Abstract
Pancreatic stellate cells mediate fibrosis in chronic pancreatitis. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs)-1 and -2 are crucial modulators of fibrosis. Transforming growth factor-β (TGF-β) is a key regulator of extracellular matrix production and myofibroblast proliferation. We have examined MMP and TIMP synthesis by transformed cultured pancreatic stellate cells and their regulation by TGF-β1. By Northern analysis they expressed mRNAs for procollagen 1, TIMP-1, TIMP-2, and MMP-2. Expression of membrane type-1 MMP was confirmed by Western blotting. By immunohistochemistry these enzymes localized to fibrotic areas in human chronic pancreatitis. Active TGF-β1 constitutes 2 to 5% of total TGF-β1 secreted by pancreatic stellate cells; they express TGF-β receptors I and II. Exogenous TGF-β1 (10 ng/ml) significantly increased procollagen-1 mRNA by 69% and collagen protein synthesis by 34%. Similarly TGF-β1 at 0.1, 1, and 10 ng/ml significantly reduced cellular proliferation rate by 37%, 44%, and 44%, respectively, whereas pan-TGF-β-neutralizing antibody increased proliferation by 40%. TGF-β1 (10 ng/ml) down-regulated MMP-9 by 54% and MMP-3 by 34% whereas TGF-β1-neutralizing antibody increased MMP-9 expression by 39%. Pancreatic stellate cells express both mediators of matrix remodeling and the regulatory cytokine TGF-β1 that, by autocrine inhibition of MMP-3 and MMP-9, may enhance fibrogenesis by reducing collagen degradation.
Increasing evidence suggests that pancreatic stellate cells (PSCs) are the major mediators of fibrosis in chronic pancreatic injury and inflammation. 1 PSCs demonstrate many similar features to hepatic stellate cells and glomerular mesangial cells. 2-4 These features include the acquisition of a myofibroblast-like phenotype after injury, a process termed activation. In the activated state, PSCs express α-smooth muscle actin (α-SMA), proliferate, and secrete fibrillar collagens, including collagen I, that characterize chronic pancreatic fibrosis. As such the PSCs probably represent the wound-healing myofibroblasts of the pancreas. 1
The matrix metalloproteinases (MMPs) are a family of calcium-dependent proteinases, which have an important role in extracellular matrix degradation. 5,6 The accumulation of extracellular matrix may result not only from increased synthesis but also from changes in the pattern of repair and degradation. MMP-2 and MMP-9 (gelatinase A and B, respectively) may be particularly important in regulating fibrogenesis and scar degradation. They degrade partially degraded collagens I and III (gelatins) and basement membrane collagen (collagen IV). Although these gelatinases require the initial activity of interstitial collagenases such as MMP-1 or MMP-13, recent studies suggest that a wider range of MMPs including the gelatinases and membrane type-1 (MT1)-MMP have interstitial collagenolytic activity. 7 MMP-3 (stromelysin 1) also exhibits the ability to degrade a broad range of substrates such as collagen III, IV, gelatins, laminin, and MMP-3 itself may activate MMP-1, which plays an important role in the cleavage of native interstitial collagens. 8 The activity of MMPs is regulated at the levels of transcription, proenzyme activation, or inhibition of activated enzyme by tissue inhibitors of metalloproteinases (TIMPs). 9,10 PSCs may play a central regulatory role in pancreatic fibrosis by controlling matrix degradation in chronic pancreatitis through the expression of MMPs and TIMPs. Transcriptional regulation of MMPs and TIMPs is mediated by cytokines and growth factors.
Transforming growth factor-β (TGF-β) plays a pivotal role in the development of fibrosis in the liver and other organs. 11-14 TGF-β is a member of a family of dimeric polypeptide growth factors and three isoforms have been described in mammals: TGF-β1, TGF-β2, and TGF-β3. As a family TGF-βs have a wider role in regulation for cell growth and differentiation. 15 TGF-β regulates these cellular processes by binding to three high-affinity cell-surface receptors types I, II, and III. The receptors type I and II are linked to serine-threonine protein kinases and after ligand stimulation, 16 intracellular signaling is initiated via transcription factors known as SMADs. 17 TGF-β1 up-regulates collagens, TIMP-1, and MMP-2 while down-regulating TIMP-2, interstitial collagenases, and stromelysins in fibroblasts. 18-20 Increasing evidence suggests that PSCs also can be activated by paracrine profibrogenic cytokines such as platelet-derived growth factor and TGF-β derived from migrating macrophages. 21 Moreover, PSCs produce ECM components in response to TGF-β suggesting that this cytokine has an important role in pancreatic fibrogenesis. 1,22
In both human and experimental pancreatic fibrosis, TGF-β has been observed in the atrophic acinar cells adjacent to areas of fibrosis suggesting a paracrine secretion of TGF-β. 23 Although PSCs produce TGF-β1 autonomously, whether it has an autocrine effect in the regulation of matrix homeostasis is a crucial unanswered question. 24 If autocrine expression occurs, it is likely to be important to TGF-β-regulated gene expression because of the high local concentration of the cytokine in the extracellular milieu adjacent to secreting cells.
In this study, we have analyzed the expression of MMP-2, MT1-MMP, TIMP-1, and TIMP-2 in vivo during chronic pancreatitis in archival human pancreatic resections. We demonstrate production of TGF-β by PSCs and show that TGF-β1 influences PSC proliferation, collagen 1 expression, MMP-2, MMP-3, and MMP-9 in an autocrine manner.
Materials and Methods
Materials
Chemicals were obtained from the following sources: Hanks’ balanced salt solution (HBSS), Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum (FCS), penicillin, and streptomycin were from Gibco Life Technologies (Paisley, UK); and pronase, collagenase P, and deoxyribonuclease were from Roche Diagnostics (Mannheim, Germany).
Monoclonal antibodies for immunostaining of the pancreatic tissue were provided for MMP-2 by Calbiochem CN Biosciences (Nottingham, UK), for TIMP-1 by Chemicon International Ltd. (Harrow, UK), and for TIMP-2 by Santa Cruz Biotechnology. Antibodies to α-SMA for Western blotting were provided by Sigma (Poole UK) for MMP-3 and MT1-MMP and by Chemicon International Ltd. TGF-β1, neutralizing antibody to pan-TGF-β, and neutralizing antibody to TGF-β1 were provided by R&D Systems (Abingdon, Oxon, UK); CLPS collagenase was from Worthington Biochemical Corporation (Lakewood, NJ); Picogreen dsDNA quantification reagent was from Molecular Probes, Eugene, OR); and 3H-thymidine, 3H-proline, and α32P-ATP were from Amersham International (Amersham, UK). All other immunochemicals were from Sigma, and molecular biology grade reagents were from Promega (Southampton, UK).
Pancreatic Stellate Cell Extraction and Culture
Rat PSCs were extracted by a modification of the method described. 2 Briefly, the dissected pancreas was finely minced and placed in a solution of HBSS with pronase (0.05%) and collagenase P (0.03%). The pancreas in the digest solution was shaken for 30 minutes at 37°C. The digested tissue was then filtered through nylon mesh and deoxyribonuclease (0.1%) was added. The cell suspension was centrifuged to obtain a pellet that was then resuspended with Optiprep 12% v/v (Sigma) and placed into centrifuge tube with a layer of HBSS placed on top. This was centrifuged for 20 minutes at 2000 rpm at 4°C. The stellate cells were collected from the band between the Optiprep/HBSS interface. After washing with HBSS once, the isolated cells were counted using a hemocytometer and the viability was assessed by trypan blue. Cells were plated onto plastic culture flasks and onto Nunc-chamber slides and maintained in 16% fetal calf serum in DMEM and antibiotics at 37°C in a humidifying incubator with 5% CO2.
RNA Extraction
RNA from cultured rat PSCs was extracted with a proprietary kit (RNAeasy, Qiagen, Crawley, UK) according to the manufacturer’s instruction. Extracted RNA was quantified by absorbance spectrometry at 260 nm and the integrity of the preparation was confirmed by the demonstration of undegraded 28S and 18S ribosomal bands after electrophoresis on a 1% agarose gel and ethidium bromide staining.
Northern Analysis
Ten μg of total RNA from passaged rat PSCs was separated by electrophoresis on a 1% agarose denaturing gel and transferred to Hybond N-nylon membranes with vacugene pump (Amersham International). The PSC-RNA bound to the nylon membranes were hybridized with the α32P-ATP labeled cDNA probe overnight before being subjected to stringency washes and autoradiography exactly as previously described. 10
Collection of Conditioned Media
Passaged PSCs cultured in serum-containing media were washed three times in serum-free DMEM and left in this media for an hour after the final wash. The cells were incubated in fresh serum-free media containing antibiotics and 0.01% bovine serum albumin for 24 hours or 48 hours. In experiments to examine the effects of exogenous agents either TGF-β1 (0.1, 1, 10 ng/ml) or neutralizing antibody to pan-TGF-β (20 μg/ml), were included to the conditioned media. The media were then collected at the specified time points and frozen at −20°C until used for subsequent analysis.
Zymography for MMP-2 and MMP-9
PSC-conditioned media were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on gelatin-containing acrylamide gels (8% acrylamide and 0.1% gelatin) under nonreducing conditions. After electrophoresis, gels were washed three times in 2.5% Triton X-100 for 10 minutes to remove the sodium dodecyl sulfate. After rinsing the gels in water, they were incubated overnight in 37°C in proteolysis buffer (50 mmol/L Tris/HCl, pH 7.8, 50 mmol/L CaCl2, 0.5 mol/L NaCl). The gels were stained with Coomassie brilliant blue (0.5% Coomassie blue, 50% methanol, 10% acetic acid) for 30 minutes. The gels were destained with 10% acetic acid/10% methanol until zones of proteolysis had cleared. Gelatinolytic activity appeared as colorless zones against a blue background.
Western Analysis for α-SMA, MT1-MMP, and MMP-3
α-SMA and MT1-MMP Western analysis used PSCs (passages 2 to 3) protein lysates whereas conditioned media were used in the MMP-3 Western analysis. Protein content of lysates or conditioned media was determined by a commercially available kit bicinchoninic acid protein assay (Sigma). Equivalent protein quantities were then size-fractionated on 10% sodium dodecyl sulfate-polyacrylamide gel. The separated samples were transferred to polyvinylidene difluoride membranes that were blocked with 500 mmol/L NaCl, 100 mmol/L Tris (pH 7.5), 0.1% Tween-20 (TTBS) containing 5% low-fat milk for 1 hour. The membrane was washed twice in TTBS and the membranes incubated overnight with monoclonal anti-mouse α-SMA antibodies (1:500 dilution) (Sigma), polyclonal anti-rabbit MT1-MMP antibodies (1:2000 dilution) and polyclonal anti-rabbit MMP-3 antibodies (1:2500). After a TTBS wash, the membranes were incubated for 1 hour in 1:2000 dilution of horseradish peroxidase-conjugated anti-mouse antibody for α-SMA and 1:3000 dilution of horseradish peroxidase-conjugated goat anti-rabbit antibody for MT1-MMP and MMP-3 (Dako, Cambridgeshire, UK). The membranes were developed using a commercial ECL detection system (Promega).
Immunolocalization of MMP-2, MT1 MMP, TIMP-1, and TIMP-2 in Samples of Normal and Chronic Pancreatitis
Six samples of normal pancreas were obtained either from the pancreatic resection margin at pancreatectomy for small pancreatic neoplasms or from pancreas resected as a result of extensive resection for nonpancreatic disease. In each case, routine histology had confirmed the absence of inflammation, pancreatic duct dilatation, or abnormal fibrosis in these tissues. In addition, six samples of pancreas from patients with chronic pancreatitis were obtained at pancreatic resection. Sequential sections from each sample were deparaffinized and subjected to pronase digestion for antigen retrieval before being immunostained. Sections were then washed in Tris-buffered saline (TBS), pH 7.6, before the addition of the primary antibodies at optimal dilutions as follows: α-SMA (1:40,000), MMP-2 (1:100), MT-MMP (1:400), TIMP-1 (1:200), and TIMP-2 (1:400). For negative controls, the primary antibody was replaced with nonimmune IgG and TBS alone. After incubation with the primary antibodies, sections were washed in TBS (3 × 5 minutes) and incubated with biotinylated anti-mouse antiserum (Roche Diagnostics) for 30 minutes. Sections were washed as before, then incubated with streptavidin complexed with biotinylated horseradish peroxidase (DAKO, Abingdon, Oxon, UK) for 30 minutes. After washing with TBS as described above, sections were exposed to 3′,3′-diaminobenzidine (Sigma) for 8 minutes, followed by TBS rinse. Finally the sections were counter-stained in Harris’ hematoxylin, dehydrated through graded alcohol, and mounted.
Determination of Cell Proliferation in Response to TGF-β1 and Neutralizing Antibody to TGF-β
Cultured rat PSCs (passages 2 to 4) were passaged onto 24-well plates. Cultured PSCs were incubated in DMEM with 0.5% FCS for 24 hours then exposed to the following treatments: TGF-β1 (0.1, 1, 10 ng/ml) and neutralizing antibody to pan-TGFβ (4, 20 μg/ml) with a positive control (5% FCS and DMEM) and negative control (0.5% FCS and DMEM) for 24 hours. A positive control of 5% FCS was included in all of the experiments to ensure that the cells had a normal proliferative response (data not shown). PSCs were pulsed with 3H-thymidine for 16 hours (1 μCi/well). At harvest, the cells were washed with cold HBSS and fixed with ice-cold absolute methanol and incubated at −20°C overnight. After three washes with HBSS (15 minutes each) on ice, 500 μl of cell dissolution solution (0.25 mol/L NaOH and 0.2% sodium dodecyl sulfate) was added into each well followed by 30 μl of 5 N HCl after 15 minutes. The mixture was transferred to the scintillation vials with 3 ml of scintillant. The radioactivity of the samples was measured using a Wallac 1217 Rackbeta liquid scintillation counter.
Determination of Collagen Synthesis in Response to TGF-β1 and TGF-β-Neutralizing Antibodies
PSCs cultured on 12-well plates were washed three times with HBSS and treated with TGF-β1 (1, 10 ng/ml), neutralizing antibody to pan-TGF-β (20 μg/ml), neutralizing antibody to TGF-β1 (10 μg/ml) in media containing ascorbic acid (25 μg/ml), bovine serum albumin (0.01%), and DMEM for 24 hours. Nonimmune IgG (20 μg/ml) or conditioned media minus treatment were used are negative controls. After 24 hours of treatment, 3H proline at a concentration of 1 μCi per well was added for a further 24 hours. At the end of the incubation period, all supernatants were collected into centrifuge tubes. The plates of cells were kept at −20°C for DNA analysis using Pico-green. Aliquots of supernatants were plated as 100-μl quadruplicates per condition onto 96-well plate. Two aliquots per condition were incubated with collagenase solution (containing 50 mmol/L N-ethylmaleimide and 0.2 mol/L Tris/0.3 mol/L CaCl2) and the other two aliquots were incubated with the same solution in the absence of collagenase for 90 minutes at 37°C. After the incubation, each aliquot was washed with ice-cold 50% TCA to precipitate the protein and then the plate was then left on ice for 1 hour. The plate was washed with 10% TCA and then left to dry at room temperature. Once dried, 50 μl of scintillant was added to each well and the radioactivity was counted by β-scintillation counting (Wallac 1450 Microbeta). The incorporation of 3H proline into collagen synthesized by PSCs was calculated by comparing the degraded (collagenase-containing) with the nondegraded (collagenase-free) values expressed per μg of DNA determined according to the manufacturer’s instruction. (Picogreen dsDNA quantification reagent, Molecular Probes).
Reverse-Transcriptase-Polymerase Chain Reaction (RT-PCR)
One μg of total RNA was reverse-transcribed using random hexamer primers. The integrity of the resultant cDNA was confirmed by PCR using primers for the housekeeping gene β-actin. The primers used to amplify β-actin were: 5′-TGT ACG TAG CCA TCC AGG CT and 5′-TTC TTC AGG GAG GAA GAG GA in the presence of 1 mmol/L MgCl2. PCR was performed for 35 cycles in the following temperature profile: 94°C, 20 seconds; 54°C, 30 seconds; 72°C, 1 minute, final extension at 72°C for 10 minutes. Rat TGF-βRI (680 bp) was amplified and detected using primers: 5′-CGT CTG CAT TGC ACT TAT GC and 5′-CTG TGG CAG AAT CAT GTC TC. PCR was performed in the following conditions: 95°C, 30 seconds; 60°C, 15 seconds; 72°C, 10 seconds; final extension at 72°C for 10 minutes. Rat TGF-βRII (650 bp) primers were: 5′-CAC TGT CCA CTT GTG ACA AC and 5′-GGT CTC AAA CTG CTC TGA AG; PCR was performed using the following conditions: 95°C, 30 seconds; 50°C, 15 seconds; 72°C, 10 seconds; final extension at 72°C for 10 minutes. The products of amplification reactions were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. For each of the RT-PCR procedure negative controls were included.
Detection of Latent and Active TGF-β1 Using Enzyme-Linked Immunosorbent Assay (ELISA)
Latent and activated TGF-β1 was detected in PSC-conditioned media using a commercially available ELISA kit entirely according to the manufacturer’s instruction. (R&D, Abingdon, Oxon, UK)
Statistical Analysis
Student’s paired t-tests were used to compare the significance of differences between data. Values of P < 0.05 were considered statistically significant.
Results
Characterization of PSCs
One to two million PSCs were isolated per pancreas (n = 5). Viability of PSCs, assessed by trypan blue exclusion, was >80%. Activated PSCs were found to express the myofibroblastic marker α-SMA by Western analysis and immunocytochemistry (Figure 1, A and B) ▶ . α-SMA was observed in all cells, suggesting that nonmyofibroblastic pancreatic cells were not present as contaminants.
Figure 1.
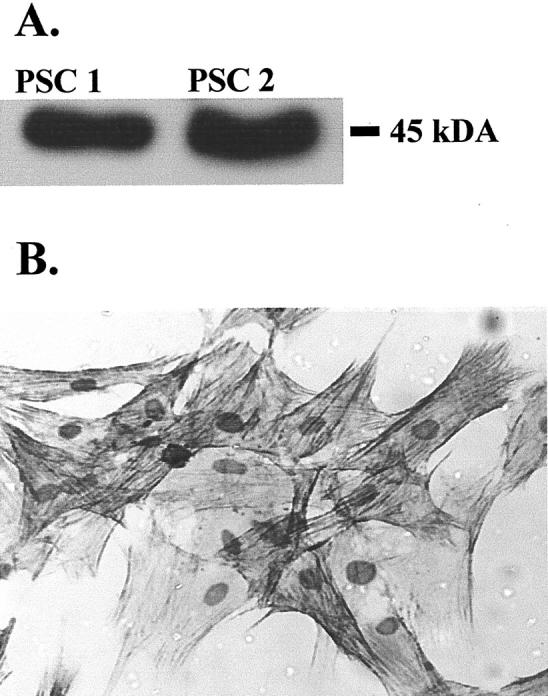
A: α-SMA was detected by Western analysis of 2 μg of protein extracts of two separate preparations (PSC1, PSC2) of activated PSCs. After autoradiography, a single signal of appropriate molecular weight (45 kd) was observed. Data are representative of three further PSC preparations. B: Activated PSCs (passage 2) express α-SMA. Cell cultures were fixed and immunostained for α-SMA using a monoclonal antibody. Data are representative of three separate preparations.
Activated PSCs Express mRNA for α-SMA, Procollagen Type 1, MMP-2, TIMP-1, and TIMP-2
By Northern analysis, the mRNA for α-SMA, procollagen type 1, MMP-2, TIMP-1, and TIMP-2 were readily detectable in total RNA extracted from two independent cultures of passaged PSCs. In each case a single hybridization signal was obtained of the reported molecular size. TIMP-2 mRNA was detected at 1 kb and 3.4 kb which is in accord with previous reports of splice variants for this transcript 25 (Figure 2) ▶ . α-SMA mRNA was detected at 1.8 kb in both PSC samples.
Figure 2.

Total cellular RNA was examined by Northern blotting for the expression of α-SMA, collagen-I, MMP 2, TIMP-1, and TIMP-2. A single signal of appropriate size was observed for each of the mRNAs for α-SMA, procollagen-1, MMP 2, and TIMP-1 whereas TIMP-2 mRNA was expressed as two splice variants of 1.0 and 3.4 kb from activated PSCs passaged two to four times. Similar results were observed in four separate preparations of PSCs.
Activated PSCs Express MT1-MMP
To examine PSC expression of MT1-MMP, protein lysates from passages 2 to 4 rat PSCs (PSC1 and PSC2) (n = 4) and one population of passage 3 human PSCs (PSC3) were subjected to Western analysis as described (Figure 3) ▶ . A single signal of the reported molecular weight of 45 kd for MT1-MMP was observed. This confirms the presence of activated MT1-MMP as previously described in hepatic stellate cells. 26
Figure 3.
Protein extracts (15 μg) from two separate preparations of passages 2 to 4 rat PSCs (PSC1, PSC2) and one preparation of passage 3 human PSCs (PSC3) were subjected to Western analysis for MT1-MMP. After autoradiography a single signal consistent with presence of active MT1-MMP of 45 kd was observed.
Activated PSCs in Vivo Express MMP-2, MT1 MMP, TIMP-1, and TIMP-2
To confirm that the pattern of MMP and TIMP expression observed in activated PSCs reflected expression by these cells in injured and fibrotic pancreas, six cases of chronic pancreatitis and six normal controls underwent immunohistochemical staining for MMP-2, MT1-MMP, TIMP-1, TIMP-2, and α-SMA. All of the normal controls showed expression of MMP-2, MT1-MMP, TIMP-1, and TIMP-2 within the periductal and perivascular connective tissue. In contrast, all of the chronic pancreatitis cases exhibited intense MMP-2, MT1-MMP, TIMP-1, and TIMP-2 expression within the areas of abnormal peri- and intra-acinar fibrosis. These areas of abnormal fibrosis also showed clear localization of α-SMA consistent with activated PSCs being the source of these proteins. (Figure 4) ▶ .
Figure 4.
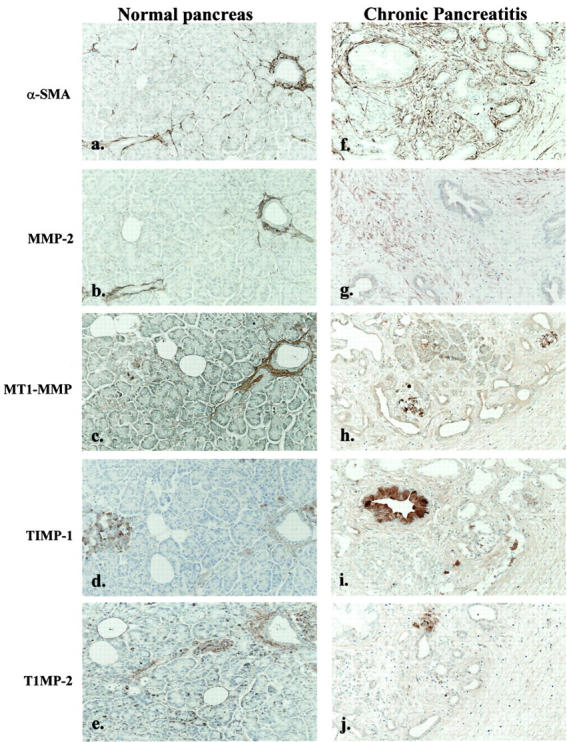
Representative sections of normal pancreas from one patient were immunostained for α-SMA, MMP-2, MT1-MMP, TIMP-1, and TIMP-2 as described in Materials and Methods. Positive staining for α-SMA (a), MMP-2 (b), and MT1-MMP (c) was observed in the vascular and perivascular stroma. In addition, islets also stained strongly for TIMP-1 (d) and TIMP-2 (e). Representative sections of resected samples from a chronic pancreatitis patient were immunostained for α-SMA (f), MMP-2 (g), MT1-MMP (h), TIMP-1 (i), and TIMP-2 (j). The vascular and perivascular stroma was positive for all of the proteins. In addition, each of the fibrosis mediators displayed a similar distribution pattern to α-SMA in the fibrosis area. (f–j). Original magnifications, ×25.
TGF-β1 Increases the Expression of Procollagen Type I mRNA by PSCs
Northern analysis showed procollagen type I mRNA was detected in activated PSCs. However, addition of TGF-β1 (10 ng/ml) to PSCs for 24 hours up-regulated collagen I expression by 69 ± 9% relative to untreated cells (control) (P < 0.05; n = 3). Representative data from one of these PSC preparation are shown (Figure 5A) ▶ . As net production of collagen protein by PSCs might be influenced by concurrent expression of MMPs and TIMPs (Figure 2) ▶ , the mRNA studies were complemented by studies of collagen protein production, using 3H-proline incorporation assay. These studies showed that TGF-β1 (10 ng/ml) significantly increased the amount of collagen protein production by 34 ± 7% above the control (Figure 5B) ▶ . The addition of neutralizing antibody to pan-TGF-β1, -β2, and -β3 isoforms and neutralizing antibody to TGF-β1 to passaged PSCs resulted in significantly reduced collagen synthesis, by 56 ± 12% and 40 ± 5%, respectively, relative to cells treated with nonimmune IgG (control) (n = 6).
Figure 5.
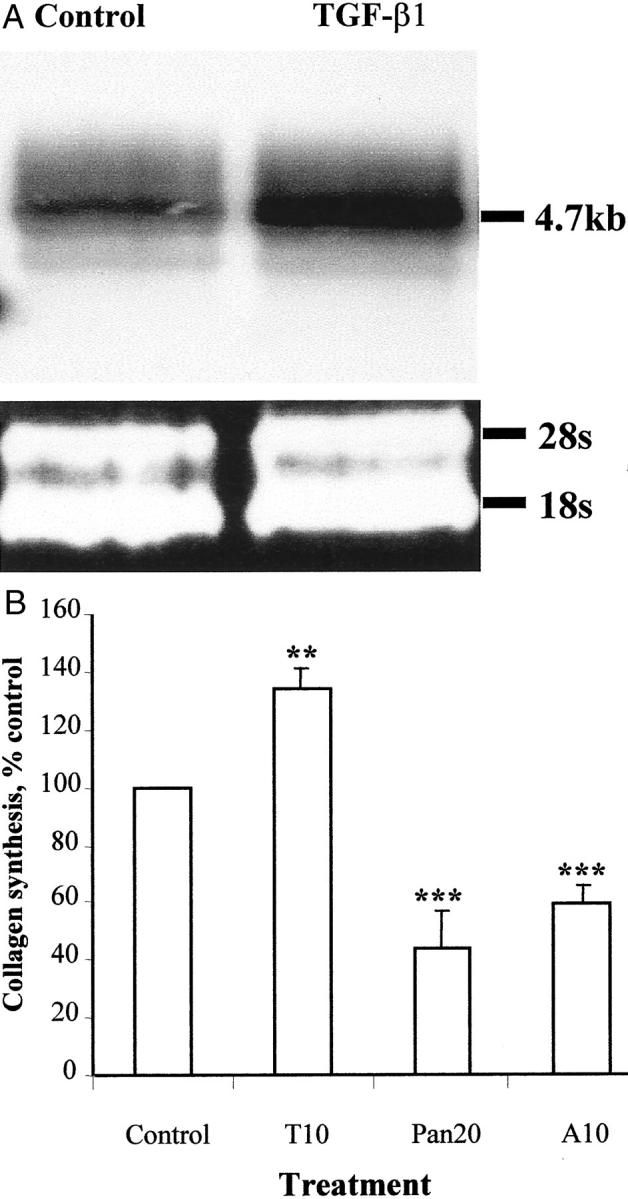
Effect of TGF-β1 on the PSC collagen synthesis. A: Northern analysis compared procollagen type 1 mRNA in cells treated for 24 hours with TGF-β1 (10 ng/ml) and untreated control. The ribosomal bands confirmed equal loading of total mRNA in the experiments. Results were representative of three independent experiments. B: Effects of TGF-β1 10 ng/ml (T10), neutralizing antibody to pan-TGF-β 20 μg/ml (Pan20), and neutralizing antibody to TGF-β1 10 μg/ml (A10) on collagen protein synthesis. Results are expressed as a percentage (mean ± SE) of control values pooled from six separate PSC preparations. **, P < 0.05; ***, P < 0.005.
TGF-β1 has been shown to increase TIMP-1 in a variety of cells. 10 However, Northern analysis showed that the expression of mRNA for TIMP-1 was unaltered by exogenous TGF-β1 (data not shown).
TGF-β1 Reduces PSC Proliferation
By 3H-thymidine incorporation, the rate of proliferation of passaged PSCs was significantly reduced by 36 ± 9%, 44 ± 10%, and 44 ± 7%, respectively, relative to control after the addition of exogenous TGF-β1 (0.1, 1, 10 ng/ml) to PSCs for 24 hours (n = 10) (Figure 6) ▶ . Addition of pan-neutralizing antibody to TGF-β, -β2, and -β3 (20 μg/ml) increased the rate of proliferation by 40 ± 8% (P < 0.005).
Figure 6.
Effect of TGF-β1 at concentrations of 0.1 (T0.1), 1 (T1), and 10 (T10) ng/ml and neutralizing antibody to pan-TGF-β at 20 μg/ml (Pan20) and nonimmune IgG at 20 μg/ml (IgG) on proliferation rate of activated PSCs passaged two to four times. Results were expressed as a percentage of control values and were representative of 10 separate PSC preparations. ***, P < 0.005.
PSCs Express Both Latent and Active TGF-β1 and TGF-β Receptors
We investigated the expression of both the latent and active form of TGF-β1 by PSCs using ELISA. Latent TGF-β1 production by PSCs was 1076 ± 225 pg/ml/24 hours and the amount of active TGF-β1 was 42 ± 8 pg/ml. The percentage of TGF-β1 present in the active form consistently represented a small proportion of the total ranging between 2 to 5% (n = 6). After 48 hours of incubation in serum-free media there was no significant increase in the amount of latent or active TGF-β1 present in the PSC culture (Figure 7, A and B) ▶ .
Figure 7.
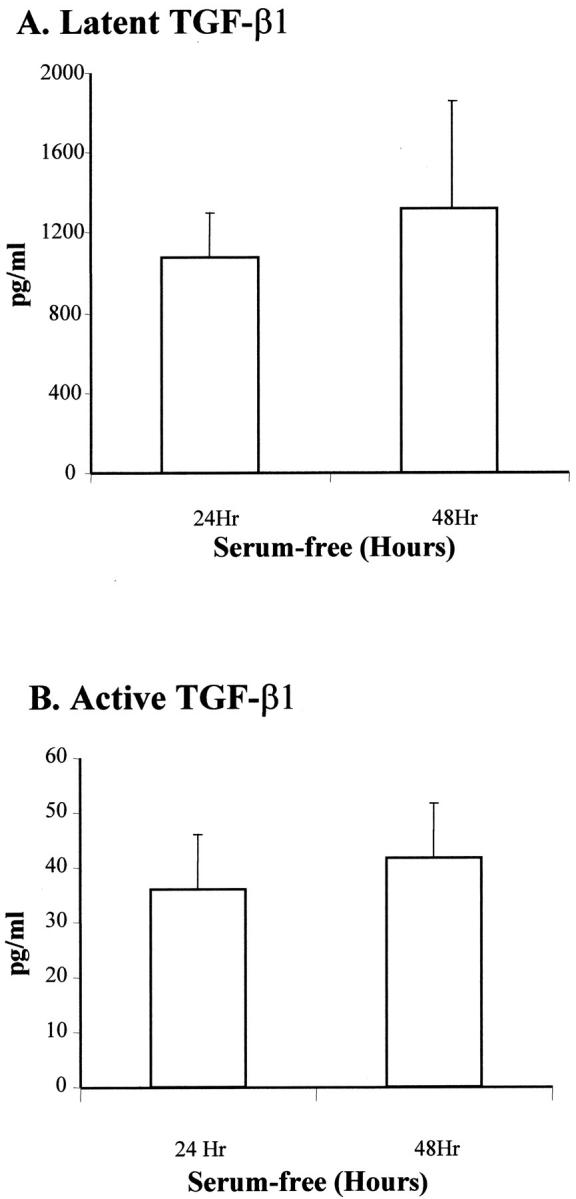
A: Quantification of latent TGF-β1 in supernatant of PSCs cultured for 24 hours and 48 hours in serum-free conditioned media. Using commercial ELISA, latent TGF-β1 was determined after hydrochloric acid activation. Data were representative of six separate PSC preparations. B: Quantification of active TGF-β1 in supernatant of PSCs cultured for 24 and 48 hours in serum-free conditioned media. Active TGF-β1 was measured directly by ELISA. Data were representative of six separate PSC preparations. The amount of active TGF-β1 ranged from 2 to 5% of the total TGF-β1 expressed by PSCs.
Using RT-PCR, we demonstrated that activated PSCs and hepatic stellate cells express TGF-β receptor types I and II as shown by the detection of the expected amplification products of 680 and 650 bp, respectively (Figure 8) ▶ .
Figure 8.
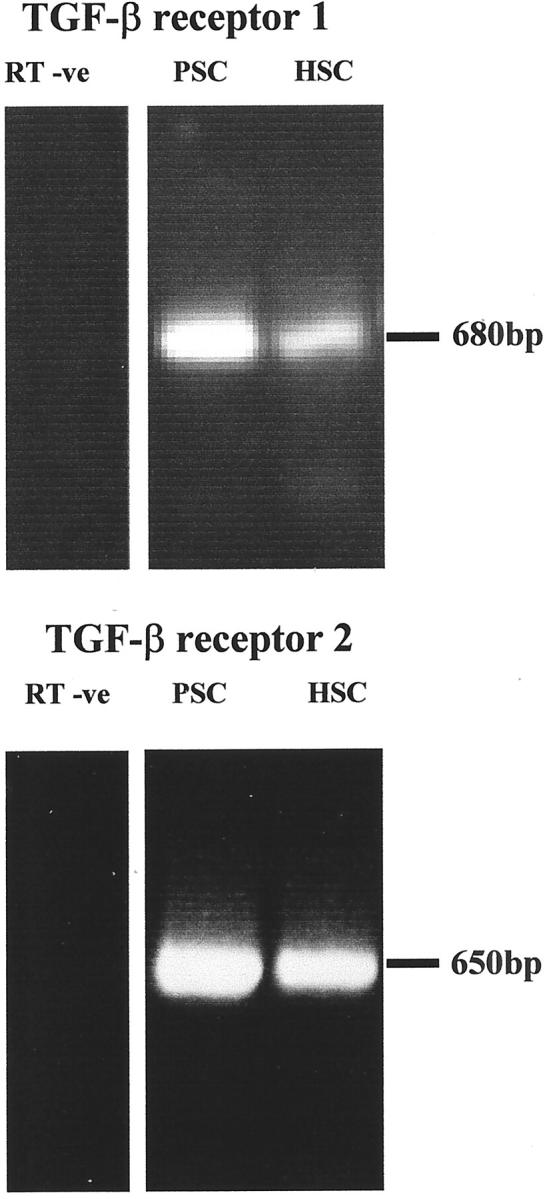
Expression of TGF-β receptors I and II by PSCs. RT-PCR showed that mRNA of TGF-β receptors I and II were detectable in activated PSCs and mRNA from activated hepatic stellate cells were used as a positive control. Data were representative of four separate PSC preparations.
PSCs Down-Regulate MMP-3 and MMP-9 in Response to TGF-β1
Western blotting showed that TGF-β1 (1, 10 ng/ml) significantly down-regulated MMP-3 concentration in conditioned media by 23 ± 4% and 34 ± 5% (Figure 9, A and B) ▶ . Gelatin zymography demonstrated that TGF-β1 significantly down-regulated MMP-9 by 54 ± 2% whereas; neutralizing antibodies to TGF-β1 significantly increased MMP-9 expression. Gelatin zymography also showed the expression of MMP-2 by PSCs, but exogenous TGF-β1 did not alter its expression (Figure 10, A and B) ▶ .
Figure 9.

A: A representative Western blot demonstrating the regulation of MMP3 by TGF-β1 at 10 ng/ml and 1 ng/ml. Equal quantity of serum-free conditioned media protein were used in all of the experiments. Similar results were observed in three separate PSC preparations. B: Densitometric analysis of the Western blots demonstrating the effect of TGF-β1 at concentrations of 1 ng/ml (T1) and 10 ng/ml (T10) on the regulation of MMP3. The results were expressed as a percentage (mean ± SE) of the control values with data pooled from three separate PSC preparations. Results were corrected for the small variation in DNA in the culture of each condition. **, P < 0.05.
Figure 10.
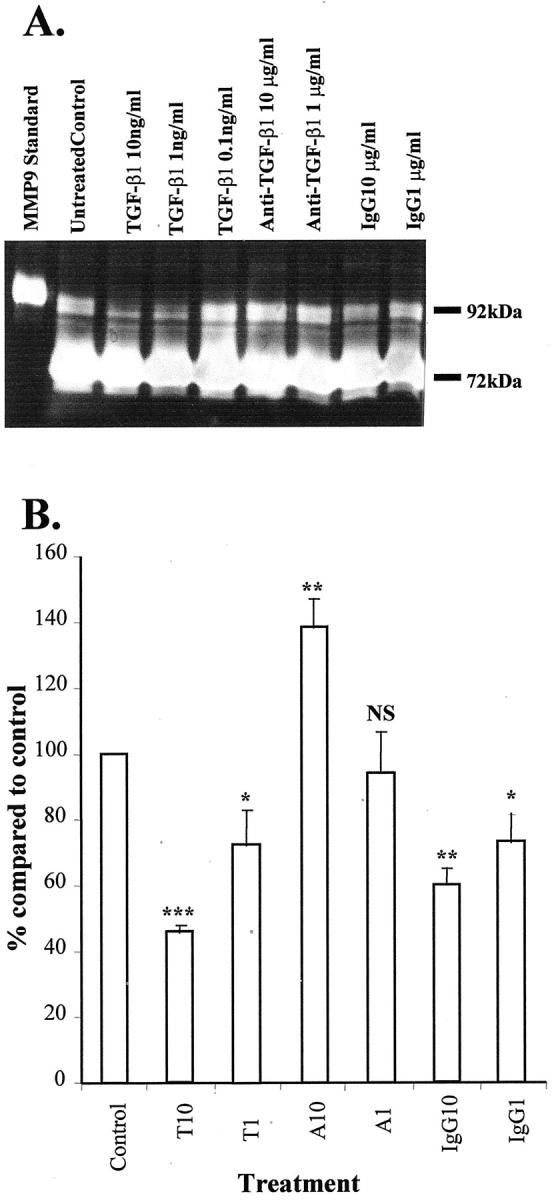
A: A representative gelatin zymography demonstrating the expression of MMP9 and MMP2 at 92 kd and 72 kd, respectively, by PSCs. This also illustrates the effect of TGF-β1 in the regulation of MMP9 at the concentration shown. Eight μl was used in each lane. Similar results were observed in three separate PSC preparations. B: Densitometric analysis of the gelatin zymography demonstrating the effects of TGF-β1 at 10 ng/ml (T10) and 1 ng/ml (T1) and neutralizing antibody to TGF-β1 at 10 μg/ml (A10) and 1 μg/ml (A1) on the regulation of MMP9. A nonimmune IgG at 10 μg/ml (IgG10) and 1 μg/ml (IgG1) were included as a control. The results were expressed as percentage (mean ± SE) of the control (nontreatment) values. Results were corrected for the small variations in DNA in the culture of each condition. Data were pooled from three separate PSC preparations. ***, P < 0.005; **, P < 0.05; *, P < 0.1).
Discussion
Previous studies by our group on collagen homeostasis in liver fibrosis suggest fibrosis results from an imbalance of collagen synthesis versus degradation. 5,27 We and others have demonstrated that matrix-regulating molecules such as MMPs and TIMPs deriving from hepatic stellate cells are likely to be critical in dictating whether fibrosis proceeds or recedes. 28,29 Using tissue culture and in vivo models, we have demonstrated that myofibroblastic cells may play a generic role in fibrogenesis in both liver and pancreas. Our studies show that PSCs express not only collagen-1 but also MMP-2, MT1-MMP, TIMP-1, and TIMP-2. This is the first description of this facet of the activated PSC phenotype and demonstrates that these cells may play an important and hitherto unappreciated role in regulating matrix homeostasis in the pancreas.
We examined MMP-2 and MMP-9 expression by zymography and demonstrated that PSCs secrete MMP-2 exclusively as its inactive proform of 72 kd. Like all metalloproteinases MMP-2 requires activation by removal of its N-terminal. 9 The major mechanism mediating the first stage of this activation has recently been defined and demonstrated to require proteolytic cleavage of MMP-2 by MT1-MMP. The cleavage of the propiece is achieved via a trimolecular complex in which TIMP-2, bound to pro-MMP-2, facilitates the interaction with MT1-MMP on the cell surface. 26,30,31 We have further demonstrated that activated PSCs express MT1-MMP and TIMP-2 and therefore should have the capacity to activate MMP-2, and the lack of detectable activated MMP-2 was unexpected, especially as we have shown that both 66-kd- and 62-kd-activated forms are readily detectable in cultures of transformed hepatic stellate cells. 26 Nonethe-less, the observation of MMP-2 secretion by PSCs is important as fibrotic disorders in other organs are also characterized by increased expression of MMP 2, 32-37 thus MMP-2 may be a common marker of myofibroblast activation in fibrotic tissues. It has further been suggested that MMP-2 might contribute to fibrosis because it is a mitogen for mesangial cells and hepatic stellate cells 26,38 and that the persistent remodeling of pericellular matrix by MMP-2 may be profibrotic by disrupting cell/matrix interactions that is normally keeping a check on proliferation. 26,39
We have demonstrated that PSCs in both tissue culture models and in vivo during fibrosis are sources of TIMP-1 and TIMP-2. As described above, TIMP-2 is required for the activation of pro-MMP-2 by MT1-MMP. However in the presence of excess TIMP-2, MT1-MMP becomes inhibited and MMP-2 is not activated. Both TIMP-1 and TIMP-2 have potent inhibitory effects on all activated MMPs including those with interstitial collagenase activity (MMP-1 and MMP-13). TIMP production by PSCs might therefore promote fibrogenesis by preventing collagen degradation, which would contribute to fibrosis in chronic pancreatitis. With regards to collagen degradation, it is important to recognize that both MT1-MMP and MMP-2 have been reported to have collagenase activity, 35 although they are also subjected to TIMP inhibition. The clear localization of TIMP-1 and TIMP-2 to the areas of fibrosis in each example of chronic pancreatitis suggests that these proteins are being expressed by activated PSCs. Further support for this was derived from our studies on serial sections of each example, demonstrating that these areas were populated by α-SMA-positive cells. These data therefore reinforce and give biological relevance to the observations that we have made in tissue culture. TIMP expression in vivo appears to be localized to the area in which matrix is being laid down.
TGF-β1 has been shown to have a profibrotic role in a variety of organs including liver, skin, and kidneys. 11-14 Our studies indicate that to this list we can now add a role for TGF-β1 in the fibrosis of chronic pancreatitis. Previous studies have emphasized the role of TGF-β1 in pancreas injury and fibrosis. 40 There is an increase of TGF-β within 24 hours of pancreatic injury and during recovery from pancreatitis. The expression of TGF-β is enhanced in the acinar and stromal cells of rat pancreas, highlighting the role of TGF-β1 in the extracellular matrix remodeling after pancreatic injury. 40 Moreover, in human chronic pancreatitis tissue, expression of TGF-β1 and TGF-β receptor I and II have been demonstrated. 41 Previous studies have emphasized the role of TGF-β1 as a paracrine mediator 23,40,42 but our work demonstrates that the autocrine effect of TGF-β1 may regulate PSC response to pancreatic injury. We have demonstrated that PSCs produce latent and active TGF-β1 and, by demonstrating that these cells possess TGF-β receptors, we suggest that TGF-β1 has an autocrine effect on PSCs. This is confirmed by the observation that inhibition of TGF-β1 in PSC cultures, using neutralizing antibodies, results in a decrease in collagen secretion and an increase in PSC proliferation. Exogenous TGF-β1 also increased the expression of procollagen type 1 at the mRNA level and production of collagen protein assessed using 3H-proline incorporation assay. It was noted that the increase in collagen protein was less than that of the mRNA levels. However collagen I is subjected to extensive posttranscriptional regulation, 43 which might lead to a poor correlation between mRNA and protein expression. It was important to show that net collagen protein deposition was increased as PSCs also concurrently produce MMP-2, MMP-3, MMP-9, and MT1-MMP, which enhance collagen degradation. Our studies suggest that TGF-β1 promotes fibrogenesis not only by increasing collagen production but also by inhibiting MMPs in the pathway of collagen degradation.
TGF-β1 reduces the expression of MMP-3 as well as MMP-9 although has little effect on MMP-2. Initial cleavage of mature collagen requires interstitial collagenase activity. A number of MMPs are known to have such activity including more recently MT1-MMP and MMP-2, both expressed by PSCs. MMP-3 activates the interstitial collagenase MMP-1, and MMP-9 is a gelatinase that mediates the breakdown of partly degraded collagen. 7,8 Therefore, concurrent inhibition of MMP-3 and MMP-9 by TGF-β1 would be expected to promote collagen accumulation (Figure 11) ▶ . As previously reported for hepatic stellate cells, TGF-β1 plays a role in repressing proliferation of PSCs. At face value this observation seems at odds with the known proliferation of the PSCs after pancreatic injury. However, within the pancreas in vivo, PSCs will be influenced by many cytokines and growth factors deriving from inflammatory and resident cells. These complex interactions are still poorly understood.
Figure 11.
A schematic diagram proposing how TGF-β1 supports a net accumulation of collagen in pancreatic fibrosis through inhibition of MMPs and stimulation of collagen synthesis.
In conclusion, we have demonstrated that activated PSCs express mediators of matrix turnover. These data reinforce the central role of PSCs in the fibrotic process within the injured pancreas. Activated PSCs also express activated TGF-β1, which up-regulates collagen-1 expression while reducing the expression of MMP-3 and MMP-9, but did not have a significant effect on TIMP-1 expression. The pattern of expression of the mediators studied is similar to that described in parallel cell types in fibrosis of the liver and kidney and reinforces the hypothesis that there may be generic aspects to wound healing in organs.
Footnotes
Address reprint requests to David R. Fine, Division of Infection, Liver Fibrosis Research Group, Mail Pt 811, Level D, S Block, Tremona Rd., Southampton, UK SO16 6YD. E-mail: drf@soton.ac.uk.
Supported by the Digestive Disorders Foundation (Amelie Waring Training Fellowship to F. W. S.), the Mason Medical Trust, the Wessex Medical Trust, and the British Medical Research Council (Senior Clinical Fellowship to J. P. I.).
D. R. F. and J. P. I. contributed equally to the design and supervision of this work.
References
- 1.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS: Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 1999, 44:534-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS: Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998, 43:128-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G: Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115:421-432 [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL: Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 5.Arthur MJ: Collagenases and liver fibrosis. J Hepatol 1995, 22:43-48 [PubMed] [Google Scholar]
- 6.Arthur MJ, Friedman SL, Roll FJ, Bissell DM: Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest 1989, 84:1076-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur MJ: Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol 2000, 279:G245-G249 [DOI] [PubMed] [Google Scholar]
- 8.Murphy G, Segain JP, O’Shea M, Cockett M, Ioannou C, Lefebvre O, Chambon P, Basset P: The 28-kDa N-terminal domain of mouse stromelysin-3 has the general properties of a weak metalloproteinase. J Biol Chem 1993, 268:15435-15441 [PubMed] [Google Scholar]
- 9.Arthur MJ, Stanley A, Iredale JP, Rafferty JA, Hembry RM, Friedman SL: Secretion of 72 kDa type IV collagenase/gelatinase by cultured human lipocytes. Analysis of gene expression, protein synthesis and proteinase activity. Biochem J 1992, 287:701-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G: Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 1996, 24:176-184 [DOI] [PubMed] [Google Scholar]
- 11.Friedman SL, Yamasaki G, Wong L: Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem 1994, 269:10551-10558 [PubMed] [Google Scholar]
- 12.Jones CL, Buch S, Post M, McCulloch L, Liu E, Eddy AA: Renal extracellular matrix accumulation in acute puromycin aminonucleoside nephrosis in rats. Am J Pathol 1992, 141:1381-1396 [PMC free article] [PubMed] [Google Scholar]
- 13.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993, 122:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieg T, Braun-Falco O: Fibrocytes and fibroblasts. Definition and significance in wound healing and fibrotic diseases of the skin. Z Hautkr 1989, 64:775-781 [PubMed] [Google Scholar]
- 15.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 16.Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL: Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993, 75:671-680 [DOI] [PubMed] [Google Scholar]
- 17.Heldin CH, Miyazono K, ten Dijke P: TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390:465-471 [DOI] [PubMed] [Google Scholar]
- 18.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK: Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 1987, 6:1899-1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overall CM, Wrana JL, Sodek J: Transforming growth factor-beta regulation of collagenase, 72 kDa-progelatinase, TIMP and PAI-1 expression in rat bone cell populations and human fibroblasts. Connect Tissue Res 1989, 20:289-294 [DOI] [PubMed] [Google Scholar]
- 20.Overall CM, Wrana JL, Sodek J: Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem 1991, 266:14064-14071 [PubMed] [Google Scholar]
- 21.Gressner AM: Hepatic fibrogenesis: the puzzle of interacting cells, fibrogenic cytokines, regulatory loops, and extracellular matrix molecules. Z Gastroenterol 1992, 30(Suppl 1):5-16 [PubMed] [Google Scholar]
- 22.Gress TM, Muller-Pillasch F, Lerch MM, Friess H, Buchler M, Beger HG, Adler G: Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in chronic pancreatitis. Z Gastroenterol 1994, 32:221-225 [PubMed] [Google Scholar]
- 23.Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS: Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 1999, 155:1087-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruse ML, Hildebrand PB, Timke C, Folsch UR, Schmidt WE: TGFbeta1 autocrine growth control in isolated pancreatic fibroblastoid cells/stellate cells in vitro. Regul Pept 2000, 90:47-52 [DOI] [PubMed] [Google Scholar]
- 25.Stetler-Stevenson WG, Brown PD, Onisto M, Levy AT, Liotta LA: Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem 1990, 265:13933-13938 [PubMed] [Google Scholar]
- 26.Benyon RC, Hovell CJ, Da Gaca M, Jones EH, Iredale JP, Arthur MJ: Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology 1999, 30:977-986 [DOI] [PubMed] [Google Scholar]
- 27.Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJ: Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest 1992, 90:282-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ: Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996, 110:821-831 [DOI] [PubMed] [Google Scholar]
- 29.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ: Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998, 102:538-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler Stevenson WG, Seiki M: Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP). FEBS Lett 1996, 385:238-240 [DOI] [PubMed] [Google Scholar]
- 31.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G: The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem 1996, 271:17119-17123 [DOI] [PubMed] [Google Scholar]
- 32.Li YY, McTiernan CF, Feldman AM: Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 2000, 46:214-224 [DOI] [PubMed] [Google Scholar]
- 33.Ishihara T, Hayasaka A, Yamaguchi T, Kondo F, Saisho H: Immunohistochemical study of transforming growth factor-beta 1, matrix metalloproteinase-2,9, tissue inhibitors of metalloproteinase-1,2, and basement membrane components at pancreatic ducts in chronic pancreatitis. Pancreas 1998, 17:412-418 [DOI] [PubMed] [Google Scholar]
- 34.Murphy G, Willenbrock F, Ward RV, Cockett MI, Eaton D, Docherty AJ: The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem J 1992, 283:637-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theret N, Lehti K, Musso O, Clement B: MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology 1999, 30:462-468 [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, O’Malley Y, Robbins ME: Irradiation of rat mesangial cells alters the expression of gene products associated with the development of renal fibrosis. Radiat Res 1999, 152:160-169 [PubMed] [Google Scholar]
- 37.Zhang X, O’Malley Y, Robbins ME: Angiotensin II-induced modulation of rat mesangial cell phenotype. Radiat Res 1999, 151:725-735 [PubMed] [Google Scholar]
- 38.Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH: Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J Biol Chem 1996, 271:15074-15083 [DOI] [PubMed] [Google Scholar]
- 39.Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM: Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem 1989, 264:10756-10762 [PubMed] [Google Scholar]
- 40.Gress T, Muller-Pillasch F, Elsasser HP, Bachem M, Ferrara C, Weidenbach H, Lerch M, Adler G: Enhancement of transforming growth factor beta 1 expression in the rat pancreas during regeneration from caerulein-induced pancreatitis. Eur J Clin Invest 1994, 24:679-685 [DOI] [PubMed] [Google Scholar]
- 41.di Mola FF, Friess H, Martignoni ME, Di Sebastiano P, Zimmermann A, Innocenti P, Graber H, Gold LI, Korc M, Buchler MW: Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg 1999, 230:63-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Laethem JL, Deviere J, Resibois A, Rickaert F, Vertongen P, Ohtani H, Cremer M, Miyazono K, Robberecht P: Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 1995, 108:1873-1881 [DOI] [PubMed] [Google Scholar]
- 43.Stefanovic B, Hellerbrand C, Brenner DA: Post-transcriptional regulation of collagen alpha 1(I) mRNA in hepatic stellate cells. Nucleic Acids Symp Ser 1995, 33:212-214 [PubMed] [Google Scholar]





