Abstract
We previously reported that ligand-independent signaling by highly expressed CD30 in Hodgkin-Reed-Sternberg (H-RS) cells is responsible for constitutive activation of NF-κB. In the present study, we characterize the intracellular localization of tumor necrosis factor (TNF) receptor associated factor (TRAF) proteins in H-RS cells. Confocal immunofluorescence microscopy of cell lines derived from H-RS cells and HEK293 transformants highly expressing CD30 revealed aggregation of TRAF2 and TRAF5 in the cytoplasm as well as clustering near the cell membrane. In contrast, TRAF proteins were diffusely distributed in the cytoplasm in cell lines unrelated to Hodgkin’s disease (HD) and control HEK293 cells. Furthermore, the same intracellular distribution of TRAF proteins was demonstrated in H-RS cells of lymph nodes of HD, but not in lymphoma cells in lymph nodes of non-Hodgkin’s lymphoma. Dominant-negative TRAF2 and TRAF5 suppressed cytoplasmic aggregation along with constitutive NF-κB activation in H-RS cell lines. Confocal immunofluorescence microscopy also revealed co-localization of IKKα, NIK, and IκBα with aggregated TRAF proteins in H-RS cell lines. These results suggest involvement of TRAF protein aggregation in the signaling process of highly expressed CD30 and suggest they function as scaffolding proteins. Thus, cytoplasmic aggregation of TRAF proteins appears to reflect constitutive CD30 signaling which is characteristic of H-RS cells.
Tumor necrosis factor receptor (TNFR)-associated factor (TRAF) proteins are adapter molecules that associate with the cytoplasmic region of TNFR superfamily members, which lack intrinsic catalytic activity in their cytoplasmic domains. Thus far, six TRAF proteins, TRAF1 to TRAF6, have been identified, of which TRAF4 is not known to interact with any receptors. 1,2 Ligation of TNF superfamily members with their cognate receptors leads to recruitment of a defined set of TRAF proteins to the receptors and transduces signals to downstream effectors which are transcription factors in the NF-κB and AP-1 family. 3-5 These transcription factors can induce expression of target genes involved in various aspects of cellular and immune functions. In addition, activation of NF-κB and AP-1 has been shown to protect cells from apoptosis via transcription of anti-apoptotic genes. 6,7
Hodgkin’s disease (HD) is a malignant lymphoma characterized by a small number of tumor cells, ie, Hodgkin/Reed-Sternberg (H-RS) cells, in a background of reactive cells. 8-10 H-RS cells represent an expansion of a single clone originating from germinal center B-cells. 11-14 However, biological mechanisms of growth, regulation, and death of H-RS cells have remained unclear for a long period. Recently, constitutively activated nuclear factor-κB (NF-κB) (p50/p65) was reported to be a unique and common characteristics of H-RS cells, which prevent them from undergoing apoptosis and trigger proliferation. 15-20 Constitutive NF-κB activation is considered to be a molecular basis for aberrant growth and cytokine gene expression of H-RS cells, which, in turn, is a basis for clinical and histological characteristics of Hodgkin’s disease.
CD30 is a member of the TNFR superfamily, 21-23 originally discovered as a marker protein highly expressed on the surface of H-RS cells. 24 Other investigators and our group have shown that CD30 signals leading to NF-κB activation are mediated by interactions with TRAF2 and TRAF5. 25-28 We recently discovered that high expression of CD30 results in ligand-independent constitutive activation of NF-κB. 29 Our results indicate that high expression of CD30 leads to self-aggregation and recruitment of TRAF2 and TRAF5, causing constitutive activation of NF-κB. Furthermore, constitutive NF-κB activation in H-RS cells could be inhibited by adenovirus vector-mediated transduction of a decoy mutant of CD30, a mutant lacking the cytoplasmic region, which down-regulates IL-13 expression and induces apoptosis of H-RS cells. These results suggest that constitutive CD30 signaling is the basis for growth of tumor cells as well as for characteristic clinical and histological features of HD. In the course of this study, we observed cytoplasmic aggregation of TRAF proteins as well as membrane co-localization with CD30 in H-RS cells. The latter appeared to reflect recruitment of TRAF protein to the cytoplasmic region of highly expressed and self-aggregated CD30. However, significance of the cytoplasmic aggregation of TRAF proteins remains to be clarified. In the present study, we examined whether or not this finding is characteristic to H-RS cells, and is associated with signaling process of CD30. The results showed that cytoplasmic aggregation of TRAF2 and TRAF5 proteins depends on constitutive signaling by CD30 and is a unique and common characteristic of H-RS cells in lymph nodes and cell lines derived from them. Cytoplasmic aggregates of TRAF proteins co-localize with IKKα, NIK, and IκBα, suggesting that they serve as a docking platform for CD30 signaling.
Materials and Methods
Cell Cultures
Jurkat, Raji, U-937, HL-60, K-562, HEK293, and HEK293T cell lines were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan) and Fujisaki Cell Biology Center (Okayama, Japan). HD-MYZ, KMH-2, L428, L540, and Karpas 299 cell lines were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Non-adherent cell lines were cultured in RPMI 1640 and adherent cells in Dulbecco’s modified Eagle’s medium (DMEM) with supplementation of recommended concentrations of fetal calf serum (FCS) and antibiotics.
Isolation of Stable Transformants Expressing the Wild-Type or Mutant CD30
Establishment of HEK293 transformants expressing CD30 or its mutant were described previously. 29 Briefly, expression vectors for the wild-type human CD30 (pME-hCD30wt), CD30 lacking the cytoplasmic tail (pME-hCD30d178) or a vacant vector were transfected with pRSV-Neo into HEK293 cells. Cells were selected with 400 μg/ml of G418 (Invitrogen, Tokyo), and surviving cells were cloned by limiting dilution. Transformants expressing the wild-type CD30 were named 293CD30, whereas those transfected with a vacant vector were referred to as 293vec.
Plasmids and cDNA
The expression vector for human CD30 was described elsewhere. 28,30 Plasmids that express FLAG-tagged TRAF2 and TRAF5, and those for truncated TRAF2 and TRAF5 proteins retaining only the TRAF domain were also described elsewhere. 28
Immunohistochemistry
Immunohistochemical analysis of cultured cells and biopsied lymph nodes was done as described, 29 using a confocal microscope (Radiance 2000, Bio-Rad, Hercules, CA), Primary antibodies were as follows: anti-TRAF2 (C-20) rabbit antibody (Santa Cruz, Santa Cruz, CA), anti-N-terminal TRAF2 (N-19) rabbit antibody (Santa Cruz), anti-C-terminal TRAF2 (H-10) mouse monoclonal antibody (Santa Cruz), anti-TRAF5 (C-19) goat antibody (Santa Cruz), anti-CD30 mouse monoclonal antibody (BerH2) (DAKO, Kyoto, Japan), anti-C-terminal CD30 goat antibody (Santa Cruz), anti-IKKα (B-8) mouse monoclonal antibody (Santa Cruz), anti-NIK (A-12) mouse monoclonal antibody (Santa Cruz), and anti-IκBα (H-4) mouse monoclonal antibody (Santa Cruz). Secondary antibodies with a fluorochrome used in these studies are as follows: FITC-labeled anti-mouse immunoglobulin sheep antibody, Texas Red-labeled anti-mouse immunoglobulin sheep antibody, FITC-labeled anti-rabbit donkey antibody, Texas Red-labeled anti-rabbit donkey antibody (all from Amersham Bioscience, Piscataway, NY), and FITC-labeled anti-goat donkey antibody (Santa Cruz). To detect TRAF proteins in lymph node samples, a modification of the tyramide signal amplification (TSA) system (Perkin Elmer, Tokyo, Japan) was used to facilitate use of streptavidin-FITC or streptavidin-Texas Red instead of peroxidase-conjugated streptavidin.
Immunoblotting
Immunoblotting experiments to detect expression of TRAF2 in soluble and insoluble fractions were done as described. 31 Separation of both fractions was confirmed by detection of lamin A. Antibodies used were as follows: anti-TRAF2 rabbit antibody (Santa Cruz), anti-lamin A (C-20) goat antibody (Santa Cruz), and anti-FLAG monoclonal antibody (M2) (Kodak, Rochester, NY). Secondary antibodies used are as follows: peroxidase-conjugated anti-goat immunoglobulin rabbit antibody (Sigma), HRP-linked anti-mouse immunoglobulin sheep antibody and HRP-linked anti-rabbit donkey antibody (both from Amersham Bioscience). Antibody binding was detected, using ECL chemiluminescence kits (Amersham Bioscience).
Reporter Gene Assays
Activation of NF-κB was tested by a reporter gene assay in HEK293, its transformants and H-RS cell lines, using a κB-site dependent luciferase vector, [κB]6-TK-Luc, as described. 28,30 Renilla luciferase expression vector driven by the herpes simplex virus thymidine kinase promoter, pRL-TK, (Promega, Madison, WI) was co-transfected to standardize each experiment. Luciferase activity was measured by Dual Luciferase assay kit (Promega).
Electrophoretic Mobility Shift Analysis
Detection of NF-κB by electrophorectic mobility shift analysis (EMSA) was done as described, 28,30 using a double-stranded oligonucleotide probe purchased from Promega.
Results
Aggregation of TRAF2 and TRAF5 in the Cytoplasm
In previous experiments, we observed cytoplasmic aggregates of TRAF2 and TRAF5, as well as co-localization of TRAF proteins with CD30 near the cell membrane, in two H-RS cell lines, L428 and HDLM2. 29 To determine whether this cytoplasmic aggregation of TRAF proteins is characteristic of H-RS cells, we screened their expression in other H-RS cell lines and cell lines unrelated to HD, as well as in lymph node samples of patients with HD or non-Hodgkin’s lymphoma (NHL). All H-RS cell-derived cell lines displayed cytoplasmic aggregates of TRAF 2 and TRAF5 (Figure 1A) ▶ , whereas in non-H-RS cell-derived cell lines both proteins were diffusely distributed in the cytoplasm (Figure 1B) ▶ . Immunoblot analysis showed no differences in levels of TRAF protein expression between H-RS cell derived cell lines and those unrelated to H-RS cells (Figure 1C) ▶ . Aggregation of TRAF2 and TRAF5 was also observed in H-RS cells in the biopsied lymph nodes of HD patients (Figure 1D ▶ , left), whereas no aggregation was observed in lymphoma cells of NHL (Figure 1D ▶ , right). The same results were confirmed in four HD samples and five samples of NHL (diffuse large B cell lymphoma). These results suggest that cytoplasmic aggregation of TRAF proteins may be a unique and characteristic feature of H-RS cells in vivo and in vitro.
Figure 1.
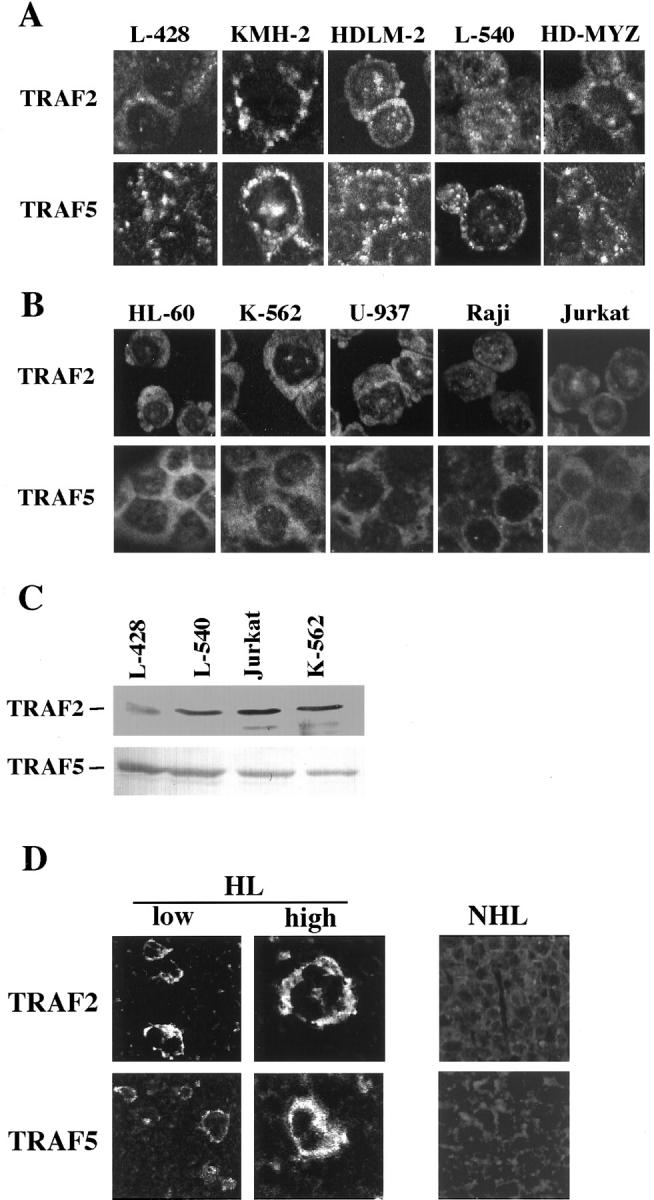
A: Laser confocal immunofluorescence microscopy for intracellular distribution of TRAF2 and TRAF5 proteins. H-RS derived cell lines. Aggregation of TRAF2 and TRAF5 in the cytoplasm is clearly shown. Original magnification, ×400. B: Hematopoietic and lymphoid cell lines unrelated to Hodgkin’s disease. Both TRAF proteins are diffusely distributed in the cytoplasm. Original magnification, ×400. For A and B, following antibodies are used. First antibodies: anti-TRAF2 (C-20) rabbit antibody (Santa Cruz) and anti-TRAF5 (C-19) goat antibody. Secondary antibodies: FITC-labeled anti-rabbit donkey antibody (Amersham Pharmacia Biotech), FITC-labeled anti-goat donkey antibody (Santa Cruz). C: Immunoblot analysis of TRAF2 and TRAF5 showing little difference in the amounts of expressed TRAF proteins. D: Laser confocal immunofluorescence microscopy of biopsied lymph nodes for intracellular distribution of TRAF2 and TRAF5 proteins. HL, a lymph node of Hodgkin’s lymphoma; NHL, a lymph node of non-Hodgkin’s lymphoma (diffuse large B cell type). Instead of secondary antibodies, streptavidin-FITC or streptavidin-Texas Red was used in modified TSA system (NEN Life Science). Original magnification, ×200.
Cytoplasmic Aggregation of TRAF2 and TRAF5 Is Involved in CD30-Mediated Constitutive NF-κB Activation
We recently reported that high expression of CD30 is responsible for constitutive activation of NF-κB in H-RS cells. 29 Thus, we next examined whether or not aggregation of TRAF proteins depends on activation of the CD30-NF-κB signaling pathway. First we examined aggregation of TRAF proteins in HEK293 transformants overexpressing CD30 and having constitutively activated NF-κB (293CD30). Confocal immunofluorescence microscopy revealed cytoplasmic aggregation of TRAF 2 and TRAF5 in these cells, whereas no aggregation of TRAF proteins was observed in control HEK293 transformants with empty vector (293vec), which showed a diffuse cytoplasmic distribution (Figure 2A) ▶ . The expression levels of TRAF proteins were almost the same in 293CD30 and 293vec cells (Figure 2B) ▶ . The difference in distribution patterns between 293CD30 and 293vec cells corresponds to that between H-RS cells and other cell lines. These results suggest that the cytoplasmic aggregation may depend on signaling by highly expressed CD30.
Figure 2.
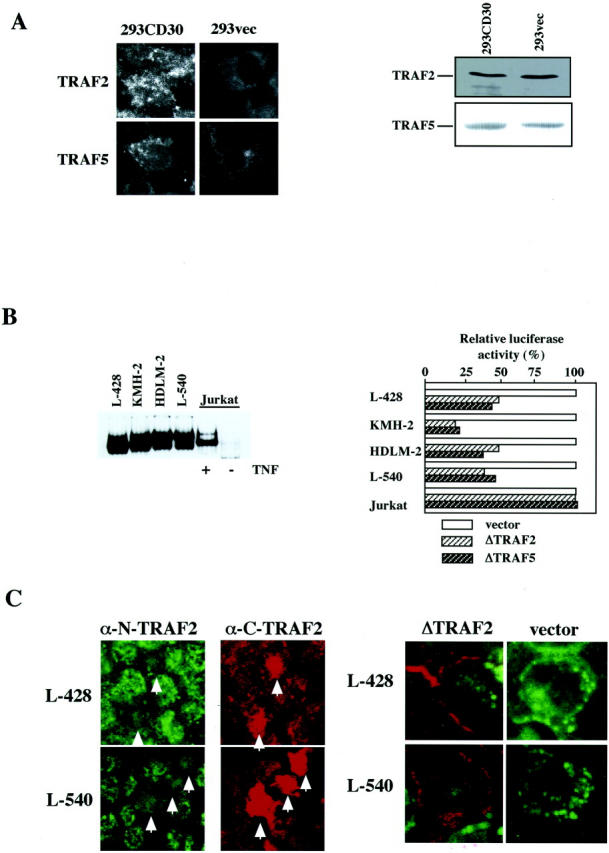
Blocking CD30 signaling by dominant-negative TRAF proteins in H-RS cell lines. A: Left, aggregation of TRAF2 and TRAF5 in HEK293 cells highly expressing CD30. TRAF2 and TRAF5 were aggregated in HEK293 cells highly expressing CD30 and retaining constitutive activation of NF-κB (293CD30), but not in HEK293 cells with an empty vector (293vec). Immunofluorescence studies were done as described in Figure 1, A and B ▶ . Right, immunoblot analysis of TRAF2 and TRAF5. The levels of TRAF protein expression are almost the same between 293CD30 and 293vec cells. B: Left, electrophoretic mobility shift analysis (EMSA) with an NF-κB probe. H-RS cell derived cell lines show constitutive activation of NF-κB. Jurkat cells treated with TNF-α were used as a positive control. Right, down-regulation of basal activities of NF-κB-driven luciferase by dominant TRAF proteins in H-RS cell lines, but not in Jurkat cells. Luciferase activities are expressed as relative levels of triplicated experiments where those co-transfected with a vacant expression vector are expressed as 100%. Transfection efficiencies were corrected by dual luciferase assays using pRL-TK-Luc. C: Expression of a dominant-negative TRAF2 abrogates cytoplasmic aggregation of endogenous TRAF proteins in L-428 and L-540 cells. Transfected ΔTRAF2 was detected by the C-terminal anti-TRAF2 mouse monoclonal antibody (Santa Cruz) and the endogenous TRAF2 by N-terminal anti-TRAF2 rabbit antibody (Santa Cruz). Secondary antibodies used are Texas Red-labeled anti-mouse immunoglobulin sheep antibody and FITC-labeled anti-rabbit immunoglobulin donkey antibody (both from Amersham Pharmacia Biotech). Strong staining of the highly expressed ΔTRAF2 protein by the C-terminal antibody made it possible to discriminate it from the endogenous TRAF2 protein. In the left panel, figures show the results with anti-N-terminal TRAF2 (N-19) (α-N-TRAF2) rabbit antibody and anti-C-terminal TRAF2 (H-10) (α-C-TRAF2) mouse monoclonal antibody (both from Santa Cruz). Arrows indicate cells that express transduced ΔTRAF2. In the right panel, merged figures of immunofluorescence by anti-N-terminal and anti-C-terminal TRAF2 antibodies. Secondary antibodies used are: FITC-labeled anti-rabbit donkey antibody and Texas Red-labeled anti-mouse immunoglobulin sheep antibody (both from Amersham Pharmacia Biotech). Original magnification, ×400.
As reported previously, electrophoretic mobility shift analysis (EMSA) of nuclear extracts of H-RS cell lines clearly demonstrated constitutive NF-κB activation (Figure 2C ▶ , left). When these cells were transduced with a dominant-negative form of TRAF 2 (ΔTRAF2) or that of TRAF5 (ΔTRAF5), NF-κB-driven promoter activity was down-regulated, whereas in Jurkat cells no down-regulation of basal promoter activity was observed (Figure 2B ▶ , right). Taken together, these results indicate that TRAF2 and TRAF5 are involved in constitutive NF-κB activation in H-RS cells and suggest that their aggregation in the cytoplasm may represent a signaling process of the CD30-TRAF protein-NF-κB pathway.
If above hypothesis is correct, blockade of the CD30-TRAF protein-NF-κB signaling pathway will lead to abrogation of their aggregation in H-RS cells. Therefore, we examined the effects of a dominant-negative form of TRAF2 (ΔTRAF2) on the distribution of endogenous TRAF2 in H-RS cell lines. We used anti-N-terminal TRAF2 antibody to specifically detect endogenous TRAF2, and anti-C-terminal TRAF2 to detect transduced ΔTRAF2 that is highly expressed and strongly stained. Results demonstrated that transduction of ΔTRAF 2 in L428 and L540 cell lines markedly decreased cytoplasmic aggregation of endogenous TRAF2 which showed diffuse cytoplasmic staining (Figure 2C ▶ , left). On the other hand, nuclear aggregation showed little change (Figure 2C ▶ , right). Notably, in these cells transduced ΔTRAF 2 was distributed near the cell membrane (Figure 2C ▶ , right), suggesting its association with the cytoplasmic tail of CD30. Taken together, these results suggest that cytoplasmic aggregates of TRAF 2 and TRAF5 in H-RS cells represent a signaling process emanating from highly expressed CD30.
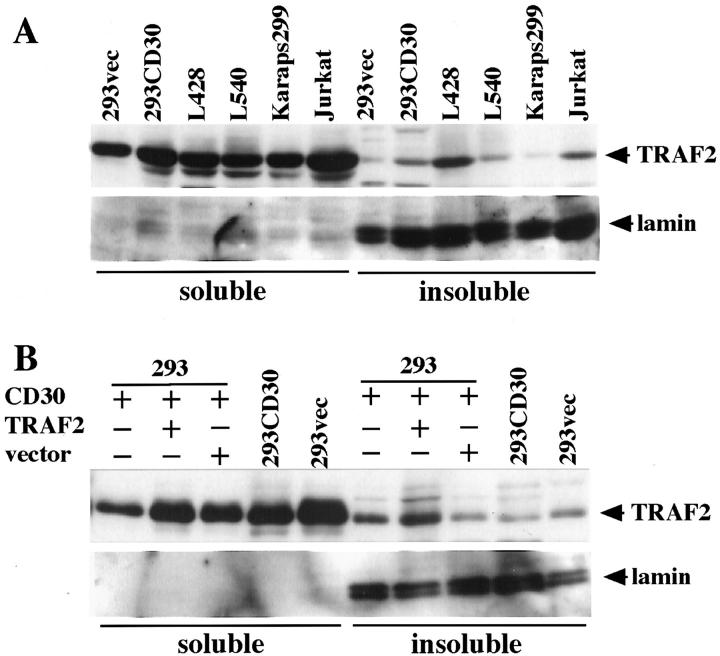
TRAF2 Is Localized in the Detergent-Soluble Fraction of H-RS Cells
A recent report suggested that CD30-mediated TRAF2 activation results in re-distribution of TRAF2, leading to depletion of the cytoplasmic soluble form and production of cytoplasmic insoluble aggregates of TRAF2. 31 To address the question whether or not the cytoplasmic aggregates that we observed in H-RS cells correspond to the cytoplasmic insoluble aggregates in the previous report, we analyzed TRAF2 protein in the soluble or insoluble fraction by immunoblotting. Results showed that in H-RS cell lines as well as in other cell lines tested, TRAF2 protein was mainly found in the soluble fraction and only a small proportion was detected in the insoluble fraction. (Figure 3A) ▶ . It appeared that distribution of TRAF2 protein between these fractions does not change depending on CD30 signaling, since no significant difference was observed in the amounts of TRAF2 protein in each fraction between H-RS cells and other cell lines. These observations contrast with those reported previously, and do not support the notion that TRAF2 redistributes from the soluble to insoluble fractions as a result of CD30 signaling.
Figure 3.
TRAF2 is in the soluble fraction irrespective of CD30 signaling. A: Distribution of TRAF2 in the soluble fraction. In all cell lines examined, the majority of TRAF2 protein was found in the soluble fraction by immunoblot analysis with anti-TRAF2 (top). Successful fractionation was confirmed by blotting of the same membrane with an anti-lamin A antibody (Santa Cruz), where laminA was detected solely in the insoluble fraction (bottom). B: Subcellular distribution of TRAF2 protein in HEK293 cells transiently co-transfected with CD30. Majority of TRAF2 protein was also found in the soluble fraction by immunoblot analysis with anti-TRAF2 antibody (top). Successful fractionation was confirmed by blotting of the same membrane with an anti-lamin A antibody (bottom).
Next we examined the possibility that the different results may be due to transient high expression of CD30 and/or TRAF2 in the previous report. When CD30 and/or TRAF2 was transiently highly expressed in HEK293 cells as was done in the previous report, TRAF2 was mainly found in the soluble fraction (Figure 3B) ▶ . However, the amount of TRAF2 in the insoluble fraction appeared to be slightly increased when compared with that in 293CD30 (Figure 3B ▶ , top, lanes 7 and 9). Taken collectively, cytoplasmic aggregates of TRAF2 observed in H-RS cells appears to be in the soluble fraction, which does not support the notion that cytoplasmic aggregates in H-RS cell lines represents redistribution of TRAF proteins from soluble to insoluble fractions.
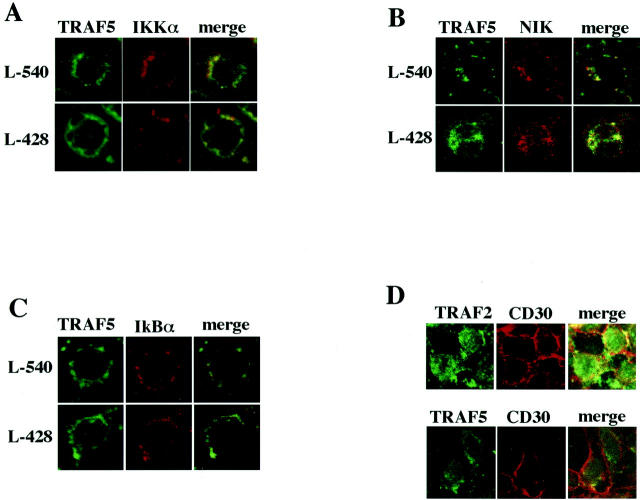
TRAF Proteins Co-Localize with Downstream Signal Transducers in H-RS Cells
If the cytoplasmic aggregation of TRAF proteins reflects constitutive signaling by highly expressed CD30, it may form a complex with downstream signal transducers such as IKKα, NIK, and IκBα. To test this possibility, co-localization of these signal transducers with TRAF proteins in two H-RS cell-derived cell lines, L-540 and L-428, was examined by confocal immunofluorescence microscopy. As shown in Figure 4 A to C ▶ , co-localization of TRAF5 with these signal transducers was clearly demonstrated. Similar results were obtained with TRAF2 (data not shown). However, CD30 did not co-localize with cytoplasmic aggregates of TRAF proteins (Figure 4D) ▶ . These results suggest that the cytoplasmic aggregated TRAF proteins form a complex with downstream signal transducers. Thus, it is likely that the cytoplasmic TRAF proteins serve as a docking platform for downstream kinases and their regulators.
Figure 4.
Co-localization of signal transducers with TRAF5 in H-RS cell derived cell lines. A to C: Laser confocal immunofluorescence microscopy showing co-localization of TRAF5 with IKKα, NIK, and IκBα, respectively. First antibodies used are as follows: anti-TRAF5 (C-19) goat antibody, anti-IKKa (B-8) mouse monoclonal antibody, anti-NIK (A-12) mouse monoclonal antibody and anti-IκBα (H-4) mouse monoclonal antibody (all from Santa Cruz). Secondary antibodies are: FITC-labeled anti-goat donkey antibody and Texas Red-labeled anti-mouse immunoglobulin sheep antibody (both from Santa Cruz). Original magnification, ×400. D: Laser confocal immunofluorescence microscopy reveals that CD30 does not co-localize with cytoplasmic aggregates of TRAF proteins in 293CD30 cells. First antibodies are anti-TRAF2 (C-20) rabbit antibody, anti-TRAF5 (C-19) goat antibody (Santa Cruz), and anti-CD30 mouse monoclonal antibody (BerH2) (DAKO). Secondary antibodies are: FITC-labeled anti-rabbit donkey antibody, FITC-labeled anti-goat donkey antibody, and Texas Red-labeled anti-mouse immunoglobulin sheep antibody (all from Santa Cruz). Original magnification, ×400.
Discussion
In the present study, we demonstrate by confocal immunofluorescence microscopy that cytoplasmic aggregation as well as membrane clustering of TRAF2 and TRAF5 is a unique characteristic of H-RS cells and HEK293 transformants highly expressing CD30. Abrogation of aggregates by a dominant-negative TRAF2 suggests that the cytoplasmic aggregation reflects a dynamic ligand-independent signaling process by CD30 activation in these cells. Furthermore, co-localization of TRAF proteins and kinases such as IKKα and NIK in H-RS cells suggests that TRAF proteins function as molecular scaffolds for CD30 signaling.
We show by confocal immunofluorescence microscopy for the first time that cytoplasmic aggregation of TRAF proteins is a unique and common characteristic of H-RS cells in vivo and in vitro. To date, there has been little information as to the intracellular distribution of endogenous TRAF proteins in lymphoma and leukemia cells. An immunohistochemical screening study for expression of TRAF1 and TRAF2 proteins in Hodgkin’s disease has recently been reported. 32 This study showed frequent expression of TRAF2 in H-RS cells, and noted occasional punctate or diffuse cytoplasmic staining as well as localization to the cell membrane. Our results are partly in line with this observation, and clearly demonstrated that the cytoplasmic aggregation is unique to H-RS cells in vivo and in vitro.
For the TRAF2 molecule, a proteolytic degradation has been suggested. It has been reported that transient high expression of CD30 and TNFR2 degrades co-transfected TRAF2. 33 More recently, a ligand-induced degradation and/or depletion of TRAF2 was also found for various stimuli targeting CD40 34 and TNFR2. 35 However, in the present study we observed almost the same amount of TRAF proteins in cells with constant signaling by CD30 such as H-RS cells and 293CD30 cells as in those without CD30 signaling such as Jurkat and K562 (Figure 1C) ▶ . Discrepancies between our observation and that reported previously may depend on transient or continuous signaling by CD30. In the H-RS cells highly expressed CD30 continuously triggers signaling. 29 Therefore, if signal-induced proteolysis of TRAF2 takes place, the amount of TRAF proteins in H-RS cells appears to be maintained by an enhanced and balanced rate of transcription, translation, and degradation of TRAF proteins. However, kinetics of these parameters remain to be determined in future studies.
Similarly, it has been reported that CD40 activation of NF-κB is mediated by proteolysis of TRAF3, which can be blocked by treatment of pepstatin-A. 36 We did not directly addressed whether TRAF3 is degraded in the H-RS cells we used, however, our results do not exclude possibilities that other receptors are involved in NF-κB activation in H-RS cells. In this regard, it was intriguing that the HDMyZ cell line which does not express CD30 showed the same intracellular distribution of TRAF proteins and constitutive activation of NF-κB (data not shown). We found transduction of a dominant-negative TRAF2 or TRAF5 in this cell line down-regulated NF-κB activity (data not shown), which provides evidence supporting our notion that cytoplasmic aggregation may represent constitutive signaling mediated by TRAF proteins. However, the membrane receptor(s) responsible for activating the signaling pathway in HDMyZ cells remains to be identified.
We showed that the cytoplasmic aggregation of TRAF proteins in H-RS cells is dependent on CD30 signaling. Previously, Arch et al 31 reported perinuclear clustered aggregates of transduced TRAF2 in HEK293 cells in the presence of CD30 signaling transiently co-transfected with a chimeric CD28-CD30. Our results did not show perinuclear aggregates of TRAF proteins. The differences might be explained by transient versus constitutive signaling of CD30, because HEK293 cells were used in both experiments. In the cells with aggregated TRAF proteins and with constant CD30 signaling, ie, H-RS cells and 293CD30, expression of a dominant-negative form of TRAF protein abrogated aggregation of TRAF proteins concurrently with inhibition of NF-κB activation (Figure 2, B and C) ▶ . The results suggest that cytoplasmic aggregation of TRAF proteins depends on CD30 signaling, although the mechanisms of relocation from the cytoplasmic tail of CD30, where they had been recruited, to the cytoplasm and also those of aggregation within the cytoplasm remain to be elucidated. We also noted a possible down-regulation of endogenous TRAF2 expression by blockade of TRAF signaling pathway (Figure 2C) ▶ . This suggests a possibility that expression of TRAF2 may depend on TRAF signaling, which also remain to be examined.
We demonstrated that TRAF proteins are mainly localized within the detergent-soluble fraction irrespective of CD30 signaling (Figure 3) ▶ . However, Arch et al 31 reported that cytoplasmic aggregation of TRAF proteins coincided with a shift of TRAF proteins from the soluble to insoluble fraction of cells. The discrepancies in the results could only be accounted for by the differences in the experimental design. Arch et al 31 analyzed distribution of transduced TRAF2 in HEK293 cells with transient overexpression of a CD28-CD30 chimera along with TRAF2. In contrast, we characterized the detergent solubility of endogenous TRAF proteins using cell lines with or without constitutive CD30 signaling (Figure 3A) ▶ . We also examined the effects of transient high expression of TRAF2 along with CD30 in HEK293 cells (Figure 3B) ▶ . In neither experiment could we reproduce the results reported by Arch et al. 31 In HEK293 cells, we observed a small increase in the amount of TRAF2 in the insoluble fraction; however, most TRAF2 protein remained in the soluble fraction (Figure 3B) ▶ . Thus, we believe that formation of the cytoplasmic aggregates does not represent transition of TRAF2 into the detergent-insoluble fraction.
Although little is known about the relationship between membrane microdomains and CD30 signaling, accumulating evidence has suggested a model of CD40 signaling. In this model, CD40 in the membrane rafts undergo an allosteric shift on binding the ligand, which recruits TRAF proteins and triggers signaling. 34,37 In this model, no idea has been proposed as to the relocation of TRAF proteins after recruitment to the CD40. To date, as described above, what is reported as to the fate of TRAF proteins after signal dependent recruitment to the membrane receptors is proteolytic degradation 33-35 and/or transition to the detergent-insoluble fraction. 31 Transient high expression of CD30 and TNFR-2 induced degradation of co-transfected TRAF2. 33 Recently, a ligand-induced degradation and/or depletion of TRAF2 was also found for various stimuli targeting CD40 34 and TNFR-2. 35 In the present study, we revealed a complex composed of cytoplasmic TRAF proteins and signal transducers such as IKKα, NIK, and IκBα by confocal immunofluorescence microscopy. The results suggest that TRAF proteins serve as a scaffolding proteins providing a docking platform for several components situated in the signaling pathway. Scaffolding proteins ensure precise regulation of signaling by co-localization of successive molecules of the cascade. Future studies will elucidate regulatory roles of the TRAF-containing complex in TRAF-NF-κB signaling in H-RS cells. We show in the present work that CD30 is found on the membrane, but not in the cytoplasm, and TRAF proteins are distributed near the membrane, in the cytoplasm and nuclei. This observation raises the possibility that, on signaling, TRAF proteins are first recruited to the cytoplasmic tail of CD30, form multimers, and then dissociate from CD30 to form a multiprotein complex in the cytoplasm. However, signal-induced translocation of TRAF proteins has not yet been examined. Thus, to address this question, H-RS cells may provide a good model.
Footnotes
Address reprint requests to Toshiki Watanabe, M.D., Ph.D., Division of Pathology, The Institute of Medical Science, The University of Tokyo, 4–6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan. E-mail: tnabe@ims.u-tokyo.ac.jp.
Supported in part by a Grant-in-Aid for Scientific Research and a Grant-in-Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, to T. Watanabe, and by The Mochida Memorial Foundation for Medical and Pharmaceutical Research to R. Horie.
References
- 1.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T: Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 2000, 254:14-24 [DOI] [PubMed] [Google Scholar]
- 2.Wajant H, Henkler F, Scheurich P: The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases, and their regulators. Cell Signal 2001, 13:389-400 [DOI] [PubMed] [Google Scholar]
- 3.Malinin NL, Boldin MP, Kovalenco AV, Wallach D: MAP3K-related kinase involved in NF-κB induction by TNF. Nature 1997, 385:540-544 [DOI] [PubMed] [Google Scholar]
- 4.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H: ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 1998, 2:389-395 [DOI] [PubMed] [Google Scholar]
- 5.Baud V, Liu Z, Bennett B, Suzuki N, Xia Y, Karin M: Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev 1999, 13:1297-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg AA, Baltimore D: An essential role for NF-κB in preventing TNF-α-induced cell death. Science 1996, 274:782-784 [DOI] [PubMed] [Google Scholar]
- 7.Mimden A, Karin M: Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta 1997, 1333:F85-F104 [DOI] [PubMed] [Google Scholar]
- 8.Grüss HJ, Kadin ME: Pathophysiology of Hodgkin’s disease: functional and molecular aspects. Baillieres Clin Haematol 1996, 9:417-446 [DOI] [PubMed] [Google Scholar]
- 9.Harris NL: Hodgkin’s lymphomas: classification, diagnosis, and grading. Semin Hematol 1999, 36:220-232 [PubMed] [Google Scholar]
- 10.Kadin ME, Liebowitz DN: Cytokines and cytokine receptors in Hodgkin’s disease. Mauch PM Armitage JO Diehl V Hoppe RT Weiss LM eds. Hodgkin’s Disease. 1999, :pp 139-157 Lippincott Williams and Wilkins, Philadelphia [Google Scholar]
- 11.Kuppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, Hansmann ML: Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci USA 1994, 91:10962-10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanzler H, Kuppers R, Hansmann ML, Rajewsky K: Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med 1996, 184:1495-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossman J, Annunziata CM, Barash S, Staudt L, Dillon P, He WW, Ricciardi-Castagnoli P, Rosen CA, Carter KC: Reed-Sternberg cell genome expression supports a B-cell lineage. Blood 1999, 94:411-416 [PubMed] [Google Scholar]
- 14.Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, Lammert H, Demel G, Theil J, Wirth T, Stein H: Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000, 95:1443-1450 [PubMed] [Google Scholar]
- 15.Bargou RC, Leng C, Krappmann D, Emmerich F, Mapara MY, Bommert K, Royer HD, Scheidereit C, Dürken B: High-level nuclear NF-κB and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood 1996, 87:4340-4347 [PubMed] [Google Scholar]
- 16.Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, Dürken B: Constitutive nuclear factor-κB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 1997, 100:2961-2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood KM, Roff M, Hay RT: Defective IκBα in Hodgkin cell lines with constitutively active NF-κB. Oncogene 1998, 16:2131-2139 [DOI] [PubMed] [Google Scholar]
- 18.Cabannes E, Khan G, Aillet F, Jarrett RF, Hay RT: Mutation in IκBα gene in Hodgkin’s disease suggest a tumor suppressor role for IκBα. Oncogene 1999, 18:3063-3070 [DOI] [PubMed] [Google Scholar]
- 19.Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F, Mathas S, Krappmann D, Scheidereit C, Stein H, Dürken B: Overexpression of IκBα without inhibition of NF-κB activity and mutations in the I κ B α gene in Reed-Sternberg cells. Blood 1999, 94:3129-3134 [PubMed] [Google Scholar]
- 20.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dürken B, Scheidereit C: Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene 1999, 18:943-953 [DOI] [PubMed] [Google Scholar]
- 21.Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H: Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell 1992, 68:421-427 [DOI] [PubMed] [Google Scholar]
- 22.Smith CA, Farrah T, Goodwin RG: The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 1994, 76:959-962 [DOI] [PubMed] [Google Scholar]
- 23.Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, Martelli MF, Stein H: CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995, 85:1-14 [PubMed] [Google Scholar]
- 24.Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, Diehl V: Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature 1982, 299:65-67 [DOI] [PubMed] [Google Scholar]
- 25.Lee SY, Lee SY, Kandala G, Liou M-L, Choi Y: CD30/TNF receptor-associated factor interaction: NF-κB activation and binding specificity. Proc Natl Acad Sci USA 1996, 93:9699-9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gedrich RW, Gilfillan MC, Ducket CS, Van Dongen JL, Thompson CB: CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J Biol Chem 1996, 271:12852-12858 [DOI] [PubMed] [Google Scholar]
- 27.Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB: Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol 1997, 17:1535-1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T: Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFκB activation. J Biol Chem 1997, 272:2042-2045 [DOI] [PubMed] [Google Scholar]
- 29.Horie R, Watanabe T, Morishita Y, Ito K, Ishida T, Kanegae Y, Saito I, Higishihara M, Mori S, Kadin M, Watanabe T: Ligand-independent signaling by overexpressed CD30 drive NF-κB activation in Hodgkin-Reed-Sternberg Cells. Ongogene (in press) [DOI] [PubMed]
- 30.Horie R, Aizawa S, Nagai M, Ito K, Higashihara M, Ishida T, Inoue J, Watanabe T: A novel domain in the CD30 cytoplasmic tail mediates NFκB activation. Int Immunol 1998, 10:203-210 [DOI] [PubMed] [Google Scholar]
- 31.Arch RH, Gedrich RW, Thompson CB: Translocation of TRAF proteins regulates apoptotic threshold of cells. Biochem Biophys Res Commun 2000, 272:936-945 [DOI] [PubMed] [Google Scholar]
- 32.Murray PG, Flavell JR, Baumforth KRN, Toomey SM, Lowe D, Crocker J, Ambinder RF, Young LS: Expression of the tumor necrosis factor receptor-associated factors 1 and 2 in Hodgkin’s disease. J Pathol 2001, 194:158-164 [DOI] [PubMed] [Google Scholar]
- 33.Duckett CS, Thompson CB: CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev 1997, 11:2810-2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hostager BS, Catlett IM, Bishop GA: Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J Biol Chem 2000, 275:15392-15398 [DOI] [PubMed] [Google Scholar]
- 35.Chan FK, Lenardo MJ: A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol 2000, 30:652-660 [DOI] [PubMed] [Google Scholar]
- 36.Annunziata CM, Safiran YJ, Irving SG, Kasid UN, Cossman J: Hodgkin disease: pharmacologic intervention of the CD40-NF-kB pathway by a protease inhibitor. Blood 2000, 96:2841-2848 [PubMed] [Google Scholar]
- 37.Kaykas A, Worringer K, Sugden B: CD40 and LMP-1 both signal from lipid rafts but LMP-1 assembles a distinct, more efficient signaling complex. EMBO J 2001, 20:2641-2654 [DOI] [PMC free article] [PubMed] [Google Scholar]