Abstract
Cadherins are calcium-dependent cell adhesion molecules involved in the regulation of various biological processes such as cell recognition, intercellular communication, cell fate, cell polarity, boundary formation, and morphogenesis. Although previous studies have shown E-cadherin expression during rodent or human odontogenesis, there is no equivalent study available on N-cadherin expression in dental tissues. Here we examined and compared the expression patterns of E- and N-cadherins in both embryonic and adult (healthy, injured, carious) human teeth. Both proteins were expressed in the developing teeth during the cap and bell stages. E-cadherin expression in dental epithelium followed an apical-coronal gradient that was opposite to that observed for N-cadherin. E-cadherin was distributed in proliferating cells of the inner and outer enamel epithelia but not in differentiated cells such as ameloblasts, whereas N-cadherin expression was up-regulated in differentiated epithelial cells. By contrast to E-cadherin, N-cadherin was also expressed in mesenchymal cells that differentiate into odontoblasts and produce the hard tissue matrix of dentin. Although N-cadherin was not detected in permanent intact teeth, it was re-expressed during dentin repair processes in odontoblasts surrounding carious or traumatic sites. Similarly, N-cadherin re-expression was seen in vitro, in cultured primary pulp cells that differentiate into odontoblast-like cells. Taken together these results suggest that E- and N-cadherins may play a role during human tooth development and, moreover, indicate that N-cadherin is important for odontoblast function in normal development and under pathological conditions.
Tooth development involves a series of interactions between the oral epithelium and cranial neural crest-derived mesenchymal cells. These interactions progressively transform the tooth primordia into complex mineralized structures. During advanced stages of odontogenesis, mesenchymal cells differentiate into odontoblasts that synthesize the dentin matrix, whereas epithelial cells differentiate into ameloblasts that are responsible for enamel matrix formation. These epithelial-mesenchymal interactions have been extensively investigated in relation to regulatory mechanisms by transcription factors, growth factors, extracellular matrix molecules, and cell adhesion molecules. 1-5
Cell adhesion molecules are cell surface glycoproteins involved in diverse biological processes such as cell adhesion, cell recognition, control of cell division, and migration, differentiation, and morphogenesis. 6-8 Cell adhesion molecules are classified into four protein families: the cadherins, the integrins, the selectins, and the immunoglobulin superfamily proteins. Cadherins make up a large superfamily that can be divided into classical cadherins, desmosomal cadherins, protocadherins, seven transmembrane cadherins, and FAT-like cadherins. E-cadherin (epithelial cadherin), N-cadherin (neuronal cadherin), and P-cadherin (placental cadherin) represent the most well-characterized classical cadherins and which were the first cadherins to be described. 9-11 Although their names imply tissue specificity, the accumulated knowledge shows that the expression of the different cadherins is not completely tissue-specific. N-cadherin, for example, is expressed also in mesenchymal tissues, muscle, fibroblasts, and vessel endothelial cells. Several cadherins can also be co-expressed in the same cell types. Furthermore, all cadherins are dynamically expressed in specific spatiotemporal patterns during embryonic development. 8-10 Classical cadherins function through a Ca2+-dependent homophilic binding mediated by their extracellular domains. 9-11 In their cytoplasmic domain, they interact with the catenins that connect them to the actin filaments of the cytoskeleton. 12 In many cell types, the cadherin/catenin complexes are connected to actin-based adherens junctions and this connection to the cytoskeleton is crucial for efficient cell-cell adhesion. 13,14 In addition to its structural role in adherens junctions, β-catenin is also found in the cytosol, where it interacts with other proteins and is an integral part of the Wnt/Wingless signaling pathway. 15 Tight regulation of the level of cytoplasmic β-catenin is important because it can interact with members of the high mobility group family of transcription factors, such as LEF-1, and alter transcription and cell fate. 16 Several studies have correlated changes in cadherin expression or function with the onset of processes that control cell migration and cell differentiation. 17,18
During embryonic development, cadherins show characteristic tissue-specific expression patterns and have been proposed to act as morphogenetic regulators. 7,9,19-21 Misregulated cadherin expression or function disrupts embryonic morphogenesis and can alter the characteristics of differentiated cells. 22,23 The influence of cadherins on cell motility seems to be important because it has been found that E-cadherin mediates contact inhibition of cell migration. 24 Furthermore, many studies have correlated changes in cadherin expression or function with human malignancies and the onset of processes that control aggravated cancer cell invasion and metastasis. 25 E-cadherin was studied extensively in experimental tumor cell systems and was found to act as an invasion suppressor. 26,27 In human tumors, mutational disruption of E-cadherin has been observed in invasive breast cancer, prostate cancer, and gastric carcinomas. 28,29 However, the roles of other cadherins in pathological conditions is less extensively investigated. A few studies show increased N-cadherin expression on down-regulation of E-cadherin in cancer cells. 30,31
Despite the significance of intercellular communication in morphogenesis and pathology, few studies have been made on the expression patterns of cadherins during odontogenesis. 32-39 To date, there is no available study on the expression of N-cadherin during tooth development. It also remains uncertain if both E-cadherin and N-cadherin are important for dentin repair after either carious or traumatic lesions.
In the light of the dual role of cadherins in adhesion and signaling, it is clearly of interest to examine their possible roles in expression in human teeth, both during development and under physiological and pathological conditions. Here we present the expression patterns of both E-cadherin and N-cadherin in embryonic, and in permanent intact, injured, and carious human teeth. We also report on E- and N-cadherin expression in a culture system of human pulp cells in vitro.
Materials and Methods
Materials
Antibodies
Preparation and characterization of a rabbit antiserum against mouse E-cadherin has already been described. 40 This antiserum was demonstrated to react specifically with E-cadherin in human tissues both in immunohistochemistry and in Western blots, and does not cross-react with other cadherins. 41 A rabbit polyclonal antibody raised against baculovirus-expressed mouse N-cadherin was kindly provided by Dr. Thomas K. Borg (University of South Carolina, School of Medicine, Columbia, SC). This antibody was demonstrated to react specifically with N-cadherin in immunohistochemistry of human tissues (T. K. Borg, personal communication), and we demonstrated that it reacted specifically with N-cadherin in Western blotting of human PC-3 cells, without any cross-reactivity with E-cadherin (data not shown).
Chemicals
Vector Vectastain ABC kit was purchased from Biosys (Compiègne, France). For the preparation of culture media, all materials were purchased from Life Technologies Inc. (Grand Island, NY). Other chemicals were obtained from Sigma (St. Louis, MO).
Culture Medium
For cultures, minimum essential medium was supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B.
Recombinant Proteins and Beads
Recombinant fibroblast growth factor 4 (FGF4; British Biotechnology Products) and bone morphogenetic protein 4 (BMP4; Genetics Institute, Cambridge, MA) were used to preload Affi-gel blue agarose beads (100 to 200 mesh, 75- to 150-μm diameter; Bio-Rad). Control beads were incubated in 0.1% bovine serum albumin in phosphate-buffered saline (PBS).
Embryonic Tissues
Human fetal tissues were obtained from legal abortions. The material comprised teeth from five fetuses (8 to 30 gestational weeks). The gestation age was estimated from the fetal foot length and from the last menstruation of the mother. Embryos were healthy, and all tissues were macroscopically and microscopically normal. The fetuses were fixed immediately by the obstetrician in 10% buffered formalin for 48 hours to 5 days according to their size. The maxillary and mandibular processes from 8-week-old embryos were embedded in Paraplast at 56°C. The samples, ranging in age from 16 to 30 gestational weeks, were decalcified for 3 weeks in formic acid/10% formalin before cryosectioning. Ten-μm-thick sections were used for immunohistochemistry. This study was performed in compliance with French legislation, after approval of the Regional Ethics Committee of the Hospital Center of Marseille (CCPPRB Marseille I).
Permanent Teeth
Permanent intact teeth extracted for orthodontic reasons, and carious teeth of 40-year-old patients were used in this study. The teeth were freshly extracted and used in this study with the patient’s informed consent. The extracted teeth were fixed in 10% neutral-buffered formalin for 7 days, demineralized in sodium formiate for 21 days, and then embedded in paraffin wax. They were serially sectioned (6-μm thick) and then processed for immunohistochemistry.
Cavity Preparation and Tooth Processing
Cavities were prepared in intact first premolars scheduled for extraction, at the Dental Care Center of Marseille. Cavities 2- to 3-mm wide and 1- to 1.3-mm deep were then cut into the dentin with a bur using the least possible pressure. The pulp chambers were not exposed during the preparation of the cavities. The walls of the cavities were immediately conditioned with a 3% hydrogen peroxide solution and dried with an extremely light air stream. The cavities were restored with a calcium hydroxide product (Dycal; Dentsplay, USA) that was covered by a temporary filling material (IRM; De Trey Dentsplay IG, Zurich, Switzerland). After a postoperative interval of 9 weeks, the teeth were extracted using a local anesthetic with the patient’s informed consent. Teeth with cavities or deep dentinal caries were fixed in 10% neutral-buffered formalin for 7 days, demineralized in sodium formiate for 21 days, and then embedded in paraffin wax.
Cultures of Human Dental Pulp Cells, Periodontal Ligament Cells, and Dental Pulp Explants
Immediately after extraction of the first premolars of a 13-year-old patient, the teeth were washed with sterile PBS. The dental pulps were gently removed with forceps, minced with scalpels, and then rinsed with PBS. Cultures of human dental pulp cells were performed as previously described. 42,43 Cultures of periodontal ligament cells were performed in a similar way. Briefly, cells were obtained from the periodontal ligament of extracted molar teeth. The periodontal ligament cells were mechanically removed by scraping the middle third of the root surface with a curette. The cells were put in 100-mm tissue culture plates and were incubated at 37°C in a humidified incubator with 5% CO2. The culture medium was replaced every other day. Cells were dissociated with 0.25% trypsin and 0.05% ethylenediaminetetraacetic acid, and were subdivided into T-25 flasks for experimentation between the second and the fifth passages.
Explants were cultured in minimum essential medium at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Beads loaded with BMP4 or FGF4 (100 to 250 μg/ml) or control beads in minimum essential medium were transferred on top of the explants. Each explant received 1 μl of a solution containing 10 beads. After 14 hours of culture the explants were fixed in 4% paraformaldehyde for 2 hours at 4°C and processed for whole-mount immunohistochemistry as described previously. 43,44
Immunohistochemistry of Sections and Cell Cultures
Immunoperoxidase staining of sections was done as previously described. 43,45 Briefly, the sections were deparaffinized, exposed to a 0.3% solution of hydrogen peroxide in methanol, and then incubated overnight at 4°C with the primary antibody against either E-cadherin or N-cadherin. The E-cadherin antibody was incubated at a dilution of 1:150 in PBS containing 0.2% bovine serum albumin and 5% normal goat serum. The N-cadherin antibody was diluted 1:50 in PBS for cryosections and cell cultures and 1:10 for paraffin sections. Peroxidase was detected by incubation with 3-amino-9-ethylcarbazole reaction solution. After staining, the slides were mounted with Aquamount (BDH, Gurr, UK). In control sections the primary antibodies were either omitted or replaced by a nonimmune rabbit serum. Cultured cells were fixed in 4% paraformaldehyde for 30 minutes and permeabilized for 15 minutes with 0.5% Triton X-100 in PBS before immunohistochemistry.
Results
E-Cadherin and N-Cadherin Expression in the Developing Deciduous Human Tooth Germs
At the eighth gestational week the ingrowth of the stomodeal epithelium into the underlying neural crest-derived mesenchyme gives rise to the dental bud of the future deciduous tooth. At this stage, immunoreactivity for E- and N-cadherins was not detected either in the epithelial or in the mesenchymal components (data not shown).
At the 16th gestational week, the dental epithelium acquires the cap configuration and forms the enamel organ consisting of three distinct layers: the inner enamel epithelium, the outer enamel epithelium, and the stellate reticulum. The mesenchyme progressively forms the dental papilla. A strong E-cadherin immunostaining was observed in the enamel organ, whereas N-cadherin staining was weak in the epithelial and some dental papilla cells (data not shown).
From the 18th to 21st gestational weeks, increasing growth of the enamel epithelium gives rise to an early bell-shaped structure. A fourth epithelial cell layer, called stratum intermedium, is formed, overlying the cells of the inner enamel epithelium. The oral epithelium overlying the tooth germs thickens and acquires several layers of cells with distinct differentiation status (Figure 1A) ▶ . E- and N-cadherin staining was found in different cell layers of the oral epithelium: N-cadherin was detected in proliferating cells located in the basal layer (Figure 1Cb) ▶ , whereas the newly differentiated cells of the overlying layer were positive for E-cadherin (Figure 1Ca) ▶ . These complementary and distinct patterns of E- and N-cadherin distribution were also observed in the developing tooth germs. By contrast to the oral epithelium, E-cadherin staining was detected in proliferating inner enamel epithelium cells (ie, cervical loop area; Figure 1, B and Ea ▶ ), whereas in inner enamel epithelium cells that have acquired a higher degree of differentiation (ie, pre-ameloblasts at the tip of the cusp) the staining decreased and was progressively lost 9Figure 1, B and Da) ▶ . The inverse gradient was observed for N-cadherin: the immunoreactivity was very faint in proliferating inner enamel epithelium cells (Figure 1Eb) ▶ and strong in pre-ameloblasts (Figure 1Db) ▶ . Cells of the outer enamel epithelium exhibited a strong staining for E-cadherin (Figure 1Fa) ▶ , whereas the N-cadherin reactivity was less pronounced (Figure 1Fb) ▶ . Cells of the stellate reticulum were also positive for E-cadherin (Figure 1, Da and Fa) ▶ , whereas the staining for N-cadherin was weak and sporadic (Figure 1, Db and Fb) ▶ . In dental papilla mesenchyme, a very faint, hardly detectable N-cadherin labeling was observed in cells located in the cusp region (Figure 1Db) ▶ , whereas no specific E-cadherin staining could be observed (Figure 1Da) ▶ . Some cells of the dental follicle, the future periodontal ligament, exhibited E- and N-cadherin immunostaining (Figure 1F) ▶ .
Figure 1.
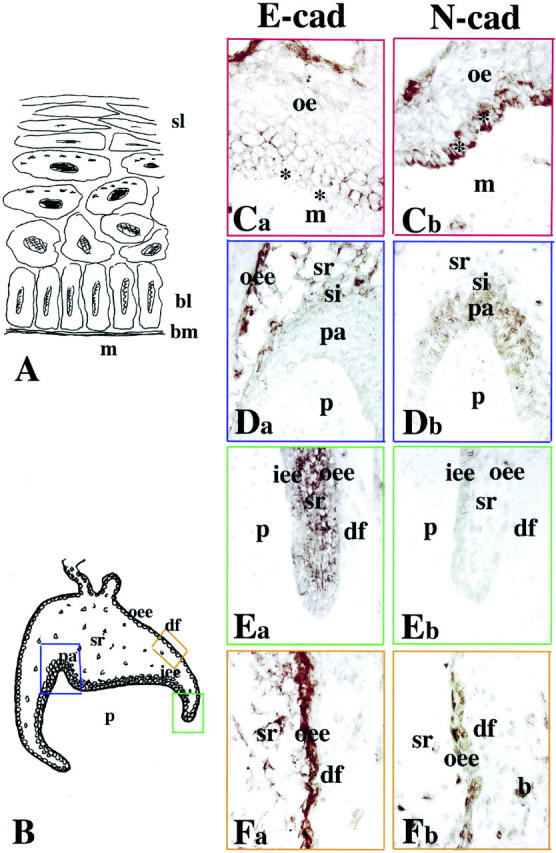
Comparison between the patterns of E- and N-cadherin immunostainings in oral epithelium and deciduous first molars. Ca, Da, Ea, and Fa: E-cadherin labeling; Cb, Db, Eb, and Fb: N-cadherin labeling. A: Schematic illustration of the oral epithelium. Note the presence of different epithelial cell layers. B: Schematic representation of a deciduous human first molar at the early bell stage of development (18 gestational weeks). The colored frames show the areas used for the comparative study. Ca and Cb: Oral epithelium (red frames). E-cadherin is distributed at the surface of cells overlying the proliferating N-cadherin-positive cells of the basal layer (asterisks). Da and Db: Cusp area (blue frames). Combinatory patterns of E- and N-cadherins: E-cadherin immunoreactivity is evident in outer enamel epithelium and stellate reticulum, whereas N-cadherin is observed in pre-ameloblasts and stratum intermedium. Note the weak N-cadherin staining in some dental pulp cells. Ea and Eb: Cervical loop area (green frames). Strong E-cadherin and faint N-cadherin staining in epithelial cells. Fa and Fb: Intermediate area (orange frames). Strong E-cadherin and weaker N-cadherin reactivity in outer enamel epithelium and stellate reticulum. Note also a positive staining in several mesenchymal cells surrounding the tooth germ. Abbreviations: b, bone forming cells; bl, basal layer; bm, basement membrane; de, dental epithelium; df, dental follicle; iee, inner enamel epithelium; m, mesenchyme; oe, oral epithelium; oee, outer enamel epithelium; p, dental papilla (pulp); pa, pre-ameloblasts; si, stratum intermedium; sl, squamus layer; sr, stellate reticulum.
During the 30th gestational week, the tooth germs are in the late bell stage of development. Dentinogenesis and amelogenesis have already started at the tip of the cusps (Figure 2A) ▶ . Pulp cells adjacent to the inner enamel epithelium layer differentiate into odontoblasts that secrete the organic matrix components of dentin. Pre-ameloblasts differentiate into ameloblasts that synthesize the enamel matrix proteins.
Figure 2.
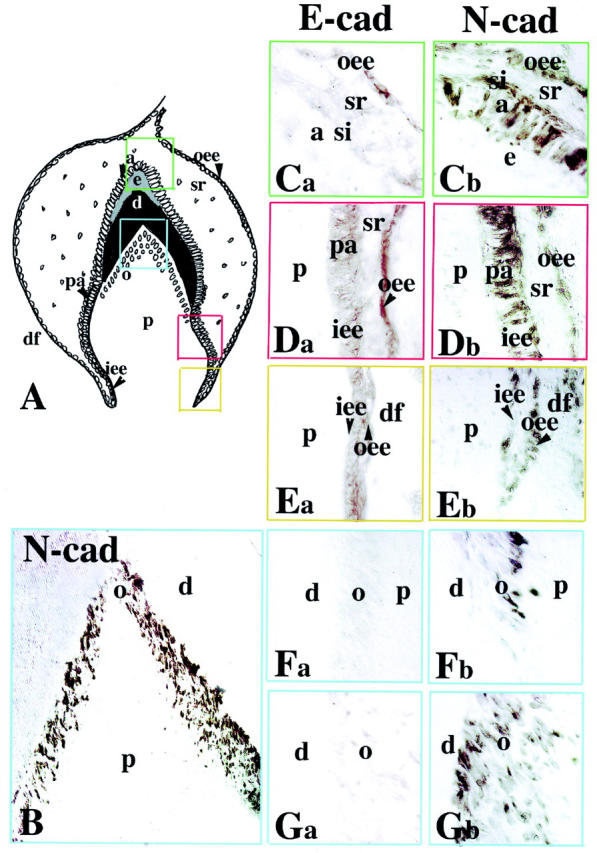
Comparison between the E- and N-cadherin staining patterns in deciduous first incisors. Ca, Da, Ea, Fa, and Ga: E-cadherin labeling; B, Cb, Db, Eb, Fb, and Gb: N-cadherin labeling. A: Schematic illustration of a deciduous human first incisor at the late bell stage of development (30 gestational weeks). The colored frames represent the areas used for the comparative study. B: Mesenchymal part of the cusp area (blue frame). N-cadherin staining is present in odontoblasts and absent from pulp fibroblasts. Note that the gradient of N-cadherin staining in odontoblasts follows their maturation degree. Ca and Cb: Epithelial part of the cusp area (green frames). E-cadherin reactivity is restricted in outer enamel epithelium, whereas N-cadherin is detected in ameloblasts, stratum intermedium, and outer enamel epithelium. Note that in stellate reticulum the E- and N-cadherin immunoreactivities are weak. Da and Db: Intermediate area (red frames). Strong E-cadherin staining in outer enamel epithelium and weaker in pre-ameloblasts and inner enamel epithelium. Heavy N-cadherin labeling in pre-ameloblasts and weaker in outer enamal epithelium and inner enamel epithelium. Ea and Eb: Cervical loop area (orange frames). E- and N-cadherin reactivities in dental epithelial cells. Note a positive staining in mesenchymal cells forming the alveolar bone. Fa and Fb: Mesenchymal part of the cusp area (blue frames). Presence of N-cadherin and absence of E-cadherin staining in newly differentiated odontoblasts. Ga and Gb: Mesenchymal part of the cusp area (blue frames). Strong N-cadherin and very faint E-cadherin reactivities in functional odontoblasts. Abbreviations: a, ameloblasts; d, dentin; df, dental follicle; e, enamel; iee, inner enamel epithelium; o, odontoblast; oee, outer enamel epithelium; p, dental pulp; pa, pre-ameloblasts; si, stratum intermedium; sr, stellate reticulum.
As in the early bell stage, a decreasing gradient of E-cadherin staining was observed in the dental epithelium following the apical (proliferation area) to coronal (differentiation area) direction. Thus, E-cadherin immunoreactivity was detected in inner enamel epithelium and outer enamel epithelium cells of the cervical loop (Figure 2Ea) ▶ and median areas (Figure 2Da) ▶ of the tooth germ, whereas the staining was completely absent in ameloblasts and weaker in outer enamel epithelium cells of the cusp area (Figure 2Ca) ▶ . A faint staining was observed in the stellate reticulum. By contrast to the E-cadherin staining, the immunoreactivity for N-cadherin in the dental epithelium was gradually increased in a coronal direction. A moderate N-cadherin staining was found in inner enamel epithelium and outer enamel epithelium cells of the cervical loop (Figure 2Eb) ▶ , whereas the labeling was strong in the pre-ameloblasts (Figure 2Db) ▶ and ameloblasts (Figure 2Cb) ▶ . Although the staining was somewhat patchy it gave the impression that the entire surface of the pre-ameloblasts and ameloblasts were positive for N-cadherin. However, there was a tendency for stronger staining of the pre-ameloblast surfaces toward the stellate reticulum, whereas the ameloblasts showed an accumulation of N-cadherin staining in distinct areas in the opposite cellular pole, ie, toward the enamel. Cells of the stratum intermedium were positive for N-cadherin (Figure 2Cb) ▶ , whereas the staining was absent from stellate reticulum cells. In the pulp, a strong N-cadherin immunoreactivity was detected in the differentiating (Figure 2, B and Fb) ▶ and functional odontoblasts (Figure 2, B and Gb) ▶ , whereas the staining was absent from pulp fibroblasts (Figure 2B) ▶ . Subodontoblastic cells (referred to as Höhl’s cells) were also stained for N-cadherin (Figure 2, B and Gb) ▶ . E-cadherin staining was very weak in functional odontoblasts (Figure 2Ga) ▶ and absent in differentiating odontoblasts and pulp fibroblasts (Figure 2Fa) ▶ .
E-Cadherin and N-Cadherin Expression in Intact, Carious, and Injured Permanent Human Teeth
E- and N-cadherin immunoreactivities were absent from odontoblasts and pulp fibroblasts of intact permanent human teeth of a 40 year-old patient (data not shown).
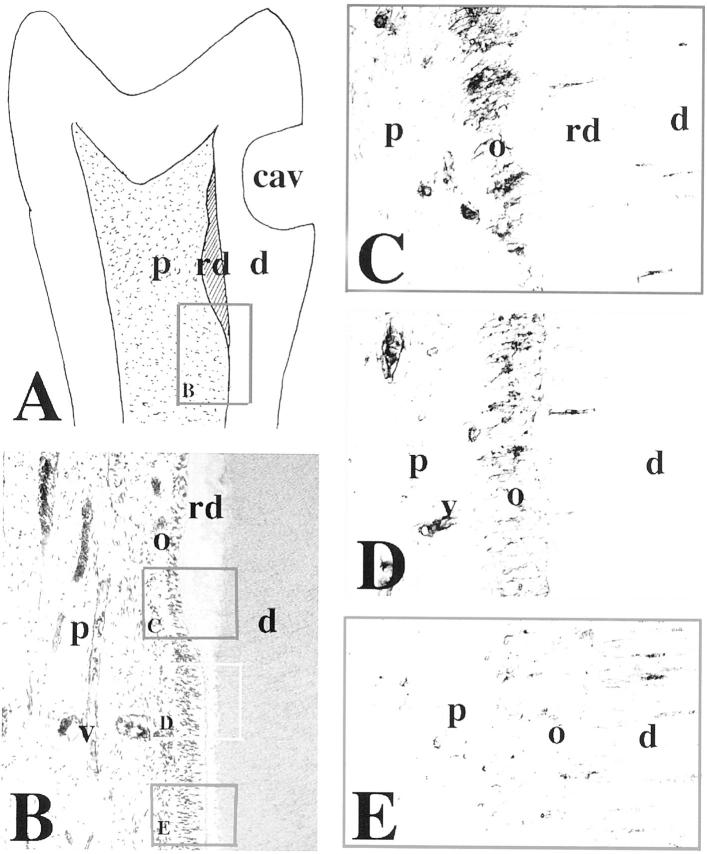
In permanent teeth with carious lesions (Figure 3, A and B) ▶ , the secretory activity of the odontoblasts is stimulated to produce tertiary dentin (hypercalcification). In response to an increased irritation, the degenerated odontoblasts are replaced by newly formed odontoblast-like cells that elaborate the reparative dentin. N-cadherin staining was observed in odontoblasts facing the carious lesion (Figure 3, C and E) ▶ , which produce the reparative dentin (Figure 3D) ▶ . The staining was detected in both the odontoblast cell bodies and the odontoblast processes. The pulp fibroblasts were negative for N-cadherin under these conditions (Figure 3, C and E) ▶ . In advanced carious lesion (Figure 3B) ▶ , inflammation was observed in the pulp of the teeth. Under these conditions, N-cadherin staining was also observed in the dilated blood vessels (Figure 3, E and G) ▶ . In odontoblasts far away from or surrounding the carious lesion, N-cadherin immunoreactivity was restricted to cell bodies (Figure 3G) ▶ . By contrast to N-cadherin, E-cadherin staining was not observed in the pulp of carious teeth (data not shown).
Figure 3.
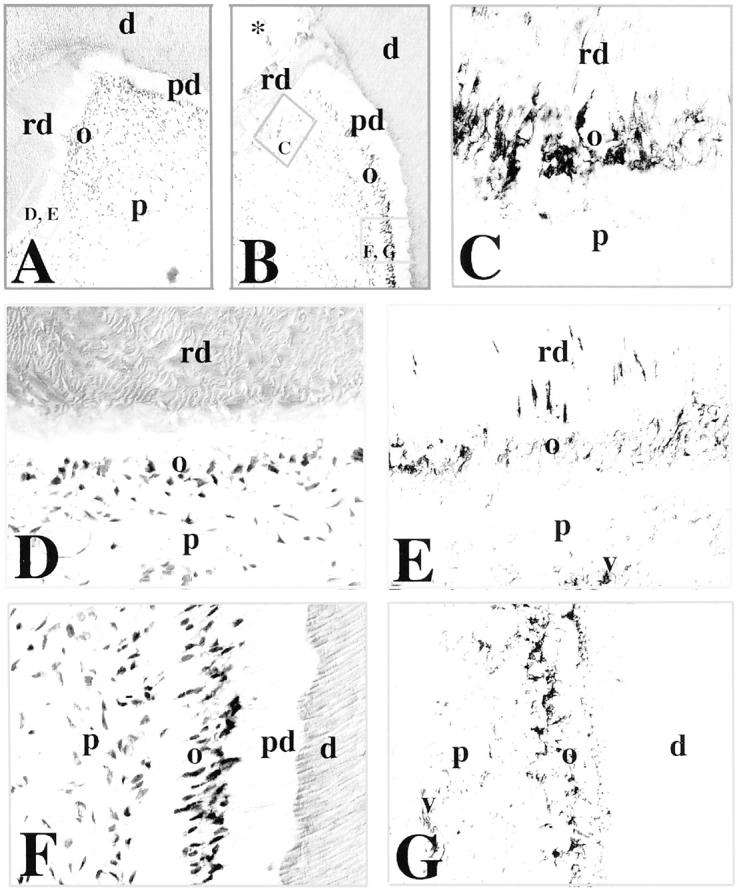
Immunohistochemical localization of N-cadherin in sections of carious permanent human teeth. A and B: H&E staining. In carious teeth, tertiary or reparative dentin is seen near the sites of the carious lesions (A and B; asterisk in B). The frames represent the sites shown in the following panels. C: Higher magnification of the framed area in B. N-cadherin reactivity is found in odontoblasts involved in reparative dentin production. D and E: Higher magnification of the framed area in A (D), showing N-cadherin immunostaining in odontoblasts producing the reparative dentin (E). Note that the staining is distributed in both odontoblast cell bodies and processes. F and G: Higher magnification of the framed area in B (F), showing N-cadherin immunoreactivity in odontoblasts at a distance from the carious lesion (G). Note that the staining in odontoblasts is restricted to the odontoblast cell bodies. Cells in dilated blood vessels are also positive for N-cadherin. Abbreviations: d, dentin; o, odontoblasts; p, pulp; pd, predentin; rd, reparative dentin; v, vessel.
Nine weeks after the cavity preparation involving the dentin, the odontoblasts facing the injury site produce tertiary dentin (Figure 4, A and B) ▶ . N-cadherin staining was seen in these odontoblasts, both in cell bodies and in processes (Figure 4C) ▶ . The N-cadherin reactivity decreased in odontoblasts that were located in the proximity of the injury site (Figure 4D) ▶ . The staining was very faint in the cell bodies of the odontoblasts at a distance from the cavity preparation (Figure 4E) ▶ and was mainly distributed in their processes. Immunoreactivity for N-cadherin was also observed in blood vessels (Figure 4D) ▶ . Furthermore, N-cadherin-positive fibers, most likely nerve fibers, were observed in the pulp (Figure 4D) ▶ , although it was not possible to unambiguously distinguish these fibers from putative odontoblast processes with the present technique (Figure 4D) ▶ . E-cadherin staining was absent in all odontoblasts and pulp fibroblasts after cavity preparation (data not shown).
Figure 4.
Immunohistochemical localization of N-cadherin in sections of permanent human premolars after cavity preparation. A: Schematic illustration of a premolar showing reparative dentin production, 9 weeks after class V cavity preparation. The frame represents the area shown in the B. B: H&E staining. The frames indicate the areas shown in C, D, and E. C: A strong N-cadherin labeling is observed in odontoblasts producing the reparative dentin. Note that the staining is detected in both odontoblast bodies and odontoblast processes. D: N-cadherin reactivity is decreased in odontoblasts near to the injury site, but not involved in reparative dentin synthesis. Note that blood vessels are N-cadherin-positive. E: N-cadherin staining is very faint in odontoblasts far away from the injury site. Note that the staining persists in some of the odontoblast processes. Abbreviations: cav, cavity; d, dentin; o, odontoblasts; p, pulp; rd, reparative dentin; v, vessel.
E- and N-Cadherin Expression in Cultured Human Tooth Periodontal Ligament and Pulp Cells in Vitro
Previous experiments have shown that cultured dental pulp cells produce nestin, osteonectin, type I collagen, and dentin sialoprotein, which make them a good model to study odontoblast differentiation and dentin formation in vitro. 42,43,46 Pulp and periodontal ligament cells from healthy human teeth that have wide apices were cultured for 3 to 4 weeks either in the presence or absence of β-glycerophosphate. β-glycerophosphate is known to stimulate hard tissue formation. At the fourth week, deposition of mineral crystals was detected only in the cultures of pulp cells treated with β-glycerophosphate. E-cadherin immunoreactivity was detected in a restricted number of periodontal ligament cells treated with β-glycerophosphate (Figure 5A) ▶ , whereas N-cadherin was absent from these cells (Figure 5B) ▶ . Pulp cells cultured in the absence of β-glycerophosphate were negative for both E- and N-cadherins (data not shown and Figure 5C ▶ ). Only N-cadherin staining was detected in the surface of pulp cells treated with β-glycerophosphate that will form the mineralization nodules (data not shown and Figure 5D ▶ ).
Figure 5.

E- and N-cadherin immunoreactivities in human dental pulp explants, pulp cells, and periodontal ligament cells cultured in vitro. A: Confluent periodontal ligament cells exhibit E-cadherin staining. B: Confluent periodontal ligament cells are negative for N-cadherin. C: Confluent pulp cells cultured without β-glycerophosphate are negative for N-cadherin. D: A cell-surface staining for N-cadherin is observed in pulp cells implicated in the formation of the nodules after β-glycerophosphate treatment. E: FGF4 beads have no effect on N-cadherin expression in human dental pulp explants cultured for 14 hours. Note the positive N-cadherin staining in nerve fibers (arrowhead). F: N-cadherin immunoreactivity is not found in pulp cells surrounding the BMP4 beads. Note the positive staining of the nerve fibers (arrowheads). Abbreviation: b, bead.
BMP4 and FGF4 Do Not Alter N-Cadherin Expression in Human Pulp Explants
Because BMPs and FGFs are important for almost all stages of tooth development, 2-4 these signaling molecules are good candidates for triggering N-cadherin up-regulation in dental pulp cells after traumatic or carious irritation. To test this hypothesis, we placed beads releasing BMP4 or FGF4 on the top of dental pulp explants and followed the expression of N-cadherin by whole-mount immunohistochemistry. Analysis of the explants showed restricted N-cadherin immunoreactivity in neuronal structures, but not in the pulp cells surrounding the beads (Figure 5, E and F) ▶ .
Discussion
E- and N-cadherins are cell-cell adhesion molecules involved in both organ morphogenesis and pathology. 6,7,9,20,26-31 In the developing human teeth, E- and N-cadherins exhibited specific and dynamic spatiotemporal expression patterns. Both proteins were down-regulated in adult teeth, but N-cadherin was re-expressed in the dental pulp of carious and injured teeth.
E- and N-Cadherins in Developing and Intact Adult Human Teeth
Although both N- and E-cadherin proteins were detected in embryonic dental tissues, pronounced differences were observed in the expression patterns of the two cadherins. E-cadherin was initially expressed in the dental epithelium at the cap stage, and was down-regulated when ameloblast differentiation started at the bell stage. N-cadherin showed an inverse gradient compared with E-cadherin and was up-regulated in differentiated epithelial cells. By contrast to E-cadherin, N-cadherin was also detected in the dental pulp and its expression was restricted to differentiated odontoblasts and Höhl’s cells. This expression pattern indicates that odontoblasts and Höhl’s cells have acquired a higher degree of differentiation than other pulp cells. In addition, both cadherins were expressed in the oral epithelium overlying the dental organ, with N-cadherin preferentially expressed in the basal proliferating cells and E-cadherin in the suprabasal layer of cells.
The expression pattern of E-cadherin in human teeth presents some differences from that previously reported in rodent teeth. 34,35,37,38 In the developing human molars, E-cadherin was initially expressed in the epithelium at the cap stage, whereas in mouse molars, it was first detected at an earlier stage (bud stage). 34,35,37 In mice, E-cadherin was weakly expressed in the odontoblasts of the incisors, 39 which contrasted to the lack of E-cadherin in odontoblasts of human teeth. The reasons for these species differences are not known, but it may either be a consequence of different detection sensitivities in the two species, or might be related to the difference in life span between rodents and humans, the constant growth of rodent incisors and/or the differences in usage of teeth.
The expression of E-cadherin and N-cadherin during embryonic tooth morphogenesis and their down-regulation in adult dental tissues indicate that these cadherins have important roles in the development and shaping of the dental organ. It is well established that both E- and N-cadherins play important roles in morphogenetic events; they regulate cell movement and maintain segregation and positioning of cell groups. 20,21 A differential role for E- and N-cadherins in regulation of dental epithelial cell growth may be suggested, because E-cadherin expression was pronounced in proliferating cells (inner enamel epithelium cells), whereas N-cadherin was up-regulated in differentiating cells (ameloblasts). However, the inverse gradient was observed in the oral epithelium, where E-cadherin was expressed in the nonproliferating cells and N-cadherin in the proliferating cells, thus suggesting that the molecular mechanisms controlling the growth of the tooth and the oral epithelium differ. The findings of selective and reciprocal patterns of E- and N-cadherin expression rather point to a role for these cadherins in cellular organization and segregation of different cell populations during odontogenesis. These expression patterns are at the principal level identical to the mutually exclusive expression patterns of various cadherins that are seen in a number of morphogenetic events, such as neurulation and placode formation. 9,17,21,47
E-cadherin down-regulation and N-cadherin up-regulation in ameloblasts indicate that this specific cadherin expression pattern may also be important for secretion of enamel matrix. During ameloblast differentiation from inner enamel epithelium cells an interesting cellular morphological metamorphosis and polarization change occur. The inner enamel epithelial cells are initially basal cells in a stratified epithelium attached to a basement membrane. During ameloblast maturation, the basement membrane disappears and the epithelial cell surface facing the basement membrane transforms into an apical, secretory surface. Close to the apical surface, the secretory cells are now joined by adherens junctions and an underlying terminal web. 48 The adhesive molecules in adherens junctions are either E-cadherin or N-cadherin. 12 Because the ameloblasts express N-cadherin but not E-cadherin their adherens junctions most likely are formed by N-cadherin. This agrees with our observation that N-cadherin was concentrated toward the apical surfaces in mature ameloblasts. These data suggest that increased expression and apical localization of N-cadherin play an important role in ameloblast transformation and polarization that is a prerequisite for enamel matrix secretion.
A rich plexus of small unmyelinated nerve fibers is formed beneath and between the odontoblasts, but conflicting information exists regarding the presence of direct contacts between these two structures. 49,50 The observation that both neural crest-derived odontoblasts and nerve fibers express N-cadherin may, however, suggest that N-cadherin could mediate direct binding between odontoblasts and the forming nerve plexus. This is in agreement with other studies that have shown that N-cadherin is expressed in Schwann cells that are in contact with unmyelinated nerve fibers and that adhesion of Schwann cells to neurites or to other Schwann cells is mediated through N-cadherin. 41-54 Furthermore, it has been demonstrated that N-cadherin is expressed by developing myotubules during the period of initial nerve-muscle contact, and that this expression is down-regulated within days after innervation. 55
N-Cadherin in Adult Human Teeth Exposed to a Lesion
During the decalcification and proteolytic processes of the carious dentin, the dentinal tubuli gradually become calcified, provided that the odontoblasts remain vital. The secretory activity of the odontoblasts facing the decay is stimulated to elaborate reactionary dentin. 56 In the case that the irritation increases, the odontoblasts degenerate and are replaced by newly formed odontoblasts secreting the reparative dentin. N-cadherin was re-expressed in odontoblasts surrounding the carious lesion. A similar effect was observed in odontoblasts that elaborate reactionary dentin after cavity preparations. Odontoblasts that are not involved in reactionary dentin production, but were located near the injury site, also expressed N-cadherin. However, N-cadherin was not re-expressed in odontoblasts located at a distance from the cavity area. These results suggest a role for N-cadherin in reparative processes and most notably in the secretory activity of odontoblasts after dental injury.
The origin of the newly formed odontoblasts during reparative dentinogenesis is not yet known. It has been suggested that cells of the immune system, Höhl’s cells, and pulp fibroblasts may give rise to new odontoblasts. 56,57 The observation that Höhl’s cells expressed N-cadherin during odontogenesis may suggest that these cells are the precursors for the newly formed odontoblasts. Regardless of their origin, however, pre-odontoblastic cell migration is important for their participation in the repair of the tooth tissues. Thus, the expression of N-cadherin in these cells suggests that this molecule may have an additional role in stimulating motility of the cells giving rise to new odontoblasts, in analogy with the role of N-cadherin in squamous carcinoma cells. Cells in oral squamous carcinomas expressing N-cadherin rather than E-cadherin no longer display an epithelial morphology, but appear fibroblastic. 31 Forced expression of N-cadherin in these cells was characterized by increased cell motility and invasion. 30,31
In response to a profound irritation of the dental pulp because of either deep cavity preparation or of advanced carious lesion, new blood vessels are formed near the injury site. N-cadherin and VE-cadherin are the two major cadherins of endothelial cells and play essential roles in angiogenesis and maintenance of blood vessel structural integrity. 58-60 N-cadherin expression by endothelial cells represents an initial and transient signal that may be involved in the commitment of early regenerating blood vessels. 59 Accordingly, neovascularization is accompanied by N-cadherin re-expression in endothelial cells. The observation that N-cadherin became expressed in blood vessels in the pulp of injured teeth caused by deep cavity preparation thus suggests that these vessels represent newly formed blood vessels. N-cadherin expression was also observed in dilated blood vessels in the inflamed tissue of carious lesions, indicating a correlation between up-regulation of N-cadherin and inflammatory events. Recently, such a relation has been demonstrated between VE-cadherin expression in endothelial cells and inflammatory cells accumulating at the pathological site. 61
BMPs and FGFs are expressed in dental tissues, and BMPs have been shown to induce odontoblast differentiation in vitro. 2,62 During dentin decalcification after a carious irritation, BMPs and FGFs may be released from the matrix and diffuse to reach adjacent cells, 56,57 triggering dental pulp cells in the vicinity of the lesion to differentiate into odontoblast-like cells, which would start secretion and deposition of the reparative dentin matrix. Therefore, we investigated if the differentiating effects of BMP4 and FGF4 might include stimulation of N-cadherin expression in human dental pulp explants. However, we were not able to detect any N-cadherin expression under these conditions, indicating either that other factors and/or culture conditions are needed for N-cadherin expression. It has indeed been demonstrated that both extracellular matrix components and growth factors are important for induction of odontoblast differentiation. 2 Even if the factors regulating odontoblast differentiation are incompletely understood, we found that induction of mineralization in vitro caused up-regulation of N-cadherin in dental pulp cells that had differentiated to odontoblast-like cells. This is consistent with the in vivo observations in carious and injured teeth, and suggests a role for N-cadherin in dentin hard tissue synthesis.
Footnotes
Address reprint requests to Thimios A. Mitsiadis, Faculté d’Odontologie, Université de la Méditerranée, F-13385 Marseille, Cedex 05, France. E-mail: mitsiadis.e@odontologie.univ-mrs.fr.
Supported by grants from the Association pour la Recherche sur le Cancer, the Swedish Medical Research Council [project 05200 and project K2000-24F-13358-01], the Swedish Cancer Foundation (project 3957), the Swedish Dental Society, the Erik and Edith Fernströms stiftelse, and the Karolinska Institutet.
References
- 1.Peters H, Balling R: Teeth. Where and how to make them. Trends Genet 1999, 15:59-65 [DOI] [PubMed] [Google Scholar]
- 2.Ruch JV, Lesot H, Begue-Kirn C: Odontoblast differentiation. Int J Dev Biol 1995, 39:51-68 [PubMed] [Google Scholar]
- 3.Thesleff I, Nieminen P: Tooth differentiation and cell differentiation. Curr Opin Cell Biol 1996, 8:844-850 [DOI] [PubMed] [Google Scholar]
- 4.Thesleff I, Sharpe P: Signalling networks regulating dental development. Mech Dev 1997, 67:111-123 [DOI] [PubMed] [Google Scholar]
- 5.Zeichner-David M, Diekwisch T, Fincham A, Lau E, MacDougall M, Moradian-Oldak J, Simmer J, Snead M, Slavkin HC: Control of ameloblast differentiation. Int J Dev Biol 1995, 39:69-92 [PubMed] [Google Scholar]
- 6.Edelman GM: Morphoregulatory molecules. Biochemistry 1988, 27:3533-3543 [DOI] [PubMed] [Google Scholar]
- 7.Edelman GM: Morphoregulation. Dev Dyn 1992, 193:2-10 [DOI] [PubMed] [Google Scholar]
- 8.Edelman GM, Crossin Kl: Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem 1991, 60:155-190 [DOI] [PubMed] [Google Scholar]
- 9.Takeichi M: The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 1988, 102:639-655 [DOI] [PubMed] [Google Scholar]
- 10.Takeichi M: Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 1990, 59:237-252 [DOI] [PubMed] [Google Scholar]
- 11.Yagi T, Takeichi M: Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev 2000, 14:1169-1180 [PubMed] [Google Scholar]
- 12.Yamada KM, Geiger B: Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol 1997, 9:76-85 [DOI] [PubMed] [Google Scholar]
- 13.Nagafuchi A, Takeichi M: Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J 1988, 7:3679-3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozawa M, Ringwald M, Kemler R: Uvomorulin-catenin complex formation is regulated by a specific domain of the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA 1990, 87:4246-4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadigan KM, Nusse R: Wnt signaling: a common theme in animal development. Genes Dev 1997, 11:286-305 [DOI] [PubMed] [Google Scholar]
- 16.Bienz TCF: Transcriptional activator or repressor? Curr Opin Cell Biol 1998, 10:366-372 [DOI] [PubMed] [Google Scholar]
- 17.Marrs JA, Nelson WJ: Cadherin cell adhesion molecules in differentiation and embryogenesis. Int Rev Cytol 1996, 165:159-205 [DOI] [PubMed] [Google Scholar]
- 18.Knudsen KA, Frankowski C, Johnson KR, Wheelock MJ: A role for cadherins in cellular signaling and differentiation. J Cell Biochem 1998, 31:S168-S176 [DOI] [PubMed] [Google Scholar]
- 19.Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84:345-357 [DOI] [PubMed] [Google Scholar]
- 20.Takeichi M: Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 1995, 7:619-627 [DOI] [PubMed] [Google Scholar]
- 21.Tepass U, Truong K, Godt D, Ikura M, Peifer M: Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 2000, 1:91-100 [DOI] [PubMed] [Google Scholar]
- 22.Burdsal CA, Damsky CH, Pedersen RA: The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development 1993, 118:829-844 [DOI] [PubMed] [Google Scholar]
- 23.Larue L, Ohsugi M, Hirchenhain J, Kemler R: E-cadherin null mutant embryos fail to form trophectoderm epithelium. Proc Natl Acad Sci USA 1994, 91:8263-8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Öbrink B: Cell-cell contacts mediated by E-cadherin (uvomorulin) restrict invasive behavior of L-cells. J Cell Biol 1991, 114:319-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W: E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Hofler H, Becker KF: Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 1999, 18:4301-4312 [DOI] [PubMed] [Google Scholar]
- 27.Vos CB, Cleton-Jansen AM, Berx G, de Leeuw WJ, ter Haar NT, van Roy F, Cornelisse CJ, Peterse JL, van de Vijver MJ: E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer 1997, 76:1131-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berx G, Nollet F, van Roy F: Dysregulation of the E-cadherin/catenin complex by irreversible mutations in human carcinomas. Cell Adhes Commun 1998, 6:171-184 [DOI] [PubMed] [Google Scholar]
- 29.Li LC, Zhao H, Nakajima K, Oh BR, Filho LA, Carroll P, Dahiya R: Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. J Urol 2001, 166:705-709 [PubMed] [Google Scholar]
- 30.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA: Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000, 148:779-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR: Expression of N-cadherin by human squamous carcinoma cell induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol 1996, 135:1643-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymann R, Kallenbach S, Alonso S, Carroll P, Mitsiadis TA: Dynamic expression patterns of the new protocadherin families CNRs and Pcdh-gamma during mouse odontogenesis: comparison with reelin expression. Mech Dev 2001, 106:181-184 [DOI] [PubMed] [Google Scholar]
- 33.Leonardi R: Spatio-temporal expression of E-cadherin during human odontogenesis. An immunohistochemical study. Minerva Stomatol 1999, 48:325-331 [PubMed] [Google Scholar]
- 34.Lüning C, Rass A, Rozell B, Wroblewski J, Öbrink B: Expression of E-cadherin during craniofacial development. J Craniofac Genet Dev Biol 1994, 14:207-216 [PubMed] [Google Scholar]
- 35.Obara N, Suzuki Y, Nagai Y, Takeda M: Expression of E- and P-cadherin during tooth morphogenesis and cytodifferentiation of ameloblasts. Anat Embryol 1998, 197:469-475 [DOI] [PubMed] [Google Scholar]
- 36.Obara N, Suzuki Y, Nagai Y, Takeda M: Immunofluorescence detection of cadherins in mouse tooth germs during root development. Arch Oral Biol 1999, 44:415-421 [DOI] [PubMed] [Google Scholar]
- 37.Palacios J, Benito J, Berraquero R, Pizzaro A, Cano A, Gamallo C: Differential spatiotemporal expression of E- and P-cadherin during mouse tooth development. Int J Dev Biol 1995, 39:663-666 [PubMed] [Google Scholar]
- 38.Sorkin BC, Wang MY, Dobeck JM, Albergo KL, Skobe Z: The cadherin-catenin complex is expressed alternately with the adenomatous polyposis coli protein during rat incisor amelogenesis. J Histochem Cytochem 2000, 48:397-406 [DOI] [PubMed] [Google Scholar]
- 39.Terling C, Heymann R, Rozell B, Öbrink B, Wroblewski J: Dynamic expression of E-cadherin in ameloblasts and cementoblasts in mice. Eur J Oral Sci 1998, 106(Suppl 1):S137-S142 [DOI] [PubMed] [Google Scholar]
- 40.Peyrièras N, Louvard D, Jacob F: Characterization of antigens recognized by monoclonal and polyclonal antibodies directed against uvomorulin. Proc Natl Acad Sci USA 1985, 82:8067-8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathman M, Lanerolle P, Ohayon H, Gounon P, Sansonetti P: Myosin light chain kinase plays an essential role in S. flexneri dissemination. J Cell Sci 2000, 113:3375-3386 [DOI] [PubMed] [Google Scholar]
- 42.About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA: Human dentin production in vitro. Exp Cell Res 2000, 258:33-41 [DOI] [PubMed] [Google Scholar]
- 43.About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA: Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol 2000, 157:287-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitsiadis TA, Muramatsu T, Muramatsu H, Thesleff I: Midkine (MK), a heparin-binding growth/differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J Cell Biol 1995, 129:267-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsiadis TA, Dicou E, Joffre A, Magloire H: Immunohistochemical localization of nerve growth factor (NGF) and NGF receptor (NGF-R) in the developing first molar tooth of the rat. Differentiation 1992, 49:47-61 [DOI] [PubMed] [Google Scholar]
- 46.About I, Camps J, Mitsiadis TA, Butler W, Franquin JC: Influence of resinous monomers on the differentiation in vitro of human pulp cells into odontoblasts. J Biomed Mater Res (in press) [DOI] [PubMed]
- 47.Hatta K, Takeichi M: Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 1986, 320:447-449 [DOI] [PubMed] [Google Scholar]
- 48.Fawcett DW: Bloom and Fawcett, a Textbook of Histology. 1994:pp 578-592 Chapman & Hall, New York
- 49.Holland GR: The odontoblast process: form and function. J Dent Res 1985, 64:499-514 [DOI] [PubMed] [Google Scholar]
- 50.Fried K, Mitsiadis TA, Guerrier A, Haegerstrand A, Meister B: Combinatorial expression patterns of the connexins 26, 32, and 43 during development, homeostasis, and regeneration of rat teeth. Int J Dev Biol 1996, 40:985-995 [PubMed] [Google Scholar]
- 51.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW: Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron 2000, 28:245-259 [DOI] [PubMed] [Google Scholar]
- 52.Letourneau PC, Roche FK, Shattuck TA, Lemmon V, Takeichi M: Interactions of Schwann cells with neurites and with other Schwann cells involve the calcium-dependent adhesion molecule, N-cadherin. J Neurobiol 1991, 22:707-720 [DOI] [PubMed] [Google Scholar]
- 53.Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V: Nerve growth cone migration onto Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol 1990, 138:430-442 [DOI] [PubMed] [Google Scholar]
- 54.Shibuya Y, Mizoguchi A, Takeichi M, Shimada K, Ide C: Localization of N-cadherin in the normal and regenerating nerve fibers of the chicken peripheral nervous system. Neuroscience 1995, 67:253-2617477906 [Google Scholar]
- 55.Hahn CG, Covault J: Neural regulation of N-cadherin gene expression in developing and adult skeletal muscle. J Neurosci 1992, 12:4677-4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H: Reactionary dentinogenesis. Int J Dev Biol 1995, 39:273-280 [PubMed] [Google Scholar]
- 57.Tziafas D, Smith AJ, Lesot H: Designing new treatment strategies in vital pulp therapy. J Dent 2000, 28:77-92 [DOI] [PubMed] [Google Scholar]
- 58.Blaschuk OW, Rowlands TM: Cadherins as modulators of angiogenesis and the structural integrity of blood vessels. Cancer Metastasis Rev 2000, 19:1-5 [DOI] [PubMed] [Google Scholar]
- 59.Navarro P, Ruco L, Dejana E: Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 1998, 140:1475-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B: Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci 1992, 102:7-17 [DOI] [PubMed] [Google Scholar]
- 61.Bobryshev YV, Cherian SM, Inder SJ, Lord RS: Neovascular expression of VE-cadherin in human atherosclerotic arteries and its relation to intimal inflammation. Cardiovasc Res 1999, 43:1003-1017 [DOI] [PubMed] [Google Scholar]
- 62.Nakashima M, Nagasawa H, Yamada Y, Reddi AH: Regulatory role of transforming growth factor-β, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins on differentiation of dental pulp cells. Dev Biol 1994, 162:18-28 [DOI] [PubMed] [Google Scholar]