Abstract
Under normoxic conditions, macrophages from C57BL mice produce low levels of vascular endothelial growth factor (VEGF). Hypoxia stimulates VEGF expression by ∼500%; interferon-γ (IFN-γ) with endotoxin [lipopolysaccharide (LPS)] also stimulates VEGF expression by ∼50 to 150% in an inducible nitric oxide synthase (iNOS)-dependent manner. Treatment of normoxic macrophages with 5′-N-ethyl-carboxamido-adenosine (NECA), a nonselective adenosine A2 receptor agonist, or with 2-[p-(2-carboxyethyl)-phenylethyl amino]-5′-N-ethyl-carboxamido-adenosine (CGS21680), a specific adenosine A2A receptor agonist, modestly increases VEGF expression, whereas 2-chloro-N6-cyclopentyl adenosine (CCPA), an adenosine A1 agonist, does not. Treatment with LPS (0 to 1000 ng/ml), or with IFN-γ (0 to 300 U/ml), does not affect VEGF expression. In the presence of LPS (EC50 < 10 ng/ml), but not of IFN-γ, both NECA and CGS21680 synergistically up-regulate VEGF expression by as much as 10-fold. This VEGF is biologically active in vivo in the rat corneal bioassay of angiogenesis. Inhibitors of iNOS do not affect this synergistic induction of VEGF, and macrophages from iNOS−/− mice produce similar levels of VEGF as wild-type mice, indicating that NO does not play a role in this induction. Under hypoxic conditions, VEGF expression is slightly increased by adenosine receptor agonists but adenosine A2 or A1 receptor antagonists 3,7-dimethyl-1-propargyl xanthine (DMPX), ZM241385, and 8-cyclopentyl-1,3-dipropylxanthine (DCPCX) do not modulate VEGF expression. VEGF expression is also not reduced in hypoxic macrophages from A3−/− and A2A−/− mice. Thus, VEGF expression by hypoxic macrophages does not seem to depend on endogenously released or exogenous adenosine. VEGF expression is strongly up-regulated by LPS/NECA in macrophages from A3−/− but not A2A−/− mice, confirming the role of adenosine A2A receptors in this pathway. LPS with NECA strongly up-regulates VEGF expression by macrophages from C3H/HeN mice (with intact Tlr4 receptors), but not by macrophages from C3H/HeJ mice (with mutated, functionally inactive Tlr4 receptors), implicating signaling through the Tlr4 pathway in this synergistic up-regulation. Finally, Western blot analysis of adenosine A2A receptor expression indicated that the synergistic interaction of LPS with A2A receptor agonists does not involve up-regulation of A2A receptors by LPS. These results indicate that in murine macrophages there is a novel pathway regulating VEGF production, that involves the synergistic interaction of adenosine A2A receptor agonists through A2A receptors with LPS through the Tlr4 pathway, resulting in the strong up-regulation of VEGF expression by macrophages in a hypoxia- and NO-independent manner.
During wound healing macrophages play a key role in the induction of new blood vessel formation by producing the angiogenic growth factor vascular endothelial cell growth factor (VEGF). 1-4 Although resting macrophages produce low levels of VEGF under normoxic conditions, hypoxia strongly stimulates VEGF production by macrophages. 1,5 Moreover, when normoxic macrophages are stimulated with interferon (IFN)-γ and lipopolysaccharide (LPS), they also up-regulate their expression of VEGF. 3 This up-regulation depends, in part, on the production of nitric oxide (NO) by the inducible NO synthase (iNOS) in activated macrophages.
In response to a variety of stimuli, including hypoxia, many different tissues and cell types release the purine nucleoside adenosine. 6-8 Adenosine, acting at both internal sites and external receptors, modulates a variety of macrophage functions. Acting at an intracellular site adenosine affects the production of NO. 9 Via occupancy of cell surface G-protein-coupled receptors adenosine modulates a variety of cellular functions, including phagocytosis; antigen presentation; target cell killing; production of IL-6, IL-10, and IL-12; and expression of MHC class II molecules. 10-15 Four different adenosine receptors, A1, A2A, A2B, and A3 receptors, have been characterized at the molecular level. 16-21 Ligation of adenosine receptors regulates cellular function via cAMP-dependent and -independent pathways. 22,23 A number of different highly receptor-specific agonists and antagonists of adenosine receptors have been developed that either mimic or block the effects of adenosine. 24-26 Adenosine mediates the anti-inflammatory effects of a number of currently available and experimental agents in the treatment of immune-mediated diseases such as rheumatoid arthritis and models of endotoxin shock, nephritis, and uveitis. 27-30 More recently adenosine A2A receptor-specific agonists have been used to diminish inflammation in animal models of inflammation. 31-33
Adenosine also plays a role in the promotion of angiogenesis. Adenosine applied to the chick chorioallantoic membrane induces growth of new microvascular blood vessels. 34 Regulation of expression of the angiogenic growth factor VEGF via adenosine receptors has been demonstrated in several cell types, including endothelial cells, smooth muscle cells, and the human U937 cell line. 35-41 As macrophages play a key role in inducing angiogenesis, we have studied the role of adenosine receptors in mediating the production of VEGF by primary macrophages. Our observations indicate that agonists of adenosine A2A receptors stimulate increased expression of VEGF by macrophages. Up-regulation of VEGF production by adenosine A2A receptor ligation is synergistically enhanced by exposure of macrophages to low levels of endotoxin (LPS). Adenosine A2A receptor/LPS-stimulated up-regulation of VEGF expression is at least as strong as that induced by hypoxia alone, and considerably stronger than that induced by IFN-γ with LPS. The synergistic up-regulation of VEGF expression is absent in macrophages from mice that lack the adenosine A2A but not the A3 receptor, confirming the specificity of this response. Similarly, the response is absent in macrophages from C3H/HeJ mice that lack functional Tlr4 receptors because of a mutation in their cytoplasmic domain, 42 indicating a critical role for the Tlr4 receptor in the signaling pathway.
Materials and Methods
Reagents
5′-N-ethylcarboxamidoadenosine (NECA), 2-[p-(2-carboxylethyl)-phenylethyl amino]-5′-N-ethyl-carboxamido-adenosine (CGS 21680), 2-chloro-N6-cyclopentyl adenosine (CCPA), 3,7-dimethyl-1-propargyl xanthine (DMPX), 8-cyclopentyl-1,3-dipropylxanthine (DCPCX), 8-sulfophenyltheophylline (8-SPT), U1026, SB203580, KT5720, recombinant murine interferon-γ (IFN-γ), LPS (Escherichia coli serotype 055:B5), aminoguanidine, and Ng-nitro-l-arginine-methyl-ester (L-NAME) were purchased from Sigma (St. Louis, MO). CMI (4-cyano-3-methyl-isoquinoline) was purchased from Calbiochem (San Diego, CA). ZM241385 was purchased from Tocris-Cookson (Bristol, UK). Polyclonal antibodies to adenosine A2A receptors were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Protease inhibitor cocktail for use with mammalian cell and tissue extracts, containing 4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin-A, E-64, bestatin, leupeptin, and aprotinin was purchased from Sigma. Hydron was purchased from Interferon Sciences (New Brunswick, NJ).
Animals
C57BL and C3H/HeJ mice (female, 7 to 12 weeks) were purchased from Jackson Laboratories (Bar Harbor, ME), C3H/HeN mice were purchased from Charles River (Wilmington, MA). Mice with a targeted disruption of the iNOS gene (iNOS−/− mice) were derived from an original homozygous breeding pair, and were kindly provided by Drs. John MacMicking and Dr. Carl Nathan (Cornell University Medical College, Ithaca, NY), and Dr. John Mudgett (Merck Research Laboratories, West Point, PA). These mice were derived from C57BL/6J × 129Sv/Ev lines originally generated at Merck, and were housed in the University of Medicine and Dentistry of New Jersey (UMDNJ) animal facility. Mice with a targeted disruption of the gene for the adenosine A2A and A3 receptor have been described in detail elsewhere. 43,44 The mice used in these experiments were derived from four original heterozygous breeding pairs for each mouse. Adenosine A2A receptor knockout mice (A2A−/−) and their wild-type controls (A2A+/+) were bred on a C57BL/6 × 129SvEv-STEEL background; adenosine A3 receptor knockout mice (A3−/−) and their wild-type controls were also bred on a C57BL background. These mice were housed in the New York University (NYU) animal facility. Mice described as wild type were specific for the related knockout mice. All mice were fed regular mouse chow and given access to drinking water ad libitum. All procedures described below were reviewed and approved by the Institutional Animal Care and Use Committee of NYU Medical Center and the UMDNJ and performed under the supervision of the facility veterinary staff.
Polymerase Chain Reaction (PCR) Confirmation of Mouse Genotype
DNA was extracted from the tips of mouse tails using a standard protocol. Briefly, tail tips were lysed in 500 μl of lysis buffer (100 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 8.0, 10 mmol/L ethylenediaminetetraacetic acid, 0.5% sodium dodecyl sulfate, 400 μg/ml proteinase K) overnight at 55°C. To the lysed tips 300 μl of a saturated solution of NaCl was added and, after 10 minutes on ice, tubes were centrifuged (16,000 × g, 4°C for 10 minutes). Genomic DNA present in the supernatant was precipitated by addition of 800 μl of isopropanol. Precipitates were washed once with 70% ethanol, vacuum dried, and resuspended in 30 μl of 10 mM Tris-1 mM EDTA (TE) buffer, pH 8.0. Using a method originally worked out by the laboratory of Dr. M. Sitkovsky (personal communication) the genomic DNA was then subjected to PCR using the following primers 5′-AGCCAGGGGTTACATCTGTG-3′ (upstream) and 5′-TACAGACAGCCTCGACATGTG-3′ (downstream), which detect a 163-bp band for the wild-type A2A allele; and 5′-AGACAATCGGCTGCTCTGAT-3′ (upstream) and 5′-CAAGCTCTTCAGCAATATCACG-3′ (downstream), which detect a 618-bp band for the mutated A2A allele; 5′-ACTTCTGGGCAGAAGTCTGACAAGA-3′ (upstream) and 5′-TTCGTCAACCCTGTTACCTGACTGT-3′ (downstream), which detect a 570-bp band for the wild-type A3 allele and 5′-ACTTCTGGGCAGAAGTCTGA CAAGA-3′ (upstream) and 5′-AGATCTATAGATCTCTCGTGGGATC-3′ (downstream), which detect a 260-bp band for the mutated A3 allele. To perform the PCR, 0.3 μg of genomic DNA was used in 30 μl of final reaction. The PCR was performed in a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer, Branchburg, NJ) under the following conditions: 95°C for 2 minutes followed by 40 cycles (94°C for 1 minute, 55°C for 20 seconds, and 72°C for 1 minute) and a final extension of 72°C for 10 minutes.
Preparation and Treatment of Peritoneal Macrophages
Mice were injected intraperitoneally with 2.5 ml of sterile Brewer’s thioglycolate broth (3% w/v) (Difco Laboratories, Detroit, MI). Four days later, the mice were sacrificed and peritoneal exudate cells were harvested using phosphate-buffered saline (PBS). Cells were centrifuged at 300 × g for 5 minutes at 4°C, washed twice with serum-free medium (RPMI), and resuspended in RPMI containing 10% fetal calf serum and 50 μg/ml of gentamicin (RPMI-10% fetal calf serum). Cells were seeded into Falcon multiwell six-well tissue culture plates (2 × 106 cells/well; Becton-Dickinson Labware, Franklin Lakes, NJ) in 2 ml of medium. Dishes were then incubated at 37°C in a humidified incubator in 95% air/5% CO2 overnight to allow the cells to adhere. Nonadherent cells were removed by washing with serum-free RPMI, and the cells were refed with RPMI containing 1% fetal calf serum (RPMI-1% fetal calf serum).
To test the effects of hypoxia, macrophages were incubated in 95% N2/4% CO2/1% O2 at 37°C. To test the effects of IFN-γ and LPS, macrophages were treated with IFN-γ alone (0 to 300 U/ml), LPS alone (0 to 1000 ng/ml), or a combination of IFN-γ (100 U/ml) and LPS (100 ng/ml), and incubated under normoxic conditions (95% air/5% CO2). The various test compounds (adenosine agonists and antagonists) were added to the macrophages within 30 minutes of the addition of IFN-γ and/or LPS. iNOS inhibitors were added before the addition of IFN-γ and/or LPS. Kinase inhibitors were added 2 hours before addition of IFN-γ and/or LPS. Conditioned media were harvested 24 hours after the initiation of treatments. Within each experiment, each test condition was assayed in duplicate. Each experiment was performed at least three times. Results of a typical experiment are shown in each figure.
Assay of VEGF Protein Levels by Enzyme-Linked Immunosorbent Assay (ELISA)
VEGF protein in conditioned media was assayed using a sandwich ELISA kit (Quantikine M; R&D Systems, Minneapolis, MN), following the manufacturer’s protocol. This assay detects murine VEGF in the range of 3 to 500 pg/ml. Samples with VEGF concentrations greater than this range were diluted with RPMI and re-assayed. All samples were assayed in duplicate. Results are presented as means ± SD.
Assay of Nitrite Production
To determine the production of NO by the macrophages under various conditions tested, the conditioned media were analyzed for nitrite using the Griess reaction, as described previously. 1 All samples were assayed in duplicate. Results are presented as means ± SD.
Assay of Macrophage Viability by the MTT Assay
To assess macrophage viability after incubation under the various test conditions, media were removed from each well and replaced with 1 ml of methyl thiazole tetrazolium (MTT) assay medium (RPMI-1640 without serum and phenol red, containing 50 μg/ml MTT). The plates were then incubated at 37°C for 2 hours. Medium was then removed and the cells were washed (two times) with PBS. One ml of cold 95% ethanol was then added, and the plates were incubated at 4°C for 5 minutes to allow the blue formazan product to dissolve. An aliquot (100 μl) of the solution from each well was then transferred to a 96-well plate, and absorption read at 560 nm with a 690-nm reference wavelength.
Isolation of Total Cellular RNA
Total cellular RNA was isolated from macrophages as described previously. 1 Medium was removed from the cells, and TRI reagent (Molecular Research Center, Inc., Cincinnati, OH) was added directly to the culture dishes. The cell lysate was passed several times through a 21-gauge syringe needle. Samples were stored at room temperature for 5 minutes, 0.2 ml of chloroform was then added per ml of lysis reagent, and the mixture was vortexed for 15 seconds and then incubated at room temperature for 10 minutes. The resultant mixture was centrifuged at 12,000 × g for 15 minutes at 4°C. The aqueous (upper) phase was transferred to a fresh microfuge tube, and RNA was precipitated by adding 0.5 ml of isopropanol per 1 ml of TRI reagent used for the original extraction. Samples were incubated at room temperature for 5 minutes and then centrifuged at 12,000 × g for 10 minutes at 4°C. The RNA pellets were washed with 75% ethanol, air-dried for 5 minutes, and dissolved in RNase-free water.
Quantitation of VEGF mRNA Steady-State Levels by Ribonuclease Protection Assay (RPA)
VEGF mRNA levels were determined by means of a RPA. A 360-bp fragment of the VEGF-coding sequence, between exon 1 and exon 4 (present in all isoforms of VEGF) was amplified by PCR, and subcloned into the PCRScript vector (Stratagene, La Jolla, CA) in an anti-sense orientation. The plasmid was linearized with EcoRI, and in vitro-transcribed with T3 RNA polymerase to obtain anti-sense RNA. The probe was labeled with α32P-UTP (specific activity, 3000 Ci/mmol; NEN Life Science Products, Boston, MA). Specific activity of the labeled probe was 7 × 108 cpm/μg. The probe was diluted to a concentration of 105 cpm/ml, and 1 ml was used for each sample in the RPA. The RPA procedure followed the manufacturer’s protocol (Ambion, Austin, TX). Twenty μg of total RNA were hybridized with the labeled probe at 68°C overnight. RNaseA/T1 cocktail was diluted 1:25 in RNase digestion buffer at 65°C for 30 minutes. Samples were then run on a 6% polyacrylamide denaturing gel (GelMix-6; Life Technologies, Inc., Rockville, MD) in 1× TBE buffer at 250 V for 1.5 hours. The gel was then dried, exposed in a PhosphorImager cassette and analyzed using a PhosphorImage Analyzer (Molecular Dynamics, Sunnyvale, CA). VEGF mRNA levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (G3PDH), as a control housekeeping gene.
RT-PCR Analysis of VEGF mRNA Isoforms
RT-PCR analysis of VEGF mRNA isoforms was performed as described previously. 1 Oligonucleotide primers in exons 3 and 8 were used to amplify the regions of the three differentially spliced VEGF mRNA isoforms formed from the VEGF gene. These VEGF mRNA isoforms are derived from a gene containing eight exons. The largest, VEGF-1, is formed using all eight exons. VEGF-2 lacks exon 7, and VEGF-3 lacks exons 6 and 7. Using PCR primers in exons 3 and 8, the three different isoforms of VEGF generate PCR amplification products of different sizes, and because they amplify from the same primers, the ratio of intensities of the three bands gives an estimate of the relative abundance of the three differentially spliced mRNA isoforms.
Analysis of Adenosine A2A Receptor Expression by Western Blotting
Western blot analyses were performed on cell membrane preparations after 24 hours of treatment of macrophages. Crude membrane proteins were isolated after sonication of cells as described previously. 45 Briefly, cells were washed (two times) with phosphate-buffered saline (PBS) at 4°C and suspended in 50 mmol/L of Tris/HCl, pH 7.4, containing 1% protease inhibitor cocktail (Sigma) and 1 mmol/L of ethylenediaminetetraacetic acid. The cells were sonicated (four times) on ice for 10 seconds each. The extract was then cleared by centrifugation at 300 × g for 10 minutes at 4°C. The supernatant containing crude membranes was centrifuged at 20,000 × g for 20 minutes at 4°C in polycarbonate tubes. The membrane pellet was resuspended in 50 μl of RIPA buffer containing 1% protease inhibitor cocktail, and stored at −20°C until analysis. Proteins (18 μg/lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electrophoretically transferred to nitrocellulose membranes. Nonspecific antibody binding to the membrane was blocked with 3% nonfat milk in PBS containing 0.3% TWEEN-20 (PBS-T). The membranes were then incubated overnight at 4°C in primary antibody (goat polyclonal antibody against adenosine A2A receptors). After several washes with PBS-T, the membranes were incubated for 1 hour at room temperature in alkaline phosphatase-conjugated secondary antibody (donkey anti-goat IgG). After several washes, the blots were exposed to a fluorescent-enhanced chemifluorescence substrate (Santa Cruz), and scanned using a FluorImage Analyzer (Molecular Dynamics, Sunnyvale, CA). Band densities were then directly quantitated using ImageQuant software (Molecular Dynamics).
Assay of Angiogenic Activity
Angiogenic activity of conditioned media from macrophage cultures was determined using the rat corneal bioassay of angiogenesis, as described previously. 1 Briefly, conditioned media were concentrated (20 times) using Amicon centrifugal spin filters (3-kd cutoff) (Amicon, Beverly, MA), and diafiltered. Aliquots of concentrated media were combined with an equal volume of slow-release Hydron (12% w/v in ethanol) and 10-ml droplets were allowed to dry and form pellets. These pellets were implanted aseptically in pockets within rat corneal stromas. Angiogenic responses were assessed 7 days after implantation. Four corneal implants were assessed per test sample, and the responses graded as previously described. 1 To determine the contribution of VEGF to the angiogenic activity, an affinity-purified neutralizing polyclonal antibody to VEGF (gift of Dr. Napoleone Ferrara, Genentech, Inc., South San Francisco, CA) was used, as previously described. 1
Statistics
Data are presented as means ± SD. Statistical analysis of results was performed using the unpaired Student’s t-test. Differences with a P value of <0.05 were considered significant.
Results
Effects of Adenosine Receptor Agonists on VEGF Production by Murine Peritoneal Macrophages
We examined the effects of a nonselective and an A2A-selective adenosine receptor agonist on VEGF production by murine peritoneal macrophages under various conditions in vitro. Macrophages were incubated with agonists, either under normoxic conditions (95% air/5% CO2), under hypoxic conditions (94% N2/5% CO2/1% O2), or under normoxic conditions after activation with either IFN-γ alone (0 to 300 U/ml), LPS alone (0 to 1000 ng/ml), or a combination of IFN-γ (100 U/ml) and LPS (100 ng/ml).
Normoxia
Under normoxic conditions, macrophages produced low levels of VEGF. The nonselective adenosine receptor agonist NECA and the adenosine A2A-specific agonist CGS21680 induced minor increases in VEGF production in a dose-dependent manner, with a maximal increase to 50 to 80% above baseline (Figure 1A) ▶ . The potency of the agonists used was consistent with that expected for an adenosine A2A receptor-mediated phenomenon; the EC50 for NECA was <100 nmol/L, and for CGS21680 the EC50 was <10 nmol/L, and CCPA, a selective adenosine A1 receptor agonist, had no effect on VEGF production. 8-SPT and theophylline, poorly selective adenosine receptor antagonists, also had little effect on VEGF production (Figure 1A) ▶ .
Figure 1.
Effect of adenosine receptor agonists on VEGF production by murine peritoneal macrophages. Cells were incubated under conditions of normoxia (A), hypoxia (1% O2) (B), or normoxia and treated with IFN-γ (100 U/ml) and LPS (100 ng/ml) (C). Conditioned media were harvested 24 hours after treatment, and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown, and are presented as means ± SD. *, Values that are significantly different from control (P < 0.05).
Hypoxia
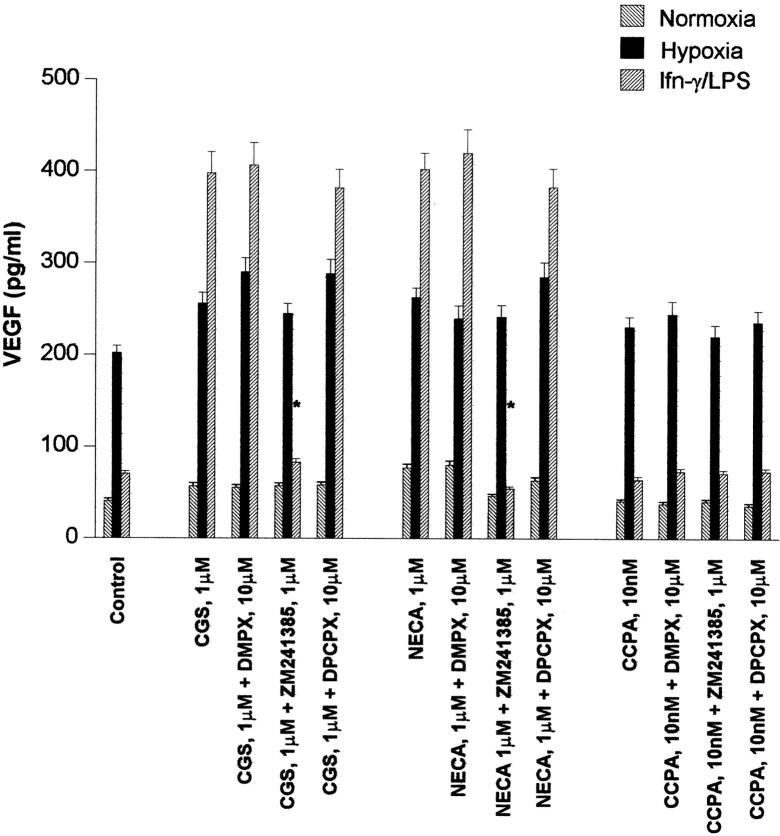
Under hypoxic conditions, VEGF production increased to ∼500% of that produced by normoxic macrophages (Figure 1B) ▶ . CGS21680 and NECA further increased the production of VEGF under hypoxic conditions although the increase was modest (∼25% above baseline, Figure 1B ▶ ). The adenosine A2 antagonist DMPX, the A2A-specific antagonist ZM241384, and the A1 antagonist DPCPX did not significantly affect VEGF production induced by hypoxia (Figure 2) ▶ , a finding that is not consistent with a role for endogenously-generated adenosine in the regulation of VEGF expression by hypoxic macrophages.
Figure 2.
Effect of adenosine receptor antagonists on VEGF production by macrophages. Cells were incubated under normoxic or hypoxic conditions, and under normoxic conditions in the presence of IFN-γ and LPS. Macrophages were treated with CGS21680, NECA, or CCPA, and the adenosine receptor antagonists DMPX, ZM241384, or DPCPX were added to the cultures at the indicated concentrations. Conditioned media were harvested 24 hours after addition of test compounds, and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown, and are presented as means ± SD. *, ZM241385 significantly reduced the synergistic stimulatory effects of CGS and NECA with IFN-γ and LPS on VEGF production (P < 0.001). DMPX and DPCPX did not significantly reduce VEGF production.
IFN-γ and LPS
Neither IFN-γ (100 μ/ml) nor LPS (100 ng/ml) alone stimulated increased VEGF production. However, treatment with IFN-γ in combination with LPS (IFN-γ/LPS) stimulated VEGF production by macrophages to 50 to 150% of control, as we have previously reported. 1 The effect of IFN-γ/LPS on VEGF production is considerably weaker than that of hypoxia (500% greater than normoxic cells). Surprisingly, treatment of IFN-γ/LPS-activated macrophages with adenosine analogs profoundly increased their production of VEGF (Figure 1C) ▶ , increasing VEGF production by an additional 500 to 600% (Figure 1C) ▶ . DPCPX, an A1 receptor antagonist, did not antagonize the action of CGS21680 or NECA in stimulating VEGF production (Figure 2) ▶ . DMPX, a nonspecific A2 receptor antagonist had little effect, but ZM241385, a selective A2A antagonist, strongly inhibited the effects of LPS/NECA and LPS/CGS on VEGF production. Alloxazine, a selective A2B antagonist, had no effect on VEGF production.
The effects of adenosine receptor occupancy on macrophage VEGF production were similar in the presence of either LPS alone or LPS plus IFN-γ, but not in the presence of IFN-γ alone (Figure 3) ▶ . Treatment with IFN-γ alone (0 to 300 U/ml) did not increase VEGF expression and adenosine agonists did not increase VEGF production in the presence of IFN-γ. In contrast, treatment with LPS alone (0 to 1000 ng/ml) had little effect on VEGF expression; however treatment with LPS plus either NECA or CGS21680 strongly increased VEGF expression. This effect was apparent at LPS concentrations as low as 10 ng/ml, and was maximal at 50 to 100 ng/ml (EC50 < 10 ng/ml) (Figure 3) ▶ .
Figure 3.
Effects of LPS and IFN-γ on VEGF production by murine peritoneal macrophages. Cells were incubated with the indicated concentrations of either LPS alone or IFN-γ alone, in the presence or absence of NECA (1 μmol/L). Media were harvested after 24 hours and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown and are presented as means ± SD. *, Values that are significantly different from control (P < 0.05).
Role of NO in the Up-Regulation of VEGF by LPS and Adenosine A2A Agonists
Effect of iNOS Inhibitors on VEGF Production
To determine whether up-regulation of VEGF by the combination of LPS and adenosine A2A agonists depended on expression of NO by iNOS, macrophages from wild-type (iNOS+/+) mice were incubated with LPS (100 ng/ml) plus NECA or CGS21680, in the presence of either L-NAME (1.5 mmol/L) or aminoguanidine (1 mmol/L), agents that strongly inhibit NO production. L-NAME and aminoguanidine reduced VEGF production by IFN-γ/LPS-treated macrophages by at least 80%. However, no effect of these inhibitors was observed on the production of VEGF by LPS/NECA-treated macrophages (Figure 4) ▶ .
Figure 4.
Effects of NO on VEGF production by murine peritoneal macrophages. Macrophages were prepared from either normal (iNOS+/+) mice or mice with a targeted disruption of the iNOS gene (iNOS−/−). Macrophages were incubated under normoxic conditions either with or without IFN-γ and LPS, and stimulated with CGS21680 (1 μmol/L). The iNOS inhibitors AG or L-NAME were added to the cultures at the indicated concentrations. Media were harvested after 24 hours, and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown and are presented as means ± SD. *, Values that are significantly different from control (P < 0.05).
VEGF Production by Macrophages from iNOS−/− Mice
Macrophages from iNOS−/− mice did not show significant up-regulation of VEGF production after treatment with IFN-γ/LPS, in contrast to wild-type (iNOS+/+) mice, which showed an ∼80% increase (Figure 4) ▶ . On the other hand, macrophages from iNOS−/− mice showed strong up-regulation of VEGF expression after NECA/LPS treatment, comparable to that observed in iNOS+/+ macrophages. These results clearly indicate that while the IFN-γ/LPS-induced up-regulation of VEGF expression is NO-dependent, the LPS/adenosine A2A receptor agonist-mediated up-regulation of VEGF expression is independent of iNOS and NO production.
Effect of Specific Kinase Inhibitors on VEGF Production
Previous results have indicated that adenosine A2A receptor occupancy modulates cellular function via either adenylate cyclase/protein kinase A-dependent pathways or stimulation of MAP kinase activation. To determine the signal transduction pathways involved in the LPS/A2A agonist-mediated pathway VEGF up-regulation, U1026 (a MAP ERK kinase inhibitor), SB203580 (a p38 MAP kinase inhibitor), CMI, and KT5720 (protein kinase A inhibitors) were tested. The inhibitors were added to the macrophages 2 hours before stimulation with LPS and either NECA or CGS21680. None of these inhibitors significantly affected the production of VEGF by the macrophages (Figure 5) ▶ . Inhibitors alone, in the absence of LPS and/or A2A agonists, did not modulate VEGF production in the nonstimulated cells. These results indicate that other signal transduction pathways, yet to be determined, must be involved in the up-regulation of VEGF expression in this system.
Figure 5.
Effects of specific kinase inhibitors on VEGF production by murine peritoneal macrophages. Macrophages were incubated under normoxic conditions either in the presence or absence of IFN-γ and LPS, and stimulated with either CGS21680 (1 μmol/L) or NECA (1 μmol/L). The cells were treated with U1026 (a MAP-ERK kinase inhibitor), SB203580 (a p38 MAP kinase inhibitor), CMI, or KT5720 (protein kinase A inhibitors), as indicated. Media were harvested after 24 hours and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown, and are presented as means ± SD. The U1026-, SB203580-, CMI-, and KT5720-treated cells did not show a significant change in VEGF production in relation to the IFN-γ- and LPS-treated cells in either the CGS- or NECA-treated groups.
VEGF Production By Macrophages from Adenosine Receptor-Deficient Mice
The pharmacological studies with adenosine receptor agonists presented above are most consistent with the hypothesis that up-regulation of VEGF expression was mediated by signaling through the adenosine A2A receptor. To test this hypothesis further, we examined production of VEGF by macrophages from adenosine receptor A2A and A3 knockout mice (A2A−/− and A3−/− mice). VEGF expression by normoxic and hypoxic macrophages from A2A−/− and A3−/− mice was similar to that observed in their wild-type littermate controls (Figure 6, A and B) ▶ . These results clearly demonstrate that the adenosine A2A and A3 receptors are not involved in the hypoxic up-regulation of VEGF expression in these cells. The adenosine receptor agonist NECA in combination with IFN-γ/LPS did not up-regulate VEGF expression in macrophages from A2A−/− mice beyond that induced by IFN-γ/LPS-treatment alone (Figure 6A) ▶ . In contrast, macrophages from A3−/− mice responded to IFN-γ/LPS and NECA in the same way as wild-type mice (Figure 6B) ▶ . These results provide strong evidence that adenosine A2A receptor occupancy mediates the increase in VEGF production by IFN-γ/LPS- or LPS-stimulated macrophages.
Figure 6.

VEGF production by macrophages from normal mice versus mice with targeted disruptions in the genes for the adenosine A2A or the adenosine A3 receptors. Media were harvested after 24 hours. At least two replicates were studied for each condition, and each sample was analyzed in duplicate for VEGF by ELISA. Results of a typical experiment are shown, and are presented as means ± SD. A: Macrophages from control (A2A+/+) and A2A receptor knockout (A2A−/−) mice were incubated under conditions of normoxia, hypoxia, or normoxia in the presence of IFN-γ and LPS. The cells were then treated with NECA (1 μmol/L) or CGS21680 (1 μmol/L). B: Macrophages from control (A3+/+) and A3 receptor knockout (A3−/−) mice were treated as in A.
VEGF Production by Macrophages from C3H/HeJ Mice that Lack Functional Tlr4 Receptors
LPS signaling in macrophages in terms of the induction of inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 is mediated primarily through the CD14-Tlr4 receptor signaling pathway. 42 However, other pathways mediated by β2 integrins, 46 scavenger receptors, 47 and l-selectin 48 have also been implicated in mediating some of the effects of LPS on macrophages. To determine the role of the Tlr4 receptor-mediated pathway in the synergistic interaction between LPS and adenosine A2A-receptor agonists, we studied macrophages from C3H/HeJ mice. C3H/HeJ mice have a mutation in the cytoplasmic domain of Tlr4 42 that functionally inactivates signaling by this receptor, resulting in a dominant-negative phenotype. C3H/HeN mice contain the normal, nonmutated, functional Tlr4 receptor, and were used as controls. VEGF production by hypoxia was strongly induced in macrophages from both C3H/HeN and C3H/HeJ mice. Expression of VEGF by macrophages from C3H/HeN mice was strongly up-regulated by NECA in combination with LPS (Figure 7) ▶ . In contrast, macrophages from C3H/HeJ mice did not respond to LPS and NECA beyond the response to NECA alone. This result indicates clearly that the role of LPS in the synergistic induction of VEGF with adenosine A2A agonists is mediated through the Tlr4 signaling pathway. It is of interest to note that the response of macrophages from C3H/HeN mice to NECA was considerably stronger than that of macrophages from C57BL mice (Figure 7 ▶ versus Figure 1 ▶ ). Similarly, the C3H/HeN response was much stronger than that observed in the iNOS+/+ and iNOS−/− mice (Figure 4) ▶ , which are also bred on a C57BL background. The A2A+/+ and A2A−/− mice, and the A3+/+ and A3−/− mice are also bred on a C57BL background, and responded to NECA to the same extent as did C57BL mice (Figure 6) ▶ .
Figure 7.
VEGF production by macrophages from C3H/HeN mice (intact, functional Tlr4 receptor) versus macrophages from C3H/HeJ mice (mutated, nonfunctional Tlr4 receptor). Cells were incubated under either normoxic or hypoxic conditions. Cells were treated with LPS, NECA, NECA plus LPS, or LPS plus IFN-γ, at the indicated concentrations. Media were harvested after 24 hours, and assayed for VEGF by ELISA. At least two replicates were studied for each condition, and each sample was analyzed for VEGF in duplicate. Results of a typical experiment are shown, and are presented as means ± SD.
Effects of Adenosine A2A Receptor Agonists and LPS on VEGF mRNA Isoforms
Three isoforms of VEGF were produced by both nonactivated, IFN-γ/LPS-activated macrophages, and NECA/LPS and CGS/LPS-activated macrophages. These isoforms corresponded to VEGF-1 (652 bp), VEGF-2 (580 bp), and VEGF-3 (448 bp). Little change in the relative proportions of these isoforms was seen after activation (data not shown).
Effects of Adenosine A2A Receptor Agonists and LPS on VEGF mRNA Steady-State Levels
VEGF steady-state mRNA levels were determined using an RPA with a 360-bp probe that recognizes all isoforms of murine VEGF. A typical example of this RPA is shown in Figure 8 ▶ . LPS alone did not increase VEGF mRNA levels. NECA/LPS, on the other hand, induced a strong increase in VEGF steady-state mRNA levels. This increase was apparent by 4 hours, and peaked at 6 to 10 hours. By 24 hours, VEGF steady-state mRNA decreased to close to baseline levels.
Figure 8.

Ribonuclease protection assay (RPA) of VEGF mRNA levels in normoxic control (C), LPS-treated (100 ng/ml) (L), and LPS (100 ng/ml)- plus NECA (1 μg/ml)-treated (L/N) murine peritoneal macrophages. Macrophages were harvested 4, 6, and 10 hours after stimulation. RNA was isolated and RPA was performed as described in Materials and Methods. The probe used to detect VEGF mRNA is a 360-bp anti-sense probe spanning exons 1 to 4, and detects all known isoforms of murine VEGF. Similar results were found in three separate experiments.
Analysis of Adenosine A2A Receptor Expression by Western Blotting
To determine whether treatment of macrophages with LPS up-regulates the expression of adenosine A2A receptors in macrophages, the level of expression of A2A receptors was determined by Western blot analysis. Crude membrane fractions were prepared from untreated macrophages, and from macrophages after overnight treatment with either LPS alone (100 ng/ml), NECA (1 μmol/L), or LPS (100 ng/ml) plus NECA (1 μmol/L). Equal amounts of protein from the crude membrane preparations were electrophoresed in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose membranes, and the adenosine A2A receptors were localized and quantified using a specific polyclonal antibody to adenosine A2A receptors. A typical Western blot showing the results of this analysis is shown in Figure 9 ▶ . The adenosine A2A receptor localized as a single band with an apparent molecular size of 44 kd. Expression of A2A receptors was virtually identical in untreated and LPS-treated macrophages. These results indicate that the synergistic up-regulation of VEGF expression by LPS and adenosine A2A receptor agonists is not simply because of the up-regulation of expression of adenosine A2A receptors by LPS.
Figure 9.
Western blot analysis of adenosine A2A receptor expression. Crude membrane proteins were isolated from untreated and treated macrophages, as described in Materials and Methods, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were electrophoretically transferred to nitrocellulose membranes and A2A receptor expression was assessed using a polyclonal antibody against A2A receptors. A: Representative Western blot for adenosine A2A receptor protein. C, Untreated cells; L, LPS (100 ng/ml)-treated cells. B: Quantitation of protein expression by densitometric analysis of Western blots. Data are expressed as mean percentage of control ± SD (n = 3). *, P < 0.05.
Angiogenic Activity of Macrophage-Conditioned Media
To determine whether the VEGF produced by macrophages treated with NECA (1 μmol/L), LPS (100 ng/ml), or LPS (100 ng/ml) plus NECA (1 μmol/L) is biologically active, conditioned media from the macrophage cultures were concentrated (20 times), diafiltered, and assayed for angiogenic activity using the rat corneal bioassay. Results are presented in Table 1 ▶ . Medium from untreated macrophages did not induce angiogenesis. Medium from LPS-treated macrophages also failed to induce an angiogenic response. Medium from NECA-treated macrophages induced a mild angiogenic response, whereas medium from LPS/NECA-treated macrophages induced a strong angiogenic response. In both NECA-treated and LPS-NECA-treated macrophage-conditioned media, a neutralizing antibody to murine VEGF abrogated the angiogenic activity.
Table 1.
Angiogenic Responses Induced in Rat Corneas by Conditioned Media from Macrophages Cultured under Various Conditions in Vitro
| Group | Macrophage culture conditions* | Angiogenic score† |
|---|---|---|
| 1 | Normoxia | 1 |
| 2 | Hypoxia | 9 |
| 3 | NECA (1 μmol/L) alone | 5 |
| 4 | LPS (100 ng/ml) alone | 1 |
| 5 | NECA (1 μmol/L) + LPS (100 ng/ml) | 12 |
| 6 | rVEGF165 (20 ng) | 11 |
| 7 | Group 5+ anti-VEGF Ab (10 μg/ml) | 2 |
| 8 | Group 6+ anti-VEGF Ab (10 μg/ml) | 2 |
*Macrophages were incubated for 24 hours under the indicated conditions, concentrated (20×) and diafiltered using Centricon 3 (3000 Mr cutoff) filters (Amicon). Samples were then combined with equal volumes of Hydron (Interferon Sciences Inc.). Ten μl droplets were then allowed to dry on the cut ends of 2-mm diameter Teflon rods. These pellets were then implanted aseptically in the corneas of rats.
†Angiogenic responses were evaluated 7 days after implantation, and assessed on a graded scale. 0, No response, or slight budding of the limbal vasculature that regresses rapidly; 1, formation of a few capillary buds and sprouts that progress less than 0.2 mm from the limbus and start to regress; 2, persistent growth of a network of capillary buds and sprouts that grow at least 1 mm toward the implant, but do not reach and invade the implant; 3, strong growth of a network of capillary buds and sprouts that reaches and surrounds the implant. The angiogenic score represents the sum of the graded angiogenic responses from four individual corneas for each test sample. A maximal response would score 12, a minimal response 0.
Discussion
Vascular endothelial growth factor (VEGF) is an endothelial cell mitogen and permeability factor that plays an important role in the regulation of angiogenesis in embryonic development, wound healing, inflammatory fibrosis, and the growth of solid tumors. 49-55 VEGF is produced by several different cell types, and its expression is regulated by hypoxia via the expression of an oxygen-regulated transcription factor, HIF-1α. 56-58 We have shown previously that macrophages produce VEGF and that expression of VEGF by macrophages is strongly regulated by hypoxia. 1 VEGF expression is also regulated in macrophages by an iNOS-dependent pathway after activation by IFN-γ and LPS. NO-dependent regulation of VEGF expression has also been demonstrated in other cell types. 59-61 In macrophages, iNOS-dependent up-regulation of VEGF production is weaker than that observed with hypoxia. It has been suggested that this iNOS-dependent pathway is mediated at least in part through the hypoxia response element in the VEGF upstream promoter region that responds to the HIF-1 transcriptional regulator. 60
The purine nucleoside adenosine is produced and released into the extracellular milieu by cells under normoxic conditions, and its production is markedly increased under stress conditions such as hypoxia and ischemia, as a result of increased ATP turnover. 6,7 Adenosine, acting at its receptors, is a potent immunomodulator, exerting effects both intracellularly (after uptake via specific transporters) and extracellularly via interaction with four different types of G-protein-coupled cell surface receptors, termed the A1, A2A, A2B, and A3 adenosine receptors. Adenosine up-regulates IL-10 and down-regulates IL-12 production in murine and human macrophages through the A2A receptor. 15 Adenosine also regulates TNF-α, IL-8, NO, free radical and arachidonic acid metabolite production in macrophages and human monocytes, 9-15 and IL-6 expression in epithelial cells. 62 In addition to its anti-inflammatory effects adenosine A2A receptor ligation promotes more rapid wound closure in murine models. 63 Promotion of VEGF release by adenosine may play an important role in the promotion of wound healing as well as the anti-inflammatory effects of A2A receptor agonists.
Previous studies have also implicated adenosine receptors in the regulation of VEGF expression in various cell types. 35,36,39-41,64,65 In PC12 pheochromocytoma cells, the adenosine A2A receptor agonist CGS21680 markedly depressed VEGF expression. 64 In bovine microvascular retinal endothelial cells, on the other hand, adenosine induced VEGF expression through the adenosine A2A receptor, and activation of protein kinase-A was implicated in this induction. 39 In porcine brain-derived microvascular endothelial cells, the adenosine receptor antagonist 8-phenyl theophylline (8-PT) reduced the hypoxia-induced expression of VEGF by PK-C-dependent and -independent pathways. 35,40 In bovine retinal pericytes and endothelial cells, adenosine induced VEGF expression via A2A receptors through a PK-A-dependent pathway. In rat aortic smooth muscle cells, adenosine up-regulated VEGF expression 2.2-fold, and this up-regulation was independent of hypoxia-induced up-regulation of VEGF. 37 In canine myocardial vascular smooth muscle cells, adenosine A2 agonists also stimulated VEGF protein levels by 51 to 132% and VEGF mRNA levels by 44 to 90%. 36 In a human macrophage cell line (U937 cells), adenosine A2 receptor antagonists inhibited hypoxic induction of VEGF mRNA, suggesting that in these cells hypoxic induction of VEGF mRNA is mediated, at least in part, by endogenously released adenosine. 41 Consistent with the capacity of A2A receptor occupancy to promote VEGF expression, NECA also up-regulated VEGF mRNA in U937 cells. Interestingly, in U937 cells both hypoxia and adenosine increased VEGF mRNA expression posttranscriptionally by enhancing the stability of VEGF mRNA. Thus in most cell types studied, adenosine, acting usually at A2A receptors, promotes VEGF production and may play a role in hypoxia-induced VEGF release.
Normoxic macrophages treated with the adenosine A2A-specific agonist CGS21680, showed a marked increase (50 to 150%) in their expression of VEGF. NECA, a nonselective adenosine A2 receptor agonist, also stimulated VEGF production of normoxic macrophages. The adenosine A1 receptor antagonist DPCPX had no effects on this VEGF production. The nonspecific adenosine A2 receptor antagonist DMPX also had little effect, but the adenosine A2A-specific antagonist ZM241385 strongly inhibited VEGF production. Alloxazine, an A2B-specific antagonist also had no effect on VEGF production (data not shown). The concentrations of adenosine receptor agonists and antagonists required to stimulate or block VEGF production, as well as their relative potencies, were most consistent with occupancy of an A2A receptor. This was clearly confirmed by the observation that the adenosine receptor agonists did not stimulate any increase in VEGF expression in A2A receptor knockout mice, but stimulated a marked increase in VEGF expression in the wild-type littermate controls and the A3 receptor-defective mice.
Whereas CGS21680 and NECA stimulated VEGF production in normoxic macrophages, this effect was greatly enhanced in the presence of concentrations of LPS that alone, did not stimulate any VEGF production. In the presence of LPS (EC50 < 10 ng/ml) the adenosine A2A receptor agonists increased VEGF production by as much as 12-fold more than that produced by nontreated macrophages. Interestingly, IFN-γ played no role in stimulating this increased VEGF production, as demonstrated by the observation that treatment of macrophages with IFN-γ alone (100 to 300 U/ml), either with or without adenosine A2A agonists had no effect on VEGF production beyond that of the agonists alone. We have recently observed that TNF-α and IL-1, agents that like LPS, provoke translocation of nuclear factor-κB to the nucleus, increase expression and function of A2A receptors, but IFN-γ diminishes both expression and function of the A2A receptors. In other studies IFN-γ has been shown to up-regulate the expression of the adenosine A2B receptor on macrophages, and this has been proposed as a mechanism for macrophage deactivation. 11 In our experiments, the up-regulation of VEGF expression seems to be mediated through the A2A receptor rather than the A2B receptor, and this effect is independent of IFN-γ. When IFN-γ (100 U/ml) was used together with LPS (100 ng/ml), in the presence of NECA or CGS21680, the up-regulation of VEGF expression was equivalent to that observed in cells treated with LPS alone. Also, increasing concentrations of IFN-γ (up to 300 U/ml) did not block the stimulatory effects of LPS together with NECA or CGS (data not shown). Thus, the enhancement of A2A receptor expression and function stimulated by LPS is greater than any diminution induced by IFN-γ.
VEGF steady-state mRNA levels increased several fold 4 to 10 hours after treatment of macrophages with LPS (100 ng/ml) together with NECA (1 μmol/L) in comparison to cells treated with LPS alone (Figure 8) ▶ . This increase is comparable to or more than that observed after hypoxic incubation of macrophages, which is mediated through the HIF-1 transcription factor. However, we do not yet know if this increase is mediated transcriptionally or posttranscriptionally. Preliminary results using a VEGF promoter construct regulating the expression of luciferase in an expression plasmid transfected into RAW264.7 cells (a macrophage cell line), indicate that whereas hypoxia and IFN-γ/LPS strongly up-regulate luciferase expression under the control of the VEGF promoter, LPS/NECA does not (data not shown). This result suggests that VEGF expression is regulated at least in part by posttranscriptional up-regulation.
Our results also suggest that the adenosine A2A-mediated pathway does not play a significant role in the induction of VEGF by hypoxia and that the hypoxia-mediated and the LPS/A2A receptor-mediated pathways of induction of VEGF are independently regulated. Hypoxia induced a >500% increase in VEGF expression, and the adenosine receptor antagonists DPCPX, DMPX, alloxazine, and ZM241385 did not significantly reduce this induction. Also, in both A2A−/− and A3−/− mice, hypoxic up-regulation of VEGF expression was the same as that in control, wild-type mice, indicating that neither of these receptors plays a role in the hypoxic induction of VEGF expression. It will be of interest to determine whether the adenosine A2A receptor/LPS-mediated pathway of VEGF induction also involves the hypoxia response element and HIF1, or whether this is an independent regulatory mechanism controlling VEGF expression. The induction of VEGF by IFN-γ together with LPS that we have reported previously was found to require at least in part, an iNOS-dependent pathway. 1 This was confirmed in the current experiments using macrophages from mice with a targeted disruption of the iNOS gene (iNOS−/−). In these macrophages, IFN-γ/LPS does not induce VEGF expression. In contrast, LPS together with adenosine A2A receptor agonists strongly induced VEGF expression in macrophages from iNOS−/− mice, to the same extent as that observed in macrophages from wild-type (iNOS+/+) mice. Similarly, inhibitors of iNOS (L-NAME and aminoguanidine) had no effect on the LPS/NECA-induced VEGF production in macrophages from iNOS+/+ mice, confirming the lack of dependence of this induction on iNOS and NO.
Induction of cytokines such as TNF-α and IL-1 by E. coli LPS is mediated through the CD-14-Tlr4 receptor-signaling pathway. 42 However, LPS signaling through β2 integrins, 46 scavenger receptors, 47 and L-selectin 48 has also been demonstrated. In this study, we used C3H/HeJ mice that lack a functional Tlr4 receptor to study the role of Tlr4 in the synergistic up-regulation of VEGF expression by LPS and adenosine A2A receptor agonists. In C3H/HeJ mice, NECA/LPS did not induce VEGF expression beyond that induced by NECA alone, whereas in C3H/HeN mice (with intact Tlr4 receptors), strong up-regulation of VEGF was observed. This clearly indicates that E. coli LPS signals through the Tlr4 pathway in this synergistic up-regulation. It will clearly be of interest to determine whether agonists of other Tlr receptors can also synergize with adenosine A2A receptor agonists to up-regulate specific gene expression in macrophages or other cell types. Interestingly, macrophages from C3H/HeN mice show a stronger response to adenosine A2A receptor agonists than do macrophages from C57BL mice or those bred on a C57BL background. This suggests that there are differences in the sensitivity of macrophages from different mouse strains to adenosine A2A agonists. The basis for this differential sensitivity is as yet unclear.
The signal transduction pathways involved in the synergistic up-regulation of VEGF expression by LPS together with A2A receptor agonists are not yet elucidated. We have investigated whether treatment of macrophages with LPS might up-regulate expression of adenosine A2A receptors, thus synergistically increasing the response of the cells to adenosine A2A receptor agonists. Western blot analysis indicated that this is not the case. The level of expression of adenosine A2A receptor protein was found to be similar in untreated and in LPS-treated macrophages (Figure 9) ▶ , suggesting that mechanisms other than the regulation of the level of receptor expression are involved in this synergistic interaction. In terms of signal transduction pathways involved in the downstream signaling, initial experiments indicate that U0126, a specific inhibitor of MEK1 and MEK2 (MAP kinase kinase), and SB203580, a p38 MAP kinase inhibitor, had no effect on VEGF production in this system. In addition 4-cyano-3-methyl-isoquinoline and KT5720, both specific inhibitors of PK-A, also had no effect on VEGF production, suggesting that the pathway of VEGF induction is PK-A-independent. Further experiments are required to elucidate the signal transduction pathways involved in this induction of VEGF expression.
The regulation of macrophage VEGF expression through the LPS/adenosine A2A receptor-mediated pathway may be an example of an alternative pathway of macrophage activation, different from the classic IFN-γ/LPS-mediated pathway, 66 and may play a key role in the local, microenvironmental regulation of macrophage phenotype. As reviewed by Gordon 67 recently, activation of macrophages with IFN-γ/LPS results in an inflammatory, cytotoxic phenotype, with the up-regulation of expression of reactive oxygen species, IL-1, TNF-α, and iNOS. In contrast, the adenosine A2A receptor-mediated activation results in an angiogenic, healing phenotype, with down-regulation of IL-12, TNF-α, iNOS, and reactive oxygen species, and the up-regulation of VEGF expression. This pathway is strongly enhanced by synergy with LPS. It will be interesting to determine what other factors are regulated through this LPS/adenosine receptor-mediated pathway, and the relationship of these factors to the angiogenic, healing phenotype apparent in these experiments.
In summary, we have identified a novel pathway of transcriptional up-regulation of VEGF expression in macrophages. This pathway involves a synergistic interaction between signaling through the adenosine A2A receptor and LPS. Macrophages from mice lacking the adenosine A2A receptor do not respond to LPS with adenosine receptor agonists with increased VEGF production, clearly indicating the critical role of the A2A receptor in this pathway. In addition, macrophages from C3H/HeJ mice, which lack functional Tlr4 receptors, do not respond, indicating a critical role for the Tlr4 receptor in this pathway. This pathway is independent of hypoxia and NO production. We have previously demonstrated that application of topical adenosine A2A receptor agonists promotes wound healing, 63 and the observation that occupancy of adenosine A2A receptors stimulates VEGF production synergistically with LPS through the Tlr4 signaling pathway may be critical for this response.
Footnotes
Address reprint requests to Samuel Joseph Leibovich, Ph.D., Dept. of Cell Biology and Molecular Medicine, New Jersey Medical School, UMDNJ, 185 South Orange Ave., Newark, NJ 07103. E-mail: leibovic@umdnj.edu.
Supported in part by the United States Public Health Service [grants RO1-GM57982 (to S. J. L.), GM56268 (to B. C.), and AR41911 (to B. C.)], a focused giving grant from Johnson and Johnson (to S. J. L.), and a grant from King Pharmaceuticals Inc. (to B. C.).
References
- 1.Xiong M, Elson G, Legarda D, Leibovich SJ: Production of vascular endothelial growth factor by murine macrophages. Am J Pathol 1998, 153:587-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA: Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998, 152:1445-1452 [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N, Chen H, Davis Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH: Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 1998, 4:336-340 [DOI] [PubMed] [Google Scholar]
- 4.Perez Ruiz M, Ross J, Morales Ruiz M, Navasa M, Colmenero J, Ruiz Delarbol L, Cejudo P, Claria J, Rivera F, Arroyo V, Rodes J, Jimenez W: Vascular endothelial growth factor production in peritoneal macrophages of cirrhotic patients: regulation by cytokines and bacterial lipopolysaccharide. Hepatology 1999, 29:1057-1063 [DOI] [PubMed] [Google Scholar]
- 5.Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ: Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen 2000, 8:353-360 [DOI] [PubMed] [Google Scholar]
- 6.Van Belle H, Goossens F, Wynants J: Formation and release of purine catabolites during hypoperfusion, anoxia and ischemia. Am J Physiol 1987, 252:H886-H893 [DOI] [PubMed] [Google Scholar]
- 7.Matherne GP, Headrick JP, Coleman SD, Berne RMZ: Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res 1990, 28:348-353 [DOI] [PubMed] [Google Scholar]
- 8.Dubyak GR, El-Moatassim C: Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 1993, 265:C577-C606 [DOI] [PubMed] [Google Scholar]
- 9.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton Thomas JR, Shuman MA, Rosenberg S: Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res 1996, 56:2428-2433 [PubMed] [Google Scholar]
- 10.Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen BN, Erlanger BF: Human mononuclear phagocytes express adenosine A1 receptors. J Immunol 1993, 151:2775-2785 [PubMed] [Google Scholar]
- 11.Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, Celada A: IFN-γ Up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol 1999, 162:3607-3614 [PubMed] [Google Scholar]
- 12.Priebe T, Platsoucas CD, Nelson JA: Adenosine receptors and modulation of natural killer cell activity by purine nucleosides. Cancer Res 1990, 151:2775-2785 [PubMed] [Google Scholar]
- 13.Ritchie PK, Spangelo BL, Krzymowsk DK, Rossiter TB, Kurth E, Judd AM: Adenosine increases interleukin 6 release and decreases tumor necrosis factor release from rat adrenal zona glomerulosa cells, ovarian cells, anterior pituitary cells, and peritoneal macrophages. Cytokine 1997, 9:187-198 [DOI] [PubMed] [Google Scholar]
- 14.Le Moine O, Stordeur P, Schandene L, Marchant A, de Groote D, Goldman M, Deviere J: Adenosine enhances IL-10 secretion by human monocytes. J Immunol 1996, 156:4408-4414 [PubMed] [Google Scholar]
- 15.Hasko G, Kuhel DG, Chen JF, Schwarzschild A, Deitch EA, Mabley JG, Marton A, Szabo C: Adenosine inhibits IL-12 and TNF-α production via adenosine A2A receptor-dependent and independent mechanisms. FASEB J 2000, 14:2065-2074 [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M: Nomenclature and classification of purinoreceptors. Pharmacol Rev 1994, 46:143-156 [PMC free article] [PubMed] [Google Scholar]
- 17.Dalzie HH, Westfall DP: Receptors for adenine nucleotides: subclassification, distribution, and molecular characterization. Pharmacol Res 1994, 46:449-466 [PubMed] [Google Scholar]
- 18.Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Jr, Gerfen CR, Sibley DR: Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol 1991, 40:1-7 [PubMed] [Google Scholar]
- 19.Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, Simons MJ, Dumont JE, Vassart G: Selective amplification and cloning of four new members of the G-protein-coupled receptor family. Science 1989, 244:569-572 [DOI] [PubMed] [Google Scholar]
- 20.Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM: Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol 1992, 6:384-393 [DOI] [PubMed] [Google Scholar]
- 21.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O: Molecular cloning and characterisation of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA 1992, 89:7432-7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronstein BN: Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol 1993, 76:5-13 [DOI] [PubMed] [Google Scholar]
- 23.Daval JL, Nicolas F, Doriat JF: Adenosine physiology and pharmacology: how about A2 receptors? Pharmacol Ther 1996, 71:325-335 [DOI] [PubMed] [Google Scholar]
- 24.Cusack NJ: 5′-N-ethylcarboxamidoadenosine—a potent inhibitor of human platelet aggregation. Br J Pharmacol 1981, 72:443-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis MF, Schulz R, Hutchinson AJ, Do UH, Sills MA, Williams M: [3H]-CGS21680C, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 1989, 251:888-893 [PubMed] [Google Scholar]
- 26.Trivedi BK, Bridges AJ, Bruns RF: Structure-Activity Relationships of Adenosine A1 and A2 Receptors. 1990:pp 57-103 Humana Press, Clifton
- 27.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G: Suppression of inflammatory protein (MIP)-1 alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol 1998, 125:379-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parmely MJ, Zhou WW, Edwards CK, III, Borcherding DR, Silverstein R, Morrison DC: Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-α production and protect mice against endotoxin challenge. J Immunol 1993, 15:389-396 [PubMed] [Google Scholar]
- 29.Poelstra K, Heynen ER, Baller JF, Hardonk MJ, Bakker WWW: Modulation of anti-Thy1 nephritis in the rat by adenine nucleotides: evidence for an anti-inflammatory role for nucleotidase. Lab Invest 1988, 66:555-563 [PubMed] [Google Scholar]
- 30.Marak GE, Jr, de Kozak Y, Faure JP, Rao NA, Romero JL, Ward PA, Till GO: Pharmacological modulation of acute ocular inflammation. I. Adenosine. Ophthalmic Res 1988, 20:220-226 [DOI] [PubMed] [Google Scholar]
- 31.Ross SD, Tribble CG, Linden J, Gangemi JJ, Lanpher BC, Wang AY, Kron IL: Selective adenosine A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant 1999, 18:994-1002 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan GW, Linden J, Buster B, Scheld WM: Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with type IV phosphodiesterase inhibitor, rolipram. J Infect Dis 1999, 180:1550-1560 [DOI] [PubMed] [Google Scholar]
- 33.Okusa MD, Linden J, Huang L, Rieger JM, MacDonald TL, Huynh LP: A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol 2000, 279:F809-F818 [DOI] [PubMed] [Google Scholar]
- 34.Dusseau JW, Hutchins PM, Malbasa DS: Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res 1986, 59:163-170 [DOI] [PubMed] [Google Scholar]
- 35.Fischer S, Knoll R, Renz D, Karlicek GF, Schaper W: Role of adenosine in the hypoxic induction of vascular endothelial growth factor in porcine brain derived microvascular endothelial cells. Endothelium 1997, 5:155-165 [DOI] [PubMed] [Google Scholar]
- 36.Gu JW, Brady AL, Anand V, Moore MC, Kelly WC, Adair TH: Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol 1999, 277:H595-H602 [DOI] [PubMed] [Google Scholar]
- 37.Pueyo ME, Chen Y, D’Angelo G, Michel JB: Regulation of vascular endothelial growth factor expression by cAMP in rat aortic smooth muscle cells. Exp Cell Res 1998, 238:354-358 [DOI] [PubMed] [Google Scholar]
- 38.Fischer S, Knoll R, Renz D, Karliczek GF, Schaper W: Role of adenosine in the hypoxic induction of vascular endothelial growth factor in porcine brain derived microvascular endothelial cells. Endothelium 1997, 5:155-165 [DOI] [PubMed] [Google Scholar]
- 39.Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP: Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci 1996, 37:1311-1321 [PubMed] [Google Scholar]
- 40.Fischer S, Sharm HS, Karliczek GF, Schaper W: Expression of vascular permeability factor/vascular endothelial growth factor in pig cerebral microvascular endothelial cells and its upregulation by adenosine. Brain Res 1995, 28:141-148 [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto E, Kage K, Ogita T, Nakaoka T, Matusuoka R, Kira Y: Adenosine as an endogenous mediator of hypoxia for induction of vascular endothelial growth factor mRNA. Biochem Biophys Res Commun 1994, 204:318-324 [DOI] [PubMed] [Google Scholar]
- 42.Beutler B, Poltorak A: Positional cloning of LPS, and the general role of toll-like receptors in the innate immune response. Eur Cytokine Network 2000, 11:145-152 [PubMed] [Google Scholar]
- 43.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA: A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 1999, 19:9192-9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA: Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 2000, 275:4429-4434 [DOI] [PubMed] [Google Scholar]
- 45.Khoa DN, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN: Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Immunol 2001, 167:4026-4032 [DOI] [PubMed] [Google Scholar]
- 46.Ingalls RR, Heine H, Lien E, Yoshimura A, Golenbock D: Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect Dis Clin North Am 1999, 13:341-353 [DOI] [PubMed] [Google Scholar]
- 47.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR: Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 1991, 352:342-344 [DOI] [PubMed] [Google Scholar]
- 48.Malhotra R, Priest R, Bird MI: Role for L-selectin in lipopolysaccharide-induced activation of neutrophils. Biochem J 1996, 320:589-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flamme I, Frolich T, Risau W: Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol 1997, 173:206-210 [DOI] [PubMed] [Google Scholar]
- 50.Brown LF, Yeo KT, Berse BK, Yeo TK, Senger DR, Dvorak HF, Van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fehrenbach H, Kasper M, Haase M, Schuh D, Muller M: Differential immunolocalization of VEGF in rat and human adult lung, and in experimental rat lung fibrosis. Light, fluorescence, and electron microscopy. Anat Rec 1999, 254:61-73 [DOI] [PubMed] [Google Scholar]
- 52.Fava RA, Olsen NJ, Spencer Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF: Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med 1994, 180:341-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrara N: The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 1995, 36:127-137 [DOI] [PubMed] [Google Scholar]
- 54.Kim HK, Lee HS, Chang KT, Ko TH, Baek KJ, Kwon NS: Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-kappa B. J Immunol 1995, 154:4741-4748 [PubMed] [Google Scholar]
- 55.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A: Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 1994, 367:576-579 [DOI] [PubMed] [Google Scholar]
- 56.Levy AP, Levy NS, Wegner S, Goldberg MA: Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 1995, 270:13333-13334 [DOI] [PubMed] [Google Scholar]
- 57.Liu YX, Cox SR, Morita T, Kourembanas S: Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells—identification of a 5′ enhancer. Circ Res 1995, 77:638-643 [DOI] [PubMed] [Google Scholar]
- 58.Semenza GL: HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 2000, 88:1474-1480 [DOI] [PubMed] [Google Scholar]
- 59.Chin K, Kurashima Y, Ogura T, Tajiri H, Yoshida S, Esumi H: Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene 1997, 15:437-442 [DOI] [PubMed] [Google Scholar]
- 60.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, Addeo R, Makuuchi M, Esumi H: Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 2000, 95:189-197 [PubMed] [Google Scholar]
- 61.Frank S, Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J: Nitric oxide triggers enhanced induction of vascular endothelial growth factor expression in cultured keratinocytes (HaCaT) and during cutaneous wound repair. FASEB J 1999, 13:2002-2014 [PubMed] [Google Scholar]
- 62.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL: Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 2001, 107:861-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN: Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med 1997, 186:1615-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olah ME, Roudabush FL: Down-regulation of vascular endothelial growth factor expression after A2A adenosine receptor activation in PC12 pheochromocytoma cells. J Pharmacol Exp Ther 2000, 293:779-787 [PubMed] [Google Scholar]
- 65.Pueyo ME, Chen Y, D’Angelo G, Michel JB: Regulation of vascular endothelial growth factor expression by cAMP in rat aortic smooth muscle cells. Exp Cell Res 1998, 238:354-358 [DOI] [PubMed] [Google Scholar]
- 66.Nathan CF, Murray HW, Wiebe ME, Rubin BY: Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 1983, 158:670-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon S: Development and distribution of mononuclear phagocytes. Gallin JI Snyderman R eds. Inflammation: Basic Principals and Clinical Correlates. 1999, :pp 35-48 Lippincott Williams & Wilkins, Philadelphia [Google Scholar]