Abstract
There is limited information about the molecular changes involved in the pathogenesis of gallbladder carcinoma (GBC). Our recent allelotyping analyses have indicated that chromosome 3p loss of heterozygosity (LOH), including the fragile histidine triad (FHIT) candidate tumor-suppressor gene locus at 3p14.2, is frequently detected in this neoplasm. To investigate the role of the FHIT abnormalities in the multistage sequential development of GBC, 33 formalin-fixed paraffin-embedded invasive GBC specimens and 76 accompanying histologically normal (n = 43) and dysplastic (n = 33) epithelia were examined by immunostaining for expression of Fhit protein. Allele loss at the FHIT gene locus (3p14.2) was studied in all GBCs and in a subset of accompanying gallbladder epithelia by polymerase chain reaction-based LOH analysis, using three 3p14.2 microsatellite markers. In addition, histologically normal epithelium from chronic cholecystitis (n = 19) and dysplasia (n = 13) from gallbladder specimens without cancer were examined for immunostaining and LOH. There was a progressive increase in both the frequency of loss of Fhit expression and LOH at FHIT with increasing severity of histopathological changes. FHIT abnormalities were occasionally demonstrated in histologically normal gallbladder epithelium. Dysplastic foci demonstrated frequent reduction or absence of Fhit immunostaining (38 to 55%) and FHIT allelic loss (33 to 46%). In invasive tumors, these abnormalities were even higher, with 79% reduction or absence of Fhit immunostaining and 76% FHIT allele loss. A high correlation (70%) was observed between Fhit immunostaining abnormalities and allele loss in GBC specimens (P < 0.05). Although a high frequency of FHIT locus breakpoints were detected in both invasive and dysplastic gallbladder specimens, no intronic homozygous deletions on FHIT were detected in GBCs. FHIT gene abnormalities are nearly universal in GBC and these changes are detected early in the sequential development of this neoplasm. Our findings indicate that the FHIT gene is one of the chromosome 3p putative tumor suppressor genes involved in the pathogenesis of this highly malignant neoplasm.
Gallbladder carcinoma (GBC) is a relatively uncommon neoplasm that demonstrates considerable geographic and gender variation in incidence. 1 It is one of the most frequent neoplasms in Chile, where it is the leading cause of cancer deaths in females. 1 GBC is a highly malignant neoplasm, which is usually diagnosed at advanced clinical stages. It has been well established that invasive GBC is preceded by preinvasive lesions, including dysplastic changes of the gallbladder epithelium. 2 Although the development of molecular markers for early detection and the prediction of response to adjuvant therapies would be beneficial, there is very limited information about the molecular changes involved in the pathogenesis of GBC. 3
It is now well recognized that epithelial tumorigenesis is a multistep process resulting from the accumulation of sequential genetic alterations, including the inactivation of one or more tumor suppressor genes (TSGs). 4 Allele loss, manifested as loss of heterozygosity (LOH) at polymorphic loci flanking TSGs, is recognized as a hallmark of cancers, with inactivation of the second allele by point mutations or by some other mechanism. 5 These changes result in the absence of the normal gene protein in the affected cells.
The fragile histidine triad (FHIT) gene, a candidate TSG, has been identified at the 3p14.2 region, spanning the FRA3B common fragile site. 6 Because the occurrence of intragenic mutations in FHIT is very rare, some researchers have argued that FHIT abnormalities may only represent alterations in the 3p14.2 FRA3B fragile site. However, frequent allelic losses as well as homozygous deletions have been described at the FHIT locus in several human cancers arising from epithelial cells, making FHIT a strong candidate TSG. 7 Similarly, several groups have demonstrated that introduction of a wild-type FHIT gene suppresses tumorigenicity. 8 In addition, tumor-specific promoter methylation and epigenetic inactivation of the FHIT gene that are absent in the corresponding normal tissues have also established the important role of FHIT in tumorigenesis. 9,10
Although FHIT abnormalities have been observed in a variety of human tumors, there is no information about its abnormalities in gallbladder tumorigenesis. As a result of our recent allelotyping analyses on GBC, we identified 3p regions with frequent allelic loss in this tumor, including the FHIT (3p14.2) region. 11 Based on our previous findings, the present study was undertaken to investigate the frequency of FHIT gene abnormalities during multistage pathogenesis of GBC, and to compare Fhit immunostaining expression abnormalities and FHIT locus allele loss in invasive GBC and corresponding histologically normal and dysplastic epithelium. Using archival paraffin-embedded tissue, we studied the immunohistochemical expression of the Fhit protein and the presence of allelic loss at the FHIT gene locus in invasive GBC and accompanying histologically normal and preinvasive gallbladder epithelia.
Materials and Methods
Archival Tumor Specimens
Formalin-fixed paraffin-embedded material from 33 primary GBCs was obtained from cholecystectomy specimens resected between 1990 and 1998 at the Catholic University Medical School Hospital, Santiago, Chile. The patients consisted of 24 women and 9 men ranging in age from 51 to 85 years (mean age, 68 years). All tumors were invasive GBCs diagnosed using established histological criteria, 2 and consisted of 18 (55%) well-differentiated, 3 (9%) moderately differentiated, and 12 (36%) poorly differentiated adenocarcinomas. Based on architectural pattern, there were 13 (39%) tubular adenocarcinomas, 1 (3%) papillary adenocarcinoma, 18 (55%) tubulopapillary adenocarcinomas, and 1 (3%) adenosquamous carcinoma. Most of the tumors were advanced GBCs with invasion of the gallbladder serosa (6 cases, 18%) or subserosa (20 cases, 61%), and the rest of the cases (7 cases, 21%) were early GBCs, with invasion of the gallbladder muscularis propia.
Histologically Normal Epithelium and Dysplastic Lesions
From the 33 GBCs selected for this study we identified 76 discrete foci consisting of histologically normal (n = 43) and dysplastic (n = 33) gallbladder epithelium for Fhit immunohistochemical analysis. A subset of those epithelial foci used for immunostaining (26 dysplastic and 16 histologically normal epithelia) were selected for FHIT LOH analysis, and microdissected as described below. In addition, histologically normal epithelium from chronic cholecystitis (n = 19) and dysplasia (n = 13) from gallbladder specimens without cancer were examined for both immunostaining and LOH. All these gallbladder specimens were histologically mapped to rule out the presence of invasive carcinoma. The dysplastic lesions were scored as low- and high-grade lesions using published criteria for the histopathological identification of dysplasia arising in the gallbladder epithelium. 2
Microdissection and DNA Extraction
Serial 5-μm sections were cut from the archival, formalin-fixed paraffin-embedded tissues. All slides were stained with hematoxylin and eosin, and one of the slides was coverslipped. The coverslipped slide was used as guide to localize regions of interest for microdissection of the other slides. Microdissection from archival paraffin-embedded tissues was precisely performed under microscopic visualization using a micromanipulator, as described previously. 12 From four sections of each case a total of 800 sectioned tumor cells were microdissected. DNA extraction was performed as described previously. 12 Dissected lymphocytes or normal stromal cells from the same slide were used as a source of constitutional DNA from each case. After DNA extraction, 5 μl of the proteinase K-digested samples containing DNA from at least 200 cells were used for each polymerase chain reaction (PCR).
Microsatellite DNA Markers and PCR-LOH Analysis
To evaluate LOH, we used primers flanking three highly polymorphic microsatellite markers within the FHIT gene, including D3S1234 (intron 8), D3S4103 (intron 5), and D3S1300 (intron 5). A two-round PCR strategy was used to amplify each marker, as described previously. 12 The final product was separated on a 6% denaturing polyacrylamide gel and subjected to autoradiography. LOH was scored by visual detection of complete absence of one allele. Because artifacts resulting from PCR amplification may be mistaken for LOH, especially when minute amounts of input DNA are used, all examples of LOH from the histologically normal epithelia and nearly half from the dysplasia were repeated for confirmation.
Fhit Protein Immunostaining
One representative paraffin block containing GBC and accompanying histologically normal and dysplastic epithelia was retrieved for each case. Five-μm paraffin sections were reacted with rabbit polyclonal anti-Fhit antibody (Zymed Laboratories Inc, San Francisco, CA) at a 1:200 dilution for 1 hour at room temperature. 13 The sections were then incubated with biotinylated anti-rabbit IgG diluted 1:200 followed by streptavidin-biotinylated peroxidase complex. Diaminobenzidine was used as chromogen, and hematoxylin as counterstain. Normal breast acinar tissue, which demonstrates strong Fhit immunoreactivity, was used as positive control whereas the primary antibody was replaced by normal rabbit serum IgG as negative control.
Both the extent and intensity of immunopositivity were considered when scoring Fhit protein expression, as previously described. 13 The extent of positivity was scored as follows: 0, <5%; 1, >5 to 25%; 2, >25 to 50%: 3, >50 to 75%; and 4, >75% of the gallbladder epithelial cells in the respective lesion. The intensity was scored as follows: 0, negative; 1+, weak; 2+, moderate; and 3+, as strong as positive control. The final score was obtained by multiplying the extent of positivity and intensity scores, producing a range from 0 to 12. 13 Scores 9 to 12 were defined as preserved or strong staining pattern, scores 6 to 8 were defined as mildly reduced staining pattern, and scores 0 to 4 were defined as severely reduced or absent expression.
Identification of FHIT Homozygous Deletions
To identify the presence of FHIT exon 3 to 9 homozygous deletions we performed multiplex PCR with seven different primer sets amplifying exons 3, 4, 5, and 8 (four sets) or exons 6, 7, and 9 (three sets) in 10 microdissected GBC specimens, according to previously published methodology. 14
Results
FHIT Abnormalities (Reduction/Loss of Immunostaining and LOH) in GBC
A very high incidence of reduction or absence (score 0 to 8) of Fhit expression (26 of 33 cases, 79%) and FHIT allelic loss (25 of 33 cases, 76%) were detected in GBC specimens (Table 1 ▶ , Figures 1 and 2 ▶ ▶ ). Most tumors (21 of 26, 81%) demonstrating Fhit immunostaining abnormalities showed a severe reduction or absence (score 0 to 4) of expression (Table 1) ▶ . Thirty-one GBC specimens (94%) demonstrated either reduced or absent Fhit immunostaining or allelic loss. Of these, 21 cases (68%) showed both changes. In the 25 cases with LOH, 21 showed reduced or absent and 4 cases showed normal Fhit immunostaining. On the other hand, among the eight cases with no LOH, six showed reduced or absent and two showed normal Fhit immunostaining. The correlation between immunostaining and LOH status was 70% in invasive GBC (P < 0.05).
Table 1.
FHIT Abnormalities in the Pathogenesis of GBC
| Histology | Fhit Immunostaining Patterns | FHIT locus (3p14.2) LOH | |||||
|---|---|---|---|---|---|---|---|
| Samples, n | Normal, score 9 to 12 | Reduced | Samples, n | LOH | |||
| Mild (score 6–8) | Severe/absent (score 0–4) | Total (score 0–8) | |||||
| Chronic cholecystitis, normal epithelium | 19 | 18 (95%) | 1 (5%) | 0 | 1 (5%) | 16 | 2 (13%) |
| Dysplasia without cancer | |||||||
| Dysplasia | 13 | 8 (62%) | 2 (15%) | 3 (23%) | 5 (38%) | 12 | 4 (33%) |
| Low-grade | 9 | 7 (78%) | 1 (11%) | 1 (11%) | 2 (22%) | 8 | 2 (25%) |
| High-grade | 4 | 1 (25%) | 1 (25%) | 2 (50%) | 3 (75%) | 4 | 2 (50%) |
| Gallbladder carcinoma | |||||||
| Normal epithelium | 43 | 39 (91%) | 4 (9%) | 0 | 4 (9%) | 16 | 0 |
| Dysplasia | 33 | 15 (46%) | 3 (9%) | 15 (46%) | 18 (55%) | 26 | 12 (46%) |
| Low-grade | 14 | 7 (50%) | 2 (14%) | 5 (36%) | 7 (50%) | 10 | 4 (40%) |
| High-grade | 19 | 8 (42%) | 1 (5%) | 10 (53%) | 11 (58%) | 16 | 8 (50%) |
| Invasive carcinoma | 33 | 7 (21%) | 5 (15%) | 21 (64%) | 26 (79%) | 33 | 25 (76%) |
Figure 1.
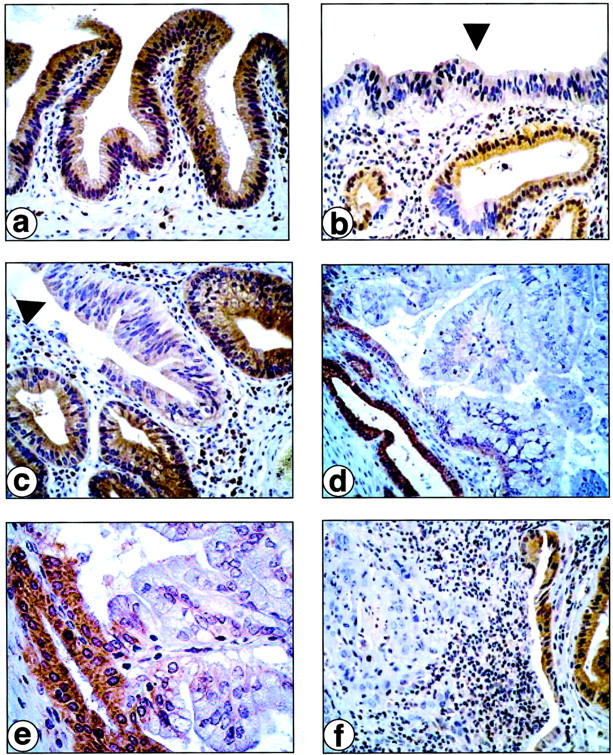
Immunohistochemical staining for Fhit on GBCs. a: Histologically normal gallbladder epithelium. b and c: Dysplastic gallbladder epithelia (arrowhead). d and e: Well-differentiated (papillary) invasive GBC. f: Poorly differentiated invasive GBC. Note positive Fhit immunostaining in histologically normal gallbladder epithelium (a), weak immunostaining in dysplastic lesions (b and c), and negative immunostaining in invasive carcinomas (d, e, and f). Normal epithelia from normal glands adjacent to dysplastic and neoplastic foci show positive Fhit immunostaining (b–f).
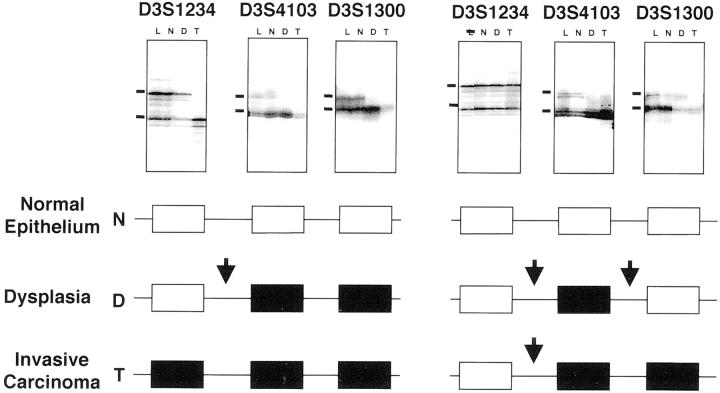
Figure 2.
Representative autoradiographs of microsatellite analyses for FHIT locus LOH at 3p14.2 using three polymorphic markers in microdissected invasive GBCs and accompanying histologically normal and dysplastic epithelia. Horizontal bars on the left indicate the main allelic bands. L, lymphocytes or normal stromal cells; N, normal epithelium; D, dysplasia; T, tumor. Open boxes, retention of heterozygosity; closed boxes, allelic loss. Breakpoints at the FHIT gene locus are represented by arrows.
There was no significant correlation between Fhit immunostaining expression or FHIT allelic loss and clinicopathological features of GBC (data not shown). Also, we did not find a correlation between FHIT gene abnormalities and the presence of microsatellite instability phenomenon measured by the frequency of microsatellite instability obtained in our genome-wide allelotyping analysis on GBC. 11
FHIT Changes in Histologically Normal and Dysplastic Gallbladder Epithelium
Nearly half of the dysplastic lesions accompanying GBC demonstrated reduction or absence (score 0 to 8) of Fhit immunostaining (18 of 33 samples, 55%) and FHIT allelic loss (12 of 26 samples, 46%) (Table 1 ▶ , Figures 1 and 2 ▶ ▶ ). Most of those dysplastic lesions (15 of 18, 83%) demonstrating Fhit immunostaining abnormalities showed a severe reduction or absence (score 0 to 4) of Fhit expression. Dysplastic lesions from gallbladder specimens without GBC demonstrated a relatively lower frequency of reduction or absence of Fhit immunostaining (5 of 13 samples, 38%) and FHIT LOH (4 of 12 samples, 33%) (Table 1) ▶ .
Although 22 of 38 (58%) dysplastic lesions tested for both FHIT abnormalities demonstrated either immunostaining abnormality or allelic loss, 9 (23%) of these dysplasias showed both changes. In the 16 cases with LOH, 9 dysplasias showed reduced or absent and 7 samples showed normal Fhit immunostaining. In contrast, among the 22 cases with no LOH, 11 showed reduced or absent and the other half showed normal Fhit immunostaining. The correlation between immunostaining and LOH status in dysplastic gallbladder epithelium was 52%. No significant differences were detected in the Fhit-negative immunostaining and in the FHIT LOH between low-grade and high-grade dysplastic lesions (Table 1) ▶ .
Although no LOH was detected in histologically normal epithelium accompanying GBC cases, four (9%) of those normal samples demonstrated mild reduced pattern (score 6 to 8) of the Fhit immunostaining. All those samples were accompanying invasive GBC and corresponding dysplastic lesions with markedly reduced or absence Fhit immunostaining. Similarly, histologically normal epithelium from chronic cholecystitis without cancer demonstrated very infrequent FHIT immunostaining (5%) and LOH (13%) abnormalities (Table 1) ▶ .
Patterns of FHIT Abnormalities in GBC and Accompanying Gallbladder Epithelia
In GBC specimens, one or both FHIT abnormalities (reduced or loss of Fhit immunostaining and FHIT LOH) were detected in 55 of 75 (73%) normal, dysplastic, and neoplastic foci examined for both FHIT abnormalities. We used these changes to determine whether the foci in individual cases were molecularly related. Of interest, all dysplastic lesions and histologically normal epithelium showing negative Fhit immunostaining and FHIT locus LOH demonstrated the same abnormality in the corresponding invasive GBC.
Breakpoints at the FHIT Gene Locus
We identified a high frequency of breakpoints in the FHIT locus in the dysplastic and invasive GBC specimens. Breakpoints were defined as the junction between a marker showing LOH and an adjacent marker retaining heterozygosity in a given sample DNA (Figure 2) ▶ . Only 24% (6 of 25) of invasive GBCs and none of the dysplastic specimens examined demonstrated allelic loss at all informative markers tested. In contrast, 76% (19 of 25) of invasive GBCs and all 16 dysplastic lesions showed restricted deletions at the FHIT locus demonstrating breakpoints at this site. We examined in all these specimens the location of the breakpoints. Of interest, in 14 of 20 (70%) cases in which all of the three FHIT markers were informative the breakpoint was located between markers D3S1234 (intron 8) and D3S4103 (intron 5).
FHIT Homozygous Exonic Deletions in GBC
Using a multiplex PCR technique previously described, 14 we did not identify any exonic homozygous deletion in 10 microdissected GBC specimens examined. All these cases demonstrated LOH in at least one 3p14.2 microsatellite marker examined.
Discussion
As a result of our recent allelotyping analysis in GBC, we identified multiple areas of frequent discontinuous LOH in chromosome 3p regions in this neoplasm, including the FHIT gene locus at 3p14.2. 11 In the present study, we have demonstrated that 79% and 76% of the GBC tumors have markedly reduced expression of Fhit protein and FHIT locus allele loss, respectively. Similar high incidences of FHIT gene abnormalities have been reported in other human tumors such as lung, 15,16 cervical, 17,18 renal, 6 pancreatic, 19 head and neck, 20 breast, 14,21 colon, 13 and esophageal carcinomas. 22 The frequent loss of FHIT expression, the expression of aberrant FHIT transcripts, and numerous deletions within the FHIT gene suggest that this gene is a candidate TSG common to many cancers. 7 Our study found evidence that FHIT is also important in the pathogenesis of GBC.
Nearly half of the dysplastic gallbladder lesions accompanying invasive tumors demonstrated reduction or absence of Fhit protein by immunostaining and allele loss at the FHIT locus. A relatively lower frequency of both FHIT gene abnormalities was detected in dysplasias from gallbladder specimens without gallbladder cancer. These findings indicate that FHIT inactivation is a frequent and early event in the sequential development of GBC. Our data showing that only occasional FHIT abnormalities are detected in histologically normal gallbladder epithelium from patients with and without GBC suggests that the FHIT gene may play an important role in the malignant transformation of the gallbladder epithelium. Alterations of the FHIT gene and/or its expression have been also reported in premalignant lesions of the lung, 15,16,23 cervix, 17,18 colon, 13 breast, 14,21 and esophagus. 22 The stage of the sequential development of tumors in which FHIT gene abnormalities appears varies among different tumor types. Although those changes have been detected as a very early event in cervical carcinoma, 17,18 they have been reported as a relatively late event in lung cancer pathogenesis. 16,23,24 On the other hand, no differences in the FHIT gene abnormalities were detected between our histologically high-grade and low-grade gallbladder dysplasias, suggesting that both lesions may have similar malignant potential.
The mechanisms leading to the reduction of Fhit expression and the manner in which this reduction promotes tumorigenesis remain obscure. Homozygous deletions of exons within the FHIT gene may result in changes of protein expression. No such exonic homozygous deletions were detected in 10 microdissected GBCs examined in the present study. A good correlation has been reported between heterozygous FHIT gene alterations (allele loss and intronic deletions) and reduction of Fhit protein expression. 7 Our invasive GBCs showed a good correlation between both allele loss and reduction or absence of Fhit protein expression. Although allele loss explains inactivation of one parental homologue, the mechanism of inactivation of the second allele is unlikely to occur by Knudson’s classic “two-hit” mechanism. 5 Missense, nonsense, or frame-shift mutations in the FHIT gene are rare in primary tumors. 7 On the contrary, hypermethylation of a 5′ CpG island seem to inactivate the FHIT gene in esophageal 9 and lung cancers, 10 and has emerged as the major epigenetic mechanism for gene silencing in FHIT.
The progression in the overall frequency of FHIT abnormalities with histological progression, as well as the presence of identical patterns of LOH and Fhit protein immunostaining in precursor dysplastic lesions and their corresponding invasive gallbladder tumors, suggest a molecular relationship through sequential changes between both noninvasive and malignant gallbladder lesions. Of interest, all dysplastic lesions and histologically normal epithelium showing negative Fhit immunostaining and FHIT locus allele loss demonstrated the same abnormality in the corresponding invasive GBC. Our recent unpublished findings based on allele loss pattern suggest that histologically normal and dysplastic gallbladder epithelia accompanying GBC arise as independent clones. Thus, the identical pattern of FHIT abnormalities detected in the present study may be related to a field cancerization phenomenon. 25
We identified a high frequency of discontinuous LOH and breakpoints in the FHIT locus in the dysplastic and invasive GBC specimens. Breakpoints were defined as the junction between a marker showing LOH and an adjacent marker retaining heterozygosity in a given sample DNA. Seventy-six percent of invasive GBCs and all dysplastic lesions with LOH showed restricted deletions at the FHIT locus involving breakpoints at this site. The 3p14.2 FHIT region harbors the most common known aphidicolin-inducible fragile site in the genome (FRA3B). 26 A high incidence of such breakpoints in this region have been recently demonstrated in invasive lung 24 and breast 21 tumors as well as in their corresponding noninvasive epithelia. Our findings indicate that the FHIT 3p14.2 site represents a highly unstable region undergoing frequent allele loss associated with breakpoints in the gallbladder epithelium undergoing malignant transformation. Although a correlation between high-grade microsatellite instability phenomenon and FHIT homozygous deletions has been suggested in pancreatic cancer, 27 no correlation between FHIT gene abnormalities and the presence of microsatellite instability was detected in our GBC cases.
In summary, FHIT gene abnormalities, expressed by considerable reduction or loss of Fhit immunostaining and allele loss are nearly universal in GBC and these changes are detected early in the sequential development of this neoplasm. Our findings suggest that the FHIT gene is one of the chromosome 3p putative TSGs involved in the pathogenesis of this highly malignant neoplasm.
Footnotes
Address reprint requests to Ignacio I. Wistuba, M.D., Department of Anatomic Pathology, P. Universidad Catolica de Chile, Marcoleta 367, P.O. Box 114-D, Santiago, Chile. E-mail: iwistuba@med.puc.cl.
Supported by grant 1990489 from the Fondo Nacional de Desarrollo Cientifico y Tecnologico (to I. I. W.).
References
- 1.Lazcano-Ponce E, Miquel JF, Ferrecio C, Wistuba I, Muñoz N, Alonso de Ruiz P, Urista G, Herrero R, Nervi F: Epidemiology and molecular pathology of gallbladder cancer. Ca Cancer J Clin 2001, 51:349-364 [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Henson DE: ed. 3 Tumors of Gallbladder and Extrahepatic Bile Ducts, fascicle 23, 2000, Armed Forces Institute of Pathology, Washington DC
- 3.Wistuba II, Albores-Saavedra J: Genetic abnormalities involved in the pathogenesis of gallbladder carcinoma. J Hepatobiliary Pancreat Surg 1999, 6:237-244 [DOI] [PubMed] [Google Scholar]
- 4.Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 5.Knudson AG: Hereditary cancer, oncogenes, and antioncogenes. Cancer Res 1985, 45:1437-1443 [PubMed] [Google Scholar]
- 6.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K: The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell 1996, 84:587-597 [DOI] [PubMed] [Google Scholar]
- 7.Croce CM, Sozzi G, Huebner K: Role of FHIT in human cancer. J Clin Oncol 1999, 17:1618-1624 [DOI] [PubMed] [Google Scholar]
- 8.Siprashvili Z, Sozzi G, Barnes LD, McCue P, Robinson AK, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, Schwartz G, Pierotti MA, Croce CM, Huebner K: Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc Natl Acad Sci USA 1997, 94:13771-13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka H, Shimada Y, Harada H, Shinoda M, Hatooka S, Imamura M, Ishizaki K: Methylation of the 5′ CpG island of the FHIT gene is closely associated with transcriptional inactivation in esophageal squamous cell carcinomas. Cancer Res 1998, 58:3429-3434 [PubMed] [Google Scholar]
- 10.Zochbauer-Muller S, Fong KM, Maitra A, Lam S, Geradts J, Ashfaq R, Virmani AK, Milchgrub S, Gazdar AF, Minna JD: 5′ CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer. Cancer Res 2001, 61:3581-3585 [PubMed] [Google Scholar]
- 11.Wistuba II, Tang M, Maitra A, Alvarez H, Troncoso P, Pimentel F, Gazdar AF: Genome-wide allelotyping analysis reveals multiple sites of allelic loss in gallbladder carcinoma. Cancer Res 2001, 61:3795-3800 [PubMed] [Google Scholar]
- 12.Wistuba II, Behrens C, Milchgrub S, Virmani AK, Jagirdar J, Thomas B, Ioachim HL, Litzky LA, Brambilla EM, Minna JD, Gazdar AF: Comparison of molecular changes in lung cancers in HIV-positive and HIV-indeterminate subjects. JAMA 1998, 279:1554-1559 [DOI] [PubMed] [Google Scholar]
- 13.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP: Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res 2000, 60:18-21 [PubMed] [Google Scholar]
- 14.Ahmadian M, Wistuba II, Fong KM, Behrens C, Kodagoda DR, Saboorian MH, Shay J, Tomlinson GE, Blum J, Minna JD, Gazdar AF: Analysis of the FHIT gene and FRA3B region in sporadic breast cancer, preneoplastic lesions, and familial breast cancer probands. Cancer Res 1997, 57:3664-3668 [PubMed] [Google Scholar]
- 15.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti MA, Croce CM, Pilotti S: Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res 1998, 58:5032-5037 [PubMed] [Google Scholar]
- 16.Fong KM, Biesterveld EJ, Virmani A, Wistuba I, Sekido Y, Bader SA, Ahmadian M, Tiong Ong S, Rassool FV, Zimmerman PV, Giaccone G, Gazdar AF, Minna JD: FHIT and FRA3B allele loss are common in lung cancer and preneoplastic bronchial lesions and are associated with cancer-related FHIT cDNA splicing aberrations. Cancer Res 1997, 57:2256-2267 [PubMed] [Google Scholar]
- 17.Wistuba II, Montellano FD, Milchgrub S, Virmani AK, Behrens C, Chen H, Ahmadian M, Nowak JA, Muller C, Minna JD, Gazdar AF: Deletions of chromosome 3p are frequent and early events in the pathogenesis of uterine cervical carcinoma. Cancer Res 1997, 57:3154-3158 [PubMed] [Google Scholar]
- 18.Birrer MJ, Hendricks D, Farley J, Sundborg MJ, Bonome T, Walts MJ, Geradts J: Abnormal Fhit expression in malignant and premalignant lesions of the cervix. Cancer Res 1999, 59:5270-5274 [PubMed] [Google Scholar]
- 19.Sorio C, Baron A, Orlandini S, Zamboni G, Pederzoli P, Huebner K, Scarpa A: The FHIT gene is expressed in pancreatic ductular cells and is altered in pancreatic cancers. Cancer Res 1999, 59:1308-1314 [PubMed] [Google Scholar]
- 20.Mao L, Fan YH, Lotan R, Hong WK: Frequent abnormalities of FHIT, a candidate tumor suppressor gene, in head and neck cancer cell lines. Cancer Res 1996, 56:5128-5131 [PubMed] [Google Scholar]
- 21.Maitra A, Wistuba II, Washington C, Virmani AK, Ashfaq R, Milchgrub S, Gazdar AF, Minna JD: High-resolution chromosome 3p allelotyping of breast carcinomas and precursor lesions demonstrates frequent loss of heterozygosity and a discontinuous pattern of allele loss. Am J Pathol 2001, 159:119-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael D, Beer DG, Wilke CW, Miller DE, Glover TW: Frequent deletions of FHIT and FRA3B in Barrett’s metaplasia and esophageal adenocarcinomas. Oncogene 1997, 15:1653-1659 [DOI] [PubMed] [Google Scholar]
- 23.Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, Gazdar AF: Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene 1999, 18:643-650 [DOI] [PubMed] [Google Scholar]
- 24.Wistuba II, Behrens C, Virmani AK, Mele G, Milchgrub S, Girard L, Fondon JW, III, Garner HR, McKay B, Latif F, Lerman MI, Lam S, Gazdar AF, Minna JD: High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and three regions of frequent breakpoints. Cancer Res 2000, 60:1949-1960 [PubMed] [Google Scholar]
- 25.Strong MS, Incze J, Vaughan CW: Field cancerization in the aerodigestive tract—its etiology, manifestation, and significance. J Otolaryngol 1984, 13:1-6 [PubMed] [Google Scholar]
- 26.Boldog F, Gemmill RM, West J, Robinson M, Robinson L, Li EF, Roche J, Todd S, Waggoner B, Lundstrom R, Jacobson J, Mullokandov MR, Klinger H, Drabkin HA: Chromosome 3p14 homozygous deletions and sequence analysis of FRA3B. Hum Mol Genet 1997, 6:193-203 [DOI] [PubMed] [Google Scholar]
- 27.Hilgers W, Koerkamp BG, Geradts J, Tang DJ, Yeo CJ, Hruban RH, Kern SE: Genomic FHIT analysis in RER+ and RER− adenocarcinomas of the pancreas. Genes Chromosom Cancer 2000, 27:239-243 [PubMed] [Google Scholar]