Abstract
Basic and acidic fibroblast growth factor (bFGF and aFGF, respectively) and vascular endothelial growth factor (VEGF) exert angiogenic actions and have a role in wound healing, inflammation, and tumor growth. Monocytes and endothelial cells are involved in these processes, but the effect of FGF and VEGF on monocyte-endothelial cell interactions has not been defined. We observed that monocyte adhesion to resting or cytokine (tumor necrosis factor-α or interleukin-1α)-stimulated human umbilical vein endothelial cells (HUVECs) was markedly inhibited (40 to 65%) by culture (1 to 6 days) of HUVECs with aFGF or bFGF. Monocyte transendothelial migration induced by C5a and chemokines (MCP-1, SDF-1α, RANTES, MIP-1α) was also suppressed (by 50 to 75%) on bFGF-stimulated HUVECs. VEGF did not have these effects at the concentrations used (10 to 20 ng/ml), although like bFGF, it promoted HUVEC proliferation. Culture of HUVECs with bFGF and aFGF significantly down-regulated intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression on resting or tumor necrosis factor-α-stimulated HUVECs, but had no influence on platelet endothelial cell adhesion molecule (PECAM)-1 and VE-cadherin expression. bFGF also inhibited MCP-1 production by HUVECs. The inhibitory effects of bFGF on monocyte transendothelial migration and adhesion molecule expression were reversed by SU6668, an anti-angiogenic agent and bFGF receptor tyrosine kinase inhibitor. Our results suggest that bFGF and aFGF may suppress endothelial-dependent monocyte recruitment and thus have an anti-inflammatory action during angiogenesis in chronic inflammation but inhibit the immunoinflammatory tumor defense mechanism. However, SU6668 is an effective agent to prevent this down-regulatory action of bFGF on monocyte-endothelial cell interactions.
A fundamental feature of inflammation involves the adhesion of leukocytes to vascular endothelium and their emigration into inflamed tissues. 1,2 Angiogenesis is the formation of new blood vessels from pre-existing vasculature and is a feature during chronic inflammation, eg, in arthritis, wound healing, and tissue remodeling or tumor growth. Angiogenesis is a complex process regulated by interactions of endothelial cells with growth factors and cytokines as well as with extracellular matrix proteins via adhesion molecules. 3
Monocytes play an important role in collateral vessel formation (arteriogenesis) by attaching to activated endothelium and by invading the walls of innate collateral vessels, where they produce growth factors. Previous studies have demonstrated that this process can be promoted by several chemokines and growth factors. 4 Recruitment of monocytes has been suggested to be important in the angiogenic cascade. 5 Furthermore, the cytokines basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) produced by monocytes, as well as other cells, are among the most potent mediators of angiogenesis. These factors are frequently present at sites of physiological and pathological angiogenesis and growing tumors are dependent on this process. 6-9
During inflammation, the cytokines tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1α and β) have been shown to induce leukocyte infiltration in part by up-regulating the expression of leukocyte adhesion molecules on vascular endothelial cells. 1,10 It is well established that the recruitment and emigration of circulating leukocytes is dependent on a multistep cascade of events involving leukocyte tethering, rolling, firm adhesion, and emigration and these steps are mediated by distinct adhesion molecules and activation pathways. 1,11 The selectins (L-, P-, and E-selectin) and α4-integrins (α4β1 and α4β7) 11,12 mediate rolling of leukocytes in postcapillary venules of the systemic circulation, whereas firm adhesion and emigration of rolling leukocytes including monocytes is mostly dependent on two members of the CD18 (β2) integrin family, CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1) as well as α4β1, 13 which engage intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 and VCAM-1 on endothelium. 14-16 These findings suggest that modulation of the adhesion molecule expression on endothelium can influence the trafficking of leukocytes into tissues.
In comparison with our understanding of the mechanisms in normal vessels, relatively little is known about factors that regulate monocyte or other leukocyte adhesion and transmigration across angiogenic vessels in inflammation or in tumors. Recent studies in vivo have suggested that rolling, adhesion, and transmigration of leukocytes in angiogenic blood vessels may be impaired. 17,18 Previous studies have shown that many tumor cells produce bFGF and VEGF, 19,20 and freshly isolated tumor endothelium exhibits decreased expression of ICAM-1 as compared to endothelium in normal tissue. 21 Furthermore, endothelial VCAM-1 expression is suppressed in melanomas and carcinomas. 22 High levels of endothelial growth factors are often found in plasma of patients with various tumors. 19,20,23 The potential impact of these factors on leukocyte-endothelial cell adhesion and emigration has not been fully evaluated. Griffioen and colleagues 24 has observed a decreased expression of ICAM-1 on bFGF-treated human umbilical vein endothelial cells (HUVECs) in vitro. However these studies did not investigate the effects on monocyte adhesion or transendothelial migration (TEM). Furthermore, most previous studies have used HUVECs grown to confluence with endothelial cell growth supplements that contain a mixture of growth factors and these may influence the basal and stimulated endothelial responses. 25
In this study we examined the effect of bFGF, aFGF, and VEGF on endothelial cell interaction with monocytes resulting in monocyte adhesion and TEM. The effects were further analyzed on basal and inflammatory cytokine-stimulated ICAM-1, VCAM-1, and E-selectin expression and chemokine production by endothelium. The effects of a potent anti-angiogenic agent (SU6668) 26 on reversing growth factor-induced changes was also determined.
Materials and Methods
Materials
Recombinant human bFGF, VEGF, and IL-8 were purchased from Peprotech Inc. (Rocky Hill, NJ); TNF-α was from Genetech Inc. (San Francisco, CA); interferon-γ was from InterMune Pharmaceutical Inc. (Palo Alto, CA); IL-1α was a gift from Immunex Corp. (Seattle, WA); human serum albumin (HSA) (pyrogen-free) was from Connaught Laboratories (Downsview, Ontario, Canada). Monoclonal antibodies (mAbs) used were mouse mAb R6.5 to ICAM-1 (gift from Dr. TA Springer, Boston, MA); mAb 4B9 to VCAM-1 and BB11 to E-selectin (gifts from R Lobb, Biogen Inc., Cambridge, MA); 3H11B9 to pertussis toxin (gift from Dr. T Issekutz, Halifax, Canada); 5H2 to PECAM-1 (in-house generated). mAb to VE-cadherin was from Chemicon (Temecula, CA); peroxidase-conjugated goat anti-mouse and goat anti-rabbit IgG were from Bio-Can Scientific (Mississauga, Ontario, Canada); peroxidase conjugate streptavidin was from Amersham (Oakville, Ontario, Canada), fluorescein isothiocyanate-conjugated sheep F(ab)2 anti-mouse IgG was from Sigma Chemical Co. (St. Louis, MO) and Alexa 488-conjugated goat anti-mouse IgG was from Molecular Probes (Eugene, OR). SU6668 (kind gift from Dr. J Cherrington, SUGEN, Inc., South San Francisco, CA) was dissolved in dimethyl sulfoxide at a stock concentration of 50 mmol/L. Recombinant human C5a, a gift from CIBA-Geigy Pharmaceuticals (Summit, NJ) and human monocyte chemotactic protein-1 (MCP-1), RANTES, stromal-derived factor-1α (SDF-1α), bFGF, aFGF, VFGF165, and macrophage inflammatory protein-1α (MIP-1α) were from Preprotech.
Human Monocyte Purification and Labeling
Human monocytes were purified as described previously 27 from ethylenediaminetetraacetic acid (EDTA)/acid citrate dextrose (ACD) anti-coagulated venous blood of healthy donors. Briefly, red cells were sedimented with 6% dextran-saline (Abbott Laboratories, Montreal, Canada), leukocyte-rich plasma was collected and after centrifugation (150 × g for 10 minutes at 22°C), the leukocyte pellet was resuspended in Ca2+, Mg2+-free Tyrode’s solution with 5% autologous platelet-poor plasma and labeled with Na251CrO4 (Amersham) for 30 minutes at 37°C. During this incubation, the osmolarity of the medium was gradually increased in three steps from 290 to 360 mOsm by addition of 9% NaCl. This improved the monocyte purity and did not affect cell viability or function, as shown previously. 28 The labeled leukocytes were washed once with Ca2+, Mg2+-free Tyrode’s solution-5% platelet-poor plasma (360 mOsm), and resuspended in Ca2+, Mg2+-free Tyrode’s solution (360 mOsm) containing 0.2% EDTA, 10% platelet-poor plasma, and 56% Percoll (Pharmacia Fine Chemicals, Dorval, PQ). The leukocytes were separated on a discontinuous Percoll gradient of 73%, 62%, 56% (containing the labeled leukocytes), 50%, 46%, and 40% by centrifugation (400 × g for 25 minutes at 22°C). The purest monocyte faction was recovered at the 46 to 40% Percoll interphase. The monocytes were resuspended for migration studies at 7 × 105/ml in RPMI 1640, 0.5% HSA containing 10 mmol/L Hepes (pH 7.4). This method yielded monocytes of ≥92% purity with no neutrophil contamination based on neutral red and Wright’s staining and >98% viability by trypan blue exclusion. 27
Endothelial Cell Cultures
HUVECs were isolated and cultured in flasks 29 and grown on filters as previously described. 30 Briefly, endothelial cells were detached from human umbilical veins by treatment with 0.5 mg/ml of collagenase (Cooper Biomedical, Ontario, Canada) in 0.01 mol/L of phosphate-buffered saline (PBS) (pH 7.4) and grown in basal medium composed of RPMI 1640 (Sigma Chemical Co.) containing 2 mmol/L of l-glutamine, 2-mercaptoethanol, sodium pyruvate, penicillin/streptomycin, and supplemented with 20% heat-inactivated human AB serum (ABS). To initially establish the cultures, endothelial cell growth supplement (12.5 μg/ml) (Collaborative Research, Lexington, MA) and 45 μg/ml of heparin (Sigma) were added to the basal medium. Cells were cultured in 2% gelatin (Difco Inc., Detroit, MI)-coated culture flasks (NUNC, Life Technologies, Mississagua, Ontario, Canada). The HUVECs were harvested using 0.025% trypsin and 0.01% EDTA (Sigma) and cultured in gelatin-coated 96-well plates at 2.5 × 104 cells/well without growth factor or 1.5 × 104/well with added growth factors as indicated until confluence at 3 days and used for enzyme-linked immunosorbent assays (ELISA) or adhesion assays.
For monocyte TEM, HUVECs were seeded on polyvinyl pyrolidene (PVP)-free polycarbonate filters (5-μm pores) in Transwell culture plate inserts (6.5-mm diameter; Fisher Scientific, Ottawa, Ontario, Canada) up to the third passage. The filters were pretreated with 0.01% gelatin (37°C, 18 hours) followed by 3 μg of human fibronectin (Collaborative Research) in 50 μl of water at 37°C for 2 hours. Fibronectin was then replaced by HUVECs (1.5 × 104 or 2.5 × 104 in basal medium with or without the indicated growth factor, respectively), added above the filter in 0.1 ml and 0.6 ml of basal medium was added to the lower compartment beneath the filter. On filters, the HUVECs required 5 to 6 days to form a tight permeability barrier that was evaluated by 125I-labeled-HSA diffusion as previously described. 30 Under all conditions, <1.5% labeled HSA diffused across the HUVEC/filter unit in 45 minutes with 1-mm applied positive hydrostatic pressure, whereas bare filters showed ≥30% diffusion of 125I-labeled HSA in this test.
Monocyte Adhesion to HUVECs
The HUVECs were grown in 96-well culture plates under the indicated conditions and 51Cr-labeled monocytes were added at a concentration of 105 cells/well for 45 minutes at 37°C. Afterward, HUVECs were gently washed three times with prewarmed RPMI 1640 medium. Bound 51Cr monocytes were harvested from wells by addition of 1 N of NaOH and quantified with γ-spectrometer (Wallac LKB-1282, Fisher Scientific). The percentage of added monocytes adherent to the HUVECs was calculated. Adhesion was measured with triplicate or quadruplicate replicates.
Monocyte Transendothelial Migration Assay
HUVECs were cultured on gelatin/fibronectin-coated polycarbonate filters with (1.5 × 104/well) or without (2.5 × 104/well) an indicated growth factor for 6 days. Migration assays were performed as described previously. 30,31 Briefly, HUVEC monolayers on the filters and the lower compartments were washed with RPMI 1640 and then were transferred to a new, clean well of a 24-well plate (lower compartment). When the HUVECs were prestimulated with cytokines, the well contained RPMI 1640 to 10% AB serum and stimulation was for 4 hours. After washing, 0.6 ml of RPMI 1640, 10 mmol/L HEPES, and 0.5% HSA was added to the well containing as indicated the chemotactic stimulus. Before immersion of the HUVEC filter unit, 0.1 ml of medium containing 1 × 105 51Cr-labeled monocytes were added above the HUVECs. After incubation (75 minutes at 37°C, 5% CO2), migration was stopped by washing the upper compartment twice with 0.1 ml of RPMI 1640 to remove nonadherent monocytes. The undersurface of the filter was wiped with a cotton swab saturated with an ice-cold PBS-0.2% EDTA solution and this was added to the lower compartment. The cells that spontaneously detached from the undersurface of the filter or were removed by the swab were lysed by the addition of 0.5% Triton X-100 and all of the 51Cr released in the lower compartment and from the swab was quantitated with a γ-spectrometer. The results are expressed as the percentage of the total 51Cr monocytes added above the HUVECs that migrated through the HUVEC filter unit.
The monocyte adhesion to the HUVECs was quantified by lysis with 0.5 N NaOH of the 51Cr-monocytes that remained on the HUVEC monolayer after three washes of the monolayer/filter unit with warm RPMI 1640. The 51Cr in this NaOH lysate was quantified and is expressed as the percentage of the total 51Cr monocytes added above the HUVECs that adhered on the HUVEC monolayer. All experiments were performed with triplicate replicates.
Quantitation of Adhesion Molecule Expression on HUVECs by ELISA, Cytometry, and Microscopy
The expression of ICAM-1, VCAM-1, and E-selectin on HUVECs was determined with whole-cell ELISA as described previously with minor modification. 31 Briefly, HUVEC monolayers in 96-well plates were incubated with (1.2 × 104/well) or without (2 × 104/well) a specific growth factor at various concentrations for various times. In some experiments the HUVEC monolayers were treated with TNF-α or IL-1α for 4 hours. For SU6668 treatment, varying concentrations of this compound were added 1 hour before bFGF was added to the wells. Before ELISA, medium was removed and HUVECs were washed twice with warm RPMI medium. The HUVEC monolayer was fixed by adding 50 μl of 1% paraformaldehyde for 15 minutes at room temperature and then 50 μl of 0.05 mol/L Tris/0.1 mol/L glycine (pH 7.2) was added for an additional 15 minutes at room temperature. After washing, 100 μl of RPMI 1640 to 5% fetal calf serum (FCS)-0.1% NaN3 containing mAb to ICAM-1, to VCAM-1, E-selectin, PECAM-1, VE-cadherin, or control mAb was added. After 60 minutes (37°C, 5% CO2), the monolayers were washed four times and then 100 μl of peroxidase-conjugated goat anti-mouse IgG (1:4000 in RPMI 1640 to 5% FCS) was added for 60 minutes (37°C, 5% CO2). The monolayers were washed four times and then 100 μl of substrate (o-phenylenediamine, 12.5 mg/ml; 0.1 mol/L citrate-phosphate buffer, pH 5; 0.012% H2O2) was added. The enzyme reaction was stopped by adding 100 μl of 4 N H2SO4, and absorbance at the 490 nm was measured. Results are expressed as OD × 1000.
The expression of endothelial adhesion molecules was also determined by immunofluorescence flow cytometry using a standard immunofluorescence protocol. 32 Briefly, HUVECs were detached by brief treatment with 0.01% trypsin and 0.02% EDTA. Cell surface expression of ICAM-1, VCAM-1, and E-selectin was assessed using mAb R6.5, 4B9, and BB11 (5 μg/ml each), respectively. Binding was assessed by secondary detection with fluorescein isothiocyanate conjugated to sheep F(ab)2 anti-mouse IgG (Sigma). Nonspecific fluorescence was assessed by substituting the primary mAb with a nonbinding isotype-matched control mAb (3H11B9). Analysis was performed on a FACScan (Becton Dickinson, Mountain View, CA). Results are expressed as fluorescence histograms plotted on a log scale.
Immunofluorescence microscopy was performed on HUVECs to localize junctional proteins PECAM-1 and VE-cadherin. The HUVECs were grown on gelatin/fibronectin-coated Perplex culture slide chambers (Labtek, Life Technologies, Mississauga, Ontario, Canada) in the indicated growth media. The monolayers were stained with primary mAb (anti-PECAM-1 or anti-VE-cadherin or isotype control 3H11B9) in RPMI 1640 to 10% FCS, 0.1% NaN3 (45 minutes, 37°C). After washing, HUVECs were fixed with 1% paraformaldehyde (15 minutes, 22°C) followed by sequential blocking with 0.05 mol/L Tris and 0.1 mol/L glycine (pH 7.2) and 10% FCS-PBS (15 minutes each at 22°C). Secondary goat anti-mouse IgG conjugated with Alexa 488 was applied for detection. Slides were examined and photographed with a Nikon fluorescence microscope.
MCP-1 and IL-8 Quantitation
MCP-1 and IL-8 concentrations were quantified by sandwich ELISA as previously described. 33 MCP-1 in the HUVEC culture supernatants was captured with mAb E11 immobilized on ELISA plates. The secondary antibody was polyclonal rabbit IgG anti-human MCP-1 (kind gifts from T Yoshimura, National Cancer Institute, Frederick, MD). This was detected with goat anti-rabbit IgG alkaline-phosphatase conjugate. Sensitivity of this assay was down to 300 pg/ml of MCP-1. For IL-8 capture, mAb 3IL-8-h10 was used and the secondary was biotinylated mAb 2IL-8-HIA (kind gifts from JS Kenney, Antibody Solutions, Palo Alto, CA). This was detected with peroxidase conjugated to streptavidin. The detection limit for this assay was 600 pg/ml.
Endothelial Cell Proliferation Assay
The HUVECs were cultured in 96-well plates at a density of 104 cells/well with or without bFGF or VEGF in RPMI 1640 to 20% ABS for 2 days. In some wells, SU6668 was added 30 minutes before growth factor addition. On the third day, the medium was changed to RPMI 1640 with 5% ABS and the above agents were added again as above. 3H-thymidine (Amersham, Oakville, Ontario, Canada) (1 μCi) was added to each well for an additional 6 hours at 37°C. 3H-thymidine-labeled DNA was then quantitated by harvesting the cells onto Whatman GF/B paper filter mats using a cell harvester. The filter content of 3H was measured with a Beckman liquid scintillation counter. Results are expressed as cpm of 3H-thymidine incorporated into DNA.
Statistical Analysis
One-way analysis of variance, Student’s t-test, or paired t-test was used for statistical analysis of the data as indicated. P values exceeding 0.05 were not considered significant.
Results
Effect of bFGF, aFGF, and VEGF on Monocyte-Endothelial Cell Adhesion
Initially, we examined the effect of bFGF, aFGF, and VEGF on the adhesion of monocytes to unstimulated TNF-α (25 U/ml) and IL-1α (0.25 ng/ml) stimulated (for 4 hours) HUVECs. As shown in Figure 1 ▶ , constitutive and TNF-α- or IL-1α-enhanced HUVEC adhesive interaction with monocytes was significantly decreased by culture of HUVECs (for 3 days) with bFGF and aFGF. In contrast, VEGF had no inhibitory effect on monocyte-HUVEC adhesion.
Figure 1.
Effect of bFGF, aFGF, and VEGF on monocyte adhesion to endothelium. HUVECs were cultured at 1.5 × 104 cells/well with 10 ng/ml of bFGF, aFGF, or VEGF, respectively, for 3 days. Unstimulated HUVECs were seeded at 2.5 × 104/well so that comparable confluence HUVEC monolayers were present with all conditions. IL-1α (0.25 ng/ml) or TNF-α (25 U/ml) were added for 4 hours as indicated. The results are expressed as the percentage of the total 51Cr-monocytes that adhered to the HUVEC monolayers. Values are means ± SD of four experiments performed with triplicate replicates. **, P < 0.01 compared to control group.
Effect of bFGF on Chemotactic Factor-Induced Monocyte Transendothelial Migration
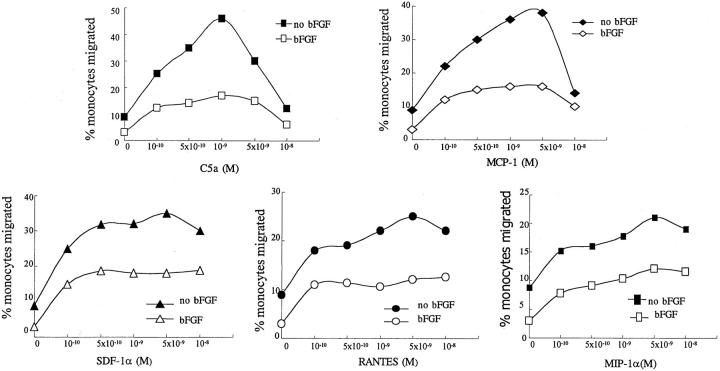
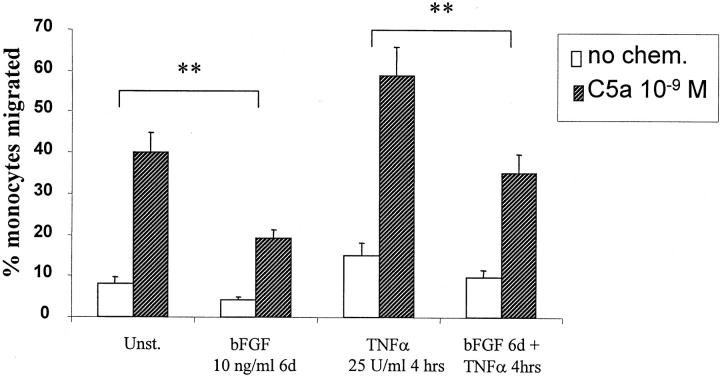
The results with bFGF and VEGF on monocyte-HUVEC adhesion prompted us to investigate whether bFGF or VEGF may effect monocyte TEM. HUVECs were cultured on gelatin/fibronectin-coated polycarbonate filters with (1.5 × 104/well) or without (2.5 × 104/well) growth factor for 6 days, respectively. HUVECs formed a monolayer with a comparable permeability barrier (see Materials and Methods) after 6 days whether growth factor was added for 6 days or not, because seeding density was adjusted to compensate for the enhanced growth observed with bFGF or VEGF. The monocyte migration assay was performed without or with addition of various concentrations (10−10 mol/L to 10−8 mol/L) of the monocyte chemotactic factors, C5α, MCP-1, RANTES, MIP-1α, or SDF-1α shown in Figure 2 ▶ . bFGF significantly decreased monocyte TEM in response to concentrations of chemotactic factor that elicited maximal TEM across HUVECs cultured without added growth factors. This depression of monocyte TEM by bFGF exposure of the endothelium was observed over a wide concentration range of chemoattractant as shown in Figure 3 ▶ , indicating that the inhibitory effect of bFGF was not associated with a shift in the dose-response curve. We also examined whether bFGF effected monocyte TEM through TNF-α-stimulated endothelial monolayers. After 6 days of pretreatment of HUVECs with bFGF, there was significant inhibition of constitutive and of C5a (10−9 mol/L)-enhanced monocyte TEM across TNF-α (25 U/ml)-activated HUVECs (Figure 4) ▶ . Culture of HUVECs with VEGF (10 to 20 ng/ml) had no effect on monocyte TEM under any of the above conditions (not shown), despite VEGF having comparable effects to bFGF in stimulating HUVEC proliferation at comparable concentrations (10 to 20 ng/ml) shown in Figure 8 ▶ . Under all conditions tested, the barrier integrity of the HUVEC monolayers on the filters was comparable as quantitated by permeability to 125I-HSA as measured previously. 30
Figure 2.
Effect of bFGF on monocyte TEM. HUVECs were cultured at 2.5 × 104 cells/filter without bFGF and 1.5 × 104 cells/filter with 10 ng/ml of bFGF for 6 days, so that comparable confluence and permeability barriers were present with all conditions. TNF-α (25 U/ml) stimulation of HUVECs was for 4 hours. No chemotactic factor (no Chem.) or C5a (10−9 mol/L), MCP-1 (5 × 10−9 mol/L), SDF-1α (5 × 10−9 mol/L), RANTES (5 × 10−9 mol/L), or MIP-1α (5 × 10−9 mol/L) were added to the lower compartment, as indicated, at the time of monocyte addition. Data (means ± SD) are the percentage of added monocytes that migrated from four experiments performed with triplicate replicates. **, P < 0.01 compared to the group without bFGF treatment.
Figure 3.
Effect of bFGF on monocyte TEM in response to different concentrations of chemotactic factor. HUVECs were cultured at 2.5 × 104 cells/filter without bFGF and 1.5 × 104 cells/filter with 10 ng/ml of bFGF for 6 days, so that comparable confluence and permeability barriers were present with all conditions. The chemotactic factors C5a, MCP-1, SDF-1α, RANTES, or MIP-1α were added to the lower compartment in different concentrations, respectively, as indicated, at the time of monocyte addition. Data (means) are the percentage of added monocytes that migrated from four experiments performed with triplicate replicates.
Figure 4.
The effect of bFGF on monocyte TEM of cytokine-stimulated endothelium. HUVECs were cultured on filters with or without growth factor as in Figure 3 ▶ for 6 days. TNF-α (25 U/ml) stimulation of HUVECs was for 4 hours. C5a (10−9 mol/L) was added to the lower compartment, as indicated, at the time of monocyte addition. Data (means ± SD) are the percentage of added monocytes that migrated from four experiments performed with triplicates. **, P < 0.01 comparison between groups with and without bFGF pretreatment.
Figure 8.
Effect of SU6668 on bFGF- and VEGF-induced endothelial cell proliferation. HUVECs were cultured in 96-well plates at a density of 104 cells/well with or without growth factors for 3 days. In some wells, SU6668 was added 30 minutes before growth factor was added. The 3H-thymidine incorporation assay was done as described in Materials and Methods. Results are expressed as cpm of 3H-thymidine incorporated into DNA. Values are means ± SD of triplicates from one representative experiment of three. ++, P < 0.01 compared to the control group without growth factor treatment; **, P < 0.01 compared to the group without SU6668 pretreatment.
Effect of bFGF on Endothelial MCP-1 and IL-8 Production
Stimulation of HUVECs with IL-1 or TNF-α is known to induce chemokine production most notably MCP-1 and IL-8 and these mediators may contribute to leukocyte TEM. Therefore, the effect of bFGF and VEGF on these responses was also evaluated. HUVECs were grown in 24-well plates with 0.5 ml of culture medium for 3 days with or without bFGF or VEGF. Basic FGF dramatically down-regulated constitutive MCP-1 production as shown in Figure 5 ▶ . VEGF inhibited MCP-1 production, but to a much lesser extent. There was no effect of either factor on the minimal constitutive IL-8 production by HUVECs. TNF-α treatment (4 hours) increased both MCP-1 and IL-8 production. The MCP-1 response was partially inhibited by bFGF treatment (++, P < 0.01) whereas VEGF had no significant effect on the TNF-α-induced response. Neither growth factor altered IL-8 production in response to TNF-α (Figure 5) ▶ .
Figure 5.
Effect of bFGF and VEGF on endothelial cell MCP-1 and IL-8 production. HUVECs were cultured at 1.5 × 104 cells/well with 10 ng/ml of bFGF and VEGF, respectively, for 3 days. Unstimulated HUVECs were seeded at 2.5 × 104/well so that comparably confluent monolayers were present with all conditions. Medium was exchanged and in some wells TNF-α (25 U/ml) was added for an additional 4 hours. This culture medium was harvested and the concentration of MCP-1 and IL-8 was quantified by double-antibody sandwich ELISA. *, P < 0.05; **, P < 0.01 relative to control; ++, P < 0.01 relative to TNF-α on control HUVECs. Values are the means of three experiments ± SD.
Effect of bFGF, aFGF, and VEGF on ICAM-1, VCAM-1, E-selectin, PECAM-1, and VE-Cadherin Expression on Endothelium
It is known that monocyte adhesion and TEM in vitro is mediated in part by E-selectin, ICAM-1, and VCAM-1. 34,35 The adhesion molecule expression on HUVECs cultured with or without 10 ng/ml of various angiogenic factors aFGF, bFGF, VEGF, platelet-derived growth factor (PDGF), or IL-8 for 3 days was examined by ELISA. Because these angiogenic factors have a strong mitogenic effect on HUVECs, the plating density of cells was carefully selected so that at the time of the whole-cell ELISA assay, the HUVECs were of comparable confluence and density. As shown in Figure 6 ▶ , culture of HUVECs for 3 days with aFGF or bFGF resulted in a significant decrease in basal ICAM-1, VCAM-1, and E-selectin expression. Interestingly, VEGF, PDGF, and IL-8 did not alter ICAM-1, VCAM-1, or E-selectin expression.
Figure 6.
Effect of various angiogenic factors on endothelial cell ICAM-1, VCAM-1, and E-selectin expression. HUVEC monolayers in 96-well plates (2 × 104/well) were cultured with bFGF, aFGF, VEGF, IL-8, or PDGF, respectively, for 3 days at the concentrations indicated. In some wells, TNF-α or IL-1α was added for an additional 4 hours. The expression of ICAM-1, VCAM-1, and E-selectin was measured by ELISA using mAbs R6.5, 4B9, and BB11, respectively, as described in Materials and Methods. mAb 3H11B9 was used as isotype-matched negative control and this background optical density (OD mean, 0.07) was subtracted from all values. One representative experiment of three is shown. Values are means ± SD of triplicates. **, P < 0.01 compared to control group.
Up-regulation of endothelial adhesion molecules is well documented in response to IL-1 or TNF-α stimulation of endothelium in vitro or in vivo. 1,2 We therefore examined the effect of these angiogenic factors on this response. HUVECs were treated with 10 ng/ml of aFGF, bFGF, VEGF, PDGF, or IL-8 for 3 days until confluent and then the cells were stimulated with 25 U/ml TNF-α or 0.25 ng/ml IL-1α for 4 hours. As shown in Figure 6 ▶ , aFGF and bFGF significantly inhibited the up-regulation of ICAM-1, VCAM-1, and E-selectin on TNF-α- and IL-1α-stimulated HUVECs. In contrast to aFGF and bFGF, VEGF, PDGF, and IL-8 had no inhibitory effect on up-regulation of HUVEC ICAM-1, VCAM-1, or E-selectin expression.
Of the above factors, VEGF and bFGF are critical and pathologically prominent angiogenic factors. Therefore, although VEGF treatment for 3 days did not alter adhesion molecule expression, in separate experiments the effect of VEGF on bFGF modulation of ICAM-1 and VCAM-1 was examined. Simultaneous stimulation with VEGF and bFGF (10 ng/ml each as in Figure 6 ▶ ) did not alter the suppression by bFGF of ICAM-1 and VCAM-1 expression on HUVECs determined by ELISA (absorbance with bFGF: ICAM-1 = 0.101 ± 0.022 and VCAM-1 = 0.035 ± 0.011; VEGF + bFGF: ICAM-1 = 0.110 ± 0.020 and VCAM-1 = 0.026 ± 0.005; n = 3). Furthermore, the bFGF inhibition of up-regulation of ICAM-1 or VCAM-1 by TNF-α (25 U/ml for 4 hours) stimulation was also not modified by VEGF (bFGF: ICAM-1 = 0.315 ± 0.04 and VCAM-1 = 0.208 ± 0.03; VEGF + bFGF: ICAM-1 = 0.307 ± 0.05; VCAM-1 = 0.200 ± 0.03; n = 3).
The effect of bFGF and VEGF on the expression of three other adhesion molecules, ie, ICAM-2, PECAM-1, and VE-cadherin was determined by ELISA. Neither growth factor altered ICAM-2 expression under the above conditions (data not shown). Furthermore, bFGF and VEGF had no effect on PECAM-1 and VE-cadherin expression and both of these proteins were localized to endothelial cell junctions as visualized by immunofluorescence staining, as shown in Figure 7 ▶ .
Figure 7.
Effect of bFGF and VEGF on VE-cadherin or PECAM-1 expression and localization on endothelial monolayers. After culture of HUVECs in AB serum (ABS) alone or with VEGF or bFGF (20 ng/ml) for 3 days, HUVEC monolayers were fixed and stained with mAb to human VE-cadherin and PECAM-1. The mAb binding was detected by indirect immunofluorescence with sheep anti-mouse IgG-Alexa488 conjugate.
The suppressive effect of bFGF on TNF-α- or IL-1α-induced adhesion molecule expression was overcome by high concentrations of TNF-α (200 U/ml) or IL-1α (1 ng/ml) (data not shown). In contrast to bFGF, VEGF at comparable concentrations had no effect on up-regulation of the above adhesion molecules by high or low concentrations of the above cytokines (data not shown).
Effect of SU6668 on bFGF- or VEGF-Induced HUVEC Proliferation
To evaluate the effect of the anti-angiogenic agent SU6668 on the above effects of bFGF, we first determined the concentration of SU6668 required to antagonize the mitogenic action of bFGF and VEGF on HUVECs. The 3H-thymidine incorporation into DNA was monitored. SU6668 significantly inhibited the mitogenic effects on HUVECs of both bFGF and VEGF in a dose-dependent manner, with 25 μmol/L completely blocking the effect of 10 ng/ml of either growth factor (Figure 8) ▶ .
Effect of SU6668 on Monocyte Transendothelial Migration
To assess the effect of SU6668 on monocyte TEM, HUVECs were cultured on gelatin/fibronectin-coated polycarbonate filters at 1.5 × 104/filter in the presence of bFGF or at 2.5 × 104/filter without bFGF or when SU6668 was also present. The HUVECs formed a monolayer with a comparably tight permeability barrier (see Materials and Methods) after 6 days under these conditions independent of whether SU6668 or bFGF was present or not because the seeding density compensated for the enhanced growth rate with bFGF, or the anti-proliferative effect of SU6668. As shown in Figure 9 ▶ , SU6668 nearly completely prevented the inhibitory effect of bFGF on monocyte TEM induced by C5a or MCP-1 across unstimulated HUVECs. Furthermore, SU6668 also blocked the bFGF-induced inhibition of monocyte TEM through TNF-α-stimulated endothelial monolayers. The residual dimethyl sulfoxide solvent (<0.05%) had no effect on any of the parameters measured.
Figure 9.
The effects of SU6668 on monocyte TEM. HUVECs were cultured on filters with growth factor (seeding 1.5 × 104/filter) as in Figure 2 ▶ and Figure 3 ▶ for 6 days. Filters without growth factor or those with SU6668 (25 μmol/L added to HUVECs 30 minutes before bFGF) received 2.5 × 104 cells/filter so that comparable permeability barriers formed. Monocyte TEM was assessed as described in Materials and Methods. No chemoattractant (no Chem.) or C5a (10−9 mol/L) or MCP-1 (5 × 10−9 mol/L) were added beneath the filters at time of 51Cr-monocyte addition above the HUVEC/filter unit. Data (means ± SD) are the percentage of added monocytes that migrated from three experiments performed with triplicates. **, P < 0.01 compared to the group without SU6668.
Flow Cytometry Analysis of Adhesion Molecule Expression on bFGF- and SU6668-Treated Endothelium
Adhesion molecule expression on HUVECs was also quantitated by immunofluorescence flow cytometry to allow analysis of expression on individual cells rather than on the whole HUVEC population as in the ELISA system. This was felt to be important because there was an observed trend for a slightly higher cell density at confluence with HUVECs exposed to bFGF, eg, in a 2-cm2 24-well, cell number without growth factor at confluence = 1.6 × 105 cells with bFGF = 1.9 × 105 and with bFGF + SU6668 = 1.8 × 105 cells. This trend might have underestimated differences by ELISA performed on monolayers. The effect of bFGF on expression of ICAM-1 and VCAM-1 on resting, SU6668, TNF-α, or TNF-α- and SU6668-treated HUVECs was determined as shown in Figure 10 ▶ . HUVECs cultured with SU6668 alone tended to express slightly more ICAM-1 and VCAM-1 constitutively and after TNF-α stimulation. The degree of ICAM-1 and VCAM-1 expression on the whole cell population was diminished by culture of HUVECs with bFGF. This inhibitory effect of bFGF on CAM expression on resting or TNF-α-stimulated HUVECs was prevented by co-culture with SU6668.
Figure 10.

Flow cytometric analysis of the effect of SU6668 on ICAM-1 and VCAM-1 expression on resting or bFGF-stimulated endothelium. The HUVECs were cultured in 6-well plates to confluence with bFGF ± SU6668 (25 μmol/L) as indicated. Where indicated, TNF-α (25 U/ml) was added (4 hours) to stimulate the HUVECs. The cells were harvested with trypsin/EDTA and ICAM-1 and VCAM-1 expression was detected with mAb R6.5 or 4B9. The broken vertical line represents the 95th percentile fluorescence value with nonbinding isotype-matched mAb as negative control. Bound primary mAb was detected with fluorescein isothiocyanate-conjugated F(ab)2 sheep anti-mouse IgG. The histograms are plotted on a log scale of fluorescence intensity. One representative experiment of three is shown.
Discussion
The regulation by angiogenic growth factors of the recruitment of monocytes into wounds, tumor stroma, or areas of chronic inflammation is poorly understood, although in such lesions growth factor production and leukocyte emigration are often simultaneous. The present study is the first to our knowledge to address the influence of endothelial growth factors on monocyte adhesion to endothelium, monocyte TEM, and MCP-1 production by endothelium, all important mechanisms in inflammation in vivo. Firstly, we demonstrate that exposure of HUVECs to bFGF or aFGF for 24 hours to 6 days significantly reduced monocyte adhesion to resting or cytokine (TNF-α and IL-1α)-stimulated HUVECs, but in contrast, VEGF did not have this effect. (Figure 1) ▶ . Furthermore, bFGF but not VEGF markedly inhibited monocyte transmigration across resting, and IL-1- or TNF-α activated HUVECs, even in response to potent chemotactic factors (C5a, MCP-1, SDF-1, MIP-1α, and RANTES) (Figures 2 and 3) ▶ ▶ . These effects of bFGF were unrelated to its endothelial growth-promoting effects, because although VEGF did not affect monocyte adhesion, TEM, or adhesion molecule expression, VEGF and bFGF had similar growth promoting effects (Figure 8) ▶ .
The induction of cell adhesion molecules on vascular endothelium by cytokines is an important mechanism for leukocyte capture, rolling, and firm adhesion to the microvasculature in vivo, essential for subsequent leukocyte emigration. 36 The adhesion molecules mediating monocyte migration across endothelium include the integrins CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, E-selectin, and other ligands on endothelium. 34,35,37 Therefore, the effect of bFGF and of other angiogenic factors on HUVEC adhesion molecule expression was investigated. Prolonged exposure (24 hours to 6 days) to bFGF or aFGF induced a marked down-regulation of ICAM-1, VCAM-1, and E-selectin expression on both resting and cytokine-stimulated HUVECs (Figure 6) ▶ . In contrast, VEGF, PDGF, or IL-8 did not effect the adhesion molecule expression, demonstrating that bFGF and aFGF, which share the same receptor system, 38 are unique in modulating endothelial activation. This was not a global effect of bFGF, because ICAM-2 expression was unaffected (not shown). The vascular endothelial (VE) cadherin and PECAM-1 localizes to adherens junctions of endothelial cells and may play a critical role in the TEM of circulating blood leukocytes. 39 However, neither bFGF nor VEGF altered HUVEC surface PECAM-1 and VE-cadherin expression (see text) or distribution (Figure 7) ▶ . These findings suggest that the effect of bFGF on monocyte TEM is not because of modification of endothelial gap junction organization. Stimulation of endothelial cells with TNF-α or IL-1α increases monocyte adhesion and TEM because of up-regulated expression of ICAM-1, VCAM-1, and E-selectin. 13,35 Our results suggest that bFGF decreases the sensitivity of endothelium to these inflammatory cytokines, because TNF-α- or IL-1-induced ICAM-1, VCAM-1, and E-selectin expression was always diminished by the presence of bFGF for longer than 24 hours. These observations are in agreement with previous studies. 24,25 However, we also show that even the low constitutive expression of these molecules was markedly suppressed by bFGF (Figure 6) ▶ and that this is associated with marked attenuation of monocyte TEM in response to a broad range of chemoattractants (Figure 3) ▶ . Such an effect of bFGF, which is produced by many tumors, 40,41 may interfere with immune and inflammatory/effector cell infiltration and attack on tumor cells.
Both VEGF and bFGF are reported to induce angiogenesis by a direct effect on human endothelial cells through different high-affinity heparin-binding receptors for VEGF (Flk and Flt) 42 or bFGF (FGFR-1 and -2). 38 However, there is a synergistic interaction between these growth factors in the induction of endothelial cell proliferation 43 and angiogenesis in vitro 44,45 as well as in vivo. 46 Indeed, it is believed that these two growth factors interact also in development of the arteriolar tree. 47 There is also evidence that bFGF in part exerts its angiogenic actions by inducing VEGF production in an autocrine manner 48 and that FGF up-regulates expression of both VEGF receptors on endothelium. 49 These results suggest that bFGF may promote angiogenesis both by a direct effect on endothelial cells and also indirectly via VEGF-dependent mechanisms. However an interplay between FGF and VEGF actions on endothelium does not apply to regulation of adhesion molecule expression because VEGF did not enhance or inhibit bFGF-induced down-regulation of ICAM-1 or VCAM-1 (see text). Because VEGF alone had no effect on expression of these molecules by HUVECs, bFGF-induced suppression seems to be mediated independent of VEGF mechanisms.
Previous studies have shown that freshly isolated endothelium derived from the vasculature of human solid tumors exhibits a decreased expression of ICAM-1 as compared to endothelium derived from normal tissue vessels, 21 and endothelial VCAM-1 expression is suppressed in melanomas and carcinomas. 22 VCAM-1 expression was also noted to be diminished in tumors on small but not on large vessels, which is relevant because leukocyte emigration occurs primarily in the small postcapillary venules. The plasma and urine levels of bFGF and VEGF have both been reported to be elevated in patients with a variety of cancers and these findings may have prognostic value. 49-51 Our findings would suggest that it is the chronically elevated levels of bFGF rather than VEGF that may contribute to this adhesion molecule deficiency on tumor vessels.
These effects of bFGF and aFGF on monocyte-TEM and endothelial adhesion molecules were likely observed because the HUVECs used in the assays were grown without supplemental growth supplements such as endothelial cell growth supplement that is commonly used to culture HUVECs. This was possible because HUVECs cultured in 20% human AB serum at around 50% confluent seeding density did not require additional growth factors to grow to confluence, something that could not be consistently achieved in 20% FCS (unpublished observations). We observed that culture with endothelial cell growth supplements caused many of the same effects on adhesion molecule expression and TEM as did bFGF (unpublished observation).
An inflammatory response requires that circulating monocytes bind to and migrate across the endothelium of the vessel wall to gain access to inflamed sites. Various chemoattractants and cytokines have been shown to initiate and regulate the margination and extravasation of monocytes. The CC chemokine MCP-1 is constitutively produced by HUVECs, but is also up-regulated by inflammatory cytokines and acts as a potent regulator of monocyte trafficking. 52,53 Endothelial cell-derived MCP-1 may play a critical role in recruiting monocytes. 54 Our results show that prolonged bFGF, but not VEGF significantly inhibited TNF-α-induced MCP-1 production by HUVECs (Figure 5) ▶ . Neither bFGF nor VEGF had an effect on IL-8 production (Figure 5) ▶ , a potent chemotactic factor for neutrophils, demonstrating that the effect of bFGF was not a global suppression of endothelial chemokine production. This result suggests that bFGF may inhibit monocyte emigration in part by suppression of MCP-1 release by endothelium. This result further supports the concept that bFGF may act as a regulator of endothelial cell activity and by down-modulating not only adhesion molecule expression but also monocyte chemoattractant production, it may potently and possibly synergistically suppress monocyte recruitment in vivo. The high circulating level of bFGF detected in patients with different cancers 19,20,23,40,41 may thus have a strong anti-inflammatory and tumor protective role because monocytes and monocyte-derived activated tissue macrophages are important immune effector cells in tumor cell killing via various mechanisms. 55-57
In general, bFGF has some immediate and early effects on endothelium such as activation of MAP kinase, phospholipase C, and so forth, 38 that require only a short exposure time to bFGF. However, our data shows that a prolonged exposure (>24 hours) of HUVECs to bFGF is required for suppression of MCP-1 production and CAM expression. The cellular responses that are elicited on prolonged exposure to bFGF remain primarily unknown. The FGF receptor family consists of four transmembrane receptor tyrosine kinases (TKs). The significance of these multiple isoforms is not fully understood. 38 VEGF, bFGF, and their cognate receptor TKs are strongly implicated in angiogenesis associated with solid tumors. SU6668 is a potent anti-angiogenic small-molecule being evaluated in clinical trials against a variety of solid tumors. 58 This compound is an inhibitor of several receptor TKs, including those of the bFGF, VEGF, and PDGF receptor families. 58,59 Simultaneously targeting TK receptors that are present on endothelial cells (VEGF, bFGF, and PDGF) represents a novel approach for anti-angiogenic therapy. Here we demonstrate that SU6668 not only inhibits bFGF-induced HUVEC proliferation (Figure 8) ▶ , but that SU6668 also blocks the bFGF-induced inhibition of monocyte TEM and of HUVEC adhesion molecule expression despite continued presence of bFGF (Figures 9 and 10) ▶ ▶ . This result suggests that: the suppression of endothelial activation by bFGF is mediated via the FGF receptor TK because VEGF and PDGF did not effect HUVEC adhesion molecule expression and, in addition to its anti-angiogenic effect, SU6668 may also increase monocyte emigration at sites of angiogenesis. Further studies with selective inhibitors of FGF receptor TK is warranted to follow-up on these observations, especially using in vivo models.
Perhaps the best characterized pathway involved in induction of endothelial cell ICAM-1, VCAM-1, E-selectin, and MCP-1 gene expression involves the nuclear factor-κB nuclear transcription factor. 60,61 Activation of nuclear factor-κB requires phosphorylation, dissociation, and proteolytic degradation of IκB before nuclear translocation of nuclear factor-κB and activation of gene transcription. 62 Our preliminary results indicate that prolonged bFGF treatment does not induce IκB phosphorylation in HUVECs, and IκB was phosphorylated to the expected degree after TNF-α stimulation, ie, bFGF had no influence on TNF-α-induced phosphorylation of IκB (data not shown). This suggests that the inhibitory effect of bFGF on TNF-α-induced ICAM-1, VCAM-1, E-selectin, and MCP-1 production may not be because of inhibition of the activation of the IκB/nuclear factor-κB pathway. Other mechanisms involved in this process are being investigated to elucidate the sustained inhibitory effects of bFGF on endothelial cell activation.
In conclusion, our results show that prolonged treatment of HUVECs with bFGF or aFGF, but not VEGF markedly decreases endothelial cell-monocyte adhesive interaction and the ability of endothelium to facilitate monocyte transmigration. This effect is associated with down-regulation of basal and stimulated endothelial cell expression of ICAM-1, VCAM-1, E-selectin, and MCP-1 production. All of these effects likely contribute to impaired monocyte TEM and strongly suggest that bFGF is a potent down-regulator of inflammatory cell trafficking at sites of tumor growth, inflammation, and angiogenesis. This may contribute to the protection of growing tumors from monocyte/macrophage invasion. However, the effect of bFGF on monocyte TEM and HUVEC CAM expression can be prevented by the receptor TK inhibitor SU6668 suggesting that this agent may not only inhibit angiogenesis but also promote the host inflammatory response, eg, in tumors. Further understanding of regulation of the endothelium and its multiple cellular interactions by growth factors and angiogenesis inhibitors may facilitate development of novel therapeutic strategies in cancer, inflammatory, and vascular diseases.
Acknowledgments
We thank the colleagues listed in Materials and Methods who supplied valuable antibodies and reagents for these studies, Mr. D. Rowter and Ms. C. Jordan for outstanding technical assistance, and Ms. M. Hopkins for expert secretarial help.
Footnotes
Address reprint requests to Dr. Andrew C. Issekutz, Dalhousie University, 8 East Research Labs, IWK Health Center, 5850 University Ave., Halifax, NS B3J 3G9. E-mail: andrew.issekutz@iwk.nshealth.ca.
Supported by grant MT-7684 from the Canadian Institutes of Health Research. H. Z. was the recipient of Fellowships from the Canadian Institutes of Health Research and the Nova Scotia Research Foundation.
References
- 1.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994, 76:301-314 [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM: Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91:3527-3561 [PubMed] [Google Scholar]
- 3.Iruela-Arispe ML, Dvorak HF: Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 1997, 78:672-677 [PubMed] [Google Scholar]
- 4.Heil M, Clauss M, Suzuki K, Buschmann IR, Willuweit A, Fischer S, Schaper W: Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur J Cell Biol 2000, 79:850-857 [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Florio T, Giunciuglio D, Masiello L, Carlone S, Corsaro A, Thellung S, Cai T, Noonan DM, Schettini G: Somatostatin controls Kaposi’s sarcoma tumor growth through inhibition of angiogenesis. FASEB J 1999, 13:647-655 [DOI] [PubMed] [Google Scholar]
- 6.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS 1997, 79:233-269 [DOI] [PubMed] [Google Scholar]
- 7.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D: Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325:257-259 [DOI] [PubMed] [Google Scholar]
- 8.McCourt M, Wang JH, Sookhai S, Redmond HP: Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg 1999, 134:1325-1332 [DOI] [PubMed] [Google Scholar]
- 9.Yeh CH, Peng HC, Huang TF: Cytokines modulate integrin alpha(v)beta(3)-mediated human endothelial cell adhesion and calcium signaling. Exp Cell Res 1999, 251:57-66 [DOI] [PubMed] [Google Scholar]
- 10.van de Stolpe A, van der Saag PT: Intercellular adhesion molecule-1. J Mol Med 1996, 74:13-33 [DOI] [PubMed] [Google Scholar]
- 11.Butcher EC: Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991, 67:1033-1036 [DOI] [PubMed] [Google Scholar]
- 12.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC: Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 1995, 80:413-422 [DOI] [PubMed] [Google Scholar]
- 13.Luscinskas FW, Cybulsky MI, Kiely JM, Peckins CS, Davis VM, Gimbrone MA, Jr: Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol 1991, 146:1617-1625 [PubMed] [Google Scholar]
- 14.Issekutz AC, Rowter D, Springer TA: Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol 1999, 65:117-126 [DOI] [PubMed] [Google Scholar]
- 15.Smith CW: Leukocyte-endothelial cell interactions. Semin Hematol 1993, 30:45-54 [PubMed] [Google Scholar]
- 16.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC: Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA 1991, 88:7538-7542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromblad S, Cheresh DA: Integrins, angiogenesis and vascular cell survival. Chem Biol 1996, 3:881-885 [DOI] [PubMed] [Google Scholar]
- 18.Borgstrom P, Hughes GK, Hansell P, Wolitsky BA, Sriramarao P: Leukocyte adhesion in angiogenic blood vessels. Role of E-selectin, P-selectin, and beta2 integrin in lymphotoxin-mediated leukocyte recruitment in tumor microvessels. J Clin Invest 1997, 99:2246-2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolomecki K, Stepien H, Narebski JM: Vascular endothelial growth factor and basic fibroblast growth factor evaluation in blood serum of patients with hormonally active and inactive adrenal gland tumours. Cytobios 2000, 101:55-64 [PubMed] [Google Scholar]
- 20.Komorowski J, Jankewicz J, Stepien H: Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and soluble interleukin-2 receptor (sIL-2R) concentrations in peripheral blood as markers of pituitary tumours. Cytobios 2000, 101:151-159 [PubMed] [Google Scholar]
- 21.Griffioen AW, Tromp SC, Hillen HF: Angiogenesis modulates the tumour immune response. Int J Exp Pathol 1998, 79:363-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH: Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med 1995, 181:811-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrmann-Benzakein E, Ma MN, Rubbia-Brandt L, Mentha G, Ruefenacht D, Sappino AP, Pepper MS: Elevated levels of angiogenic cytokines in the plasma of cancer patients. Int J Cancer 2000, 85:40-45 [DOI] [PubMed] [Google Scholar]
- 24.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G: Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996, 56:1111-1117 [PubMed] [Google Scholar]
- 25.Zhang H, Issekutz AC: Growth factor regulation of neutrophil-endothelial cell interactions. J Leukoc Biol 2001, 70:225-232 [PubMed] [Google Scholar]
- 26.Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, Fong TA, Strawn LM, Sun L, Tang C, Hawtin R, Tang F, Shenoy N, Hirth KP, McMahon G, Cherrington JM: SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res 2000, 60:4152-4160 [PubMed] [Google Scholar]
- 27.Chuluyan HE, Issekutz AC: VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest 1993, 92:2768-2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyum A: Isolation of human blood monocytes with Nycodenz, a new non-ionic iodinated gradient medium. Scand J Immunol 1983, 17:429-436 [DOI] [PubMed] [Google Scholar]
- 29.Jaffe EA, Nachman RL, Becker CG, Minick CR: Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 1973, 52:2745-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issekutz AC, Chuluyan HE, Lopes N: CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol 1995, 57:553-561 [DOI] [PubMed] [Google Scholar]
- 31.Issekutz AC, Lopes N: Endotoxin activation of endothelium for polymorphonuclear leucocyte transendothelial migration and modulation by interferon-gamma. Immunology 1993, 79:600-607 [PMC free article] [PubMed] [Google Scholar]
- 32.Issekutz TB, Wykretowicz A: Effect of a new monoclonal antibody, TA-2, that inhibits lymphocyte adherence to cytokine stimulated endothelium in the rat. J Immunol 1991, 147:109-116 [PubMed] [Google Scholar]
- 33.Chuluyan HE, Lang BJ, Yoshimura T, Kenney JS, Issekutz AC: Chemokine production and adhesion molecule expression by neural cells exposed to IL-1, TNF alpha and interferon gamma. Life Sci 1998, 63:1939-1952 [DOI] [PubMed] [Google Scholar]
- 34.Chuluyan HE, Osborn L, Lobb R, Issekutz AC: Domains 1 and 4 of vascular cell adhesion molecule-1 (CD106) both support very late activation antigen-4 (CD49d/CD29)-dependent monocyte transendothelial migration. J Immunol 1995, 155:3135-3134 [PubMed] [Google Scholar]
- 35.Meerschaert J, Furie MB: The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol 1995, 154:4099-4112 [PubMed] [Google Scholar]
- 36.Luscinskas FW, Gimbrone MA, Jr: Endothelial-dependent mechanisms in chronic inflammatory leukocyte recruitment. Annu Rev Med 1996, 47:413-421 [DOI] [PubMed] [Google Scholar]
- 37.Meerschaert J, Furie MB: Monocytes use either CD11/CD18 or VLA-4 to migrate across human endothelium in vitro. J Immunol 1994, 52:1915-1926 [PubMed] [Google Scholar]
- 38.Friesel RE, Maciag T: Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J 1995, 9:919-925 [DOI] [PubMed] [Google Scholar]
- 39.Allport JR, Muller WA, Luscinskas FW: Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J Cell Biol 2000, 148:203-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer GE, Yu E, Siegal JA, Petteway JC, Blumenstein BA, Brawer MK: Serum basic fibroblast growth factor in men with and without prostate carcinoma. Cancer 1995, 76:2304-2311 [DOI] [PubMed] [Google Scholar]
- 41.Chopra V, Dinh TV, Hannigan EV: Angiogenin, interleukins, and growth-factor levels in serum of patients with ovarian cancer: correlation with angiogenesis. Cancer J Sci Am 1996, 2:279. [PubMed] [Google Scholar]
- 42.Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT: Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 1993, 90:7533-7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hata Y, Rook SL, Aiello LP: Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes 1999, 48:1145-1155 [DOI] [PubMed] [Google Scholar]
- 44.Pepper MS, Mandriota SJ: Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp Cell Res 1998, 241:414-425 [DOI] [PubMed] [Google Scholar]
- 45.Goto F, Goto K, Weindel K, Folkman J: Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest 1993, 69:508-517 [PubMed] [Google Scholar]
- 46.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM: Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 1995, 92:II365-II371. [DOI] [PubMed] [Google Scholar]
- 47.Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS: Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res 2001, 88:1135-1141 [DOI] [PubMed] [Google Scholar]
- 48.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P: Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 1998, 141:1659-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JH, Wang XC, Kan M, Sato JD: Effect of FGF-1 and FGF-2 on VEGF binding to human umbilical vein endothelial cells. Cell Biol Int 2001, 25:257-260 [DOI] [PubMed] [Google Scholar]
- 50.Graeven U, Andre N, Achilles E, Zornig C, Schmiegel W: Serum levels of vascular endothelial growth factor and basic fibroblast growth factor in patients with soft-tissue sarcoma. J Cancer Res Clin Oncol 1999, 125:577-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, Kantoff P, Shuman MA, Small EJ: Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res 2001, 61:2533-2536 [PubMed] [Google Scholar]
- 52.Lukacs NW, Strieter RM, Elner V, Evanoff HL, Burdick MD, Kunkel SL: Production of chemokines, interleukin-8 and monocyte chemoattractant protein-1, during monocyte: endothelial cell interactions. Blood 1995, 86:2767-2773 [PubMed] [Google Scholar]
- 53.Chuluyan HE, Schall TJ, Yoshimura T, Issekutz AC: IL-1 activation of endothelium supports VLA-4 (CD49d/CD29)-mediated monocyte transendothelial migration to C5a, MIP-1 alpha, RANTES, and PAF but inhibits migration to MCP-1: a regulatory role for endothelium-derived MCP-1. J Leukoc Biol 1995, 58:71-79 [DOI] [PubMed] [Google Scholar]
- 54.Wempe F, Lindner V, Augustin HG: Basic fibroblast growth factor (bFGF) regulates the expression of the CC chemokine monocyte chemoattractant protein-1 (MCP-1) in autocrine-activated endothelial cells. Arterioscler Thromb Vasc Biol 1997, 17:2471-2478 [DOI] [PubMed] [Google Scholar]
- 55.Snyderman R, Pike MC, Blaylock BL, Weinstein P: Effects of neoplasms on inflammation: depression of macrophage accumulation after tumor implantation. J Immunol 1976, 116:585-589 [PubMed] [Google Scholar]
- 56.Kleinerman ES, Zwelling LA, Howser D, Barlock A, Young RC, Decker JM, Bull J, Muchmore AV: Defective monocyte killing in patients with malignancies and restoration of function during chemotherapy. Lancet 1980, 2:1102-1105 [DOI] [PubMed] [Google Scholar]
- 57.Matthews N: Human monocyte killing of tumor cells: contribution of an extracellular cytotoxin. Adv Exp Med Biol 1982, 155:721-729 [DOI] [PubMed] [Google Scholar]
- 58.Liekens S, De Clercq E, Neyts J: Angiogenesis: regulators and clinical applications. Biochem Pharmacol 2001, 61:253-270 [DOI] [PubMed] [Google Scholar]
- 59.Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE, Bucana CD, McMahon G, Ellis LM: Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res 2001, 61:1464-1468 [PubMed] [Google Scholar]
- 60.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T: Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 1995, 9:899-909 [PubMed] [Google Scholar]
- 61.Chen CC, Manning AM: Transcriptional regulation of endothelial cell adhesion molecules: a dominant role for NF-kappa B. Agents Actions 1995, 47:S135-S141 [DOI] [PubMed] [Google Scholar]
- 62.Thanos D, Maniatis T: NF-kappa B: a lesson in family values. Cell 1995, 80:529-532 [DOI] [PubMed] [Google Scholar]