indicated to the left of each panel here and in Figures 4 and 5 ▶ ▶ .
Figure 4.
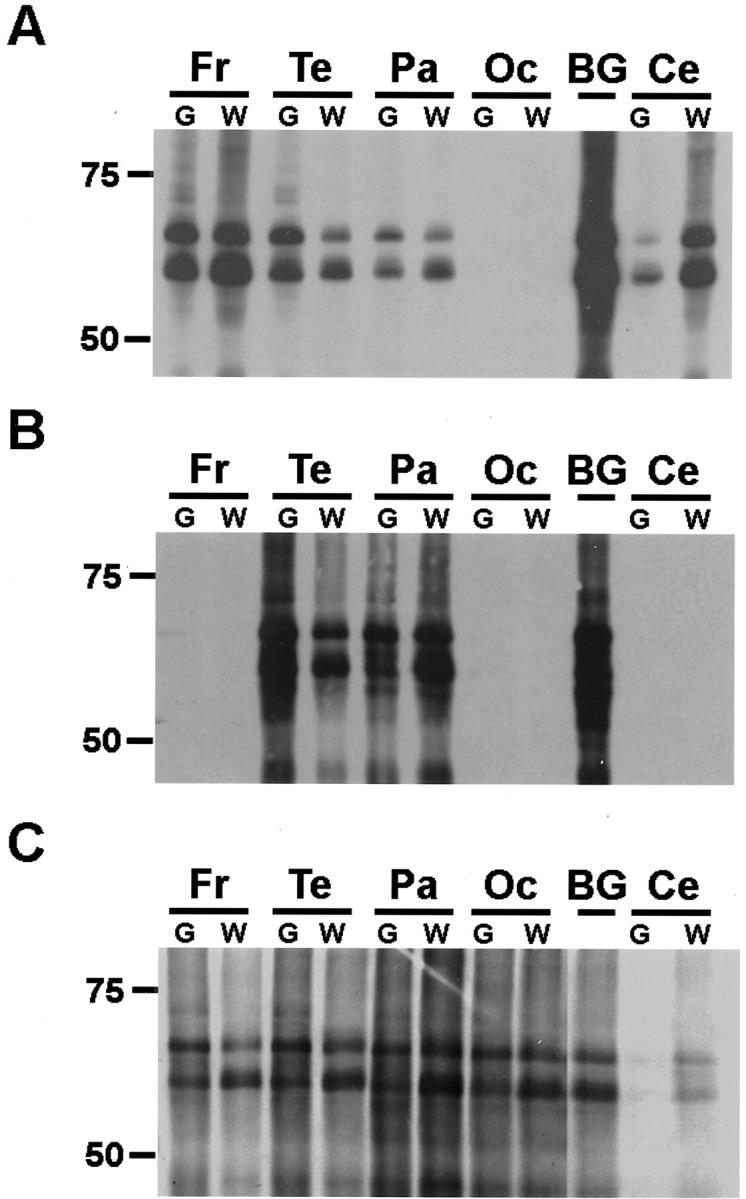
Western blot analysis of insoluble tau in CBD shows marked regional variability. Insoluble tau fractions from the brain regions indicated were resolved by SDS-PAGE and immunoblotted with PHF1. The insoluble tau protein from all brain regions is composed predominantly of two major proteins of 64 and 68 kd. Patient 2 (A), who presented clinically with an unclassified dementia, shows the most severe pathology in the frontal and temporal lobes. Patient 1 (B), who was diagnosed clinically with AD, shows the most severe pathology in the parietal and temporal lobes. Prolonged exposure of the immunoblot revealed mild tau pathology in frontal and occipital lobes as well as cerebellum as reflected in Table 4 ▶ . In contrast, patient 4 (C), who was diagnosed clinically with frontotemporal dementia, shows moderate pathology in all neocortical regions. Fr, frontal lobe; Te, temporal lobe; Pa, parietal lobe; Oc, occipital lobe; BG, basal ganglia; Ce, cerebellum; G, gray matter; W, white matter.
Similar to IHC, a Western blot analysis of the topographic pattern of insoluble tau showed extensive tau pathology in both gray and white matter throughout the brain of all patients (Figure 4 ▶ and Table 4 ▶ ). The basal ganglia consistently demonstrate robust tau pathology. Also consistent with IHC, the neocortex showed mild to marked pathology in the frontal, parietal, and temporal lobes of all patients. In contrast, the occipital lobe and cerebellum were the only brain regions examined wherein tau pathology occasionally was absent. The biochemical distribution of insoluble tau pathology loosely correlated with the distribution of tau pathology revealed by IHC with a Spearman’s rank order correlation coefficient equal to 0.37 [S(38) = 0.37; P < 0.02]. The differences most likely reflect sampling because tau pathology in CBD is often focal and asymmetric. 18 However, the present findings of tau pathology in both cerebral hemispheres argue that CBD is a bilateral disorder with an asymmetric distribution of the severity of the tau pathology that may account for the specific clinical phenotypes.
Table 4.
Insoluble Tau Pathology in CBD, Biochemistry
| Patient | Frontal lobe | Temporal lobe | Parietal lobe | Occipital lobe | Basal ganglia | Cerebellum | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grey matter | White matter | Grey matter | White matter | Grey matter | White matter | Grey matter | White matter | Folia | White matter | ||
| 1 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 1 | 3 | 1 | 1 |
| 2 | 3 | 3 | 3 | 2 | 2 | 2 | 0 | 0 | 3 | 1 | 2 |
| 3 | 3 | 3 | 2 | 1 | 3 | 3 | 2 | 2 | 3 | 0 | 1 |
| 4 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 2 |
| 5 | 2 | 2 | 2 | 3 | 2 | 3 | 0 | 0 | 3 | 0 | 0 |
| 6 | 1 | 2 | 1 | 1 | 3 | 3 | 0 | 0 | 2 | 0 | 0 |
| 7 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 2 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 2 |
| 9 | 1 | 1 | NA | NA | 2 | 2 | 2 | 2 | 3 | 2 | 2 |
| 10 | 2 | 1 | NA | NA | 2 | 2 | 1 | 2 | 3 | NA | 2 |
| 11 | 1 | 2 | NA | NA | 1 | 2 | 0 | 0 | 3 | 1 | 2 |
| 12 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 0 | 2 |
0, absent; 1, mild density; 2, moderate density; 3, high density; NA, not available.
Gray and White Matter Demonstrate Qualitative Differences in Tau Phosphorylation
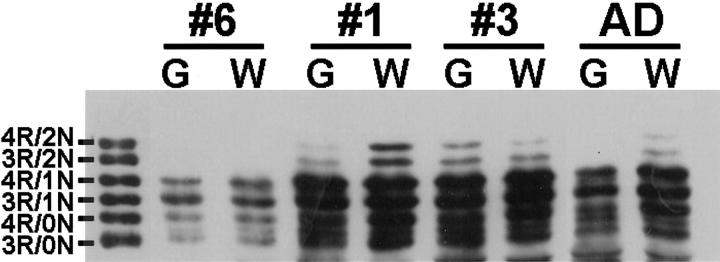
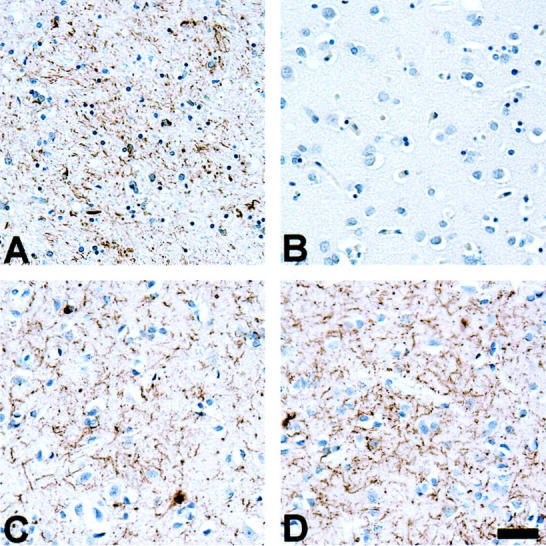
To further characterize the biochemical properties of insoluble tau in gray and white matter of CBD patients, we performed Western blot analysis with a panel of antibodies specific for phosphorylation-dependent tau epitopes. The monoclonal antibodies PHF1, 12E8, and AT270 that are specific for Ser-396/Ser-404, Ser-262, and Thr-181, respectively, show similar patterns of phosphorylation in both gray and white matter (Figure 5 ▶ and data not shown). The antibody PHF6 that recognizes Thr-231 shows a relative increase in tau phosphorylation in gray matter; however, these differences are highly variable between cases (data not shown). In contrast, the antibody AT8 that recognizes Ser-202/Thr-205 demonstrates a relative increase in tau phosphorylation in white matter in most cases (Figure 5) ▶ . The exceptions were two cases (patients 2 and 12) that biochemically demonstrate marked (3+) tau pathology in the frontal lobes. Similarly, by immunohistochemical analysis, AT8 preferentially recognizes the tau pathology in the white matter relative to other phosphorylation-dependent antibodies including PHF1 (Figure 6) ▶ . The explanation for this observation is unclear, but the distinct pattern of phosphorylation may reflect differences in the subcellular localization (ie, cell bodies versus cell processes) of the tau pathology as AT8 preferentially recognizes the white matter pathology consisting primarily of threads derived from multiple cell lineages including neuronal axons and glial processes. 25,36
Figure 5.
Western blot analysis of insoluble tau shows distinct phosphorylation profiles in white matter. Insoluble tau fractions from gray and white matter of the frontal lobe of CBD and AD patients were resolved by SDS-PAGE and immunoblotted with the epitope-specific, phosphorylation-dependent antibodies PHF1 and AT8 as indicated. PHF1 (B) detects similar patterns of phosphorylation in both gray and white matter. In contrast, AT8 (A), specific for phosphorylated Ser 202 and Thr 205, detects increased tau pathology in the white matter of the majority (10 of 12) of the cases. G, gray matter; W, white matter.
Figure 6.
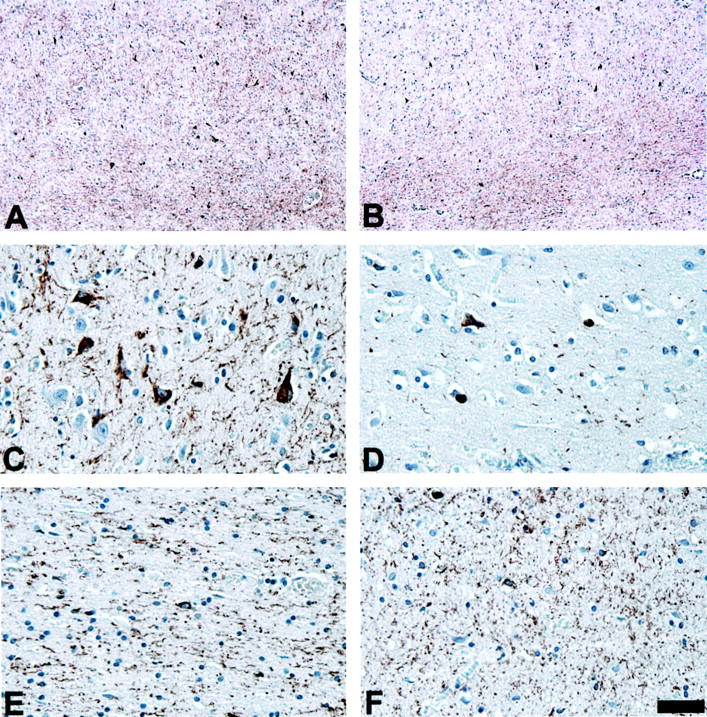
AT8 preferentially detects tau pathology in the white matter of CBD patients. Adjacent sections from the mid-frontal gyrus of patient 7 were immunostained with PHF1 (A, C, and E) or AT8 (B, D, and F). Although PHF1 detects abundant tau pathology in both the gray (C) and white (E) matter, AT8 preferentially detects the white matter pathology (F). A and B: Low-power photomicrographs of frontal lobe immunostained with PHF1 (A) and AT8 (B). C–F: High-power photomicrographs of cortex (C and D) and white matter (E and F) represented in A and B immunostained with PHF1 (C and E) and AT8 (D and F). Scale bars: 200 μm (A and B); 40 μm in (C–F).
To determine whether the observed differences in gray and white matter reflect distinct patterns of tau isoform expression, we performed Western blot analysis of soluble tau extracts from gray and white matter. Dephosphorylated soluble tau extracts showed similar levels of tau isoform expression in both gray and white matter (Figure 7) ▶ . The relative proportions of the six tau isoforms was similar to that observed in both AD (Figure 6) ▶ and normal (data not shown) brain tissue as previously reported with a ratio of 3R:4R-tau of ∼1:1. 37
Figure 7.
Western blot analysis of soluble tau shows similar levels of tau isoform expression in gray and white matter. Soluble tau fractions from gray and white matter of CBD and AD patients were dephosphorylated with E. coli alkaline phosphatase, resolved by SDS-PAGE, and immunoblotted with T14. The soluble extracts showed similar levels of tau isoform expression in both gray and white matter, similar to that observed in AD patients. Recombinant tau isoforms (rTau) are as indicated.
Discussion
In this study, we assessed the distribution of tau pathology by both IHC and biochemistry in 12 patients with pathologically confirmed CBD. Similar to previous studies on CBD, there was heterogeneous and variable involvement of all cortices, although Armstrong and colleagues 38 reported similar pancortical tau pathology in CBD patients using a detailed morphometric analysis. In general, there was concordance between the biochemical and immunohistochemical data. We attribute the observed differences to the fact that the biochemical and IHC analysis were performed on contralateral hemispheres and thus reflects the well-defined asymmetrical nature of the disease. In contrast, the widespread distribution of tau pathology did not correlate with the clinical presentation of the patient. Several reports have demonstrated a correlation of neuron loss with clinical phenotype. 9,15,39 The absence of correlation in our study is probably due to several factors including: 1) the selection of specific brain regions to provide uniform analysis across all of the cases, rather than selecting the most affected region; 2) the analysis was performed at end-stage when the neurodegenerative process becomes more generalized; and 3) the tau pathology predates the cell loss that correlates with clinical phenotype.
The composition of the insoluble tau aggregates in CBD is characterized by predominantly 4R-tau in both the neuronal and glial inclusions. 40-43 This is similar to that detected in PSP, but distinct from other disorders with extensive tau pathology such as AD and PiD in which aggregated tau is composed of either all six CNS tau isoforms (AD) or predominantly 3R-tau (PiD). 26,44 However, no previous studies of CBD included biochemical analysis of insoluble and soluble tau from multiple brain regions of many patients. In all 12 cases, we consistently detected insoluble tau composed of predominantly 4R-tau in both the gray and white matter of all brain regions with the exception of variable and typically mild pathology in the occipital lobe and cerebellum. Furthermore, similar to that observed in the frontal lobe, semiquantitative analysis indicated that there were typically equal, if not greater, amounts of pathology in the subcortical white matter relative to the gray matter of the same lobe. 40 The majority of the tau pathology in the white matter of CBD is in the form of threads, which implies that a significant majority of the insoluble tau pathology precipitates within processes rather than cell bodies in CBD. Consistent with this notion, Feany and Dickson 23 demonstrated that the thread pathology in CBD, particularly in white matter, was significantly greater than that observed in either PSP or PiD. Furthermore, in contrast to AD, co-localization studies suggest that a significant proportion of the thread pathology is within astrocytes and oligodendrocytes rather than neurons. 25,36
In all tauopathies, including CBD, PSP, PiD, and AD, the insoluble tau is hyperphosphorylated and the vast majority of tau phosphoepitopes are identified in all of these disorders. 27,45 However, there appears to be a relative predominance of a subset of these phosphoepitopes in the white matter of CBD patients (Figure 5) ▶ . Specifically, phosphorylated Ser-202/Thr-205 is relatively increased in white matter. Although the specific role of tau phosphorylation in its aggregation is not clear, we speculate that the abnormal phosphorylation of tau occurs by distinct mechanisms both within different cell types of the CNS and within distinct subcellular compartments (ie, cellular processes), thus accounting for the relative differences observed in the gray and white matter. Indeed, given recent evidence that in vitro phosphorylation of all six brain tau isoforms promotes filament formation, it is possible that in CBD the preferential accumulation of phosphorylated 4R-tau isoforms in glial tangles reflects a hitherto unexpected differential activity of specific kinases that preferentially target 4R-tau for phosphorylation. 46 However, the mechanisms for the observed differences in tau phosphorylation in gray and white matter remain to be elucidated.
The preferential aggregation of 4R-tau isoforms in CBD, as well as PSP, remains enigmatic. In FTDP-17, specific mutations that effect the splicing of exon 10 result in the aggregation of predominantly 4R-tau isoforms. However, this reflects the preferential expression of 4R-tau rather than a specific predilection of these isoforms to aggregate. 26-28 In both CBD and PSP, there is no overt alteration in splicing of CNS tau relative to normal individuals or patients with tauopathies wherein all six tau isoforms (ie, AD) or preferentially 3R-tau (ie, PiD) aggregate. Interestingly, in both CBD and PSP, there is overrepresentation of the A0 allele, one component of the H1 tau haplotype. 47-49 The H1 haplotype consists of a series of polymorphisms in the tau gene, some of which are located within intron 10, that are in linkage disequilibrium. 47 The effect, if any, of the H1 haplotype or A0 allele is unknown, but it is possible that one or a group of polymorphisms specific to the H1 haplotype alter the biochemical properties or splicing of tau. However, to date, no association of the tau polymorphisms and splicing have been identified.
In conclusion, this present study supports the hypothesis that CBD has a distinct pathological phenotype characterized by the widespread aggregation of 4R-tau throughout both the gray and white matter of the CNS. However, although 4R-tau aggregates in a wide variety of cells, including astrocytes, oligodendrocytes, as well as neurons, the relative contribution of the glial pathology to the neurodegenerative process remains unknown.
Acknowledgments
We thank the families of the many patients studied for making the research reviewed here possible.
Footnotes
Address reprint requests to John Q. Trojanowski, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 3400 Spruce St., Maloney Building, 3rd Floor, Philadelphia, PA 19104. E-mail: trojanow@mail.med.upenn.edu.
Supported by grants from the National Institutes of Health [the National Institute of Aging and a Mentored Clinical Scientist Development Award AG20073-01 (to M. S. F.)], the Dana Foundation, and the Alzheimer’s Association.
V.M.-Y.L. is the John H. Ware Third Chair of Alzheimer’s disease research at the University of Pennsylvania.
References
- 1.Rebeiz JJ, Kolodny EH, Richardson EP, Jr: Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 1968, 18:20-33 [DOI] [PubMed] [Google Scholar]
- 2.Riley DE, Lang AE, Lewis A, Resch L, Ashby P, Hornykiewicz O, Black S: Cortical-basal ganglionic degeneration. Neurology 1990, 40:1203-1212 [DOI] [PubMed] [Google Scholar]
- 3.Gibb WR, Luthert PJ, Marsden CD: Corticobasal degeneration. Brain 1989, 112:1171-1192 [DOI] [PubMed] [Google Scholar]
- 4.Rinne JO, Lee MS, Thompson PD, Marsden CD: Corticobasal degeneration. A clinical study of 36 cases. Brain 1994, 117:1183-1196 [DOI] [PubMed] [Google Scholar]
- 5.Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, Poewe W, Jellinger K, Ray CK, D’Olhaberriague L, Pearce RK: Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 1998, 64:184-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC: Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999, 53:795-800 [DOI] [PubMed] [Google Scholar]
- 7.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG: The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000, 55:1368-1375 [DOI] [PubMed] [Google Scholar]
- 8.Paulus W, Selim M: Corticonigral degeneration with neuronal achromasia and basal neurofibrillary tangles. Acta Neuropathol 1990, 81:89-94 [DOI] [PubMed] [Google Scholar]
- 9.Bergeron C, Pollanen MS, Weyer L, Black SE, Lang AE: Unusual clinical presentations of cortical-basal ganglionic degeneration. Ann Neurol 1996, 40:893-900 [DOI] [PubMed] [Google Scholar]
- 10.Arima K, Uesugi H, Fujita I, Sakurai Y, Oyanagi S, Andoh S, Izumiyama Y, Inose T: Corticonigral degeneration with neuronal achromasia presenting with primary progressive aphasia: ultrastructural and immunocytochemical studies. J Neurol Sci 1994, 127:186-197 [DOI] [PubMed] [Google Scholar]
- 11.Lippa CF, Smith TW, Fontneau N: Corticonigral degeneration with neuronal achromasia. A clinicopathologic study of two cases. J Neurol Sci 1990, 98:301-310 [DOI] [PubMed] [Google Scholar]
- 12.Ikeda K, Akiyama H, Iritani S, Kase K, Arai T, Niizato K, Kuroki N, Kosaka K: Corticobasal degeneration with primary progressive aphasia and accentuated cortical lesion in superior temporal gyrus: case report and review. Acta Neuropathol 1996, 92:534-539 [DOI] [PubMed] [Google Scholar]
- 13.Litvan I, Grimes DA, Lang AE: Phenotypes and prognosis: clinicopathologic studies of corticobasal degeneration. Adv Neurol 2000, 82:183-196 [PubMed] [Google Scholar]
- 14.Mathuranath PS, Xuereb JH, Bak T, Hodges JR: Corticobasal ganglionic degeneration and/or frontotemporal dementia? A report of two overlap cases and review of literature. J Neurol Neurosurg Psychiatry 2000, 68:304-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimes DA, Lang AE, Bergeron CB: Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999, 53:1969-1974 [DOI] [PubMed] [Google Scholar]
- 16.Clark AW, Manz HJ, White CL, III, Lehmann J, Miller D, Coyle JT: Cortical degeneration with swollen chromatolytic neurons: its relationship to Pick’s disease. J Neuropathol Exp Neurol 1986, 45:268-284 [DOI] [PubMed] [Google Scholar]
- 17.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS: Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 1997, 48:959-969 [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K: Basic pathology of corticobasal degeneration. Neuropathology 2001, 17:127-133 [Google Scholar]
- 19.Dickson DW, Liu WK, Ksiezak-Reding H, Yen SH: Corticobasal degeneration: neuropathologic and molecular considerations. Adv Neurol 2000, 82:9-27 [PubMed] [Google Scholar]
- 20.Dickson DW: Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999, 246(Suppl 2):II6-II15 [DOI] [PubMed] [Google Scholar]
- 21.Dickson DW, Yen SH, Suzuki KI, Davies P, Garcia JH, Hirano A: Ballooned neurons in select neurodegenerative diseases contain phosphorylated neurofilament epitopes. Acta Neuropathol 1986, 71:216-223 [DOI] [PubMed] [Google Scholar]
- 22.Smith TW, Lippa CF, de Girolami U: Immunocytochemical study of ballooned neurons in cortical degeneration with neuronal achromasia. Clin Neuropathol 1992, 11:28-35 [PubMed] [Google Scholar]
- 23.Feany MB, Dickson DW: Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol 1996, 40:139-148 [DOI] [PubMed] [Google Scholar]
- 24.Komori T: Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 1999, 9:663-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feany MB, Dickson DW: Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 1995, 146:1388-1396 [PMC free article] [PubMed] [Google Scholar]
- 26.Forman MS, Lee VM-Y, Trojanowski JQ: New insights into genetic and molecular mechanisms of brain degeneration in tauopathies. J Chem Neuroanat 2000, 20:225-244 [DOI] [PubMed] [Google Scholar]
- 27.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR: Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev 2000, 33:1-36 [DOI] [PubMed] [Google Scholar]
- 28.Lee VM-Y, Goedert M, Trojanowski JQ: Neurodegenerative tauopathies. Ann Rev Neurosci 2001, 24:1121-1159 [DOI] [PubMed] [Google Scholar]
- 29.Cleveland DW, Hwo SY, Kirschner MW: Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 1977, 116:207-225 [DOI] [PubMed] [Google Scholar]
- 30.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW: A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 1975, 72:1858-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 32.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA: Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 1989, 8:393-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee VM-Y, Wang J, Trojanowski JQ: Purification of paired helical filament tau and normal tau from human brain tissue. Methods Enzymol 1999, 309:81-89 [DOI] [PubMed] [Google Scholar]
- 34.Bergeron C, Davis A, Lang AE: Corticobasal ganglionic degeneration and progressive supranuclear palsy presenting with cognitive decline. Brain Pathol 1998, 8:355-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S: Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol 1994, 87:545-553 [DOI] [PubMed] [Google Scholar]
- 36.Ikeda K, Akiyama H, Haga C, Kondo H, Arima K, Oda T: Argyrophilic thread-like structure in corticobasal degeneration and supranuclear palsy. Neurosci Lett 1994, 174:157-159 [DOI] [PubMed] [Google Scholar]
- 37.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y: Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282:1914-1917 [DOI] [PubMed] [Google Scholar]
- 38.Armstrong RA, Cairns NJ, Lantos PL: A quantitative study of the pathological lesions in the neocortex and hippocampus of twelve patients with corticobasal degeneration. Exp Neurol 2000, 163:348-356 [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya K, Ikeda K, Uchihara T, Oda T, Shimada H: Distribution of cerebral cortical lesions in corticobasal degeneration: a clinicopathological study of five autopsy cases in Japan. Acta Neuropathol (Berl) 1997, 94:416-424 [DOI] [PubMed] [Google Scholar]
- 40.Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu WK, Yen SH, Weidenheim K, Dickson DW: Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol 1994, 145:1496-1508 [PMC free article] [PubMed] [Google Scholar]
- 41.Sergeant N, Wattez A, Delacourte A: Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem 1999, 72:1243-1249 [DOI] [PubMed] [Google Scholar]
- 42.Buée-Scherrer V, Hof PR, Buée L, Leveugle B, Vermersch P, Perl DP, Olanow CW, Delacourte A: Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick’s disease. Acta Neuropathol 1996, 91:351-359 [DOI] [PubMed] [Google Scholar]
- 43.Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL: Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 2001, 101:167-173 [DOI] [PubMed] [Google Scholar]
- 44.Buée L, Delacourte A: Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathol 1999, 9:681-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billingsley ML, Kincaid RL: Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 1997, 323:577-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K: Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 2001, 98:6923-6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M: Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 1999, 8:711-715 [DOI] [PubMed] [Google Scholar]
- 48.Conrad C, Andreadis A, Trojanowski JQ, Dickson DW, Kang D, Chen X, Wiederholt W, Hansen L, Masliah E, Thal LJ, Katzman R, Xia Y, Saitoh T: Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol 1997, 41:277-281 [DOI] [PubMed] [Google Scholar]
- 49.Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P: Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol 2000, 47:374-377 [DOI] [PubMed] [Google Scholar]
- 50.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y: Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 1999, 24:751-762 [DOI] [PubMed] [Google Scholar]
- 51.Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G: Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1988, 1:817-825 [DOI] [PubMed] [Google Scholar]
- 52.Goedert M, Jakes R, Crowther RA, Cohen P, Vanmechelen E, Vandermeeren M, Cras P: Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer’s disease: identification of phosphorylation sites in tau protein. Biochem J 1994, 301:871-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM-Y: The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci USA 1993, 90:5066-5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann R, Lee VM-Y, Leight S, Varga I, Otvos L, Jr: Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry 1997, 36:8114-8124 [DOI] [PubMed] [Google Scholar]
- 55.Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ, Lee VM-Y: Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem 1995, 270:18917-18922 [DOI] [PubMed] [Google Scholar]
- 56.Greenberg SG, Davies P: A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 1990, 87:5827-5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee VM-Y, Otvos L, Jr, Carden MJ, Hollosi M, Dietzschold B, Lazzarini RA: Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci USA 1988, 85:1998-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jakes R, Crowther RA, Lee VM-Y, Trojanowski JQ, Iwatsubo T, Goedert M: Epitope mapping of LB509, a monoclonal antibody directed against human alpha-synuclein. Neurosci Lett 1999, 269:13-16 [DOI] [PubMed] [Google Scholar]