Abstract
Peritonitis, a common complication of peritoneal dialysis, is followed by acute changes in the function of the peritoneum. The role of inflammatory cytokines in these processes is not clearly identified. We used adenoviral-mediated gene transfer to transiently overexpress interleukin (IL)-1β (AdIL-1β) or tumor necrosis factor (TNF)-α (AdTNF-α) in the rat peritoneum then used a modified equilibrium test to study the histological and functional changes. Overexpression of IL-1β or TNF-α led to an acute inflammatory response. Both inflammatory cytokines induced an early expression of the angiogenic cytokine, vascular endothelial growth factor, along with increased expression of the profibrotic cytokine, transforming growth factor-β1, along with fibronectin expression and collagen deposition in peritoneal tissues. Both inflammatory cytokines induced angiogenesis, increased solute permeability, and ultrafiltration dysfunction at earlier time points. Changes in structure and function seen in AdTNF-α-treated animals returned to normal by 21 days after infection, whereas AdIL-1β-treated animals had persistently increased vasculature with submesothelial thickening and fibrosis. This was associated with up-regulation TIMP-1. TNF-α or IL-1β both induce acute changes in the peritoneum that mimic those seen in peritoneal dialysis patients who experience an episode of peritonitis. These functional changes were associated with early angiogenesis that resolved rapidly after exposure to TNF-α. IL-1β exposure, however, led to a different response with sustained vascularization and fibrosis. IL-1β inhibition may be a therapeutic goal in acute peritonitis to prevent peritoneal damage.
Peritonitis is a common complication of peritoneal dialysis. 1 The response of the peritoneum to infective organisms involves the inflammatory cytokines and the interaction between resident cell populations: macrophages, mesothelial cells, and fibroblasts. 2,3 The earliest response involves tumor necrosis factor (TNF)-α and interleukin (IL)−1 3 derived from macrophages in response to bacterial products. 4 What follows is a complex interaction between inflammatory cytokines, such as IL-6, chemokines and subsequent leukocyte transmigration, prostaglandins, nitric oxide, and adhesion molecules. There is also an anti-inflammatory response consisting of soluble receptors to TNF-α, 5 IL-1 receptor antagonist, 6 and repair molecules such as hyaluronan. 7
In the first month after peritonitis, patients develop an acute dysfunction of the peritoneum as a dialysis membrane. 8 This is characteristically associated with increased transport of small molecular weight solutes and ultrafiltration failure. The mechanism for this is not well established, but vascular effects of nitric oxide 9 or prostaglandins 10 have been hypothesized to play a role.
Recurrent peritonitis has been shown, in some studies, to be associated with long-term peritoneal membrane changes. 11,12 As in the acute changes after peritonitis, long-term changes are associated with increased solute transport and decreased ultrafiltration. In this setting, human biopsy studies 13-15 and animal experiments 16 have identified an increase in the peritoneal-associated vasculature, which seems to be the primary cause of increased solute transport. A fibrogenic response and associated vasculopathy have also been identified in patients on long-term peritoneal dialysis. 17
The association between peritoneal inflammation, angiogenesis, and fibrosis is not clear. IL-1β has been identified in vivo as having strong fibrogenic properties through up-regulation of transforming growth factor (TGF)-β. 18 This has been confirmed in in vitro mesothelial cell culture work. 19 Both IL-1β and TNF-α have angiogenic properties as has been demonstrated in studies using rat mesenteric window assays 20,21 and TNF-α has been associated with up-regulation of the angiogenic cytokine vascular endothelial growth factor (VEGF) in cell culture. 22
We have previously described the use of adenovirus-mediated gene transfer of cytokines and growth factors as an effective tool in elucidating functional and morphological changes in the peritoneum. 23 In the following experiments, we compared the effects of transient overexpression of TNF-α or IL-1β on the peritoneum of rats using adenovirus-mediated gene transfer (AdTNF-α or AdIL-1β) and controlled with a null adenovirus (AdDL70). Four days after infection with either AdTNF-α or AdIL-1β we saw increased vascularity of the peritoneum, increased solute transport, and decreased ultrafiltration. By day 21, the animals treated with AdTNF-α returned to normal peritoneal morphology and function, but the AdIL-1β-treated animals remained quite abnormal with peritoneal fibrosis and persistent vascularization.
Materials and Methods
Adenovirus
The construction of the adenovirus vectors AdIL-1β, 18 AdTNF-α, 24 and AdDL70 25 have been previously described. Adenovirus preparations were purified by CsCl gradient centrifugation and PD-10 Sephadex chromatography (Amersham Pharmacia, Baie d’Urfe, Canada) and plaque-titered on 293 cells as previously described. 26
Animals
All animal studies were performed according to the Canadian Council on Animal Care Guidelines. Three groups of female Sprague-Dawley rats (Harlan, Indianapolis, IN), 200 to 250 g (corresponding to 6 to 8 weeks of age 27 ) were studied. The first group (n = 27) received an intraperitoneal injection of AdIL-1β. The second group (n = 20) received AdTNF-α and the third group (n = 28) received a null control virus (AdDL70). All were administered adenovirus at a dose of 1.5 to 2.0 × 10 9 plaque-forming units/ml diluted to 100 μl in phosphate-buffered saline on day 0. Animals did not receive anti-inflammatory agents during the experimental protocol. Animals from each group were sacrificed on days 4, 7, 21, and 28 after adenovirus administration. Before sacrifice, 0.09 ml/g of 2.5% Dianeal (Baxter Health Care, McGaw Park, IL) was administered intraperitoneally. Four hours later, the peritoneum was opened and the entire fluid content removed for accurate ultrafiltration measurement. Net ultrafiltration was the volume of fluid removed after 4 hours minus the volume of fluid administered. Blood samples were drawn and the entire anterior abdominal wall was removed after skin and subcutaneous tissue was removed. The lower portion of this tissue was stored in formalin and the upper portion taken for RNA extraction. Mesenteric tissue was taken and frozen in liquid nitrogen.
Whole blood was centrifuged at 5000 rpm for 10 minutes and the serum removed. Peritoneal fluid samples were centrifuged at 1500 rpm for 5 minutes. Samples were analyzed on a Hitachi 917 automated chemistry analyzer (Roche Diagnostics, Laval, Canada) for glucose and albumin. Mass transport of glucose out of the peritoneum was calculated as (initial dialysate glucose × initial volume) − (final dialysate glucose × final volume). Albumin clearance was calculated as mass transport divided by the serum solute concentration. All values were corrected for animal weight at sacrifice.
Cell number in the peritoneal fluid was counted with a hemocytometer. The fluid was plated to a glass slide by cytospin (Shandon Inc., Pittsburgh, PA) and stained (Biochemical Science Inc., Swedesboro, NJ). Cell differential was counted on at least 300 cells.
Histology
Tissue samples at sacrifice from the lower anterior abdominal wall or omentum were taken and fixed in a sufficient amount of 4% phosphate-buffered formaldehyde for 24 hours. The tissue samples were then paraffin-processed, embedded, and 5-μm sections cut. Cut sections were then stained for Masson’s trichrome and immunohistochemistry was performed with antibodies to Factor VIII-von Willebrand factor (vWF) antigen (DAKO Corp., Carpinteria CA). Negative control sections were run in parallel with nonimmune mouse or rabbit serum. All sections were deparaffinized in xylene followed by 100% ethanol and then placed in a methanol H2O2 solution for 30 minutes to block endogenous peroxidase activity. After hydration to water with graded alcohols, the sections were placed in 0.05 mol/L Tris-buffered saline, pH 7.6. The sections were digested with 0.05% Pronase (Sigma Chemical Co., St. Louis, MO) in Tris-buffered saline with calcium chloride for 17 minutes at room temperature then blocked in 5% normal goat serum followed by a 1-hour incubation in the 1:500 rabbit anti-human factor VIII in 1% normal goat serum. Sections were then incubated in a prediluted kit of a biotinylated goat anti-rabbit followed by a streptavidin/peroxidase conjugate (Zymed Labs, San Francisco, CA) as per the manufacturer’s instructions. Immunohistochemistry incubations were performed at room temperature and sections were washed in between incubations 3 × 5 minutes with 0.05 mol/L Tris-buffered saline, pH 7.6, except before the addition of primary antibody. Sections were rinsed in 0.05 mol/L acetate buffer, pH 5.0, before development in an 3-amino-9-ethylcarbazole (AEC) chromogen substrate for 15 minutes. Sections were counterstained in Mayer’s hematoxylin for 2 minutes before mounting with glycerin gelatin.
Quantitative Immunohistochemistry
Low-power fields from factor VIII-immunostained sections of the anterior abdominal wall were digitized using a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany). All sections were analyzed by the same image-processing algorithm using Leica Qwin Image Processing Software (Leica Imaging Systems, Cambridge, England). Results are reported as number of vessels/mm 2 of peritoneal tissue. We were able to estimate the total vessel cross-section area in each digitized image and we could therefore calculate an average cross-sectional area per vessel for each slide analyzed.
Hydroxyproline Assay
A portion of mesentery was taken and frozen for a hydroxyproline assay, modified from Woessner’s method. 28 Tissues were weighed, homogenized in water, centrifuged at 1000 rpm for 5 minutes, and the superficial fatty material removed by vacuum suction. Solid material was precipitated with trichloroacetic acid with centrifugation at 1500 rpm for 15 minutes at 4°C. Samples were hydrolyzed overnight in 6 N HCl at 110°C. Hydroxyproline content is quantified by Erlich’s reagent (Sigma) and assayed by measuring the optical density at 557 nm. A hydroxyproline standard sample (Sigma) was used to create a standard curve.
Cytokine Analysis
Peritoneal fluid taken at sacrifice was analyzed for the following cytokines using enzyme-linked immunosorbent assay (ELISA) (all R&D Systems, Minneapolis, MN) as directed by the manufacturer: human TGF-β (cross-reactive with rat), murine VEGF (cross-reactive with rat), rat IL-6, rat TNF-α (cross-reactive with murine), human IL-1β (not cross-reactive with rat), and murine TNF-soluble receptor II (TNFsrII, cross-reactive with rat). To measure total TGF-β, samples were first activated using 1 mol/L HCl for 10 minutes then normalized with 1 N NaOH to dissociate TGF-β from its latency-associated binding protein.
Frozen omental tissue was taken and homogenized in Trizol reagent (Life Technologies, Burlington, Ontario, Canada). Protein was extracted from the phenol layer after centrifugation according to the manufacturer’s protocol. Total protein in this extract was assayed using a standard protein assay method (BioRad DC Protein Assay; BioRad Laboratories, Mississauga, Canada). Equal quantities of protein were then assayed using murine VEGF ELISA (R&D Systems).
RNase Protection Assay
The peritoneal surfaces of the anterior abdominal wall sections were immersed for 15 minutes in Trizol reagent. The parietal peritoneum was gently scraped and the Trizol collected and processed according to the manufacturer’s instruction for isolation of RNA. The concentration of RNA resuspended in RNase-free water was measured by optical density at 260 nm. RNA (8 μg) was then hybridized overnight with a custom probe set (Pharmingen, Mississauga, ON) labeled with α-32P-UTP (New England Nuclear, Boston, MA). The custom probe set contains different length probes for fibronectin, TGF-β1, tissue inhibitor of metalloproteinase-1 (TIMP-1), and housekeeping genes GAPDH and L32 for loading control. The hybridized samples were extracted using phenol/chloroform and acetate precipitation and then washed with ethanol. The extracted bound RNA was then run on a 5% polyacrylamide gel, transferred to blotting paper, dried, and exposed for 4 days to film (Eastman Kodak, Rochester, NY).
The images obtained were digitized and analyzed for band density using Scion Image software (Scion Corp, Frederick, MD). Densities were standardized to L32.
Statistics
Data are presented ± SEM unless otherwise noted. Comparison between groups was made by t-test. We used regression analysis to compare the cytokine concentration on day 4 in the peritoneal fluid and subsequent vascularization of the peritoneal membrane and combined all three treatment groups (AdIL-1β, AdTNF-α, AdDL70) in these results.
Results
Adenoviral Response
In previous work, we have identified an early (48 hour) inflammatory response to AdDL70 in similar dosage used in these experiments. 29 There were no adenoviral effects identified by 4 days, 23 the earliest time point used in our experiments. We therefore did not include a nonadenoviral control group in these experiments.
We have previously shown that adenovirus is effectively taken up, and the transgene product expressed, by mesothelial cells in the peritoneum. 23 The peak of expression is 4 to 7 days after infection, and the total duration of expression is 10 to 14 days. In these experiments, we confirmed the high levels of expression of the transgene product by analysis of the peritoneal dialysis fluid with the appropriate ELISA. Four days after infection peritoneal fluid was taken after a 4-hour dwell and analyzed by ELISA. Animals treated with AdIL-1β had 326 ± 89 pg/ml of human IL-1β and animals treated with AdTNF-α had 2076 ± 1131 pg/ml of rodent TNF-α measured in the peritoneal fluid (Table 1) ▶ .
Table 1.
Cytokine Concentration in the Peritoneal Fluid and Tissue 4, 7, and 21 Days after Infection with Control Adenovirus, AdTNF-α, or AdIL-1β
| Day 4 | Day 7 | Day 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AdDL70 | AdTNF-α | AdIL-1β | AdDL70 | AdTNF-α | AdIL-1β | AdDL70 | AdTNF-α | AdIL-1β | |
| Human IL-1β (pg/ml) | <10 | <10 | 326 ± 89*† | <10 | <10 | 77 ± 105 | <10 | <10 | <10 |
| Rat IL1β (pg/ml) | 32 ± 10 | 302 ± 255* | 310 ± 186* | 31 ± 10 | 114 ± 92 | 638 ± 800 | 46 ± 21 | 28 ± 2 | 251 ± 301 |
| TNF-α (pg/ml) | 69 ± 8 | 2076 ± 1131* | 69 ± 7† | 58 ± 14 | 365 ± 482* | 83 ± 12† | nd | 66 ± 11 | 69 ± 9 |
| Total TGF-β (pg/ml) | 131 ± 80 | 186 ± 82 | 756 ± 441*† | 88 ± 50 | 306 ± 137* | 1056 ± 610*† | 63 ± 2 | 64 ± 18 | 166 ± 101 |
| VEGF (pg/ml) | 75 ± 44 | 172 ± 130* | 210 ± 173* | 85 ± 49 | 205 ± 133* | 262 ± 157* | 63 ± 24 | 30 ± 4 | 47 ± 17 |
| Tissue VEGF (pg/ml) | 43 ± 10 | 72 ± 40 | 76 ± 16 | 47 ± 14 | 45 ± 21 | 78 ± 8* | nd | nd | nd |
*P < 0.05 compared with AdDL70 control.
†P < 0.05 compared with AdTNF-α.
Data shown ± SD.
nd, No data.
Inflammatory Response
The inflammatory response after intraperitoneal administration of AdTNF-α, AdIL-1β, or control adenovirus was measured from various markers in the peritoneal fluid and the results are shown in Table 2 ▶ . The cellular response to overexpression of IL-1β and TNF-α was dramatic with a significant increase in the total number of cells and a disproportionate increase in neutrophils measured in the peritoneal fluid. There was also an associated weight loss that was more dramatic in the AdIL-1β-treated animals compared to control adenovirus-treated animals. Rodent IL-6 was measured by ELISA in peritoneal fluid and was elevated after exposure to IL-1β or TNF-α (Table 1) ▶ . Finally, we measured rodent TNFsrII by ELISA in peritoneal fluid and this was significantly elevated 4 and 7 days after infection with AdIL-1β and AdTNF-α (Table 1) ▶ .
Table 2.
Inflammatory Changes Measured 4 and 7 Days after Infection with Control Adenovirus, AdTNF-α, AdIL-1β
| Day 4 | Day 7 | |||||
|---|---|---|---|---|---|---|
| AdDL70 | AdTNF-α | AdIL-1β | AdDL70 | AdTNF-α | AdIL-1β | |
| White blood cells (104/ml) | 77 ± 69 | 158 ± 81* | 952 ± 275*† | 132 ± 164 | 262 ± 148 | 763 ± 541*† |
| Monocyte, % | 19.4 | 15.3 | 8.0* | 28.9 | 20.3 | 13.2 |
| Macrophage, % | 46.2 | 41.4 | 30.7 | 50.5 | 49.4 | 27.1 |
| Neutrophil, % | 4.2 | 31.7* | 48.3* | 3.5 | 25.9* | 58.3*† |
| Eosinophil, % | 27.4 | 9.8* | 11.3* | 19.4 | 3.8 | 1.1 |
| Change in weight from day 0 (g) | 1.3 ± 5.3 | −2.1 ± 2.8 | −5.6 ± 4.1 | 2.8 ± 6.1 | 2.2 ± 3.9 | −5.9 ± 4.2 |
| IL-6 (pg/ml) | 59.1 ± 0.3 | 988 ± 857 | 1451 ± 497 | 66 ± 2 | 74 ± 8 | 1788 ± 746 |
| TNFsrII (pg/ml) | 34 ± 16 | 604 ± 420* | 751 ± 364* | 63 ± 11 | 1156 ± 275* | 917 ± 388* |
*P < 0.05 compared with AdDL70 control.
†P < 0.05 compared with AdTNF-α.
Data shown ± SD.
We also noted a quantitative difference between the inflammatory response to AdIL-1β or AdTNF-α. Specifically the response to transient overexpression of IL-1β was more intense, as measured by total cell count and percentage of neutrophils, especially 7 days after infection. Also, two animals in the AdIL-1β group became very ill in the first week after treatment and required euthanasia. The response to IL-1β was more prolonged, with levels of TNFsrII remaining significantly elevated in AdIL-1β-treated animals 21 days after infection.
Cytokine Response after Transient Overexpression of TNF-α or IL-1β
We analyzed the peritoneal fluid after a 4-hour dwell for rat IL-1β, TNF-α, TGF-β, and VEGF using ELISA (Table 1) ▶ . Both AdTNF-α and AdIL-1β treatment induced the production of endogenous IL-1β measured using a rodent-specific assay. Interestingly, AdIL-1β-treated animals did not show an increase in peritoneal concentration of TNF-α. Overexpression of both TNF-α and IL-1β induced a significant increase in the peritoneal fluid concentration of total TGF-β1 and VEGF compared to AdDL70-treated animals 7 days after infection. We identified a threefold increase in peritoneal concentration of TGF-β1 7 days after AdIL-1β treatment compared to AdTNF-α. Increases in peritoneal VEGF concentrations were similar after exposure to IL-1β and TNF-α.
To confirm that VEGF was present in the tissues after adenovirus infection, we isolated protein from omental homogenates and assayed for VEGF. We noted an increase in tissue VEGF that was significantly elevated 7 days after treatment with AdIL-1β (Table 1) ▶ .
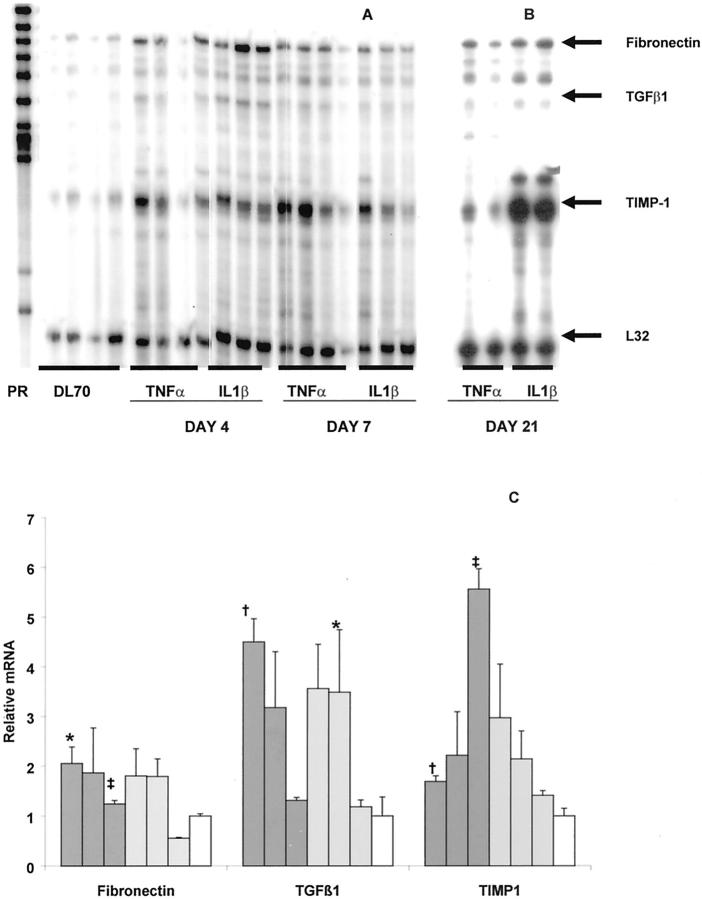
We isolated RNA from parietal peritoneal tissue of animals 4, 7, and 21 days after receiving adenovirus and analyzed it using an RNase protection assay (Figure 1) ▶ . Quantitative density analysis indexed to L32 and referenced to AdDL70-treated animals revealed that at 4 and 7 days after infection, both TNF-α and IL-1β led to an increase in mRNA signal for fibronectin and TGF-β1. These changes declined to baseline by day 21 except for a small persistent elevation in fibronectin mRNA in AdIL-1β-treated animals at day 21. Most striking was the progressive increase in TIMP-1 mRNA expression seen in AdIL-1β-treated animals at day 21 (Figure 1) ▶ .
Figure 1.
RNase protection assay. RNA was extracted from parietal peritoneal tissue and hybridized with a radiolabeled probe set. Samples were then RNase-digested, purified, and separated by polyacrylamide gel electrophoresis. A: PR is unprotected probe. Samples are from animals treated with AdDL70, AdTNF-α, or AdIL-1β 4 and 7 days after infection. B: Day 21 samples run on a separate gel. Arrows indicate signal from hybridized mRNA for fibronectin, TGF-β1, TIMP-1, and housekeeping gene L32 for loading control. C: Quantification of band density. Images were digitized and band density analyzed. Density was corrected for loading by L32 and then normalized to AdDL70-treated animals. Dark gray bars, AdIL-1β-treated animals; light gray bars, AdTNF-α-treated animals; open bars, AdDL70-treated animals. The three bars for each treatment group represent days 4, 7, and 21 after adenovirus administration. AdDL70 animals are day 4 after adenoviral infection. There was a similar up-regulation of fibronectin and TGF-β1 in AdIL-1β- and AdTNF-α-treated animals at days 4 and 7. TIMP-1 was strongly expressed at day 21 in AdIL-1β but not AdTNF-α animals. Data represents three to four animals at each data point. *, P < 0.01 compared with AdDL70 control; †, P < 0.05 compared with AdDL70; ‡, P < 0.01 for AdIL-1β compared with AdTNF-α-treated animals.
Changes in Peritoneal Membrane Morphology
Transient overexpression of both IL-1β and TNF-α led to early, significant histological changes in the parietal peritoneum compared to animals treated with the control adenovirus, AdDL70 (Figure 2) ▶ . The mesothelial cells, which normally are flattened against the basement membrane, became rounded up with enlarged nuclei. The submesothelial zone became substantially thickened with inflammatory cells and edematous changes. These changes were evident in both parietal and visceral peritoneal tissue as seen in omental tissue 7 days after infection (Figure 3) ▶ .
Figure 2.
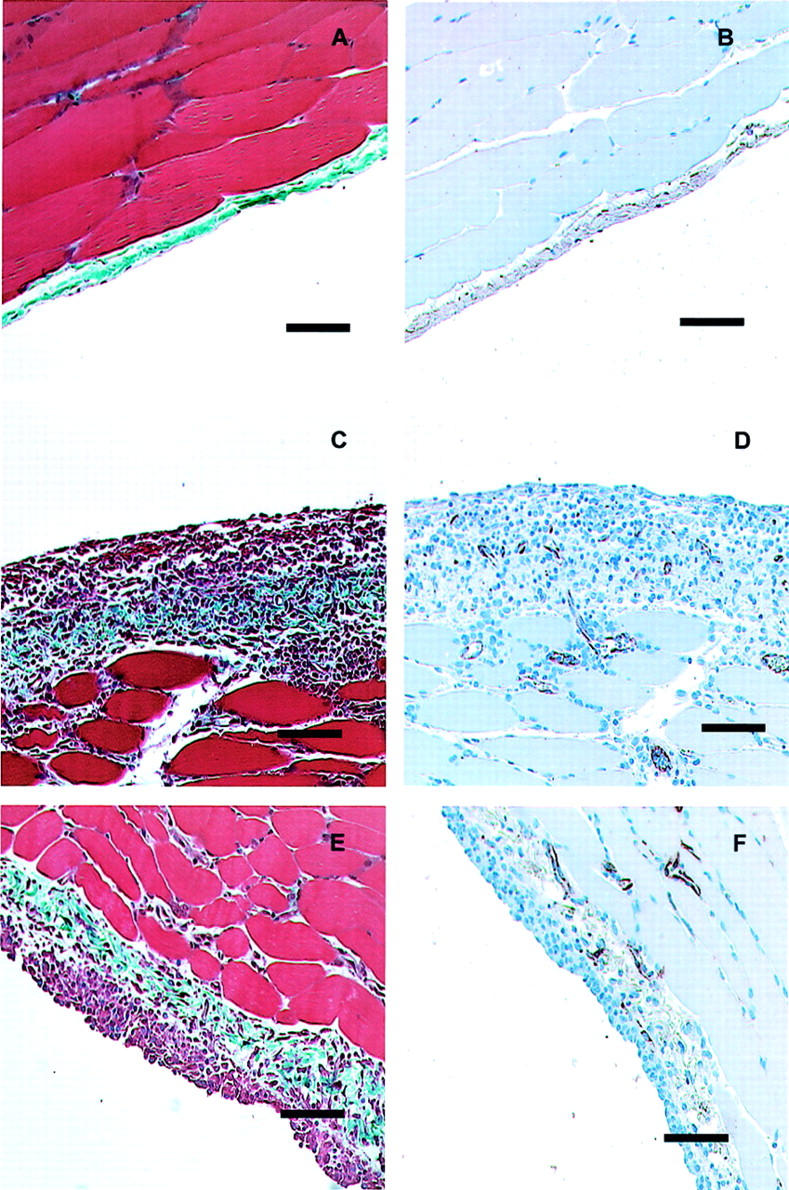
Histology of the anterior abdominal wall 7 days after infection with AdDL70 (A and B), AdIL-1β (C and D), or AdTNF-α (E and F). After control adenovirus infection, the parietal peritoneum has normal histology (A) with thin submesothelial zone with few cells, intact mesothelial cell layer, and few blood vessels (B). Seven days after infection with AdIL-1β or AdTNF-α, similar changes are observed. There is an increase in the submesothelial thickness, collagen deposition, and markedly increased cellularity (C and E). There is an associated increase in neovascularization (D and F). Scale bars, 100 μm. Masson’s trichrome (A, C, and E) and Factor VIII-vWF immunohistochemistry (B, D, and F).
Figure 3.

Histology of the visceral peritoneum (omentum) 7 days after infection with AdDL70 (A), AdIL-1β (B), or ADTNF-α (C). AdIL-1β and AdTNF-α-treated animals demonstrate increased cellular infiltration in omental tissue with collagen deposition (arrows). Scale bars, 200 μm. Masson’s trichrome.
Seven days after infection with AdIL-1β and AdTNF-α there was a dramatic angiogenesis that occurred in the peritoneum as evidenced by factor VIII-stained sections (Figure 2) ▶ . Peritoneal-associated vessels were evaluated using digital image analysis from these sections (Figure 4) ▶ . Overexpression of IL-1β or TNF-α led to significantly different kinetics of angiogenesis. Animals treated with AdIL-1β had increased peritoneal-associated blood vessels at each time point studied compared with AdDL70-treated animals. AdTNF-α-treated animals had an increase in peritoneal vasculature that was significantly elevated over AdDL70-treated animals 4 and 7 days after adenovirus infection, but by day 21, the peritoneal-associated vasculature in the AdTNF-α-treated animals had returned to baseline.
Figure 4.
Vascular density in the submesothelial zone of the parietal peritoneum was measured using image analysis from digitized Factor VIII-vWF-stained sections taken from animals exposed to AdIL-1β (black bars), AdTNF-α (gray bars), or control adenovirus (AdDL70, open bars). Animals treated with AdIL-1β demonstrated increased vascular density in the submesothelial zone at all time points. In contrast, animals treated with AdTNF-α demonstrated a transient induction of angiogenesis that resolved by 21 days after infection. *, P < 0.01 compared with AdDL70-treated animals; †, P < 0.05 compared with AdDL70-treated animals. Data represents four to six animals for each data point.
There was an alteration in morphology of blood vessels after exposure to TNF-α and IL-1β. We observed an increase in the average cross-sectional area of the blood vessels after exposure to inflammatory cytokines (IL-1β, 146 ± 18; TNF-α, 136 ± 17 versus AdDL70, 73 ± 23 μm 2 4 days after infection). This dilation of blood vessels persisted to 21 days after infection.
In both AdIL-1β- and AdTNF-α-treated animals there was an early accumulation of collagen evident by day 7 (Figures 2 and 3) ▶ ▶ . We confirmed the histological evidence of collagen accumulation by analyzing mesenteric tissue for hydroxyproline content. As shown in Figure 5 ▶ , AdIL-1β-treated animals had persistent elevation in hydroxyproline, and therefore, collagen content, of mesenteric tissue at each time point evaluated. AdTNF-α-treated animals showed a significant early accumulation of collagen but by day 21, this had resolved.
Figure 5.
Hydroxyproline analysis of mesenteric tissue after infection with AdIL-1β (black bars), AdTNF-α (gray bars), or control adenovirus (AdDL70, open bars). AdIL-1β-treated animals demonstrated sustained fibrosis measured by hydroxyproline content of mesenteric tissue whereas TNF-α-treated animals demonstrated a transient accumulation of collagen by day 7 that disappeared by day 21. *, P < 0.01 compared with AdDL70; †, P < 0.05 compared with AdDL70. Data represents three to six animals at each time point.
The most significant histological difference after exposure to TNF-α or IL-1β can be seen in the day 21 samples (Figure 6) ▶ . AdIL-1β-treated animals had submesothelial thickening, now with collagen deposition (Figure 6A) ▶ . There was persistent angiogenesis seen in the factor VIII-stained section (Figure 6B) ▶ . The AdTNF-α-treated animals had peritoneal histology indistinguishable from normal rat peritoneum 21 days after infection and demonstrated thin, compact submesothelial tissue and virtual absence of peritoneal-associated vessels (Figure 6, C and D) ▶ .
Figure 6.
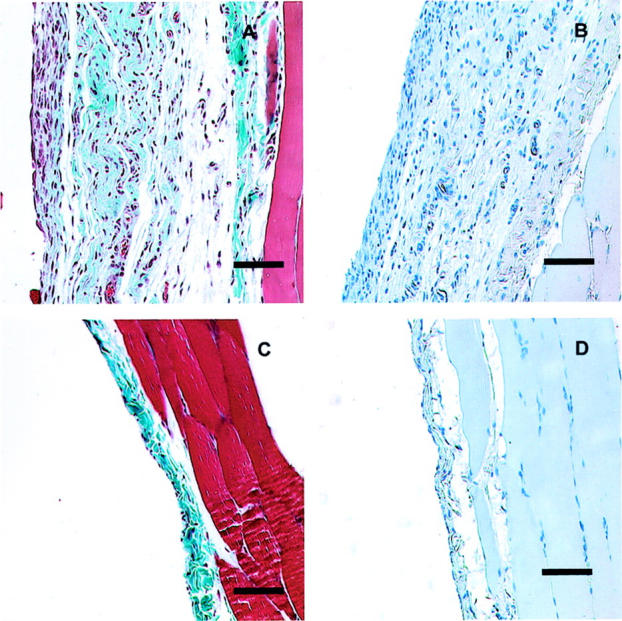
Histology of the anterior abdominal wall 21 days after infection with AdIL-1β (A and B) or AdTNF-α (C and D). Animals infected with AdIL-1β show persisting thickening of the submesothelial zone with collagen deposition (A) and persisting vascularization (B). After infection with AdTNF-α, the parietal peritoneum returned to normal histology (C) with absence of vascularization (D). Scale bars, 100 μm. Masson’s trichrome (A and C); Factor VIII-vWF immunohistochemistry (B and D).
Using regression analysis of all treatment groups combined, we could find a weak but significant correlation between dialysate VEGF concentration 4 days after adenovirus administration and subsequent vascularization (Figure 7A) ▶ . Of note, there was a stronger correlation between TGF-β peritoneal fluid concentration on day 4 and subsequent vascularization (Figure 7B) ▶ .
Figure 7.
Correlation between peritoneal cytokine concentrations in all three treatment groups and vascularization measured by vessels/mm2. A: Day 4 peritoneal VEGF concentration correlates weakly with vascularization (r = 0.35, P = 0.02). B: A stronger correlation exists between peritoneal TGF-β1 concentration and vascularization (r = 0.45, P = 0.01).
Peritoneal Membrane Function
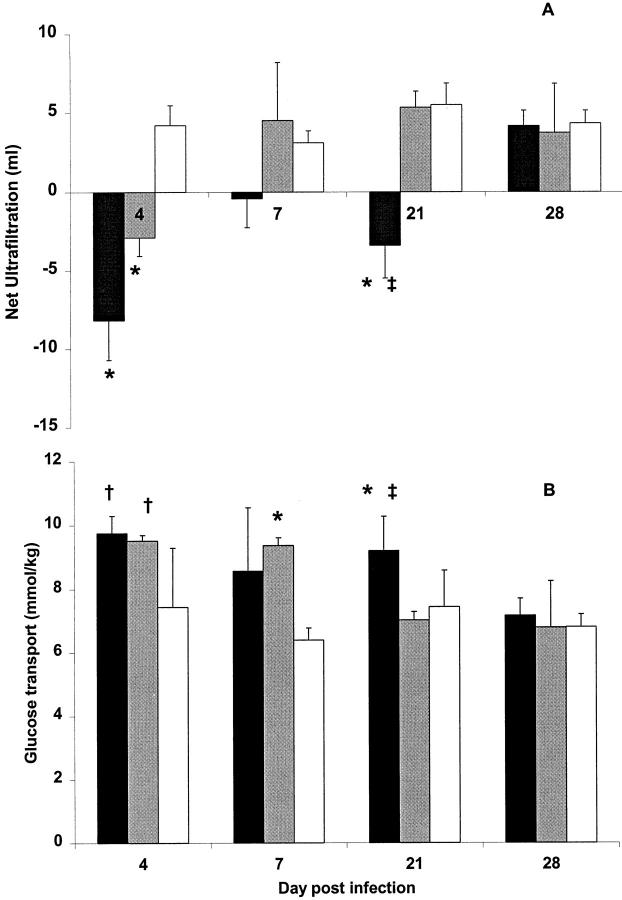
We studied the function of the peritoneum as a dialysis membrane using net ultrafiltration, glucose mass transport, and albumin clearance, after a 4-hour dwell of 2.5% Dianeal. The net ultrafiltration (Figure 8A) ▶ reveals a significant impairment 4 days after infection with AdTNF-α and AdIL-1β compared with AdDL70-treated animals. The ultrafiltration function of the AdTNF-α-treated animals recovered by day 7, but remained impaired in IL-1β-treated animals until day 28. The glucose transport (Figure 8B) ▶ showed a similar trend with early increased glucose transport after overexpression of both inflammatory cytokines, but with increased glucose transport persisting until day 28 for AdIL-1β-treated animals.
Figure 8.
Peritoneal membrane function after adenovirus infection with AdIL-1β (black bars), AdTNF-α (gray bars), or control adenovirus (AdDL70, open bars) represented by net ultrafiltration (A) and mass transport of glucose transport across the peritoneal membrane (B). AdIL-1β-treated animals displayed impaired peritoneal function with decreased ultrafiltration and increased solute transport until day 28. AdTNF-α-treated animals demonstrated a more transient membrane dysfunction with resolution of ultrafiltration failure at day 7 and normalization of solute transport by day 21 after infection. *, P < 0.01 compared with AdDL70; †, P < 0.05 compared with AdDL70; ‡, P < 0.05 AdIL-1β compared with AdTNF-α. Data represents three to six animals for each data point.
Transient overexpression of TNF-α not only caused significant peritoneal vascularization by day 7, but albumin clearance suggests an increased permeability to macromolecules at this time point. The albumin clearance in AdTNF-α-treated animals, 7 days after adenovirus infection, was 30 ± 23 L/kg, which was significantly elevated over AdDL70-treated animals (3 ± 1 L/kg, P = 0.03), and elevated over AdIL-1β-treated animals (7 ± 1 L/kg, P = ns).
Discussion
Adenovirus-mediated gene transfer is a versatile tool to analyze the effects of transient overexpression of cytokines on peritoneal morphology and function. 23 Both inflammatory cytokines IL-1β and TNF-α induced an early acute inflammatory response not seen in control adenovirus-treated animals. This response consisted of increased peritoneal inflammatory cell infiltration, notably by neutrophils. There was a secondary up-regulation of IL-6 and TNFsrII, as has been seen in patients with peritonitis. 5
IL-1β and TNF-α also induced functional changes similar to those seen in patients who develop peritonitis. Specifically, we saw an early ultrafiltration failure with increased transport of glucose and loss of albumin. These functional changes corresponded to increased expression of VEGF and TGF-β1 and increased peritoneal angiogenesis. We, and others, have demonstrated that angiogenesis is a key component in increased glucose transport and ultrafiltration dysfunction 16,30 and we suggest that, in this model, angiogenesis is an integral factor causing the acute peritoneal dysfunction. Others have observed a role for nitric oxide 9 or prostaglandins 10 in acute peritoneal dysfunction associated with peritonitis. We did not assay for these compounds in our experiments and they may play a role, but they are likely downstream components and the inflammatory cytokines can alone initiate peritoneal angiogenesis and acute ultrafiltration failure.
The role of inflammatory cytokines in angiogenesis has been studied previously and the results are in agreement with our findings. Specifically, in a series of studies using the rat mesenteric window model, Norrby 20,21 has shown that both inflammatory cytokines induce angiogenesis, but the angiogenesis induced by IL-1β is more prolonged.
The most interesting findings from our experiments derive from the differences we observed between IL-1β and TNF-α. Specifically, TNF-α induced a transient, dramatic angiogenesis with collagen deposition and increased macromolecular permeability. These changes resolved completely by 21 days after adenovirus-mediated gene transfer. In contrast, IL-1β exposure induced a persisting vasculogenesis with associated up-regulation of profibrotic cytokines and induction of submesothelial fibrosis.
There was a quantitative difference in inflammatory response to IL-1β and TNF-α with the former leading to a more severe and prolonged inflammation measured by persisting neutrophilia and up-regulation of TNFsrII. We do not think this quantitative increased inflammatory response alone explains the qualitative fibrotic and angiogenic response seen by days 21 and 28. We saw a similar angiogenic and fibrogenic response between TNF-α and IL-1β at early time points. This was accompanied by an equal up-regulation of TGF-β and fibronectin mRNA and tissue VEGF. We hypothesize that the different fibrogenic response at later time points is related to the significant up-regulation of TIMP-1 as demonstrated by RNase protection assay (Figure 1) ▶ . Increasing evidence would suggest that persisting fibrosis is related to imbalance between collagenase proteins and their inhibitors. 31 The profibrotic nature of IL-1β has been demonstrated previously in which overexpression in the lung led to progressive fibrosis, 18 however overexpression of TNF-α in the lung led to a patchy, mild fibrosis. 32
One possible explanation for the differential angiogenic response to IL-1β and TNF-α is the recognized property of TNF-α to induce apoptosis through binding to its receptor p55. 33 IL-1β is not known to directly induce apoptosis. It is possible that the high peritoneal concentration of TNF-α seen during the first 7 days induces inflammation and angiogenesis and, as the concentration falls, there is a secondary induction of apoptosis and reversion of the membrane to normal histology by day 21.
Another possibility is that some element, or elements, of the profibrotic environment initiated by IL-1β is responsible for protecting the viability and stability of endothelial cells. Recent experiments have revealed that angiogenesis involves the growth of new immature blood vessels following a stabilization phase. 34 This stabilization involves different stimuli such as angiopoietin-1 35 along with the recruitment of perivascular cells and these factors may be enhanced in the fibrotic environment.
We cannot rule out a differential response to human IL-1β, the gene insert in the adenovirus used in these experiments. However, IL-1β has significant homology across mammalian species 36 and the receptor apparatus is known to be remarkably well preserved. 37 Only very minor differences in response to different species IL-1β has been documented previously. 38 Therefore, we do not feel that the differential effects between IL-1β and TNF-α seen in these experiments are because of the presence of human IL-1β.
We have not clearly identified the major angiogenic cytokine in this model. VEGF concentration was up-regulated in the peritoneal dialysate but this is not an accurate marker of local production. 39 Peritoneal cytokine concentration is effected by shifts of water into or out of the peritoneal cavity as well as transport of the cytokine into the peritoneum across permeable blood vessels. The tissue concentration of VEGF was elevated in animals exposed to TNF-α and IL-1β but the changes from AdDL70-treated animals were not striking (Table 2) ▶ . It could be that higher tissue VEGF levels are present at earlier time points, as angiogenesis is already present by day 4. It is also possible that other fibrosis-related cytokines such as platelet-derived growth factor or basic fibroblast growth factor are important modulators of angiogenesis in this setting of acute inflammation. 40 This could explain the observation that peritoneal fluid TGF-β1 concentration correlates more closely with vascularization than VEGF concentration (Figure 7) ▶ .
Some discrepancies exist between functional and morphological findings in the peritoneum. Seven days after infection with AdTNF-α, there is persisting angiogenesis and increased glucose transport, but the ultrafiltration has reverted to normal in AdTNF-treated animals (Figure 8) ▶ . One possible confounding variable is the extremely high albumin transport measured in TNF-α-exposed animals at this time point. The increased osmotic component of the peritoneal protein may counteract the rapid loss of glucose gradient and preservation of ultrafiltration. Functional properties of IL-1β-treated animals improved at day 28 despite ongoing evidence of angiogenesis and fibrosis. We saw a similar pattern after adenoviral-mediated gene transfer of TGF-β1. 23 It is possible that there are progressive alterations in the interstitium of the peritoneum in fibrosis, such as increased deposition of hyaluronan, that may improve the peritoneal membrane function despite ongoing angiogenesis. 41 Alternatively, the peritoneum may regulate the blood supply to the peritoneum throughout time so that, despite the histological persistence of blood vessels, the overall volume of blood supply slowly returns to a basal level.
Using adenoviral-mediated gene transfer of the proinflammatory cytokines IL-1β and TNF-α, we have shown that these cytokines individually can induce peritoneal angiogenesis and functional changes similar to those found in peritoneal dialysis patients who suffer acute peritonitis. More importantly, IL-1β and TNF-α induce differing fibrogenic and angiogenic responses. These differential responses suggest that IL-1β may have a greater and more prolonged impact on the peritoneum through induction of sustained vasculogenesis and fibrosis. IL-1β may be the link between recurrent peritonitis and longer-term changes in the function of the peritoneum. This may explain why some patients have a poor outcome after one or several episodes of peritonitis. Targeted inhibition of IL-1β, perhaps through its receptor antagonist, may preserve the peritoneum after acute peritonitis. Finally, the further study of the angiogenic response in this inflammatory model may yield insights into the supporting role of fibrosis in angiogenesis and this may have implications beyond maintenance of the peritoneum as a dialysis membrane.
Acknowledgments
We thank Duncan Chong and Xueya Feng for technical support, Mary Jo Smith for help with histology, and Drs C. Richards and A. Ingram for their helpful review.
Footnotes
Address reprint requests to Dr. J. Gauldie, Chair, Department of Pathology and Molecular Medicine, McMaster University, 1200 Main St. W, Room 2N16, Hamilton, Ontario, Canada, L8N 3Z5. E-mail: gauldie@mcmaster.ca.
Supported by Baxter Health Care, the Canadian Institutes of Health Research, and the Kidney Foundation of Canada.
This paper was presented in abstract form at the IX Congress of the International Society for Peritoneal Dialysis, Montreal, PQ, Canada, June 2001.
References
- 1.Bunke CM, Brier ME, Golper TA: Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997, 52:524-529 [DOI] [PubMed] [Google Scholar]
- 2.Topley N, Liberek T, Davenport A, Li FK, Fear H, Williams JD: Activation of inflammation and leukocyte recruitment into the peritoneal cavity. Kidney Int 1996, 56:S17-S21 [PubMed] [Google Scholar]
- 3.Jorres A, Ludat K, Sander K, Dunkel K, Lorenz F, Keck H, Frei U, Gahl GM: The peritoneal fibroblast and the control of peritoneal inflammation. Kidney Int 1996, 56:S22-S27 [PubMed] [Google Scholar]
- 4.Visser CE, Brouwer-Steenbergen JJ, Struijk G, Krediet RT, Beelen RH: Production of IL-1 beta and TNF-alpha by peritoneal macrophages depends on the bacterial species and the inoculum. Adv Perit Dial 1997, 13:201-204 [PubMed] [Google Scholar]
- 5.Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT: Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 1994, 46:1422-1430 [DOI] [PubMed] [Google Scholar]
- 6.Brauner A, Hylander B, Wretlind B: Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 receptor antagonist in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996, 27:402-408 [DOI] [PubMed] [Google Scholar]
- 7.Yung S, Coles GA, Davies M: IL-1 beta, a major stimulator of hyaluronan synthesis in vitro of human peritoneal mesothelial cells: relevance to peritonitis in CAPD. Kidney Int 1996, 50:1337-1343 [DOI] [PubMed] [Google Scholar]
- 8.Ates K, Koc R, Nergizoglu G, Erturk S, Keven K, Sen A, Karatan O: The longitudinal effect of a single peritonitis episode on peritoneal membrane transport in CAPD patients. Perit Dial Int 2000, 20:220-226 [PubMed] [Google Scholar]
- 9.Combet S, Van Landschoot M, Moulin P, Piech A, Verbavatz JM, Goffin E, Balligand JL, Lameire N, Devuyst O: Regulation of aquaporin-1 and nitric oxide synthase isoforms in a rat model of acute peritonitis. J Am Soc Nephrol 1999, 10:2185-2196 [DOI] [PubMed] [Google Scholar]
- 10.Steinhauer HB, Schollmeyer P: Prostaglandin-mediated loss of proteins during peritonitis in continuous ambulatory peritoneal dialysis. Kidney Int 1986, 29:584-590 [DOI] [PubMed] [Google Scholar]
- 11.Davies SJ, Bryan J, Phillips L, Russell GI: Longitudinal changes in peritoneal kinetics: the effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant 1996, 11:498-506 [PubMed] [Google Scholar]
- 12.Lin CY, Chen WP, Yang LY, Chen A, Huang TP: Persistent transforming growth factor-beta 1 expression may predict peritoneal fibrosis in CAPD patients with frequent peritonitis occurrence. Am J Nephrol 1998, 18:513-519 [DOI] [PubMed] [Google Scholar]
- 13.Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, Krediet RT: Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 1999, 19:517-525 [PubMed] [Google Scholar]
- 14.Plum J, Hermann S, Fussholler A, Schoenicke G, Donner A, Rohrborn A, Grabensee B: Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int 2001, 59(Suppl 78):S42-S47 [DOI] [PubMed] [Google Scholar]
- 15.Combet S, Miyata T, Moulin P, Pouthier D, Goffin E, Devuyst O: Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol 2000, 11:717-728 [DOI] [PubMed] [Google Scholar]
- 16.Margetts PJ, Kolb M, Yu L, Hoff CM, Gauldie J: A chronic inflammatory infusion model of peritoneal dialysis in rats. Perit Dial Int 2001, 21:S319-S323 [PubMed] [Google Scholar]
- 17.Honda K, Nitta K, Horita S, Yumura W, Nihei H: Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996, 72:171-176 [DOI] [PubMed] [Google Scholar]
- 18.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J: Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001, 107:1529-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WS, Kim BS, Lee SK, Park JS, Kim SB: Interleukin-1beta stimulates the production of extracellular matrix in cultured human peritoneal mesothelial cells. Perit Dial Int 1999, 19:211-220 [PubMed] [Google Scholar]
- 20.Norrby K: Interleukin-1-alpha and de novo mammalian angiogenesis. Microvasc Res 1997, 54:58-64 [DOI] [PubMed] [Google Scholar]
- 21.Norrby K: TNF-alpha and de novo mammalian angiogenesis. Microvasc Res 1996, 52:79-83 [DOI] [PubMed] [Google Scholar]
- 22.Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, Kuwano M: Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem 1996, 271:28220-28228 [DOI] [PubMed] [Google Scholar]
- 23.Margetts PJ, Kolb M, Hoff CM, Shockley TR, Gauldie J: Gene transfer of transforming growth factor beta 1 to the rat peritoneum: effects on membrane function. J Am Soc Nephrol 2001, 12:2029-2039 [DOI] [PubMed] [Google Scholar]
- 24.Marr RA, Addison CL, Snider D, Muller WJ, Gauldie J, Graham FL: Tumour immunotherapy using an adenoviral vector expressing a membrane-bound mutant of murine TNF alpha. Gene Ther 1997, 4:1181-1188 [DOI] [PubMed] [Google Scholar]
- 25.Bett AJ, Haddara W, Prevec L, Graham FL: An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA 1994, 91:8802-8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing Z, Ohkawara Y, Jordana M, Graham F, Gauldie J: Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest 1996, 97:1102-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleman CL, Mas RM, Rodeiro I, Noa M, Hernandez C, Menendez R, Gamez R: Reference database of the main physiological parameters in Sprague-Dawley rats from 6 to 32 months. Lab Anim 1998, 32:457-466 [DOI] [PubMed] [Google Scholar]
- 28.Woessner JF, Jr: The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961, 93:440-447 [DOI] [PubMed] [Google Scholar]
- 29.Hoff CM, Piscopo D, Inman KL, Shockley TR: Adenovirus-mediated gene transfer to the peritoneal cavity. Perit Dial Int 2000, 20:128 [Google Scholar]
- 30.Krediet RT, Zweers MM, van der Wal AC, Struijk DG: Neoangiogenesis in the peritoneal membrane. Perit Dial Int 2000, 20(Suppl 2):S19-S25 [PubMed] [Google Scholar]
- 31.Benyon RC, Arthur MJ: Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis 2001, 21:373-384 [DOI] [PubMed] [Google Scholar]
- 32.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J: Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol 1998, 153:825-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA: Proinflammatory cytokines. Chest 2000, 118:503-508 [DOI] [PubMed] [Google Scholar]
- 34.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J: Vascular-specific growth factors and blood vessel formation. Nature 2000, 407:242-248 [DOI] [PubMed] [Google Scholar]
- 35.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD: Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000, 6:460-463 [DOI] [PubMed] [Google Scholar]
- 36.Howard RD, McIlwraith CW, Trotter GW, Nyborg JK: Cloning of equine interleukin 1 receptor antagonist and determination of its full-length cDNA sequence. Am J Vet Res 1998, 59:712-716 [PubMed] [Google Scholar]
- 37.O’Neill LA, Greene C: Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol 1998, 63:650-657 [PubMed] [Google Scholar]
- 38.Dejana E, Breviario F, Erroi A, Bussolino F, Mussoni L, Gramse M, Pintucci G, Casali B, Dinarello CA, Van Damme J, Mantovani A: Modulation of endothelial cell functions by different molecular species of interleukin 1. Blood 1987, 69:695-699 [PubMed] [Google Scholar]
- 39.Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT: Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med 1999, 134:124-132 [DOI] [PubMed] [Google Scholar]
- 40.Kalluri R, Sukhatme VP: Fibrosis and angiogenesis. Curr Opin Nephrol Hypertens 2000, 9:413-418 [DOI] [PubMed] [Google Scholar]
- 41.Breborowicz A, Polubinska A, Moberly J, Ogle K, Martis L, Oreopoulos D: Hyaluronan modifies inflammatory response and peritoneal permeability during peritonitis in rats. Am J Kidney Dis 2001, 37:594-600 [DOI] [PubMed] [Google Scholar]