Abstract
The Jagged and Delta family of transmembrane proteins are ligands for Notch receptors, which control the proliferation and/or differentiation of many cell lineages. Expression and localization of these ligands in the adult human liver has not been fully elucidated, nor whether dysregulation of these proteins contributes to liver disease processes. We have examined expression of the five known Notch ligands in human liver. Expression of Jagged-1 and Delta-4 mRNA was seen in normal and diseased liver tissue, whereas Jagged-2, Delta-1, and Delta-3 mRNA was undetectable. In primary liver cell isolates, Jagged-1 expression was found in all cell types, whereas Delta-4 was present in biliary epithelial and liver endothelial cells, but absent in hepatocytes. Interestingly, Jagged-1 mRNA expression was significantly up-regulated in diseased liver tissue. By immunohistochemistry, Jagged-1 expression was present on most structures in normal tissue. However in disease, strikingly strong Jagged-1 immunoreactivity was observed on many small neovessels and bile ductules. The expression of downstream modulators and effectors of Notch signaling was also detectable in purified cell isolates. This, together with aberrant Jagged-1 expression suggests that the Notch signaling pathway may play a role in the neovascularization and biliary defects observed in the liver during the development of cirrhosis.
The Notch signaling pathway plays an essential role in cellular specification, proliferation, and differentiation in a wide variety of organisms ranging from worms to man. 1 The Notch gene family encodes large type 1 transmembrane proteins that function as receptors in a highly conserved signal transduction pathway. 2 In man, four Notch genes have been identified so far (Notch-1, -2, 3, and -4) and they are expressed in numerous tissue types. 3-6 Signaling is activated after receptor binding to type 1 transmembrane ligands expressed on adjacent cells. The ligands are characterized by two conserved motifs in the extracellular region, the DSL (Delta, Serrate, Lag-2) domain, important for receptor binding, and a series of epidermal growth factor-like repeats that may function to stabilize or modify ligand receptor interactions. 7
Two Notch ligand families have been identified in man based on the presence of a cysteine-rich region in the extracellular domain. Those with the cysteine-rich domain belong to the Serrate/Jagged family and include Jagged-1 and -2. 8,9 The Delta family, which lack the cysteine-rich motif, include Delta-like-1, -3, and -4. 10-12 The ligands also possess a poorly conserved cytoplasmic domain, the function of which is unknown. However, deletion studies have revealed that this domain is essential for normal wild-type ligand function. 13 The ligands all display distinctive expression patterns in human organs and tissues. During early human development the Jagged-1 gene is expressed in various tissues and cell types. 14 In adult tissues strong Jagged-1 mRNA expression is detectable in heart, placenta, and kidney with lower levels in lung muscle and pancreas. 10 Jagged-2 transcripts are also detectable in heart and placenta, together with skeletal muscle. 10 The Delta-1 gene is highly expressed in heart and pancreas, with weak expression also detectable in brain and muscle tissue. 10 Little is known about the tissue distribution of Delta-3 in adult organs, however during mouse embryogenesis, expression implicates Delta-3 with a role in somitogenesis and neurogenesis. 15 In contrast, Delta-4 gene expression is seen in most adult and fetal tissues. 12
Mutations in two of the five known human Notch ligands have been associated with developmental disorders in man. Lesions in the human Delta-like-3 gene give rise to spondylocostal dysostosis, a group of vertebral malsegmentation syndromes resulting from axial skeletal defects. Spondylocostal dysostosis is a disorder with autosomal-dominant and autosomal-recessive modes of inheritance, which is characterized by multiple hemivertebrae, rib fusions, and deletions with a nonprogressive lateral curving of the spine. 11 In patients with spondylocostal dysostosis, two mutations in the human Delta-3 gene were identified within the conserved extracellular domain and are predicted to give rise to truncated proteins. A third missense mutation present in a highly conserved glycine residue of the fifth epidermal growth factor-like repeat highlights the functional importance of this domain. 11
Mutations in the Jagged-1 gene give rise to Alagille syndrome an autosomal-dominant developmental disorder that affects structures in the liver, skeleton, eye, heart, kidney, and other organs. 8,16,17 In the liver it is characterized by cholestatic liver disease related to paucity of intrahepatic bile ducts. The disease can vary in its degree of severity from an apparently normal phenotype to chronic cases in which cirrhosis and liver failure result in the requirement for liver transplantation in early childhood. Analysis of DNA samples from Alagille syndrome patients has revealed three frame-shift mutations, two splice donor mutations, and one mutation that abolishes RNA expression from the altered allele. Thus the phenotype observed for Alagille syndrome and spondylocostal dysostosis may be the result of haplo-insufficiency or dominant-negative effects of the Jagged-1 and Delta-like-3 gene products. 8,11,16
We have previously shown that all four of the human Notch genes are expressed in adult human liver and the expression, together with the distribution of these receptors, is altered in liver disease. 18 To determine whether these perturbations in Notch receptor expression are the result of altered ligand expression, we have analyzed the expression profile of all of the Notch ligands together with downstream targets and modulators of the Notch pathway in normal and diseased liver tissue. Taken together with our previous data on the localization of Notch receptor gene expression, these results provide valuable insight into possible ligand/receptor interactions occurring in liver cell types in the normal liver as well as during disease processes. 18
Materials and Methods
Liver Tissue
Diseased liver tissue was obtained from the adult liver transplant program at University Hospital Birmingham, National Health Science Trust. Hepatectomy specimens were obtained from patients undergoing transplantation for primary biliary cirrhosis (PBC) (n = 5; age range, 19 to 45 years), primary sclerosing cholangitis (PSC) (n = 5; age range, 27 to 46 years), and alcoholic liver disease (n = 5; age range, 18 to 46 years). Donor tissue excess to surgical requirements, served as normal controls (n = 5; age range, 20 to 43 years). For immunohistochemistry and tissue reverse transcriptase-polymerase chain reaction (RT-PCR), tissue was snap-frozen and stored at −70°C. For cell isolations, tissue was stored in Dulbecco’s modified Eagles medium (Gibco, Paisley, UK) at 4°C and used within 48 hours after hepatectomy. Informed consent and local regional ethical committee approval was obtained before tissue collection.
Isolation and Culture of Specific Cell Types for RT-PCR
Hepatocytes were isolated from human hepatectomy specimens and donor livers by collagenase perfusion. 19 After isolation, cells were resuspended in William’s E medium containing insulin, glutamine, and hydrocortisone and plated as previously described. 19 Isolation of biliary epithelial cells was performed as previously described. 20,21 Endothelial cells were selected from the same Percoll gradient fraction as the biliary epithelial cells (BECs), but were further purified by immunomagnetic separation using the monoclonal antibody CD31 (endothelial cell marker). Cells were maintained in culture for a week before harvesting and RNA extraction.
Isolation of Total RNA
Total RNA was isolated from whole tissue or cultured cells using RNazol B (Biogenesis Ltd., Poole, UK) essentially as described by the manufacturer. Briefly, liver tissue (1 g) was homogenized in 6 ml of RNazol B solution. The homogenate was extracted by the addition of 600 μl of chloroform and centrifuged at 12,000 rpm for 15 minutes. The RNA-containing supernatant was then extracted again by the addition of an equal volume of phenol:chloroform:isoamyl alcohol (5:3:1) (Sigma, Poole, UK). Total RNA was then recovered by isopropanol precipitation and centrifugation at 12,000 rpm for 30 minutes. The pellet was washed once with 75% ethanol and dissolved in distilled water treated with diethyl pyrocarbonate. RNA was then incubated with RNase-free DNase I at 37°C for 20 minutes to remove traces of genomic DNA. After heat denaturation to inactivate DNase I activity, total RNA was recovered by isopropanol precipitation, subjected to a 75% ethanol wash, and resuspended in diethyl pyrocarbonate-treated water. Total RNA was extracted from cultured cells by using a scaled-down version of the procedure used for extracting total RNA from tissue. Briefly 1 ml of RNazol B was added directly to culture flasks. Cell lysates were extracted by the addition of 0.1 ml of chloroform and centrifuged at 15,000 rpm for 15 minutes. After DNase I treatment, RNA was recovered by isopropanol precipitation, overnight at −20°C, and centrifugation at 15,000 rpm for 30 minutes. Pellets were then dissolved in diethyl pyrocarbonate-treated water and stored at −70°C until required.
Semiquantitative RT-PCR Analysis and Primers
Semiquantitative RT-PCR was performed as previously described. 22,23 Briefly, first-strand cDNA was prepared from 1 μg of DNase I-treated total RNA using random hexamers and Superscript II reverse transcriptase (Gibco). Control reactions were also performed in the presence and absence of reverse transcriptase or RNA. The cDNA was then normalized for equivalent template amounts (up to a volume of 1.6 μl) by amplification with primers specific for β-actin in the presence of α-32P dGTP (Amersham-Pharmacia Biotech, Little Chalfont, UK). The cycling parameters used for β-actin amplification were as follows: an initial cycle of 94°C for 3 minutes (denaturation step), 58°C for 1 minute (annealing step), 72°C for 1 minute (extension step). The required number of cycles as specified in Table 1 ▶ were performed as for the initial cycle, but with 30 seconds for the 94°C denaturation step. A final extension step of 72°C for 5 minutes was then performed. With the exception of the annealing temperature and the cycle number, which are given in Table 1 ▶ for specific primers, these cycling parameters were used for all of the primers sets used. PCR products were then resolved on 6% nondenaturing polyacrylamide gels, dried under vacuum, and subjected to autoradiography. After autoradiography the intensities of amplified bands was quantified using the Gel Doc 2000 densitometry software (BioRad, Hemel Hempstead, UK). In control cDNA synthesis reactions RNA and reverse transcriptase were excluded and the products of these reactions were then used for PCR. In control PCR reactions cDNA was omitted and water added to the subsequent PCR reaction. 0.2 μl, 0.4 μl, 0.8 μl, and 1.6 μl are controls with the implied proportion of cDNA added to four PCR reactions to ensure that amplification was within the linear range.
Table 1.
Primer Sequences for RT-PCR Analysis
| Gene | Primer sequence (5′–3′) | Starting position in cDNA | Annealing temperature (°C) | Product amplified (bp) |
|---|---|---|---|---|
| Jagged-1A | Fw. 5′-AGTCACTGGCACGGTTGTAG-3′ | 1783 | 56 | 227 |
| (28 cycles) | Rv. 5′-TCGCTGTATCTGTCCACCTG-3′ | 2009 | ||
| Jagged-2B | Fw. 5′-GATTGGCGGCTATTACTGTG-3′ | 1355 | 56 | 600 |
| (35 cycles) | Rv. 5′-AGGCAGTCGTCAATGTTCTC-3′ | 1954 | ||
| Delta-1C | Fw. 5′-AGACGGAGACCATGAACAAC-3′ | 2079 | 55 | 382 |
| (35 cycles) | Rv. 5′-TCCTCGGATATGACGTACAC-3′ | 2460 | ||
| Delta-3D | Fw. 5′-GTGAATGCCGATGCCTAGAG-3′ | 711 | 57 | 256 |
| (35 cycles) | Rv. 5′-GGTCCATCTGCACATGTCAC-3′ | 966 | ||
| Delta-4E | Fw. 5′-TGACCACTTCGGCCACTATG-3′ | 576 | 55 | 620 |
| (27 cycles) | Rv. 5′-AGTTGGAGCCGGTGAAGTTG-3′ | 1195 | ||
| Hes-1F | Fw. 5′-TGGATGCGGAGTCTACGATG-3′ | 217 | 57 | 468 |
| (24 cycles) | Rv. 5′-TAAGGCCACTTGCCACCTTC-3′ | 684 | ||
| LnFgG | Fw. 5′-TGGAGTATGACCGCTTCATC-3′ | 238 | 57 | 460 |
| (24 cycles) | Rv. 5′-ATACCGTAGCTCAGCGTCAC-3′ | 697 | ||
| DeltexH | Fw. 5′-TGATGCCTGTGAATGGTCTG-3′ | 529 | 57 | 450 |
| (23 cycles) | Rv. 5′-TCTGCGACATGCTGTTGAAG-3′ | 978 | ||
| β-actinI | Fw. 5′-CATCACCATTGGCAATGAGC-3′ | 782 | 58 | 284 |
| (16 cycles) | Rv. 5′-CGATCCACACGGAGTACTTG-3′ | 1065 |
Fw., forward; Rv., reverse; bp, base pairs.
Genbank accession numbers: A, U61276; B, AF003521; C, AF003522; D, XM009230; E, AF253468; F, Y07572; G, U94354; H, AF053700; I, X00351.
Immunohistochemistry
Horseradish peroxidase staining was used to visualize antigens on acetone-fixed 5-μm cryostat tissue sections. Briefly, sections were incubated with 20% normal swine serum (DAKO, High Wycombe, UK) for 20 minutes. Sections were then incubated with a Jagged-1 primary goat polyclonal antibody (Santa Cruz Biotechnologies, Inc., Santa Cruz, CA), (diluted 1:100 in 10% normal swine serum) for 1 hour. Sections were washed twice in Tris- buffered saline and then incubated with a rabbit anti-goat peroxidase conjugate (DAKO) (diluted 1:100 in 10% normal swine serum) for 45 minutes. Signals were enhanced by the addition of a goat anti-rabbit peroxidase conjugate (DAKO) (diluted 1:100 in 10% normal swine serum) for a further 45 minutes. Slides were then washed twice in Tris-buffered saline and staining was visualized using the diaminobenzidine color substrate (Sigma, Poole, UK). Before mounting in DEPEX mountant, slides were counterstained with Mayer’s hematoxylin (BDH, Lutterworth, UK). In control sections the primary antibody was omitted.
Dual Immunofluorescence Staining
Double immunostaining for studies on antigen co-localization was performed with selected antibodies using the fluorescent conjugates alexaFluor 594 (red fluorescence, rabbit anti-mouse 1:200; Cambridge Bioscience) and alexaFluor 488 (green fluorescence, donkey anti-goat, 1:50; Cambridge Bioscience). The primary antibody combinations consisted of either cytokeratin 19 (monoclonal antibody recognizing BECs, 1:100; DAKO) or CD31 (monoclonal antibody recognizing endothelial cells, 1:100; DAKO), coupled with Jagged-1 (goat polyclonal, 1:50; Santa Cruz Biotechnologies, Inc.). Primary antibodies were incubated overnight at 4°C on 5-μm cryostat sections, and visualized using the alexaFluor conjugates on an Axiovert fluorescence microscope (CarlZeiss, UK). Images were captured using a digital camera and Axiovision software (Carl Zeiss, UK).
Histological Assessment
Staining of sections was assessed by two independent observers using a validated semiquantitative scale, where − denotes absence of staining; ±, occasional weak staining on some structures; +, weak staining; ++, moderate staining, and +++, strong staining. 24
Results
Semiquantitative RT-PCR Analysis of Notch Ligand Expression
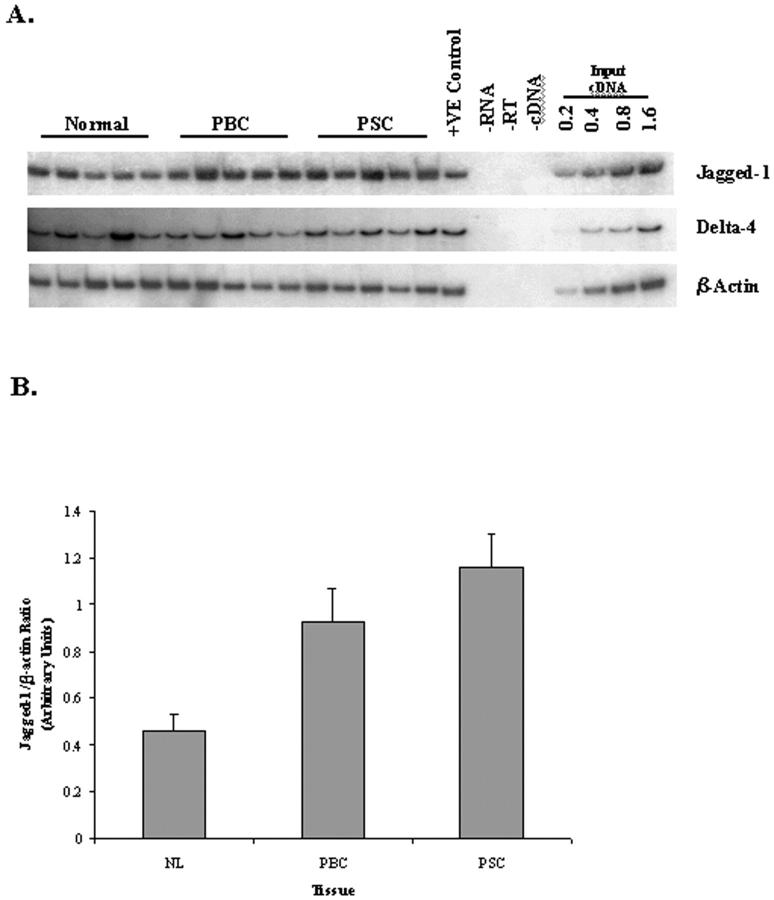
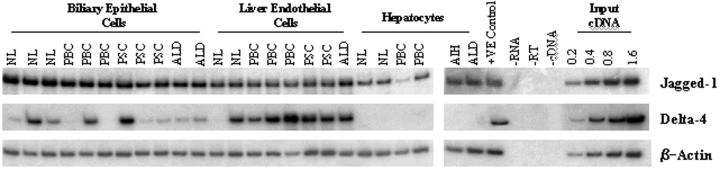
To identify which Notch ligand genes were expressed in the adult liver, semiquantitative RT-PCR was performed initially on total RNA extracted from normal and diseased (PBC and PSC) adult liver tissue. The expression of Jagged-1 and Delta-4 mRNA was seen in normal and diseased liver tissue (Figure 1A) ▶ . However, the expression of Jagged-2, Delta-1, and Delta-3 was not detectable (data not shown). Interestingly, the expression of Jagged-1 seemed to be elevated in liver tissue with PBC and PSC, when compared to normal liver (Figure 1B) ▶ and was statistically significant (PBC, P < 0.05; PSC, P < 0.01; paired Student’s t-test). The up-regulation in Jagged-1 mRNA expression was confirmed by performing RT-PCR on RNA extracted from a second sample of tissue from the same donor/patient groups (data not shown). We then investigated Jagged-1 and Delta-4 mRNA expression in cell isolates derived from normal and diseased liver tissue. Semiquantitative RT-PCR was performed on total RNA extracted from BECs, liver endothelial cells (LECs), and hepatocytes (Figure 2) ▶ . The Jagged-1 gene was detectable in all BECs, LECs, and hepatocyte cell isolates. Delta-4 mRNA however displayed a more interesting expression pattern. Although Delta-4 mRNA was undetectable in hepatocytes, it was strongly expressed in LECs, with the exception of one normal liver cell preparation. Delta-4 expression was also seen in BEC isolates although this expression was somewhat variable (Figure 2) ▶ .
Figure 1.
A: RT-PCR analysis of Jagged-1 and Delta-4 gene expression in normal and diseased liver tissue. First-strand cDNA prepared from total RNA extracted from NIH 3T3 cells expressing the human Jagged-1 gene was used as a positive and linearity control. For Delta-4 PCR, cDNA prepared from human umbilical vein endothelial cells was used as a positive and linearity control. B: Densitometric analysis of Jagged-1 gene expression in normal and diseased liver tissue. The graph shows values for Jagged-1 mRNA levels obtained by densitometry normalized with respect to β-actin signals. Data represents the mean densitometric value for Jagged-1 ±SEM (n = 5). A paired Student’s t-test shows that the data for PBC (P < 0.05) and PSC (P < 0.01) is significantly different.
Figure 2.
RT-PCR analysis of Jagged-1 and Delta-4 mRNA expression in cell isolates. RT-PCR analysis was performed on RNA extracted from primary cell cultures. NL, normal liver; AIH, autoimmune hepatitis.
Immunohistochemistry
To localize the expression of Jagged-1 within the adult liver immunohistochemistry was performed on normal and diseased liver tissue sections. Results are summarized in Table 2 ▶ . In normal liver most portal tract structures were positive for Jagged-1 immunoreactivity (Figure 3B ▶ , Table 2 ▶ ). Weak staining of hepatocyte membranes and the endothelium of hepatic veins was also seen in the parenchyma of normal liver tissue (Figure 3C ▶ , Table 2 ▶ ). In diseased liver, the immunolocalization of Jagged-1 in PBC, PSC, and alcoholic liver disease was similar. There was weak to moderate staining of bile ducts and portal vessels, as seen in normal liver. However, in portal and septal regions strong staining of bile ductules (Figure 3, D and F ▶ ; Table 2 ▶ ) and other structures that appeared to resemble small vessels was evident (Figure 3, E and F ▶ ; Table 2 ▶ ). In the parenchyma, Jagged-1 immunoreactivity was localized to hepatocyte cell membranes in all three diseases although this appeared to be slightly higher in PBC tissue (Table 2) ▶ .
Table 2.
Summary of Immunohistochemical Analysis for Jagged-1 Expression in Normal and Diseased Adult Liver Tissue
| Portal tract | Parenchyma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bile ducts | B.D. | Vessels | I.C. | Hepatocytes | Sinusoids | Hepatic veins | ||||
| P.V. | H.A. | S.N.V. | K cells | SECs | ||||||
| NORMAL | + | NA | ++ | ++ | NA | − | + | − | − | + |
| PBC | + | +++ | ++ | ++ | +++ | + | ++ | + | − | + |
| PSC | + | +++ | + | +++ | +++ | − | + | − | − | + |
| ALD | + | ++ | + | ++ | +++ | − | + | − | − | − |
B.D., bile ductules; H.A., hepatic artery; P.V., portal vein; I.C., inflammatory cells; S.N.V., small neovessels; K cells; Kupffer cells; SECS., sinusoidal endothelial cells; NA., not applicable.
−, Negative; +/−, occasional weak staining on some structures; +, weak staining; ++, moderate staining; +++, strong staining.
Figure 3.
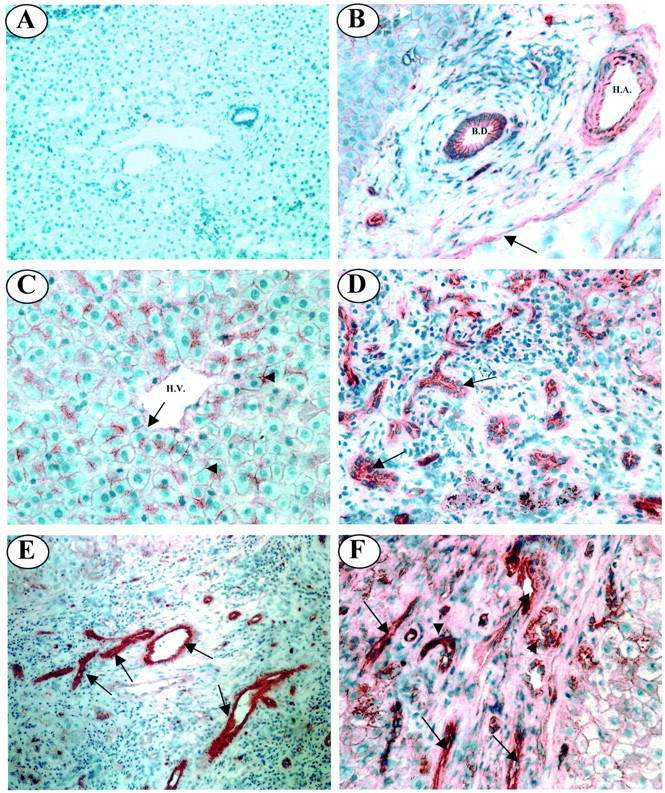
Immunolocalization of Jagged-1 expression in normal and diseased adult liver tissue. A: No primary antibody control. B: In the portal tract of normal liver tissue Jagged-1 staining is present on the endothelium of the portal vein (black arrow), together with the walls and endothelium of the hepatic artery. Jagged-1 expression is also evident on the epithelium of bile ducts. C: In the parenchyma of normal liver tissue, Jagged-1 is localized to the cell membrane of hepatocytes (arrowheads) and the endothelium of the hepatic vein (arrow). D: Strong Jagged-1 immunoreactivity is associated with bile ductules in PBC liver tissue (black arrows). E: In PSC liver strong Jagged-1 staining is seen on numerous small vessels in fibrous septa (black arrows). F: In alcoholic liver disease liver, strong Jagged-1 expression was evident on bile ductules (arrowheads) and numerous small vessels (black arrows) within the portal tract. B. D., bile duct; H. A., hepatic artery. Original magnifications: ×100 (A and E); ×200 (B, D, and F); ×400 (C).
To confirm that Jagged-1 expression was indeed localized to bile ductules in diseased tissue, dual-immunofluorescence staining using cytokeratin 19 (a biliary cell marker) and Jagged-1 was used. In normal tissue, both CK19 and Jagged-1 were found to stain the epithelial cells of bile ducts, but also co-localized to these cells (Figure 4A) ▶ . In PBC tissue, both markers were co-expressed by bile ductules (Figure 4C) ▶ . Jagged-1 was also seen to stain numerous structures in portal regions of diseased tissue that resembled small blood vessels (Figure 3E ▶ , Table 2 ▶ ). Immunofluorescence staining using CD31 (an endothelial cell marker) and Jagged-1 was performed to confirm that this was the case. In normal tissue, both CD31 and Jagged-1 co-localized to the vascular endothelium in portal regions (Figure 4B) ▶ . In PBC tissue, CD31 and Jagged-1 were co-expressed on numerous neovessel-like structures (Figure 4D) ▶ , confirming that they were indeed blood vessels.
Figure 4.
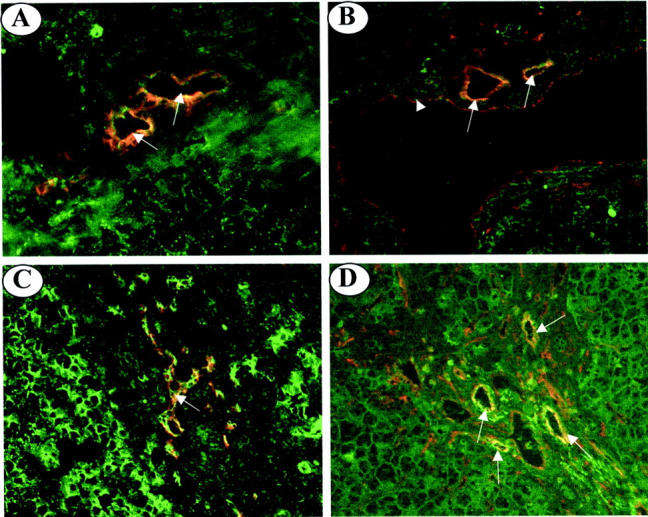
Dual-immunofluorescence staining of frozen liver sections from normal and PBC livers. A: In normal liver, double-immunofluorescent staining with CK19 (red fluorescence) and Jagged-1 (green fluorescence) reveals co-localization on bile ducts (yellow fluorescence, arrows). B: In normal liver, double-immunofluorescent staining with CD31 (red fluorescence) and Jagged-1 (green fluorescence) reveals co-localization (yellow fluorescence) to the endothelium of the hepatic arteries (arrows) and the endothelium of the portal vein (arrowhead). C: CK19 (red fluorescence) and Jagged-1 (green fluorescence) staining co-localizes to bile ductules (yellow fluorescence, arrow) in PBC liver tissue. D: CD31 (red fluorescence) and Jagged-1 (green fluorescence) co-localize (yellow fluorescence, arrows) to the endothelium of neovessels in the portal tract of PBC liver tissue (arrows). Original magnifications, ×200.
Semiquantitative RT-PCR Analysis of Downstream Modulators of Notch Signaling
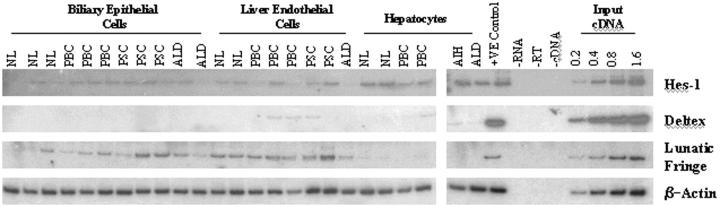
To gain insights into whether Notch signaling can occur between similar cell types in liver tissue, we have analyzed the expression of two downstream targets of Notch activation (Hes-1 and Deltex) in addition to an extracellular modulator of Notch activity (Lunatic fringe). We examined the expression of Hes-1, a basic helix-loop-helix transcription factor, directly regulated by the Notch signaling pathway. 25 The expression of Hes-1 was present in most of the cell isolates examined and did not display any restricted pattern of expression to a given cell type (Figure 5) ▶ . Deltex is a cytoplasmic protein that physically interacts with the Notch intracellular domain and may function as a positive regulator of Notch signaling. 26 No Deltex expression was evident in any BEC isolates, but weak expression was seen in two PBC and one PSC endothelial cell isolate (Figure 5) ▶ . Weak expression was also evident in hepatocyte isolates derived from diseased tissue (Figure 5) ▶ . Lunatic fringe (Lfng) is one of the vertebrate homologues of Drosophila Fringe and has recently been shown to function as a glycosyltransferase that regulates the activation Notch receptors by the various Notch ligands. 27,28 The expression of Lfng was detectable in most BEC isolates, but interestingly the levels were consistently higher in all LEC isolates. No LnFg expression was discernable in hepatocyte isolates (Figure 5) ▶ .
Figure 5.
The expression of downstream modulators of Notch signaling in cell isolates. RT-PCR analysis was performed on RNA extracted from primary cell cultures. First-strand cDNA prepared from total RNA extracted from Jurkat cells was used as a positive and linearity control for Deltex and Hes-1 PCR. For Lunatic Fringe PCR, cDNA prepared from human umbilical vein endothelial cells was used as a positive and linearity control. NL, normal liver; AIH, autoimmune hepatitis.
Discussion
In man five members of the Notch ligand family have been described to date. Notch signaling will occur only where both ligand and receptor are expressed. Our previous studies have revealed that the adult human liver expresses all four of the known Notch receptor genes. 18 Therefore, a comprehensive understanding of Notch signaling in the adult liver also requires the analysis of expression patterns for the ligands. We have examined the expression of the Notch ligands by quantitative RT-PCR and have found that the Jagged-1 and Delta-4 genes are both expressed in normal and diseased liver tissue whereas expression of Jagged-2, Delta-1, and Delta-3 was not detectable. Interestingly the expression of Jagged-1 was found to be significantly elevated in PBC and PSC livers when compared to normal livers.
To localize the expression of the two ligands in adult liver, we performed RT-PCR analysis on primary cell isolates. Jagged-1 expression was seen in all BECs, LECs, and hepatocyte isolates (Figure 2) ▶ . These findings were in agreement with the results obtained for Jagged-1 immunolocalization studies in which Jagged-1 expression was seen on bile ducts, hepatocytes, and the vasculature (Figure 3, B and C) ▶ . Furthermore, no Jagged-1 immunoreactivity was seen on the sinusoids, therefore, the Jagged-1 mRNA expression seen in LEC isolates is derived solely from vascular endothelium. Thus, our findings agree with and extend those of others, who have also been able to detect Jagged-1 expression in adult liver. 17
Although Delta-4 mRNA was absent in hepatocytes, it was clearly present in most BEC preparations examined (Figure 2) ▶ . The variability in intensity may reflect the fact that the cells (including the LEC preparations) were maintained in culture for up to 7 days. It is likely therefore that the expression may not accurately reflect in vivo levels. Nevertheless the demonstration that these purified human liver cells express this ligand is a significant step toward defining the role of the Notch receptor pathway in human hepatic biology. The relative contribution of vascular or sinusoidal endothelium to the strong Delta-4 mRNA expression in all but one of the LEC isolates is of particular interest. However because both cell types express CD31, the antigen used to isolate LECs and a specific antibody to Delta-4 is not yet available, this remains unresolved. 29
The reason for the observed increase in Jagged-1 gene expression (Figure 1) ▶ is not clear, but may possibly be because of the combination of two factors specific to the diseased tissues examined. Firstly, strong Jagged-1 expression is seen on bile ductules in PBC, PSC, and alcoholic liver disease livers (Figure 3, D and F) ▶ . Secondly, strong Jagged-1 expression was localized to small neovessels in the portal tracts of diseased livers (Figure 3E ▶ , Table 2 ▶ ). The strong expression of Jagged-1 on the aforementioned structures in diseased tissue may explain the increased mRNA expression seen in PBC and PSC liver tissue.
To gain further insight into the signaling events between similar cell types and the significance of the Jagged-1 and Delta-4 expression patterns, we analyzed the expression of genes for downstream targets and modulators of Notch activity. The expression of Hes-1, a transcription factor directly regulated by the Notch signaling pathway, was detectable in most cell isolates examined, indicating that signaling events were being transduced in BECs, LECs, and hepatocytes (Figure 5) ▶ . 25 In cell isolates, the absence of Delta-4 gene expression in hepatocytes suggests that Hes-1 gene induction is likely to be initiated by Jagged-1 (Figures 1 and 2) ▶ ▶ . It is possible that this is also the case in vivo, because no other Jagged-1-expressing structures appear to be in direct contact with parenchymal hepatocytes. The exact nature of the receptor(s) regulating Hes-1 gene activity is not clear. However, Notch-2 and -3 seem to be the most likely candidates because they are both expressed by hepatocytes in culture and in vivo. 18 The expression of Deltex was only detectable in two PBC and one PSC LEC isolate (Figure 5) ▶ . Deltex is thought to act by potentiating Notch signaling, and suggests that Deltex may possibly have a role in this cell type during disease. 30
Recent studies have shown that Lfng may differentially modify the Notch extra-cellular domain and thus alter events downstream of ligand binding by either potentiating or conversely inhibiting Notch receptor activation and signaling. 28 The co-expression of Jagged-1, Delta-4, and Lfng, together with Notch-1, -2, and -3 in normal BECs opens the way for complex signal modulation (Figures 2 and 5) ▶ ▶ . 18 Lfng inhibits Jagged-1 signaling through Notch-1, but potentiates signaling through Notch-2. 28 The effect of Lfng on Notch-3 function is not known. Interestingly, expression of Jagged-1 was seen on abnormal bile ductules in disease in the absence of Notch receptor expression, suggesting that normal signaling between bile duct epithelial cells is not occurring (Figure 3D) ▶ . 18 We are currently pursuing the hypothesis that loss of Notch signaling may in part be responsible for the abnormal growth and inability to form normal luminal structures during disease progression.
In the liver, characteristic processes associated with the disease states examined include neovascularization of portal regions and capillarization of the sinusoids. 31 Because the Notch signaling pathway plays a pivotal role in vascular development and homeostasis, 12,32,33 we speculate that neovascularization may be under the control of Notch signaling. 18 Several observations support this hypothesis. Together with increased Jagged-1 expression on these vessels (Figure 3, E and F) ▶ we previously reported that they also co-express Notch-2 and Notch-3. 18 Furthermore, the detection of Deltex and Lfng mRNA in LECs also suggests that active Notch signaling may be important in regulating the responses of these cells (Figure 5) ▶ . The expression of Lfng could for example potentiate signaling through Notch-2, which may in turn be able to override the normal inhibitory activity of Notch-3. 34
Our previous studies have also shown increased Notch-1 and -2 expression on sinusoidal endothelial cells in diseased tissue. 18 However, the absence of Jagged-1 on the sinusoids of diseased livers (Figures 3 and 4) ▶ ▶ suggests that another Notch ligand may be interacting with these receptors. It is reasonable to speculate that Delta-4 fulfills this role because it is expressed in the early embryonic vasculature, where it is thought to play a role in vascular morphogenesis. 32 Further studies are ongoing to address this interesting question.
In conclusion, these findings together with our previous analyses of Notch receptor expression, indicate that all of the necessary components for mediating functional Notch signaling are present in the normal adult liver. Dysregulation of signaling potential may well be an important contributing factor during ductule formation, sinusoidal capillarization, and the neovascularization of portal regions during disease.
Footnotes
Address reprint requests to Professor Alastair J Strain, Liver Research Laboratories, Clinical Research Block, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH, UK. E-mail: a.j.strain@bham.ac.uk.
Supported by the Wellcome Trust.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ: Notch signaling: cell fate control and signal integration in development. Science 1999, 284:770-776 [DOI] [PubMed] [Google Scholar]
- 2.Mumm JS, Kopan R: Notch signaling: from the outside in. Dev Biol 2000, 228:151-165 [DOI] [PubMed] [Google Scholar]
- 3.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J: TAN-1, the human homologue of the Drosophila gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991, 66:649-661 [DOI] [PubMed] [Google Scholar]
- 4.Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S: Human homologs of a Drosophila enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 1992, 2:119-127 [DOI] [PubMed] [Google Scholar]
- 5.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis E-A, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E: Notch3 mutations in CADASIL, an hereditary adult onset condition causing stroke and dementia. Nature 1996, 383:707-710 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Huang GM, Banta AB, Deng Y, Smith T, Dong P, Friedman C, Chen L, Trask BJ, Spies T, Rowen L, Hood L: Cloning, characterization, and the complete 56.8-kilobase DNA sequence of the human Notch4 gene. Genomics 1998, 51:51:45-58 [DOI] [PubMed] [Google Scholar]
- 7.Weinmaster G: The ins and outs of Notch signaling. Mol Cell Neurosci 1997, 9:91-102 [DOI] [PubMed] [Google Scholar]
- 8.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC: Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 1997, 16:235-242 [DOI] [PubMed] [Google Scholar]
- 9.Luo B, Aster JC, Hasserjian RP, Kuo F, Sklar J: Isolation and functional analysis of a cDNA for human Jagged2, a gene encoding a ligand for the Notch1 receptor. Mol Cell Biol 1997, 17:6057-6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray GE, Mann RS, Mitsiadis E, Henrique D, Carcangiu ML, Banks A, Leiman J, Ward D, Ish-Horowitz D, Artavanis-Tsakonas S: Human ligands of the Notch receptor. Am J Pathol 1999, 154:785-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD: Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet 2000, 24:438-441 [DOI] [PubMed] [Google Scholar]
- 12.Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL: Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev 2000, 14:1313-1318 [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Artavanis-Tsakonas S: The intracellular deletions of Delta and Serrate define dominant negative forms of the Drosophila Notch ligands. Development 1996, 122:2465-2474 [DOI] [PubMed] [Google Scholar]
- 14.Crosnier C, Attie-Bitachi T, Encha-Razavi F, Audollent S, Soudy F, Hadchouel M, Meunier-Rotival M, Vekemans M: JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 2000, 32:574-581 [DOI] [PubMed] [Google Scholar]
- 15.Dunwoodie SL, Henrique D, Harrison SM, Beddington RS: Mouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development 1997, 124:3065-3076 [DOI] [PubMed] [Google Scholar]
- 16.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Lin Kuo W, Cochran J, Costa T, Pierpont MEM, Rand EB, Picoli DA, Hood L, Spinner NB: Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997, 16:243-251 [DOI] [PubMed] [Google Scholar]
- 17.Louis AA, Van Eyken P, Haber BA, Hicks C, Weinmaster G, Taub R, Rand EB: Hepatic jagged-1 expression studies. Hepatology 1999, 30:1269-1275 [DOI] [PubMed] [Google Scholar]
- 18.Nijjar S, Crosby HA, Wallace L, Hubscher SG, Strain AJ: Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularisation. Hepatology 2001, 34:1184-1192 [DOI] [PubMed] [Google Scholar]
- 19.Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, McMaster P: Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest 1991, 87:1853-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joplin R, Strain AJ, Neuberger JM: Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell Dev Biol 1989, 25:1189-1192 [DOI] [PubMed] [Google Scholar]
- 21.Joplin R, Hishida T, Tsubouchi H, Daikuhara Y, Ayres R, Neuberger JM, Strain AJ: Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest 1992, 90:1284-1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson C, Clements D, Friday RV, Stott D, Woodland HR: Xsox17alpha and beta mediate endoderm formation in Xenopus. Cell 1997, 91:397-405 [DOI] [PubMed] [Google Scholar]
- 23.Brennan HC, Nijjar S, Jones EA: The specification and growth factor inducibility of the pronephric glomus in Xenopus laevis. Development 1999, 126:5847-5856 [DOI] [PubMed] [Google Scholar]
- 24.Adams DH, Hubscher SG, Shaw J, Johnson GD, Bubbs C, Rothlein R, Neuberger JM: Increased expression of ICAM-1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology 1991, 14:426-432 [PubMed] [Google Scholar]
- 25.Scott HS, Chen HS, Rossier C, Lalioti MD, Antonarakis S: Isolation of a human gene (HES1) with homology to an E. coli and a zebrafish protein which maps to chromosome 21q22.3. Hum Genet 1997, 5:616-623 [DOI] [PubMed] [Google Scholar]
- 26.Matsuno K, Eastman D, Mitsiades T, Quinn AM, Carcanciu ML, Ordentlich P, Kadesch T, Artavanis-Tsakonas S: Human Deltex is a conserved regulator of Notch signaling. Nat Genet 1998, 19:74-78 [DOI] [PubMed] [Google Scholar]
- 27.Johnston SH, Rauskolb C, Wilson R, Prabhakaran B, Irvine KD, Vogt TF: A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development 1997, 124:2245-2254 [DOI] [PubMed] [Google Scholar]
- 28.Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G: Fringe differentially modulates Jagged-1 and Delta1 signaling through Notch1 and Notch2. Nat Cell Biol 2000, 2:515-520 [DOI] [PubMed] [Google Scholar]
- 29.McNab G, Afford SC, Morland CM, Strain AJ, Joplin R, Adams DH: Cultured human hepatic sinusoidal endothelial cells express and secrete adhesion molecules and chemokines that are important for leukocyte recruitment to the liver. Wisse E Knook DL Balabaud C eds. Cells of the Hepatic Sinusoid. 1997, :pp 123-127 The Kupffer Cell Foundation, Leiden [Google Scholar]
- 30.Xu T, Artavanis-Tsakonas S: Deltex, a locus interacting with the neurogenic genes, Notch, Delta and Mastermind in Drosophila melanogaster. Genetics 1990, 126:665-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rappaport AM, McPhee PJ, Fisher MM, Phillips MJ: The scarring of the liver acini (cirrhosis). Tridimensional and microcirculatory considerations. Virchows Arch (A) 1983, 402:107-137 [DOI] [PubMed] [Google Scholar]
- 32.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T: Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 2000, 14:1343-1352 [PMC free article] [PubMed] [Google Scholar]
- 33.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T: Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 1999, 8:723-730 [DOI] [PubMed] [Google Scholar]
- 34.Beatus P, Lundkvist J, Oberg C, Lendahl U: The Notch-3 intracellular domain represses Notch-1 mediated activation through Hairy/Enhancer of Split(HES) promoters. Development 1999, 126:3925-3935 [DOI] [PubMed] [Google Scholar]