Abstract
We have begun to study the genetic basis of deterioration of cardiac function in the fruit fly Drosophila melanogaster as an age-related cardiac disease model. For this purpose we have developed heart function assays in Drosophila and found that the fly's cardiac performance, as that of the human heart, deteriorates with age: aging fruit flies exhibit a progressive increase in electrical pacing-induced heart failure as well as in arrhythmias. The insulin receptor and associated pathways have a dramatic and heart-autonomous influence on age-related cardiac performance in flies, suggestive of potentially similar mechanisms in regulating cardiac aging in vertebrates. Compromised KCNQ and KATP ion channel functions also seem to contribute to the decline in heart performance in aging flies, suggesting that the corresponding vertebrate gene functions may similarly decline with age, in addition to their conserved role in protecting against arrhythmias and hypoxia/ischemia, respectively. The fly heart is thus emerging as a promising genetic model for studying the age-dependent decline in organ function.
Keywords: heart, aging, arrhythmia, heart failure, hypoxia, KATP channel
Introduction
Age-related diseases are becoming increasingly more prevalent in industrialized societies that have increasingly longer average life expectancies. These diseases are difficult to treat, in part because we do not sufficiently understand the distinction between ‘normal’ and disease-associated aging. By studying genetically tractable model systems it has become clear in recent years that both aging-related processes and disease progression may be influenced and regulated by specific genes. However, the mechanisms underlying the aging process in a normal or mutant organism and how the functional and morphological decline of organ systems is initiated, coordinated and executed is almost completely unknown. Since cardiac dysfunction is the most common cause of death in the elderly, an understanding of the progression and control of age-related changes in heart function becomes increasingly important as our societies become increasingly older. To date virtually nothing is known about the genetic mechanisms that control the age-dependent deterioration of the heart in health and disease. We have recently implemented assays for monitoring the cardiac stress response, arrhythmias and other dysfunctions in the simple Drosophila system, the only invertebrate genetic model with a heart. Given the high degree of parallel genetic functions between flies and vertebrates in cardiogenesis (Bodmer, 1995; Cripps and Olson, 2002; Zaffran and Frasch, 2002; Bodmer, et al., 2005), and given the strikingly common mechanisms that determine lifespan and rate of overall aging (Guarente and Kenyon, 2000; Helfand and Rogina, 2003; Finch and Ruvkun, 2001; Partridge and Gems, 2002; Barbieri, et al., 2003; Tatar, et al., 2003), it is likely that the genetic basis of age-related changes in cardiac performance is also conserved. Thus, Drosophila is an ideal system to study the genetic basis of cardiac performance decline with age and disease. Below, we summarize the approaches we have taken and some of the insights that we have recently obtained.
Age-dependent increase in heart failure in flies
Chronic heart disease often accelerates the decline of cardiac function in older people (Ribera-Casado, 1999; McLaughlin, 2001; Busby, et al., 1989; Maurer, et al., 1995). The human heart has been demonstrated to undergo changes in function with advancing age, including increased prevalence of rhythmic abnormalities. These changes cause an age-related increase in the risk for congestive heart failure and contribute additional risk factors to other cardiac pathologies (Lakatta and Levy, 2003). As the life expectancy continues to increase, these age-related risk factors become increasingly clinically relevant. Among the age-related changes in the cardiovascular system are decreases in myocyte number, accumulation of fibrosis and collagen and decreases in stress-induced cardiac function, and many other alterations (reviewed in Khan et al., 2002). Mammalian models of age-related cardiac dysfunction and heart failure are often of limited value or are unpractical for uncovering fundamental mechanisms, mainly because normal heart function is essential for viability, and overall lifespan is prohibitively long. Therefore, we have developed Drosophila as a model for studying genetic mechanisms of cardiac aging and disease progression, since a fly's life span is only weeks (compared to years in rodents) and its cardiac function can be significantly compromised without causing premature death (Wessells et al., 2004), in part because oxygen supply is provided by a tracheal system that is separate from the circulatory system.
Pacing-induced heart failure
Cardiac stress in humans, induced by exercise and electrical pacing, coupled with echocardiography is a useful tool in the detection of certain forms of disease (reviewed in Gligorova and Agrusta, 2005). To gage cardiac performance in Drosophila, our lab recently developed methods for assessing cardiac performance in Drosophila. In the external electrical pacing paradigm the fly heart is physically stressed by accelerating its baseline rate, which at rest is 3-4 Hz, to 6 Hz for 30 seconds (methods described in Wessells et al., 2004; Wessells and Bodmer, 2004). When monitoring young wildtype flies immediately after this pacing regime, the fraction of wildtype hearts that exhibit arrest or uncoordinated fibrillation is relatively low (20-35% electrical cardiac ‘failure’ in 1-week old flies, Fig. 1), and this rate increases progressively with age (65-85% failure in 5- to 7-week old flies; Wessells, et al., 2004). Interestingly, these results parallel the increased risk of heart failure in the aging human population (see Lakatta and Levy, 2003), although in mammals heart failure is defined as a mechanical failure to pump blood, whereas in our assay it is a failure of the fly's heart to produce repeated, productive contractions when stressed by electrical pacing.
Figure 1.
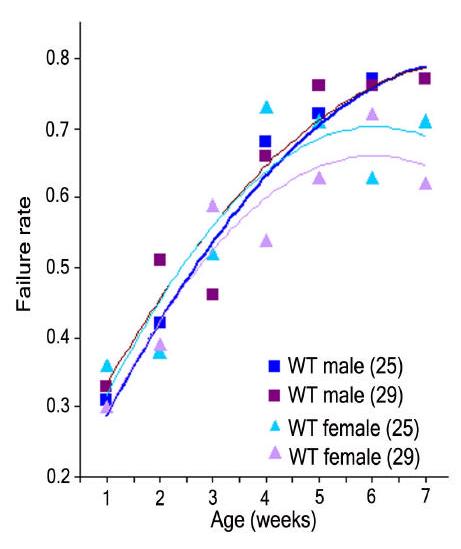
Pacing-induced heart failure as a function of age following external electrical pacing from outbred wild type offspring (yw x Canton S). Experiments were performed at both 25°C and 29°C for a period of seven weeks. Test temperature alone has no effect on failure rate (Χ2= 0.05, p=0.82). Pacing-induced failure rate is age-dependent for both genders at both temperatures (Χ2>40, p<0.0001). More than 90 flies were used for each sample point. From Wessells et al. (2004).
Insulin signaling and cardiac aging
By examining mutants known to affect overall aging, we found that reducing Insulin Receptor (InR) or Target Of Rapamycin (TOR) signaling dramatically lowers the risk of pacing-induced heart failure in old flies (Wessells, et al., 2004; Luong, et al., 2006). Moreover, heart-specific expression of InR and TOR signal transduction components also alters cardiac performance (Wessells, et al., 2004; R.J. Wessells and R.B., unpublished), demonstrating that at least part of the effect of InR-TOR signaling is cardiac autonomous. Thus, cardiac performance, in particular the age-dependent rate of heart failure brought on by pacing-induced stress, is highly sensitive to changes in these signaling pathways. The ability to manipulate gene functions specifically in heart cells of Drosophila makes this system ideal for studying the genetics of age-dependent influences on cardiac function.
Age-dependent increase in arrhythmias in flies
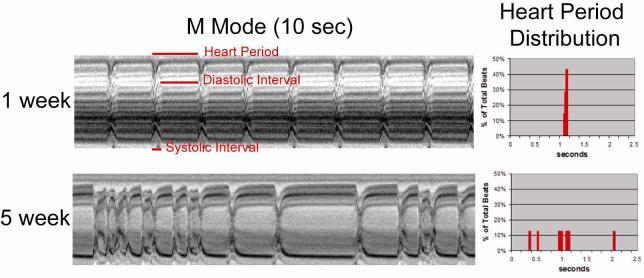
Although pacing-induced cardiac arrest or fibrillation is indicative of cardiac dysfunction it does not provide information as to the specific underlying causes or mechanisms. In order to further characterize the heart's contractile properties we used a high speed video camera to capture heart wall movements in a semi-intact fly preparation in which the heart is surgically exposed and most neuronal inputs to the heart are disrupted. We created M-mode records from these recordings and found that the majority of young, 1-week old wildtype fly hearts (yw, w1118) show regular rhythmic contractions with constant rates (∼2 Hz) that last for over an hour in oxygenated supplemented artificial hemolymph (Figure 2, top). This regular, rhythmic beating pattern deteriorates as the fly ages, and by 5-7 weeks a majority of wildtype flies exhibit non-rhythmical heart contraction patterns (Figure 2, bottom).
Figure 2.
(left hand panels) M-mode traces prepared from high speed movies of dissected flies with exposed hearts at one week (top) and five weeks of age (bottom). A 1 pixel-wide region with both edges of the heart tube is defined in a single movie frame. Same regions are electronically cut from all of the frames in the movie and aligned horizontally to produce the trace. (right hand panels) Histograms of the heart period lengths from the M-mode traces. Note the regular heart beat and corresponding narrow heart period distribution at one week; the incidence and severity of arrhythmicity increases with age (eg. at five weeks) and the heart period distribution broadens.
KCNQ-induced arrhythmias
The observed age-dependent increase in arrhythmicity correlates with an age-related decrease in the RNA expression level of the single Drosophila KCNQ-type K+ channel gene (T.A., K.O. & R.B., unpublished) and a KATP - channel gene (dSUR; Akasaka et al., 2006); see below Fig. 4A). Mutations in human KCNQ genes, encoding the pore-forming subunits underlying IKs, cause type 1 long QT syndrome (LQT1) and associated ventricular arrhythmias. Deletion mutants of the Drosophila KCNQ gene are viable but show increased pacing-induced heart failure (K.O., R.J. Wessells & R.B., unpublished). High-speed video recording and display of heart wall movements in M-mode (as in Fig. 2) show episodes of prolonged heart contraction and fibrillation in these KCNQ deletion mutants and this phenotype gets progressively more severe as the flies age. Our observation that the incidence of cardiac arrhythmia increases with age, concomitant with a decrease in KCNQ gene expression, suggests that alterations in these and other channels (eg. Seizure / HERG) involved in heart muscle repolarization may be responsible for some of the effects of aging on heart function. Because flies seem to develop congenital and age-dependent arrhythmias, as is observed in mammals, this genetic model can serve as a tool to study the genetics underlying arrhythmias with likely relevance to humans.
Figure 4.
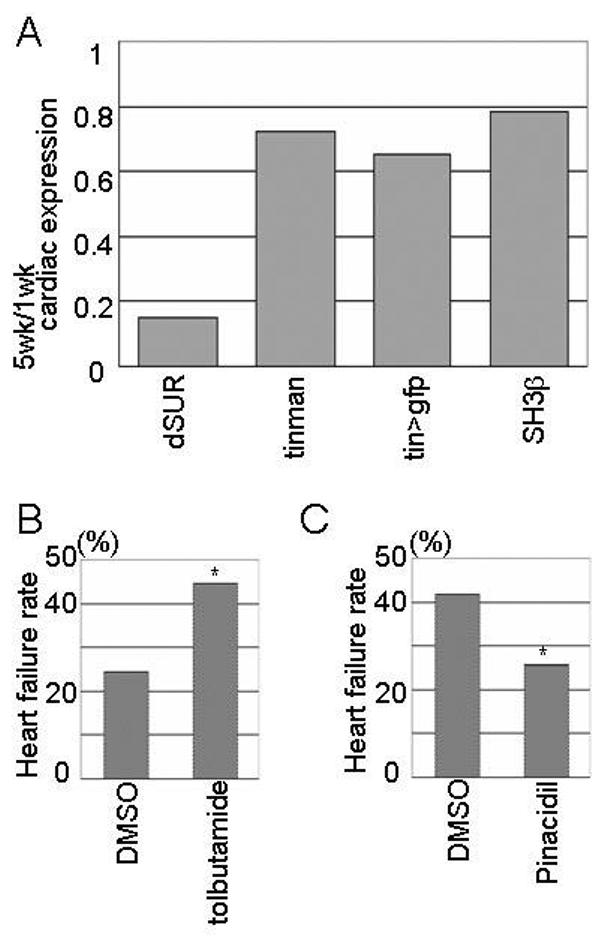
dSUR KATP channel expression declines with senescence. (A) Relative expression of dSUR (0.15), tin (0.72), GFP (0.65) and SH3β (0.78) genes in five-week old hearts compared to hearts at one-week of age. Note the dramatic reduced dSUR RNA level with age compared to controls. (B,C) Electrical pacing-induced heart failure in flies treated with drugs that affect the mammalian sulfonylurea receptors. (B) One-week old yw flies fed with SUR blocker tolbutamide showed a higher heart failure rate than DMSO control-treated yw flies (45% and 25% respectively; p<0.01). (C) 3.5-week old yw flies fed with the KATP channel opener pinacidil showed a lower failure rate than DMSO-controls (26% and 42% respectively; p<0.02). From Akasaka et al. (2006).
KATP channel function and aging
The incidence of ischemic heart disease is strikingly on the rise in the modern world (Giordano, 2005). It is thus important to elucidate the molecular basis of the heart's response to hypoxia/ischemia and determine how this response changes with age, in order to devise preventive and therapeutic strategies. KATP channels are thought to sense the intracellular metabolic state; they are regulated by intracellular ATP/ADP ratio, which in turn is affected by metabolic stresses including hypoxia (Babenko et al., 1998). One consequence of KATP channel activation is an increased hyperpolarization of cardiomyocytes resulting in shortened action potential durations, thus preventing a Ca2+ overload. One of the mammalian KATP channels in cardiac muscle is a heterooctamer of two subunits: a sulfonylurea receptor (SUR2A) and an inward rectifier (Kir6.2). Drosophila dSUR has been identified as a functional homolog of the mammalian SUR2 gene (Nasonkin et al., 1999). We have recently shown that dSUR plays a critical role in providing tolerance against hypoxia in Drosophila, similar to the role of SUR2 in mammals (Akasaka, et al., 2006). In addition, cardiac dSUR knockdown results in an increased rate of pacing-induced heart failure (Fig. 3), as in KCNQ mutants (see above). Similar heart dysfunction is seen in vertebrates when KATP channels are perturbed: Kir6.2 mutant hearts exhibit diminished electrical tolerance against catecholamine-induced ventricular arrhythmia because of a failure to achieve action potential shortening and by causing early after-depolarization (Liu, et al., 2004). Thus, the elevated heart failure rate in dSUR knockdown hearts may be due to KATP channel insufficiency.
Figure 3.
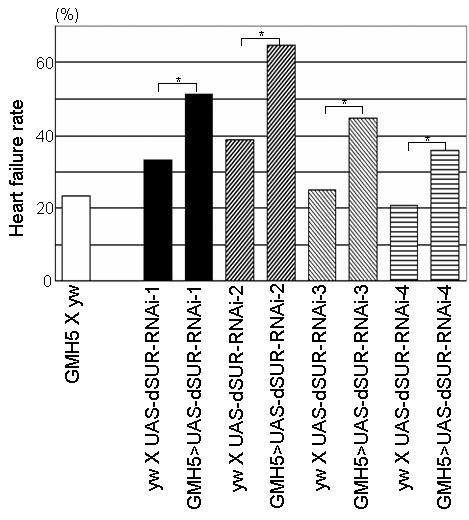
Cardiac dSUR function protects heart performance against electrical pacing. Heart failure rate after 30-second high frequency (6Hz) external pacing in one-week old RNAi-mediated knockdown mutants (progeny of UAS-dSUR-RNAi flies with the heart-specific GMH5 driver. Controls (progeny of GMH5 crossed with yw and yw crossed with UAS-dSUR-RNAi lines) show a low rate of heart failure. Conversely, cardiac dSUR knockdown results in significantly elevated levels of pacing-induced heart failure (*<0.01). Adapted from Akasaka et al. (2006).
Since an elevation of the pacing-induced heart failure rate is also observed during the natural age-related decline in fly cardiac function (Fig. 1), it is possible that KATP channel insufficiency is an important contributor to the increased risk of heart failure with age. Indeed, dSUR RNA levels, as determined by real-time RT-PCR with RNA from isolated hearts, show a dramatic decline in dSUR expression compared to control genes (Fig. 4A). Thus, the increase in heart failure rate with age correlates with a decrease in dSUR expression. Pharmacological manipulation of dSUR activity supports this conclusion: dietary administration of tolbutamide, a KATP channel inhibitor, causes a higher incidence of heart failure compared to the non-treated flies (Fig. 4B). In contrast, exposure of older flies to pinacidil, a KATP channel activator, shows a lower than normal pacing-induced failure rate (Fig. 4C). These results suggest that increased dSUR activity may improve cardiac performance and lends further support to the idea that loss-of-dSUR-function contributes to declining cardiac performance with age. Interestingly, ischemic preconditioning, a treatment that protects the myocardium from hypoxic injury by applying repetitive pre-exposure to hypoxia, is no longer observed in older human patients (Ishihara et al., 2000), and in old guinea pigs with reduced ventricular SUR2A expression when compared to tissue from young animals (Ranki et al., 2002). Moreover, mutations in human SUR2 were found in two independent families with middle age onset of dilated cardiomyopathy (Bienengraeber et al., 2004). These mutations result in structural abnormalities of the KATP channel and impair ATP-dependent channel gating. Taken together, reduced dSUR-function seems to be an indicator of cardiac aging, and Drosophila with its genetics and short life span may provide a unique model for age-dependent responses to hypoxia, in addition to other age-related human diseases (see above).
In sum, Drosophila is emerging as an excellent genetic model for various aspects of organ-specific aging, especially that of the heart, which ages remarkably similar to humans. Aided by its short lifespan, genetic versatility and our simple but robust assays of (cardiac) physiology, the fly can now be used for large-scale screening for new genes that affect organ (heart)-specific aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akasaka T, Klinedinst S, Ocorr K, Bustamante EL, Kim SK, Bodmer R. The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc Natl Acad Sci U S A. 2006;103:11999–2004. doi: 10.1073/pnas.0603098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–87. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–71. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Bodmer R. Heart development in Drosophila and its relationship to vertebrate systems. Tends in Cardiovascular Medicine. 1995;5:21–27. doi: 10.1016/1050-1738(94)00032-Q. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Johnson E, Dowse D. Heart Development and Function. In: Gilbert L,IK, Gill S, editors. Comprehensive Insect Science. Elsevier; Amsterdam: 2005. In press. [Google Scholar]
- Busby MJ, Shefrin EA, Fleg JL. Prevalence and long-term significance of exercise-induced frequent or repetitive ventricular ectopic beats in apparently healthy volunteers. J Am Coll Cardiol. 1989;14:1659–65. doi: 10.1016/0735-1097(89)90012-0. [DOI] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–62. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Sato H, Tateishi H, Kawagoe T, Shimatani Y, Ueda K, Noma K, Yumoto A, Nishioka K. Beneficial effect of prodromal angina pectoris is lost in elderly patients with acute myocardial infarction. Am Heart J. 2000;139:881–8. doi: 10.1016/s0002-8703(00)90021-8. [DOI] [PubMed] [Google Scholar]
- Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O'Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–8. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- Luong N, Davies C, Wessells R, Graham S, King M-T, Veech R, Bodmer R, S. O. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metabolism. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Maurer MS, Shefrin EA, Fleg JL. Prevalence and prognostic significance of exercise-induced supraventricular tachycardia in apparently healthy volunteers. Am J Cardiol. 1995;75:788–92. doi: 10.1016/s0002-9149(99)80412-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin MA. The aging heart. State-of-the-art prevention and management of cardiac disease. Geriatrics. 2001;56:45–9. quiz 50. [PubMed] [Google Scholar]
- Nasonkin I, Alikasifoglu A, Ambrose C, Cahill P, Cheng M, Sarniak A, Egan M, Thomas PM. A novel sulfonylurea receptor family member expressed in the embryonic Drosophila dorsal vessel and tracheal system. J Biol Chem. 1999;274:29420–5. doi: 10.1074/jbc.274.41.29420. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–75. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Ranki HJ, Crawford RM, Budas GR, Jovanovic A. Ageing is associated with a decrease in the number of sarcolemmal ATP-sensitive K+ channels in a gender-dependent manner. Mech Ageing Dev. 2002;123:695–705. doi: 10.1016/s0047-6374(01)00415-8. [DOI] [PubMed] [Google Scholar]
- Ribera-Casado JM. Ageing and the cardiovascular system. Z Gerontol Geriatr. 1999;32:412–9. doi: 10.1007/s003910050138. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Wessells R, Bodmer R. Screning assays for heart function mutants in Drosophila. Bio Techniques. 2004;37:2–7. doi: 10.2144/04371ST01. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–81. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–69. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]