Abstract
Amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) is a progressive neurodegenerative disorder of Chamorro residents of Guam and the Mariana Islands, characterized by abundant neuron loss and tau neurofibrillary pathology similar to that observed in Alzheimer’s disease (AD). A variety of neurodegenerative diseases with tau pathology including ALS/PDC also have α-synuclein positive pathology, primarily in the amygdala. We further characterized the tau and α-synuclein pathology in the amygdala of a large series of 30 Chamorros using immunohistochemical and biochemical techniques. Tau pathology was readily detected in both affected and unaffected Chamorros. In contrast, α-synuclein pathology was detected in 37% of patients with PDC but not detected in Chamorros without PDC or AD. The α-synuclein aggregates often co-localized within neurons harboring neurofibrillary tangles suggesting a possible interaction between the two proteins. Tau and α-synuclein pathology within the amygdala is biochemically similar to that observed in AD and synucleinopathies, respectively. Thus, the amygdala may be selectively vulnerable to developing both tau and α-synuclein pathology or tau pathology may predispose it to synuclein aggregation. Furthermore, in PDC, tau and α-synuclein pathology occurs independent of β-amyloid deposition in amygdala thereby implicating the aggregation of these molecules in the severe neurodegeneration frequently observed in this location.
Amyotrophic lateral sclerosis-parkinsonism-dementia complex (ALS/PDC) is a neurodegenerative disorder that afflicts the Chamorro population on the island of Guam and the Mariana Islands. 1,2 It is characterized clinically by either motor neuron disease or progressive cognitive dysfunction with extrapyramidal signs or both. While the etiology of the disorder remains unknown, both genetic and environmental factors have been implicated. 3,4 Neuropathologically, ALS/PDC is characterized by cortical atrophy and neuronal loss with numerous neurofibrillary tangles (NFTs) widely distributed throughout the central nervous system, similar both biochemically and immunohistochemically to that observed in Alzheimer’s disease (AD). 5-8 The NFTs in both AD and Guam ALS/PDC are composed primarily of abnormally phosphorylated tau, a phosphoprotein that regulates the assembly and stability of microtubules. 9,10 In the central nervous system (CNS), six tau isoforms are produced by alternative splicing 11,12 and all six isoforms are present in the NFTs of AD and ALS/PDC patients. 5,6 However, ALS/PDC is distinguished from AD by the laminar distribution of these NFTs, 13,14 the prominent glial pathology in ALS/PDC including granular hazy astrocytic inclusions, 15 as well as the absence of amyloid plaques in most, but not all, cases. 16,17
α-Synuclein, a small, highly conserved pre-synaptic protein, is the major constituent of Lewy bodies (LBs) and Lewy neurites as well as the glial cytoplasmic inclusions of multiple system atrophy. 18 More recently, α-synuclein positive pathology was identified, primarily in the amygdala, in a variety of disorders with extensive tau pathology including familial AD, 19 Down’s syndrome with AD, 20 neurodegeneration with brain iron accumulation, 21-23 as well as up to 60 to 70% of sporadic AD. 24,25 In Guamanian PDC patients, LBs have been reported in the substantia nigra 8 and, more recently, α-synuclein positive pathology was identified in the amygdala. 26 In this study, we further characterized the tau and α-synuclein pathology in the amygdala of a large series of 30 Chamorros patients using both immunohistochemical and biochemical techniques.
Materials and Methods
Patients
Amygdala from 30 Chamorros were used in this study. The age, gender, and pathological diagnosis of these patients are given in Table 1 ▶ . These included 13 patients with PDC, 3 patients with pre-clinical or early PDC, and 6 patients with PDC plus additional neurological disorder(s) (PDC+) including one patient with PDC/ALS and one patient with early PDC/ALS. The distinction between PDC, PDC/ALS, and ALS is based largely on clinical criteria, ie, the presence of dementia and parkinsonism (PDC), amyotrophy (ALS), or both (ALS/PDC), because spinal cords are not available in the majority of cases. Pre-clinical PDC refers to cases that show widespread tau pathology in the absence of a clinical history of dementia while early PDC refers to similar patients but with mild neurological dysfunction that is insufficient for a clinical diagnosis of PDC. In addition, 3 patients without neurological disease and 5 control patients with a neurological disorder other than PDC were also available.
Table 1.
Clinical Data and Pathological Findings of Chamorro Patients
| Case no. | Pathological diagnosis | Age (yr) | Gender | LBs | NFTs | Tau+ glia | Gliosis | CNS, NFTs |
|---|---|---|---|---|---|---|---|---|
| 1 | PDC | 66 | F | − | 3+ | 2+ | 2+ | + |
| 2 | PDC | 67 | F | − | 2+ | 2+ | 1+ | + |
| 3 | PDC | 67 | M | 2+ | 3+ | 1+ | 3+ | + |
| 4 | PDC | 69 | F | − | 3+ | 3+ | 2+ | + |
| 5 | PDC | 72 | M | 2+ | 3+ | 2+ | 3+ | + |
| 6 | PDC | 72 | M | 2+ | 2+ | 3+ | 2+ | + |
| 7 | PDC | 72 | M | − | 3+ | 1+ | 2+ | + |
| 8 | PDC | 74 | M | − | 1+ | 2+ | 1+ | + |
| 9 | PDC | 74 | F | − | 2+ | 1+ | 2+ | + |
| 10 | PDC | 78 | F | 3+ | 3+ | 3+ | 3+ | + |
| 11 | PDC | 79 | F | − | 3+ | 1+ | 2+ | + |
| 12 | PDC | 80 | F | − | 2+ | 1+ | 1+ | + |
| 13 | Preclinical PDC | 81 | M | − | 3+ | − | 2+ | + |
| 14 | Preclinical PDC/ischemic CVD | 64 | M | − | 3+ | 1+ | 1+ | + |
| 15 | Early PDC/ALS | 71 | F | − | 2+ | 1+ | 2+ | + |
| 16 | PDC/ALS | 56 | M | − | 1+ | − | 1+ | + |
| 17 | PDC/AD | 76 | F | 2+ | 2+ | 1+ | 2+ | + |
| 18 | PDC/CVD | 74 | F | − | 3+ | 1+ | 3+ | + |
| 19 | PDC/ischemic CVD | 76 | F | − | 1+ | 3+ | 2+ | + |
| 20 | PDC/metastasis | 71 | F | 2+ | 2+ | 2+ | 3+ | + |
| 21 | PDC/MID/CVD | 75 | M | 2+ | 3+ | 1+ | 3+ | + |
| 22 | PDC, clinical (pathology pending) | 80 | F | − | 1+ | 2+ | 2+ | + |
| 23 | ALS | 61 | M | − | 1+ | − | − | + |
| 24 | MID | 63 | M | − | − | − | 2+ | − |
| 25 | AD | 82 | F | − | 2+ | 1+ | 1+ | + |
| 26 | AD | 86 | F | 1+ | 2+ | − | 1+ | + |
| 27 | Ischemic CVD | 71 | M | − | 1+ | − | 2+ | + |
| 28 | Normal | 41 | M | − | − | − | − | − |
| 29 | Normal | 71 | M | − | 2+ | 1+ | 1+ | + |
| 30 | Normal | 73 | F | − | 1+ | − | − | + |
Histochemistry and Immunohistochemistry
The tissues were fixed in 10% formalin, paraffin-embedded, and cut into 6-μm-thick sections. For thioflavin-S staining, rehydrated sections were immersed in 0.05% KMNO4 in phosphate-buffered saline (PBS) for 20 minutes and differentiated in 0.2% K2S2O5/0.2% oxalic acid in PBS. Subsequently, the slides were immersed in 1% thioflavin-S in the dark for 3 minutes and further differentiated in 50% ethanol in PBS, rinsed, and mounted. Immunohistochemistry was performed as previously described using the ABC method (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine (DAB) as chromogen. 6,27 The following primary antibodies were used: monoclonal antibodies (mAb) Syn202 (1:20,000) and Syn303 (1:500) to α-synuclein and oxidized α-synuclein, respectively; 28,29 mAb to tau included AT8 (1:1000), 30,31 PHF1 (1:1000), the generous gift of Peter Davies (Albert Einstein College of Medicine, New York, NY), 32 12E8 (1:500), 33 T14 (1:3000) and T46 (1:1000); 34 polyclonal antiserum 17026 (1:10,000) to recombinant tau, 35 and a polyclonal antiserum to glial fibrillary acidic protein (GFAP; 1:10,000) was purchased from Dako Corp. (Carpinteria, CA). Tau and α-synuclein pathology was assessed semiquantitatively as absent (−), mild, (1+) moderate (2+) or marked (3+) based on the number of tau or α-synuclein-positive inclusions in the area of highest density. For tau immunostaining, mild (1+), ≤ 2 inclusions/10× field; moderate (2+), 2 to 10 inclusions/10× field; and marked (3+) ≥, 10 inclusions/10× field. For α-synuclein, mild, 1 inclusion/10× field; moderate, 2 to 4 inclusions/10× field; and marked, > 4 inclusions/10× field (Figure 2) ▶ . Double-labeling immunofluorescence studies were performed by incubating sections with the antibodies PHF1 and GFAP. Following extensive washes, sections were labeled using Alexa Fluor 488 and 594 conjugated secondary antibodies (Molecular Probes, Eugene, OR) and coverslipped with Vectashield-DAPI-mounting medium (Vector Laboratories). Sections containing α-synuclein-positive LBs were double-stained with Congo red, 36 dehydrated and coverslipped with Cytoseal (Stephens Scientific, Kalamazoo, MI). The sections were viewed in a Nikon FXA microscope equipped with bright-field and fluorescence light sources. The Congo red fluorescence was observed using a Texas red filter set. From the double-stained preparations both bright-field and fluorescent images were obtained from the same field using a Coolsnap camera (BioVision Technologies, Exton, PA). For publication the Congo red image was converted to black and white, brightened, and superimposed onto the bright-field image of the DAB stain in Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA). The images were printed on a Fuji Pictography 3000 printer (Fuji Photo Film Co., Ltd., Tokyo, Japan).
Figure 2.
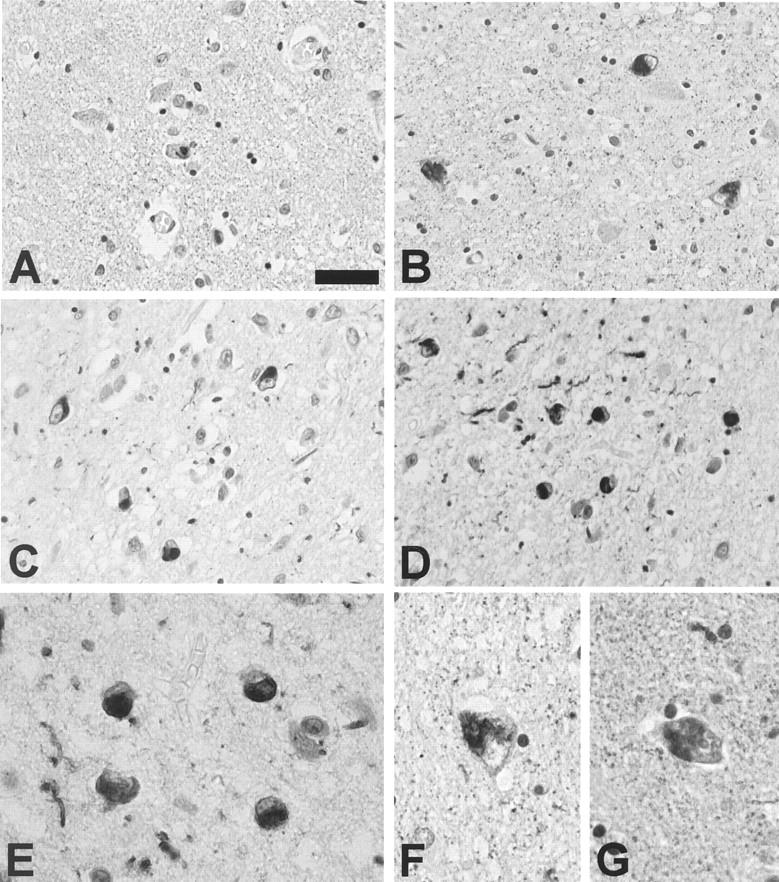
α-Synuclein-positive pathology in the amygdala of Guam PDC patients. A–G: Variable density of Lewy bodies and neuritic pathology in the amygdala of PDC patients immunostained with mAb Syn202. Patient 26 (A), diagnosed with AD, was assessed as having mild (1+) α-synuclein pathology; patients 3 (B) and 20 (C) were assessed as moderate; (2+) patient 10 (D) was assessed as having a marked (3+) density of α-synuclein pathology. E–G: Variable morphology of α-synuclein pathology including LBs that appear fragmented within the neuron (F, G). A–D: Scale bar, 40 μm. E–G: Scale bar, 20 μm.
Tau and α-Synuclein Protein Extraction
Fresh, frozen brain tissue from the amygdala of seven Guam PDC patients were used for biochemical analysis. In addition, brain tissue from non-Chamorro normal and LB variant of AD patients was obtained through the University of Pennsylvania Alzheimer’s Disease Center. Heat-stable soluble tau and α-synuclein proteins were extracted as described previously. 37 Insoluble tau and α-synuclein proteins were extracted as described. 19,38 Briefly, the insoluble pellets were homogenized in 1 mol/L sucrose in RAB buffer (0.1 mol/L morpholineethane sulfonic acid, 1 mmol/L EGTA, 0.5 mmol/L MgSO4, pH 7.0) and centrifuged at 50,000 × g to remove myelin. The resulting pellets were extracted with 1 ml/g RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% NP40, 5 mmol/L EDTA, 0.5% sodium deoxycholate, and 0.1% SDS, pH 8.0) and centrifuged at 50,000 × g to generate RIPA-soluble samples. The RIPA-insoluble fractions were subsequently extracted with 1 ml/g 70% formic acid and disrupted with sonication. Formic acid was evaporated in an Automatic Environmental SpeedVac system (Savant Instruments, Holbrook, NY). The dried pellets were resuspended in 1 ml/g sample buffer and centrifuged at 50,000 × g for 30 minutes. Where indicated, tau was dephosphorylated by dialyzing a sample of the soluble or insoluble fraction into 50 mmol/L Tris, 0.2 mmol/L EDTA (pH 8.0) and treatment with Escherichia coli alkaline phosphatase (Sigma, St. Louis, MO) at 67°C for 1 hour. For Western blot analysis, nitrocellulose replicas were prepared from 7.5% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) slab gels containing either the soluble, RIPA-soluble or insoluble protein samples and probed with antibodies for tau or α-synuclein as indicated. For all Western blots, 10 μl of heat-stable soluble, RIPA-soluble or -insoluble fractions was used corresponding to 10 mg of the extracted amygdala. mAb were detected with horseradish peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotechnologies). Immunoreactive proteins were revealed used the ECL chemiluminescence (NEN Life Science, Boston, MA) and/or DAB detection systems.
Results
NFTs were identified in the amygdala of all but three of the Chamorros, regardless of pathological diagnosis (Table 1) ▶ . A 41-year-old normal control patient and a 63-year-old with multi-infarct dementia (MID) had no neurofibrillary pathology. In addition, a 71-year-old normal control patient had a moderate density of pre-tangles, but no thioflavin-S-positive or congophilic NFTs in the amygdala or the rest of the brain. In the third normal Chamorro patient, a 73-year-old female, there were rare NFTs in the amygdala as well as in several additional brain areas. In contrast, in all but one of the PDC, PDC+, and preclinical PDC patients, NFTs were variably abundant in the amygdala as well as the remainder of the brain (Table 1 ▶ and Figure 1 ▶ ). In addition, tau-positive astroglia occurred in 20 of 22 PDC, PDC plus additional neurological disorder(s) (PDC+) and preclinical PDC cases (Table 1 ▶ and Figure 1 ▶ ). These tau-positive astrocytes showed highly variable density and morphology with many resembling the hazy, granular inclusions described by Oyanagi et al. 15 Some of the tau-positive astrocytes were small, but more frequently they resembled reactive astrocytes and these were often seen in clusters. Immunostaining for GFAP on adjacent sections showed accompanying moderate to severe gliosis of the amygdala (Figure 1 ▶ and Table 1 ▶ ). Double-labeling experiments with antibodies to tau and GFAP confirmed the astrocytic lineage of the hazy, granular inclusions. A low density of tau-positive astroglia with associated mild gliosis was also detected in one AD and one normal control case.
Figure 1.
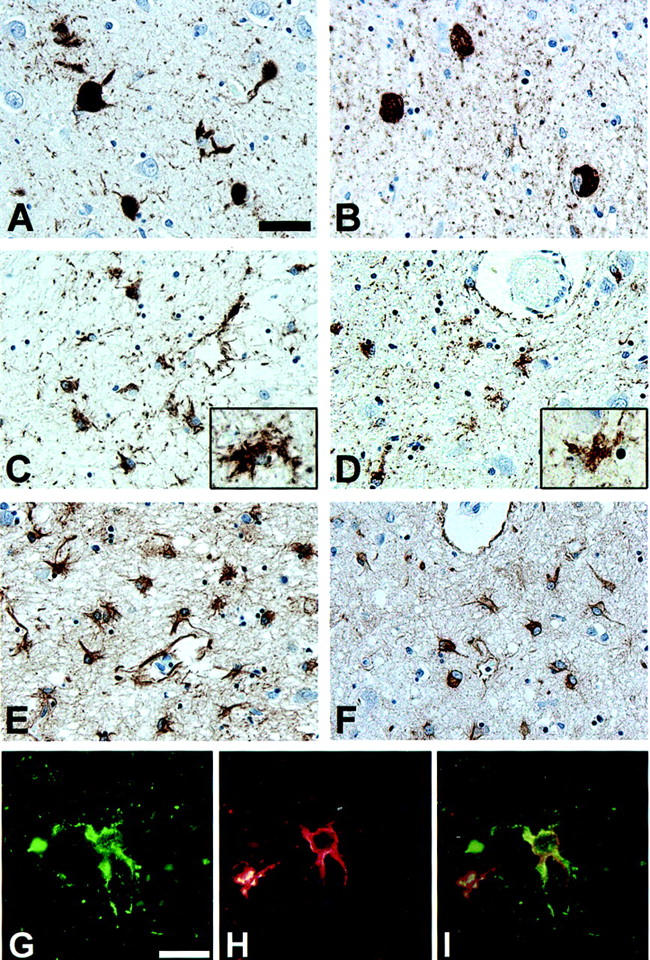
Tau and GFAP positive pathology in the amygdala of Guam PDC patients. A and B: NFTs in the amygdala of PDC patients 21 (A) and 3 (B), immunostained with the phosphorylation-dependent tau mAb AT8. C and D: Numerous tau-positive astrocytes in the amygdala of PDC patients 10 (C) and 6 (D), immunostained with the mAb AT8. Inset shows high power magnification of tau-positive astrocytes with morphology resembling the hazy, granular astrocytes described by Oyanagi et al. 15 E and F: Moderate to severe astrocytosis in the same amygdala of the patients illustrated in C and D, (patient 10, E; patient 6, F), immunostained with GFAP polyclonal antisera. G–I: Tau-positive hazy, granular astrocytes co-express GFAP. Double-labeling immunofluorescence of the amygdala of patient 5 stained with the mAb PHF1 (G, green) and GFAP polyclonal antisera (H, red). I represents an overlay of G and H. A–F: Scale bar, 40 μm. G–I: Scale bar, 20 μm.
In 7 of 19 patients with PDC or PDC+ (37%), a moderate to marked density of α-synuclein-positive LBs and neurites was identified in the amygdala (Table 1 ▶ and Figure 2 ▶ ). Furthermore, in all seven of these cases at least a small number of neurons harbored both a NFT and a LB within the same cell (Figure 3) ▶ . α-Synuclein positive pathology was not identified in the amygdala of any of the Chamorro patients with ALS, preclinical PDC, or control Chamorro patients with the exception of one AD patient who showed a low density of α-synuclein pathology (Figure 2 ▶ and Table 1 ▶ ). Although intracellular and extracellular NFTs were abundant in these PDC cases, LBs were only associated with intracellular NFTs. The LBs within the amygdala were irregular in shape and occasionally appeared fragmented within some cells (Figure 2) ▶ . Moreover, in cells with both tau and α-synuclein pathology, the NFTs were frequently seen as wisps of fibrillar tau immunoreactivity at the periphery of the LB (Figure 3) ▶ .
Figure 3.
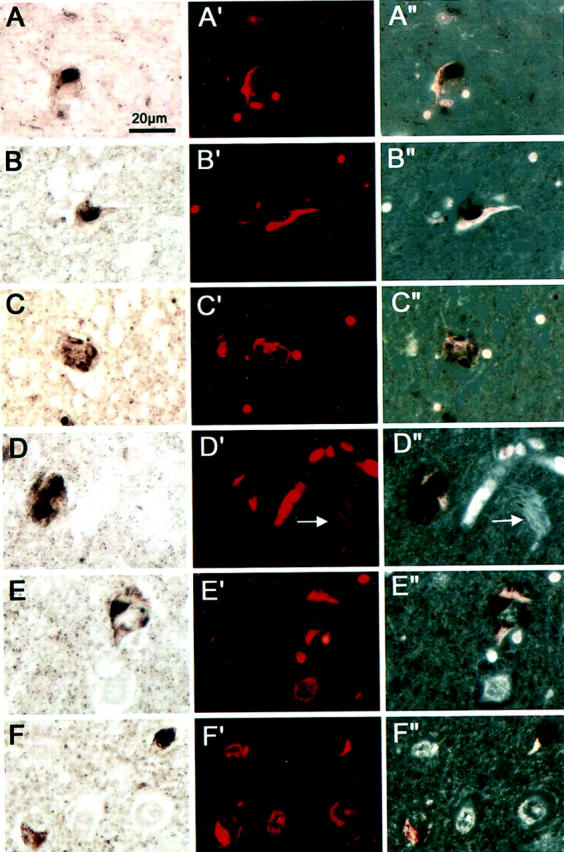
Colocalization of LBs and NFTs in neurons of the amygdala of Guam PDC patients. Double-stained preparations for LBs and NFTs are as follows: A–F: Bright-field images of LBs immunostained with Syn202 using the ABC procedure and DAB as chromogen. A′-F′: Fluorescent images of Congo red stained NFTs in the same sections as shown in A–F. A″-F″: Fluorescent images after conversion to black and white superimposed onto the bright-field images. In A (patient 10), B (patient 3), and F (patient 6, upper right), three neurons are depicted in which the LB and NFT are located side by side. In C (patient 3), D and E (patient 21) and F (bottom left) the neurons show NFTs intermingled with LBs. The arrows in D′ and D″ point to extracellular NFTs which were not associated with LBs. Scale bar, 20 μm.
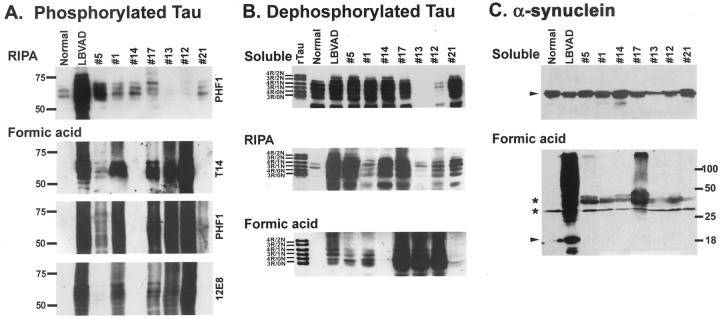
To further analyze the tau and α-synuclein pathology, we performed Western blot analysis of both soluble and insoluble (formic acid extractable) fractions from the amygdala of Guam PDC patients. Fresh, frozen tissue from the amygdala of the hemispheres contralateral to those used for immunohistochemical analysis was available from 7 of the 19 patients with the pathological diagnosis of PDC or PDC+, including 3 of the patients with α-synuclein positive pathology detected by immunohistochemistry. In sequential extractions with both RIPA buffer (1% NP40/0.1% SDS) and 70% formic acid, insoluble tau was composed of four major protein bands ranging from 60- to 72-kd (Figure 4A) ▶ . In formic acid extracts, the insoluble tau was also extensively aggregated, appearing as a smear of immunoreactivity with several phosphorylation-dependent tau antibodies (Figure 4A) ▶ . The insoluble tau co-migrated with insoluble tau extracted from the brain of a non-Chamorro patient with the pathological diagnosis of Lewy body variant of AD (LBVAD) and was absent from a non-Chamorro normal control. On dephosphorylation, these major protein bands co-migrated with all six tau isoforms expressed in the adult brain (Figure 4B) ▶ . The amount of insoluble tau was highly variable between individuals, presumably related to the severity of the neurodegenerative process. In contrast, variable levels of soluble tau were detected in both control and affected individuals (Figure 4B) ▶ and there appears to be an inverse correlation between soluble and insoluble tau. Cases with high levels of insoluble tau contain little or no soluble tau and, conversely, cases with abundant soluble tau contain little formic acid extractable tau. Immunoblotting the same fractions with antibodies to α-synuclein revealed formic acid extractable, high molecular weight, insoluble aggregates similar to that observed in LBVAD in two of the three Chamorro patients with α-synuclein pathology detected by immunohistochemistry (Figure 4C) ▶ . The other Guam PDC patients without α-synuclein pathology as well as the non-Chamorro normal control brain lacked the high molecular weight aggregates of α-synuclein. As with tau, the relative quantity of insoluble, aggregated α-synuclein was highly variable. Moreover, both affected and control brains demonstrated abundant soluble synuclein protein (Figure 4C) ▶ .
Figure 4.
Western blot analysis of soluble and insoluble tau and α-synuclein in the amygdala of Guam PDC patient. A: RIPA and insoluble (FA extractable) fractions were resolved by SDS-PAGE and immunoblotted with both phosphorylation-dependent (PHF1 and 12E8) and phosphorylation-independent (T14) tau antibodies as indicated. The insoluble tau from the Chamorro patients was composed of four major bands ranging from 60- to 72-kd similar to that observed in the non-Chamorro LBVAD control patient. In the formic acid extracts, the tau was also extensively aggregated. Molecular weight standards are indicated to the left of A. B: Aliquots of both soluble and insoluble (RIPA and formic acid extracts) were dephosphorylated with E. coli alkaline phosphatase, resolved by SDS-PAGE, and immunoblotted with T14. The soluble extracts were composed of all six tau isoforms that are normally expressed in adult brain. The RIPA and formic acid extracts from the Chamorro patients were also composed of all six tau isoforms similar to that observed in the non-Chamorro LBVAD patient. Recombinant tau isoforms (rTau) are indicated to the left of B. C: Soluble and formic acid fractions were resolved by SDS-PAGE and immunoblotted with Syn303. High molecular weight aggregates of insoluble synuclein was detected in two of three Chamorro patients with α-synuclein pathology detected by immunohistochemistry similar to that observed in the non-Chamorro LBVAD control. Non-aggregated α-synuclein is indicated by the arrowheads. The asterisks indicate proteins that are variably detected in both affected and normal control patients. Molecular weight standards are indicated to the right of C.
Discussion
Guam ALS/PDC is a chronic neurodegenerative disease characterized by the widespread deposition of hyperphosphorylated tau protein in neurons and glia in the relative absence of senile plaques and thus falls into the category of diseases termed “tauopathies.” However, NFTs are not only found in large numbers of Chamorros with ALS/PDC, but most Chamorros without neurological disease above age 50 also have some NFTs in the brain and spinal cord. 15,39,40 Rare LBs were described in the substantia nigra of approximately 10% of PDC patients and these often co-localized within neurons containing NFTs. 2,8 More recently, Yamazaki et al 26 described the presence of α-synuclein pathology in 5 of 13 PDC patients primarily within the amygdala. In the present study, we found α-synuclein pathology in the form of both LBs and Lewy neurites in the amygdala of 37% of PDC and PDC+ Chamorro patients. LBs were not detected in the amygdala in any of the patients with ALS and only 1 of 8 Guamanian control patients, ranging in age from 41 to 86 years. This Chamorro was an 86-year-old female diagnosed with AD. In contrast, similar to previous reports, tau pathology was found in six of these control Chamorros. 15,40 Thus, unlike NFTs, LBs do not occur in older Chamorros without PDC or other synucleinopathies. Furthermore, it is argued that PDC and ALS of Guam are distinct disease entities, rather than a single disorder with different clinical and pathological features. 2 The absence of α-synuclein pathology in all of the patients with ALS might support the two-disease hypothesis. However, the limited number of cases with ALS and the identification of synuclein pathology in only a subset of PDC patients preclude this conclusion until additional cases are analyzed.
α-Synuclein pathology has been identified primarily in the amygdala in several disorders with prominent tau pathology including sporadic AD, 24,25 familial AD, 19 Down’s syndrome with AD, 20 and neurodegeneration with brain iron accumulation. 21-23 While there are rare reports of coexistent Parkinson’s disease/dementia with LBs and tau disorders such as progressive supranuclear palsy and corticobasal degeneration, 41-45 Guam PDC represents the first example of a primary tauopathy with synuclein aggregation in a large subset of patients. In all of these disorders with both α-synuclein and tau pathology there is often co-localization of the aggregates within at least a subset of neurons in the amygdala. 23,26,46-49 It is unclear whether the coexistence of both pathologies within the same neuron represents a chance event in a highly affected brain region or if tau and synuclein interact to promote their mutual aggregation. However, the selective vulnerability of the neurons within the amygdala to develop both LBs and NFTs probably plays a mechanistic role in the severe neurodegeneration often observed in the amygdala. The reason for the convergence of tau and α-synuclein pathology in the neurons of the amygdala remains largely unknown. One recent report suggests an interaction between these two molecules whereby α-synuclein binds directly to tau and stimulates the phosphorylation of tau by protein kinase A. 50 The authors proposed that by modulating the phosphorylation state of tau, this kinase affects the stability of axonal microtubules, but it is unclear how this would lead to the aggregation of both tau and α-synuclein.
The neurofibrillary pathology in Guam ALS/PDC is composed of insoluble, hyperphosphorylated tau that is similar biochemically and ultrastructurally to what is observed in AD. 5,6 In this study, we also characterized tau and α-synuclein pathology biochemically. We detected a pattern of insoluble tau similar to that of AD in the amygdalas of Guam PDC patients which was composed of all six tau isoforms and was hyperphosphorylated and heavily aggregated. In addition, in the brains of 2 of 3 patients with α-synuclein pathology detected by immunohistochemistry, we observed aggregated α-synuclein similar to that observed in LBVAD and other synucleinopathies. 19,51 It is unclear why case 21 which showed evidence of α-synuclein pathology by immunohistochemistry did not show biochemical evidence of insoluble aggregated synuclein. However, the amygdala tissue obtained for the biochemical analysis was procured from the hemisphere contralateral to that used for immunohistochemical analysis and it is not known whether there was α-synuclein pathology in this hemisphere. Furthermore, similar to tau, the amount of insoluble α-synuclein detected is highly variable and may simply be below the level of detection of the Western blot.
Despite the similarities of both the biochemical and immunohistochemical characteristics of tau and α-synuclein to that observed in AD and LBVAD, in Guam PDC, both proteins aggregate independent of β-amyloid suggesting a common disease mechanism in all of these disorders. However, a recent study in transgenic mice suggests that Aβ deposits may augment LB formation. 52 This notwithstanding, it is clear that further studies are needed to elucidate mechanisms of aggregation of these molecules and facilitate an understanding of their role in the neurodegenerative process.
Acknowledgments
We thank the Chamorro families of the many Guamanian patients studied here for making this research possible.
Footnotes
Address reprint requests to John Q. Trojanowski, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 3400 Spruce Street, Maloney Building, 3rd Floor, Philadelphia, PA 19104. E-mail: trojanow@mail.med.upenn.edu.
Supported by National Institute of Health postdoctoral training grant T32 AG00255 (to M.S.F.) and by grants from the National Institute of Aging of the National Institutes of Health, the Dana Foundation, and the Alzheimer’s Association.
V.M.-Y.L. is the John H. Ware third Chair of Alzheimer’s disease research at the University of Pennsylvania.
References
- 1.Murakami N: Parkinsonism-dementia complex on Guam. J Neurol 1999, 246(Suppl 2):II16-II18 [DOI] [PubMed] [Google Scholar]
- 2.Oyanagi K, Wada M: Neuropathology of parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 1999, 246(Suppl 2):II19-II27 [DOI] [PubMed] [Google Scholar]
- 3.Kurland LT: Amyotrophic lateral sclerosis and Parkinson’s disease complex on Guam linked to an environmental neurotoxin. Trends Neurosci 1988, 11:51-54 [DOI] [PubMed] [Google Scholar]
- 4.Poorkaj P, Tsuang D, Wijsman E, Nemens E, Garruto R, Craig UK, Anderson L, Bird T, Plato CC, Weiderholt W, Galasko D, Schellenberg GD: Tau is a candidate gene for amyotropic lateral sclerosis-parkinsonism dementia complex. Arch Neurol 2001, 58:1871-1878 [DOI] [PubMed] [Google Scholar]
- 5.Buée-Scherrer V, Buée L, Hof PR, Leveugle B, Gilles C, Loerzel AJ, Perl DP, Delacourte A: Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam: immunochemical characterization of tau proteins. Am J Pathol 1995, 146:924-932 [PMC free article] [PubMed] [Google Scholar]
- 6.Mawal-Dewan M, Schmidt ML, Balin B, Perl DP, Lee VM-Y, Trojanowski JQ: Identification of phosphorylation sites in PHF-TAU from patients with Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex. J Neuropathol Exp Neurol 1996, 55:1051-1059 [PubMed] [Google Scholar]
- 7.Hirano A, Dembitzer HM, Kurland LT, Zimmerman HM: The fine structure of some intraganglionic alterations: neurofibrillary tangles, granulovacuolar bodies, and “rod-like” structures as seen in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J Neuropathol Exp Neurol 1968, 27:167-182 [PubMed] [Google Scholar]
- 8.Hirano A, Malamud N, Elizan TS, Kurland LT: Amyotrophic lateral sclerosis and parkinsonism-dementia complex on Guam: further pathologic studies. Arch Neurol 1966, 15:35-51 [DOI] [PubMed] [Google Scholar]
- 9.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR: Tau protein isoforms, phosphorylation, and role in neurodegenerative disorders. Brain Res Rev 2000, 33:1-36 [DOI] [PubMed] [Google Scholar]
- 10.Forman MS, Lee VM-Y, Trojanowski JQ: New insights into genetic and molecular mechanisms of brain degeneration in tauopathies. J Chem Neuroanat 2000, 20:225-244 [DOI] [PubMed] [Google Scholar]
- 11.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA: Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 1989, 8:393-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA: Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3:519-526 [DOI] [PubMed] [Google Scholar]
- 13.Hof PR, Perl DP, Loerzel AJ, Steele JC, Morrison JH: Amyotrophic lateral sclerosis and parkinsonism-dementia from Guam: differences in neurofibrillary tangle distribution and density in the hippocampal formation and neocortex. Brain Res 1994, 650:107-116 [DOI] [PubMed] [Google Scholar]
- 14.Hof PR, Perl DP, Loerzel AJ, Morrison JH: Neurofibrillary tangle distribution in the cerebral cortex of parkinsonism-dementia cases from Guam: differences with Alzheimer’s disease. Brain Res 1991, 564:306-313 [DOI] [PubMed] [Google Scholar]
- 15.Oyanagi K, Makifuchi T, Ohtoh T, Chen KM, Gajdusek DC, Chase TN: Distinct pathological features of the gallyas- and tau-positive glia in the parkinsonism-dementia complex and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 1997, 56:308-316 [DOI] [PubMed] [Google Scholar]
- 16.Gentleman SM, Perl D, Allsop D, Clinton J, Royston MC, Roberts GW: β(A4)-amyloid protein and parkinsonian-dementia complex of Guam. Lancet 1991, 337:55-56 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt ML, Lee VM-Y, Saido T, Perl D, Schuck T, Iwatsubo T, Trojanowski JQ: Amyloid plaques in Guam amyotrophic lateral sclerosis/parkinsonism-dementia complex contain species of Aβ similar to those found in the amyloid plaques of Alzheimer’s disease and pathological aging. Acta Neuropathol (Berl) 1998, 95:117-122 [DOI] [PubMed] [Google Scholar]
- 18.Duda JE, Lee VM-Y, Trojanowski JQ: Neuropathology of synuclein aggregates. J Neurosci Res 2000, 61:121-127 [DOI] [PubMed] [Google Scholar]
- 19.Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM-Y, Iwatsubo T, Trojanowski JQ: Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 1998, 153:1365-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippa CF, Schmidt ML, Lee VM-Y, Trojanowski JQ: Antibodies to α-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol 1999, 45:353-357 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi S, Akasaki Y, Morimura Y, Takauchi S, Sato M, Miyoshi K: An autopsy case of late infantile and juvenile neuroaxonal dystrophy with diffuse Lewy bodies and neurofibrillary tangles. Clin Neuropathol 1992, 11:1-5 [PubMed] [Google Scholar]
- 22.Wakabayashi K, Fukushima T, Koide R, Horikawa Y, Hasegawa M, Watanabe Y, Noda T, Eguchi I, Morita T, Yoshimoto M, Iwatsubo T, Takahashi H: Juvenile-onset generalized neuroaxonal dystrophy (Hallervorden-Spatz disease) with diffuse neurofibrillary and Lewy body pathology. Acta Neuropathol (Berl) 2000, 99:331-336 [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Kawai M, Inoue K, Sasaki R, Arai H, Nanba E, Kuzuhara S, Ihara Y, Kanazawa I, Murayama S: Widespread expression of α-synuclein and tau immunoreactivity in Hallervorden-Spatz syndrome with protracted clinical course. J Neurol Sci 2000, 177:48-59 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton RL: Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol 2000, 10:378-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazee AM, Han LY: Cortical Lewy bodies in Alzheimer’s disease. Arch Pathol Lab Med 1995, 119:448-453 [PubMed] [Google Scholar]
- 26.Yamazaki M, Arai Y, Baba M, Iwatsubo T, Mori O, Katayama Y, Oyanagi K: α-Synuclein inclusions in amygdala in the brains of patients with the parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 2000, 59:585-591 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt ML, Carden MJ, Lee VM-Y, Trojanowski JQ: Phosphate-dependent and -independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest 1987, 56:282-294 [PubMed] [Google Scholar]
- 28.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VM-Y: A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res 2000, 59:528-533 [DOI] [PubMed] [Google Scholar]
- 29.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM-Y: Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 2000, 290:985-989 [DOI] [PubMed] [Google Scholar]
- 30.Goedert M, Jakes R, Vanmechelen E: Monoclonal antibody AT8 recognizes tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 1995, 189:167-169 [DOI] [PubMed] [Google Scholar]
- 31.Mercken M, Vandermeeren M, Lubke U, Six J, Boons J, Van d V, Martin JJ, Gheuens J: Monoclonal antibodies with selective specificity for Alzheimer tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol (Berl) 1992, 84:265-272 [DOI] [PubMed] [Google Scholar]
- 32.Greenberg SG, Davies P: A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 1990, 87:5827-5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ: Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem 1995, 270:18917-18922 [DOI] [PubMed] [Google Scholar]
- 34.Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM-Y, Lee G: Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1988, 1:817-825 [DOI] [PubMed] [Google Scholar]
- 35.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM-Y: Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282:1914-1917 [DOI] [PubMed] [Google Scholar]
- 36.Puchtler H, Sweat F, Levine M: On the binding of Congo red by amyloid. J Histochem Cytochem 1962, 10:355-364 [Google Scholar]
- 37.Lee VM-Y, Wang J, Trojanowski JQ: Purification of paired helical filament tau and normal tau from human brain tissue. Methods Enzymol 1999, 309:81-89 [DOI] [PubMed] [Google Scholar]
- 38.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y: Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron 1999, 24:751-762 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt ML, Garruto R, Chen J, Lee VM-Y, Trojanowski JQ: Tau epitopes in spinal cord neurofibrillary lesions in Chamorros of Guam. Neuroreport 2000, 11:3427-3430 [DOI] [PubMed] [Google Scholar]
- 40.Chen L: Neurofibrillary change on Guam. Arch Neurol 1981, 38:16-18 [DOI] [PubMed] [Google Scholar]
- 41.Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS: Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 1997, 48:959-969 [DOI] [PubMed] [Google Scholar]
- 42.Mori H, Yoshimura M, Tomonaga M, Yamanouchi H: Progressive supranuclear palsy with Lewy bodies. Acta Neuropathol (Berl) 1986, 71:344-346 [DOI] [PubMed] [Google Scholar]
- 43.D’Amato CJ, Sima AF, Foster NL, Dickson DW, Hicks SP: Cerebral Lewy bodies with progressive supranuclear palsy. J Neuropathol Exp Neurol 1991, 50:308-314 [Google Scholar]
- 44.Gearing M, Olson DA, Watts RL, Mirra SS: Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology 1994, 44:1015-1024 [DOI] [PubMed] [Google Scholar]
- 45.Judkins AR, Forman MS, Uryu K, Hinkle DA, Asbury AK, Lee VM-Y, Trojanowski JQ: Co-occurrence of Parkinson’s disease with progressive supranuclear palsy. Acta Neuropathol (Berl) 2002, 103:103:526-530 [DOI] [PubMed] [Google Scholar]
- 46.Iseki E, Marui W, Kosaka K, Ueda K: Frequent coexistence of Lewy bodies and neurofibrillary tangles in the same neurons of patients with diffuse Lewy body disease. Neurosci Lett 1999, 265:9-12 [DOI] [PubMed] [Google Scholar]
- 47.Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Ueda K, Ikeda K, Kawai M: Cellular co-localization of phosphorylated tau- and NACP/α-synuclein-epitopes in Lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res 1999, 843:53-61 [DOI] [PubMed] [Google Scholar]
- 48.Marui W, Iseki E, Ueda K, Kosaka K: Occurrence of human α-synuclein immunoreactive neurons with neurofibrillary tangle formation in the limbic areas of patients with Alzheimer’s disease. J Neurol Sci 2000, 174:81-84 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt ML, Martin JA, Lee VM-Y, Trojanowski JQ: Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer’s disease and Lewy body disorders. Acta Neuropathol (Berl) 1996, 91:475-481 [DOI] [PubMed] [Google Scholar]
- 50.Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R: α-Synuclein binds to tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem 1999, 274:25481-25489 [DOI] [PubMed] [Google Scholar]
- 51.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM-Y, Trojanowski JQ, Iwatsubo T: Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 1998, 152:879-884 [PMC free article] [PubMed] [Google Scholar]
- 52.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L: β-Amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA 2001, 98:12245-12250 [DOI] [PMC free article] [PubMed] [Google Scholar]