Abstract
Inflammatory mechanisms are thought to contribute to lesion pathogenesis and neuronal cell death in Alzheimer’s disease. Transforming growth factor-β (TGF-β) plays a central role in the response of the brain to injury, and is increased in the brain in Alzheimer’s disease. In this study we determine whether expression of TGF-β is abnormal in the microvasculature in Alzheimer’s disease and whether TGF-β affects vascular production of pro-inflammatory cytokines, interleukin (IL)-1β, and tumor necrosis factor (TNF)-α. Microvessels isolated from the cortices of Alzheimer’s disease patients and age-matched controls are analyzed for microvessel-associated and released TGF-β. Results from Western blot analysis and enzyme-linked immunosorbent assay indicate a higher level of TGF-β in Alzheimer’s disease vessels compared to controls. To determine whether TGF-β affects vascular release of inflammatory factors, cultured brain endothelial cells are treated with TGF-β and levels of IL-1β and TNF-α determined. Both enzyme-linked immunosorbent assay and Western blot analyses show that untreated endothelial cells express little IL-1β or TNF-α, but incubation with TGF-β results in robust expression of these factors by brain endothelial cells. Our results suggest that vessel-derived TGF-β contributes to inflammatory processes in the Alzheimer brain.
Inflammatory mechanisms, especially in the later stages of Alzheimer’s disease (AD), are thought to contribute to lesion pathogenesis and neuronal cell death. 1-5 This hypothesis is supported by observations in human and animal studies that anti-inflammatory drugs reduce the risk of AD pathology and, in some instances, enhance cognitive performance. 6-9 A large number of cytokines and chemokines are elevated in the AD brain and have been implicated in lesion development. Transforming growth factor-β (TGF-β) plays a central role in the response of the brain to injury and is elevated in the AD brain. 10-14
TGF-β is a multifunctional protein family that regulates cell growth, differentiation, migration, and extracellular matrix production and is involved in tissue remodeling and inflammation. 15,16 In the central nervous system, TGF-β is an important regulator of survival/death decisions in neurons and has been shown to be cytoprotective as well as injurious to neurons. 14,17-20 This pleiotropic cytokine has been implicated in the pathophysiology of stroke and in several aspects of AD pathogenesis. 21-24 In AD patients, TGF-β levels in plaques, cerebrospinal fluid, and serum are higher than in nondemented elderly controls. 11,12,21 Also, TGF-β immunoreactivity is elevated along the cerebral vasculature in AD but not control brains. 25 Transgenic mice overexpressing TGF-β in astrocytes show high levels of cerebrovascular amyloid deposition, suggesting that TGF-β induces cerebrovascular amyloidosis and contributes to microvascular degeneration in AD. 24
Microvascular endothelial cells are a rich source of both cytokines and chemokines and release inflammatory factors in response to a wide variety of stimuli. 26 Previous work from our laboratory has shown that in the AD brain endothelial cells express inflammatory mediators such as CAP37 (cationic antimicrobial protein, MW 37 kd) and MCP-1 (monocyte chemoattractant protein-1) on their surface and release significantly higher levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. 27,28 Also, we have shown high levels of inducible nitric oxide synthase, an isoform usually up-regulated in inflammation, in microvessels isolated from AD brains. 29 Taken together, these data implicate the cerebral microvasculature as a source of inflammatory factors in AD.
The objectives of this study are to determine whether TGF-β expression is abnormal in the microvasculature in AD and whether TGF-β affects vascular production of inflammatory factors such as IL-1β and TNF-α.
Materials and Methods
Human Microvessel Isolation
Human autopsy brains were obtained ∼4 to 15 hours postmortem and frozen at −70°C until dissection. The average postmortem interval was similar for both AD and control samples. The clinical diagnosis of primary AD was confirmed by neuropathological examination. Control samples from age-matched patients without evidence of neuropathology were also collected. The age ranges for AD (69 to 86 years) and control samples (66 to 75 years) were similar. Microvessels were isolated from pooled temporal, parietal, and frontal cortices using filtration through a 210-μm sieve and collection on a 53-μm sieve, as we have previously described. 28-32 This procedure yields ∼6 to 10 mg of microvessel protein from 15 g of human cortex. The isolation procedure is characterized by extensive sieving and washing and the resulting microvessels are unlikely to contain trapped cells or plasma-derived proteins within the lumen. 33 A separate microvessel preparation was isolated from each human brain. The purity of the microvessel preparations was routinely monitored by phase-contrast microscopy. Microvessels were resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY), containing 10% fetal calf serum and 10% dimethyl sulfoxide and stored frozen in liquid nitrogen until used.
Preparation of Conditioned Media and Lysates
Microvessels were quick-thawed at 37°C and centrifuged at 2000 × g for 15 minutes. Microvessels were washed three times with cold Hanks’ balanced salt solution and resuspended in serum-free DMEM containing 1% lactalbumin hydrogenase. Microvessels (50 μg/sample) were incubated in 1 ml for 4 to 6 hours at 37°C in a 95% CO2/5% O2 incubator, and then centrifuged (2000 × g). The conditioned media were collected and used for enzyme-linked immunosorbent assay (ELISA). To determine microvessel-associated TGF-β, microvessels were lysed using 2% sodium dodecyl sulfate (SDS) containing ethylenediaminetetraacetic acid (0.5 mol/L), and lysates used for Western blots analyses. 28 To determine the effect of microvessel homogenization on TGF-β release, we compared the TGF-β released into microvessel-conditioned media to that obtained after homogenizing the microvessels. For these experiments, vessels were incubated in 1% collagenase for 1 hour at 37°C and homogenized by both polytron and sonication.
Rat brain microvessels were isolated as previously described 33,34 and seeded as explant cultures. Microvascular endothelial cells were isolated from the resulting mixed endothelial cell and smooth muscle cell cultures using cloning penicylinders. The endothelial identity of these cultures was confirmed using antibodies to endothelial cell factor VIII, as previously described. 35 Confluent endothelial cells were incubated in serum-free DMEM with 0.1% bovine serum albumin and then exposed to different concentrations of TGF-β for 24 hours. Control (unstimulated) cells were incubated in media alone. Supernatants were assayed for IL-1β and TNF-α by ELISA. Endothelial cells were washed, scraped, and samples lysed using 2% SDS containing ethylenediaminetetraacetic acid (0.5 mol/L) and lysates used for Western blot analyses. 28
Cytokine Analysis Using ELISA
Levels of TGF-β in conditioned media from AD and non-AD samples were measured by ELISA. ELISA kits to detect IL-β and TNF-α were obtained from Boehringer Mannheim (Indianapolis, IN) and the TGF-β kit from R&D systems (Minneapolis, MN). Levels of biologically active TGF-β, IL-β, and TNF-α were determined using a color-based assay. For the determination of IL-β and TNF-α, samples were bound by a biotin-labeled antibody and the peroxidase-conjugated detection antibody. This complex bound via the biotin-labeled antibody to the streptavidin-coated microtiter plate. The plate was incubated at room temperature for 2 hours, washed, and bound, peroxidase was developed by adding tetramethylbenzidine as a substrate. For the determination of TGF-β, samples were bound by immobilized TGF-β receptor that had been precoated onto microtiter plates. An enzyme-linked polyclonal antibody specific for TGF-β1 was added to the wells then incubated at room temperature for 2 hours, washed, and the substrate solution added. The developed color (read at 450 nm) was proportional to the concentration of the IL-β, TNF-α, or TGF-β bound in the initial step. The measuring range for the IL-1β and TNF-α kits is between 5 to 700 pg/ml and for TGF-β kits the range is between 31.2 to 2000 pg/ml. All samples measured within the range of the standard curve.
Cytokine Detection by Western Blot
Microvessels (50 μg/lane) or endothelial cell (20 to 30 μg/lane) lysates were loaded for SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes for Western blot analyses. The blots were washed two times using Tris-buffered saline with Tween 20 (TBST), incubated in milk solution (TBST with 3% nonfat dry milk) for 1 hour, and washed two times with TBST. The blots were then incubated in the primary antibody (TGF-β1: catalog no. sc-398, R&D Systems; TNF-α: catalog no. sc-8301, Santa Cruz Biotechnology, Santa Cruz, CA; and IL-1β: catalog no. sc-7884, Santa Cruz Biotechnology) in milk solution (dilution 1:500) for 1 hour, washed three times with TBST, incubated with the secondary antibody (Bio-Rad no. 1706515 for TGF-β, TNF-α, and IL-1β; Bio-Rad, Richmond, CA) in milk solution (dilution, 1:4000) for 1 hour, and washed three times with TBST. The blots were then enhanced (Bio-Rad Immun-Star Kit, catalog no. 170-5011) and visualized on film. Relative quantitation of band optical density was performed using a GEL DOC 2000. An intensity profile for each band was generated by averaging the value of each horizontal row of pixels and the number of pixel rows between top and bottom within a set bracket. 28 The limits of detection for the Western blot, using our current protocols and antibodies, was 6 pmol/L of TGF-β.
Results
Both Released and Microvessel-Associated TGF-β Are Elevated in AD Microvessels
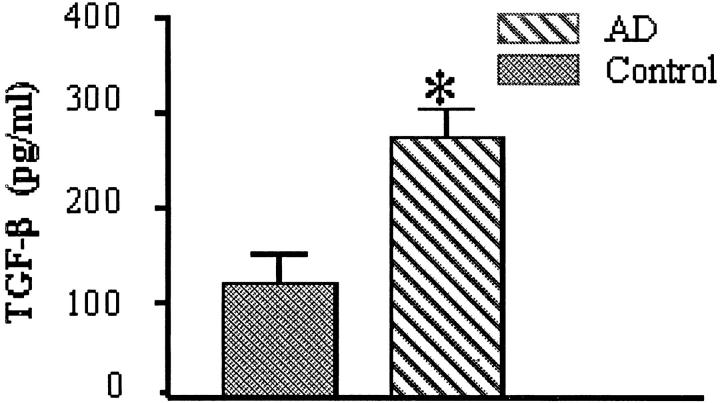
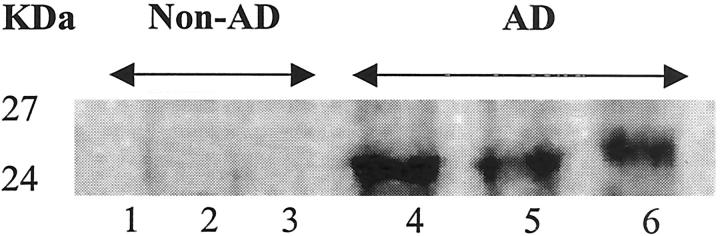
AD and control microvessels were analyzed for release of soluble as well as microvessel-associated TGF-β. Incubation of isolated microvessels in serum-free medium for 4 to 6 hours resulted in a significantly (P < 0.05) higher (150%) release of TGF-β into the media by AD-derived microvessels compared to control-derived vessels (Figure 1) ▶ . Western blot analysis of microvessel-associated TGF-β showed strong reactivity of AD-derived vessels to TGF-β antibody with no detectable expression in control microvessels (Figure 2) ▶ .
Figure 1.
Microvessels were isolated from the brains of patients with AD (n = 10) or nondemented elderly controls (n = 5). Brain microvessels were washed and incubated for 6 hours with serum-free DMEM containing 1% lactalbumin hydrogenase, centrifuged, and the supernatant assayed for TGF-β using an ELISA-based assay. *, P < 0.05, significantly different from control-derived microvessels.
Figure 2.
Microvessels were isolated from the brains of patients with AD or nondemented elderly controls. Microvessel lysates (50 μg/lane) were loaded for SDS-polyacrylamide gel electrophoresis, and Western blot analyses and were performed using antibodies to TGF-β. Lanes 1–3, non-AD control-derived microvessels; lanes 4–6, AD-derived vessels.
Disruption of AD microvessels by enzyme and mechanical homogenization resulted in a 104% increase in the amount of TGF-β detected in the media compared to the amount detected in the conditioned media from intact microvessels.
TGF-β Stimulates Release of Pro-Inflammatory Cytokines from Cultured Brain Endothelial Cells
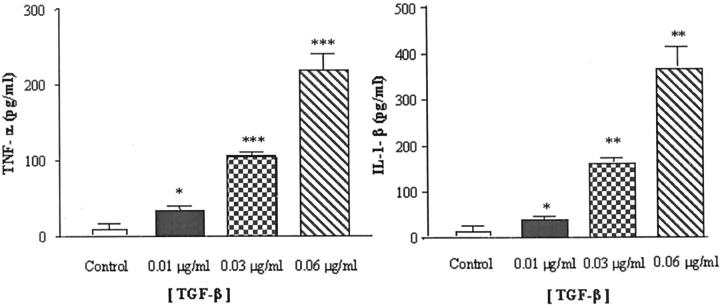
ELISA analysis of conditioned medium from cultured brain endothelial cells collected after 24 hours showed barely detectable levels of the pro-inflammatory cytokines IL-1β and TNF-α (Figure 3) ▶ . In contrast, incubation of endothelial cells with TGF-β caused a dose-dependent increase in release of both cytokines. Levels of IL-1β were significantly (P < 0.05) elevated in response to 0.01 μg/ml TGF-β and increased at higher TGF-β concentrations (P < 0.01) (Figure 3 ▶ , right). Similarly, incubation with TGF-β produced a dose-dependent increase in the release of TNF-α that was significant at 0.01 μg/ml of TGF-β and increased at higher TGF-β concentrations (P < 0.005) (Figure 3 ▶ , left).
Figure 3.
Confluent brain endothelial cells were incubated in serum-free DMEM with 0.1% bovine serum albumin and then exposed to different concentrations of TGF-β for 24 hours. Control (unstimulated) cells were incubated in media alone. The supernatants were assayed for IL-1β (right) and TNF-α (left) using an ELISA-based assay. *, P < 0.05; **, P < 0.01;***, P < 0.005, significantly different from unstimulated endothelial cells.
Increased Expression of Cell-Associated IL-1β and TNF-α in Cultured Brain Endothelial Cells Treated with TGF-β
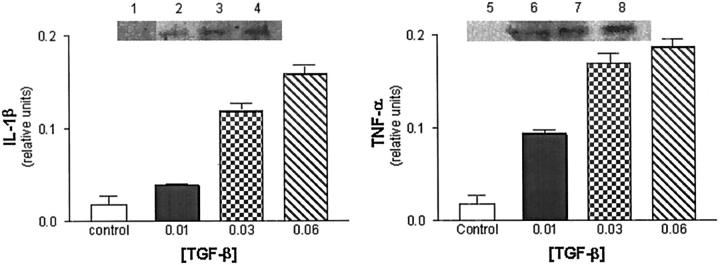
Western blot analyses of brain endothelial cell lysates collected from untreated endothelial cells showed little detectable expression of either IL-1β or TNF-α (Figure 4) ▶ . In contrast, incubation of cultured brain endothelial cells with several doses of TGF-β resulted in a dose-dependent increase in expression of IL-1β (Figure 4 ▶ , left) and TNF-α (Figure 4 ▶ , right), as determined by increased immunoreactivity to cytokine antibodies.
Figure 4.
Confluent endothelial cells were incubated in serum-free DMEM with 0.1% bovine serum albumin and then exposed to different concentrations of TGF-β for 24 hours (lanes 2–4 and 6–8). Control (unstimulated) cells were incubated in media alone (lanes 1 and 5). Endothelial cells were washed, scraped, and samples prepared for SDS-polyacrylamide gel electrophoresis. Western blot analysis was performed using antibodies to IL-1β (left) and TNF-α (right).
Discussion
Recent studies have implicated pro- and anti-inflammatory cytokines as integral to AD pathogenesis. Among them, TGF-β, increased in the central nervous system in AD, is emerging as an important factor that could contribute to several aspects of AD pathogenesis. 10-14,21-24 For example, in transgenic mice where astrocytes overproduce TGF-β, cerebrovascular amyloidosis, accumulation of basement membrane proteins, and degenerative changes in microvascular cells have been observed. 15 TGF-β expression in these mice is widespread but is most prominent in perivascular locations. Also, staining of blood vessels in TGF-β transgenic mice is similar to the staining of blood vessels in AD patients. 25 The observation that TGF-β triggers AD-like cerebrovascular amyloidosis and microvascular degeneration supports a relevant role for TGF-β in AD pathology.
TGF-β could also contribute to vascular and neuronal deleterious changes in the AD brain via interactions and/or regulation of inflammatory mediators. It is clear soluble inflammatory factors are important in the AD brain because virtually all cytokines and chemokines that have been studied in AD are up-regulated. 3 Despite the presence of large numbers of inflammatory mediators in the AD brain, it is unclear what factors regulate expression of these inflammatory proteins. Our data showing that TGF-β is elevated in AD microvessels and that it stimulates release of the pro-inflammatory cytokines IL-1β and TNF-α from brain endothelial cells suggests that TGF-β is a pivotal factor in the control of inflammatory mediators and processes in AD.
In AD, amyloid-β is heavily deposited in the blood vessel wall and both brain endothelial cells and astrocytes release inflammatory proteins. 36-40 In this study we demonstrate that AD brain microvessels express and release high levels TGF-β. However, it is not clear whether the vessels synthesize TGF-β. There is evidence that both astrocytes and endothelial cells can produce TGF-β. 25,41 Indeed, astrocyte-derived TGF-β induces amyloid-β deposition in cerebral blood vessels of aged transgenic mice. 25 Although the TGF-β measured could reflect an astrocyte-derived cytokine that is adherent to the vessel wall, we believe this is unlikely because the microvessel isolation procedure is characterized by extensive sieving and washing. It is possible that some of the TGF-β is astrocyte-derived protein that has been internalized by brain endothelial cells. Alternatively, the TGF-β measured could be vascular-derived. Endothelial cells have been shown to synthesize TGF-β and this protein in response to increased shear stress or cell injury. 41 The amount of TGF-β detected by ELISA in microvessel-conditioned media is much lower than the level of cytokine apparently required to induce an effect in cultured endothelial cells. In part, this apparent discrepancy may reflect differences between isolated microvessels and cultured cells. In this regard, we have new preliminary evidence showing that cultured endothelial cells treated with an inflammatory cocktail release higher levels of TGF-β than that detected in microvessel-conditioned media (data not shown). Although it is difficult to draw conclusions as to what occurs in vivo, it is likely that both endothelial cells and astrocytes contribute perivascular TGF-β.
The inflammatory hypothesis of AD, that is supported both by basic laboratory evidence and epidemiological studies, suggests that treatment with anti-inflammatory drugs may reduce the risk or slow the progression of AD. 1,6-9 Inflammatory mediators co-localize in the AD brain with those regions that exhibit high levels of AD pathology. 42 Our previous results showing an up-regulation of vascular-derived inflammatory mediators in AD are significant because of the topographic association of capillaries with neuritic plaques and the co-localization of vascular-derived heparan sulfate proteoglycan deposits with senile plaques. 43,44 In our current study we report that TGF-β is elevated in AD microvessels and stimulates release of pro-inflammatory cytokines IL-1β and TNF-α from brain endothelial cells. These results suggest that vessel-derived TGF-β could initiate and/or propagate a destructive inflammatory cycle, contribute to neuronal cell death and may be a therapeutic target in AD.
Acknowledgments
We thank Theresa Rush for secretarial assistance, and the Department of Pathology, University of California, San Diego, California, for the procurement of tissue samples.
Footnotes
Address reprint requests to Paula Grammas, Ph.D., Department of Pathology, University of Oklahoma Health Sciences Center, BRC, Room 262, 975 NE 10th Street, Oklahoma City, OK 73104. E-mail: paula-grammas@ouhsc.edu.
Supported in part by grants from the National Institutes of Health (AG15964 to P. G.) and the Alzheimer’s Association (to P. G.).
P. G. is the recipient of the Presbyterian Health Foundation Chair in Neuroscience.
References
- 1.McGeer PL, Schulzer M, McGeer EG: Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology 1996, 47:425-432 [DOI] [PubMed] [Google Scholar]
- 2.Cooper NR, Bradt BM, O’Barr S, Yu JX: Focal inflammation in the brain: role in Alzheimer’s disease. Immunol Res 2000, 21:159-165 [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboon P, Emmerling M, Fiebich BL, et al: Inflammation and Alzheimer’s disease. Neurobiol Aging 2000, 21:383-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RW: Inflammation and Alzheimer’s disease. Lancet 2001, 358:436-437 [DOI] [PubMed] [Google Scholar]
- 5.Yagami T, Ueda K, Asakura K, Sakaeda T, Kuroda T, Hata S, Kambayashi Y, Fujimoto M: Effects of S-2474, a novel nonsteriodal anti-inflammatory drug, on amyloid beta protein-induced neuronal cell death. Br J Pharmacol 2001, 134:673-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hull M, Fiebich BL, Schumann G, Lieb K, Bauer J: Anti-inflammatory substances—a new therapeutic option in Alzheimer’s disease. Drug Discov Today 1999, 4:275-282 [DOI] [PubMed] [Google Scholar]
- 7.Halliday G, Robinson SR, Shepherd C, Kril J: Alzheimer’s disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol 2000, 27:1-8 [DOI] [PubMed] [Google Scholar]
- 8.Halliday GM, Shepherd CE, McCann H, Reid WG, Grayson DA, Broe GA, Kril JJ: Effect of anti-inflammatory medications on neuropathological findings in Alzheimer disease. Arch Neurol 2000, 57:831-836 [DOI] [PubMed] [Google Scholar]
- 9.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM: Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 2000, 20:5709-5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM: TGF-β1 is an organizer of responses to neurodegeneration. J Cell Biochem 1993, 53:314-322 [DOI] [PubMed] [Google Scholar]
- 11.Chao CC, Hu S, Frey WH, II, Ala TA, Tourtellotte WW, Peterson PK: Transforming growth factor beta in Alzheimer’s disease. Clin Diag Lab Immunol 1994, 1:109-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB: Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology 1995, 45:1561-1569 [DOI] [PubMed] [Google Scholar]
- 13.Peress NS, Perillo E: Differential expression of TGF-beta 1, 2 and 3 isotypes in Alzheimer’s disease: a comparative immunohistochemical study with cerebral infarction, aged human and mouse control brains. J Neuropathol Exp Neurol 1995, 54:802-811 [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP, Barger SW, Furukawa K, Bruce AJ, Wyss-Coray T, Mark RJ, Mucke L: Cellular signaling roles for TGF beta, TNF alpha and beta APP in brain injury responses and Alzheimer’s disease. Brain Res Rev 1997, 23:47-61 [DOI] [PubMed] [Google Scholar]
- 15.Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L: Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol 1995, 147:53-67 [PMC free article] [PubMed] [Google Scholar]
- 16.Bottner M, Krieglstein K, Unsicker K: The transforming growth factor-[beta]s: structure, signaling, and roles in nervous system. J Neurochem 2000, 75:2227-2240 [DOI] [PubMed] [Google Scholar]
- 17.Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K: GDNF requires TGF-β for establishing its neurotrophic activity. J Neurosci 1998, 18:9822-9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieglstein K, Richter S, Farkas L, Schuster N, Dunker N, Oppenheim RW, Unsicker K: Reduction of endogenous transforming growth factor beta prevents ontogenetic neuron death. Nat Neurosci 2000, 3:1085-1090 [DOI] [PubMed] [Google Scholar]
- 19.Nagatsu T, Mogi M, Ichinose H, Togari A: Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl 2000, 60:277-290 [DOI] [PubMed] [Google Scholar]
- 20.Hailer NP, Wirjatijasa F, Roser N, Hischebeth GT, Korf HW, Dehghani F: Astrocytic factors protect neuronal integrity and reduce microglial activation in an in vitro model of N-methyl-D-aspartate-induced excitotoxic injury in organotypic hippocampal slice cultures. Eur J Neurosci 2001, 14:315-326 [DOI] [PubMed] [Google Scholar]
- 21.Van der Wal EA, Gomez-Pinilla F, Cotman CW: Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport 1993, 4:69-72 [DOI] [PubMed] [Google Scholar]
- 22.Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E: Chronic overproduction of transforming growth factor-β1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 2000, 156:139-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali C, Docagne F, Nicole O, Lesne S, Toutain J, Young A, Chazalviel L, Divoux D, Caly M, Cabal P, Derlon JM, MacKenzie ET, Buisson A, Vi D: Increased expression of transforming growth factor-beta after cerebral ischemia in the baboon: an endogeneous marker of neuronal stress? J Cerebral Blood Flow Metab 2001, 21:820-827 [DOI] [PubMed] [Google Scholar]
- 24.Masliah E, Ho G, Wyss-Coray T: Functional role of TGFbeta in Alzheimer’s disease microvascular injury: lessons from transgenic mice. Neurochem Int 2001, 39:393-400 [DOI] [PubMed] [Google Scholar]
- 25.Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L: Amyloidogenic role of cytokine TGF-beta 1 in transgenic mice and in Alzheimer’s disease. Nature 1997, 389:603-606 [DOI] [PubMed] [Google Scholar]
- 26.Gimbrone MA, Jr: Vascular endothelium, hemodynamic forces and atherogenesis. Am J Pathol 1999, 155:1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira HA, Kumar P, Grammas P: Expression of CAP37, a novel inflammatory mediator in Alzheimer’s disease. Neurobiol Aging 1996, 17:753-759 [PubMed] [Google Scholar]
- 28.Grammas P, Ovase R: Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 2001, 22:837-842 [DOI] [PubMed] [Google Scholar]
- 29.Dorheim MA, Tracey WR, Pollock JS, Grammas P: Nitric oxide is elevated in Alzheimer’s brain microvessels. Biochem Biophys Res Commun 1994, 205:659-665 [DOI] [PubMed] [Google Scholar]
- 30.Grammas P, Roher AE, Ball MJ: Decreased α-adrenergic receptors at the blood-brain barrier in Alzheimer’s disease. Iqbal K McLachlan DRC Winblad B Wisniewski HM eds. Alzheimer’s Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies. 1991, :pp 129-136 John Wiley & Sons, Ltd., Chicester, UK [Google Scholar]
- 31.Grammas P, Roher AE, Ball MJ: Increased accumulation of cAMP in cerebral microvessels in Alzheimer’s disease. Neurobiol Aging 1994, 15:113-116 [DOI] [PubMed] [Google Scholar]
- 32.Cashman RE, Grammas P: cAMP-dependent protein kinase in cerebral microvessels in aging and Alzheimer disease. Mol Chem Neuropathol 1995, 26:247-258 [DOI] [PubMed] [Google Scholar]
- 33.Diglio CA, Grammas P, Giacomelli F, Wiener J: Primary culture of rat cerebral microvascular endothelial cells. Lab Invest 1982, 46:554-563 [PubMed] [Google Scholar]
- 34.Diglio CA, Grammas P, Giacomelli F, Wiener J: Rat cerebral microvascular smooth muscle cells in culture. J Cell Physiol 1986, 129:131-141 [DOI] [PubMed] [Google Scholar]
- 35.Diglio CA, Liu W, Grammas P, Giacomelli F, Wiener J: Isolation and characterization of cerebral resistance vessel endothelium in culture. Tissue Cell 1993, 25:833-846 [DOI] [PubMed] [Google Scholar]
- 36.Sawada M, Kondo N, Suzumura A, Marunouchi T: Production of TNF-alpha by microglia and astrocytes in culture. Brain Res 1989, 491:394-397 [DOI] [PubMed] [Google Scholar]
- 37.Ko LW, Sheu KF, Blass JP: Immunohistochemical colocalization of amyloid precursor protein with cerebrovascular amyloid of Alzheimer’s disease. Am J Pathol 1991, 139:523-533 [PMC free article] [PubMed] [Google Scholar]
- 38.Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M: Alzheimer’s β-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res 1998, 807:110-117 [DOI] [PubMed] [Google Scholar]
- 39.Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN: IL-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci 1999, 19:5236-5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies TA, Long HJ, Eisenhauer PB, Hastey R, Cribbs DH, Fine RE, Simons ER: Beta amyloid fragments derived from activated platelets deposit in cerebrovascular endothelium: usage of a novel blood brain barrier endothelial cell model system. Amyloid 2000, 7:153-165 [DOI] [PubMed] [Google Scholar]
- 41.Cucina A, Sterpetti AV, Borrelli V, Pagliei S, Cavallaro A, D’Angelo LS: Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery 1998, 123:212-217 [PubMed] [Google Scholar]
- 42.Lue LF, Brachova L, Civin WH, Rogers J: Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol 1996, 55:1083-1088 [PubMed] [Google Scholar]
- 43.Miyakawa T, Shimoji A, Kumamoto R, Higuchi Y: The relationship between senile plaques and cerebral blood vessels in Alzheimer’s disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch (Cell Pathol) 1982, 40:121-129 [DOI] [PubMed] [Google Scholar]
- 44.Buee L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM: Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol 1994, 87:469-480 [DOI] [PubMed] [Google Scholar]