Abstract
The development and progression of glomerulosclerosis (GS) is determined by the genetic background. The incidence of end-stage renal disease is increased in postmenopausal women, suggesting that estrogen deficiency may play a role in the accumulation of extracellular matrix by mesangial cells (MCs), which are primarily responsible for the synthesis and degradation of this matrix. Using mouse models that are prone or resistant to the development of GS, we compared the expression of estrogen receptor (ER)-α and ER-β subtypes in GS-prone and GS-resistant glomeruli and isolated MCs, and examined the effects of estrogens on ER, collagen, and matrix metalloproteinase (MMP) expression in MCs. Glomeruli and MCs from GS-prone mice had decreased expression of ER-α and ER-β subtypes and ER transcriptional activity was also decreased in their MCs. Importantly, although 17β-estradiol treatment resulted in decreased collagen accumulation and increased MMP-9 expression and activity in MCs from GS-resistant mice, there was, paradoxically, no effect on collagen accumulation and decreased MMP-9 expression and activity in MCs from GS-prone mice. Thus, GS susceptibility is associated with diminished ER expression in MCs. The renal protective effects of estrogens, including decreased collagen accumulation and increased MMP-9 expression, seem to be blunted in GS-prone MCs.
Glomerulosclerosis (GS), a process characterized by the accumulation of extracellular matrix (ECM) in the mesangium, is thought to result from an imbalance between ECM synthesis and degradation. 1 Mesangial cells (MCs) have a central role in this process because they synthesize both the structural components of ECM, mainly collagens and matrix metalloproteinases (MMP) that are enzymes that degrade ECM. 2,3 The propensity of developing GS in humans as well as in mice is genetically determined. 4 GS susceptibility is inherited in a recessive manner in mice involving at least 8 to 10 loci. 5 We found that the expression of mRNA, proteins, and enzyme activity involved in the synthesis and degradation of ECM found in vivo were also present in MCs isolated from the glomeruli. This was true of MCs from both GS-resistant and GS-prone mice. Thus, MCs isolated from the glomeruli of these mouse strains [C57BL6J (B6) or C57BL6/SJLF1/J (B6SJL) and ROP/Le-+Es1b/ES1a (ROP)] can be used to study the influence of the genetic background on GS. 6 We used MCs to determine whether estrogen responsiveness was dependent on the level of ER subtype expression and to study the effects of estrogens on collagen accumulation and MMP-9 expression and activity.
Before menopause, diabetic women have a lower risk of developing end-stage renal disease (ESRD) than age-matched male diabetics (female:male ratio, 0.68). 7 However, after menopause, this protection is lost (female:male ratio, 1.04). Furthermore, the relative risk is even higher in postmenopausal African-Americans (female:male ratio, 1.33). These data provided the impetus for the current study. We hypothesized that estrogens may protect against GS and that MCs may represent an important target for estrogens in preventing the development or progression of GS.
The effects of estrogens are primarily mediated via two estrogen receptor (ER) subtypes, α and β. 8 We reported that MCs isolated from a GS-resistant B6SJL mouse strain expressed both ER subtypes and that estrogens positively regulated their transcription and translation. We found that 17β-estradiol (E2) up-regulated the expression and activity of MMP-9, an enzyme that degrades ECM components, suggesting that estrogens may act to reduce the accumulation of ECM. 9 Estrogens have also been shown to decrease the synthesis of type I and IV collagens by MCs, which may also contribute to decreased glomerular ECM accumulation. 10,11 In the present study, we compared the expression of the ER subtypes α and β in intact glomeruli and in MCs isolated from GS-resistant and GS-prone mice. We compared the effects of E2 on the expression of ER-α and ER-β subtypes, collagen type I and type IV accumulation, and on MMP expression and activity in MCs derived from both mouse strains. We found that glomeruli and MCs isolated from GS-prone mice had decreased expression of ER-α and ER-β subtypes. There was decreased ER transcriptional activity in MCs from GS-prone mice. Surprisingly, we found that although collagen accumulation was down-regulated and MMP-9 expression and activity was up-regulated by estrogens in MCs isolated from GS-resistant mice, there was no effect on collagen accumulation and a decrease in MMP-9 expression and activity in MCs isolated from GS-prone mice. Thus, MCs from GS-prone mice have two estrogen-related abnormalities, a reduced ER expression and a prosclerotic response after estrogen treatment.
Materials and Methods
Materials
Mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All reagents used for real-time polymerase chain reaction (PCR) were purchased from Perkin Elmer Applied Biosystems (Foster City, CA). Culture media, supplements, and primers were obtained from Life Technologies, Inc. (Grand Island, NY). Charcoal-stripped fetal bovine serum (FBS) was purchased from Hyclone (Pittsburgh, PA). 17β-estradiol (E2), tamoxifen, anti-α-smooth muscle actin and β-galactosidase substrate were purchased from Sigma (St. Louis, MO). ICI 182,780 (ICI) was obtained from Tocris (Ballwin, MO). First Strand cDNA Synthesis Kit for Reverse Transcriptase (RT)-PCR (AMV) and Taq polymerase were purchased from Roche (Indianapolis, IN). Pierce BCA Assay to measure protein concentrations and prestained molecular weight marker was purchased from Bio-Rad Laboratories (Hercules, CA). Human recombinant proteins for ER-α and ER-β were purchased from Panvera (Madison, WI). ER antibodies and their respective blocking peptides (ER-α, MC-20, H-184, and ER-β Y-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Nitrocellulose membranes (Hybond ECL) and films (Hyperfilm ECL) for chemiluminescence detection were from Amersham Pharmacia Biotech (Buckinghamshire, England). TransFast and lysis buffer were purchased from Promega (Madison, WI). Zymography gels were purchased from Invitrogen (San Diego, CA) and zymography standards were from Chemicon (Temecula, CA). To perform enzyme-linked immunosorbent assays, standard collagen type I and type IV were from Collaborative Biomedical Products (Bedford, MA) and collagen type I and type IV antibodies were purchased from Biodesign International (Kennebunk, ME). The secondary biotinylated goat anti-rabbit IgG was from Biosource International (Camarillo, CA).
Isolation of Glomeruli
Kidneys from 8-week-old female C57BL/6J (B6) (n = 4) and ROP/Le-+Es1b/ES1a (ROP) (n = 4) were perfused and 100 glomeruli were isolated from each animal by microdissection at 4°C in a solution containing RNase inhibitors as described previously. 12 Total RNA was extracted using Tri-Reagent 13 and used to assess ER-α and ER-β mRNA levels.
Real-Time PCR
Primer Express (Perkin Elmer Applied Biosystems) was used to determine the optimal primer pairs and probe sequences for mouse ER-α and ER-β. Primer pairs were selected so that they amplified different exons (exons 3 and 4 and exons 5 and 6, for ER-α and ER-β primers, respectively) to prevent the amplification of contaminating genomic DNA. The sequence of probe for ER-α was 5′-TGGGCATGATGAAAGGCGGCA-3′ and labeled FAM (6-carboxyfluorescein) fluorescent spectrum as a reporter. The amplification primer pairs were 5′-GGCTGCGCAAGTGTTACGA-3′ and 5′-TCCTCGGCGGTCTTTCC-3′ for ER-α. For ER-β the sequence of the probe was 5′-TGCACATGATTGGCTGGGCCA-3′ labeled with the TET (tetrachloro-6-carboxyfluorescein) fluorescent spectrum as reporter. The sequences of the primers used for ER-β amplification were 5′-AAGCTGGCTGACAAGGAACTG-3′ and 5′-CCACAAAGCCAGGGATTTTC-3′. RT-PCR reactions were performed using the TaqMan One-step RT PCR Master Mix reagents kit and the ABI Prism 7700 sequence detection system (Perkin Elmer Applied Biosystems) in a total volume of 50 μl of reaction mixture. The TaqMan ribosomal RNA control reagents kit was used to detect 18S ribosomal RNA gene, which represented an endogenous control. Each sample was normalized to the 18S transcript content. The primer probe mixture was purchased from Perkin Elmer Applied Biosystems and used as specified by the manufacturer’s protocol. The standard curves for ER-α, ER-β, and 18S were generated using serially diluted solutions (0.001 to 100 ng) of mRNA from mouse uterus. PCR assays were conducted in duplicate for each sample. Data are expressed in percentage of glomeruli isolated from B6 strain and represent the mean ± SEM of four animals for each group.
Cell Culture
MCs were isolated from microdissected glomeruli of B6SJL and ROP female mice 14 and have been previously characterized. 6 MCs were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 containing phenol red [3/1 (v/v)], supplemented with 20% FBS and used between passages 19 and 26. A second cell line independently isolated from the same strain was used to confirm ER subtypes mRNA and protein expression. To examine ER regulation, MCs were transferred into phenol red-free medium supplemented with charcoal-stripped FBS, as previously described. 15 Proliferation was assessed by culturing MCs in phenol red-free medium supplemented with 20% charcoal-stripped FBS in the presence of increasing concentrations of E2 (0, 0.1, 1, and 10 nmol/L) for 5 days. Cell number, determined every 2 days with a Coulter cell counter (Hialeah, FL), was not affected by the presence of E2 in either MC type (data not shown). For all experiments the number of MCs initially plated was adjusted to obtain a similar cell density at the end of the experiment. The function of endogenous ER, was assessed by transfection experiments, as described previously. 9 To study the regulation of ER subtype mRNA and protein expression, as well as collagen type I and type IV accumulation, and the regulation of MMP expression and activity by E2, MCs were plated and maintained for 3 days in phenol red-free medium supplemented with 20% charcoal-stripped FBS. The medium was replaced by 0.1% charcoal-stripped FBS with increasing concentrations of E2 (0.1 and 1 nmol/L) or with vehicle (ethanol 0.001%) for 24 hours. When the cell layers reached confluency they were harvested for RNA and/or protein collection. The supernatants were saved for the measurement of MMP activity. All experiments (duplicate wells for each condition) were performed in triplicate.
Transfection and Luciferase Assays
Before transfection, MCs were cultured 4 days in phenol red-free medium containing 20% charcoal-stripped FBS. Subsequently, MCs were transfected with the reporter construct, 4ERE-TATA-Luc (0.25 μg/well; generous gift from Dr. D. Shapiro, Department of Biochemistry, University of Illinois, Urbana IL 16 ) as previously described. 9 The TATA-Luc vector, which does not contain an ERE, served as a control. Transfection efficiency was adjusted by co-transfection with pRSV-βgal (0.25 μg/well). MCs were incubated for an additional 24 hours in the presence of 0.1 or 10 nmol/L of E2, 1 μmol/L of ICI, or vehicle (ethanol 0.001%). Three experiments were performed in triplicate. Results are expressed as percentage of vehicle-treated MCs.
Isolation of mRNA and RT-PCR
Total RNA was extracted from confluent cell cultures using Tri-Reagent. 13 RT was performed on 2 μg of total RNA in a total volume of 20 μl. RNA analysis was performed as described previously. 9 Briefly, to assess ER-α and ER-β mRNA expression, cDNA was amplified by PCR using previously described primers. 9,17,18 PCR products were, respectively, 408 and 409 bp. Murine MMP-2, MMP-9, and GAPDH primer pairs were used as previously described, 19 which resulted in PCR productsof 760 bp, 414 bp, and 561 bp in length, respectively. PCR data obtained for ER-α, ER-β, MMP-2, and MMP-9 were normalized to GAPDH signals as previously described. 19 Samples from at least three independent experiments were run in duplicate.
Western Blots
ER-α and ER-β protein expression were assessed by Western blot as described previously. 9 Confluent MC layers were washed once in phosphate-buffered saline and protein was extracted with a lysis buffer. Equal amounts of protein lysates (ER-α) or immunoprecipitates (for ER-β) from each experimental condition were run on a 10% polyacrylamide gel. Experiments were performed in the presence of ER-α and ER-β human recombinant peptides as positive controls, whereas the specificity of the signal was demonstrated by incubating blots with an excess of the corresponding specific immunizing peptide. Three independent experiments were performed in duplicate. Densitometry was performed using ImageJ 1.17 (National Institutes of Health, Bethesda, MD), to determine relative ER-α and ER-β amounts. Results are expressed as percentage of vehicle-treated MCs.
Assessment of Collagens
Samples were collected and enzyme-linked immunosorbent assays were performed as previously described. 20 Briefly, the medium was incubated for 2 hours at 37°C, then in blocking solution for an additional 30 minutes. Incubation with antibody against collagen type IV (1:3000) or collagen type I (1:2000) was performed overnight at 4°C. After the washes, a biotinylated goat anti-rabbit IgG was applied for 2 hours. The concentrations of the type I standards as well as the type IV standards were 0 to 3 ng/well. Final values were expressed as ng/105 cells and results are expressed as percentage of vehicle-treated MCs.
MMP Activity
Cell supernatants were collected 24 hours after treatment. MMP-2 and MMP-9 activities were assessed as described previously. 9 Standards were electrophoresed in parallel. Gels were incubated 18 or 40 hours, respectively, for MMP-2 and MMP-9 in 50 mmol/L of Tris buffer allowing determination of total proteolytic MMP activities with no interference from their associated tissue inhibitors. 21,22 Densitometry was performed using ImageJ 1.17 (National Institutes of Health) to determine relative MMP-2 and MMP-9 activities.
Statistical Analysis
Shown are the mean ± SEM of at least three independent experiments, performed either in duplicate or triplicate(as indicated). Data are expressed as percentage of vehicle-treated MCs. One-way analysis of variance and Dunnett’s multiple comparison post hoc test or Student’s t-test were performed for the statistical analysis.
Results
Microdissected Glomeruli from GS-Prone Mice Express Lower Levels of ER Subtypes mRNA than Those from GS-Resistant Mice
The ER-α and ER-β mRNA levels in glomeruli isolated from GS-prone ROP mice were respectively 50.9 ± 8.9% and 39.9 ± 13.8% of the glomeruli isolated from GS-resistant B6 mice as measured by real-time PCR 23 (Figure 1) ▶ .
Figure 1.
Glomeruli isolated from GS-prone mice express lower levels of ER-α and ER-β subtype mRNA than glomeruli isolated from GS-resistant mice by real-time PCR. Total RNA was obtained from 100 glomeruli. ER-α, ER-β, and 18S transcripts were analyzed by real-time PCR. Graphs show ER-α and ER-β mRNA expression, normalized to 18S. Data are expressed as percentage of glomeruli isolated from the B6 strain. Shown are means ± SEM of samples run in duplicate from four animals for each strain. *, P < 0.05; **, P < 0.01.
MCs from GS-Prone Mice Express Lower Levels of ER Subtypes mRNA and Protein than Those from GS-Resistant Mice
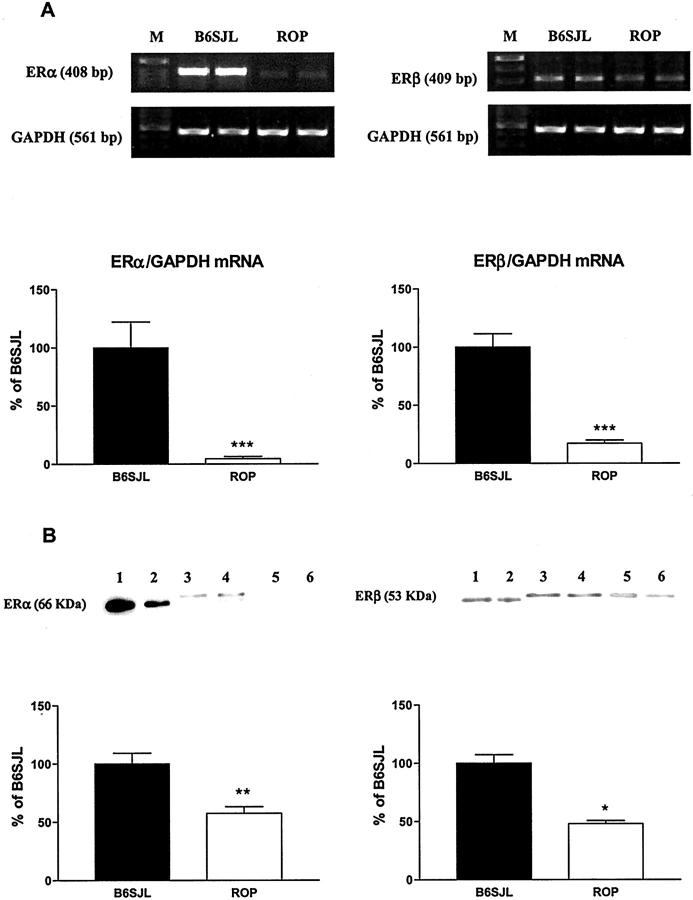
MCs from ROP mice expressed less ER mRNA of both subtypes than did MCs from B6SJL mice (Figure 2) ▶ . The levels of ER-α and ER-β mRNA expression in MCs from ROP mice were 4.5% and 17.2% of MCs from B6SJL mice, respectively (Figure 2A) ▶ . The expression of mouse ER-α (∼67 kd) and ER-β (∼55 kd) protein was examined by Western analysis, using human recombinant ER-α (66 kd) and ER-β (53 kd) peptides as positive controls (Figure 2B ▶ , lanes 1 and 2). The levels of ER-α and ER-β protein expression were lower (57.5% and 48.1% of B6SJL, respectively) in the MCs from ROP mice (Figure 2B ▶ , lanes 5 and 6) than in MCs from B6SJL mice (Figure 2B ▶ , lanes 3 and 4). The corresponding signals were abrogated by preincubation with the respective immunizing peptide (data not shown). Thus, MCs isolated from GS-prone mice express less ER than those isolated from GS-resistant mice. These in vitro results are comparable to the data obtained in isolated glomeruli.
Figure 2.
MCs isolated from GS-prone mice express lower levels of ER-α and ER-β subtypes than MCs isolated from GS-resistant mice. A: Total RNA was collected from MCs grown in DMEM/F12 medium supplemented with 20% FBS. ER-α, ER-β, and GAPDH transcripts were analyzed by RT-PCR. Representative amplicons of ER-α, ER-β, GAPDH, and molecular weight standards (M) were run in parallel. Graphs show ER-α (left) and ER-β (right) mRNA expression normalized to GAPDH. Data are expressed as percentage of MCs isolated from B6SJL mouse. Shown are means ± SEM of three independent experiments. ***, P < 0.001. B: Mouse MC homogenates (ER-α) or immunoprecipitates (ER-β) were analyzed by Western blotting using the ER-α MC-20 antiserum and the ER-β Y-19 antiserum, respectively. Twenty μg of MC homogenates or 25 μl of ER-β immunoprecipitates were loaded, respectively. The 66-kd human recombinant ER-α peptide (10 ng and 5 ng) and the 53-kd human recombinant ER-β peptide (1 μg and 0.5 μg) served as positive controls (lanes 1 and 2). Signals corresponding to the molecular weight of the wild-type ∼67-kd mouse ER-α, the ∼55-kd ER-β for B6SJL-derived MCs (lanes 3 and 4), and from ROP-derived MCs (lanes 5 and 6) were detected in the immunoblots. Graphs show ER-α (left) and ER-β (right) protein expression. Data are expressed as percentage of MCs isolated from B6SJL mouse. Shown are means ± SEM of three independent experiments. Statistical significance is indicated by * and ** (P < 0.05 and P < 0.01, respectively) when compared to MCs isolated from B6SJL mouse.
ER Transcriptional Activity Is Lower in MCs from GS-Prone than in Those from GS-Resistant Mice
The ability of endogenous ER to modulate ER transcriptional activity was assessed using an ERE-containing promoter. A concentration of 10 nmol/L of E2 induced an approximately twofold increase in luciferase activity in MCs from ROP mice, whereas a similar response occurred at a concentration of 0.1 nmol/L of E2 in MCs from B6SJL mice. ICI, an antagonist of both ER-α and ER-β, did not alter baseline transcriptional activity (Figure 3) ▶ . In summary, endogenous ER maintain their function as ligand-regulated transcription factors in MCs from both GS-prone and GS-resistant mice. However, it required a supraphysiological concentration of E2 to induce an increase in transcriptional activity in MCs from GS-prone mice.
Figure 3.
Higher E2 concentration was required to initiate transcriptional activity of ER in MCs from GS-prone mice. MCs were grown in phenol red-free DMEM/F12 supplemented with 20% charcoal-stripped FBS for 4 days. After transfection, MCs were treated with 0 or 0.1 nmol/L of E2, 10 nmol/L of E2, or 1 μmol/L of ICI for 24 hours in phenol red-free medium containing 10% charcoal stripped FBS. Data are expressed as percentage of vehicle-treated MCs from three independent experiments performed in triplicate.
E2 Up-Regulates ER-α mRNA and Protein Expression in MCs from GS-Prone and GS-Resistant Mice
We confirmed our previous observations that E2 induces an increase in ER-α mRNA levels in MCs from B6SJL mice and now show that a similar regulation is present in MCs from ROP mice. 9 ER-α mRNA levels peaked at 1 nmol/L of E2 in MCs from ROP mice. ER-β mRNA expression was not changed in either MCs from ROP or B6SJL mice (Table 1) ▶ . E2 treatment increased ER-α and ER-β protein expression in MCs from both ROP and B6SJL mice. In ROP-derived MCs, 0.1 nmol/L of E2 increased both ER-α and ER-β protein expression after 24 hours of treatment. In B6SJL-derived MCs, 0.1 nmol/L of E2 increased by twofold ER-α protein expression whereas 1 nmol/L of E2 was required to increase ER-β protein expression (Table 1) ▶ . In summary, the response of ER-α and ER-β to E2 treatment did not differ between MCs from GS-prone and GS-resistant mice.
Table 1.
E2 Up-Regulates ER-α and ER-β mRNA and Protein Expression in Both GS-Prone and GS-Resistant MCs.
| nM | B6SJL†, E2, nmol/L | ROP†, E2, nmol/L | ||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 0 | 0.1 | 1 | |
| ER-α mRNA | 100.0 ± 7.5 | 149.8 ± 18.3* | 183.8 ± 27.9* | 100.0 ± 4.0 | 188.1 ± 16.44*** | 196.6 ± 29.3*** |
| ER-α protein | 100.0 ± 1.3 | 201.1 ± 39.2* | 167.2 ± 39.8 | 100.0 ± 0.7 | 137.1 ± 13.6* | 84.88 ± 17.9 |
| ER-β mRNA | 100.0 ± 8.2 | 99.7 ± 9.7 | 127.4 ± 14.5 | 100.0 ± 17.9 | 124.3 ± 24.3 | 128.9 ± 7.1 |
| ER-β protein | 100.0 ± 2.3 | 152.3 ± 24.4 | 214.5 ± 15.1** | 100.0 ± 1.3 | 212.9 ± 20.7** | 171.6 ± 18.1* |
MCs were grown for 3 days in phenol red-free DMEM/F12 supplemented with 20% charcoal-stripped FBS. Medium was replaced with phenol red-free DMEM/F12 medium supplemented with 0.1% charcoal-stripped FBS containing 0, 0.1, or 1 nmol/L of E2 for 24 hours. Data are expressed as percent of vehicle-treated MCs (0.001% ethanol) for each cell type and shown are means ± SEM of at least three independent experiments. Statistical significance is indicated by *, **, and *** (P < 0.05, P < 0.01, and P < 0.001, respectively) compared to vehicle-treated MCs.
†% ± SEM
E2 Down-Regulates Collagen in MCs from GS-Resistant Mice But Not in MCs from GS-Prone Mice
We show that E2 treatment induced a slight decrease in collagen type IV accumulation in MCs from B6SJL mice (85.9 ± 5.4%, 1 nmol/L E2; Figure 4A ▶ ). In contrast, E2 did not induce changes in MCs isolated from the ROP strain (91.3 ± 5.5%, Figure 4B ▶ ). We also confirmed that a similar E2 treatment (1 nmol/L) decreased type I collagen accumulation in MCs isolated from B6SJL mice (79.2 ± 6.0%, P < 0.05) as previously described. 11 In contrast, in ROP-derived MCs, E2 treatment did not change collagen type I levels (91.1 ± 7.6%). In summary, physiological concentrations of E2 decreased type I and type IV collagen accumulation in MCs from GS-resistant mice but not in MCs from GS-prone mice.
Figure 4.
E2 down-regulated collagen type IV accumulation in MCs from GS-resistant mice, but not in MCs from GS-prone mice. MCs were grown for 3 days in phenol red-free DMEM/F12 supplemented with 20% charcoal-stripped FBS. The medium was replaced with phenol red-free DMEM/F12 medium supplemented with 0.1% charcoal-stripped FBS and E2 (1 nmol/L) for 24 hours. Collagen type IV accumulation in the presence of 1 nmol/L of E2 in MCs isolated from B6SJL mice and from ROP mice. Data are expressed as percentage of vehicle-treated MCs (0.001% ethanol, open bars) for each cell type and shown are means ± SEM of three independent experiments. Statistical significance is indicated by * (P < 0.05) for comparison to vehicle-treated MCs.
E2 Increased MMP-9 in MCs from GS-Resistant Mice But Decreased MMP-9 in MCs from GS-Prone Mice
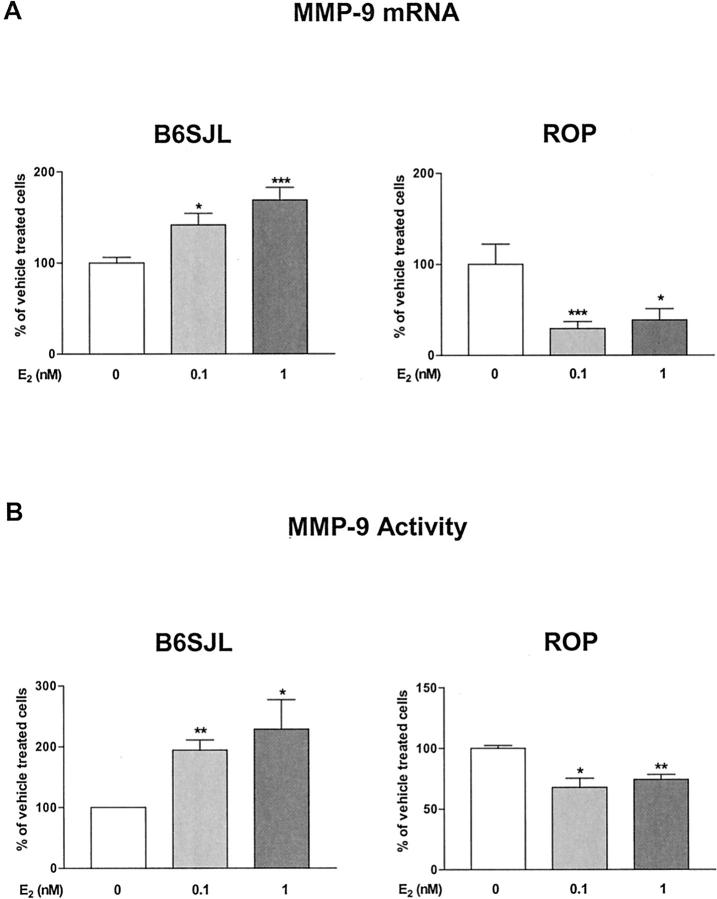
We confirmed that E2 treatment induced a dose-dependent increase in MMP-9 mRNA in MCs from B6SJL mice (169.0 ± 13.6%, 1 nmol/L E2; Figure 5A ▶ ). 9 In contrast, E2 decreased MMP-9 mRNA expression in MCs isolated from the ROP strain (29.25 ± 7.6%, 0.1 nmol/L E2; Figure 5A ▶ ).
Figure 5.
E2 up-regulated MMP-9 mRNA expression and activity in MCs from GS-resistant mice, but down-regulated them in MCs from GS-prone mice. MCs were grown for 3 days in phenol red-free DMEM/F12 supplemented with 20% charcoal-stripped FBS. The medium was replaced with phenol red-free DMEM/F12 medium supplemented with 0.1% charcoal-stripped FBS and E2 (0, 0.1, or 1 nmol/L) for 24 hours. A: MMP-9 mRNA expression in the presence of E2 in MCs isolated B6SJL and from ROP mice. B: MMP-9 activity in the presence of E2 in MCs from B6SJL and ROP MCs. Data are expressed as percentage of vehicle-treated MCs (0.001% ethanol, open bars) for each cell type and shown are means ± SEM of three independent experiments. Statistical significance is indicated by *, **, and *** (P < 0.05, P < 0.01, and P < 0.001, respectively) for comparison to vehicle-treated MCs.
Similarly, MMP-9 activity, after 24 hours of treatment with 1 nmol/L of E2 was markedly increased in MCs from B6SJL mice (228.7 ± 48.5%, Figure 5B ▶ ). Tamoxifen (1 μmol/L) and ICI (1 μmol/L) blocked this increase [107.4 ± 2.0% (NS) and 89.7 ± 21.4% (NS), respectively]. However, in MCs from ROP mice, MMP-9 activity was decreased (67.8 ± 7.5%, Figure 5B ▶ ) after treatment with 0.1 nmol/L of E2. Tamoxifen (1 μmol/L) and ICI (1 μmol/L) blocked this decrease [94.3 ± 13.1% (NS) and 102.4 ± 23.0% (NS), respectively]. Tamoxifen or ICI treatment did not affect baseline MMP-9 activity in MCs from either ROP or B6SJL mice (data not shown).
Treatment of MCs from either ROP or B6SJL mice with physiological concentrations of E2 (0.1 and 1 nmol/L) caused no changes in MMP-2 mRNA levels or activity (data not shown).
In summary, MMP-9 mRNA expression and activity were up-regulated by physiological concentrations of E2 in MCs from GS-resistant mice, but were down-regulated in MCs from GS-prone mice. Because the regulation of MMP-9 activity by E2 was abolished by tamoxifen and by the ER antagonist ICI, the regulation of MMP-9 by E2 is mediated by ER.
ER-α Mediates the Effect of E2 on MMP-9 Activity
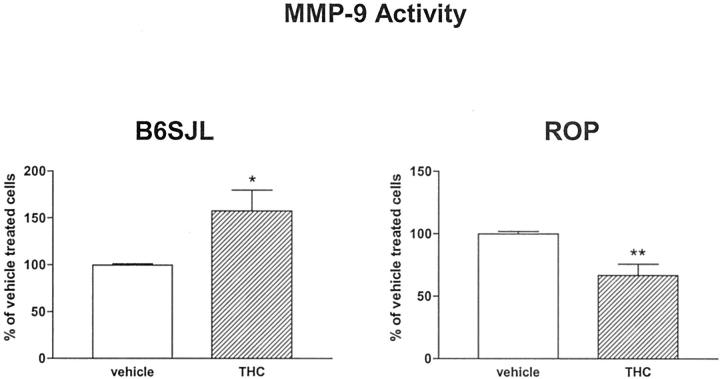
We used (R,R)-diethyl tetrahydrochrysene (THC), which is an ER-α agonist and ER-β-selective antagonist, 24 to examine MMP-9 regulation. THC treatment mimicked the effects of E2 on MMP-9 activity in MCs from both ROP and B6SJL mice. Namely, there was a significant decrease of MMP-9 activity in MCs isolated from the ROP strain after 24 hours of treatment with 1 μmol/L of THC (66.9 ± 8.9%), whereas under the same conditions MMP-9 activity was increased in MCs from B6SJL mice (157.5 ± 22.3%) (Figure 6) ▶ . These data suggest that the effects of E2 on MMP-9 activity are mediated by ER-α.
Figure 6.
E2 effects on MMP-9 activity are modulated by ER-α. Treatment of MCs with THC, an ER-α agonist, down-regulated MMP-9 activity in MCs isolated from GS-prone mice but up-regulated MMP-9 activity in GS-resistant MCs. MCs were grown for 3 days in phenol red-free DMEM/F12 supplemented with 20% charcoal-stripped FBS. The medium was replaced with phenol red-free DMEM/F12 medium supplemented with 0.1% charcoal-stripped FBS and THC (1 μmol/L) for 24 hours. Data are expressed as percentage of vehicle-treated MCs (0.001% EtOH, open bars) for each cell type and shown are means ± SEM of three independent experiments. Statistical significance is indicated by *, **, and *** (P < 0.05, P < 0.01, and P < 0.001, respectively) for comparison to vehicle-treated MCs.
Discussion
We studied the role of estrogens in the regulation of glomerular ECM deposition, by examining ER expression and regulation and resultant changes in collagen accumulation and MMP-9. We compared glomeruli and MC lines obtained from mice that were either GS-prone or GS-resistant, and found that glomeruli and MCs isolated from GS-prone mice expressed lower levels of ER-α and ER-β subtypes, than those isolated from GS-resistant mice. Using an estrogen-responsive reporter construct, we found that the nuclear ERs were transcriptionally active in MCs from both B6SJL and ROP mice. Both ER subtypes were expressed and regulated by estrogens in the MCs from B6SJL and ROP mice. E2 treatment down-regulated collagen accumulation and up-regulated MMP-9 mRNA and activity in MCs from GS-resistant B6SJL mice, whereas there were no changes in collagen accumulation and a down-regulation of MMP-9 in MCs from GS-prone ROP mice.
This study provides the first evidence that the susceptibility to GS is associated with decreased expression of both ER-α and ER-β mRNA and protein in glomeruli and MCs from mice with a GS-prone background. It also provides evidence that the decreased MMP-9 levels in response to E2 in GS-prone mice may favor ECM accumulation, including collagens.
Multiple lines of evidence suggest that there is a genetic propensity to develop GS in humans 25,26 and rats. 27 We reported that the development of GS also depends on genetic background in mice. 1,4,5 It has been shown that E2-dependent responses (such as uterine growth) are under genetic control and are organ-specific. 28,29 Thus, the genetic background may be a major determinant of E2 responsiveness.
MCs have a central role in the progression of GS and contribute to ECM accumulation by synthesizing collagens. 19 Decreased degradation of ECM has also been implicated as an important determinant of progressive GS. 2 MMP-9 belongs to a group of matrix-degrading enzymes that exhibit high activity against gelatin and native type IV collagen. 30 Increased MMP-9 levels may contribute to ECM degradation, which could have a protective role in the glomerulus. We previously found that E2 up-regulates the expression and activity of MMP-9 in MCs isolated from B6SJL mice. 9 In addition, we, in the present study, and others showed that estrogens regulate type I and IV collagen synthesis in MCs. 10,11 Therefore ECM components, including collagens and MMP-9, are important target genes for the estrogen’s effects in the mesangium.
We found that glomeruli and MCs isolated from GS-prone mice expressed lower levels of ER-α and ER-β subtypes, compared to those isolated from GS-resistant mice. Steroid hormone action is, in large part, controlled by the cellular receptor concentration. The receptor number is the limiting factor that dictates the magnitude of the steroid response. 31 For instance, in cell lines engineered to overexpress glucocorticoid receptor, there was a linear relationship between the amount of receptor and the transcriptional activation of target genes. 32 Similar studies of ER indicated that physiological levels of ER limit estrogen transcriptional activity well below the cellular capacity to respond to estrogens. 31 Thus, the expression levels of ER are important determinants of responsiveness to estrogens.
We found that physiological concentration of E2 down-regulated type I and type IV collagen accumulation in MCs from GS-resistant mice. E2 has been previously shown to suppress MC type I and type IV collagen synthesis. 10,11
We found that E2 regulated MMP-9 mRNA expression and activity in a dose-dependent manner. Both a selective ER modulator (tamoxifen) and the anti-estrogen (ICI) blocked the effect of E2 on MMP-9 activity. Thus, the regulation of MMP-9 by E2 in MCs was mediated by ER. Katzenellenbogen and colleagues 33 have developed ER ligands that are full agonists of ER-α and full antagonists of ER-β. We used THC, an ER-α agonist that is also an ER-β-selective antagonist. 24 THC regulation of MMP-9 activity was indistinguishable from that of E2, suggesting that the regulation of MMP-9 was primarily mediated by ER-α.
We found that the effects of E2 treatment depended on the genetic background. Namely, E2 treatment down-regulated type I and type IV collagen accumulation and up-regulated MMP-9 mRNA and activity in MCs from GS-resistant mice, whereas there was no change in collagen accumulation and a down-regulation of MMP-9 expression and activity in MCs from GS-prone ROP mice. Others have reported that in the pituitary the effects of estrogen on MMP-9 expression depend on the genetic background. Namely, estrogen was found to up-regulate MMP-9 expression in the pituitary in Fischer 344 rats, but not in Brown Norway rats. 34
Type I and type IV collagen promoters do not contain ERE, but the molecular mechanisms of collagens regulation by estrogens have been partly described by Neugarten and colleagues. 11,35 Namely, AP-1 activation through MAPK has been implicated in the down-regulation of collagen type I by E2 whereas down-regulation of type IV collagen by E2 seems to be mediated through a Sp1 site. 36 On the other hand, the molecular mechanisms by which estrogens regulate MMP-9 transcription in MCs are unknown. Differences in the functional expression of transcription factors as well as alterations in the structure of the MMP-9 promoter could be responsible for the opposite effects of estrogens on MMP-9 expression and activity. Comparable to collagen type I and type IV, the MMP-9 promoter does not contain a consensus ERE, but contains other important regulatory elements 37 including several AP-1 complexes and nuclear factor-κB that interact with ERs. 38,39 The proximal AP-1 complex mediates the transcriptional down-regulation of the MMP-9 promoter via c-fos, 40 whereas, at the same complex, c-jun increases MMP-9 expression at the transcriptional level. 41 This could represent a mechanism by which estrogens regulate differentially collagen type I and type IV and MMP-9 expression in MCs derived from GS-prone and GS-resistant mice.
In summary, we found that glomeruli and MCs isolated from GS-prone mice express lower levels of ER subtypes α and β than those isolated from GS-resistant mice and the transcription of both subtypes was up-regulated by estrogens. Importantly, E2 down-regulated MMP-9 in MCs from GS-prone mice while down-regulating collagen type I and type IV accumulation and up-regulating MMP-9 in MCs from GS-resistant mice. The regulation of MMP-9 was mediated by ER-α. These differences in the regulation of ECM components by E2 in MCs from GS-prone and GS-resistant mice translate into increased or reduced, respectively, accumulation of ECM in the mesangium, therefore GS progression.
This study provides the first evidence that glomeruli and MCs from mice that are prone to the development of GS have decreased expression of both ER-α and ER-β subtypes and that E2 down-regulates their expression of MMP-9. Thus, the lack of beneficial effect of E2 treatment in postmenopausal women from ethnic minority groups may be the result of both a reduced number of ERs and their aberrant response to estrogen replacement therapy.
Acknowledgments
We thank Dr. Shapiro for the 4ERE-TATA-Luc reporter constructs, Dr. Katzenellenbogen for the generous gift of the (R,R)-diethyl THC compound, and Ana Rivera and Kimberley Jaimes for technical assistance.
Footnotes
Address reprint requests to Liliane J. Striker, M.D., Vascular Biology Institute, University of Miami School of Medicine, P.O. Box 019132 (R104), Miami, FL 33101. E-mail: lstriker@med.miami.edu.
Supported by the National Institutes of Health (grants NIH/NIA R01 AG17170-01 to L. J. S. and NIH/NIA R01 AG19366-01 to G. E. S.), the American Heart Association (postdoctoral fellowship no. 0020544B to M. P.), the American Diabetes Association (Career Development Award to M. K.), the American Heart Association (grant-in-aid no. 0051513B to S. J. E.), and the Florida Department of Health (grant BM041 to S. J. E.).
References
- 1.He CJ, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ: Dissociation of glomerular hypertrophy, cell proliferation and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest 1996, 97:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenz O, Elliot SJ, Stetler-Stevenson WG: Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol 2000, 11:575-581 [DOI] [PubMed] [Google Scholar]
- 3.Nagase H: Activation mechanisms of matrix metalloproteinases. Biol Chem 1997, 378:151-160 [PubMed] [Google Scholar]
- 4.Zheng F, Striker G, Esposito C, Lupia E, Striker L: Strain differences rather than hyperglycemia determine the severity of glomerulosclerosis in mice. Kidney Int 1998, 54:1999-2007 [DOI] [PubMed] [Google Scholar]
- 5.Lenz O, Zheng F, Vilar J, Doublier S, Lupia E, Schwedler S, Striker LJ, Striker GE: The inheritance of glomerulosclerosis in mice is controlled by multiple quantitative trait loci. Nephrol Dial Transplant 1998, 13:3074-3078 [DOI] [PubMed] [Google Scholar]
- 6.Fornoni A, Lenz O, Tack I, Potier M, Elliot SJ, Striker LJ, Striker GE: Matrix accumulation in mesangial cells exposed to cyclosporine A requires a permissive genetic background. Transplantation 2000, 70:587-593 [DOI] [PubMed] [Google Scholar]
- 7.U.S.Renal Data System: USRDS 2000 Annual Data Report. Bethesda, The National Institutes of Health, NIDDK, 2000
- 8.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J: Mechanisms of estrogen action. Physiol Rev 2001, 81:1535-1565 [DOI] [PubMed] [Google Scholar]
- 9.Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M: Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 2001, 12:241-251 [DOI] [PubMed] [Google Scholar]
- 10.Lei J, Silbiger S, Ziyadeh FN, Neugarten J: Serum-stimulated alpha 1 type IV collagen gene transcription is mediated by TGF-beta and inhibited by estradiol. Am J Physiol 1998, 274:F252-F258 [DOI] [PubMed] [Google Scholar]
- 11.Silbiger S, Lei J, Neugarten J: Estradiol suppresses type I collagen synthesis in mesangial cells via activation of activator protein-1. Kidney Int 1999, 55:1268-1276 [DOI] [PubMed] [Google Scholar]
- 12.Peten EP, Garcia-Perez A, Terada Y, Woodrow D, Martin BM, Striker GE, Striker LJ: Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol 1992, 263:F951-F957 [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 14.Conti FG, Striker LJ, Lesniak MA, MacKay K, Roth J, Striker GE: Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology 1988, 122:2788-2795 [DOI] [PubMed] [Google Scholar]
- 15.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS: Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 1986, 83:2496-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattick S, Glenn K, de Haan G, Shapiro DJ: Analysis of ligand dependence and hormone response element synergy in transcription by estrogen receptor. J Steroid Biochem Mol Biol 1997, 60:285-294 [DOI] [PubMed] [Google Scholar]
- 17.Roy D, Angelini NL, Belsham DD: Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology 1999, 140:5045-5053 [DOI] [PubMed] [Google Scholar]
- 18.Skynner MJ, Sim JA, Herbison AE: Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 1999, 140:5195-5201 [DOI] [PubMed] [Google Scholar]
- 19.Lenz O, Striker LJ, Jacot TA, Elliot SJ, Killen PD, Striker GE: Glomerular endothelial cells synthesize collagens but little gelatinase A and B. J Am Soc Nephrol 1998, 9:2040-2047 [DOI] [PubMed] [Google Scholar]
- 20.Jacot TA, Striker GE, Stetler-Stevenson MA, Striker LJ: Mesangial cells from transgenic mice with progressive glomerulosclerosis exhibit stable, phenotypic changes including undetectable MMP-9 and increased type IV collagen. Lab Invest 1996, 75:791-799 [PubMed] [Google Scholar]
- 21.Leber TM, Balkwill FR: Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem 1997, 249:24-28 [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Stetler-Stevenson WG: Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 1994, 218:325-329 [DOI] [PubMed] [Google Scholar]
- 23.Bustin SA: Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000, 25:169-193 [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS: Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-alpha or estrogen receptor-beta. Endocrinology 1999, 140:800-804 [DOI] [PubMed] [Google Scholar]
- 25.Doria A, Warram JH, Krolewski AS: Genetic susceptibility to nephropathy in insulin-dependent diabetes: from epidemiology to molecular genetics. Diabetes Metab Rev 1995, 11:287-314 [DOI] [PubMed] [Google Scholar]
- 26.Striker GE, Peten EP, Carome MA, Pesce CM, Schmidt K, Yang CW, Elliot SJ, Striker LJ: The kidney disease of diabetes mellitus (KDDM): a cell and molecular biology approach. Diabetes Metab Rev 1993, 9:37-56 [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ: Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 1996, 12:44-51 [DOI] [PubMed] [Google Scholar]
- 28.Roper RJ, Griffith JS, Lyttle CR, Doerge RW, McNabb AW, Broadbent RE, Teuscher C: Interacting quantitative trait loci control phenotypic variation in murine estradiol-regulated responses. Endocrinology 1999, 140:556-561 [DOI] [PubMed] [Google Scholar]
- 29.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M: Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 1999, 285:1259-1261 [DOI] [PubMed] [Google Scholar]
- 30.Mott JD, Khalifah RG, Nagase H, Shield CF, Hudson JK, Hudson BG: Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int 1997, 52:1302-1312 [DOI] [PubMed] [Google Scholar]
- 31.Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ: The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol 1992, 6:157-167 [DOI] [PubMed] [Google Scholar]
- 32.Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR: Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol 1987, 1:68-74 [DOI] [PubMed] [Google Scholar]
- 33.Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA: Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol 2000, 74:279-285 [DOI] [PubMed] [Google Scholar]
- 34.Sclafani RV, Wendell DL: Suppression of estrogen-dependent MMP-9 expression by Edpm5, a genetic locus for pituitary tumor growth in rat. Mol Cell Endocrinol 200, 1176:145-153 [DOI] [PubMed] [Google Scholar]
- 35.Neugarten J, Medve I, Lei J, Silbiger SR: Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am J Physiol 1999, 277:F875-F881 [DOI] [PubMed] [Google Scholar]
- 36.Zdunek M, Silbiger S, Lei J, Neugarten J: Protein kinase CK2 mediates TGF-beta1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int 2001, 60:2097-2108 [DOI] [PubMed] [Google Scholar]
- 37.Munaut C, Salonurmi T, Kontusaari S, Reponen P, Morita T, Foidart JM, Tryggvason K: Murine matrix metalloproteinase 9 gene. 5′-upstream region contains cis-acting elements for expression in osteoclasts and migrating keratinocytes in transgenic mice. J Biol Chem 1999, 274:5588-5596 [DOI] [PubMed] [Google Scholar]
- 38.Cerillo G, Rees A, Manchanda N, Reilly C, Brogan I, White A, Needham M: The oestrogen receptor regulates NFkappaB and AP-1 activity in a cell-specific manner. J Steroid Biochem Mol Biol 1998, 67:79-88 [DOI] [PubMed] [Google Scholar]
- 39.McKay LI, Cidlowski JA: Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev 1999, 20:435-459 [DOI] [PubMed] [Google Scholar]
- 40.Crowe DL, Brown TN: Transcriptional inhibition of matrix metalloproteinase 9 (MMP-9) activity by a c-fos/estrogen receptor fusion protein is mediated by the proximal AP-1 site of the MMP-9 promoter and correlates with reduced tumor cell invasion. Neoplasia 1999, 1:368-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowe DL, Tsang KJ, Shemirani B: Jun N-terminal kinase 1 mediates transcriptional induction of matrix metalloproteinase 9 expression. Neoplasia 2001, 3:27-32 [DOI] [PMC free article] [PubMed] [Google Scholar]