Abstract
Oncostatin M (OSM) has been described as a bone-remodeling factor either stimulating osteoblast activity or osteoclast formation in vitro. To elucidate the in vivo effect of OSM on bone remodeling, we injected an adenoviral vector encoding murine OSM in knee joints of mice. OSM strongly induced interleukin (IL)-6 gene expression, a known mediator of osteoclast development. We investigated the OSM effect in wild-type and IL-6-deficient mice and found a similar degree of OSM-induced joint inflammation. Within the first week of inflammation, the periosteum along the femur and tibia increased in cell number and stained positive for the osteoblast marker alkaline phosphatase. At these sites bone apposition occurred in both strains as demonstrated by Goldner and Von Kossa staining. In vitro OSM enhanced the effect of bone morphogenetic protein-2 on osteoblast differentiation. Immunohistochemistry demonstrated expression of receptor activator of nuclear factor-κB ligand (RANKL) and its receptor, receptor activator of nuclear factor-κB (RANK), in the periosteum but osteoclasts were not detected at sites of bone apposition. Induced mRNA expression for the receptor activator of nuclear factor-κB ligand inhibitor osteoprotegerin probably controlled osteoclast development during OSM overexpression. Our results show that OSM favors bone apposition at periosteal sites instead of resorption in vivo. This effect was not dependent on or inhibited by IL-6.
Oncostatin M (OSM) is a 28-kd glycoprotein that belongs to the interleukin (IL)-6 family. 1 It was originally discovered by its ability to inhibit the growth of the melanoma cell line A375. 2 Subsequently, more effects were discovered and OSM was found to be a multifunctional cytokine like the other IL-6 family members. OSM can for example stimulate an acute-phase response in liver cells 3 and enhance expression of tissue inhibitor of metalloproteinase-1 4 and adhesion molecules such as ICAM-1. 5 Macrophages and activated T cells have been shown to produce OSM. 2,6
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is accompanied by destruction of joints. Elevated levels of OSM can be detected in the synovial fluid of RA patients and synovial macrophages are the source of this OSM. 7,8 Unlike its family member IL-6, OSM was not detected in the serum of RA patients. 7 This suggests a local rather than a systemic role for OSM in RA. Circumstantial evidence for a local role is the positive correlation found between concentrations of OSM and cartilage degradation markers in synovial fluid. 9 Injection of recombinant human OSM in the joints of goats induced inflammation, 10 further supporting a local and pro-inflammatory role for OSM in joint pathology. Recently, intraperitoneal administration of blocking antibodies to OSM ameliorated experimental arthritis in mice, 11 demonstrating a pro-inflammatory role for OSM in murine arthritis. A systemic effect of blocking OSM can, however, not be excluded under these experimental conditions.
The receptor complex for OSM consists of the glycoprotein gp130 that is used by all of the IL-6 family members, and a second receptor. This second receptor in the complex is either the leukemia inhibitory factor receptor-β or the OSM receptor-β. 12 The isolation of the murine OSM receptor-β showed that the use of these receptor complexes differed between species. 13 Human OSM utilizes both receptor complexes on human cells but only the gp130/leukemia inhibitory factor receptor-β complex on murine cells. Murine OSM in contrast only binds to the gp130/OSM receptor-β complex on murine cells. At present it is not known if this differential use of receptor complexes is also true for other combinations of species. The conflicting pro-inflammatory 5 and anti-inflammatory 14 effects of human OSM in mice, however, stress the need to use species-specific OSM in experimental disease models.
Injection of an adenoviral vector expressing murine OSM (AdmuOSM) in murine knee joints led to inflammation and synovial cell proliferation. 15 OSM has been shown to be a potent inducer of IL-6 gene expression. IL-6 plays a pivotal role in development and chronicity of experimental arthritis. 16-18 In the present study we have injected this same vector in the knee joints of wild-type and IL-6-deficient mice to elucidate a role for IL-6 in the AdmuOSM-induced pathology.
Bone erosion can take place in patients with RA 19 and IL-6 family members are also implicated to play a role in this process. IL-6 together with the soluble IL-6 receptor has been shown to induce the formation of osteoclast-like cells. 20 IL-6 also has been shown to enhance the resorbing activity of tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells. 21 The role of OSM during pathological bone remodeling is at present unclear. OSM can influence differentiation and proliferation of osteoblasts 22 relating it to bone development. On the other hand can OSM also induce the formation of osteoclast-like cells 20,23 relating it to bone erosion. Interestingly, we discovered apposition of new bone tissue after injection of the AdmuOSM vector. This new bone apposition took place under inflamed conditions and was not dependent on nor inhibited by IL-6.
Materials and Methods
Animals
For this study C57BL/6 mice were obtained from Charles River (Sulzfeld, Germany). IL-6-deficient mice 24 back-crossed for eight generations into C57BL/6 mice were obtained from Dr. M. Kopf (Basel, Switzerland) and breeding colonies were kept at the Central Animal Facilities of the Catholic University of Nijmegen. Male animals were used between 11 and 13 weeks of age. All mice were housed in filter-top cages under specific pathogen-free conditions and a standard diet and water were provided ad libitum. The mice were housed in isolators after adenoviral injection. Experiments were performed according to national and institutional regulations for animal use.
Adenoviral Vectors and Intra-Articular Injection
The construction of the replication-deficient E1-deleted AdmuOSM was described before. 25 As a control vector Addl70-3 (No. 5), a vector without insert, was used. For in vivo experiments the virus was diluted in physiological saline and 2.106-plaque-forming units (pfu) in a total volume of 6 μl were injected in the knee joint cavity.
Histological Evaluation of Knee Joints
Knee joints were dissected, fixed in formalin, decalcified, dehydrated, and embedded in paraffin. Standard frontal sections of 7 μm were prepared and stained with safranin-O and counterstained with fast green. Synovial inflammation was scored on five semiserial sections of the joint. Scoring on a scale from 0 up to 3 was performed in a blindfolded manner by two independent observers.
Isolation of Synovial RNA and Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Synovial mRNA was isolated and quantitated as described by van Meurs and colleagues. 26 Patellae with surrounding synovium were isolated from knee joints and two pieces of tissue adjacent to the patella were punched out with a 3-mm biopsy punch (Stiefel Laboratorium GMbH, Offenbach am Main, Germany). The tissue was immediately frozen in liquid nitrogen. Tissue samples were homogenized in a freeze mill, thawed in 1 ml of Trizol reagent, and further processed according to the manufacturer’s protocol. All reagents for RNA isolation and RT-PCR were from Life Technologies (Breda, The Netherlands). Isolated RNA was treated with DNase I before being reverse-transcribed into cDNA with MMLV reverse transcriptase. After increasing numbers of PCR cycles, samples were taken and run on an agarose gel. The cycle number at which the PCR product was first detected on the gel was taken as a measure for the amount of specific mRNA originally present in the isolated synovial RNA. PCR for glyceraldehyde-3-phosphate dehydrogenase was performed to verify that equal amounts of cDNA were used. Primers for osteoprotegerin (OPG) and receptor activator of nuclear factor-κB ligand (RANKL) 27 were used as described before. For IL-6 the following primers were used: IL-6 forward 5′TCT-GCA-AGA-GAC-TTC-CAT-CCA and reverse 5′GCA-AGT-GCA-TCA-TCG-TTG-TTC (55°C, 1 mmol/L MgCl2).
Alkaline Phosphatase (ALP) Staining
Cryostat sections of knee joints (7 μm) were stained for ALP activity with the naphthol AS-BI (Sigma, St. Louis, MO) method. 28
Von Kossa and Goldner’s Trichrome Staining
Whole formalin-fixed knee joints were embedded in plastic and 7-μm thick sections were cut. Sections were stained with von Kossa staining 29 to identify calcified bone and with a Goldner’s trichrome staining 30 to demonstrate the presence and maturation of newly formed bone.
Image Analysis of Newly Formed Bone
The surface area of the newly formed bone in wild-type and IL-6-deficient mice was measured using the Qwin image analysis system (Leica Imaging Systems Ltd., Cambridge, UK). Images of safranin-O-stained sections were captured using a JVC 3-CCD color video camera (Victor Company of Japan Ltd., Tokyo, Japan) and displayed on a computer monitor. Per joint four measurements of bone apposition on the femur were performed in a standardized manner. Both the length of the original cortical bone (marked by a precipitation line in the staining) and the volume of the newly formed bone were measured. The amount of newly formed bone is expressed as μm2 new bone/10 μm cortical bone.
TRAP Staining
Whole formalin-fixed knee joints were decalcified in 10% ethylenediaminetetraacetic acid (Titriplex III; Merck, Darmstadt, Germany)/1 mmol/L Tris-HCl (pH 7.4) for 2 weeks at 4°C. Decalcified knee joints were processed for paraffin embedding and 7-μm thick tissue sections were prepared. These sections were stained for TRAP with the leukocyte acid phosphatase kit (Sigma, St. Louis, MO) according to the manufacturer’s protocol.
Immunohistochemistry of RANKL and RANK
Tissue sections (7 μm) of paraffin-embedded whole knee joints were treated for 15 minutes with 3% H2O2/methanol at room temperature. After antigen retrieval (2 hours in 10 mmol/L of citrate, pH 6.0, at room temperature) sections were incubated with the primary antibody or normal serum for 1 hour. Antibodies used were rabbit anti-RANK (H300) at 2 μg/ml, goat anti-RANKL (N19) at 1 μg/ml, goat IgG at 1 μg/ml (all from Santa Cruz Biotechnology Inc., Santa Cruz, CA) and rabbit IgG at 2 μg/ml (DAKO, Glostrup, Denmark). After rinsing, sections were blocked for 20 minutes at room temperature with 4% normal mouse serum for RANKL and normal goat serum for RANK. Thereafter, sections for RANKL were incubated for 30 minutes with biotinylated mouse anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and detected by biotin-streptavidin/peroxidase staining (Elite kit; Vector Laboratories, Burlingame, CA). Sections for RANK were incubated for 30 minutes with horseradish peroxidase-conjugated goat anti-rabbit IgG (DAKO). Development of the peroxidase staining was done with 3′,3′diaminobenzidine. Sections were counterstained with hematoxylin.
ALP Assay on C2C12 Cells
C2C12 cells were obtained from the American Tissue Culture Collection (Rockville, MD). The cells were grown in Dulbecco’s modified Eagle’s medium/10% newborn calf serum/100 U/ml penicillin/100 μg/ml streptomycin at 37°C in a humidified atmosphere at 7.5% CO2. The cells were seeded at 6.7 × 103 cells per well in a 96-well round-bottom plate. The next day the cells were washed with phosphate-buffered saline (PBS). Subsequently, the cells were stimulated for 3 days in medium containing 5% newborn calf serum and different combinations of the following recombinant proteins: recombinant murine OSM, recombinant human bone morphogenetic protein-2 (BMP-2), recombinant human IL-6, and recombinant soluble IL-6 receptor (R&D Systems, Minneapolis, MN). IL-6 and soluble IL-6 receptors were used in a 1:1 ratio. After 3 days the cells were washed with cold Hank’s buffer and fixed for 10 minutes with 4% formalin on ice. Thereafter the cells were washed with cold PBS and incubated with substrate (1 mol/L diethanolamine, 1 mmol/L MgCl2, and 5.26 mg/ml p-nitrophenylphosphate). The plate was placed at 37°C in the dark. The reaction was stopped after 5 minutes with 0.5 mol/L of NaOH and the OD 405 nm was measured on a Ceres UV 900C spectrophotometer (Bio-Tek Instruments Inc., Winooski, VT). The effect of recombinant proteins on the number of cells was measured by Neutral Red staining 31 and measured at 550 nm.
Statistical Analysis
Statistical comparison between groups was performed with Student’s t-test. Values of P < 0.05 were considered significant.
Results
Adenoviral Overexpression of OSM Induces Joint Inflammation in Wild-Type and IL-6-Deficient Mice
Intra-articular injection of 2.106-pfu AdmuOSM, but not of the control vector Addl70-3, in the joint of wild-type mice induced an inflammation that was characterized by influx of mononuclear and polymorphonuclear cells and synovial hyperplasia. Synovitis lasted at least until week 4 after injection, the last time point studied. No signs of inflammation were macroscopically observed in the ankle or foot of the leg receiving AdmuOSM in the knee joint, indicating that OSM induced a localized joint inflammation.
OSM is a strong inducer of IL-6 gene expression and semiquantitative RT-PCR of injected knee joints showed that AdmuOSM induced IL-6 expression (Figure 1) ▶ . To elucidate the role of IL-6 in the AdmuOSM-induced inflammation, we injected this vector in knee joints of IL-6-deficient mice. Histological scoring of joint inflammation showed no difference between both strains indicating that the inflammatory effect of OSM did not depend directly on IL-6 (Figure 2) ▶ .
Figure 1.
IL-6 gene expression in AdmuOSM-injected knee joints. Semiquantitative RT-PCR showed enhanced IL-6 gene expression (data shown for 30 cycles) in the AdmuOSM-injected knee joint. IL-6 gene expression was not detected after Addl70-3 injection (not even after 40 PCR cycles). RNA was isolated from synovia of three mice on day 3 after injection of the adenoviral vectors. PCR for glyceraldehyde-3-phosphate dehydrogenase was performed to assess the amount of cDNA used (data shown for 18 cycles). PCR samples were taken during the PCR and analyzed on a 1.6% agarose gel as described in Materials and Methods. IL-6 mRNA expression in AdmuOSM-injected knee joints was first detected after 27 PCR cycles.
Figure 2.
Histological scoring for the joint inflammation at days 7 and 14 after AdmuOSM injection. ▪, Wild-type mice; □, IL-6-deficient mice. Per time point six mice per group were evaluated. Both strains did not differ in the inflammation (P > 0.05, Student’s t-test).
Bone Formation in AdmuOSM-Injected Knee Joints
OSM can influence both cell types that are involved in bone remodeling, the osteoblast and the osteoclast. Its family member IL-6, in contrast, is generally described as an erosive cytokine through the induction of osteoclast development. 20 It was therefore of interest to compare the effect of OSM and the OSM-induced inflammation on the articular bone in both wild-type and IL-6-deficient mice.
Already at day 3 of the inflammation, we observed the formation of several layers of periosteal cells along the femur and tibia of the AdmuOSM-injected knee joint (Figure 3A) ▶ . Positive staining of these layers for ALP activity showed the osteoblast-like nature of these cells (Figure 3B) ▶ . During the second week of inflammation apposition of new bone occurred next to this thickened periosteal cell layer as shown for day 14 after adenoviral injection (Figure 3C) ▶ . A Goldner staining demonstrated the most recently formed and still not completely mineralized new bone closest to the periosteal cell layer in orange (Figure 3D) ▶ . This layer could not be stained by the Von Kossa technique (Figure 3E) ▶ further demonstrating that mineralization of this bone is still not completed. The mineralization of new bone that was already completed was demonstrated by both stainings. This clearly shows that new bone is formed in AdmuOSM-injected knee joints and that the process of bone formation is still continuing at day 14 after injection. Injection of the control vector Addl70-3 did not lead to new bone formation. Periosteal bone apposition and joint inflammation were also observed with AdmuOSM when the contralateral knee joint was not injected (Dr. C. D. Richards, McMaster University, Hamilton, Ontario, Canada, unpublished observation).
Figure 3.
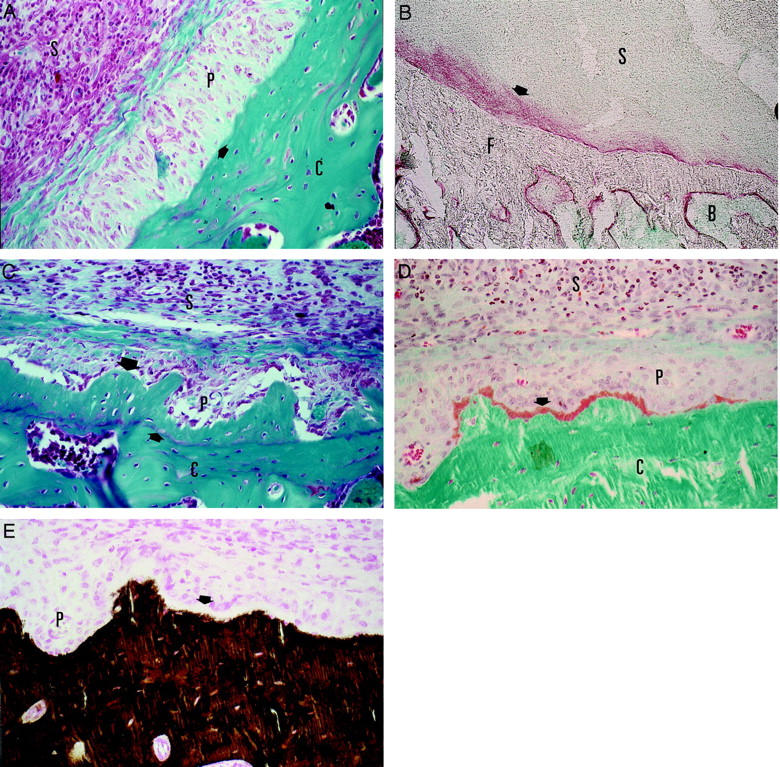
A: Activated periosteum of the femur 7 days after injection of AdmuOSM. Stained by Safranin-O. An arrow indicates the surface of the femoral cortical bone. B: Positive staining of the activated periost for ALP activity (red) is indicated with an arrow. Bone and inflammation is stained in green with methyl green. C: New bone formed on the femur of a wild-type mouse 14 days after injection of AdmuOSM (large arrow). A small arrow indicates the former surface of the bone. D: Goldner staining of the newly formed bone. The latest formed and still unmineralized bone is stained in orange and is indicated with an arrow. The mineralized bone is stained in green. E: Von Kossa staining of a section adjacent to the section of D. The mineralized bone is stained in brown. The newly formed and unmineralized bone is not stained by the Von Kossa staining (white layer indicated by an arrow). B, Bone marrow; C, cortical bone of the femur; F, femur; P, periosteum, S, inflamed synovium. Original magnifications: ×400 (A, C–E); ×180 (B).
The formation of layers of osteoblast-like cells and the deposition of new bone occurred in both wild-type and IL-6-deficient mice. The IL-6-deficient mice did not differ from wild-type mice in the amount of newly formed bone (Figure 4) ▶ . Adenoviral expression of murine IL-6 did not induce apposition of new bone in the knee joint (data not shown). This strengthens our results in the IL-6-deficient mice showing that the observed OSM effect on the articular bone is not mediated by IL-6.
Figure 4.
Quantification of newly formed bone in wild-type and IL-6-deficient mice. The surface area of newly formed bone was measured as described in Materials and Methods and was expressed as μm2 new bone/10 μm cortical bone. No significant difference was found between wild-type (n = 10) and IL-6-deficient mice (n = 8) (P = 0.469, Student’s t-test). Injection of the control vector Addl70-3 did not lead to bone apposition. Data shown are for day 14 after adenoviral injection. ▪, Wild-type mice; □, IL-6-deficient mice.
OSM Enhances BMP-2-Induced ALP Activity in Vitro
A possible direct effect of OSM on development of the ALP-positive cell layer was further investigated in vitro. For these experiments we used pluripotent murine C2C12 cells that can differentiate toward the osteoblast lineage 32 and measured ALP activity after 3 days of culture. Recombinant murine OSM alone was, in contrast to a bone-forming factor such as BMP-2, not able to induce ALP activity in these cells. Addition of OSM to a constant BMP-2 concentration, however, had a strong enhancing effect on the BMP-2-induced ALP activity (Figure 5A) ▶ . The mean induction of three experiments was 2.1 ± 0.2 for 100 ng/ml BMP-2, 3.7 ± 1.0 for 100 ng/ml BMP-2 + 0.36 ng/ml OSM (P < 0.05), and 5.8 ± 1.1 for 100 ng/ml BMP-2 + 5 ng/ml OSM (P < 0.005) (Student’s t-test, n = 6). The other concentrations of OSM tested in combination with BMP-2 also differ significantly from incubations with BMP-2 alone. Equal neutral red staining for C2C12 cells treated with BMP-2 or BMP-2 and OSM excluded an effect on cell proliferation (data not shown). The enhanced ALP activity does not seem to depend directly or indirectly on IL-6 because it was not observed after incubation with the combination of BMP-2, IL-6, and the soluble IL-6 receptor (Figure 5B) ▶ . These results suggest that the in vivo observed effect of OSM is dependent on cooperation of OSM with other bone-forming factors such as, for example, BMP-2.
Figure 5.

OSM enhances the BMP-2-induced ALP activity in C2C12 cells. A: C2C12 cells were incubated for 3 days with variable concentrations of recombinant murine OSM in the presence or absence of 100 ng/ml of recombinant human BMP-2. ALP activity was measured as described in Materials and Methods. The OD 405-nm value for cells without OSM or BMP-2 added to the medium was set at 1 and used for calculating the effect of BMP-2, OSM, and the combination of both proteins. OSM alone did not induce ALP activity in these cells even at concentrations up to 500 ng/ml (data not shown). BMP-2 alone gave a twofold induction of ALP activity. This BMP-2-induced ALP activity was greatly enhanced by OSM. This same effect of OSM was also found when 300 ng/ml of BMP-2 was used (data not shown). B: C2C12 cells were incubated for 3 days with variable concentrations of recombinant IL-6 and the soluble IL-6 receptor in the presence or absence of 100 ng/ml of recombinant human BMP-2. In contrast to OSM the IL-6/sIL-6R combination did not enhance the BMP-2-induced ALP activity. IL-6/sIL-6R alone did also not induce ALP activity in the C2C12 cells. The experiments of Figure 5, A and B ▶ , were performed in duplicate for at least three times with similar results. One representative experiment is shown. The SD in ALP activity was less than 5% between duplicate measurements.
Expression of RANKL, RANK, and OPG in the Inflamed Joint
The new deposited bone had an irregular border facing the joint cavity in wild-type mice as well as in the IL-6-deficient mice. Because in vitro results have implicated that OSM could induce osteoclast formation, 23 it was possible that OSM not only induced bone formation but also bone resorption in the inflamed joint. A key molecule involved in osteoclastogenesis is RANKL. Because IL-6-type cytokines have been shown to enhance RANKL mRNA levels, 33 we studied expression of RANKL and its receptor RANK in the AdmuOSM-induced inflammation. Both RANKL and RANK were detected immunohistochemically in the inflamed synovium and the periosteal cell layers (day 7; Figure 6, A and B ▶ ) and expression continued after apposition of the new bone (day 14; Figure 6, C and E ▶ ). No RANK or RANKL could be demonstrated in Addl70-3-injected knee joints (data not shown). A similar expression pattern for RANKL and RANK was detected in joint sections from AdmuOSM-injected wild-type and IL-6-deficient mice.
Figure 6.
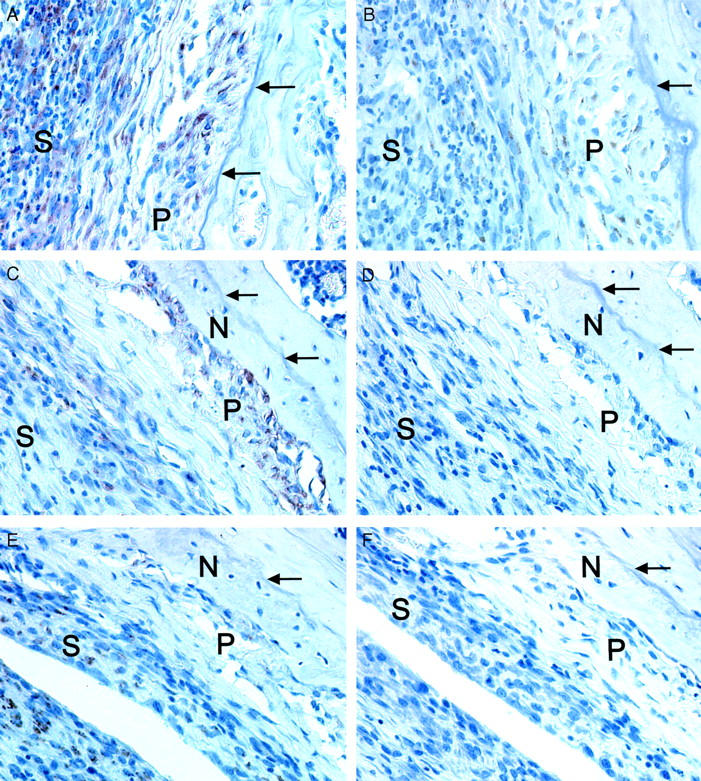
Immunohistochemistry for RANK and RANKL expression in AdmuOSM-injected knee joints. RANK (A) and RANKL (B) are detected in the periosteum and the inflamed synovium 7 days after injection of AdmuOSM. Continued expression of RANK (C) and RANKL (E) 14 days after AdmuOSM injection. Control sera for the RANK (D) or RANKL (F) antisera did not show staining of the periosteum or the inflamed synovium. Data shown are for day 14 after AdmuOSM injection. Arrows indicate the surface of the cortical bone of the femur before injection of AdmuOSM. N, Newly formed bone; P, periosteum; and S, inflamed synovium. Original magnifications, ×400.
The RANK/RANKL system and the development of osteoclasts can be controlled by the RANKL antagonist OPG. Semiquantitative RT-PCR on synovial mRNA showed that besides expression for RANKL, OPG expression also was up-regulated in the AdmuOSM-induced inflammation (Figure 7) ▶ . Induction of OPG occurred in both wild-type and IL-6-deficient mice. Gene induction for OPG could provide a counterbalance against the observed RANKL expression. TRAP staining of AdmuOSM-injected knee joints did not identify osteoclasts in the inflamed synovium or at sites of new bone formation (Figure 8) ▶ . TRAP-positive multinucleated cells were also not detected on the layer of osteoblast-like cells earlier during inflammation on day 7 (data not shown). These results indicate that the OSM-induced inflammation does not create an environment favoring osteoclast development. Our results show that OSM can induce bone formation in vivo, an effect found to be independent of IL-6.
Figure 7.
Enhanced synovial mRNA expression for RANKL and OPG at days 7 and 14 of the AdmuOSM-induced inflammation. Gene expressions for RANKL and OPG were compared between synovia from AdmuOSM-injected and contralateral Addl70-3-injected knee joints as described in Materials and Methods. Six mice per group were used and equal amounts of cDNA were used as assessed by PCR for glyceraldehyde-3-phosphate dehydrogenase. RT-PCR reactions were performed at least twice. ▪, RANKL; □, OPG.
Figure 8.
Trap staining of a wild-type knee joint 14 days after injection of AdmuOSM. TRAP-positive cells were observed in the bone marrow but not in the inflammation or on the new bone. A joint from a mouse with collagen-induced arthritis served as a positive control for the staining (data not shown). A small arrow indicates the former surface of the bone. The large arrows indicate TRAP-positive cells in the bone marrow. B, Bone marrow, C, cortical bone of the femur; N, newly formed bone; P, periosteum; S, inflamed synovium. Original magnification, ×200.
Discussion
OSM is a member of the IL-6 family of cytokines. Experiments with recombinant OSM have led to conflicting results as both pro- and anti-inflammatory properties have been published. 5,14 Most reports, however, describe OSM to be pro-inflammatory. These conflicting results could be related to the different models used. Human and murine IL-6 use the same receptor complex on murine cells. Human and murine OSM, in contrast, make use of different receptor complexes on murine cells. 13 This finding could be another complicating factor and murine OSM should therefore be used to establish its role in experimental inflammatory models in mice.
OSM can be detected in the synovial fluid but not in the serum of RA patients. 7 This suggests a local role for OSM in the inflammation and joint damage during RA. Recently, it was shown that murine OSM stimulated anchorage-independent growth of murine synovial fibroblasts in vitro and that AdmuOSM induced joint inflammation in the knee joints of mice. 15 This was not observed with a control vector. Our in vivo results confirm these data and support a local pro-inflammatory role for OSM during RA.
Adenoviral vectors have been used to express proteins of interest in the knee joint. 34-36 Advantages of these vectors are that they can infect both dividing and nondividing cells and can express a protein for days in the knee joint. Adenoviral vectors, however, can by themselves also cause inflammation. In our hands no inflammation is induced in naive knee joints injected with 1.107-pfu empty control virus or virus-expressing luciferase or β-galactosidase marker genes. In the present study we have used five times less virus and we did not observe inflammation in the contralateral knee joint injected with control virus.
The fact that no sensitive test is available specific for murine OSM makes it difficult to address the production by the AdmuOSM vector. We have tried to set up a bioassay by incubating the murine B9 cell line with washouts of AdmuOSM-injected IL-6-deficient knee joints. This IL-6-dependent cell line can also respond to OSM, although at higher concentration. 37 The detection limit for recombinant murine OSM was 2 ng/ml. This is relatively high compared to the several 100 pg/ml that we generally observe with 1.107 pfu of vectors expressing other transgenes. The AdmuOSM washouts did not show a production above the detection limit. The production of OSM is therefore less than 2 ng/ml but a precise measurement awaits the development of a more sensitive specific assay.
In our study we have found a high induction of gene expression for IL-6 after injection of AdmuOSM in naive wild-type mice. IL-6 plays a very important role during experimental arthritis. 16-18 IL-6 gene expression was, however, not necessary for development of the AdmuOSM-induced inflammation. This was demonstrated by a similar degree of inflammation when we injected the AdmuOSM vector in the joints of wild-type and IL-6-deficient mice. Injection of an adenoviral vector expressing murine IL-6 did not lead to inflammation even in a10-fold higher concentration. 15 This observation was confirmed by results in our laboratory (unpublished data). Together these data suggest that locally produced OSM plays by itself a pro-inflammatory role during joint inflammation. Secondly, it suggests that members of the IL-6 family play different roles during joint inflammation and that they cannot simply be substituted for by other family members.
Under nonpathological circumstances there is a continuous synthesis of new bone by osteoblasts and breakdown of bone by osteoclasts. This balance can be disrupted as is shown by the occurrence of bone erosion during RA. Osteoclasts have been identified as the main bone resorbing cells in RA. 38 The members of the IL-6 family can influence differentiation and activation of both osteoblasts and osteoclasts and hence influence bone homeostasis. 22 Unraveling the precise involvement of these cytokines in bone remodeling and homeostasis could not only be important for RA but also for other osteolytic diseases such as Paget’s disease and giant-cell tumors. IL-6 is generally described to stimulate bone resorption. OSM can influence both osteoblasts and osteoclasts and its role is less clear. The results observed after injecting the AdmuOSM vector in the knee joints of naive mice showed apposition of new bone in the periosteum of the inflamed joint. This phenomenon was observed in all knee joints injected with AdmuOSM in four different experiments. We studied the role that both osteoblasts and osteoclasts could play in this phenomenon.
Expression of OSM by the AdmuOSM vector not only caused inflammation but also activated the periosteal cells along the femur and tibia. These cells were positive for ALP activity, a marker for osteoblasts and osteoblast-like cells. In all wild-type and IL-6-deficient mice tested bone apposition occurred at these sites with a thickened layer of periosteal cells. The formation of this ALP-positive cell layer pointed at an important influence of OSM on osteoblastic cells in vivo. Most experiments on the relation between OSM and osteoblast function have been performed in vitro with human OSM. Recombinant human OSM has been found to activate murine osteoblasts and to inhibit basal bone resorption in vitro. 39 Also, human OSM did prevent apoptosis in both murine and human osteoblastic cell lines. 40 As described above the use of human or murine OSM on murine cells might lead to different results. To obtain additional information on the role of OSM in osteoblast differentiation and activation we have performed in vitro experiments with recombinant murine OSM on the murine C2C12 cell line. These cells can differentiate toward the osteoblast lineage and express osteoblastic markers such as ALP, osteocalcin, and Cbfa1. 32 They also showed mRNA expression of the specific OSM receptor-β (data not shown).
When ALP activity was measured in the C2C12 cells, we found that murine OSM by itself did not differentiate these cells toward the osteoblast lineage. Similarly, it was found that human OSM did not differentiate murine embryonic fibroblasts toward the osteoblast lineage. 41 The ALP activity of C2C12 cells in response to BMP-2, however, was clearly enhanced by addition of OSM and not by IL-6. This suggests that the in vivo-observed ALP activity might be an effect of OSM co-operating with a bone-forming factor. We previously reported that an intra-articular injection of recombinant BMP-2 protein induced chondrogenesis in the murine knee joint. 42 Uusitalo and colleagues 43 found that adenoviral transfection of the periosteum with BMP-2 also caused cartilage callus tissue formation and that endochondral ossification replaced most of this callus cartilage by bone. Previous studies using crude extracts of BMPs showed direct periosteal bone induction that was not preceded by chondrogenesis. 44 Further research is needed to determine whether OSM has stimulatory or (re)directing effects on BMP-2 or other bone-forming factors in vivo.
The observed bone formation could also be a direct effect of OSM on mature osteoblast-like cells in vivo. OSM can activate the signal transducer and activator of transcription (STAT)-3 in osteoblasts. 45 Activation of STAT-3 by OSM can induce expression of c-Fos as shown in the hepatoma cell line HepG2. 46 Fos proteins form together with the Jun proteins, the activator protein-1 (AP-1) transcription complex. Recently, it was shown that overexpression of the fos proteins FRA-1 47 or ΔFosB 48 led to a progressive increase in overall bone mass in an as yet unknown way. Further research on the signal transduction and gene expression of osteoblasts under influence of OSM is therefore needed to determine whether the observed new bone formation is regulated by AP-1. Another factor that could play a role in the AdmuOSM-induced bone formation is Cbfa1, the first osteoblast-specific transcription factor. It controls the rate of bone formation by differentiated osteoblasts. 49 A relation between Cbfa1 and OSM, however, has not yet been investigated.
Bone formation is besides locally also systemically controlled. 50 An effect of OSM on the endocrine system during the AdmuOSM-induced inflammation seems, however, unlikely because no activation of osteoblasts or bone formation was observed in the contralateral knee joints injected with Addl70-3.
Consistent with our findings using the adenoviral vector, excessive bone growth was observed in the femur of a transgenic mouse expressing bovine OSM. 51 However, no overt inflammation was detected surrounding this new bone (Dr. C. Clegg, personal communication). This suggests that the effect of OSM on bone formation does not depend directly on the observed pro-inflammatory properties of OSM.
The other cell type involved in bone remodeling and homeostasis, the osteoclast, can also be influenced by OSM. Recombinant murine OSM increased in vitro the formation of osteoclasts in co-cultures of murine bone marrow and calvaria cells. 23 TRAP-positive cells were, however, not detected in the inflamed synovium of AdmuOSM-injected knee joints. Only very rarely were they detected on the newly formed bone. Langdon and colleagues 15 showed with this same virus that in some joints the inflammation protruded through the bone marrow. We also observed this occasionally in our experiments, but not at sites of bone apposition. The existence of channels between the bone marrow and the synovium has been described. 52 It is possible that cell trafficking between the inflamed synovium and the bone marrow occurs through these channels. This could be accompanied by widening of these channels, although a mechanism behind this is still unknown. TRAP staining of AdmuOSM-injected knee joints did not detect osteoclasts in these channels. This makes it unlikely that in our experimental conditions osteoclasts are involved in widening of the channels. Aggressive fibroblasts could be involved in this process. Synovial fibroblasts with a transformed phenotype are present in synovial pannus tissue and their presence has been related to joint damage. 53-55 This would be in line with the observed positive effect of murine OSM on the anchorage-independent growth of mouse synovial fibroblasts. 15
OSM has been shown to induce expression of RANKL in stromal/osteoblastic cells. 33 This RANKL can interact with the receptor RANK on the precursors of osteoclasts. The RANK/RANKL system and the interaction between osteoblasts and osteoclast precursor cells are crucial for the development of osteoclasts. 56 Semiquantitative RT-PCR analysis showed increased expression of RANKL in the inflamed synovium. Immunohistochemistry showed expression of RANKL and RANK proteins in the synovium and in the activated osteoblast-like cell layers. At present, we do not have an assay to demonstrate presence of the soluble RANKL inhibitor OPG on histological sections but increased mRNA expression for OPG was found in the synovium. The ratio between RANKL and OPG expression has been implicated as a determining factor in bone resorption during RA. 57 In our experiments expression of RANKL and RANK did not induce osteoclast development in vivo. It could be that the balance between RANKL and OPG favors bone formation during the AdmuOSM-induced inflammation but this needs further experimental research. Another option is expression of an inhibitor or repression of an osteoclast activator that as yet has not been related to OSM.
We have observed formation of new bone from the periosteum in both the antigen- and the zymosan-induced arthritis in mice. 58,59 Because of the irregular shape of the newly formed periosteal bone it will not always have been recognized as bone apposition. The results of the Goldner’s trichrome staining and the absence of TRAP-positive cells in the periosteum clearly demonstrates that in our model with the AdmuOSM vector bone apposition and not erosion takes place. OSM could also play a role in bone apposition in experimental arthritis models but further research on this is needed. Expression of the pro-inflammatory cytokines IL-1 or IL-17 did induce inflammation and bone erosion but no bone apposition in naive knee joints (manuscript in preparation). This indicates that inflammation is not always accompanied by bone apposition. The cytokine environment could influence the effect of a given cytokine on bone remodeling. The effects of OSM on the periosteum, but not its pro-inflammatory properties, could be negatively influenced by other cytokines in RA. During RA, bone erosion instead of apposition occurs. In other human arthropathies the cytokine environment could have a different effect and bone apposition and erosion can even occur at different parts of the joint. Periosteal bone apposition for example takes place in diseases such as Reiter disease, 60 juvenile chronic arthritis, 61 erosive osteoarthritis, 62 and hypertrophic osteoarthropathy. 63 OSM could have a role in these diseases, as suggested by our results with the OSM adenoviral vector. Future therapies for these diseases, as well as RA, might therefore be targeted at this cytokine.
Acknowledgments
We thank Natasja Lieuwes and Dinie Versleyen for Von Kossa and Goldner stainings, Birgitte Oppers for the RANKL and RANK immunohistochemistry, Dr. C. Clegg (Seattle, WA) for unpublished data, and Dr. M. Kopf (Basel, Switzerland) for the IL-6-deficient mice.
Footnotes
Address reprint requests to Alfons S. K. de Hooge, Rheumatology Research Laboratory, University Medical Center Nijmegen, Nijmegen Center for Molecular Life Sciences, Geert Grooteplein 26-28, 6500 HB Nijmegen, The Netherlands. E-mail: a.dehooge@reuma.azn.nl.
Supported by a grant from the Dutch Arthritis Association (grant NR-97-2-402).
References
- 1.Rose TM, Bruce AG: Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci USA 1991, 88:8641-8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarling JM, Shoyab M, Marquardt H, Hanson MB, Lioubin MN, Todaro GJ: Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci USA 1986, 83:9739-9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards CD, Brown TJ, Shoyab M, Baumann H, Gauldie J: Recombinant oncostatin M stimulates the production of acute phase proteins in HepG2 cells and rat primary hepatocytes in vitro. J Immunol 1992, 148:1731-1736 [PubMed] [Google Scholar]
- 4.Richards CD, Shoyab M, Brown TJ, Gauldie J: Selective regulation of metalloproteinase inhibitor (TIMP-1) by oncostatin M in fibroblasts in culture. J Immunol 1993, 150:5596-5603 [PubMed] [Google Scholar]
- 5.Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM: Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest 1997, 100:158-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TJ, Lioubin MN, Marquardt H: Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol 1987, 139:2977-2983 [PubMed] [Google Scholar]
- 7.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z: The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum 1997, 40:1096-1105 [DOI] [PubMed] [Google Scholar]
- 8.Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, Spaull JR, Goldring MB, Koshy PJ, Rowan AD, Shingleton WD: The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum 1998, 41:1760-1771 [DOI] [PubMed] [Google Scholar]
- 9.Manicourt DH, Poilvache P, Van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ: Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum 2000, 43:281-288 [DOI] [PubMed] [Google Scholar]
- 10.Bell MC, Carroll GJ, Chapman HM, Mills JN, Hui W: Oncostatin M induces leukocyte infiltration and cartilage proteoglycan degradation in vivo in goat joints. Arthritis Rheum 1999, 42:2543-2551 [DOI] [PubMed] [Google Scholar]
- 11.Plater-Zyberk C, Buckton J, Thompson S, Spaull J, Zanders E, Papworth J, Life PF: Amelioration of arthritis in two murine models using antibodies to oncostatin M. Arthritis Rheum 2001, 44:2697-2702 [DOI] [PubMed] [Google Scholar]
- 12.Thoma B, Bird TA, Friend DJ, Gearing DP, Dower SK: Oncostatin M and leukemia inhibitory factor trigger overlapping and different signals through partially shared receptor complexes. J Biol Chem 1994, 269:6215-6222 [PubMed] [Google Scholar]
- 13.Lindberg RA, Juan TS, Welcher AA, Sun Y, Cupples R, Guthrie B, Fletcher FA: Cloning and characterization of a specific receptor for mouse oncostatin M. Mol Cell Biol 1998, 18:3357-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace PM, MacMaster JF, Rouleau KA, Brown TJ, Loy JK, Donaldson KL, Wahl AF: Regulation of inflammatory responses by oncostatin M. J Immunol 1999, 162:5547-5555 [PubMed] [Google Scholar]
- 15.Langdon C, Kerr C, Hassen M, Hara T, Arsenault AL, Richards CD: Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo. Am J Pathol 2000, 157:1187-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Hooge ASK, van De Loo FA, Arntz OJ, van Den Berg WB: Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol 2000, 157:2081-2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T: Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA 1998, 95:8222-8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, De Benedetti F, Poli V, Ciliberto G: Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med 1998, 187:461-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldring SR, Gravallese EM: Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol 2000, 12:195-199 [DOI] [PubMed] [Google Scholar]
- 20.Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T: Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA 1993, 90:11924-11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Morita I, Maruo N, Kubota T, Murota S, Aso T: Expression of IL-6 receptor and GP130 in mouse bone marrow cells during osteoclast differentiation. Bone 1998, 22:487-493 [DOI] [PubMed] [Google Scholar]
- 22.Heymann D, Rousselle AV: gp130 cytokine family and bone cells. Cytokine 2000, 12:1455-1468 [DOI] [PubMed] [Google Scholar]
- 23.Richards CD, Langdon C, Deschamps P, Pennica D, Shaughnessy SG: Stimulation of osteoclast differentiation in vitro by mouse oncostatin M, leukaemia inhibitory factor, cardiotrophin-1 and interleukin 6: synergy with dexamethasone. Cytokine 2000, 12:613-621 [DOI] [PubMed] [Google Scholar]
- 24.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G: Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994, 368:339-342 [DOI] [PubMed] [Google Scholar]
- 25.Kerr C, Langdon C, Graham F, Gauldie J, Hara T, Richards CD: Adenovirus vector expressing mouse oncostatin M induces acute-phase proteins and TIMP-1 expression in vivo in mice. J Interferon Cytokine Res 1999, 19:1195-1205 [DOI] [PubMed] [Google Scholar]
- 26.van Meurs JB, Van Lent PL, Joosten LA, Van der Kraan PM, van Den Berg WB: Quantification of mRNA levels in joint capsule and articular cartilage of the murine knee joint by RT-PCR: kinetics of stromelysin and IL-1 mRNA levels during arthritis. Rheumatol Int 1997, 16:197-205 [DOI] [PubMed] [Google Scholar]
- 27.Lubberts E, Joosten LA, Chabaud M, van Den BL, Oppers B, Coenen-De Roo CJ, Richards CD, Miossec P, van Den Berg WB: IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest 2000, 105:1697-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bancroft JD: Enzyme histochemistry. Bancroft JD Stevens A eds. Theory and Practice of Histological Techniques. 1982:pp 379-405 Churchill Livingstone, Edinburgh
- 29.von Kossa J: Nachweis von Kalk: Beitrage zur pathologischen Anatomie und zur allgemeinen Pathologie. 1901, 29:163
- 30.Goldner J: A modification of the Masson trichrome technique for routine laboratory purposes. Am J Pathol 1938, 14:237. [PMC free article] [PubMed] [Google Scholar]
- 31.Modha K, Whiteside JP, Spier RE: The determination of cellular viability of hybridoma cells in microtitre plates: a colorimetric assay based on neutral red. Cytotechnology 1993, 13:227-232 [DOI] [PubMed] [Google Scholar]
- 32.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T: Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 1994, 127:1755-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien CA, Gubrij I, Lin SC, Saylors RL, Manolagas SC: STAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kappaB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormone. J Biol Chem 1999, 274:19301-19308 [DOI] [PubMed] [Google Scholar]
- 34.Nita I, Ghivizzani SC, Galea-Lauri J, Bandara G, Georgescu HI, Robbins PD, Evans CH: Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum 1996, 39:820-828 [DOI] [PubMed] [Google Scholar]
- 35.Lubberts E, Joosten LA, van Den BL, Helsen MM, Bakker AC, Xing Z, Richards CD, van Den Berg WB: Intra-articular IL-10 gene transfer regulates the expression of collagen-induced arthritis (CIA) in the knee and ipsilateral paw. Clin Exp Immunol 2000, 120:375-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH, Robbins PD: Adenovirus-mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum 2000, 43:2563-2570 [DOI] [PubMed] [Google Scholar]
- 37.Schwabe M, Cox GW, Bosco MC, Prohaska R, Kung HF: Multiple cytokines inhibit interleukin-6-dependent murine hybridoma/plasmacytoma proliferation. Cell Immunol 1996, 168:117-121 [DOI] [PubMed] [Google Scholar]
- 38.Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR: Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 1998, 152:943-951 [PMC free article] [PubMed] [Google Scholar]
- 39.Jay PR, Centrella M, Lorenzo J, Bruce AG, Horowitz MC: Oncostatin-M: a new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology 1996, 137:1151-1158 [DOI] [PubMed] [Google Scholar]
- 40.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC: Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998, 13:793-802 [DOI] [PubMed] [Google Scholar]
- 41.Taguchi Y, Yamamoto M, Yamate T, Lin SC, Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E, Manolagas SC: Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians 1998, 110:559-574 [PubMed] [Google Scholar]
- 42.van Beuningen HM, Glansbeek HL, Van der Kraan PM, van Den Berg WB: Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage 1998, 6:306-317 [DOI] [PubMed] [Google Scholar]
- 43.Uusitalo H, Hiltunen A, Ahonen M, Kahari VM, Aro H, Vuorio E: Induction of periosteal callus formation by bone morphogenetic protein-2 employing adenovirus-mediated gene delivery. Matrix Biol 2001, 20:123-127 [DOI] [PubMed] [Google Scholar]
- 44.Hosokawa R, Kubo T, Wadamoto M, Sato Y, Kimoto T: Direct bone induction in the subperiosteal space of rat calvaria with demineralized bone allografts. J Oral Implantol 1999, 25:30-34 [DOI] [PubMed] [Google Scholar]
- 45.Levy JB, Schindler C, Raz R, Levy DE, Baron R, Horowitz MC: Activation of the JAK-STAT signal transduction pathway by oncostatin-M cultured human and mouse osteoblastic cells. Endocrinology 1996, 137:1159-1165 [DOI] [PubMed] [Google Scholar]
- 46.Botelho FM, Edwards DR, Richards CD: Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J Biol Chem 1998, 273:5211-5218 [DOI] [PubMed] [Google Scholar]
- 47.Jochum W, David JP, Elliott C, Wutz A, Plenk H, Jr, Matsuo K, Wagner EF: Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med 2000, 6:980-984 [DOI] [PubMed] [Google Scholar]
- 48.Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R: Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med 2000, 6:985-990 [DOI] [PubMed] [Google Scholar]
- 49.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G: Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997, 89:747-754 [DOI] [PubMed] [Google Scholar]
- 50.Ducy P, Schinke T, Karsenty G: The osteoblast: a sophisticated fibroblast under central surveillance. Science 2000, 289:1501-1504 [DOI] [PubMed] [Google Scholar]
- 51.Malik N, Haugen HS, Modrell B, Shoyab M, Clegg CH: Developmental abnormalities in mice transgenic for bovine oncostatin M. Mol Cell Biol 1995, 15:2349-2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakagawa S, Toritsuka Y, Wakitani S, Denno K, Tomita T, Owaki H, Kimura T, Shino K, Ochi T: Bone marrow stromal cells contribute to synovial cell proliferation in rats with collagen induced arthritis. J Rheumatol 1996, 23:2098-2103 [PubMed] [Google Scholar]
- 53.Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL: Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest 1989, 83:1267-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yocum DE, Lafyatis R, Remmers EF, Schumacher HR, Wilder RL: Hyperplastic synoviocytes from rats with streptococcal cell wall-induced arthritis exhibit a transformed phenotype that is thymic-dependent and retinoid inhibitable. Am J Pathol 1988, 132:38-48 [PMC free article] [PubMed] [Google Scholar]
- 55.Firestein GS: Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum 1996, 39:1781-1790 [DOI] [PubMed] [Google Scholar]
- 56.Teitelbaum SL: Bone resorption by osteoclasts. Science 2000, 289:1504-1508 [DOI] [PubMed] [Google Scholar]
- 57.Hofbauer LC, Heufelder AE: The role of osteoprotegerin and receptor activator of nuclear factor kappaB ligand in the pathogenesis and treatment of rheumatoid arthritis. Arthritis Rheum 2001, 44:253-259 [DOI] [PubMed] [Google Scholar]
- 58.Kruijsen MW, van Den Berg WB, van de Putte LB: Sequential alterations of periarticular structures in antigen-induced arthritis in mice. Histological observations on fibrous capsule, ligaments, bone and muscles, using whole joint sections. Br J Exp Pathol 1983, 64:298-305 [PMC free article] [PubMed] [Google Scholar]
- 59.Schalkwijk J, van Den Berg WB, van de Putte LB, Joosten LA, van der Sluis M: Effects of experimental joint inflammation on bone marrow and periarticular bone. A study of two types of arthritis, using variable degrees of inflammation. Br J Exp Pathol 1985, 66:435-444 [PMC free article] [PubMed] [Google Scholar]
- 60.Martel W, Braunstein EM, Borlaza G, Good AE, Griffin PE, Jr: Radiologic features of Reiter disease. Radiology 1979, 132:1-10 [DOI] [PubMed] [Google Scholar]
- 61.Resnick D, Sartoris D, Cone RO: Diagnostic tests and procedures in Rheumatic disease. Kelley WN Harris ED, Jr Ruddy S Sledge CB eds. Textbook of Rheumatology. 1989:pp 650-708 W.B. Saunders Philadelphia
- 62.Martel W, Stuck KJ, Dworin AM, Hylland RG: Erosive osteoarthritis and psoriatic arthritis: a radiologic comparison in the hand, wrist, and foot. AJR Am J Roentgenol 1980, 134:125-135 [DOI] [PubMed] [Google Scholar]
- 63.Altman RD, Tenenbaum J: Hypertrophic osteoarthropathy. Kelley WN Harris ED, Jr Ruddy S Sledge CB eds. Textbook of Rheumatology. 1989:pp 1666-1673 W.B. Saunders Philadelphia







