Abstract
Arpp, a protein containing an ankyrin repeat domain, PEST sequence, and proline-rich region, is a novel ankyrin-repeated protein highly homologous to Carp, which is proposed to be the putative genetic marker for cardiac hypertrophy. In this study, we comparatively analyzed expression of Arpp and Carp protein in skeletal and cardiac muscles and rhabdomyosarcomas (RMSs). In adult skeletal muscle, Arpp was preferentially expressed in the nucleus and cytoplasm of type I fibers, whereas Carp was barely detectable in skeletal muscle. On the other hand, in adult cardiac muscle, interestingly, Arpp was expressed in ventricles mostly, whereas Carp was expressed throughout the atrium and ventricle. Furthermore, although Carp was identified in fetal heart at 11 developmental weeks, Arpp was very low or undetectable in these fetal hearts. These results suggest that Arpp and Carp are differentially expressed and function in both skeletal and cardiac muscle of fetus and adult. We found that Arpp expression was induced during the differentiation of C2C12 cells in vitro, suggesting that Arpp-expression may be associated with the differentiation stage during myogenesis. Both Arpp and Carp were found to be expressed in all of the RMS cases studied. Because the expression patterns of Arpp in RMS were different from those of muscle actin or desmin, Arpp may be detectable in RMS cases that do not express other existing RMS markers.
We recently isolated from a human esophageal carcinoma cell line, TE-1, a novel gene, Arpp, encoding an ankyrin-repeated protein composed of 333 amino acids. 1 The main structural features of the Arpp protein are the presence of four ankyrin-like repeat motifs in the middle portion, a lysine-rich sequence (KKRK) similar to a nuclear localization signal, a PEST-like sequence in the N-terminal region, and a proline-rich region in the C-terminal region. Analysis using multiple tissue expression array revealed that Arpp mRNA was restrictedly expressed in skeletal muscle and heart, suggesting that Arpp may play a functional role in skeletal and cardiac muscle. 1 Recently, expression of the mouse gene, Ankrd2, whose protein product is homologous to Arpp in its amino acid sequence, was found to be induced in stretched skeletal muscle. 2 Because of its structural similarity, the Ankrd2 gene was thought to be a murine counterpart of the Arpp gene.
Arpp’s amino acid sequence is highly homologous to that of human cardiac ankyrin-repeated protein (Carp) (52% identical). It has been reported that expression of mouse Carp mRNA is high in fetal heart but down-regulated after birth and is only found at trace levels in adult skeletal muscle. 3 Recent reports have proposed that Carp is a genetic marker for cardiac hypertrophy, based on the finding that Carp expression is significantly increased in the mouse model of hypertrophic heart induced by pressure overload. 4 It has been reported that, in primary cultured cardiomyocytes of rat neonate, Carp protein was localized in the nucleus, 3,5 whereas tissue distributions and intracellular localization of Carp and Arpp proteins in skeletal muscle and heart are not yet known.
In this study, we immunohistochemically analyzed the expression of Arpp and Carp proteins in skeletal muscle and heart, using anti-Arpp antibody (Ab) and anti-Carp Ab, and found that Arpp is preferentially expressed in the nuclei and cytoplasm of type I skeletal muscle fibers and in cardiac muscle fibers of the ventricles but not those of the atria. Carp was found to be expressed in the cytoplasm of cardiac ventricles and atria, but it was barely detectable in skeletal muscle. In addition, although Carp was expressed in fetal heart, Arpp was barely detectable. Furthermore, when C2C12 myoblasts were induced to differentiate to myocytes, Arpp expression was significantly increased, suggesting that Arpp may be expressed during the differentiation process of skeletal muscle. Next, we analyzed the expression of Arpp and Carp in rhabdomyosarcoma (RMS) and found that Arpp- and Carp-expressing RMS cells were detectable in all cases of RMS that we examined. Furthermore, Arpp was not detected in leiomyosarcoma (LMS).
Materials and Methods
Cell Lines and Tissues
C2C12, a murine myoblast cell line, and HeLa, a human cervical cancer cell line were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum. The paraffin-embedded tissues used in this study for diagnostic purposes were obtained by autopsy or surgical operation. Ten cases of skeletal muscle, five cases of tongue, two cases of diaphragm, and five cases of heart were subjected to the immunohistochemistry (Table 1) ▶ . Five cases of fetus at 10, 11, and 14 developmental weeks were selected for immunohistochemistry. All of them were diagnosed as intrauterine fetal death (Table 2) ▶ . Tissue samples from 24 cases of RMS and 11 cases of LMS were selected from the files of the First Department of Pathology, Tottori University, and the Department of Pathology, University of Tokyo. Histological diagnosis was based on the classification described by Weiss and colleagues. 6 The 24 cases of RMS comprised 12 cases of the embryonal type, 6 cases of the alveolar type, and 6 cases of the pleomorphic type. Histological subtypes, tumor sites, and the sex and age of patients are listed in Table 3 ▶ . Use of these tissue samples for this study was approved by the Institutional Review Board of Tottori University (permission no. 2001-149).
Table 1.
Expression of Arpp and Carp Protein in Adult
| Arpp | Carp | |
|---|---|---|
| Skeletal muscle | ||
| Biceps brachii (1) | + | +/− |
| Supinator (1) | + | − |
| Pronator quadratus (1) | + | − |
| Trapezius (1) | ++ | +/− |
| Latissimus dorsi (1) | ++ | +/− |
| Quadriceps femoris (2) | + | − |
| Biceps femoris (1) | ++ | − |
| Iliopsoas (1) | ++ | +/− |
| Tongue (5) | + | +/− |
| Diaphragm (2) | ++ | + |
| Heart (5) | ||
| Atrium | +/− | ++ |
| Ventricle | ++ | ++ |
Numbers of cases are parenthesized.
Immunoreactivities were classified based on the Arpp-positive rate. ++, <50%; +, 5 to 50%; +/−, 0 to 5%; −, unstained.
Table 2.
Expression of Arpp and Carp Protein in Fetus
| Developmental weeks | Arpp | Carp | ||
|---|---|---|---|---|
| Heart | Skeletal muscle | Heart | Skeletal muscle | |
| 1. 10W | N | + | N | + |
| 2. 11W | − | ++ | ++ | ++ |
| 3. 11W | − | + | + | + |
| 4. 14W | N | ++ | N | ++ |
| 5. 14W | N | ++ | N | ++ |
Immunoreactivities were classified based on the Arpp-positive rate. ++, <50%; +, 5 to 50%; +/−, 0 to 5%; −, unstained.
N, Not identified.
Table 3.
Expression of Arpp and Carp Protein in RMS
| Histology | Age | Sex | Site | Arpp | HHF35 | Myoglobin | Desmin | Carp |
|---|---|---|---|---|---|---|---|---|
| 1. Alveolar | 38 | M | Nasal | ++ | ++ | + | + | ++ |
| 2. Alveolar | 7 | F | Muscle | ++ | + | +/− | + | ++ |
| 3. Alveolar | 20 | F | ++ | + | + | NT | ++ | |
| 4. Alveolar | 2 | F | Nasal | + | ++ | + | + | NT |
| 5. Alveolar | 3 | F | Nasal | ++ | ++ | − | + | NT |
| 6. Alveolar | 16 | M | Hip | + | ++ | + | ++ | ++ |
| 7. Embryonal | 48 | M | Soft tissue | ++ | ++ | + | + | ++ |
| 8. Embryonal | 18 | M | Lymph node | ++ | + | +/− | + | ++ |
| 9. Embryonal/botryoid | 1.8 | M | Ureter | ++ | ++ | + | +/− | ++ |
| 10. Embryonal/botryoid | 55 | M | Rectum/bladder | + | + | + | + | ++ |
| 11. Embryonal | 12 | F | Soft tissue | + | ++ | + | + | ++ |
| 12. Embryonal | 25 | M | Soft tissue | + | ++ | + | NT | ++ |
| 13. Embryonal | 8 | M | Bladder | + | ++ | +/− | +/− | ++ |
| 14. Embryonal | 4 | M | Lung | +/− | ++ | +/− | + | + |
| 15. Embryonal | 22 | F | Maxillary | + | ++ | − | ++ | NT |
| 16. Embryonal | 4 | M | Pelvic cavity | ++ | − | − | +/− | NT |
| 17. Embryonal | 13 | M | Hand | ++ | ++ | − | + | NT |
| 18. Embryonal | 8 | M | Retroperitoneum | + | ++ | − | + | NT |
| 19. Pleomorphic | 26 | M | Prostate/bladder | + | ++ | + | + | ++ |
| 20. Pleomorphic | 20 | F | Leg | ++ | ++ | + | ++ | NT |
| 21. Pleomorphic | 48 | F | Thigh | + | ++ | − | ++ | NT |
| 22. Pleomorphic | 53 | M | Lumbar region | ++ | ++ | +/− | ++ | + |
| 23. Pleomorphic | 62 | F | Ovary | ++ | ++ | + | + | ++ |
| 24. Pleomorphic | 38 | M | Cervical region | + | ++ | +/− | ++ | NT |
Immunoreactivities were classified based on the Arpp-positive rate. ++, <50%; +, 5 to 50%; +/−, 0 to 5%; −, unstained.
NT, Not tried.
Antibodies
Anti-Arpp Ab [α-Arpp(N) Ab] recognizing the N-terminal 84 amino acids of Arpp protein (5 to 88) and anti-Carp Ab [α-Carp(N) Ab] recognizing the N-terminal 69 amino acids of Carp protein (1 to 69) were generated as follows. First, the cDNA encoding the N-terminal region (5 to 88) of Arpp was amplified by polymerase chain reaction (PCR) with the forward primer, Arp-f1, 5′-TCTCGAGATGGAGGACTCCGAGGCGGTG-3′ and the reverse primer, Arp-r1, 5′-TGCGGCCGCTGAGGTTCTGGATCCCGCC-3′ using a plasmid containing full-length Arpp cDNA as a template for PCR, as reported previously. 1 Next, the cDNA encoding the N-terminal region (1 to 69) of Carp was amplified by PCR with the forward primer Carp-f1, 5′-TGGATCCACATGATGGTACTGAAAGTAGAG-3′ and the reverse primer Carp-r2, 5′-TCTCGAGTCACTCTGCCTCTCGTTGTTTC-3′ using the human skeletal muscle cDNA library as a template. The resulting PCR products were subcloned into pGEM-T Easy vector (Promega, Madison, WI) and sequenced. The PCR products were digested with BamHI and XhoI and subcloned into the BamHI and XhoI sites of the pGEX5X-1 vector plasmid (Amersham-Pharmacia Biotech, Buckinghamshire, UK). The Arpp (5 to 88) protein and Carp (1 to 69) protein fused to glutathione S-transferase that were subsequently synthesized in Escherichia coli were purified using glutathione-Sepharose, as described previously. 7 The purities and concentrations of the eluted proteins were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anti-Arpp Ab recognizing amino acids 5 to 333 of Arpp [α-Arpp(FL) Ab] was generated as described previously. 1 The following Abs were purchased and used according to the manufacturer’s instructions: anti-slow myosin mAb [α-myosin heavy chain (MHC)(slow) Ab] (YLEM, Rome, Italy), which specifically recognizes type I MHC; anti-skeletal myosin (FAST) mAb (MY-32 Ab; Sigma, Saint Louis, MO), which specifically recognizes type II MHC [α-MHC(fast) Ab]; anti-myogenin mAb (F5D Ab; Santa Cruz, CA), [α-myogenin Ab]; anti-human desmin mAb (D33; DAKO, Carpinteria, CA) [α-desmin Ab]; anti-human muscle actin (HHF35) Ab (DAKO) [HHF35 Ab], anti-myoglobin Ab (Nichirei, Tokyo, Japan) [α-myoglobin Ab]; anti-α tubulin Ab (DM 1A; Sigma) [α-tubulin Ab]; and anti-smooth muscle actin mAb (M0851; DAKO) [α-smooth muscle actin Ab].
Plasmid Construction and Transfection
To generate an expression plasmid encoding full-length human Carp protein (1 to 319), the full-length cDNA was amplified by PCR with the forward primer Carp-f1 and the reverse primer, Carp-r1, 5′-TCTCGAGATTAAGAGTCTGTCGTTTGCC-3′, using the human skeletal muscle cDNA library (Clontech, Palo Alto, CA) as a template. The PCR product was subcloned into the pGEM-T Easy vector (Promega) and sequenced. The PCR product was digested with BamHI/XhoI and inserted into the BglII/XhoI site of pCMV-Myc vector (Clontech). The expression plasmid encoding full-length Arpp protein (pcDNA3-Arpp) generated as described previously 1 was used for the transfection experiment. Transfection experiments were performed using Trans IT-LT1 Polyamine Transfection Reagents (Takara, Tokyo, Japan) according to the manufacturer’s instructions.
Western Blotting
Cells transfected with expression plasmids were cultured for 36 hours then lysed in a similar condition to our previous report. 1 The cell lysates (50 μg) were subjected to sodium dodecyl sulfate (10% w/v)-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The resulting filter was then subjected to immunoblotting using 1 μg/ml of α-Arpp(FL) Ab, 1 μg/ml of α-Arpp(N) Ab, or 2 μg/ml of α-Carp(N) Ab as the first Ab in a similar way to our previous report. 1
Immunocytochemistry
C2C12 cells were cultured for 36 hours on coverslips coated with collagen type IV (Nitta gelatin; Nitta, Osaka, Japan). To induce differentiation to C2C12 cells, the culture medium was replaced by Dulbecco’s modified Eagle’s medium containing 3 ng/ml of insulin-like growth factor I (R&D Systems, Minneapolis, MN) and 1% w/v bovine serum albumin [differentiation medium (DM)]. After the cells had been incubated for up to 7 days, they were fixed with 4% w/v paraformaldehyde in 1× phosphate-buffered saline (PBS) for 10 minutes. Subsequently, they were washed with 1× PBS and permeabilized with 0.3% v/v Triton X-100 in 1× PBS for 15 minutes. After washing four times for 5 minutes each with 1× PBS containing 0.2% v/v Tween 20, the cells were then incubated for 16 hours at 4°C with a mixture of α-Arpp(FL) Ab (1:500) and α-myogenin Ab (1:200), or with a mixture of α-Arpp(FL) Ab (1:500) and either α-fast MHC Ab (1:500) or α-slow MHC Ab (1:50) in 1× PBS containing 3% bovine serum albumin. After being washed a further three times for 5 minutes each with 1× PBS containing 0.2% v/v Tween 20, the cells were incubated for 2 hours with a mixture of Alexa Fluor 488-conjugated goat anti-rabbit Ab (1:500) (Molecular Probes, Eugene, OR) and Alexa Fluor 546-conjugated goat anti-mouse Ab (1:500) in 1× PBS containing 3% w/v bovine serum albumin. The coverslips were washed three times for 5 minutes each with 0.2% v/v Tween 20 and mounted with gel mount (Biomedia Corp., Foster City, CA). The mounted coverslips were then observed using a fluorescence microscope (Eclipse E800; Nikon, Tokyo, Japan), and the images processed with the MRC-1024 confocal system (Bio-Rad, Hercules, CA).
Immunohistochemistry
Tissue sections were thoroughly deparaffinized and rehydrated using standard protocols. For antigen retrieval, sections immersed in 10 mmol/L of sodium citrate buffer, pH 6.0 (Iatron Co., Tokyo, Japan), were autoclaved at 120°C for 10 minutes and cooled to room temperature. Sections were then treated with 3% H2O2 for 5 minutes at room temperature to inactivate endogenous peroxidase activity, then blocked with 10% goat serum (Nichirei Co., Tokyo, Japan) for 20 minutes at room temperature. Subsequently, they were incubated with α-Arpp(FL) Ab diluted to 1:1000 with diluting solution (DAKO) for 60 minutes at room temperature. The other first Abs were used in the following dilution ratios, α-Carp(N) Ab (1:500), α-Arpp(N) Ab (1:1000), HHF35 Ab (1:1), α-myoglobin Ab (1:1), and α-desmin Ab (1:50). Next, sections were washed with 1× PBS and incubated for 20 minutes with biotinylated goat anti-rabbit IgG (Nichirei Co.). After being washed with 1× PBS, the sections were incubated with a solution of avidin-conjugated horseradish peroxidase (Vectastain Elite ABC kit; Vector Laboratories Inc., Burlingame, CA) for 10 minutes, according to the manufacturer’s recommendations, and then washed with 1× PBS for 5 minutes. Peroxidase activity was detected with H2O2/diaminobenzidine substrate solution and the sections were counterstained with hematoxylin before dehydration and mounting.
Double-Labeled Immunohistochemistry (Arpp Versus Fast or Slow Specific Myosin, Arpp Versus Desmin or HHF35)
Deparaffinized sections were immersed in 10 mmol/L of sodium citrate buffer, pH 6.0 (Iatron Co.), and autoclaved at 120°C for 10 minutes. Subsequently, they were blocked with 10% normal goat serum (Nichirei Co.) for 30 minutes at room temperature. The sections were then incubated for 60 minutes at room temperature with a mixture of α-Arpp(FL) Ab diluted to 1:1000 and one of the following first Abs: α-MHC(slow) Ab diluted to 1:50; α-MHC(fast) Ab diluted to 1:500; HHF35 Ab without dilution, or α-desmin Ab diluted to 1:50. After being washed with 1× PBS, the sections were incubated for 2 hours at room temperature with a mixture of Alexa Fluor 488-conjugated goat anti-rabbit Ab diluted to 1:200 and Alexa Fluor 546-conjugated goat anti-mouse Ab (Molecular Probes) diluted to 1:200 in 1× PBS. The sections were then washed with 1× PBS and mounted with gel mount (Biomedia Corp.). The mounted sections were observed with a fluorescence microscope (Eclipse E800; Nikon), and the images processed with the MRC-1024 confocal system (Bio-Rad).
Results
Specificity of the Antibodies
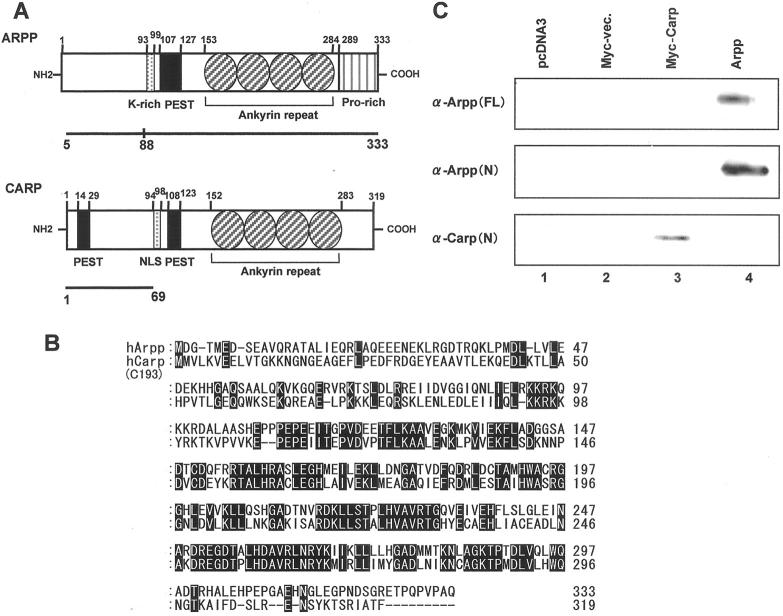
To analyze the expression of Arpp and Carp proteins in human tissues, polyclonal Abs recognizing full-length Arpp [α-Arpp(FL) Ab], N-terminal region of the Arpp [α-Arpp(N) Ab], and the N-terminal region of the Carp protein [α-Carp(N) Ab] (Figure 1, A and B) ▶ were raised as described in Materials and Methods. To confirm the specificity of the Abs, we performed Western blot analysis of HeLa cells transfected with the expression plasmids encoding the full-length Arpp, full-length Carp, or vector alone. As shown in Figure 1C ▶ , α-Arpp(FL) Ab, α-Arpp(N) Ab, and α-Carp(N) Ab were able to recognize the expressed Arpp and Carp proteins, respectively. Furthermore, α-Arpp(FL) Ab and α-Arpp(N) Ab were not cross-reactive with Carp protein, and α-Carp(N) Ab was not cross-reactive with Arpp protein expressed in HeLa cells (Figure 1C) ▶ . These results clearly show that these Abs can specifically recognize the Arpp and Carp proteins, respectively.
Figure 1.
Identification of Carp and its homologue, Arpp, by specific Abs. A: Schematic representation of the domain structures of Arpp and Carp proteins. The amino acid sequences used for immunization [Arpp(5-333) for α-Arpp(FL) Ab, Arpp(5-88) for α-Arpp(N) Ab, and Carp(1-69) for α-Carp(N) Ab] are indicated by bold bars. B: Comparison of the amino acid sequence of Arpp with that of human Carp (C-193). Shaded boxes show identical residues. C: Western blotting of Arpp- and Carp-transfected HeLa cells. HeLa cells were transfected with pcDNA3-vector (lane 1), myc-vector (lane 2), myc-Carp (lane 3), and pcDNA3-Arpp (lane 4). The cell lysates were subsequently subjected to Western blotting with α-Arpp(FL) Ab (top), α-Arpp(N) Ab (middle), and α-Carp(N) Ab (bottom).
Arpp Is Preferentially Expressed in Type I Skeletal Muscle Fibers
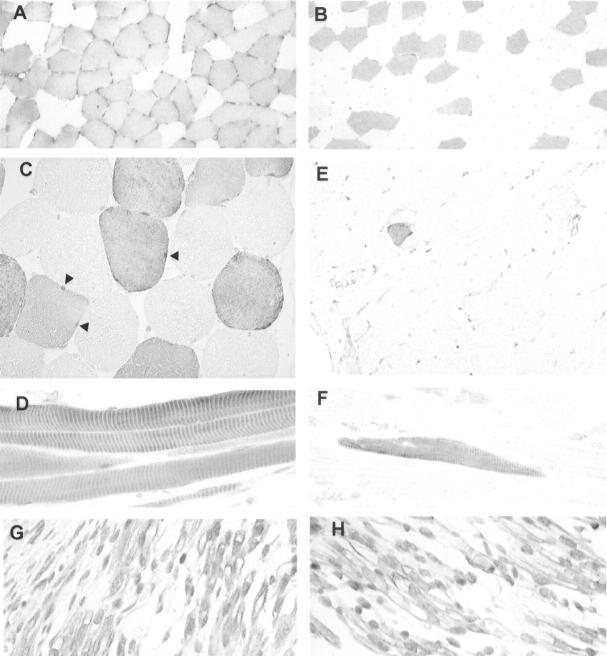
To evaluate the expression of Arpp and Carp protein in vivo, we analyzed paraffin-embedded human tissue sections by immunohistochemistry using the α-Arpp(FL) Ab and α-Carp(N) Ab (Table 1) ▶ . As shown in Figure 2 ▶ ; A to C, Arpp was detected in both cytoplasm and nucleus of skeletal muscle fibers. Distributions of Arpp-positive myofibers were not uniform; rather, positive and negative myofibers were scattered randomly in a checkerboard-like pattern (Figure 2 ▶ ; A to C). These characteristic expression patterns were also observed when the α-Arpp(N) Ab was used for the immunohistochemistry (data not shown), confirming that the both α-Arpp(N) and α-Arpp(FL) Abs can specifically detect Arpp in vivo. As shown in Figure 2, A and B ▶ , Arpp-positive rates of biceps femoris muscle are significantly higher than those of quadriceps femoris muscle, suggesting that Arpp-positive rates may be different depending on the positions of the skeletal muscle. In the longitudinal view of skeletal muscle fibers, strong immunoreaction was found to coincide with muscle striation (Figure 2D) ▶ . Immunoreactions with α-Arpp(FL) Ab were not seen in smooth muscle layers of stomach or small and large intestines (data not shown). By contrast, Carp was barely detectable in skeletal muscle, although we did observe a very small number of muscle fibers expressing Carp at a comparable level to that of Arpp (Figure 2E) ▶ . In longitudinal section of Carp-positive muscle fibers, immunoreaction was coincided with muscle striation in a similar way to Arpp (Figure 2F) ▶ . In addition, Carp-positive muscle fibers tended to be more frequently detected in tongue and diaphragm than in the other positions of skeletal muscle (Table 1) ▶ .
Figure 2.
Expression of Arpp and Carp in human skeletal muscle tissues demonstrated immunohistochemically. A: Cross-section of biceps femoris muscle immunostained with α-Arpp(FL) Ab. Arpp-positive myofibers are scattered randomly in a checkerboard-like pattern. B: Cross-section of quadriceps femoris muscle immunostained with α-Arpp(FL) Ab. C: Cross-section of skeletal muscle immunostained with α-Arpp(FL) Ab. Both nuclei (arrowheads) and cytoplasm of myofibers are positively stained. D: Longitudinal section of skeletal muscle immunostained with α-Arpp(FL) Ab. Positive immunoreactions coincide with muscle striation in Arpp-positive muscle fibers. E: Cross-section of skeletal muscle immunostained with α-Carp(N) Ab. Carp is undetectable in almost all myofibers. Very small numbers of Carp-positive myofibers were detected. F: Longitudinal section of skeletal muscle immunostained with α-Carp(N) Ab. Positive immunoreaction coincides with muscle striation in Carp-positive muscle fibers. G and H: Immunohistochemistry of skeletal muscle of fetus at 11 developmental weeks with α-Carp(N) Ab (G) and α-Arpp(FL) Ab (H). Both nuclei and cytoplasms are positively stained with α-Arpp(FL) Ab and α-Carp(N) Ab. Myocytes positively immunostained and those stained at only a trace level are observed (G, H). Original magnifications: ×100 (A, B, E); ×400 (C, D, F–H).
Based on the knowledge that type I and II muscle fibers, which are defined by MHC isoforms, are distributed in a checkerboard-like pattern, we hypothesized that Arpp may specifically be expressed in one of those two types. To confirm this possibility, double-staining analyses were performed using α-Arpp(FL) Ab in combination with α-MHC(slow) Ab or α-MHC(fast) Ab. As shown in Figure 3 ▶ , Arpp-positive myofibers coincided well with myofibers immunoreactive with α-MHC(slow) Ab, but did not coincide with α-MHC(fast) Ab, indicating that Arpp is preferentially expressed in type I skeletal muscle fibers. However, we could also identify small populations of Arpp-negative slow MHC-positive fibers, Arpp-positive fast MHC-positive fibers, and Arpp-negative fast MHC-negative fibers (data not shown), suggesting that Arpp-positive muscle fibers do not completely correspond to slow MHC-positive muscle fibers.
Figure 3.
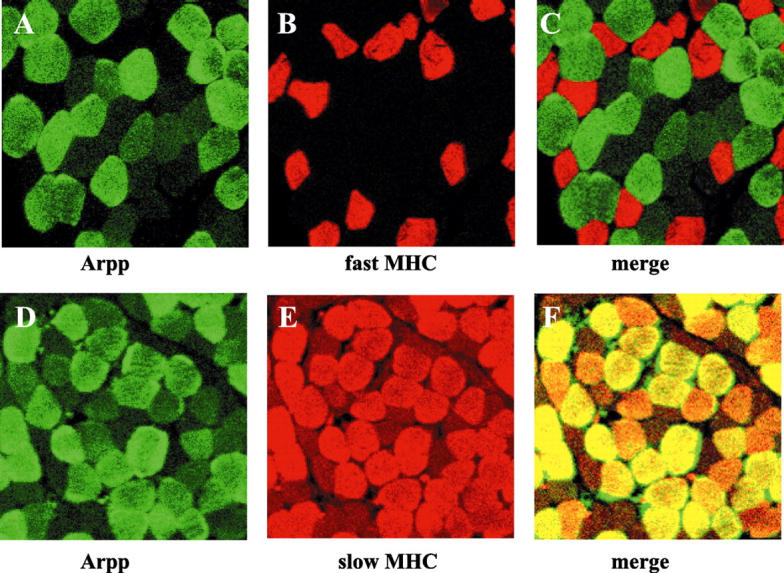
Muscle-type-specific expression of Arpp protein. Paraffin-embedded human skeletal muscle tissue sections were analyzed by double-immunostaining analysis using confocal microscopy. Skeletal muscle tissue sections were incubated with α-Arpp(FL) Ab and α-MHC(fast) Ab (A–C), or with α-Arpp(FL) Ab and α-MHC(slow) Ab (D–F). Subsequently, these first Abs were detected by Alexa Fluor 488-conjugated goat anti-rabbit secondary Ab (green) or Alexa Fluor 546-conjugated goat anti-mouse secondary Ab (red). Skeletal muscle fibers expressing Arpp (A and D, green), fast MHC (B, red) and slow MHC (E, red) were detected. When the signals reflecting the expression of Arpp and fast MHC or Arpp and slow MHC were merged (C and F), the resulting yellow signal indicated co-expression of Arpp and fast MHC or Arpp and slow MHC.
Differential Expression of Arpp and Carp Proteins in Adult Heart
To explore the expression of Arpp and Carp proteins in heart, we analyzed human heart tissue sections by immunohistochemistry. As shown in Figure 4 ▶ ; A to C, Carp-positive cardiomyocytes were diffusely distributed throughout the atria and ventricles and the expression levels in atrial and ventricular cardiomyocytes were almost similar. Although it has previously been believed that the expression of Carp is restricted to the nuclei of cardiomyocytes, 3 we unexpectedly found Carp to be strongly expressed in the cytoplasm of cardiomyocytes whereas expression levels in the nuclei were low or undetectable (Figure 4B) ▶ . Positive immunoreaction in the cytoplasm coincided with striation of cardiomyocytes in a similar manner to that seen in skeletal muscle.
Figure 4.
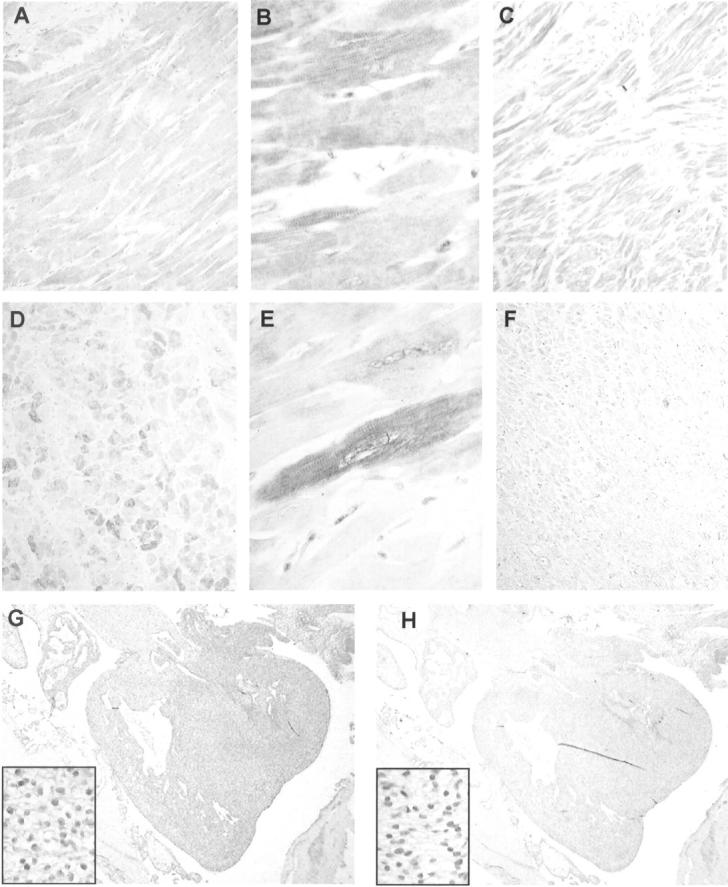
Arpp and Carp expression in human heart. Paraffin-embedded human heart tissue sections were analyzed by immunohistochemistry with α-Carp(N) Ab (A, B, C, G) and α-Arpp(FL) Ab (D, E, F, H). Carp is expressed in both ventricle (A) and atrium (C). Ventricular cardiomyocytes diffusely express Carp protein (B). Carp is also expressed in fetal heart at 11 developmental weeks (G). Arpp protein is strongly expressed in ventricular cardiomyocytes (D) but rarely expressed in the atria (F). Ventricular cardiomyocytes expressing Arpp at high, low, or undetectable levels were admixed (E). Arpp expression is barely detectable in fetal heart at 11 developmental weeks (H). Original magnifications: ×100 (A, C, D, F); ×400 (B, insets in G and H, E); ×20 (G, H).
The expression patterns of Arpp were different from those of Carp. As shown in Figure 4 ▶ , Arpp was strongly expressed in the ventricles but rarely detectable in the atria (Figure 4 ▶ ; D to F). Furthermore, strongly positive cardiomyocytes, weakly positive cardiomyocytes, and Arpp-negative cardiomyocytes were randomly admixed (Figure 4D) ▶ . With respect to intracellular localization of Arpp, strong positive immunoreactions were detected in the cytoplasm and coincided with muscle striation (Figure 4E) ▶ , similar to the coincidence seen in skeletal muscle. Expression levels of Arpp in the nuclei of positive cells were found varied. Cardiomyocytes with Arpp-positive nuclei and those with Arpp-negative nuclei were admixed (Figure 4E) ▶ .
Expression of Arpp and Carp in Fetal Heart and Skeletal Muscle
Next, to determine whether Arpp and Carp are expressed in fetal tissues, we immunohistochemically analyzed tissue sections from five cases of human fetus at 10, 11, and 14 developmental weeks. Both Arpp and Carp were detected in the skeletal muscle of fetus at 10, 11, and 14 developmental weeks (Table 2 ▶ and Figure 2, G and H ▶ ). However, in heart, Arpp and Carp expressions exhibited a remarkable contrast. In fetal heart at 11 developmental weeks, Carp was strongly expressed (Table 2 ▶ , Figure 4G ▶ ), but Arpp was undetectable or detectable at a trace level (Table 2 ▶ , Figure 4H ▶ ). Carp-positive cardiomyocytes were diffusely distributed throughout the atria and ventricles (Figure 4G) ▶ . Interestingly, in contrast to nuclei of adult cardiomyocytes, nuclei of fetal cardiomyocytes strongly expressed Carp (Figure 4G) ▶ .
Arpp Is Induced during Differentiation of C2C12 Myoblasts
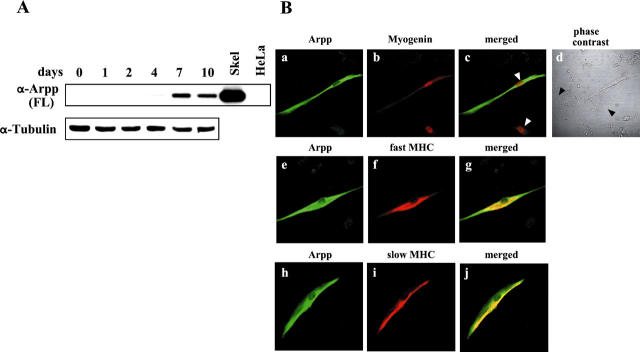
Based on the finding that Arpp expression is highly restricted to cardiac and skeletal muscles, we hypothesized that Arpp may be involved in the myogenic differentiation pathway. To test this hypothesis, we investigated the relationships between differentiation of C2C12 cells and Arpp expression. C2C12 myoblasts are well known to be inducible to terminally differentiated myotubes when they were cultured in serum-reduced media. 8 As shown in Figure 5A ▶ , when C2C12 myoblasts were cultured in the DM to induce myogenic differentiation, Arpp protein was detectable by Western blotting after 4 days (Figure 5A) ▶ . Levels were then found to increase gradually and peaked after 7 days of culture in DM, followed by a slight decrease after 10 days (Figure 5A) ▶ . This observation is consistent with recently reported data that Ankrd2 is induced during the differentiation of C2C12 cells at mRNA level. 2 We further confirmed the expression of Arpp in C2C12 cells during the differentiation by microscopic analysis. We could detect Arpp-positive cells among the Arpp-negative myoblasts after at least 2 days culture in DM (data not shown). The populations of Arpp-positive cells then increased after culture for 7 days in DM (data not shown). By contrast, in morphologically undifferentiated myoblasts, Arpp was not induced even after the culture for 7days (Figure 5B, a and d) ▶ . To confirm the expression of Arpp in differentiated C2C12 cells, we performed double immunocytochemistry for the co-expression of Arpp and other markers, including myogenin, a marker for the early differentiated stage of myocytes, or slow or fast MHC, a marker for the terminally differentiated stage of myotubes. In myogenin-positive myocytes, Arpp-positive and -negative myocytes were mingled (Figure 5B, b and c) ▶ , and the number of myocytes co-expressing Arpp and myogenin significantly increased after 7 days of culture in DM (data not shown). In addition, among myogenin-negative myoblasts, we could not find any myoblasts that expressed Arpp. Approximately half of the Arpp-positive myocytes cultured for 7 days in DM were found to co-express slow or fast MHC (Figure 5B, e to j) ▶ . Conversely, we could not find any myotubes that expressed MHC but did not express Arpp. Based on these findings, we suggest that, during myogenesis of C2C12 cells, the expression of Arpp may follow that of myogenin but precede that of slow or fast MHC.
Figure 5.
Arpp is induced during differentiation of C2C12 cells. A: Western blot analysis of differentiating C2C12 cells for Arpp expression. After culture of C2C12 cells in DM for 0, 1, 2, 4, 7, or 10 days, the cells were collected and their lysates subjected to Western blotting using α-Arpp(FL) Ab. Mouse skeletal muscle (Skel) and HeLa cells (HeLa) were subjected to the same analysis as a positive and negative control, respectively. The same amount of the lysate was subjected to Western blotting using α-tubulin Ab as a loading control. B: Arpp is expressed in differentiated C2C12 cells. After culture for 7 days in DM, C2C12 cells were fixed and incubated with a mixture of α-Arpp(FL) Ab and α-myogenin Ab (a–c), α-MHC(fast) Ab (e–g), or α-MHC(slow) Ab (h–j). These first Abs were detected by Alexa Fluor 488-conjugated goat anti-rabbit secondary Ab (green) or Alexa Fluor 546-conjugated goat anti-mouse secondary Ab (red). Arpp was detected as green signals (a, e, h). Myogenin (b), fast MHC (f), and slow MHC (i) were detected as red signals. When both signals were merged, the resulting yellow signals reflect co-expression of Arpp and myogenin (c), Arpp and fast MHC (g), or Arpp and slow MHC (j). Myogenin was detected in the differentiated myocytes (red in b). Among the myogenin-expressing myotube-like cells, Arpp-positive myotube-like cells and Arpp undetectable myotube-like cells (white arrowheads in c) were admixed. After culture of C2C12 cells for 7 days in DM, among undifferentiated myoblast-like cells (black arrowheads in d), differentiated myotube-like cells were distributed.
Arpp and Carp Are Highly Expressed in RMS
Because RMS is a malignant tumor showing a striated muscle-like differentiation, 9 we decided to determine whether RMS expresses Arpp and Carp. Therefore, we immunohistochemically analyzed tissue sections of RMS for expression of Arpp and Carp. Table 3 ▶ shows that all 24 cases of RMS examined for Arpp exhibited positive immunoreaction with α-Arpp(FL) Ab and all 15 cases tested for Carp exhibited positive immunoreaction with α-Carp(N) Ab. Furthermore, Arpp expression was found to have no relevance to histopathological subtype. By contrast, in LMS, although 4 of 11 cases exhibited a positive immunoreaction for Carp, Arpp was detectable in only 2 cases at a very low level (Table 4) ▶ . Other malignant tumors, including 15 cases of gastric cancer, 15 cases of colon cancer, and 5 cases of malignant lymphoma were not immunoreactive with α-Arpp(FL) Ab (M Osaki, unpublished observation). As shown in Figure 6 ▶ , we found that Arpp and Carp were detected in both the nucleus and cytoplasm of RMS cells, although Arpp was more frequently localized at the nucleus than Carp. We then compared the expression patterns of Arpp with other existing muscle-specific markers used as tumor markers for RMS by double-immunostaining analysis. As shown in Figure 7 ▶ , Arpp-expressing cells did not always co-express desmin (Figure 7, C and F) ▶ , fast MHC (Figure 7I) ▶ , or muscle actin detected by HHF35 Ab (data not shown), indicating that α-Arpp(FL) Ab can detect RMS cells that are undetectable by other existing tumor markers.
Table 4.
Expression of Arpp and Carp Protein in LMS
| Age | Sex | Site | Arpp | HHF35 | Desmin | SMA | Carp | |
|---|---|---|---|---|---|---|---|---|
| 1. | 62 | M | Thigh | − | ++ | ++ | ++ | − |
| 2. | 35 | M | Retroperitoneum | +/− | +/− | ++ | − | + |
| 3. | 58 | M | Arm | − | +/− | ++ | − | − |
| 4. | 33 | M | Leg | − | + | ++ | − | − |
| 5. | 63 | M | Chest | − | − | ++ | − | − |
| 6. | 74 | F | Leg | − | ++ | ++ | ++ | − |
| 7. | 58 | F | Uterus | − | ++ | ++ | ++ | − |
| 8. | 47 | F | Uterus | − | ++ | + | − | ++ |
| 9. | 69 | F | Retroperitoneum | − | + | − | + | − |
| 10. | 43 | F | Uterus | − | + | + | ++ | +/− |
| 11. | F | Uterus | + | − | − | ++ | ++ |
SMA, Smooth muscle actin.
Immunoreactivities were classified based on the Arpp-positive rate. ++, <50%; +, 5 to 50%; +/−, 0 to 5%; −, unstained.
NT, Not tried.
Figure 6.
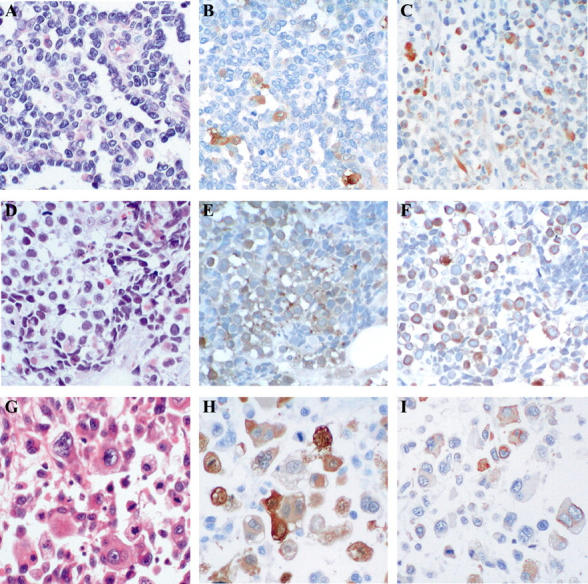
Arpp and Carp are strongly expressed in RMS cells. RMS tissues were analyzed by H&E staining (A, D, G) and immunohistochemistry with α-Arpp(FL) Ab (B, E, H) and α-Carp(N) Ab (C, F, I). In a case of alveolar-type RMS (A–C), Arpp-positive and -negative RMS cells (B) and Carp-positive and -negative RMS cells (C) are admixed. In a case of embryonal-type RMS (D–F), strongly immunoreactive RMS cells are scattered among those weakly stained (E). Population of Carp-positive RMS cells is larger than that of Arpp (F). Both nuclei and cytoplasm are positively stained (F). In a case of pleomorphic-type RMS (G–I), Arpp-positive and -negative RMS cells are scattered. Both cytoplasms and nuclei are positively immunostained (H). Carp-positive and -negative RMS cells are diffusely distributed (I). The population of Carp-positive RMS cells is lower than that of Arpp (I). Original magnifications, ×200.
Figure 7.
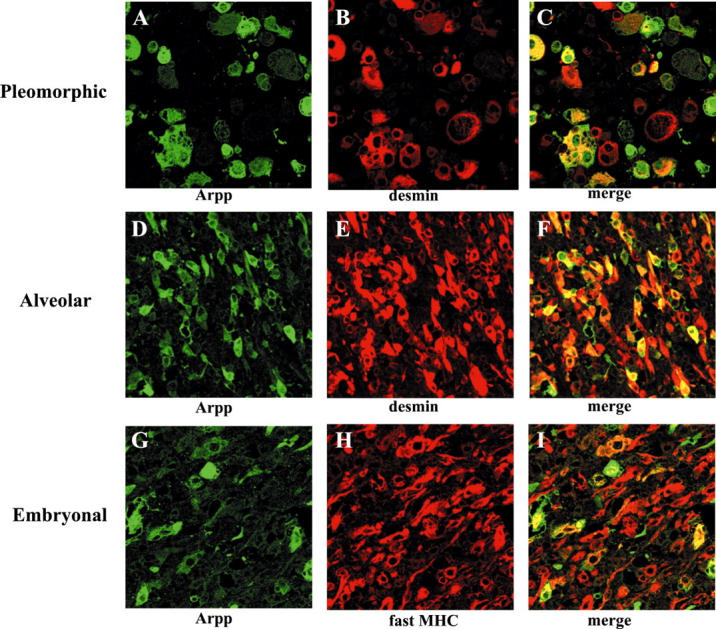
Double immunohistochemistry of Arpp and other existing muscle-specific markers for RMS. Paraffin-embedded RMS tissue sections were analyzed by double-immunostaining analysis using α-Arpp(FL) Ab together with α-desmin Ab or α-MHC(fast) Ab as the first Ab, followed by incubation with a mixture of Alexa Fluor 488-conjugated goat anti-rabbit secondary Ab (green), and Alexa Fluor 546-conjugated goat anti-mouse secondary Ab (red). In a case of pleomorphic type RMS (A–C) and a case of alveolar type RMS (D–F), RMS cells expressing Arpp (A and D, green) and desmin (B and E, red) were detected. When both the green signals and the red signals were merged, the detected yellow signals reflected co-expressions of Arpp and desmin (C and F, yellow). In a case of embryonal type RMS (G–I), RMS cells expressing Arpp (G, green) and fast myosin (H, red) were detected. When merged, the detected yellow signals reflected co-expression of Arpp and fast myosin (I, yellow).
Discussion
We previously reported that Arpp is localized at both the nucleus and cytoplasm in an esophageal carcinoma cell line, TE-1, which endogenously expresses Arpp. 1 In the present study, we found that Arpp is immunohistochemically detectable in both nucleus and cytoplasm of human cardiomyocytes and skeletal muscle fibers, indicating that Arpp protein is localized at both nucleus and cytoplasm in vivo. Interestingly, cardiomyocytes in which both nucleus and cytoplasm were positively immunostained with Arpp Ab were admixed with those in which only the cytoplasm was positively immunostained, suggesting that Arpp protein level in the nucleus may be tightly regulated via some unknown physiological mechanisms. We recently found that the Arpp protein level in the nucleus was significantly increased when Arpp-transfected HeLa cells were stimulated with serum after incubation under serum-free conditions at least overnight (T Baba, unpublished observation). Thus, it is likely that transition of the protein from cytoplasm to nucleus may be regulated. Alternatively, stability of the nuclear Arpp may be specifically regulated.
On the other hand, it has been proposed that Carp is a nuclear protein and may function as a transcriptional regulator in cardiomyocytes. 3,5,10 Indeed, Carp has been shown to be localized in the nucleus in primary-cultured cardiomyocytes of rat neonate. 3 However, our present study revealed that Carp is mainly expressed in the cytoplasm but is barely detectable in the nucleus in the adult heart. By contrast, in the fetus, we observed positive immunoreaction in nuclei of cardiomyocytes, suggesting that intracellular localization of Carp may change after birth. Recently, Bang and colleagues 11 reported that Carp is localized at sarcomeric I bands of primary-cultured skeletal and cardiac muscle fibers and that it interacts with myopalladin, which is a component of Z-disk complexes. Our recent immunoelectron microscopic study also revealed that Arpp is localized at I bands of skeletal muscle fibers. 12 These findings suggest that both Arpp and Carp may play some physiological roles not only in the nucleus but also in the cytoplasm of cardiac and skeletal muscles. In addition, their characteristic localization at I bands raises the possibilitythat they may participate in the contractile function of myofibers.
In this study, we could detect a direct evidence that Arpp is preferentially expressed in slow muscle (type I muscle), although slow muscles do not always express Arpp and Arpp-positive muscle cells were not always slow muscles. During the preparation of this manuscript, human Ankrd2 that is completely identical to Arpp in its amino acid sequences was reported. 13 The authors of this report have shown an indirect evidence that type II muscle fibers do not express Arpp/Ankrd2, 13 supporting our conclusion. The biological functions of Arpp in slow muscle are primarily unknown at this stage. However, it is conceivable that Arpp may play important physiological roles in slow muscle.
Interestingly, it has recently been reported that mouse Ankrd2 mRNA is induced in stretched skeletal muscle. 2 Furthermore, Carp expression has been shown to be associated with cardiac hypertrophy. 4,14 These findings lead us to speculate that Arpp may be induced during skeletal muscle hypertrophy. If this is the case, it will be an important issue to determine whether Arpp protein may be induced in Arpp-negative skeletal and cardiac muscle fibers or whether its expression level may be elevated only in Arpp-positive muscle fibers. Further studies will be required to address these possibilities.
With regard to the expression of Arpp and Carp in heart, we found that Carp is expressed at comparable levels in both atria and ventricles of heart. Carp has been reported to interact with YB-1 to form a complex that acts as a negative regulator of HF-1a-dependent MLC-2v transcription, suggesting that Carp may play some physiological functional role in cardiac muscles. 3,15 Because Arpp expression, in contrast to Carp, was found to be restricted to the ventricles, Arpp may regulate transcription of cardiac ventricle-specific genes. Furthermore, we found that Arpp-positive and -negative cardiomyocytes were admixed in cardiac ventricles in a pattern similar to that of skeletal muscle, although Carp was diffusely expressed in cardiac muscles. These data suggest the distinct functions of Arpp and Carp in heart. Thus, it is important to determine the physiological function of both proteins in cardiomyocytes. In this study, Arpp was found to be expressed only in adult heart, although Carp was expressed in both fetal and adult heart, suggesting that distinct mechanisms may be involved in the induction of Carp and Arpp during development of heart. It has been reported that Carp expression is induced during cardiac hypertrophy that is experimentally induced by pressure overload in mouse or rat heart. 4,14 These findings lead us to speculate that Arpp expression may also be induced in cardiomyocytes of hypertrophic heart.
Our present immunohistochemical analysis of RMS using α-Arpp(FL) Ab, α-Carp(N) Ab, and Abs to the other markers of early myogenic differentiation revealed that both Arpp- and Carp-positive RMS cells were detectable in all cases of RMS tested (Table 3) ▶ . It is well known that desmin and muscle actin, which are routinely used as markers of myogenic differentiation, are detectable not only in RMS but also in LMS. 6 However, Arpp was barely detected in LMS, suggesting that Arpp expression may be more specific for RMS than desmin or muscle actin. When C2C12 cells were induced to differentiate to myotubes, induction of Arpp expression followed the expression of myogenin, but preceded that of MHC, suggesting that Arpp expression may be correlated with the stage of differentiation. Therefore, one may speculate that the expression pattern of Arpp may reflect the differentiation stage of RMS cells.
We cannot completely rule out the possibility at this stage that Arpp may also be expressed at a high level in tumors other than RMS. Indeed, we previously reported that Arpp was detectable in esophageal carcinoma cell lines by Western blot analysis. 1 However, our preliminary immunohistochemical analysis of paraffin-embedded esophageal carcinomas revealed that their expression levels are apparently lower than that of skeletal muscle and RMS (Kase and colleagues, in preparation). To determine whether Arpp may be useful as a diagnostic marker for RMS, analysis using a larger number of cases of various tumor types is required. Because the mechanisms responsible for the induction and the effect of overexpression of Arpp in RMS cells are still unclear, further biochemical and biological studies will be required to address these questions.
Acknowledgments
We thank Mr. Itaki for his skilful technical assistance in immunohistochemistry.
Footnotes
Address reprint requests to Masatsugu Moriyama, M.D., Department of Molecular Biology, School of Life Science, Faculty of Medicine, Tottori University, 86 Nishimachi, Yonago-city, Tottori 683-8503, Japan. E-mail: moriyama@grape.med.tottori-u.ac.jp.
References
- 1.Moriyama M, Tsukamoto Y, Fujiwara M, Kondo G, Nakada C, Baba T, Ishiguro N, Miyazaki A, Nakamura K, Hori N, Sato K, Shomori K, Takeuchi K, Satoh H, Mori S, Ito H: Identification of a novel human ankyrin-repeated protein homologous to CARP. Biochem Biophys Res Comm 2001, 285:715-723 [DOI] [PubMed] [Google Scholar]
- 2.Kemp TJ, Sadusky TJ, Saltisi F, Carey N, Moss J, Yang SY, Sassoon DA, Goldspink G, Coulton GR: Identification of Ankrd2, a novel skeletal muscle gene coding for a stretch-responsive ankyrin-repeat protein. Genomics 2000, 66:229-241 [DOI] [PubMed] [Google Scholar]
- 3.Zou Y, Evans S, Chen J, Kuo H-C, Harvey RP, Chien KR: CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development 1997, 124:793-804 [DOI] [PubMed] [Google Scholar]
- 4.Kuo H, Chen J, Ruiz-Lozano P, Zou Y, Nemer M, Chien KR: Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development 1999, 126:4223-4234 [DOI] [PubMed] [Google Scholar]
- 5.Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L: A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 1997, 272:22800-22808 [DOI] [PubMed] [Google Scholar]
- 6.Weiss SW: Histological Typing of Soft Tissue Tumours, World Health Organization, ed 2 1994. Springer-Verlag, Berlin
- 7.Onizuka T, Moriyama M, Yamochi T, Kuroda T, Kazama A, Kanazawa N, Sato K, Kato T, Ota H, Mori S: BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood 1995, 86:28-37 [PubMed] [Google Scholar]
- 8.Wang J, Walsh K: Resistance conferred by Cdk inhibitors during myocyte differentiation. Science 1996, 273:359-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mietteinen M, Weiss SW: Soft-tissue tumors. Damjanov I Linder J eds. Anderson’s Pathology, ed 10 1996:pp 2504-2507 Mosby, Tokyo
- 10.Chu W, Burns DK, Swerlick RA, Presky DH: Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem 1995, 270:10236-10245 [DOI] [PubMed] [Google Scholar]
- 11.Bang ML, Mudry RE, McElhinny AS, Trombitas K, Greach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S: Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 2001, 153:413-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto Y, Senda T, Nakano T, Nakada C, Hida T, Ishiguro N, Kondo G, Baba T, Sato K, Osaki M, Mori S, Ito H, Moriyama M: Arpp, a new homolog of Carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest, in press [DOI] [PubMed]
- 13.Pallavicini A, Kojic S, Bean C, Vainzof M, Salamon M, Ievolella C, Bortoletto G, Pacchioni B, Zatz M, Lanfranchi G, Faulkner G, Valle G: Characterization of human skeletal muscle Ankrd2. Biochem Biophys Res Comm 2001, 285:378-386 [DOI] [PubMed] [Google Scholar]
- 14.Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, Nagai R: Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy. Role of M-CAT element within the promoter. Hypertension 2000, 36:48-53 [DOI] [PubMed] [Google Scholar]
- 15.Zou Y, Chien KR: EFIA/YB-1 is a component of cardiac HF-1A binding activity and positively regulates transcription of the myosin light-chain-2v gene. Mol Cell Biol 1995, 15:2972-2982 [DOI] [PMC free article] [PubMed] [Google Scholar]