Abstract
Strategies for conditional induction of transgene expression in mice are likely to be valuable for testing the role of candidate genes in disease pathogenesis. We have developed a system for lineage-specific, ligand-dependent, induction of sustained transgene expression in fibroblastic cells in mice using a chimeric gene encoding the Cre-ER(T) fusion protein, under the control of a fibroblast-specific regulatory sequence from the proα2(I)collagen gene. Cre-ER(T) operates as a tamoxifen-dependent DNA recombinase to excise fragments flanked by specific LoxP consensus sequences. To test efficiency and ligand dependency of this strategy, Cre-ER(T)-expressing mice were backcrossed with heterozygous ROSA26-LacZ reporter mice, in which a floxed-STOP cassette has been introduced upstream of a bacterial β-galactosidase (LacZ) reporter gene at a ubiquitously expressed locus. Constitutive or tamoxifen-induced LacZ expression was examined in embryonic, neonatal, and adult compound-transgenic mice. When pregnant ROSA26-LacZ females received a single dose of tamoxifen, high-level expression of LacZ in the skin was demonstrable from 24 hours after injection in double-transgenic embryos harboring both the Cre-ER(T) transgene and the target ROSA26-LacZ allele. High-level expression of LacZ was also induced postnatally by tamoxifen specifically in dermal and visceral fibroblasts. By allowing efficient embryonic or postnatal modification of alleles that have been targeted to incorporate LoxP sites, or to switch on transgenes cloned downstream of the floxed-STOP cassette, this system will allow fibroblast-specific genetic perturbations to be induced at predetermined embryonic or postnatal time points. This should greatly assist in in vivo functional studies of candidate genes in fibrotic diseases such as systemic sclerosis.
Connective tissue fibrosis is a major medical problem leading to substantial morbidity and mortality. A final pathway in the development of fibrosis seems to be the establishment of a population of fibrogenic fibroblasts in lesional tissues. 1 The autoimmune rheumatic disease systemic sclerosis is a good model for acquired fibrosis, as well as being an important medical condition in its own right with substantial mortality. Recent reports have highlighted several genes that may underlie fibroblast dysfunction in systemic sclerosis through genetic linkage or association studies, and by differential display methodologies including cDNA-based transcriptional profiling and polymerase chain reaction (PCR)-based subtractive hybridization methods. Candidate genes include protease nexin-1, 2,3 connective tissue growth factor, 4 transforming growth factor-β2, transforming growth factor-β3, and TIMP1. 5 A major challenge arising from such studies is confirmation that overexpression of these genes leads to fibrosis. Animal models, and especially the genetic modification of mice, provide the most physiological system in which to test the role of altered expression of genes in vivo. This has fuelled development of a number of different strategies to induce cell-specific genetic perturbation in a spatiotemporally regulated manner. 6
Tissue-specific promoters allow transgene expression to be restricted, but for some cell lineages, such as fibroblasts, this is difficult owing to the lack of naturally occurring lineage-specific marker genes. To overcome this, cis-acting regulatory elements subcloned from genes such as type I collagen that are expressed at an early stage in differentiating mesenchymal cells, have been used to produce promoter-reporter constructs that function as lineage-specific transgenes in these cell types. 7 One of the most powerful of these regulatory elements so far characterized is a far-upstream transcriptional enhancer from the proα2(I)collagen gene that directs expression in fibroblasts but not in other type I collagen-producing cells. 8 In conjunction with a minimal promoter this enhancer directs expression in fibroblasts at levels similar to those of the endogenous type I collagen gene and selective overexpression of these reporter genes in fibroblasts in transgenically modified type 1 tight skin mice. 9
Methods for conditional activation of transgene expression add further refinement to lineage-specific transgenesis by allowing activation of gene predetermined time points. Although a number of ligand-dependent transgenes have been reported, such as those incorporating tetracycline/doxycycline 10-12 or ecdysone-dependent 13 constructs, these have not been widely used in transgenic mice. Tetracycline-dependent repressor domains have also been used to specifically inactivate transgenes postnatally. 14 There are several limitations to these systems, for example the effect of long-term ligand administration needs to be considered, and many of these systems are not entirely inactive in the absence of ligand. 15 To circumvent this, tetracycline-dependent promoters and silencers have been combined to reduce background expression levels of transgenes. 16 An alternative system utilizes a gene-switch strategy in which a ligand-dependent transactivating protein is encoded and will activate a target transgene regulated by a responsive promoter. 17
Naturally occurring sequence-specific DNA-modifying enzymes such as the P1 bacteriophage enzyme Cre-recombinase 18 have been widely used for in vitro DNA modification and are increasingly being applied in vivo. This enzyme directs genetic recombination at specific sites termed “LoxP sequences” (palindromic sequences with a variable length spacer sequence, which must be identical for recombination to occur). Cre-recombinase excises a DNA sequence flanked by LoxP sequences in the same orientation. Tissue-specific promoters can be used to target Cre to certain tissues at particular times in development and ligand-dependent forms of Cre have been developed that allow regulated recombination at LoxP sites. In general, these have been synthesized as chimeric proteins in which the catalytic subunit of Cre is fused to a steroid receptor domain. 19 These steroid receptor domains can be mutated so that they have affinity for artificial steroids but not endogenous ligand. 20
In the present study, we have expressed a tamoxifen-dependent form of Cre-recombinase specifically in fibroblasts using a potent lineage-specific enhancer, and have used this to selectively activate a reporter gene in fibroblasts at predetermined embryonic or postnatal time points.
Materials and Methods
Cre-ER Construct
A fibroblast-specific transcriptional enhancer was used to drive the expression of a cDNA encoding a fusion protein incorporating the catalytic domain of Cre-recombinase and a mutated ligand-binding domain of the estrogen receptor having affinity for tamoxifen or 4-hydroxytamoxifen, but not for estradiol. Fibroblast specificity was determined by a 6-kb transcriptional enhancer subcloned from the far-upstream region of the mouse proα2(I)collagen gene that has previously been shown to direct high-level transgene expression in fibroblastic cells during embryonic development from E9.5. 8
To construct this expression vector for Cre-ER(T) a 2-kb fragment encoding the fusion protein was excised by EcoRI and subcloned into the EcoRI site of pBS. The 6.4-kb fragment incorporating 6 kb of the upstream 5′-flanking region of the Col1a2 gene (−19.5-kb to −13.5-kb upstream of the transcription start site was linked to an endogenous minimal promoter (−350 bp to +54 bp) was subcloned from the vector pCD3 21 into plasmid pRM lacking LacZ between NotI and SalI. Construction of the plasmid pRM has been previously described in detail. 8 It was modified from the vector pLacF 22 by addition of short linkers containing unique restriction sites and incorporates the intron and polyadenylation signal of the mouse protamine-1 gene. The donor plasmid pRM incorporates a bacterial β-galactosidase reporter gene, and this was excised during subcloning steps to introduce the Cre-ER(T) cDNA. An additional NotI site was introduced into the Nru1 site of this vector. The IRES-hpAP construct from the dicistronic expression vector pWH8 23,24 was excised using Nru1/SalI and cloned into the SmaI site of the 2-kb Cre-ER fragment in pBS. An additional SalI site was introduced into the NotI of the intermediate IRES construct. Finally, the SalI fragment including Cre-ER(T)-IRES-hpAP was cloned into the SalI site of the intermediate construct. The transgene construct is shown in Figure 1 ▶ .
Figure 1.
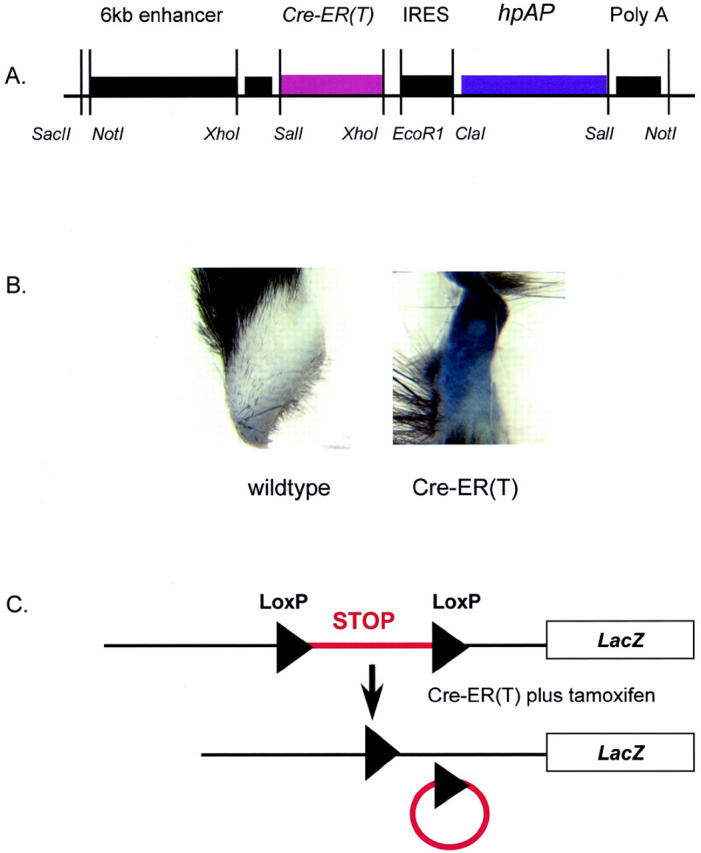
Fibroblast-specific expression of ligand-dependent Cre-recombinase. A: Transgene construct for fibroblast-directed expression of CreER(T). The coding sequence for tamoxifen-dependent Cre-recombinase is regulated by a fibroblast-specific expression cassette comprising a 6-kb fragment from the far-upstream region (−19.5-kb to −13.5-kb upstream of the transcription start site) and a minimal promoter of the Col1a2 gene. A viral IRES sequence linked to the human placental alkaline-phosphatase reporter gene (hpAP) downstream of the CreER(T) directs co-expression of this marker enzyme, from a dicistronic mRNA to identify transgene expression. Major restriction sites used for subcloning are annotated. The backbone sequence for the parent plasmid is derived from plasmid pUC18 as described in the text. B: Expression of hpAP in transgenic skin biopsy. Skin biopsy specimens taken from transgenic (right) mice or wild-type littermate controls demonstrating high-level dermal expression of placental alkaline phosphatase in transgenic skin. C: Excision of floxed STOP cassette induced by tamoxifen. Schematic showing excision of the trimeric transcription/translation blocking STOP cassette by Cre-ER(T) in the presence of tamoxifen, by homologous recombination of LoxP consensus sequences in the same orientation.
Generation of Transgenic Mice
Transgenic mice were generated by standard methods. In brief, the transgene construct was linearized the backbone bacterial sequence removed using NotI. The fragment was gel-purified and electro-eluted before purification using ultracentrifugation (60 minutes at 4°C). After dilution to 2 ng/ml final concentration in microinjection buffer, 25 DNA was microinjected into fertilized B6D2 F2 oocytes. These were transferred into CD1 foster mothers and examined at 15.5 days after conception or continued to term for examination of postnatal time points. Selected mice were bred with wild-type animals to establish transgenic lines that were maintained at heterozygosity.
To identify transgenic mice genomic DNA was extracted from tails of 10-day-old mice and genotyping performed by PCR for sequences within the Cre cDNA, using specific primers (5′-ATCCGAAAAGAAAACGTTGA-3′ and 5′-ATCCAGGTTACGGATATAGT-3′) to yield a 700-bp product. Amplification was undertaken by 35 cycles of 60 seconds annealing at 58°C, Mg++ 2.6 mmol/L, and 60 seconds extension at 72°C.
Assessment of Transgene Expression
Co-expression of the hpAP reporter gene via a viral IRES sequence facilitated screening of mice for transgene expression. This human placental isoform of alkaline phosphatase is relatively resistant to inactivation by heat and so can be readily identified in transgenic tissues after heat inactivation of endogenous murine forms of this enzyme. 24 Embryos at 15.5 days after conception or skin biopsy specimens were processed for hpAP activity. In brief, skin or tail biopsies were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde overnight at 4°C. After rinsing in PBS, samples were heated to 75°C for 30 minutes to inactivate endogenous alkaline-phosphatase activity. After further washing with PBS, samples were washed for 10 minutes with alkaline-phosphatase buffer containing 0.1 mol/L Tris-HCL (pH 9.5), 0.1 mol/L NaCl, and 10 mmol/L MgCl2. Finally, embryos were stained with BM Purple AP substrate (Boehringer Mannheim, Indianapolis, IN) at room temperature. Samples were washed with PBS plus 0.1% Tween 20 and 2 mmol/L of MgCl2, and stored at 4°C in PBS.
ROSA26-LacZ Reporter Strain for Testing Cre Activity in Vivo
To test the activity and ligand-dependency of the Cre-ER(T) transgene product transgenic males harboring the Cre-ER(T) transgene were back-crossed with heterozygous female ROSA26-STOP-LacZ reporter mice. 26,27 This line has the LacZ reporter gene introduced using a gene-trap protocol to insert a promoterless reporter gene (ROSA, reverse orientation splice acceptor) randomly into the mouse genome into a ubiquitously expressed locus. Using homologous recombination a STOP cassette flanked by LoxP sequences has been introduced upstream of the LacZ gene. 28 In the presence of functional Cre-recombinase this STOP cassette is excised and the LacZ gene is expressed. Mice harboring the conditional allele were genotyped by PCR of genomic DNA extracted from tail biopsies taken from neonatal pups or from placentas from founder embryos, using primers specific for the β-galactosidase reporter gene. 9
X-gal Staining for β-Galactosidase
Activity of LacZ in whole-mount embryos or in tissue samples was performed as described previously. 7 Briefly, whole mouse embryos or dissected tissue fragments were fixed for 60 minutes (0.1 mol/L sodium phosphate, pH 7.3, 5 mmol/L EGTA, pH 8.0, 2 mmol/L magnesium chloride, 0.2% glutaraldehyde, 0.3% formaldehyde), rinsed for 90 minutes and stained with 5-bromo-4-chloro-3-indoyl β-d-galactopyranoside (X-gal) staining solution (1 mg/ml) at room temperature. Tissue was processed through increasing concentrations of ethanol and stored in 80% ethanol at −20°C. For histological examination tissues were dehydrated, paraffin wax-embedded, and 7-μm sections were cut and counterstained with eosin.
Activation of Cre-Recombinase
The activity of the ligand-dependent Cre-ER(T) fusion protein was tested in vivo by back-crossing transgenic mice onto the ROSA26-STOP-LacZ reporter mouse. 26 This reporter line was maintained at heterozygosity, because of potentially deleterious effects of homozygosity for the targeted allele. For these experiments female heterozygote reporter mice were mated with male Cre-ER mice. Initially, activation was tested in embryos by injecting a pregnant female mouse at 13.5 days after conception with 1 mg of tamoxifen dissolved in corn oil. Mice were sacrificed at 15.5 days after conception and embryos were processed for whole-mount X-gal staining as described above. Control mice of the same genotype but not treated with tamoxifen were used for comparison to confirm ligand dependency of the Cre fusion protein.
Later experiments examined postnatal activation of the conditional Cre-recombinase. Two time points of administration of tamoxifen ligand were compared. First, mice were injected intraperitoneally for 5 consecutive days with 1 mg of tamoxifen starting at P8 or P9. At 48 hours after the final injection they were sacrificed and tissue samples processed for X-gal staining as described above. Second, to examine the effect of genetic recombination in adult mice tamoxifen administration was repeated at 5 weeks of age, followed by examination of tissue samples for LacZ expression at 6 weeks.
Histology
After processing for X-gal staining as described above, whole-mount embryos or tissue samples from neonatal or adult mice were processed for histological examination. Samples were dehydrated, paraffin wax-embedded, and 7-μm sections were cut and counterstained with eosin as described previously. 8
Results
Transgenic Mice Generated
Initial PCR genotyping identified eight pups harboring the transgene (Table 1) ▶ . Staining of tail or skin biopsy specimens from these founders or their progeny confirmed strong expression of heat-stable alkaline phosphatase in five transgenic founders or lines. Two of these mice became sick at 14 days and died at age 4 weeks. Other transgenic mice had no overt phenotype. The same appearance in two independent transgenic founders suggests that this is not a chance occurrence related to transgene integration site, although its basis and significance remains unclear. Of the two other founder mice expressing hpAP both remained healthy and transgenic lines were established for back-crossing with the ROSA26-STOP-LacZ reporter line.
Table 1.
Summary of Transgenic Mice Harboring Cre-ER(T) Transgene
| Founder | Sex | PCR | Skin expression hpAP | Transgenic line |
|---|---|---|---|---|
| 1 | m | Positive | Weak | No |
| 2 | f | Positive | None | No |
| 3 | m | Positive | Strong | Died at 4 weeks |
| 4 | m | Positive | Strong | Died at 4 weeks |
| 5 | f | Equivocal | None | No |
| 6 | f | Positive | None | No |
| 7 | m | Positive | Strong | Yes |
| 8 | f | Positive | Medium | Yes |
Conditional Genetic Recombination
When the heterozygous Cre-ER(T) founder male mice were crossed with the reporter ROSA26-STOP-LacZ strain and tamoxifen administered, 25% of the progeny have the potential for LacZ expression in fibroblasts. As expected X-gal staining of whole-mount embryos showed high-level LacZ expression in the skin (Figure 2) ▶ . Histological examination confirmed that expression was essentially restricted to fibroblastic cells of the fascia and dermis (Figure 3) ▶ . Some expression was also present in mesenchymal cells at sites of membranous ossification and in some osteoblastic cells. PCR genotyping confirmed the presence of the floxed reporter allele in half of the offspring from heterozygous females. Similarly, half of the progeny of matings harbored the Cre-ER(T) transgene. A quarter of the offspring were compound-transgenic mice harboring both the conditional allele of LacZ and the ligand-dependent recombinase. These mice have the potential to express the LacZ reporter gene after exposure to tamoxifen. In control matings in which no tamoxifen was administered there was no LacZ staining with X-gal. This confirms the efficiency of the STOP cassette and also the ligand-dependency of the Cre-ER(T).
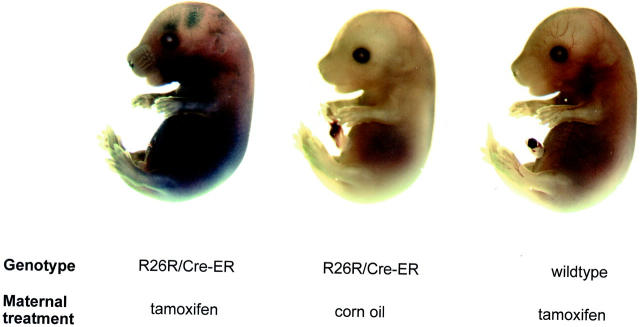
Figure 2.
Tamoxifen-induced expression of LacZ in whole-mount transgenic embryos. Heterozygote reporter mice (ROSA26-LacZ) were mated with the Cre-ER(T) male mice and treated with a single intraperitoneal injection of tamoxifen (1 mg) or corn oil vehicle at 13.5 days after conception. Embryos were stained using X-gal 48 hours later. Only compound-transgenic mice of mothers receiving tamoxifen show dermal LacZ expression.
Figure 3.
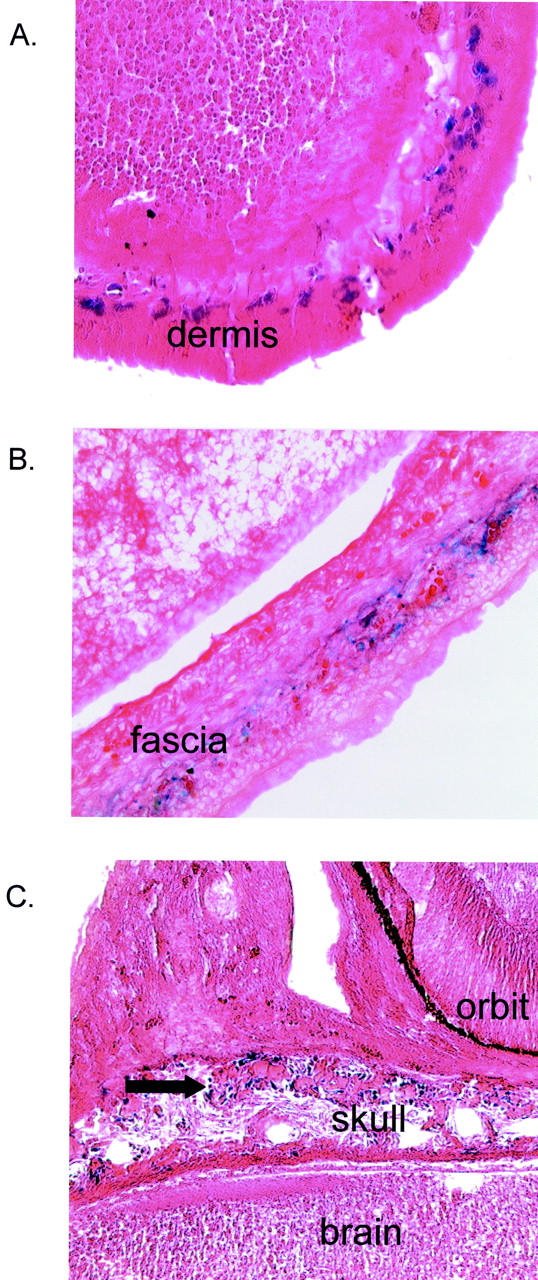
Expression of LacZ in dermis and fascia of double-transgenic embryos. Histological examination of E15.5 double-transgenic embryos after maternal treatment with tamoxifen at E14.5. Whole-mount staining with X-gal confirms expression of LacZ in fibroblastic cells of the dermis (A), fascia of lower back (B), and mesenchymal cells (arrow) at sites of membranous ossification within the skull (C).
These results confirm that genetic recombination occurred in vivo in a ligand-dependent manner in fibroblastic cells of these compound-transgenic mice. Ligand dependency postnatally was explored by injecting neonatal mice with tamoxifen. Tissues were processed for X-gal staining. Again, there was no LacZ expression in either ROSA26-lacZ mice or in compound-transgenic animals in the absence of treatment with tamoxifen. However in animals injected for a period of 5 consecutive days with tamoxifen there was high-level expression of LacZ in fibroblastic cells of all tissues examined. This was apparent macroscopically and staining was observed in all organs examined in which fibrous connective tissue was present. There was no expression in control tissues from double-transgenic mice that had not been treated with tamoxifen or in mice harboring either transgene in isolation even after treatment with tamoxifen. These data from whole-mount samples were extended and confirmed by histological examination. Thus there was demonstrable expression of bacterial β-galactosidase in connective tissue cells of the spleen, lung, skin, fascia, and perimysial tissues (Figure 4) ▶ . Expression was also observed in blood vessel walls and around smooth muscle cells of the gut wall. There was also expression in some sites of periosteum. These expression patterns recapitulate those previously observed in an allelic series of transgenic mice harboring a LacZ reporter gene driven by the 6-kb Col1a2 enhancer (−19.5 to −13.5 kb). 21 Levels of expression of LacZ were more intense following the multiple dosing regimen used postnatally than after a single injection of tamoxifen in pregnant female reporter mice, but the cell types expressing LacZ in treated compound-transgenic embryos analyzed by whole-mount staining was essentially the same. No expression was observed in littermate compound-transgenic mice treated with corn oil vehicle alone (Figure 5) ▶ .
Figure 4.
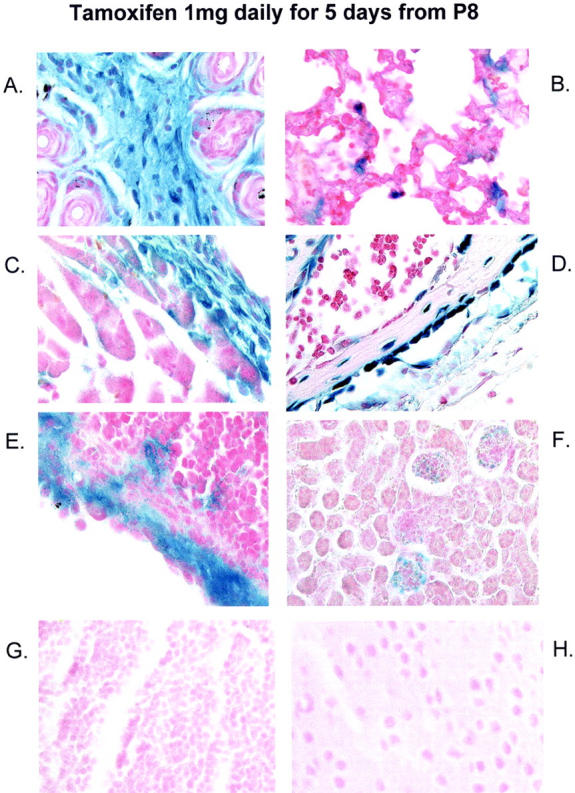
Postnatal induction of transgene expression in fibroblastic cells of neonatal mice. After postnatal administration of tamoxifen to neonatal compound-transgenic mice there was high-level expression of the LacZ reporter gene in fibroblastic structures of the dermis (A), lung (B), pericardial connective tissue (C), blood vessel wall (D), and splenic capsule (E). Expression was also present in mesangial cells of the glomerulus (F). No staining was detected in parenchymal cells of the thymus (G) or brain (H). Control tissues are shown in Figure 5 ▶ .
Figure 5.
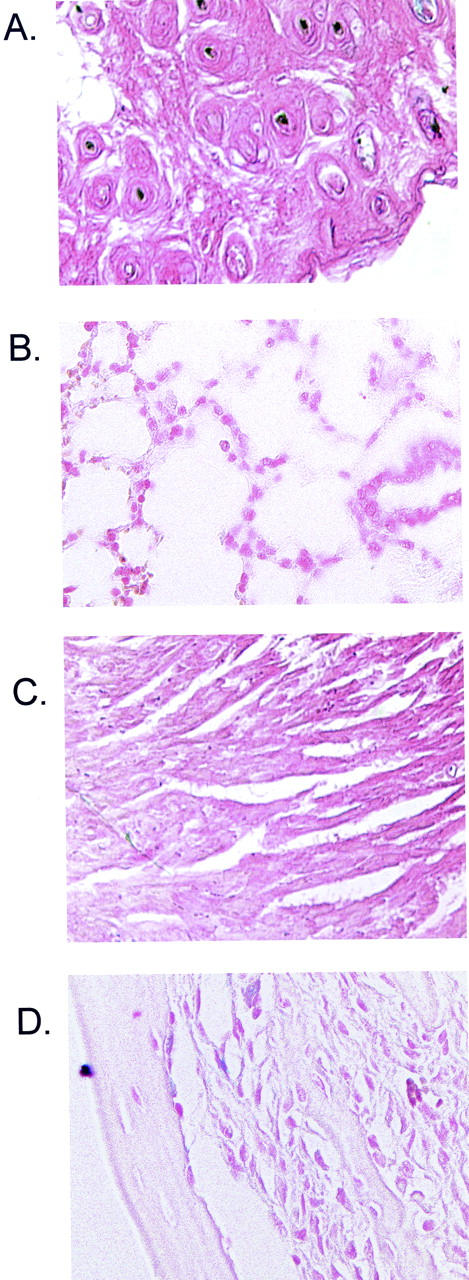
Conditional LacZ transgene is not expressed in compound-transgenic mice in the absence of tamoxifen. To confirm ligand dependency for expression of LacZ in compound-transgenic mice, ROSA26-LacZ/CreER(T) progeny were injected with corn oil on 5 consecutive days and tissues stained by X-gal 48 hours after the fifth injection. In contrast to mice receiving tamoxifen (Figure 4) ▶ no expression was observed. Representative sections from dermis (A), lung (B), heart (C), and blood vessel wall (D) are shown.
To confirm sustained transgene expression mice were treated with tamoxifen perinatally and analyzed at 6 weeks, 28 days after the last injection of tamoxifen. Consistent with previous examination of reporter mouse lines at 6 weeks positive cells were scattered through the dermis and other fibrous structures. In a third set of experiments, ligand-dependent genetic recombination at later postnatal time points after repeated ligand administration was examined in compound-transgenic mice receiving tamoxifen at 2 and 5 weeks of age. These animals showed very similar expression patterns at 6 weeks to those observed in mice injected neonatally (Figure 6) ▶ , suggesting that postnatal induction of transgene expression at later time points is feasible.
Figure 6.
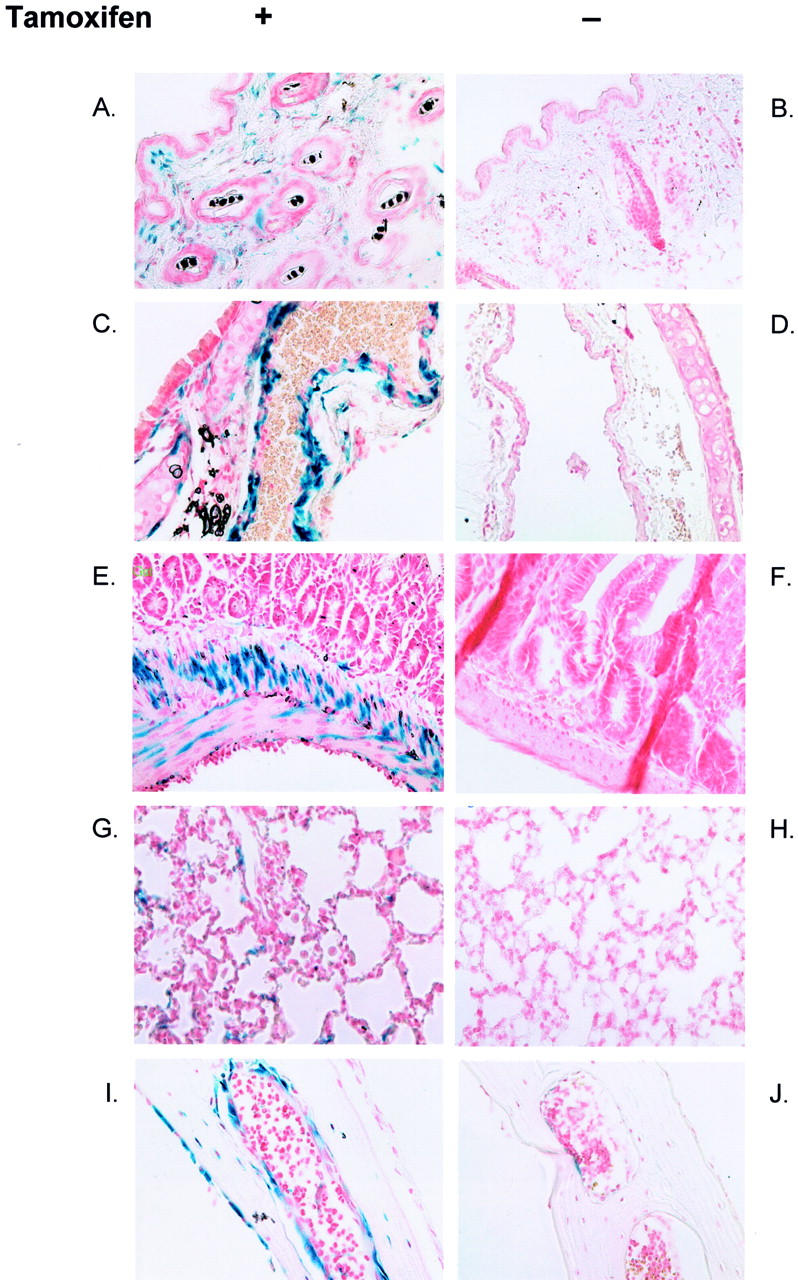
Sustained fibroblast-specific expression of LacZ in adult compound-transgenic mice. Sustained transgene expression of LacZ was observed after perinatal injection of compound-transgenic mice with tamoxifen. Mice received an additional injection of ligand at 5 weeks of age and tissues were analyzed at age 6 weeks. Expression is compared with that in the absence of tamoxifen administration in a littermate control. Expression is seen only in tamoxifen-treated mice in the skin (A and B), blood vessel wall (C and D), intestinal wall (E and F), lung (G and H), and in fibroblastic cells at sites of membranous ossification in the skull (I and J). A few cells in the control panel (J) show positive staining that is an artifact attributed to endogenous galactosidase activity.
Discussion
Conventional transgenic methodologies have proven extremely valuable in elucidating mechanisms regulating gene expression in vivo in the intact animal. 29 However, whereas reporter transgenes such as β-galactosidase, 7 firefly, 8 human placental alkaline phosphatase, 24 or enhanced green fluorescent protein 30 are not generally associated with any impairment of viability, attempts to express other biologically active transgenes are often confounded by the deleterious effects of the gene product. If the promoter selected to direct expression is not widely expressed or is only significantly active postnatally then there are likely to be few problems. In contrast, promoters that drive high-level embryonic expression are likely to have effects on embryonic viability.
The expression vector used in these experiments provides a versatile mechanism for targeting high-level transgene expression to fibroblasts and not other cell types. Previous analysis of the Col1a2 gene suggests a modular arrangement of regulatory elements directing lineage-specific expression, and a key role for a far-upstream enhancer element. Thus a −350-bp fragment is expressed at low levels in a range of collagen-producing tissues, but much lower levels than the endogenous collagen gene. A longer 2-kb fragment shows a similar distribution of gene expression but somewhat higher levels in bone, skin, and tendon fibroblasts. 31 These short promoter fragments demonstrate considerable variation in the level of expression between independently derived transgenic lines consistent with important influence of site-of-integration of the transgene. Fibroblast-specific hypersensitive sites were identified in the far-upstream region of the Col1a2 mouse gene and fragments around these hypersensitive sites appear to be powerful transcriptional enhancers for minimal promoters driving reporter transgenes. Strong sequence conservation between the mouse Col1a2 and human COL1A2 genes has been identified and the presence of a similar potent enhancer capable of driving reporter genes at high levels in fibroblasts in transgenic mice embryos has been delineated within the human COL1A2 gene. 32 These experiments suggest that far-upstream sequences of both genes are targets for DNA-binding proteins, which are active or expressed only in fibroblasts, and that transgenes under the control of these elements serve as differentiation markers for the fibroblast lineage. We have previously shown that reporter transgenes regulated by this the far-upstream enhancer are selectively activated in fibrotic states, 9 including expression at sites of cutaneous scarring, 21 and such reporter gene studies are a valuable tool for tracking high-level collagen gene expressing fibroblasts in these pathologies.
Incorporating an additional marker gene that can be readily detected in whole-mount embryos or postnatal biopsy specimens, driven by the same promoter as the CreER(T) transgene but translated via an IRES sequence from a dicistronic transgene product, simplifies evaluation of tissue-specific transgenic mice, and provides an important control in the present study. Thus, we were easily able to screen genotypically positive founder mice for the level and location of transgene expression and to compare expression of the constitutively active hpAP gene product with activity of the ligand-dependent Cre-ER(T) protein determined in back-cross experiments. The heat-stable properties of the human placental alkaline-phosphatase isoform allow it to be distinguished from endogenous activity of other murine isoforms of this enzyme by following specific staining protocols. Our findings confirm that both open-reading frames of dicistronic transgenes expressed under the control of this fibroblast-specific enhancer are expressed at high levels in fibroblasts during embryonic development and postnatally.
Our choice of a Cre-ER(T)-based method to allow inducible gene expression in transgenic mice was based on the apparent robustness of the system in vivo. 33,34 In particular there does not appear to be read through the STOP cassette and the ligand-dependent Cre-recombinase is not active in the absence of exogenous tamoxifen. Absent staining in adult compound-transgenic mice, including pregnant females with high levels of endogenous estrogen, confirms that endogenous estrogens do not activate it. However, tamoxifen is detrimental at high dose in pregnancy and our own data (not shown) and published reports suggest that 1 mg is the maximum dose consistent with a viable pregnancy. 35 This has led to the development of other ligand-dependent Cre-recombinase enzymes that may be superior for embryonic analysis. 20 For postnatal experiments a tamoxifen-dependent construct is well suited. Indeed, experiments studying connective tissue metabolism or inflammation are more likely to be confounded by dexamethasone than tamoxifen administration. In addition, the ability to induce sustained gene expression after short-term ligand administration makes the present approach particularly versatile. In addition, once genetic recombination has occurred this will be passed on to daughter cells at mitosis so that those cells expressing the transgene can multiply. This is likely to be especially relevant in longer-term studies of the effect of relatively subtle fibroblast-specific genetic perturbations. Alternative techniques that have been used include the development of ligand-dependent transactivators, the so-called “gene switch.” 17 Disadvantages of this system are its dependence on continued ligand presence and the nonphysiological regulation of expression that makes it unsuitable for many developmental studies.
A potential limitation of this system is that expression of the Cre-fusion protein at high level may not be inconsequential. Two of our high-expressing founders had stunted growth. This could have been a nonspecific effect because of disruption of genomic DNA at the time of pronuclear injection or it could be a direct effect of expression of the Cre-ER(T) fusion protein. Although Cre has been expressed in many transgenic mice without apparent consequence, this depends on the level, timing, and site of expression. In addition, it may be that high-level expression of the ligand-binding domain of the fusion molecule is detrimental. For example, the receptor binds heat shock protein (Hsp)90 in the absence of ligand and depletion of Hsp90 has been shown to be detrimental. 36
Another potential application of this mouse line is for the generation of conditional knockout mice. A number of mouse lines have already been established using gene targeting so that a floxed allele is generated. If such a mouse line is back-crossed with the Cre-ER line then there would be no effect on gene expression until the compound-transgenic progeny are exposed to the Cre-activating ligand tamoxifen. Cells expressing this transgene will however, in the presence of tamoxifen, have active Cre-recombinase and the floxed DNA sequence will be excised. This will allow the system to be used effectively for loss-of function experiments involving fibroblast-specific gene targeting as well as the gain of function strategies using transgenes expressed from a cDNA as outlined above.
Overall, these data suggest that the combination of a powerful lineage-specific promoter with a ligand-dependent Cre-recombinase enzyme will be valuable to experiments analyzing fibroblasts in vivo and also in explanted fibroblasts from these mice. Fibrosis is a major medical problem responsible for many forms of organ failure and representing a common pathological al pathway in many diseases such as liver, kidney, or lung failure. Localized vascular fibrosis may underlie important conditions such as atherosclerosis or pulmonary hypertension. The system that we describe in this article is likely to prove a powerful technique for studying some of the most intractable and currently least treatable of human diseases.
Acknowledgments
We thank Phillip Soriano (Seattle, WA) for his gift of the ROSA26-STOP-LacZ mouse reporter line, and Pierre Chambon (Strasbourg, France) for kindly providing the pCreER(T) plasmid used in the transgene construct.
Footnotes
Address reprint requests to Christopher P. Denton, Ph.D., M.R.C.P., Center for Rheumatology, University College London, Royal Free Campus, Rowland Hill St., London NW3 2PF, UK. E-mail: c.denton@rfc.ucl.ac.uk.
Supported by The Wellcome Trust, The Arthritis Research Campaign (UK), and the National Institutes of Health, including a Specialized Center of Research Grant in Scleroderma at the University of Texas-Houston Health Science Center (no. P50AR44888).
References
- 1.Denton CP, Black CM, Korn JH, de Crombrugghe B: Systemic sclerosis: current pathogenetic concepts and future prospects for targeted therapy. Lancet 1996, 347:1453-1458 [PubMed] [Google Scholar]
- 2.Strehlow D, Jelaska A, Strehlow K, Korn JH: A potential role for protease nexin 1 overexpression in the pathogenesis of scleroderma. J Clin Invest 1999, 103:1179-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feghali CA, Wright TM: Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis Rheum 1999, 42:1451-1457 [DOI] [PubMed] [Google Scholar]
- 4.Shi-wen X, Pennington D, Holmes A, Leask A, Bradham D, Beauchamp JR, Fonseca C, du Bois RM, Martin GR, Black CM, Abraham DJ: Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp Cell Res 2000, 259:213-224 [DOI] [PubMed] [Google Scholar]
- 5.Susol E, Rands AL, Herrick A, McHugh N, Barrett JH, Ollier WE, Worthington J: Association of markers for TGFbeta3, TGFbeta2 and TIMP1 with systemic sclerosis. Rheumatology 2000, 39:1332-1336 [DOI] [PubMed] [Google Scholar]
- 6.Pluck A: Conditional mutagenesis in mice: the Cre/LoxP recombination system. Int J Exp Path 1996, 77:269-278 [PMC free article] [PubMed] [Google Scholar]
- 7.Rossert J, Eberspaecher H, de Crombrugghe B: Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol 1995, 129:1421-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou-Gharios G, Garrett LA, Rossert J, Niederreither K, Eberspaecher H, Smith C, Black C, Crombrugghe B: A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol 1996, 134:1333-1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denton CP, Zheng B, Shi-wen X, Bou-Gharios G, Zhang Z, Eberspaecher H, Black CM, de Crombrugghe B: Activation of a fibroblast-specific enhancer of the proα2(I)collagen gene in tight-skin mice. Arthritis Rheum 2001, 44:712-722 [DOI] [PubMed] [Google Scholar]
- 10.Diamond I, Owolabi T, Marco M, Lam C, Glick A: Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol 2000, 115:788-794 [DOI] [PubMed] [Google Scholar]
- 11.Ye L, Chan S, Chow YH, Tsui LC, Hu J: Regulated expression of the human cftr gene in epithelial cells. Mol Ther 2001, 3:723-733 [DOI] [PubMed] [Google Scholar]
- 12.Tichelaar JW, Lu W, Whitsett JA: Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000, 275:11858-11864 [DOI] [PubMed] [Google Scholar]
- 13.Saez E, Nelson MC, Eshelman B, Banayo E, Koder A, Cho GJ, Evans RM: Identification of ligands and coligands for the ecdysone-regulated gene switch. Proc Natl Acad Sci USA 2000, 97:14512-14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron U, Bujard H: Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol 2000, 327:401-421 [DOI] [PubMed] [Google Scholar]
- 15.Manickan E, Satoi J, Wang TC, Liang TJ: Conditional liver-specific expression of simian virus 40 t antigen leads to regulatable development of hepatic neoplasm in transgenic mice. J Biol Chem 2001, 276:13989-13994 [DOI] [PubMed] [Google Scholar]
- 16.Zhu B, Benjamin D, Zheng Y, Angliker H, Thiry S, Siegmann M, Jost JP: Overexpression of 5-methylcytosine DNA glycosylase in human embryonic kidney cells EcR293 demethylates the promoter of a hormone-regulated reporter gene. Proc Natl Acad Sci USA 2001, 98:5031-5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR: Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci USA 1999, 96:8483-8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocard J, Warot X, Wendling O: Spatio-temporally controlled site-specific somatic mutagenesis in the mouse. Proc Natl Acad Sci USA 1997, 94:14559-14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger D, Clifford J, Chiba H, Chambon P: Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci USA 1995, 92:6991-6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brocard J, Feil R, Chambon P: A chimeric Cre recombinase inducible by synthetic but not by natural ligands of the glucocorticoid receptor. Nucleic Acids Res 1998, 26:4086-4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denton CP, Zheng B, Zhang Z, Black CM, de Crombrugghe B: Fibroblast-directed perturbation of TGFβ signaling in transgenic mice. Arthritis Rheum 1999, 42:S234 [Google Scholar]
- 22.Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD: The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron 1991, 7:703-716 [DOI] [PubMed] [Google Scholar]
- 23.Kim DG, Kang HM, Jang SK, Shin HS: Construction of a bifunctional mRNA in the mouse by using the internal ribosomal entry site of the encephalomyocarditis virus. Mol Cell Biol 1992, 12:3636-3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Wang W, Lukin T: Dicistronic LacZ and alkaline phosphatase reporter constructs permit simultaneous histological analysis of expression from multiple transgenes. BioTechniques 1977, 23:874-882 [DOI] [PubMed] [Google Scholar]
- 25.Hogan B, Costantini F, Lacy E: Manipulating the Mouse Embryo: A Laboratory Manual. 1980. Cold Spring Harbor Laboratory, Cold Spring Harbor
- 26.Soriano P: Generalized LacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999, 21:70-71 [DOI] [PubMed] [Google Scholar]
- 27.Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P: Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA 1997, 94:3789-3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxwell IH, Harrison GS, Wood WM, Maxwell F: A DNA cassette containing a trimerised SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. Biotechniques 1989, 7:276-280 [PubMed] [Google Scholar]
- 29.MacDonald RJ, Swift GH: Analysis of transcriptional regulatory regions in vivo. Int J Dev Biol 1998, 42:983-994 [PubMed] [Google Scholar]
- 30.Magness ST, Tugores A, Brenner DA: Analysis of ferrochelatase expression during hematopoietic development of embryonic stem cells. Blood 2000, 95:3568-3577 [PubMed] [Google Scholar]
- 31.Niederreither K, D’Souza RN, de Crombrugghe B: Minimal DNA sequences that control the cell lineage-specific expression of the pro alpha 2(I) collagen promoter in transgenic mice. J Cell Biol 1992, 119:1361-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniv TT, DeVal S, Wells D, Denton CP, Rabe C, de Crombrugghe B, Ramirez F, Bou-Gharios G: Characterization of an evolutionarily conserved far-upstream enhancer in the human alpha2(I) collagen (COL1A2) gene. J Biol Chem 2001, 276:21754-21764 [DOI] [PubMed] [Google Scholar]
- 33.Minamino T, Gaussin V, DeMayo FJ, Schneider MD: Inducible gene targeting in postnatal myocardium by cardiac-specific expression of a hormone-activated Cre fusion protein. Circ Res 2001, 88:587-592 [DOI] [PubMed] [Google Scholar]
- 34.Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D: Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res 1999, 27:4324-4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP: Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 1998, 8:1323-1326 [DOI] [PubMed] [Google Scholar]
- 36.Kang KI, Meng X, Devin-Leclerc J, Bouhouche I, Chadli A, Cadepond F, Baulieu EE, Catelli MG: The molecular chaparone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc Natl Acad Sci USA 1999, 96:1439-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]