Abstract
The aim of the current study was to examine the influence of transforming growth factor (TGF)-β1 on proximal tubular epithelial cell-cell interaction, with particular emphasis on the regulation of adherens junction complex formation. Stimulation of the proximal tubular cell line HK-2 cells by TGF-β1 led to loss of cell-cell contact and disassembly of both adherens and tight junctional complexes. Adherens junction disassembly was associated with reduction of both Triton-soluble and Triton-insoluble E-cadherin, and an increase in detergent-soluble β-catenin. Under these conditions, immunoprecipitation and Western analysis demonstrated decreased association of β-catenin, both with E-cadherin, α-catenin, and the cell cytoskeleton. Confocal microscopy after immunostaining, showed decreased intensity of peripheral E-cadherin staining, and redistribution of β-catenin expression to a perinuclear location. Tight junction disassembly was manifest by a reduction in the expression of Triton-soluble occludin and ZO-1 by Western analysis and their disassociation manifested by immunostaining and confocal microscopy. Loss of cell-cell contact and disassembly of adherens junctions were seen after addition of TGF-β1 to the basolateral aspect of the cells. Immunoprecipitation experiments demonstrated co-localization of E-cadherin, β-catenin, and TGF-β1 RII in unstimulated cells. After TGF-β1 stimulation, the TGF-β1 RII no longer associated with either E-cadherin or β-catenin. Dissociation of the adherens junction protein from the TGF-β1 receptor was associated with increased β-catenin tyrosine phosphorylation and decreased threonine phosphorylation. Furthermore after receptor ligand binding, β-catenin became associated with the TGF-β1-signaling molecules Smad3 and Smad4.
It is now clear in all renal diseases, that the progression of renal insufficiency is closely correlated to the degree of renal interstitial fibrosis. 1,2 Epithelial cells of the proximal tubule have the potential to contribute to the pathogenesis of renal fibrosis by the production of profibrotic growth factors such as transforming growth factor-β1 (TGF-β1), 3-5 and may also influence the turnover of the adjacent extracellular matrix. Recent work suggests that these cells may express fibroblast-specific markers in vitro and in vivo, 6 and furthermore de novo expression of α-smooth muscle actin (α-SMA), a marker of myofibroblast phenotype, by proximal tubular cells (PTCs), may be associated with disruption of the tubular basement membrane and migration of these cells into the corticointerstitium. 7 In vivo PTC form a polarized monolayer whose integrity is maintained by the physical interactions of neighboring cells through intercellular junctional complexes. Regulation of PTC cell-cell contact will therefore influence their migration and influence pathological events in the renal interstitium. Although there is extensive work characterizing the functional aspect of TGF-β1-mediated alterations in epithelial cell function, much less is known of the mechanism by which it affects cell-cell contact and monolayer integrity.
Intercellular junctions are important sites of regulation of cell function. Under certain physiological conditions such as wound healing or tissue morphogenesis, cell junctions may be disrupted thus allowing cell migration. Epithelial cells have discrete specialized regions of cell-cell adhesion comprising the tight junction, which forms the main barrier to paracellular traffic and adherens junctions. Adherens junctions are composed of cadherin-catenin complexes linked to the actin cytoskeleton. In the epithelial cell E-cadherin, a single pass trans-membrane molecule, mediates Ca2+-dependent homotypic interactions between adjacent cells. The cytoplasmic tail of the cadherins binds either β- or γ-catenin. α-Catenin binds with β-catenin and γ-catenin, forming the link with the actin cytoskeleton. 8 Evidence now suggests that the adherens junction is an important target for the regulation of intracellular signaling pathways. 9 Furthermore other junctions such as the tight junctions, are dependent on previous formation of the adherens junction, and may therefore be subject to indirect modulation by adherens junction function. 10 Data obtained in the Drosophila and Xenopus systems suggest an additional cadherin adhesion-independent role for β-catenin involving its translocation to the nucleus, preceded by its accumulation in a stabilized form in the cytoplasm. 11,12 Subsequent studies have also demonstrated accumulation of a pool of cytoplasmic β-catenin during human epithelial cell migration. 13,14 Generation of stabilized cytoplasmic β-catenin has therefore been implicated in transcriptional regulation of specific genes particularly those involved in embryonic development and cell differentiation.
In the current study we have examined the effect of TGF-β1 on cell-cell contact and in particular on its regulation of adherens complex structure. In addition we have investigated that mechanism by which this occurs. The results suggest that alterations in epithelial cell morphology on TGF-β1 stimulation are associated with adherens junction disassembly, loss of attachment from the cell cytoskeleton, and an increase in the stabilized cytoplasmic pool of β-catenin. Furthermore we show that these events are polarized and likely to be the result of the co-localization of the TGF-β1 type II receptor with the adherens junction complex. Activation of the latter results in the generation of a stabilized form of β-catenin that becomes associated to the TGF-β1-signaling molecule Smad4. Recent studies suggest that there are co-operative effects in terms of cell signaling mediated by the TGF-β1 and Wnt pathways. The data thus supports such an association in renal proximal tubular epithelial cells.
Materials and Methods
Materials and Antibodies
Murine monoclonal anti-cytokeratin was purchased from DAKO (Cambridgeshire, UK). Mouse monoclonal antibody against human E-cadherin and α-catenin were purchased from Transduction Laboratories (Lexington, KY). Rabbit polyclonal antibody recognizing a 69-kd fusion protein corresponding to amino acids 463 to 1109 of human ZO-1 cDNA and rabbit monoclonal anti-mammalian β-catenin and occludin were obtained from Zymed Laboratories Inc. (San Francisco, CA). Rabbit polyclonal antibody against TGF-β receptor II and mammalian Smad2, Smad3, and Smad4 were purchased from Santa Cruz Biotechnology, Inc. (Wiltshire, UK). Mouse monoclonal anti-phosphothreonine antibody and anti-SMA antibody were purchased from Sigma (Poole, UK). Mouse monoclonal anti-phosphotyrosine antibody was purchased from Upstate Biotechnology (Buckingham, UK). For immunoblotting, peroxidase-conjugated secondary antibodies that are reactive with rabbit or mouse immunoglobulins were purchased from Sigma. For immunofluorescence, fluorescein isothiocyanate-conjugated antibodies against rabbit or mouse immunoglobulins (Sigma) were used. Protein A Sepharose was also purchased from Sigma.
Cell Culture
HK-2 cells (human renal proximal tubular epithelial cells immortalized by transduction with human papilloma virus 16 E6/E7 genes 15 ) were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum (Biological Industries Ltd., Cumbernauld, UK), glutamine (Life Technologies), Hepes buffer (Gibco BRL, Paisley, UK), insulin, transferrin, and sodium selenite (Sigma). Cells were grown at 37°C in 5% CO2 and 95% air. Fresh growth medium was added to cells every 3 to 4 days until confluent. Cells were grown to confluence and serum-deprived for 48 hours before experimental manipulation. In all experiments cells were stimulated with recombinant TGF-β1 under serum-free conditions.
For the determination of polarity of the effects of TGF-β, cells were seeded onto porous tissue culture inserts (0.4-μm pore size) (Becton Dickinson, Oxford, UK). Re-assembly of junctional complexes and integrity of the monolayer were demonstrated by serial measurements of transepithelial resistance using a commercially available system (Millicell-ERS Resistance System, Millipore) as previously described. 3 At peak trans-epithelial cell resistance, cells were serum-deprived for 72 hours before application of recombinant TGF-β1 to either the apical or basolateral compartments.
Immunoblotting and Western Analysis
Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of HK-2 cells for up to 4 days. Cells were washed once with phosphate-buffered saline (PBS) at each time point before preparation of cell lysates for immunoblot analysis. Total cell lysates were obtained by washing cells once with cold PBS, cells were subsequently detached by scraping into 5 ml of cold PBS. After centrifugation at 2500 rpm for 10 minutes, cell pellets were mixed with sodium dodecyl sulfate (SDS) sample buffer (reducing sample buffer), and stored at 20°C until use. To obtain Triton-soluble and -insoluble cell fractions, the confluent monolayers were washed once with cold PBS, scraped, and rinsed into 5 ml of cold PBS. After centrifugation at 2500 rpm for 10 minutes, cell pellets were extracted in buffer (150 mmol/L NaCl, 50 mmol/L Tris-Cl, 0.01% NaN3, 2 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L sodium orthovanadate, 10 μg/ml leupeptin, 25 μg/m aprotinin) containing 1% Triton X-100 (TX buffer) for 30 minutes on ice. Samples were centrifuged at 12,500 rpm for 30 minutes and then the supernatant (Triton-soluble component including membrane and cytosolic fraction) was transferred to a separate tube and kept at −70°C until use. The remaining pellets were repeatedly pipetted in the same buffer containing 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS and kept on ice for 30 minutes. Then, samples were centrifuged at 12,500 rpm for 30 minutes. The supernatant (Triton-insoluble component containing cytoskeleton) was kept at −70°C till use. To optimize immunoblot analysis, the volume of lysis buffer was minimized by lysis of cells after detachment. Direct cell lysis did not affect the expression of the proteins assessed (data not shown).
Immunoblot analysis of lysate samples was performed by standard methodologies. Briefly cell extracts were prepared in SDS sample buffer and boiled for 5 minutes at 95°C. Cell protein (100 μg) were loaded onto 5 to 10% SDS-polyacrylamide gel electrophoresis gradient gels and electrophoresis performed under reducing conditions according to the procedure of Laemmli. 16 After electrophoresis the separated proteins were transferred to a nitrocellulose membrane (Amersham, Little Chalfont, UK). The membrane was blocked with Tris-buffered saline containing 5% nonfat powdered milk for 1 hour and then incubated with the appropriate primary antibody (see Materials and Antibodies) in Tris-buffered saline containing 1% bovine serum albumin and 0.1% Tween 20 (Tris-buffered saline-Tween) for 1 hour at room temperature. The blots were subsequently washed in Tris-buffered saline-Tween and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (Sigma) in Tris-buffered saline-Tween. Proteins were visualized using enhanced chemiluminescence (Amersham) according to the manufacturer’s instructions.
Immunoprecipitation
Immunoprecipitation was performed by standard methodologies. Briefly cell protein samples were precleared with 50 μl of packed protein A crosslinked 4% beaded agarose (Sigma) at 4°C for 1 hour. The beads were removed by centrifugation (13,000 rpm, 10 minutes) and the supernatant collected. Primary antibody (2 μg/ml) was added to the cleared supernatant and incubated at 4°C with constant mixing for 12 hours. The immune complex was captured by the addition of packed agarose protein A beads (50 μl/500 μl supernatant) for 2 hours at 4°C. Separation of the beads was achieved by centrifugation (13,000 × g for 25 minutes) and the supernatant removed. Subsequently samples were subject to immunoblot/Western analysis as described above. Specificity of immunoprecipitation was confirmed by negative control reactions performed with either no primary antibody or rabbit IgG control.
Immunohistochemistry
Cells were grown in 8-well multichamber slides (In Vitrogen Ltd., Paisley, UK) under serum-free media for 48 hours and then stimulated with recombinant TGF-β (10 ng/ml) for up to 4 days. At each time point, cells were rinsed three times in PBS for 5 minutes each, before fixation in 3% paraformaldehyde for 15 minutes at room temperature and subsequent permeabilized with 0.2% Triton in PBS for 5 minutes at room temperature. After a blocking step (1% bovine serum albumin/PBS for 1 hour), cells were incubated with the primary antibodies (1:80 dilution of murine monoclonal anti-cytokeratin; 1:100 dilution of rabbit anti-TGF-β1 receptor II and mouse anti-E-cadherin; 1:150 dilution of rabbit anti-β-catenin, ZO-1, and occludin) for 1 hour at room temperature followed by the incubation of fluorescein isothiocyanate-conjugated secondary antibodies [1:50 dilution of goat anti-mouse immunoglobulin (Sigma) or 1:30 dilution of rabbit anti-mouse immunoglobulin (DAKO); 1:80 dilution of goat anti-rabbit immunoglobulin (Sigma)]. After washing with PBS, cells were mounted with fluorSave reagent (Calbiochem, Nottingham, UK) and analyzed by confocal microscope (Leica TCS 4D).
Results
Morphological Alterations
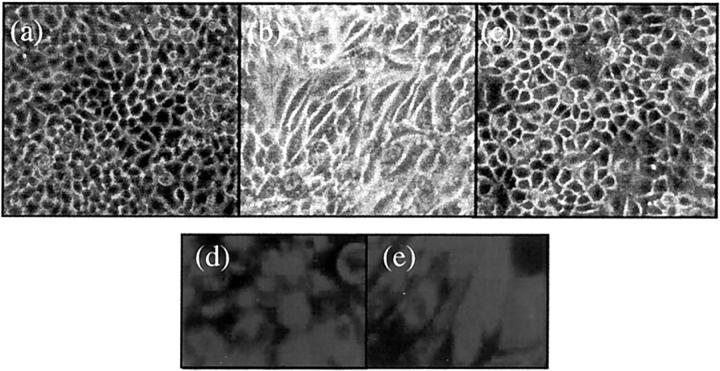
Addition of recombinant TGF-β1 (10 ng/ml) to serum-deprived confluent monolayers of HK-2 cells resulted in a marked alteration in cell morphology (Figure 1) ▶ . Cells developed marked hypertrophy, becoming elongated and spindle-shaped and losing the cobblestone pattern. These effects were apparent within 2 days of addition of TGF-β1. Alterations in cell phenotype were reversible on removal of TGF-β1, suggesting that TGF-β1 did not induce a stable alteration or terminal differentiation of cell phenotype (Figure 1c) ▶ .
Figure 1.
Morphological alterations after TGF-β1 stimulation. Appearance of control HK-2 cells grown on a glass surface without any treatment after serum deprivation for 7 days (a). Cells grow as a typical cobblestone monolayer. Stimulation of confluent monolayers of HK-2 cells with recombinant TGF-β1 (10 ng/ml) under serum-free conditions results in marked changes in cell morphology at day 2 (b). Cells lose their regular cuboidal appearance becoming elongated and spindle shaped. Reversibility of incorporation of α-SMA into stress fibers was determined by removal of supernatant containing TGF-β1 after 72 hours, before washing the cells with phosphate-buffered saline. Subsequently medium supplemented with 10% fetal calf serum was added to the cells for a further 48 hours (c). By immunohistochemistry, both unstimulated (d) and TGF-β1-treated (e) HK-2 cells stain positively for the epithelial cell marker cytokeratin.
TGF-β1-Mediated Alterations in Adherens Junction Organization
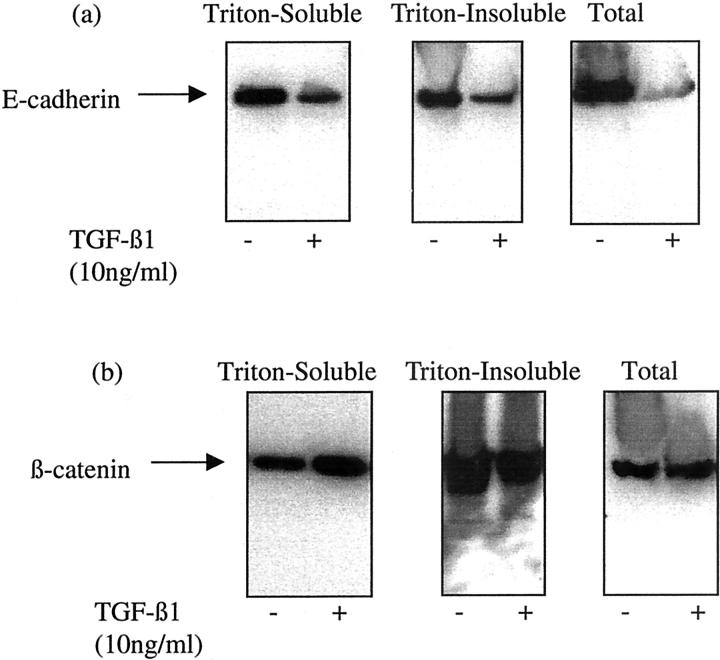
Western blot analysis of cell extract after stimulation of confluent monolayers of cells with TGF-β1 demonstrated decreased Triton-soluble (membrane and cytoplasmic), Triton-insoluble (cytoskeletal) and total cell-associated E-cadherin (Figure 2a) ▶ . These effects were first apparent on day 2 after addition of TGF-β1, and were maximal by day 4. In contrast there was an increase in the expression of the Triton-soluble β-catenin, and a concomitant decrease in the Triton-insoluble fraction with no change in its total expression (Figure 2b) ▶ . The time course of alterations in β-catenin expression mirrored those observed for E-cadherin, with maximal alterations in expression seen at day 4 after addition of TGF-β1.
Figure 2.
TGF-β1-induced alteration in E-cadherin and β-catenin expression. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3, cells were either lysed by the addition of SDS sample buffer (total lysates), or extracted by the sequential addition of 1% Triton alone (Triton-soluble extract) and 1% Triton/0.5% deoxycholate and 0.1% SDS (Triton-insoluble extract) as described in Materials and Methods. Subsequently Western blot analysis for either E-cadherin (a) or β-catenin (b), of each of the extract samples was performed by standard methodologies. In control experiments serum-free medium alone was added to the confluent monolayers before lysis or sequential extraction. One representative experiment of at least four individual replicate experiments is shown.
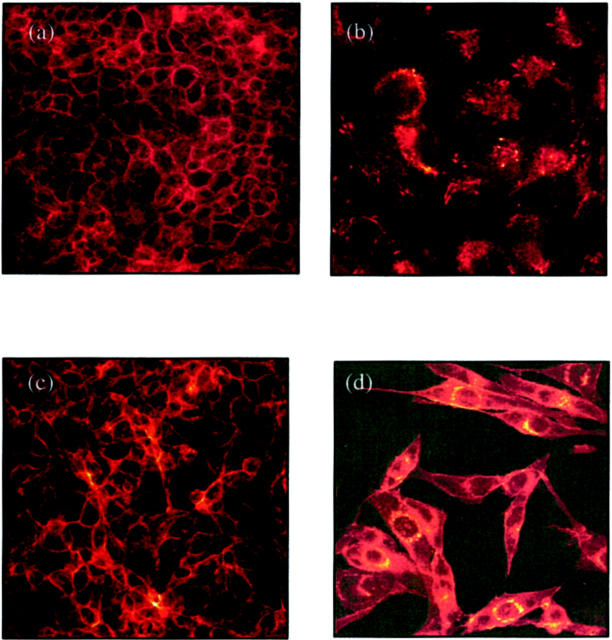
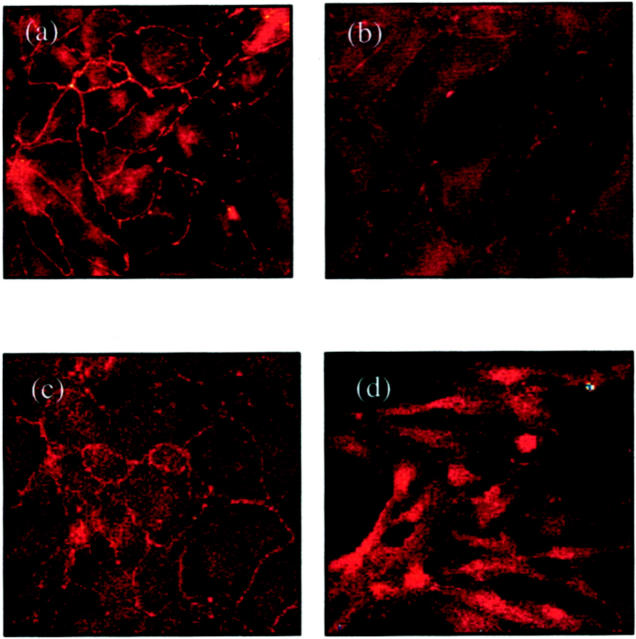
Examination of fluorescence immunostaining for E-cadherin under a confocal laser scanning microscope clearly outlined cell contours in control cultures (Figure 3a) ▶ . Immunofluorescence of E-cadherin decreased and became discontinuous after incubation with TGF-β1 (Figure 3b) ▶ . Immunostaining for β-catenin also clearly outlined cell contours in unstimulated cells (Figure 3c) ▶ . After addition of TGF-β1 there was a marked increase in β-catenin expression in the perinuclear region (Figure 3d) ▶ .
Figure 3.
Immunohistochemistry for E-cadherin (a and b) and β-catenin (c and d). Confluent monolayers of HK-2 cells were stimulated with TGF-β1 for 4 days before fixation and analysis by immunohistochemistry as described in Materials and Methods. Under control conditions (serum-free medium alone) both E-cadherin (a) and β-catenin (c) clearly outline the cell contour. After stimulation with recombinant TGF-β1 E-cadherin staining intensity becomes weaker and discontinuous (b). β-catenin staining after addition TGF-β1 demonstrates a relocation from the cell periphery to a perinuclear location (d).
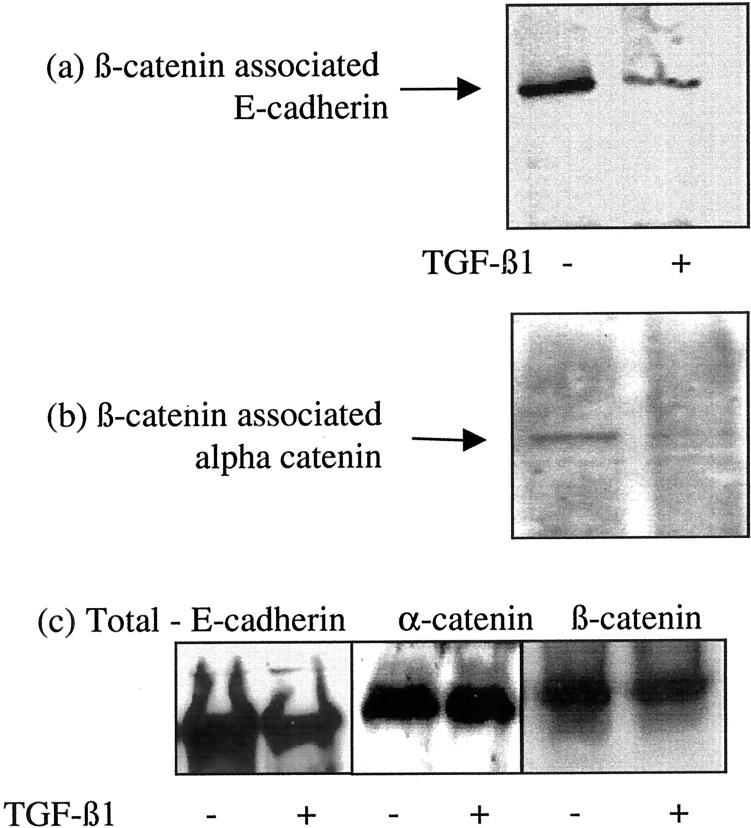
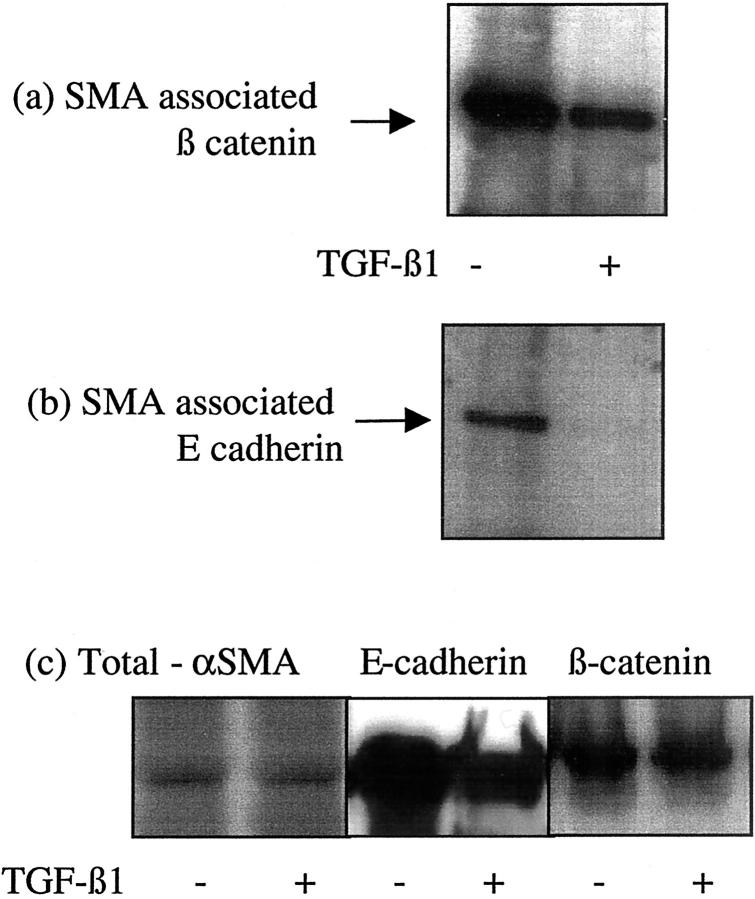
The relationship of the E-cadherin-β-catenin-α-catenin complex was examined by immunoprecipitation of β-catenin followed by analysis of its association with either E-cadherin or α-catenin by immunoblot. Immunoprecipitation of β-catenin resulted in isolation of both E-cadherin and β-catenin in unstimulated cells (Figure 4a) ▶ . After incubation with TGF-β1 there was a marked reduction in the co-immunoprecipitation of E-cadherin and β-catenin suggesting dissociation of the E-cadherin-β-catenin link (Figure 4a) ▶ . Previous studies have demonstrated that β-catenin may be linked to the cell cytoskeleton via α-catenin. Immunoprecipitation of β-catenin confirmed co-immunoprecipitation of these proteins in unstimulated cells (Figure 4b) ▶ . Addition of TGF-β1 followed by immunoprecipitation of β-catenin resulted in reduction of β-catenin-α-catenin co-immunoprecipitation (Figure 4b) ▶ . Stimulation with TGF-β1 did not influence α-catenin protein expression in the total cell lysates (Figure 4c) ▶ . Immunoprecipitation with antibodies to the cytoskeletal protein α-SMA also demonstrated decreased association of both β-catenin and E-cadherin with the cell cytoskeleton after incubation with TGF-β1 (Figure 5) ▶ . There was no change in the expression of α-SMA in the total cell lysates after TGF-β1 stimulation.
Figure 4.
Association of β-catenin with E-cadherin and α-catenin. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3 β-catenin was immunoprecipitated as described in Materials and Methods, and E-cadherin (a) or α-catenin (b) expression in the precipitate examined by Western analysis. For comparison total E-cadherin, α-catenin, and β-catenin expression is shown (c). In control experiments serum-free medium was added to the confluent monolayers on cells. One representative experiment of at least four individual replicate experiments is shown.
Figure 5.
Association of β-catenin with the actin cytoskeleton. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3 α-SMA was immunoprecipitated as described in Materials and Methods, and β-catenin (a) or E-cadherin (b) expression in the precipitate examined by Western analysis. For comparison total α-SMA, E-cadherin, and β-catenin expression is shown (c). In control experiments serum-free medium was added to the confluent monolayers on cells. One representative experiment of at least four individual replicate experiments is shown.
Influence of TGF-β1 on the Phosphorylation of β-Catenin
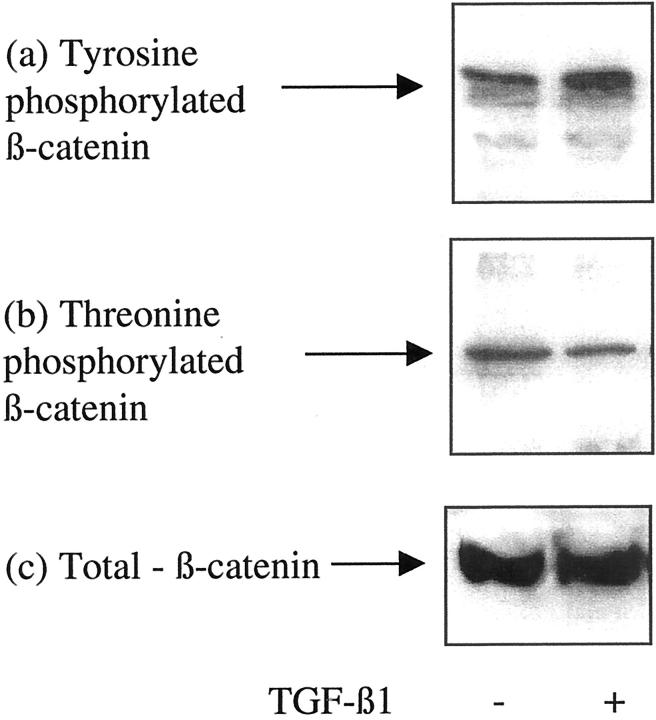
After phosphotyrosine or phosphothreonine immunoprecipitation, in the current study, β-catenin phosphorylation status was examined by immunoblot analysis. TGF-β1 increased β-catenin tyrosine phosphorylation (Figure 6a) ▶ . In contrast after addition of TGF-β1, β-catenin was dephosphorylated on threonine residues (Figure 6b) ▶ . This suggests that TGF-β1-increased stability of cytoplasmic β-catenin is associated with both an increase in tyrosine phosphorylation and dephosphorylation on threonine residues.
Figure 6.
Influence of TGF-β1 on β-catenin phosphorylation status. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3 phosphotyrosine (a) or phosphothreonine (b) antibodies were used for immunoprecipitation as described in Materials and Methods, and β-catenin expression in the immunoprecipitate examined by Western analysis. For comparison, total β-catenin expression is shown (c). In control experiments serum-free medium was added to the confluent monolayers on cells. One representative experiment of at least four individual replicate experiments is shown.
TGF-β-Mediated Alterations in Tight Junction Assembly
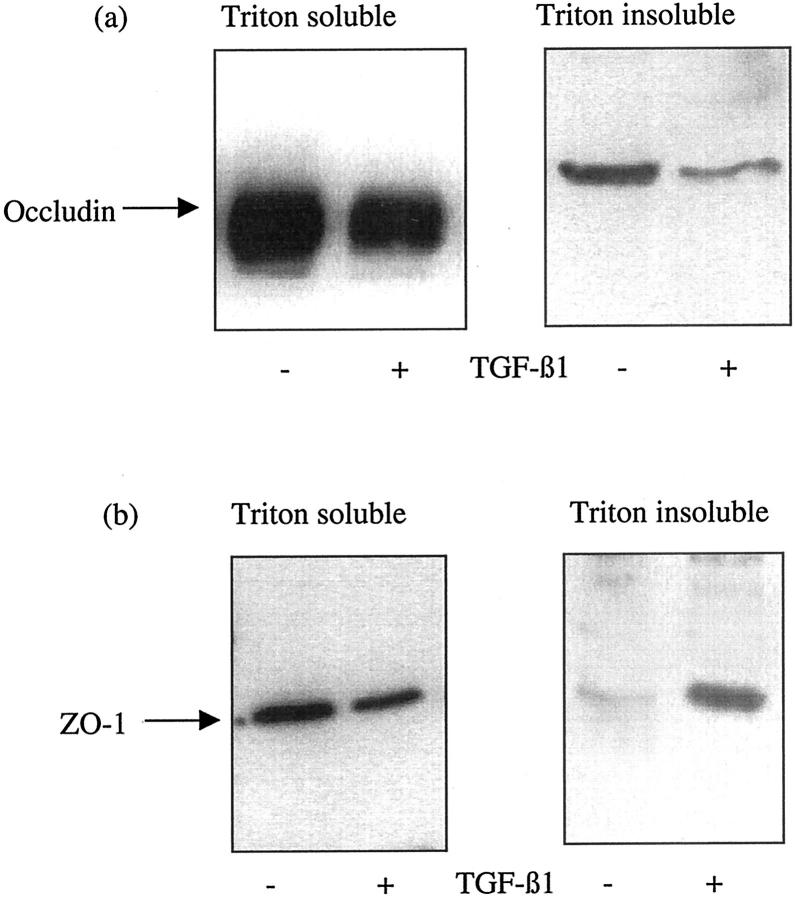
Regulation of tight junction formation was examined by assessment of the expression of occludin and ZO-1 protein by immunoblotting and their cellular location by immunostaining. Western analysis demonstrated decreased expression of both occludin and ZO-1 in the Triton-soluble (membrane and cytoplasmic) fraction. Occludin expression also decreased in the Triton-insoluble (cytoskeletal) fraction. In contrast ZO-1 expression increased in the insoluble fraction (Figure 7) ▶ . In control cells the cell outline was clearly outlined by fluorescence staining for occludin and ZO-1 (Figure 8) ▶ . Occludin expression decreased and the staining around the periphery of the cell became discontinuous, whereas ZO-1 immunostaining demonstrated a relocation of this protein to the cell cytoplasm.
Figure 7.
TGF-β1-induced alteration in occludin and ZO-1 expression. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3, cell proteins were extracted by the sequential addition of 1% Triton alone (Triton-soluble extract) and 1% Triton/0.5% deoxycholate and 0.1% SDS (Triton-insoluble extract) as described in Materials and Methods. Subsequently Western blot analysis for either occludin (a) or ZO-1 (b), of each of the extract samples was performed by standard methodologies. In control experiments serum-free medium alone was added to the confluent monolayers before lysis or sequential extraction. One representative experiment of at least four individual replicate experiments is shown.
Figure 8.
Immunohistochemistry for occludin (a and b) and ZO-1 (c and d). Confluent monolayers of HK-2 cells were stimulated with TGF-β1 for 4 days before fixation and analysis by immunohistochemistry as described in Materials and Methods. Under control conditions (serum-free medium alone) both occludin (a) and ZO-1 (c) clearly outline the cell contour. After stimulation with recombinant TGF-β1, occludin staining intensity becomes weaker and discontinuous (b). ZO-1 staining after the addition TGF-β1 demonstrates relocation from the cell periphery into the cell cytoplasm (d).
Polarity of TGF-β1-Dependent Adherens Junction Disassembly
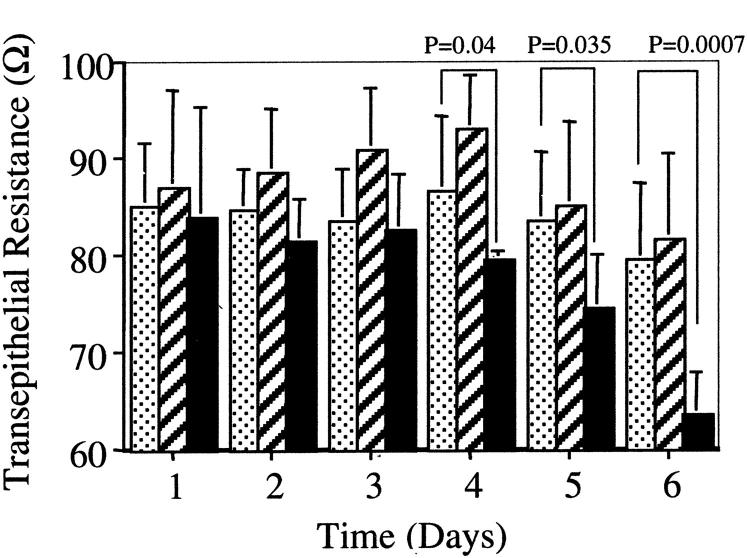
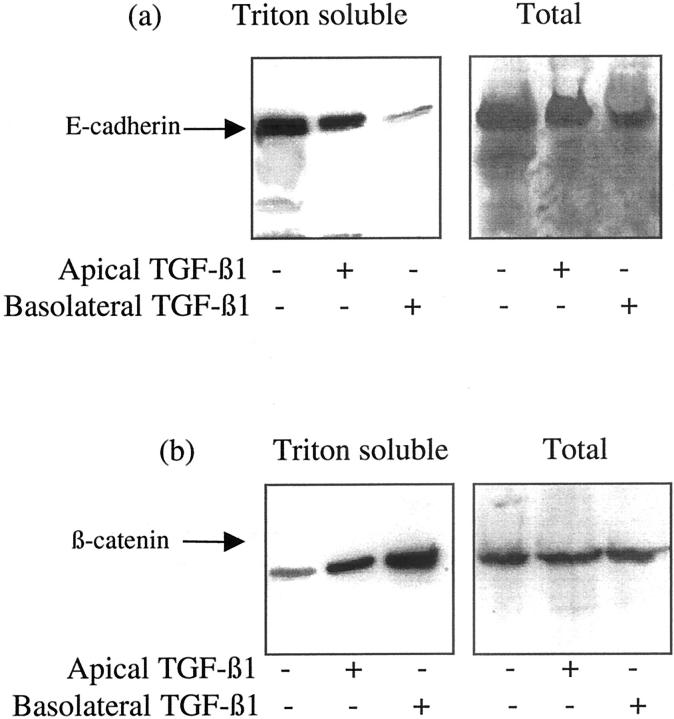
In vivo, PTCs exist as polarized monolayers. We have previously demonstrated that this cell type may respond in a polarized way to the application of stimuli. 17,18 Polarity of TGF-β1-induced loss on cell-cell contacts and disassembly of adherens junctions was examined by the culture of cells on porous tissue culture insert. At confluence TGF-β1 was applied to either the apical or basolateral aspect of the cells. Loss of cell-cell contact and integrity of the cell monolayer was assessed by serial determination of transepithelial resistance. A significant reduction in transepithelial resistance, relative to the unstimulated controls, and cells stimulated on their apical aspect, was seen when TGF-β1 was added to the basolateral aspect of the cells (Figure 9) ▶ . Similarly immunoblot analysis of cell protein extracts demonstrated greater reduction in Triton-soluble and total E-cadherin expression, and increase in Triton-soluble β-catenin expression after basolateral addition of TGF-β1 (Figure 10) ▶ .
Figure 9.
Polarity of TGF-β1-mediated alteration in transepithelial resistance. HK-2 cells were grown to confluence on porous tissue culture inserts as described in Materials and Methods. Subsequently TGF-β1 was added to either the apical (hatched bars) or basolateral (black bars) compartment on day 1. In control experiments serum-free medium alone was added to both compartments (dotted bars). Subsequently transepithelial resistance was determined daily. Each reading represents triplicate determinants of six independent experiments.
Figure 10.
Polarity of TGF-β1-mediated alteration in E-cadherin and β-catenin expression. HK-2 cells were grown to confluence on porous tissue-culture inserts as described in Materials and Methods. Subsequently TGF-β1 was added to either the apical or basolateral compartment and either the total cell protein or Triton-soluble protein extracted as described in Materials and Methods before analysis of either E-cadherin (a) or β-catenin (b) by Western analysis. One representative experiment of at least four individual replicate experiments is shown.
Characterization of the Association between the TGF-β Receptor and the Adherens Junction Complex
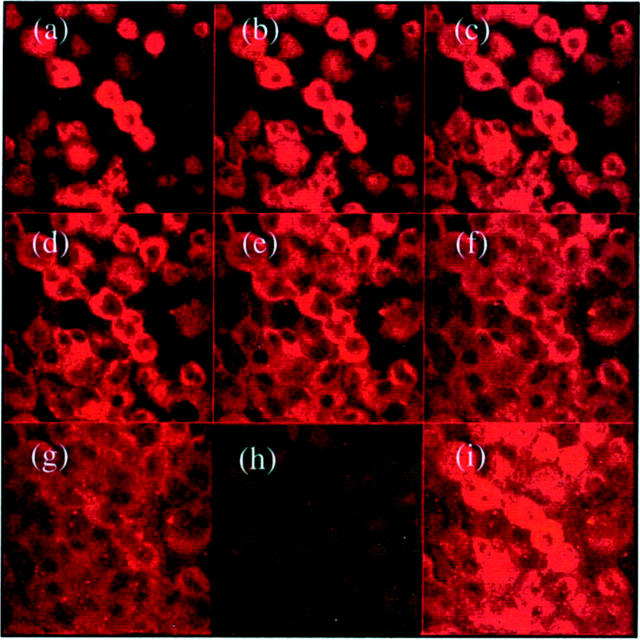
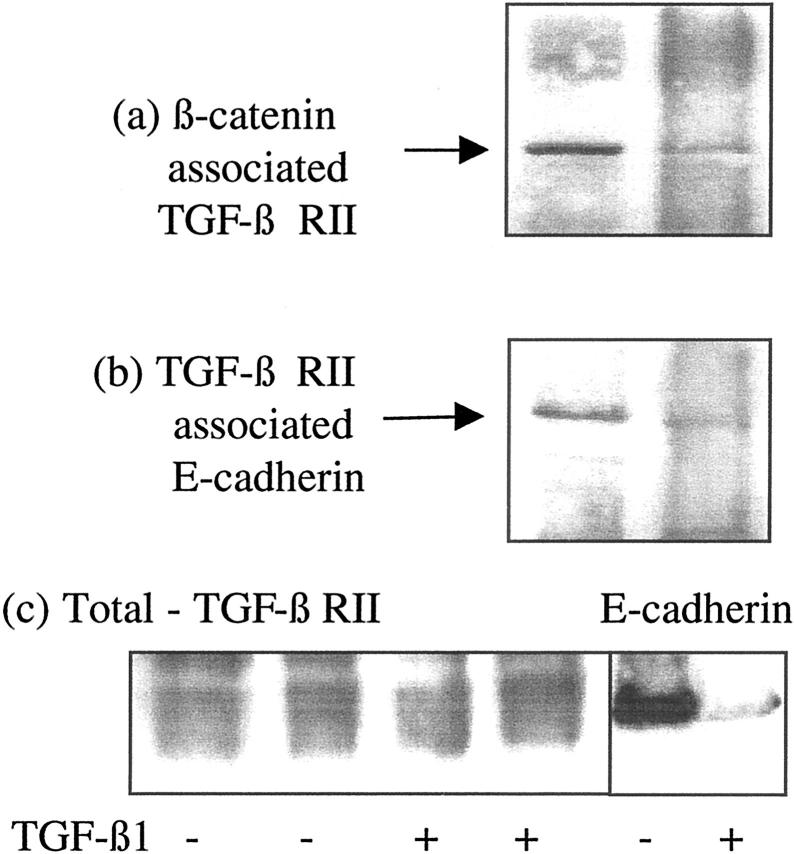
In unstimulated cells TGF-β RII immunostaining was detected on both apical and basolateral aspects of the cells, thus polarity of TGF-β1-mediated adherens junction disassembly could not be explained by asymmetric distribution of its receptor (Figure 11) ▶ . Specificity of the TGF-β1 RII antibody was confirmed by blocking immunohistochemical staining with the peptide used for immunization (Figure 11) ▶ . This suggested the possibility that polarity of TGF-β1 response may be the result of TGF-β1 influencing adherens junction disassembly via a subpopulation of TGF-β1 receptors accessible only from the basolateral aspect of the cell. The association of TGF-β1 receptors specifically with the adherens junction could explain this observation, as the intact tight junction would prevent the access of apically applied TGF-β1 to E-cadherin-associated receptor. The relationship between TGF-β1 RII and E-cadherin-β-catenin was therefore determined by immunoprecipitation of either TGF-β1 RII or β-catenin and immunoblot analysis of E-cadherin (Figure 12) ▶ . In control unstimulated cells, immunoprecipitation of either β-catenin or TGF-β1 RII resulted in co-immunoprecipitation of E-cadherin, thus confirming an association between the TGF-β1 receptor and the adherens junction complex. After incubation with TGF-β1, these proteins were no longer co-immunoprecipitated demonstrating disassociation of TGF-β1 RII from the adherens junction proteins, as disassembly of the adherens junction occurred. Addition of TGF-β1 did not alter the expression of TGF-β1 RII protein in the total cell lysates (Figure 12c) ▶ .
Figure 11.
Immunhistochemical localization of TGF-β type II receptor expression. Confluent growth-arrested monolayers of HK-2 cells were fixed before analysis of TGF-β type II receptor by immunohistochemistry as described in Materials and Methods. Subsequently localization of fluorescence was examined in eight 0.8-μm serial sections from the apical to the basolateral aspect of the cell using the confocal microscope (a–g). In addition a composite image of the superimposed images was generated after immunostaining in the presence (h) or absence (i) of blocking peptide.
Figure 12.
Association of β-catenin with the TGF-β1 RII. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3 β-catenin was immunoprecipitated as described in Materials and Methods, and TGF-β1 RII expression in the precipitate was examined by Western analysis. For comparison total TGF-β RII and E-cadherin expression is shown (c) In control experiments serum-free medium was added to the confluent monolayers on cells. One representative experiment of at least four individual replicate experiments is shown.
Association of the TGF-β1/Smad and Wnt/β-Catenin Signaling Pathways
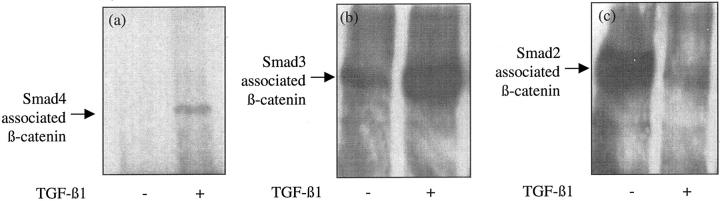
To define the relationship between adherens junction disassembly and TGF-β1 signaling, we examined alterations in the association of Smad2, Smad3, or Smad4, and β-catenin induced by TGF-β1 stimulation. After incubation of cells with TGF-β1, immunoprecipitation with Smad4 antibody and immunoblot analysis with the β-catenin antibody demonstrated a marked increase in the co-immunoprecipitation of the two proteins (Figure 13a) ▶ . Similarly there was increased co-immunoprecipitation of Smad3 and β-catenin after incubation of confluent cell monolayers with TGF-β1 (Figure 13b) ▶ . In contrast there was a decrease in association of Smad2 and β-catenin after addition of TGF-β1 (Figure 13c) ▶ .
Figure 13.
Association of β-catenin with Smad proteins. Recombinant TGF-β1 (10 ng/ml) was added to confluent monolayers of growth-arrested HK-2 cells under serum-free conditions. On day 3 Smad4 (a), Smad3 (b), or Smad2 (c) were immunoprecipitated as described in Materials and Methods, and β-catenin expression in the precipitates examined by Western analysis. In control experiments serum-free medium was added to the confluent monolayers on cells. One representative experiment of at least four individual replicate experiments is shown.
Discussion
Although TGF-β1 has been extensively characterized as a negative regulator of epithelial cell growth, the mechanisms by which TGF-β1 regulate epithelial cell monolayer integrity remain unknown. A large body of evidence suggests that TGF-β1 may be involved in tumorigenesis, and that the epithelial-mesenchymal trans-differentiation may involve TGF-β1-mediated dysregulation of E-cadherin and alterations in the β-catenin-Wnt signaling pathway. 19 These observations therefore point to TGF-β1-mediated regulation of cell-cell contacts. In the current study we have demonstrated that TGF-β1 stimulated a marked change in PTC morphology that was associated with a loss of cell-cell contact.
To further characterize the mechanism by which TGF-β1 led to these alterations we characterized its effect of both adherens and tight junctional protein complexes. The results demonstrate altered expression of adherens complex protein components. In particular, the increase in Triton-extractable β-catenin expression after incubation of cells with TGF-β1 suggests increased generation of stable β-catenin in the cytoplasm. In contrast TGF-β1 induced decreased PTC E-cadherin expression. This is consistent with observations previously described in murine mammary epithelial cells. 20 In addition the data demonstrate that addition of TGF-β1 results in the loss of β-catenin association with both E-cadherin and α-catenin, which accompanied loss of both E-cadherin and β-catenin association with the cell cytoskeleton. This is consistent with previous studies, which have suggested that cadherin/catenin complexes are linked to the actin cytoskeleton via a direct association between α-actinin and α-catenin. 21 This is further supported by our data demonstrating not only by its dissociation form both E-cadherin and α-catenin, but also by the increased expression of detergent soluble β-catenin after addition of TGF-β1, and its relocalization within the cell as seen by immunofluorescence and confocal microscopic examination.
There is a large body of evidence to support a cadherin-independent role for β-catenin. This involves translocation of β-catenin to the nucleus as was seen in our experiments after addition of TGF-β1 to cells. Translocation of β-catenin to the nucleus is preceded by its accumulation in the cytoplasm. The signals resulting in increased cytoplasmic β-catenin include binding of Wingless/Wnt to its trans-membrane receptor Frizzled and the inhibition of the serine-threonine kinase glycogen-synthase kinase, 22 which phosphorylates β-catenin on specific serine and threonine residues. Another essential component directly involved in regulating β-catenin stability is the adenomatous polyposis coli protein. Adenomatous polyposis coli and GSK can form a complex that also includes β-catenin and proteins of the axin family. 23 Phosphorylation of adenomatous polyposis coli protein and β-catenin is involved in regulating their binding to each other, and in subsequent degradation of β-catenin via the ubiquitin-proteasome pathway. 22 Furthermore hyperphosphorylation of β-catenin on serine-threonine residues has been shown to stimulate loss of cell-cell contact. 24 In addition to the negative regulatory effect of serine/threonine phosphorylation targeting cytoplasmic β-catenin for its degradation, tyrosine phosphorylation has been implicated as a positive regulator of cytoplasmic β-catenin. 13 Furthermore tyrosine phosphorylation may also regulate β-catenin transcription factor recognition and binding. 25 Reversible tyrosine phosphorylation of β-catenin may be regulated by both tyrosine kinases and tyrosine phosphatases. 13 Our data demonstrate alterations in both tyrosine phosphorylation, and threonine phosphorylation status of β-catenin after TGF-β1 receptor-ligand binding. TGF-β1 receptors themselves have intrinsic serine-threonine kinase activity. Reduced threonine phosphorylation of β-catenin therefore implies activation of a phosphatase after receptor ligand binding, which subsequently mediates the changes in phosphorylation preventing β-catenin degradation. This precedent is supported by previous studies, which have demonstrated a similar phenomenon of serine-threonine dephosphorylation of E-cadherin, after activation of the serine-threonine kinase protein kinase C. 26
In addition to the disassembly of the adherens junction, alteration in cell morphology after addition of TGF-β1 was also associated with altered expression of tight junction protein components, and their disassembly. Previous studies have suggested that the integrity of tight junctions is dependent on the previous formation of the adherens junctions. 10 It is interesting therefore to speculate that the disassembly of the adherens junctional complex is also the driving force behind the changes in tight junction complex reorganization.
Stimulation of adherens junction disassembly after application of TGF-β1 to the basolateral side implies either polarity of TGF-β1 receptors, or that the effects of TGF-β1 were mediated by only a subpopulation of the TGF-β1 receptors that are only activated after basolateral TGF-β1 application. TGF-βs elicit their signaling effects by binding mainly to three cell-surface receptors: type I (RI), type II (RII), and type III (RIII). RI and RII are serine/threonine kinases that form heteromeric complexes, and are necessary for TGF-β signaling, which is initiated when the ligand induces assembly of a heteromeric complex of type II and type I receptors. The RII kinase then phosphorylates RI on a conserved glycine-serine-rich domain. This activates the RI kinase, which subsequently recognizes and phosphorylates members of the intracellular receptor-regulated Smad (R-Smad) signal transduction pathway. For TGF-β1 these include SMAD 2 and SMAD 3. This causes dissociation of the R-Smads from the receptor, stimulates the assembly of a heteromeric complex between the phosphorylated R-Smad and the Co-Smad Smad4, and induces the nuclear accumulation of this heteromeric Smad complex. 27 Immunostaining and confocal microscopy were used to determine the distribution of the TGF-β1 type II receptor expression in PTC. We have demonstrated, by confocal microscopy, that the type II TGF-β1 receptors are expressed both on the apical and basolateral aspect of the cells. The polarity of TGF-β1-mediated loss of cell contact however is likely to be the result of interaction of TGF-β1 with a population of its receptors that are associated with the adherens junction protein E-cadherin. To our knowledge, this is the first demonstration of an association of the TGF-β receptor with adherens junction proteins. The precedent for growth factor receptor association with E-cadherin has, however, been previously established as β-catenin can mediate the interaction of the cadherin-catenin complex with the epidermal growth factor receptor. 28
Several recent studies have shown that co-operation may exist between TGF-β1 and Wnt/β-catenin pathways, both by interactions of intracellular proteins of both pathways facilitating translocation into the nucleus, 29 and also by co-operative regulation of target gene within the cell nucleus. 30 The results of our experiments suggest that TGF-β1 stimulates the interaction between the TGF-β1 signaling intermediates Smad3 and Smad4, and β-catenin within the cytoplasm after disassociation of β-catenin from the adherens junction complex. This is the first demonstration that TGF-β receptor activation leads to interaction of the Smads and β-catenin although it is consistent with a recent study demonstrating that Smad4 plays an important role in Wnt-dependent activation of target genes that occurs independently of TGF-β signaling. 29 In these studies activation of the Wnt pathway enhanced Smad4/β-catenin interaction and resulted in concomitant nuclear accumulation. This is particular intriguing as the nuclear association of Smads with Wnt stimulated transcription factors (LEF1/TCF) and β-catenin can independently or co-operatively regulate LEF1/TCF target genes. 31 Although they are structurally very similar Smad2 and Smad3 have specific and different roles in TGF-β1 signaling. Although Smad2 and Smad3 have different subsets of target genes which they activate after TGF-β1 receptor activation, 32,33 currently we can only speculate on the transcriptional events triggered by TGF-β1-induced increase, in the association of β-catenin with Smad3 and Smad4, and the decreased association with Smad2 in renal PTCs.
Acknowledgments
We thank Dr. G. Newman for the preparation of the confocal images.
Footnotes
Address reprint requests to Dr. A. O. Phillips, Institute of Nephrology, University of Wales College of Medicine, Heath Park, Cardiff CF14 4XN. E-mail: phillipsao@cf.ac.uk.
Supported by the Chang Gung Memorial Hospital, Taipei, Taiwan (research fellowship no. CMRP1059 to Y. C. T.) and Glaxo Wellcome (senior fellowship to A. O. P.).
References
- 1.Bohle A, Mackensen-Haen S, Gise H: Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol 1987, 6:421-433 [DOI] [PubMed] [Google Scholar]
- 2.Mackensen-Haen S, Bader R, Grund KE, Bohle A: Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol 1981, 15:167-172 [PubMed] [Google Scholar]
- 3.Phillips AO, Steadman R, Topley N, Williams JD: Elevated D-glucose concentrations modulate TGF-β1 synthesis by human cultured renal proximal tubular cells: the permissive role of platelet derived growth factor. Am J Pathol 1995, 147:362-374 [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AO, Topley N, Steadman R, Morrisey K, Williams J: Induction of TGF-β1 synthesis in D-glucose primed human proximal tubular cells: differential stimulation by the macrophage derived pro-inflammatory cytokines IL-1β and TNFα. Kidney Int 1996, 50:1546-1554 [DOI] [PubMed] [Google Scholar]
- 5.Phillips AO, Topley N, Morrisey K, Williams JD, Steadman R: Basic fibroblast growth factor stimulates the release of pre-formed TGF-β1 from human proximal tubular cells in the absence of de-novo gene transcription or mRNA translation. Lab Invest 1997, 76:591-600 [PubMed] [Google Scholar]
- 6.Strutz F: Novel aspects of renal fibrogenesis. Nephrol Dial Transplant 1995, 10:1526-1532 [PubMed] [Google Scholar]
- 7.Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY: Tubular epithelial myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int 1998, 54:864-876 [DOI] [PubMed] [Google Scholar]
- 8.Tsukita S, Nagafuchi A, Yonemura S: Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol 1992, 4:834-839 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ze’ev A, Geiger B: Differential molecular interactions of β-catenin and plakoglobin in adhesion, signalling and cancer. Curr Opin Cell Biol 1998, 10:629-639 [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner B, Stevenson B, Grimaldi A: The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 1988, 107:1575-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funayama N, Fagotto F, McCrea P, Gumbiner BM: Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signalling. J Cell Biol 1995, 128:959-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peifer M: Cell adhesion and signal transduction: the armadillo connection. Trends Cell Biol 1995, 5:244-229 [DOI] [PubMed] [Google Scholar]
- 13.Müller T, Choidas A, Reichmann E, Ullrich A: Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem 1999, 274:10173-10183 [DOI] [PubMed] [Google Scholar]
- 14.Behrens J, Vaskaet L, Friis R, Winterhager E, Van Roy F, Mareei MM, Birchmeier W: Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature sensitive v-SRC gene. J Cell Biol 1993, 120:757-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B: HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 1994, 45:48-57 [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK: Cleavage of structural proteins during the assembly the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 17.Phillips AO, Morrisey R, Steadman R, Williams JD: Polarity of stimulation and secretion of TGF-β1 by proximal tubular cells. Am J Pathol 1997, 150:1101-1111 [PMC free article] [PubMed] [Google Scholar]
- 18.Morrisey K, Steadman R, Williams JD, Phillips AO: Renal proximal tubular cell fibronectin accumulation in response to glucose is polyol pathway dependent. Kidney Int 1999, 55:160-167 [DOI] [PubMed] [Google Scholar]
- 19.Janji B, Melchuior C, Gouon V, Valler L, Kieffer N: Autocrine TGF-β1-regulated expression of adhesion receptors and integrin-linked kinase in HT-144 melanoma cells correlates with their metastatic phenotype. Int J Cancer 1999, 83:255-262 [DOI] [PubMed] [Google Scholar]
- 20.Miettinen PJ, Ebner R, Lopez AR, Derynck R: TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type 1 receptors. J Cell Biol 1994, 127:2021-2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ: Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol 1995, 130:67-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R: β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997, 16:3797-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A: Axin, a negative regulator of the Wnt signalling pathway, forms a complex with GSK-3β-dependent phosphorylation of β-catenin. EMBO J 1998, 17:1371-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serres M, Grangeasse C, Haftek M, Durocher Y, Dulclos B, Schmitt D: Hyperphosphorylation of β-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp Cell Res 1997, 231:163-172 [DOI] [PubMed] [Google Scholar]
- 25.Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG: Regulation of β-catenin structure and activity by tyrosine phosphorylation. J Biol Chem 2001, 276:20436-20443 [DOI] [PubMed] [Google Scholar]
- 26.Ratccliffe MJ, Rubin LL, Staddon JM: Dephosphorylation of the cadherin-associated p100/p120 proteins in response to activation of protein kinase C in epithelial cells. J Biol Chem 1997, 272:31894-31901 [DOI] [PubMed] [Google Scholar]
- 27.Heldin CH, Miyazono K, Dijke P: TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390:465-471 [DOI] [PubMed] [Google Scholar]
- 28.Hoschuetzky H, Aberle H, Kemler R: β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 1994, 127:1375-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KWY: Interaction between Wnt and TGF-β signalling pathways during formation of Spemann’s organizer. Nature 2000, 403:781-785 [DOI] [PubMed] [Google Scholar]
- 30.Letamedia A, Labbe E, Attisano L: Transcriptional regulation by SMADs: cross-talk between the TGF-β and Wnt pathways. J Bone Joint Surg 2001, 83(Suppl 1):S1-S31 [PubMed] [Google Scholar]
- 31.Labbe E, Letamedia A, Attisano L: Association of Smads with lymphoid enhancer binding factor1/T cell-specific factor mediates co-operative signalling by transforming growth factor β and Wnt pathways. Proc Natl Acad Sci USA 2000, 97:8358-8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piek E, Ju WJ, Heyer J, Escalante-Alcade D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Böttinger EP, Roberts AB: Functional characterisation of transforming growth factor β signalling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem 2001, 276:19945-19953 [DOI] [PubMed] [Google Scholar]
- 33.Dennler S, Huet S, Gouthier JM: A short amino-acid sequence in MH1 domain is responsible for functional differences between Smad2 and Smad3. Oncogene 1999, 18:1643-1648 [DOI] [PubMed] [Google Scholar]