Abstract
Integrins mediate cell adhesion to extracellular matrix components. Integrin α1β1 is a collagen receptor expressed on many mesenchymal cells, but mice deficient in α1 integrin (α1-KO) have no gross structural defects. Here, the regeneration of a fractured long bone was studied in α1-KO mice. These mice developed significantly less callus tissue than the wild-type (WT) mice, and safranin staining revealed a defect in cartilage formation. The mRNA levels of nine extracellular matrix genes in calluses were evaluated by Northern blotting. During the first 9 days the mRNA levels of cartilage-related genes, including type II collagen, type IX collagen, and type X collagen, were lower in α1-KO mice than in WT mice, consistent with the reduced synthesis of cartilaginous matrix appreciated in tissue sections. Histological observations also suggested a diminished number of chondrocytes in the α1-KO callus. Proliferating cell nuclear antigen staining revealed a reduction of mesenchymal progenitors at the callus site. Although, the number of mesenchymal stem cells (MSCs) obtained from WT and α1-KO whole marrow was equal, in cell culture the proliferation rate of the MSCs of α1-KO mice was slower, recapitulating the in vivo observation of reduced callus cell proliferation. The results demonstrate the importance of proper collagen-integrin interaction in fracture healing and suggest that α1 integrin plays an essential role in the regulation of MSC proliferation and cartilage production.
The repair of a fractured long bone in adult mammals recapitulates many of the steps of endochondral ossification, which occur during embryonic bone development. The process results in the sequential expression of specific genes and ends with complete bone recovery. Fracture healing has been thoroughly studied at the morphological and biochemical levels in mouse, rat, and man. 1-4 There is increasing evidence that during bone development the extracellular matrix (ECM) profoundly influences the major cellular programs of growth, differentiation, and apoptosis in selected cells. Integrins, a large family of cell surface receptors that mediate cell-ECM and cell-cell interactions, and ECM itself, collaborate to regulate gene expression associated with these functions. 5,6
ECM in bone and cartilage is organized around type I and type II collagen fibers, respectively. Bone collagen structure is simple and consists almost solely of type I collagen. The importance of the collagens to cartilage is emphasized by the existence of numerous collagen subtypes, such as type IX, X, and XI, in addition to the predominant collagen type II. 7 Cell adhesion to collagen is mediated by a specific subset of integrins, composed of four heterodimers sharing a common β1 subunit. The α subunits, namely α1, α2, α10, and α11, have a special collagen-binding I (inserted) domain and they are therefore structurally different when compared to integrins that are receptors for other matrix proteins. 8,9 The α10β1 and α11β1 integrins have recently been identified 10,11 and their tissue distribution has not been entirely elucidated. Integrin α1β1 is expressed on fibroblasts, chondrocytes, osteoblasts, and smooth muscle cells. 12-15 It seems to have selectivity for nonfibrillar collagens, namely basement membrane type IV and transmembrane type XIII collagens, rather than for type I and II collagens. 16,17 Thus, although α1 I domain can bind type II collagen and the α1 integrin can probably transduce signals from type II collagen, it is likely that adhesion to type II collagen of structural importance is mediated by α2β1. 16,17 Interestingly the α subunits of these two integrins have distinct intracellular domains and therefore also distinct signaling functions. 18 Integrin α1β1 can activate the Shc/Ras/mitogen-activated protein kinase pathway and promote fibroblast proliferation. 19 It also acts to inhibit type I collagen synthesis. 19-22 Integrin α2β1 induces type I collagen gene expression 18,20 and also the expression of collagenase-1 [matrix metalloproteinase-1 (MMP-1)] 20-21 and collagenase-3 (MMP-13). 23
Although α1 integrin is not required to maintain adult bone 14 we have used α1-KO mice in a fracture model to investigate the importance of α1 integrin in bone healing. We hypothesized that because a complex pattern of collagen gene expression occurs during bone repair, proper α1 integrin-collagen interactions might be of great importance. Our results indicate that α1 integrin is required for healing by endochondral bone formation.
Materials and Methods
Experimental Model
A detailed report about generation and analysis of α1 integrin-deficient mice has been published. 14 All animals used in the study were α1 knockouts or controls on the inbred 129sv/Tae background. We used 49 wild-type (WT) and 45 α1-KO mice at 10 to 12 weeks of age for fracture-healing studies. Approximately half of the animals (22 WT and 22 α1-KO mice) received a pellet diet containing 0.1 to 0.2% (w/w) β-aminopropionitrile (Sigma, St Louis, MO). 24 The other half (27 WT and 23 α1-KO) were fed with a regular pellet diet. A closed diaphyseal fracture of tibia was produced bilaterally under general anesthesia as described previously. 25 Full weight bearing was allowed immediately. The animals were killed with CO2 5, 7, 9, 14, and 22 days after operation and then weighed. Five mice (three WT and two α1-KO) were excluded because of inadequate fractures. The calluses were carefully dissected from surrounding tissue, their largest and smallest diameters were measured in the middle of the fracture spindle with a micrometer. The calluses were either fixed with formaldehyde for histology or dissected from underlying bone and frozen in liquid nitrogen for Northern analyses.
Histology
For routine histology two calluses from different animals at each time point (except samples of day 7 that were used for RNA isolation) were fixed in phosphate-buffered 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid, embedded in paraffin, and sectioned longitudinally with a microtome. The sections were stained with hematoxylin and eosin or safranin, studied with routine microscopy and photographed. The numbers of chondrocyte nuclei were counted from enlarged photographs (15 × 15 cm; original magnification ×5) using 20 KO and 28 WT equal size fields (10 × 10 mm) in the middle of the callus.
Immunohistochemistry
Immunostainings of formalin-fixed paraffin-embedded sections were performed with polyclonal antibodies against collagen types I (Research Diagnostics, Inc., Flanders, NJ), and biotinylated proliferating cell nuclear antigen (PCNA) (PC10; Zymed, South San Francisco, CA). The sections were deparaffinized and rehydrated in a descending ethanol series. Endogenous peroxidase activity was quenched by treating the sections for 30 minutes with 1% H2O2 in absolute methanol. To prevent nonspecific binding, the sections were incubated with nonimmune serum for 20 minutes. Primary antibodies were appropriately diluted in Tris-buffered saline and the sections were incubated overnight at 4°C. After rinsing the sections with Tris-buffered saline, specific binding of anti-collagen type I was detected by corresponding biotinylated secondary antibody and visualized by the avidin-biotin-peroxidase complex (ABC) technique (Vectastain ABC kit; Vector Laboratories, Inc., Burlingame, CA) using diaminobenzidine as chromogen. Biotinylated anti-PCNA was directly visualized with the ABC technique. The tissues were counterstained with hematoxylin. The specificity of the reactions was controlled by using preimmune sera in parallel sections.
RNA Extraction and Northern Analysis
For the extraction of RNA, calluses of 76 animals were frozen in liquid nitrogen, cut into pieces with a scalpel blade, ground to fine powder under liquid nitrogen, and homogenized. Total RNA was extracted using the single-step method. 26 Because of the small callus size, 6 to 10 RNA samples from each observation day were pooled to obtain an adequate amount of total RNA. Calluses of day 5 were used only for histology. At days 9 and 14, the callus size was sufficient to get enough total RNA from one callus and individual samples (n = 4 to 6) of those days, in addition to the pooled samples, and were used in Northern analysis to estimate the biological variance. RNA (7.5 μg) was separated in formaldehyde-containing agarose gels, transferred to nylon membrane (Gene Screen Plus; New England Nuclear, Boston, MA), and hybridized according to the instructions of the manufacturer with 32P-labeled cDNA inserts (labeled with Multiprime kit; Amersham, Buckinghamshire, UK). 27 The following cDNA clones were used as hybridization probes: pMCol1α1-1 collagen mRNA, 28 pMCol2α1-1 for mouse proα1(II) collagen mRNA, 28 pRGR5 for rat proα1(III) collagen, 29 pMCol9α2-1 for mouse α2(IX) collagen mRNA, 30 pMCol10α1-1 for mouse α1(X) collagen mRNA, 30 pMAgg-1 for mouse aggrecan mRNA, 31 pMBgn-1 for mouse biglycan mRNA (A M-K Säämänen and EI Vuorio, unpublished), pMDcn-1 for mouse decorin mRNA, 32 pMMP-13 for MMP-13, 33 and 341-1 for mouse 28S rRNA. 34
The cDNA-mRNA hybrids were detected with a Fuji Bas 5000 phosphoimager (Fuji, Tokyo, Japan) and quantitated using Tina 2.0 software package (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany). The results were normalized against 28S rRNA signal in the same RNA samples. For rehybridization, the previous probe was removed from the membranes as recommended by the manufacturer.
Bone Marrow-Derived Mesenchymal Stem Cells (MSCs)
MSCs were harvested and subsequent colony formation evaluated as previously reported. 35 Briefly, both femurs and tibias were harvested from six WT and six α1-KO mice and the metaphyses were removed under sterile conditions. The marrow was flushed from the bone using 10 ml of MSC media (α-minimum Eagle’s medium (Sigma), 10% fetal calf serum (Gemini, Woodland, CA), 25 μg/ml sodium ascorbate, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10 μmol/L amphotericin B all from Sigma). For colony analysis, 4 million whole-marrow cells were plated to duplicate 60-mm tissue culture plates for each of the six animals and media was changed every third day. To differentiate osteoblast-committed MSCs from pluripotent progenitors the plates were fixed on day 12 in citrate/acetone/formalin and stained for alkaline phosphatase and counterstained with phenol red using a commercially available kit (Sigma). For proliferation experiments, primary MSCs plated on 100-mm plates were harvested with 0.25% trypsin/10 mmol/L ethylenediaminetetraacetic acid, pooled, and plated at cell densities of 1 × 104 cells/cm2 in 12-well plates (six wells for each genotype). Plates were harvested on day 2 and day 6 after plating and relative cell number was determined using Cyquant (Molecular Probes, Eugene, OR) fluorescence, which was visualized on a Molecular Dynamics Storm Imager (Molecular Dynamics, Sunnyvale, CA).
Statistical Analyses
Statistical analyses of callus sizes, cell counts, PCNA labelings, and Northern hybridizations were performed using two-way analysis of variance using SAS System for Windows, version 8.2 (SAS Institute Inc., Cary, NC) or using Student’s t-tests.
Results
Diminished Callus Size in α1 Integrin-Deficient Mice
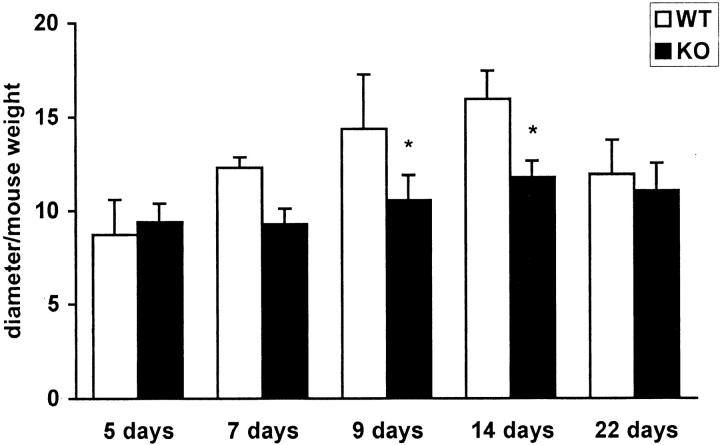
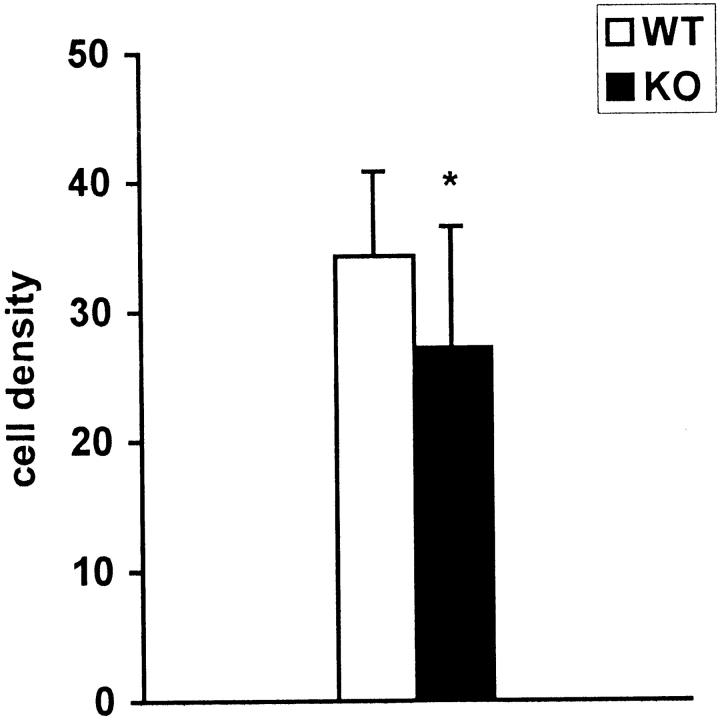
The fracture calluses were measured circumferentially and the values were plotted against the weights of the animals. Calluses in the α1-KO mice were smaller than in the WT mice (Figures 1 and 2, c and d) ▶ ▶ and their shapes were spherical whereas WT calluses were irregular and often flattened. Safranin staining of proteoglycans showed that α1-KO calluses contained less cartilage than WT controls (Figure 2, a and b) ▶ . The appearance of cartilage, new bone, and connective tissue in α1-KO calluses was strikingly regular with distinct demarcations between different tissue components in comparison with WT calluses in which tissue components were more disorganized. The proliferating cells (Figure 2, c and d ▶ ; arrows) were arranged in a streaming pattern suggesting that cartilage cells originated from bone marrow MSCs. Importantly, in α1-KO the numbers of cartilaginous cells were smaller than in the WT callus (Figure 3) ▶ .
Figure 1.
WT animals produce larger calluses than α1-KO animals. The columns represent the means of the largest and smallest diameters of calluses ± SD. Forty-six measured means from 27 WT and 44 from 23 α1-KO animals plotted against animal weights (expressed as mm/g × 100). *, P < 0.05, analysis of variance.
Figure 2.
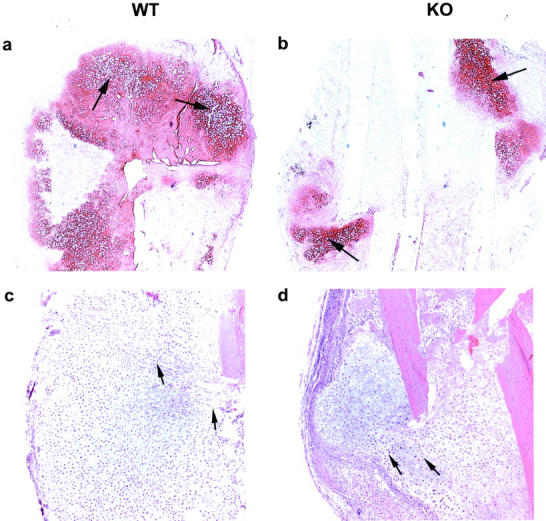
WT (left) and α1-KO (right) calluses at 9 days after fracture. Safranin stain (a and b) showing cartilaginous tissue (arrows). HE stain (c and d) indicating differences in proliferation of callus cells. The arrows point to lines of migrating cells. Original magnifications: ×2.5 (a and b); ×5 (c and d).
Figure 3.
The number of cells in cartilaginous callus in sections from WT and α1-KO animals at 9 days. Nuclei were counted from enlarged photographs using several (20 α1-KO and 28 WT) fields of equal size (10 × 10 mm) in the middle of callus. The difference is statistically significant: *, P < 0.003; t-test.
The Proliferation Rate of Mesenchymal Cells in α1-KO Callus Is Reduced
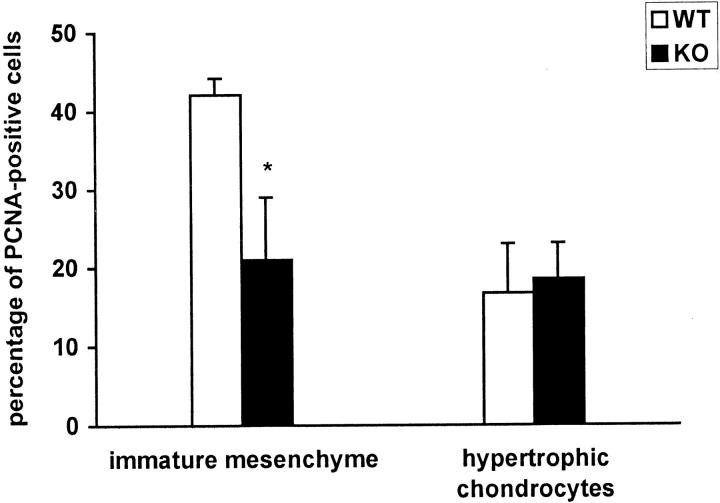
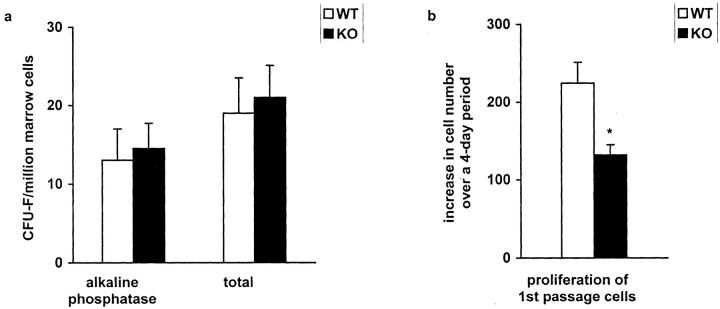
Because the histological observations suggested that the number of cartilaginous cells were reduced in the α1-KO callus, the extent of cell proliferation was studied by immunostaining with anti-PCNA antibody. The results in (Figure 4) ▶ show that the number of PCNA-positive undifferentiated cells was decreased in α1-KO callus, whereas there were no changes in the number of PCNA-positive hypertrophic chondrocytes suggesting that there was a reduced proliferation of MSCs in α1-KO animals. To determine whether there was a decrease in the pool of MSCs available for regeneration, the quantity of these cells in the marrow cavity was determined using a colony-forming assay. Cell preparations from WT and α1-KO animals produced an equal number of total fibroblast colonies (total CFU-F) and alkaline phosphatase-positive colonies (committed to osteoblasts) (Figure 5a) ▶ . However, when first passage MSCs were evaluated in culture, α1-KO cells showed decreased proliferation relative to WT cells (Figure 5b) ▶ , mimicking the in vivo observation.
Figure 4.
Proliferation of mesenchymal cells and hypertrophic chondrocytes in 9-day WT and α1-KO callus as measured by PCNA staining. Three fields were calculated from two animals. The columns represent the means ± SD for the total number of fields counted. The difference in the number of PCNA-positive immature mesenchymal cells (WT versus α1-KO) is statistically highly significant: *, P < 0.001; t-test.
Figure 5.
Proliferation of bone marrow-derived MSCs from WT and α1-KO mice. a: The equivalent numbers of MSCs are present in WT and α1-KO animals (total CFU-F) and the percentages of cells committed to the osteoblastic phenotype are equivalent (alkaline phosphatase CFU-F) (n = 6). b: The α1-KO-derived MSCs have reduced cell growth throughout a 4-day period in comparison to WT MSCs. *, P < 0.015; analysis of variance; n = 6. The columns represent the means ± SD.
Decreased Cartilage-Related Matrix Gene mRNA Levels in α1 Integrin-Deficient Mice
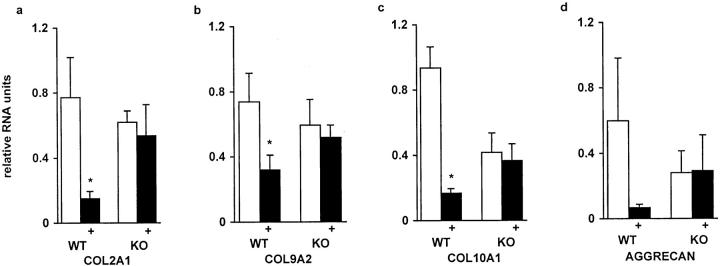
The mRNA levels of nine matrix-related genes were analyzed by Northern hybridization (Figures 6 and 8) ▶ ▶ . In addition to different collagens the mRNA levels of three proteoglycans, aggrecan, decorin, and biglycan were measured. In accordance with the histological observation that α1-KO animals have reduced amounts of cartilage in the fracture callus, the mRNA levels of cartilage collagens (type II, IX, and X), were decreased in α1-KO calluses (Figure 8 ▶ ; a to c). A similar finding was seen in mRNA levels of aggrecan, the main proteoglycan of cartilage (Figure 8d) ▶ , but the difference was not statistically significant. The levels of type III collagen and biglycan diminished faster in α1-KO calluses than in the WT controls, but the significance of this observation is unclear. The profiles of decorin mRNA expression during callus formation in WT and α1-KO mice were similar. The most striking difference in type I collagen mRNA expression was the high level observed at 22 days in two different pools of α1-KO samples (one shown in Figure 6 ▶ ). Immunolocalization of type I collagen in 22-day-old calluses showed more intense staining in α1-KO animals throughout the callus compared with WT controls (Figure 7) ▶ . This is in accordance with previous observations suggesting defective negative feed-back regulation of type I collagen synthesis in α1-KO mice. 22 In skin and experimental granuloma, this increased collagen expression is compensated by increased metalloproteinase expression. 22 However, there was no overexpression of collagenase-3 (MMP-13) in α1-KO calluses during the observation period.
Figure 6.
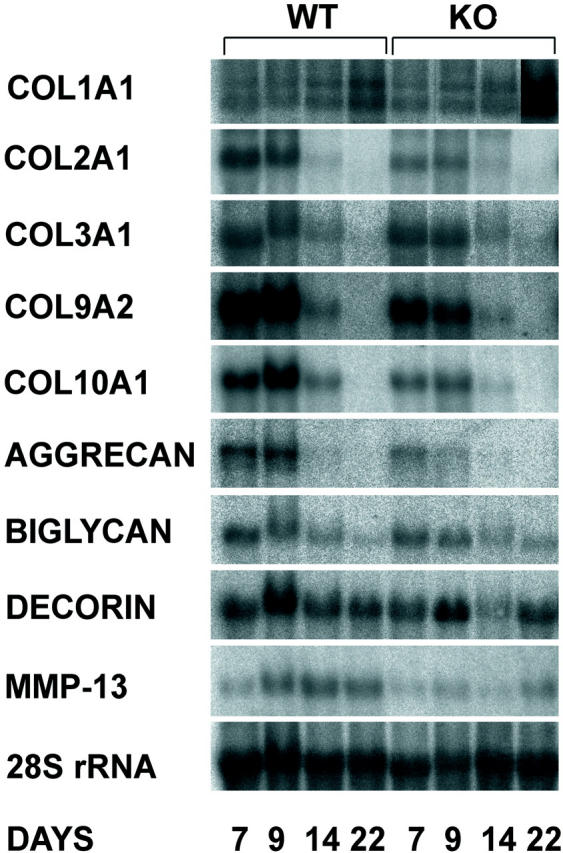
Northern analysis of connective tissue genes in 7-, 9-, 14-, and 22-day WT and α1-KO calluses.
Figure 8.
Densitometric profiles of mRNA levels of COL2A1 (a), COL9A2 (b), COL10A1 (c), and aggrecan (d) genes in WT, α1-KO, lathyritic WT (+), and lathyritic α1-KO (+) calluses 9 days after fracture measured by Northern hybridization. The mRNA levels are given as relative mRNA units corrected for loading variations with 28S rRNA levels. Columns represent mean ± SD from all analyzed samples (n = 4 to 6). *, P < 0.05; t-test.
Figure 7.
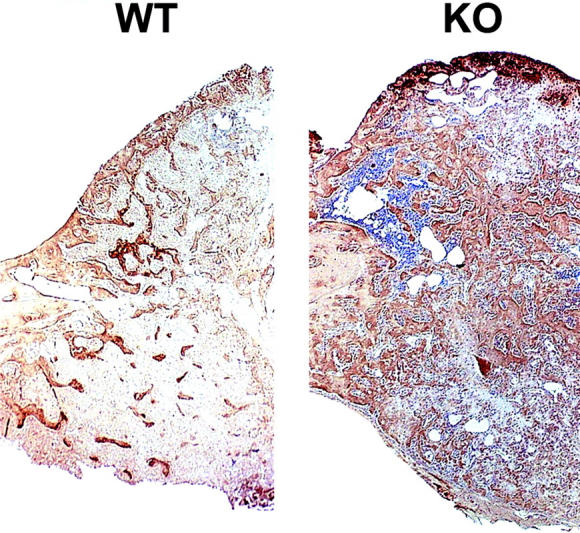
Immunolocalization of type I collagen in 22-day WT and α1-KO callus. In α1-KO specimen the immunostaining is more intense throughout the callus area.
Taken together the data suggest that α1β1 integrin is essential for proper formation of cartilage. Because the other observations indicated that the number of cartilaginous cells is diminished, the lowered mRNA levels of the cartilage genes probably reflect decreased gene expression per cell even though both mechanisms, reduced cell proliferation and decreased matrix production, may be involved.
Lathyrism Decreases the Expression of Cartilage-Related Matrix Genes
The fact that callus formation was affected in animals lacking a specific collagen receptor suggested the importance of cell-collagen interaction in fracture healing. This idea was studied further by using lathyrogenic diet (β-aminopropionitrile) to destroy the normal organization of newly formed collagenous matrix. During the second week of healing, lathyrism had a striking negative effect on the expression of all studied cartilage-specific genes (type II, IX, and X collagen and aggrecan) in WT calluses (Figure 8) ▶ . Type III collagen and biglycan mRNA levels were also low in lathyritic WT calluses (not shown). The lathyritic effect on these ECM components was, however, not detected in the calluses of the α1-KO animals. The mRNA expression of type I collagen was enhanced by lathyrism during the 2 first weeks of healing, especially in α1-KO calluses (not shown). Decorin levels were also increased at days 7 and 14 in lathyritic samples. In contrast to the rat, 24 the mouse lathyrism had no statistically significant effect on increasing callus size in this internally fixed fracture-healing model (not shown).
Discussion
Bone formation during adult fracture repair involves activation of MSCs, which then replicate and differentiate into osteoblasts and chondroblasts of the callus. MSC proliferation and the expression of genes associated with their differentiation are regulated by local growth factors. Proper cellular interactions with the ECM are required for the correct function of growth factor-initiated signaling cascades. 36 The integrin-type adhesion receptors organize focal contact sites promoting the accumulation of numerous signaling molecules. 37 In addition, integrin ligation to ECM proteins generates independent signals that regulate differentiation, growth, and survival. 36
Numerous research reports have described integrin-related functions in various cell culture models. Less is known about the significance of the integrin-matrix interaction in vivo. Production of transgenic and knockout mice for different integrins has enabled the study of integrins during differentiation and development. Many integrin knockouts (for example, animals deficient of fibronectin receptor α-subunits α4 or α5) die as embryos. 38 Some are born with structural defects, eg, αV, α3, α6, and β4 integrin knockouts, and often die during or immediately after birth. 38 Integrin α1-KO mice develop normally and they are born without obvious structural defects, 14 despite the fact that α1β1 integrin is expressed on many mesenchymal cell types, including osteoblasts 12 and chondrocytes. 13 In fact none of the integrin knockout mice have reported defects in bone development. However, overexpression of dominant-negative β1 integrin under the osteocalcin promoter results in reduced bone mass in transgenic animals. 5
Here, the bone connective tissue in α1-KO mice was challenged by creating a stabilized tibial fracture. Although α1-KO mice were able to heal, we detected a significant reduction in their capacity to make cartilaginous callus. The number of cartilage-producing cells was diminished and PCNA immunostaining confirmed the decreased proliferation of the undifferentiated mesenchymal cells, whereas the proliferation of more differentiated, chondrocyte-like cells was unaltered. The data suggest that α1β1 integrin participates in the growth control of cartilage stem cells during the period of cell expansion. Indeed we found that the basal numbers of MSCs obtained from whole-bone marrow were equivalent, but that in culture, when cell growth is enhanced, the α1-KO-derived MSCs showed reduced proliferation. We have previously reported impaired formation of experimental granulation tissue in the same animals. 22 Thus, the α1-KO animals seem to have a specific difficulty in the activation of cells responsible for the repair of connective tissues.
Previous studies have suggested mechanisms that may explain the affected cell proliferation. Most importantly, α1β1 integrin is connected to cellular signaling pathways, such as Shc/Ras/ERK-pathway, 19 that induce cell proliferation. The dermis in α1-KO animals is also hypocellular. 22 Secondly, α1-KO animals show an impaired ability to generate capillary vessels, eg, during tumor growth. 39 This is connected to increased angiostatin production because of elevated MMP activity. 39
Cell adhesion to fibrillar collagen leads to a different cellular response than adhesion to monomeric collagen, suggesting that the organization of collagenous matrix is important for integrin function. 40 The significance of the new collagenous matrix for in vivo cell behavior can be studied by using a lathyrogenic diet to prevent collagen cross-linking. In accordance with our previous observations with lathyritic rats the expression of matrix genes in mouse cartilage was reduced. 24 We have also reported that in lathyritic rats cartilage genes are expressed during a much wider time period. 24 Thus, faulty matrix organization has an effect similar to that observed in α1-KO mice. Interestingly, these lathyritic effects were only seen in WT animals but not in α1-KO animals. These results support the conclusion that proper α1β1 integrin-mediated cell-collagen interaction is required for callus growth during bone fracture healing.
Acknowledgments
We thank Ms. Päivi Auho for preparing histological sections, Mr. Jari Sundell for statistical analyses, and Drs. Hannu Järveläinen and Eero Vuorio for critical comments on the manuscript.
Footnotes
Address reprint requests to R. Penttinen, Department of Medical Biochemistry, University of Turku, Kiinamyllynkatu 10, 20520 Turku, Finland. E-mail: risto.penttinen@utu.fi.
Supported by grants from the Turku University Foundation (to R. P.), the Academy of Finland (to J. H.), the Sigrid Jusélius Foundation (to J. H.), the Finnish Cancer Association (to J. H.), and the Technology Development Center in Finland (to J. H. and R. P.).
References
- 1.McKibbin B: The biology of fracture healing in long bones. J Bone Joint Surg Br 1978, 60B:150-162 [DOI] [PubMed] [Google Scholar]
- 2.Jingushi S, Joyce ME, Bolander ME: Genetic expression of extracellular matrix proteins correlates with histological changes during fracture repair. J Bone Miner Res 1992, 7:1045-1066 [DOI] [PubMed] [Google Scholar]
- 3.Hiltunen A, Aro HT, Vuorio E: Regulation of extracellular matrix genes during fracture healing in mice. Clin Orthop 1993, 297:23-27 [PubMed] [Google Scholar]
- 4.Sandberg M, Aro HT, Vuorio EI: Gene expression during bone repair. Clin Orthop 1993, 289:292-312 [PubMed] [Google Scholar]
- 5.Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C: Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol 2000, 220:2-15 [DOI] [PubMed] [Google Scholar]
- 6.Damsky CH: Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 1999, 25:95-96 [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ, Kivirikko KI: Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 1995, 64:403-434 [DOI] [PubMed] [Google Scholar]
- 8.Kamata T, Takada Y: Direct binding of collagen to the I domain of integrin alpha 2 beta 1 (VLA-2, CD49b/CD29) in a divalent cation-independent manner. J Biol Chem 1994, 269:26006-26010 [PubMed] [Google Scholar]
- 9.Kern A, Briesewitz R, Bank I, Marcantonio EE: The role of the I domain in ligand binding of the human integrin alpha 1 beta 1. J Biol Chem 1994, 269:22811-22816 [PubMed] [Google Scholar]
- 10.Camper L, Hellman U, Lundgren-Åkerlund E: Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem 1998, 273:20383-20389 [DOI] [PubMed] [Google Scholar]
- 11.Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D: cDNA cloning and chromosomal localization of human α11 integrin. A collagen-binding, I domain-containing, β1-associated integrin α-chain present in muscle tissues. J Biol Chem 1999, 274:25735-25742 [DOI] [PubMed] [Google Scholar]
- 12.Clover J, Dodds RA, Gowen M: Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci 1992, 103:267-271 [DOI] [PubMed] [Google Scholar]
- 13.Salter DM, Godolphin JL, Gourlay MS: Chondrocyte heterogeneity: immunohistologically defined variation of integrin expression at different sites in human fetal knees. J Histochem Cytochem 1995, 43:447-457 [DOI] [PubMed] [Google Scholar]
- 14.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R: Deletion of integrin α1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol 1996, 175:301-313 [DOI] [PubMed] [Google Scholar]
- 15.Heino J: The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol 2000, 19:319-323 [DOI] [PubMed] [Google Scholar]
- 16.Kern A, Eble J, Golbik R, Kuhn K: Interaction of type IV collagen with the isolated integrins alpha 1 beta 1 and alpha 2 beta 1. Eur J Biochem 1993, 215:151-159 [DOI] [PubMed] [Google Scholar]
- 17.Nykvist P, Tu H, Ivaska J, Käpylä J, Pihlajaniemi T, Heino J: Distinct recognition of collagen subtypes by α1β1 and α2β1 integrins—α1β1 mediates cell adhesion to type XIII collagen. J Biol Chem 2000, 275:8255-8261 [DOI] [PubMed] [Google Scholar]
- 18.Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri V-M, Heino J: Integrin α2β1 mediates isoform specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J Cell Biol 1999, 147:401-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pozzi A, Wary KK, Giancotti FG, Gardner HA: Integrin α1β1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol 1998, 142:587-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kähäri V-M, Heino J: α2β1 integrin is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem 1995, 270:13548-13552 [DOI] [PubMed] [Google Scholar]
- 21.Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B: Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol 1995, 131:1903-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner H, Broberg A, Pozzi A, Laato M, Heino J: Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 1999, 112:263-272 [DOI] [PubMed] [Google Scholar]
- 23.Ravanti L, Heino J, López-Otín C, Kähäri VM: Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 1999, 274:2446-2455 [DOI] [PubMed] [Google Scholar]
- 24.Ekholm EC, Ravanti L, Kähäri V-M, Paavolainen P, Penttinen R: Expression of extracellular matrix genes, TGF-β1 and ras in tibial fracture healing of lathyritic rats. Bone 2000, 27:551-557 [DOI] [PubMed] [Google Scholar]
- 25.Hiltunen A, Vuorio E, Aro HT: A standardized experimental fracture in the mouse tibia. J Orthop Res 1993, 11:305-312 [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 27.Ekholm EC, Hietaniemi K, Määttä A, Vuorio E, Paavolainen P, Penttinen RPK: Extended expression of cartilage components in experimental pseudoarthrosis. Connect Tissue Res 1995, 31:211-218 [DOI] [PubMed] [Google Scholar]
- 28.Metsäranta M, Toman D, De Crombrugghe B, Vuorio E: Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem 1991, 266:16862-16869 [PubMed] [Google Scholar]
- 29.Glumoff V, Mäkela JK, Vuorio E: Cloning of cDNA for rat pro alpha 1(III) collagen mRNA. Different expression patterns of type I and type III collagen and fibronectin genes in experimental granulation tissue. Biochim Biophys Acta 1994, 1217:41-48 [PubMed] [Google Scholar]
- 30.Elima K, Metsäranta M, Kallio J, Perälä M, Eerola I, Garofalo S, De Crombrugghe B, Vuorio E: Specific hybridization probes for mouse alpha 2(IX) and alpha 1(X) collagen mRNAs. Biochim Biophys Acta 1992, 1130:78-80 [DOI] [PubMed] [Google Scholar]
- 31.Glumoff V, Savontaus M, Vehanen J, Vuorio E: Analysis of aggrecan and tenascin gene expression in mouse skeletal tissues by Northern and in situ hybridization using species specific cDNA probes. Biochim Biophys Acta 1994, 1219:613-622 [DOI] [PubMed] [Google Scholar]
- 32.Säämänen AM, Salminen HJ, Rantakokko AJ, Heinegård D, Vuorio EI: Murine fibromodulin: cDNA and genomic structure, and age related expression and distribution in the knee joint. Biochem J 2001, 355:577-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salminen HJ, Säämänen A-MK, Vankemmelbeke MN, Auho PK, Perälä MP: Differential expression patterns of matrix metalloproteinases and their inhibitors during development of osteoarthritis in a transgenic mouse model. Ann Rheum Dis 2002, in press [DOI] [PMC free article] [PubMed]
- 34.Iruela-Arispe ML, Hasselaar P, Sage H: Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest 1991, 64:174-186 [PubMed] [Google Scholar]
- 35.Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P: Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res 2000, 15:851-862 [DOI] [PubMed] [Google Scholar]
- 36.Clark EA, Brugge JS: Integrins and signal transduction pathways: the road taken. Science 1995, 268:233-239 [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM: Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 1995, 131:791-805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheppard D: In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol 2000, 19:203-209 [DOI] [PubMed] [Google Scholar]
- 39.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA: Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA 2000, 97:2202-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R: Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 1996, 87:1069-1078 [DOI] [PubMed] [Google Scholar]